Abstract
Infection with Helicobacter pylori induces humoral immune responses against various antigens of the bacterium. Heat shock proteins (hsps) are immunodominant antigens in various diseases including H. pylori infection. In the present study, we measured the anti-hsp antibody titers in 42 patients with H. pylori-infected peptic ulcers during a bacterial eradication study. The patients were treated with a proton pump inhibitor and antimicrobial agents to eradicate the organism. Their sera were obtained at pretreatment and at 1 month and 6 months after the eradication therapy. The titers of immunoglobulin G antibodies to the H. pylori hsp, whole-cell lysate, and urease (30-kDa subunit) antigens in serum were measured by a capture enzyme-linked immunosorbent assay. The levels of H. pylori hsp60 antibodies in sera collected 1 month after treatment had declined significantly, even when changes in the titers of antibodies to whole-cell and urease antigens were not apparent. These results suggest that measurement of antibodies to H. pylori hsp60 in serum is useful for the early monitoring of the effectiveness of eradication therapy.
Helicobacter pylori is associated with gastritis and peptic ulcer disease in humans. H. pylori infection induces the host's constitutional immune response against various antigens of this bacterium. The detection of immunoglobulin G (IgG) antibodies to H. pylori is useful for the diagnosis of infection. Some investigators reported that the titers of these antibodies declined during therapy for H. pylori eradication (1, 12, 14, 15, 17, 18). Kosunen (13) reported that a consistent decrease in the IgG antibody titer within 6 months of antimicrobial therapy reliably indicated the eradication of H. pylori (13). However, a serological test that can be used to judge the success of treatment earlier in the follow-up period has not yet been established. In this study we measured the titers of IgG antibodies to the heat shock protein (hsp) hsp60, urease, and whole-cell lysates of H. pylori in sera from patients with peptic ulcer during antimicrobial treatment of H. pylori and then assessed its usefulness for the monitoring of eradication therapy.
MATERIALS AND METHODS
Patients studied.
We investigated 20 subjects with gastric ulcer (GU) (17 men and 3 women; age range, 35 to 74 years; mean age, 52 years) and 17 subjects with duodenal ulcer (DU) (13 men and 4 women; age range, 22 to 51 years; mean age, 36.6 years). All patients underwent gastroduodenoscopy because of gastrointestinal symptoms. Examinations were performed in the First Department of Internal Medicine, Okayama University School of Medicine, and its affiliated hospitals. At the initial diagnostic endoscopy, all patients were diagnosed as having a peptic ulcer.
Status of H. pylori infection.
H. pylori infection status was evaluated by bacterial culture, measurement of urease activity, and histologic analysis. A patient was judged to be H. pylori positive if culture and/or histologic analysis of specimens retrieved endoscopically was positive for the organism; a patient was classified H. pylori negative if culture, the urease test, and histologic analysis were negative. A weakly positive urease test was not considered sufficient for the diagnosis of infection.
Antimicrobial therapy.
After informed consent was obtained, the patients were treated with dual therapy (2-week course of omeprazol at 40 mg orally twice daily and amoxicillin at 1,500 mg orally twice daily). At 1 month and 6 months after the treatment, the patients underwent endoscopic examination, and biopsies were performed to evaluate the patient's H. pylori infection status. At the same time, serum samples were taken and were stored at −30°C until they were assayed.
Preparation of antigen and antibodies.
H. pylori (ATCC 43504) was cultured in brucella broth with 7% horse serum. The cells were harvested by centrifugation (6,000 × g, 20 min) and were washed twice with phosphate-buffered saline (PBS; pH 7.4). The precipitate was resuspended in ice-cold distilled water containing 4 mM phenylmethylsulfonyl fluoride and was disrupted with a sonicator. The broken cells were separated by centrifugation (20,000 × g, 30 min), and its supernatant was designated 20S. The 20S supernatant was further centrifuged at 100,000 × g for 30 min to remove the cytoplasmic membrane fraction, and its supernatant was designated 100S. The 100S supernatant was used as the antigen in a capture enzyme-linked immunosorbent assay (ELISA).
The 66-kDa (hsp) and 30-kDa (urease α or A subunit) proteins were separated from the 20S antigen by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were electroeluted with an Elutrap apparatus (Schleicher & Schuell, Dassel, Germany). The identity of the eluted proteins was checked by N-terminal sequencing (20), and the concentrations were determined by measuring the absorbance at 280 nm. Rabbits were immunized with the eluted proteins and the 20S antigens in Freund's incomplete adjuvant to obtain antibodies against hsp, urease, and whole-cell lysates of H. pylori. Rabbit IgGs were purified with a DEAE-Sephacel column (Pharmacia, Uppsala, Sweden).
