Abstract
We examined the progressive and irreversible loss of antigen-specific lymphoproliferative responses in peripheral blood mononuclear cells (PBMC) obtained from blood exposed for prolonged periods to EDTA as an anticoagulant. The responses of these lymphocytes to interleukin-2 or to concanavalin A were, however, unaffected. The observed loss was not due to depletion of metal ions by EDTA, since the addition of several divalent cations to whole blood during storage in EDTA or to lymphocytes from EDTA-stored blood during antigen stimulation in vitro did not alleviate the defect. Reconstitution of antigen-specific T-cell lines or Percoll-purified T cells with adherent antigen-presenting cells in antigen stimulation assays revealed that the presenting cells and not the effector T-cells were the targets of EDTA-mediated damage. The anticoagulant heparin helped to circumvent this problem. Surprisingly, EGTA, another metal ion chelator, could successfully replace EDTA, with a marginal loss in antigen-specific responses. Lymphoproliferative responses to antigens of Japanese encephalitis virus (JEV) and Mycobacterium tuberculosis were both significantly preserved in EGTA. JEV antigen-specific responses of PBMC obtained from the blood of convalescent JEV patients and stored in EGTA for as long as 24 h (n = 20) were comparable to those of fresh PBMC (n = 10), while PBMC from blood stored in EDTA (n = 17) for 16 h or longer failed to respond. We recommend that EGTA be used as the anticoagulant of choice for applications that require the lymphocyte proliferation assay, especially when on-site testing facilities are not available.
Human immune responses to specific antigens have been successfully studied with peripheral blood mononuclear cells (PBMC), which comprise B and T lymphocytes, monocytes, natural killer cells, and dendritic cells. Currently used anticoagulants include EDTA, heparin, and acid-citrate-dextrose (ACD). EDTA works as an anticoagulant by chelating metal ions; it is the reagent of choice, since it preserves cellular integrity (10), and is particularly recommended for achieving high yields of monocytes (2). However, blood stored in EDTA for more than a few hours yields PBMC contaminated with red cells and granulocytes (11). Heparinized blood, while avoiding this problem, nevertheless causes platelet aggregation and changes the white cell morphology (3). This platelet clumping in turn interferes with applications such as flow cytometric analysis of PBMC samples.
In our efforts to study antigen-specific T-cell responses, we have encountered a progressive loss of proliferative responses in PBMC obtained from blood stored in EDTA for increasing lengths of time. Storage of blood with EDTA for 2 days was reported to cause alterations in the proportion of lymphocyte subsets (11, 12). Earlier studies also described EDTA-mediated inhibition of phytohemagglutinin (PHA)-stimulated blast transformation of human PBMC (1). In these studies, the cells were continuously maintained in cultures in the presence of EDTA. A study of lymphocyte proliferative responses of whole blood and separated PBMC to different stimulants after overnight storage of blood in heparin, ACD, and citrate cell preparation tubes revealed varied responses, depending on the antigen used as well as the human immunodeficiency virus (HIV) status of the individual (23). In that study, however, responses to PHA were not significantly affected.
Here we report the irreversible loss of antigen-specific but not mitogen-induced responses in PBMC obtained from blood stored in EDTA for 24 h, although no EDTA was present during the 4 days of culturing with antigen. The paucity of metal ions following exposure to EDTA was not the cause of the defect, since EGTA did not do any damage to antigen-specific responses. In addition, metal ions added to the cultures failed to restore the functional competence of the affected cells. We show conclusively that EDTA damages antigen-presenting cells but not effector T cells.
MATERIALS AND METHODS
Study population and experimental design.
Ten milliliters of blood was drawn from volunteers after informed consent was obtained; 4 mM (0.15% [wt/vol]) EDTA or EGTA or 5 U of heparin per ml was used as an anticoagulant. Blood was either processed immediately or stored for up to 24 h at room temperature prior to isolation of PBMC.
For studying the response to Mycobacterium tuberculosis antigen, PBMC from healthy purified protein derivative (PPD)-positive volunteers (n = 9) with a strong response to this antigen were used. For studies with viral antigen, convalescent Japanese encephalitis virus (JEV) patients (n = 47), 3 to 10 years old, whose blood samples were collected 3 months to 1 year after the onset of the disease (when maximal JEV antigen-specific responses are normally detected), constituted the study population. All the JEV patients had developed clinical encephalitis, confirmed by the presence of anti-JEV immunoglobulin M titers in cerebrospinal fluid at the time of admission to the hospital with clinical symptoms of encephalitis (determined by the MAC-ELISA [15]). The blood drawn from JEV patients was either processed immediately (n = 10) or stored in EDTA (n = 17) or EGTA (n = 20) as an anticoagulant and transported at room temperature from areas in which JEV is endemic to the laboratory for processing (16 to 24 h after collection). Owing to the very small volume of blood that could be obtained from these children, the three groups necessarily consisted of nonoverlapping individuals. Controls included healthy age-matched individuals (n = 8) from the same areas who had never suffered a JEV infection and who were serologically negative for antibodies to the virus.