Antigen-capture ELISA for detection of antibodies in serum.
Antibodies against H. pylori-specific proteins in serum were detected by an antigen-capture ELISA. In brief, 96-well plates (Costar, Cambridge, Mass.) were coated with rabbit IgG to the hsp (5 μg) or urease (5 μg) of H. pylori in 100 μl of 0.1 M carbonate-bicarbonate buffer (pH 9.6) overnight at 4°C. The wells were washed twice in PBS containing 0.05% Tween 20 (pH 7.4) and were blocked with PBS containing 10% skim milk (skim milk-PBS). After the wells were washed they were incubated with 5 μg of soluble antigen (100S supernatant) per 100 μl for 1 h at room temperature. Wells for the assay of hsp and urease were incubated with 100 μl of the patient's serum diluted 1:200 in skim milk-PBS. After the wells were washed they were incubated with peroxidase-conjugated rabbit anti-human IgG (specific to gamma chains; lot 115; DAKO Inc., Glostrup, Denmark) and then with o-phenylenediamine (Wako Pure Chemicals, Tokyo, Japan). Absorbances at 490 nm were measured with a NOVAPATH microplate reading spectrophotometer (Bio-Rad, Hercules, Calif.).
The patients' levels of antibodies against H. pylori whole cells were also obtained by capture ELISA with an anti-H. pylori whole-cell antibody. The wells were coated with 1 μg of anti-whole-cell IgG to catch various antigens of the bacterium and were blocked with skim milk-PBS. The wells were incubated with 100 μl of the patient's serum diluted 1:1,000 in skim milk-PBS. Continuous reactions were done in the same way as described above.
Histopathology.
Formalin-fixed and paraffin-embedded biopsy specimens were stained with hematoxylin-eosin and were examined to grade the severity of gastritis. All slides with biopsy specimens were examined by a single pathologist. Gastritis was classified according to the Sydney System (2, 16), and its activity was graded on a scale of 0 to 3 as follows, depending on the intensity of neutrophilic infiltration: 0, normal; 1, mild gastritis; 2, moderate gastritis; and 3, highly active gastritis.
Statistical analysis.
Data are expressed means ± standard errors of the means. The titers of IgG antibody to Hsp, whole-cell lysate, and urease of H. pylori were analyzed statistically by one-way analysis of variance followed by Scheffe's S procedure. Pathological parameters were assessed by the Friedman test and the Dunn procedure and by regression analysis. P values of <0.05 were considered statistically significant.
RESULTS
Characterization of antibodies.
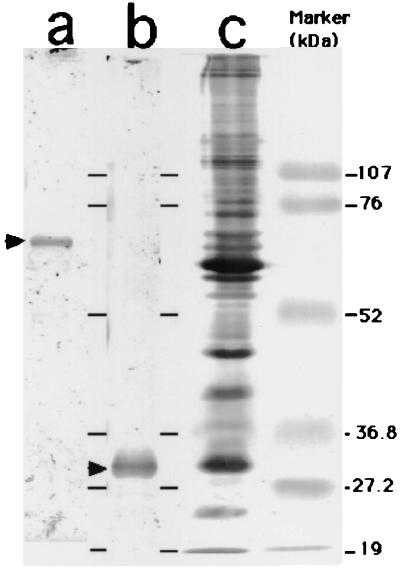
Rabbit IgG antibodies against hsp, urease, and whole-cell lysates were characterized by Western blotting (Fig. 1). Rabbit sera with anti-hsp and antiurease antibodies reacted only with the hsp and urease of the H. pylori 20S antigen, respectively. Sera with anti-whole-cell antibodies recognized many H. pylori proteins. These sera showed no cross-reactivity with Escherichia coli, Campylobacter jejuni, Campylobacter fetus, or Campylobacter coli antigens.
FIG. 1.
Western blotting of IgG antibodies to anti-H. pylori hsp (lane a), urease α subunit (lane b), and whole-cell lysate (lane c).
Reactivities of patients' sera by capture ELISA.