Cell preparation.
Blood was diluted with an equal volume of phosphate-buffered saline, layered over an equal volume of Ficoll-Hypaque (Pharmacia), and centrifuged in a table-top centrifuge with swing-out buckets at 900 × g for 30 min. The PBMC banding below the plasma were collected, washed multiple times with phosphate-buffered saline, and suspended in RPMI 1640 (Life Technologies-BRL) containing 2 mM l-glutamine, 10% human AB serum, 50 μg of streptomycin per ml, and 50 U of penicillin per ml. Cells were cultured at 0.5 × 105 cells per well for M. tuberculosis antigen and 1 × 105 cells per well for JEV antigen in 96-well flat-bottom plates (Costar). We routinely obtained 1 × 106 to 2 × 106 PBMC/ml of blood. Storing blood in the various anticoagulants for as long as 24 h resulted in no significant differences in the absolute numbers of viable lymphocytes obtained, as measured by trypan blue exclusion.
Antigen preparation and lymphocyte proliferation assays.
Mycobacterial antigen was prepared by sonicating M. tuberculosis cells for a total of 15 min, followed by centrifugation for 30 min at 10,000 × g to remove insoluble material. The clear supernatant was filtered through a 0.2-μm-pore-size filter. The viral antigen used was glutaraldehyde-fixed lysates of JEV-infected Vero cells prepared as described previously (8).
PBMC from PPD-positive volunteers were stimulated either with 1 μg of mycobacterial sonicate antigen per well or with 2 μg of concanavalin A (ConA; Sigma) per ml for 4 days. Interleukin-2 (IL-2; Boehringer Mannheim Biochemicals) was used at 20 U/ml. Divalent cations Ca2+, Mg2+, and Zn2+ were added to whole blood at concentrations of 4, 8, and 0.16 mM, respectively. Ca2+, Mg2+, Zn2+, and Fe2+ were used at 2, 0.8, 0.1, and 0.2 mM, respectively, when added singly or in combination to PBMC cultures during the 4-day antigen stimulation period. These metal ion concentrations were chosen based on previous reports (1, 18) as well as on our requirement to neutralize the EDTA concentration present. Contents of wells were pulsed with 0.5 μCi of 3H-thymidine (NEN-Dupont), harvested after 18 h, and counted in an LKB Rack Beta liquid scintillation counter. The results obtained for each volunteer were expressed either as the mean stimulation index (SI), obtained by dividing the average counts per minute incorporated by antigen-stimulated PBMC in triplicate wells by that incorporated by unstimulated PBMC, or the mean counts per minute obtained in three independent experiments ± the standard error of the mean (SEM).
JEV antigen at a 1:50 dilution of the antigen preparation was used in assays with PBMC obtained from convalescent JEV patients and corresponding control individuals (8). This concentration did not stimulate the proliferation of PBMC from seronegative donors. Uninfected Vero cell lysates treated similarly served as the control antigen. Wells were pulsed with radiolabel after 5 days of incubation with antigen. The SI was calculated for each individual by dividing the average counts per minute incorporated in triplicate wells by that in wells stimulated with control antigen at the same concentration.
Reconstitution of adherent antigen-presenting cells with Percoll-purified lymphocytes or T-cell lines.
PBMC obtained from PPD-positive individuals by the Ficoll-Hypaque method of density gradient centrifugation were separated on a preformed continuous Percoll (Sigma) gradient in order to obtain separate populations of monocytes and lymphocytes as described previously (4). Briefly, the leukocytes (20 × 106) were layered onto preformed Percoll gradients in 15-ml polycarbonate tubes and spun in swing-out buckets in a refrigerated centrifuge at 1,000 × g for 20 min. The cells from the two bands obtained were collected separately using sterile Pasteur pipettes and were found to be enriched for monocytes (upper band, 86%) and lymphocytes (lower band, 81%) by fluorescence-activated cell sorter analysis. Monocytes were seeded at 105 cells/well, washed to remove nonadherent cells, and reconstituted with lymphocytes at 1.5 × 105 cells/well, followed by the addition of mycobacterial antigen. The inability of either of these two populations to proliferate in response to ConA treatment indicated that the level of purity obtained was adequate for our experiments.
To obtain T-cell lines, PBMC from PPD-positive individuals were stimulated for 5 days with mycobacterial sonicate antigen (5 μg/ml), followed by the addition of IL-2 at 20 U/ml. The cells were cultured for an additional 10 days before being used in antigen stimulation assays at 104 cells per well of a 96-well plate with or without prior exposure to EDTA for 24 h. Adherent cells were obtained from PBMC as follows: 0.5 × 106 PBMC in RPMI 1640 containing 2% human AB serum were seeded per well of a 96-well plate. One hour later, nonadherent cells were removed by repeated washing of the wells with medium without serum, followed by the addition of T-cell lines and antigen. The results were expressed as the mean counts per minute ± SEM of triplicate cultures.
Statistical analysis.
In the experiment with convalescent JEV patients, positive responses to viral antigen were scored based on the criteria that (i) the SI was equal to or greater than 2.4, since the maximum value of the proliferative response induced in PBMC of control individuals plus 1.96 times the standard deviation of the mean was 2.388, and (ii) the total counts per minute observed on stimulation with viral antigen measured 1,500 or more. The calculation of the SEM for M. tuberculosis and JEV antigens has already been described above. The SIs for both bacterial and viral antigens were logarithmically transformed (21), and significance values were calculated using Student's t test.
RESULTS
Effect of EDTA exposure on antigen-specific responses of PBMC.
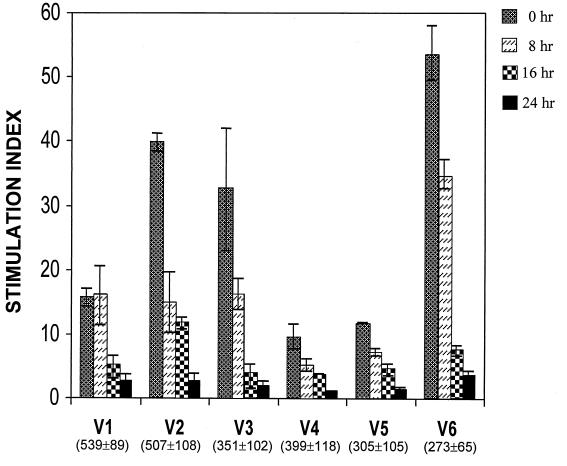
In our initial experiments, we observed a near complete loss of mycobacterial antigen-specific lymphoproliferation in PBMC obtained from the blood of nine PPD-positive individuals and stored in 4 mM EDTA as an anticoagulant for more than 20 h. The proliferative responses of PBMC from six representative individuals after various periods of exposure to EDTA are shown in Fig. 1. There was a dramatic and significant reduction in the SI, ranging from 86 to 94% in the individuals tested (P < 0.01). The results are plotted as the mean ± SEM of the SIs obtained from three independent experiments.
FIG. 1.
Progressive loss of antigen-specific proliferative responses in PBMC obtained from blood stored in EDTA for increasing periods of time. Proliferation was measured as outlined in Materials and Methods. V1 to V6 indicate the six PPD-positive volunteers participating in the study. Values in parentheses indicate the mean ± SEM counts per minute incorporated by control PBMC. The SI indicated is the mean value obtained from three independent experiments. Bars indicate standard errors.
The concentration of Ca2+ in blood is known to be between 2.2 and 2.6 mM (22). The removal by EDTA of calcium, which is required for the functional integrity of intracellular signaling pathways, appeared therefore to be the most probable cause of this loss in proliferation. Although EDTA is known to be a non-membrane-permeating chelator (16), high concentrations of EDTA (10 mM) have been reported to alter membrane fluidity (13), an action which may in turn perturb intracellular calcium levels. Alternatively, the effect of EDTA may occur through the sequestration of some other metal ions, such as Mg2+, Zn2+, and Fe2+, whose equilibrium constants for complex formation with EDTA are 8.7, 16.5, and 14.3, respectively (1). Interestingly, Zn2+ has been shown to be required for lymphocyte transformation by PHA as well as for DNA synthesis in animal cells (18, 24).
Metal ions fail to reverse EDTA-mediated damage.