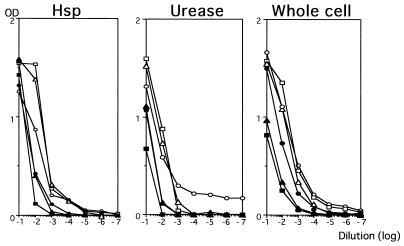
Capture ELISAs were assessed by determining the reactivities of serially diluted sera obtained from three H. pylori-infected patients or three H. pylori-negative individuals (Fig. 2). According to the dilution curve thus obtained, we determined that a 1:200 serum dilution should be used for measurement of titers of antibodies against hsp and urease and that a serum dilution of 1:1,000 should be used for measurement of titers of antibodies against whole-cell lysates. The specificities and sensitivities of three capture ELISAs were calculated for the sera obtained from the patients pretreatment and the sera obtained from the patients 6 months after eradication therapy. Cutoff optical densities (ODs) were determined as the means plus standard deviations for the sera obtained 6 months after therapy. Cutoff ODs of 0.12, 0.20, and 0.67 were chosen for hsp, urease, and whole cells, respectively. The capture ELISAs showed high sensitivities but low specificities (Table 1).
FIG. 2.
Dilution curves for sera from three H. pylori-positive patients (open symbol) and three H. pylori-negative individuals (closed symbol).
TABLE 1.
Sensitivities and specificities of capture ELISAs
| Antigen | Antibody level (mean ± SD) at:
|
Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|
| Pretreatment | 6 mo after treatment | |||
| hsp | 0.118 ± 0.098 | 0.056 ± 0.057 | 86 | 76 |
| Urease | 0.181 ± 0.100 | 0.118 ± 0.076 | 84 | 57 |
| Whole cell | 0.712 ± 0.649 | 0.285 ± 0.349 | 81 | 68 |
Histopathology.
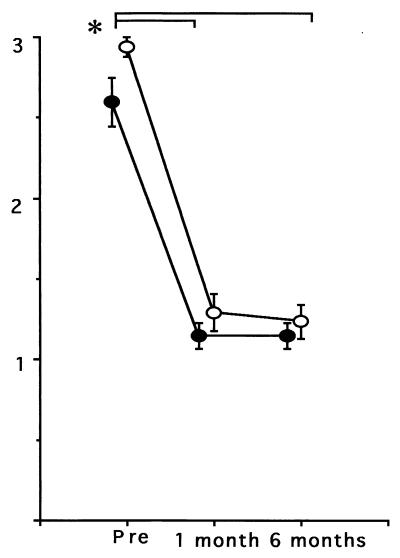
For 14 (70%) of 20 GU patients, the degree of severity of gastritis was judged to be high. Only 4 (20%) and 2 (10%) patients had gastritis of grades 2 and 1, respectively. Histopathological findings for 15 (94%) of 16 of the patients with DUs were assessed as grade 3 (highly active gastritis) before treatment. Histological grades (mean ± standard errors) before eradication therapy were 2.60 ± 0.15 (patients with GU) and 2.94 ± 0.06 (patients with DU). After 1 month of eradication therapy, no patient had gastritis of grade 3. Histological grades were significantly decreased in both patients with GUs (1.15 ± 0.08) and patients with DUs (1.29 ± 0.11). The grades after 6 months of treatment were 1.15 ± 0.08 (patients with GUs) and 1.23 ± 0.11 (patients with DUs) (Fig. 3). A regression analysis was conducted for histological grades and antibody titers. The titers of antibodies to hsp significantly (P = 0.001; R = 0.30) correlated with histological grades; however, correlations between titers of antibodies to urease (P = 0.07; R = 0.15), titers of antibodies to whole cells (P = 0.08; R = 0.23), and histological grades were not statistically significant.
FIG. 3.
Decline in histological grades (mean ± standard error) after successful eradication of H. pylori from patients with GUs (closed circle) and DUs (open circle). ∗, P < 0.05; pre, pretreatment.
Antibody levels.
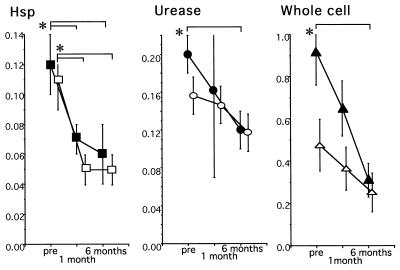
The antibody levels (means ± standard errors of the means) for patients with GUs and DUs during treatment are shown in Fig. 4. Antibody levels were expressed as absorbances at 490 nm. Anti-hsp antibody levels were significantly decreased in both GU (P = 0.047) and DU (P = 0.05) patients 1 month after treatment and remained decreased in GU (P = 0.031) and DU (P = 0.005) patients 6 months after treatment. In contrast, anti-whole-cell and antiurease antibody levels in GU and DU patients were not significantly decreased 1 month after treatment. Levels of antibodies to H. pylori whole cells and urease for both GU patients were significantly decreased at 6 months and were lower than the levels of antibodies to whole cells at 6 months for DU patients, but the differences were not significant.