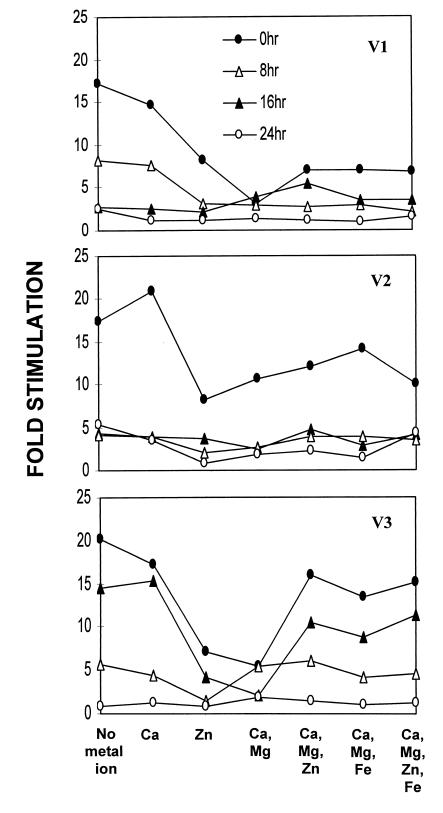
We attempted the restoration of antigen-specific lymphoproliferation by adding Ca2+, Mg2+, or Zn2+ to the blood samples during storage in EDTA. The effect of Ca2+ could not be studied, as Ca2+ resulted in clotting of the blood samples. Mg2+ did not show any beneficial effect. We observed only a marginal reversal of the EDTA effect when 0.16 mM Zn2+ was added to whole blood during the 24-h exposure to EDTA (data not shown). As an alternative means of providing depleted ions, we next carried out the lymphoproliferation assay with the metal ions added to the PBMC in cultures during the 4-day antigen exposure period. Chloride salts of Ca2+, Mg2+, Zn2+, and Fe2+ were added singly or in combination to the PBMC along with antigen. None of these metal ions helped restore the proliferative response of PBMC (Fig. 2). In view of the fact that EDTA was removed from the cells by extensive washing and the presence of adequate quantities of several divalent cations in culture medium formulations, the concentrations of the added divalent cations achieved in the cultures were perhaps in excess of the optimal levels required, as suggested by the observed suppressive effect on the SI when some of the metal ions or combinations thereof were added.
FIG. 2.
Inability of metal ions to reverse EDTA-mediated suppression of antigen-specific lymphocyte proliferation. Responses were assayed as described in the legend to Fig. 1 with various metal ions added either singly or in combinations as indicated during the 4-day exposure to antigen. The concentrations of the metal ions used were as follows: Ca2+, 2 mM; Mg2+, 0.8 mM; Zn2+, 0.1 mM; and Fe2+, 0.2 mM.
EDTA-exposed PBMC respond maximally to IL-2 and ConA.
In order to determine the overall ability of EDTA-exposed lymphocytes to proliferate in response to nonspecific stimuli, we treated the PBMC with IL-2 or ConA in place of antigen or, in the case of IL-2, in combination with antigen as well. Each of these agents was able to elicit maximum proliferation of the treated lymphocytes in an antigen-independent manner. Use of ConA or IL-2 brought about maximum levels of proliferation regardless of the period of exposure to EDTA (Table 1). The addition of IL-2 to PBMC from blood stored in EDTA as late as 6 days after they were seeded into culture wells still elicited maximum levels of thymidine incorporation (data not shown). Our results clearly indicated the presence of a fully functional DNA-synthesizing machinery in the EDTA-exposed lymphocytes. A similar differential response to mitogens as opposed to specific antigens was also reported for HIV patients (23). These results suggested to us that the effector T-cell population in the PBMC was perhaps not the target of the EDTA-mediated damage and suggested the possibility that EDTA affected the antigen-presenting cells in PBMC.
TABLE 1.
Lymphoproliferative responses of PBMC obtained from fresh and EDTA-treated blood to ConA and IL-2
| Volunteer | Mean ± SEM cpm in the following blood samples after the indicated treatmenta:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| No treatment | Fresh blood
|
EDTA-exposed bloodb
|
|||||||
| Ag | IL-2 | Ag + IL-2 | ConA | Ag | IL-2 | Ag + IL-2 | ConA | ||
| V1 | 515 ± 118 | 9,643 ± 456 | 37,154 ± 692 | 35,712 ± 1,905 | 25,243 ± 1,940 | 757 ± 415 | 24,178 ± 532 | 23,738 ± 1,466 | 46,400 ± 2,000 |
| V2 | 481 ± 148 | 13,080 ± 311 | 21,303 ± 2,844 | 22,867 ± 885 | 25,040 ± 2,964 | 2,197 ± 633 | 25,522 ± 2,303 | 29,025 ± 218 | 15,512 ± 1,609 |
| V3 | 364 ± 107 | 2,947 ± 170 | 21,994 ± 3,623 | 19,539 ± 1,297 | 34,543 ± 3,822 | 518 ± 346 | 24,047 ± 1,152 | 20,229 ± 674 | 35,747 ± 3,389 |
Data were obtained from three independent experiments. Ag, antigen.