FIG. 4.
Antibody levels (mean ± standard error) after eradication in patients with GUs (closed symbols) and DUs (open symbols). ∗, P < 0.05; pre, pretreatment.
DISCUSSION
This study has shown that anti-hsp antibody titers were significantly decreased at an early stage of eradication therapy in patients with either GUs or DUs. On the other hand, it took 6 months before anti-whole-cell and antiurease antibody titers decreased. Perez-Perez et al. (14) reported that the levels of IgG antibody to hsp of H. pylori in serum were significantly higher in infected patients and that the titer was correlated with the degree of gastric mucosal inflammation. Local IgA levels were correlated with inflammatory cell infiltration of the gastric mucosa (8). We previously reported that H. pylori hsp60 was a dominant protein and was mainly induced by heat shock treatment (20). Furthermore, some reports indicate that hsp60 is located on the surface of the bacteria (3, 4, 5, 7, 9, 19). These data indicate that hsp60 develops a tendency to be recognized by the host and may be closely related to H. pylori-induced inflammation.
The decrease in inflammation in the gastric mucosa of successfully treated patients showed a correlation with a decrease in the level of anti-hsp antibodies. Clearly, eradication of H. pylori results in the cure of gastritis and peptic ulcers. However, the relationship between inflammation and mucosal injury is not clear. hsps were detected in H. pylori-infected human gastric epithelial cells (6). Engstrand and colleagues (7) suggested that gastric γ/δ T cells were involved in autoimmunity elicited by bacterial infection and cross-reacted to autologous hsps from stressed epithelial cells and that they may play a role in H. pylori-associated gastric epithelial injuries. We recently reported that hsp60 might be involved with T-cell proliferation in gastric lymphoid follicles of patients with mucosa-associated lymphoid tissue (MALT) lymphoma and that antibody to hsp was detected in patients with MALT lymphoma (10, 11). Transplantation of lymphocytes from H. pylori-infected MALT lymphoma patients into severe combined immunodeficient mice induced ulceration in the stomachs of the mice (21). In several infections and autoimmune diseases, hsp represents the dominant antigen in the humoral and cellular immune responses (22). Therefore, characterization of the immune responses to hsp may contribute to the elucidation of the mechanism of H. pylori-related gastric mucosal injury.
In conclusion, our data suggest that measurement of anti-hsp antibody levels is useful for the early monitoring of the effectiveness of H. pylori eradication therapy. Moreover, we postulate that hsp may be involved in the immune reactions responsible for H. pylori-infected gastric mucosal injury.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the Ministry of Education, Science, Sport and Culture, Japan and by Otsuka Pharmaceutical Co. Ltd.
REFERENCES
- 1.Cutler A F, Prasad V M. Long-term follow-up of Helicobacter pylori serology after successful eradication. Am J Gastroenterol. 1996;91:85–88. [PubMed] [Google Scholar]
- 2.Dixon M F, Genta R M, Yardly J H, Correa P. Classification and grading of gastritis. Am J Sur Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Dunn B E, Roop R M D, Sung C C, Sharma S A, Perez-Perez G I, Blaser M J. Identification and purification of a cpn60 heat shock protein homolog from Helicobacter pylori. Infect Immun. 1992;60:1946–1951. doi: 10.1128/iai.60.5.1946-1951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn B E. Pathogenic mechanisms of Helicobacter pylori. Gastroenterol Clin N Am. 1993;22:43–57. [PubMed] [Google Scholar]
- 5.Dunn B E, Vakil N B, Schneider B G, Miller M M, Zitzer J B, Peutz T, Phadnis S H. Localization of Helicobacter pylori urease and heat shock protein in human gastric biopsies. Infect Immun. 1997;65:1181–1188. doi: 10.1128/iai.65.4.1181-1188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engstrand L, Scheynius A, Pahlson C, Grimelius L, Schwan A, Gustavsson S. Association of Campylobacter pylori with induced expression of class II transplantation antigens on gastric epithelial cells. Infect Immun. 1989;57:827–832. doi: 10.1128/iai.57.3.827-832.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engstrand L, Scheynius A, Pahlson C. An increased number of γ/δ T-cells and gastric epithelial cell expression of the groEL stress-protein homologue in Helicobacter pylori-associated chronic gastritis of the antrum. Am J Gastroenterol. 1991;86:976–980. [PubMed] [Google Scholar]