Blood was stored in EDTA for 24 h.
Antigen-presenting cells but not effector T cells are damaged by EDTA.
In order to determine whether effector or presenting cells were affected by EDTA, we resorted to the preparation of pure populations of these two subsets of cells and then reconstituted them for the antigen stimulation assay. Percoll gradients were used to obtain monocyte and lymphocyte populations. The data from a representative PPD-positive volunteer (Table 2) showed that while monocytes from fresh blood successfully reconstituted the antigen-specific response of lymphocytes obtained from both fresh and EDTA-stored blood, monocytes from EDTA-exposed blood were incapable of eliciting the same effect.
TABLE 2.
Inability of EDTA-treated monocytes to reconstitute antigen-specific responses of lymphocytes
| Monocytes | Mean ± SEM cpm of the following cellsa:
|
|||
|---|---|---|---|---|
| Lymphocytes
|
T-cell lines
|
|||
| Untreated | EDTA treated | Untreated | EDTA treated | |
| None | 1,477 ± 40 | 1,371 ± 115 | 764 ± 94 | 682 ± 126 |
| Untreated | 9,275 ± 2,492 | 6,631 ± 1,000 | 3,753 ± 247 | 7,616 ± 598 |
| EDTA treated | 1,443 ± 65 | 1,528 ± 46 | 961 ± 75 | 1,058 ± 177 |
Data are for triplicate cultures.
The same results were obtained when monocytes from fresh and 24-h EDTA-stored blood of a volunteer were reconstituted with mycobacterial sonicate-stimulated T-cell lines which were judged to be free of presenting cells after 15 days in cultures by the absence of thymidine incorporation when exposed to antigen in the absence of added monocytes. Adherent monocytes free of lymphocytes were obtained from PBMC by exhaustive washing of adherent cells. Table 2 shows the representative data for 3H-thymidine radiolabel incorporated by cells from one of the three volunteers tested in this reconstitution assay. Adherent monocytes from fresh blood were able to reconstitute antigen-stimulated proliferation of untreated or 24-h EDTA-treated T-cell lines, whereas those from EDTA-treated blood were wholly incompetent. Similar results were obtained with all three volunteers tested. Monocytes alone were found not to incorporate radiolabel significantly in multiple experiments. In keeping with reports documenting the total dependence of ConA responses of lymphocytes on monocytes (9, 17), the T-cell lines did not respond to ConA in the absence of monocytes (data not shown).
Exposure of blood to heparin or EGTA does not damage the antigen-specific responses of PBMC.
Anticoagulants other than EDTA were examined for their ability to preserve the antigen responsiveness of PBMC from stored blood. Heparin, which is a reagent commonly used for this purpose, as well as EGTA, also a metal ion chelator, were examined. Heparin allowed unaltered mycobacterial antigen-specific proliferation of lymphocytes from blood stored for up to 24 h for two of the four PPD-positive individuals tested (Fig. 3A). We did observe a significant reduction (>50%) in proliferation brought about by storage in heparin for the remaining two volunteers. An earlier report described deterioration in PHA-induced blastogenic responses in PBMC obtained from blood processed after 24 h of storage in heparin (7). In our studies, however, the antigen-specific proliferative response of PBMC from heparinized blood samples was vastly superior to that of PBMC from EDTA-treated blood. Surprisingly, in contrast to the results obtained with EDTA, lymphocytes from blood stored in EGTA for up to 24 h were relatively more proficient in antigen-specific proliferation (Fig. 3B). Two of the volunteers tested, V1 and V2, did, however, show a 40 to 50% reduction in antigen-specific responses. PBMC from heparinized or EGTA-treated blood also responded maximally to both ConA and IL-2 (data not shown). We have not tested cells obtained from blood stored in EGTA or heparin for periods longer than 24 h.
FIG. 3.
Heparin and EGTA do not adversely affect antigen-specific responses of PBMC from stored blood. Proliferation was assayed as described in Materials and Methods. (A) Blood stored in heparin. (B) Blood stored in EGTA. V1 to V4 indicate the four PPD-positive volunteers participating in the study. Values in parentheses indicate the mean ± SEM counts per minute incorporated by control PBMC. The SI indicated is the mean value obtained from three independent experiments. Bars indicate standard errors.
Lymphoproliferative responses to JEV antigen are not impaired on storage of blood in EGTA.
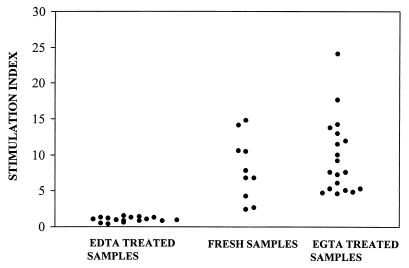
EGTA was also found to be very efficient in preserving JEV antigen-specific lymphoproliferative responses on storage of blood obtained from convalescent JEV patients for as long as 24 h after collection (Fig. 4). Blood samples stored in EGTA for 24 h (n = 20) gave results similar to those of blood samples processed at the site of collection (n = 10) (P > 0.05). All 30 samples had SIs above 2.4, the cutoff value chosen as described in Materials and Methods. The loss in response upon storage in EDTA for 16 h (n = 17) was extremely significant (P < 0.001) compared to the results for fresh samples. All 17 individuals had SIs below 2.4 on stimulation with JEV antigen, although their ConA responses were comparable to those of the other 30 volunteers (data not shown).
FIG. 4.
EGTA preserves virus-specific proliferative responses in convalescent JEV patients. Blood samples obtained from convalescent JEV patients were processed at the time of collection (n = 10) or stored in 4 mM EDTA (n = 17) or EGTA (n = 20) for 16 to 24 h at room temperature prior to processing. Samples were scored as positive if the SI was >2.4.
DISCUSSION
The analysis of lymphocyte subsets in peripheral blood often serves as a useful marker of disease progression and prognosis, especially in infections such as HIV type 1. The choice of anticoagulant is of major importance when blood samples are to be used for techniques such as immunophenotyping by flow cytometry or for analysis of antigen-specific cellular responses. The three most commonly used anticoagulants, EDTA, heparin, and ACD, have been compared by several investigators for their ability to preserve the proportion of lymphocyte subsets and their morphological features, surface markers, and ability to proliferate in response to various antigens (11, 12, 14, 19, 20, 23). Although EDTA is the anticoagulant of choice for hematology (10), it was nevertheless reported to cause alterations in the proportions of T- and B-cell subsets when blood was processed 2 days after collection compared to fresh blood samples (11, 12). In addition, PHA-induced lymphocyte transformation was also reported to be inhibited by the continuous presence of EDTA in cultures (1). The choice of the anticoagulant used has also been found to have profound effects on the surface expression of the activation markers HLA-DR and CD11b on peripheral leukocytes (19). Studies of the proliferative ability of lymphocytes from HIV-infected patients have shown that 24 h of storage of blood in heparin or ACD does affect antigen-specific responses, while mitogen-specific responses are better preserved (23). Of the anticoagulants tested, heparin preserved only cytomegalovirus responses better than ACD, while samples stored in citrate cell preparation tubes gave better results than those stored in ACD with all antigens tested. None of the anticoagulants tested was able to provide results comparable to those obtained with fresh blood.
The analysis of human antigen-specific T-cell responses in populations living in remote areas in which a pathogen is endemic often entails storage of blood samples in anticoagulants for as long as 24 h during transportation before the samples reach the laboratory for processing. Because blood is the only source of immune cells that can be obtained from human volunteers, PBMC obtained from such stored samples have to be used for lymphoproliferative as well as cytotoxic responses, the effectors for which may often be present in low proportions in peripheral blood.
Calcium is a divalent cation vital for downstream transduction of signals in lymphocytes following receptor interaction with major histocompatibility complex-bound antigen and appeared to be the most likely candidate whose loss may have been responsible for the results that we observed. Ca2+ has also been shown to restore EDTA- and citrate-mediated inhibition of PHA-induced proliferation of lymphocytes when these metal ion chelators were added to cultures (1). We were, however, unable to detect any restoration by metal ions such as Ca2+, Mg2+, Fe2+, and Zn2+, over a range of concentrations, of EDTA-induced reduction of antigen-specific lymphoproliferation. The vital factor depleted by EDTA exposure has yet to be identified.
A striking aspect of our results was the absence of proliferation inhibition when blood was stored in EGTA as an anticoagulant. In fact, such differential effects of EDTA and EGTA were also reported for the inhibition of DNA synthesis in cultured chick embryo cells (18). The affinity of EGTA for Ca2+, Zn2+, and Cu2+ is very similar to that of EDTA for these three divalent cations (18). For Ni2+, Co2+, and Mn2+, EGTA has a lower affinity than EDTA (1, 5). The benign nature of EGTA is perhaps due to the low affinity of EGTA for some trace ions vital for the functional integrity of antigen-presenting cells.