- 8.Hayashi S, Sugiyama T, Yokota K, Isogai H, Isogai E, Oguma K, Asaka M, Fujii N, Hirai Y. Analysis of immunoglobulin A antibodies to Helicobacter pylori in serum and gastric juice in relation to mucosal inflammation. Clin Diagn Lab Immunol. 1998;5:617–621. doi: 10.1128/cdli.5.5.617-621.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huesca M, Borgia S, Hoffman P, Lingwood C A. Acidic pH changes receptor binding specificity of Helicobacter pylori: a binary adhesion model in which surface heat shock (stress) proteins mediate sulfatide recognition in gastric colonization. Infect Immun. 1996;64:2643–2648. doi: 10.1128/iai.64.7.2643-2648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawahara Y, Yokota K, Mizuno M, Yunoki N, Uetsu T, Okada H, Kobayashi K, Hirai Y, Oguma K, Tsuji T. Antibodies to human gastric epithelial cell and heat shock protein 60 in Helicobacter pylori positive mucosa associated lymphoid tissue lymphoma. Gut. 1999;45:20–23. doi: 10.1136/gut.45.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi K, Yokota K, Yoshino T, Kawahara Y, Dey A, Hirai Y, Oguma K, Akagi T. Detection of Helicobacter pylori associated antigen and heat shock protein 60 on follicular dendritic cells in the germinal centres of low grade B cell lymphoma of gastric mucosa associated lymphoid tissue (MALT) J Clin Pathol. 1998;51:396–398. doi: 10.1136/jcp.51.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosunen T U, Seppala K, Sarna S, Sipponen P. Diagnostic value of decreasing IgG, IgA, and IgM antibody titres after eradication of Helicobacter pylori. Lancet. 1992;339:893–895. doi: 10.1016/0140-6736(92)90929-w. [DOI] [PubMed] [Google Scholar]
- 13.Kosunen T U. Antibody titres in Helicobacter pylori infection: implications in the follow-up of antimicrobial therapy. Ann Med. 1995;27:605–607. doi: 10.3109/07853899509002477. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Perez G I, Brown W R, Cover T L, Dunn B E, Cao P, Blaser M J. Correlation between serological and mucosal inflammatory responses to Helicobacter pylori. Clin Diagn Lab Immunol. 1994;1:325–329. doi: 10.1128/cdli.1.3.325-329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Perez G I, Cutler A F, Blaser M J. Value of serology as a noninvasive method for evaluating the efficacy of treatment of Helicobacter pylori infection. Clin Infect Dis. 1997;25:1038–1043. doi: 10.1086/516089. [DOI] [PubMed] [Google Scholar]
- 16.Price A B. The Sydney System. Histological division. J Gastroenterol Hepatol. 1991;6:209–222. doi: 10.1111/j.1440-1746.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 17.Veenendaal R A, Pena A S, Meijer J L, Endtz H P, van der Est M M C, van Duijn W, Eulderink F, Kreuning J, Lamers C B H W. Long term serological surveillance after treatment of Helicobacter pylori infection. Gut. 1991;32:1291–1294. doi: 10.1136/gut.32.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W M, Chen C Y, Jan C M, Chen L T, Perng D S, Lin S R, Liu C S. Long-term follow-up and serological study after triple therapy of Helicobacter pylori-associated duodenal ulcer. Am J Gastroenterol. 1994;89:1793–1796. [PubMed] [Google Scholar]
- 19.Yamaguchi H, Osaki T, Taguchi H, Hanawa T, Yamamoto T, Kamiya S. Flow cytometric analysis of the heat shock protein 60 expressed on the cell surface of Helicobacter pylori. J Med Microbiol. 1996;45:270–277. doi: 10.1099/00222615-45-4-270. [DOI] [PubMed] [Google Scholar]
- 20.Yokota K, Hirai Y, Haque M, Hayashi S, Isogai H, Sugiyama T, Nagamachi E, Tsukada Y, Fujii N, Oguma K. Heat shock protein produced by Helicobacter pylori. Microbiol Immunol. 1994;38:403–405. doi: 10.1111/j.1348-0421.1994.tb01799.x. [DOI] [PubMed] [Google Scholar]
- 21.Yokota K, Kobayashi K, Kawahara Y, Hayashi S, Hirai Y, Mizuno M, Okada H, Akagi T, Tsuji T, Oguma K. Gastric ulcers in SCID mice induced by Helicobacter pylori infection after transplanting lymphocytes from patients with gastric lymphoma. Gastroenterology. 1999;117:893–899. doi: 10.1016/s0016-5085(99)70348-3. [DOI] [PubMed] [Google Scholar]
- 22.Zugel U, Kaufmann H E S. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]