A 24-h delay in the processing of heparinized blood was reported earlier to cause more than a 50% decrease in PHA-stimulated DNA synthesis (7). In our studies, too, heparin caused up to 70% inhibition of proliferative responses in some individuals. Moreover, we observed a small but distinct enhancement of total thymidine incorporation in lymphocytes when blood was stored in EGTA as opposed to heparin for about half of the individuals tested, a result which translated to increased fold stimulation and therefore was more like the responses obtained for fresh blood. EGTA was able to preserve virus-specific responses in convalescent JEV patients at levels comparable to those in blood processed immediately following collection (P > 0.05). Our results suggest that EGTA can successfully replace EDTA as an anticoagulant in instances where prolonged storage of unclotted blood may become necessary before the samples can be transported to a laboratory for obtaining lymphocytes.
The observation that antigen-presenting cells were the victims of EDTA was unexpected. The ability of PBMC stored in EDTA to respond to IL-2 or ConA was the initial indication of this possibility. Similarly, PHA-specific responses in HIV patients were found not to be altered on storage in heparin and ACD (23), indicating that PHA-specific proliferation differed significantly from antigen-specific proliferation. Reconstitution of antigen-specific proliferation of either untreated or EDTA-exposed T-cell lines by fresh untreated monocytes but not by EDTA-treated monocytes established beyond a doubt that presenting cells developed a defect following prolonged exposure to EDTA. We also observed that treatment of these affected monocytes with bacterial lipopolysaccharide for 24 h failed to make them proficient in presenting antigen. The EDTA effect appeared unlikely to be brought about by a loss of major histocompatibility complex class II molecules on the antigen-presenting cells of the PBMC population, since it was shown that exposure of mouse tissues to 10% EDTA (269 mM) for as long as 14 days to achieve demineralization for histological examination did not cause any loss of class II molecules (6). The blastogenic response of human or guinea pig T cells to mitogens such as PHA or ConA has been conclusively shown to require the presence of monocytes (9, 17). It is therefore surprising that despite damage to monocytes by EDTA, the response of whole PBMC to the mitogen ConA or to IL-2 was wholly unaffected by EDTA.
ACKNOWLEDGMENTS
We thank all the volunteers for repeated generous donations of blood used in these studies. We thank Vidyanand Nanjundiah for help with statistical analyses. We are very grateful to the staff of the Pediatrics Department, Vijayanagar Institute of Medical Sciences, Bellary, Karnataka, India, for help in the collection of clinical samples from convalescent JEV patients.
P.K. is a senior research fellow of the Council of Scientific and Industrial Research.
REFERENCES
- 1.Alford R. Metal cation requirements for phytohemagglutinin-induced transformation of human peripheral blood lymphocytes. J Immunol. 1970;104:698–703. [PubMed] [Google Scholar]
- 2.Boyum A. Separation of lymphocytes, granulocytes, and monocytes from human blood using iodinated density gradient media. Methods Enzymol. 1984;108:88–102. doi: 10.1016/s0076-6879(84)08076-9. [DOI] [PubMed] [Google Scholar]
- 3.Eica C. On the mechanism of platelet aggregation induced by heparin, protamine and Polybrene. Scand J Hematol. 1972;9:248. doi: 10.1111/j.1600-0609.1972.tb00937.x. [DOI] [PubMed] [Google Scholar]
- 4.Gmelig-Meyling F, Waldmann T A. Separation of human blood monocytes and lymphocytes on a continuous Percoll gradient. J Immunol Methods. 1980;33:1–9. doi: 10.1016/0022-1759(80)90077-0. [DOI] [PubMed] [Google Scholar]
- 5.Holloway J H, Reilley C N. Metal chelate stability constants of aminopolycarboxylate ligands. Anal Chem. 1960;32:249–256. [Google Scholar]
- 6.Jonsson R, Tarkowski A, Klareskog L. A demineralization procedure for immunohistopathological use. EDTA preserves lymphoid cell surface antigens. J Immunol Methods. 1986;88:109–114. doi: 10.1016/0022-1759(86)90058-x. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan J, Nolan D, Reed A. Altered lymphocyte markers and blastogenic responses associated with 24 hour delay in processing of blood samples. J Immunol Methods. 1982;50:187–191. doi: 10.1016/0022-1759(82)90224-1. [DOI] [PubMed] [Google Scholar]
- 8.Konishi E, Kurane I, Mason P W, Innis B I, Ennis F A. Japanese encephalitis virus-specific proliferative responses of human peripheral blood T lymphocytes. Am J Trop Med Hyg. 1995;53:278–283. doi: 10.4269/ajtmh.1995.53.278. [DOI] [PubMed] [Google Scholar]
- 9.Levis W R, Robbins J H. Effect of glass-adherent cells on the blastogenic response of purified lymphocytes to phytohaemagglutinin. Exp Cell Res. 1970;61:153–158. doi: 10.1016/0014-4827(70)90269-7. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Reference leukocyte differential count (proportional) and evaluation of instrumental methods. Approved standard. NCCLS publication H20A. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 11.Nicholson J K A, Jones B M, Cross G D, McDougal J S. Comparison of T and B cell analyses on fresh and aged blood. J Immunol Methods. 1984;73:29–40. doi: 10.1016/0022-1759(84)90028-0. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson J K A, Green T A Collaborating Laboratories. Selection of anticoagulants for lymphocyte immunophenotyping. Effect of specimen age on results. J Immunol Methods. 1993;165:31–35. doi: 10.1016/0022-1759(93)90103-e. [DOI] [PubMed] [Google Scholar]
- 13.Ohba S, Hiramatsu M, Edamatsu R, Mori I, Mori A. Metal ions affect neuronal membrane fluidity of rat cerebral cortex. Neurochem Res. 1994;19:237–241. doi: 10.1007/BF00971570. [DOI] [PubMed] [Google Scholar]
- 14.Prince H E, Lape-Nixon M. Influence of specimen age and anticoagulant on flow cytometric evaluation of granulocyte oxidative burst generation. J Immunol Methods. 1995;188:129–138. doi: 10.1016/0022-1759(95)00210-3. [DOI] [PubMed] [Google Scholar]
- 15.Ravi V, Vanajakshi S, Gowda A, Chandramukhi A. Laboratory diagnosis of Japanese encephalitis using monoclonal antibodies and correlation of findings with outcome. J Med Virol. 1989;29:221–223. doi: 10.1002/jmv.1890290313. [DOI] [PubMed] [Google Scholar]
- 16.Richardson D, Ponka P, Baker E. The effect of the iron (III) chelator, desferrioxamine, on iron and transferrin uptake by the human malignant melanoma cell. Cancer Res. 1994;54:685–689. [PubMed] [Google Scholar]
- 17.Rosenstreich D L, Oppenheim J J. The role of macrophages in the activation of T and B lymphocytes in vitro. In: Nelson D S, editor. Immunobiology of the macrophage. New York, N.Y: Academic Press, Inc.; 1976. pp. 162–201. [Google Scholar]
- 18.Rubin H. Inhibition of DNA synthesis in animal cells by ethylene diamine tetraacetate and its reversal by zinc. Proc Natl Acad Sci USA. 1972;69:712–716. doi: 10.1073/pnas.69.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shalekoff S, Page-Shipp L, Tiemessen C T. Effects of anticoagulants and temperature on expression of activation markers CD11b and HLA-DR on human leukocytes. Clin Diagn Lab Immunol. 1998;5:695–702. doi: 10.1128/cdli.5.5.695-702.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shield C F, III, Marlett P, Smith A, Gunter L, Goldstein G. Stability of human lymphocyte differentiation antigens when stored at room temperature. J Immunol Methods. 1983;62:347–352. doi: 10.1016/0022-1759(83)90179-5. [DOI] [PubMed] [Google Scholar]
- 21.Snedecor G W, Cochran W G. Statistical methods. 8th ed. Ames: Iowa State University Press; 1989. pp. 273–296. [Google Scholar]
- 22.Stein J H, Kunau R T, Jr, Reineck J H. Principles of renal physiology. In: Stein J H, editor. Internal medicine. 2nd ed. Boston, Mass: Little, Brown & Company; 1987. pp. 715–723. [Google Scholar]
- 23.Weinberg A, Betensky R A, Zhang L, Ray G. Effect of shipment, storage, anticoagulant and cell separation on lymphocyte proliferation assays for human immunodeficiency virus-infected patients. Clin Diagn Lab Immunol. 1998;5:804–807. doi: 10.1128/cdli.5.6.804-807.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams R O, Loeb L A. Zinc requirement for DNA replication in stimulated human lymphocytes. J Cell Biol. 1973;58:594–601. doi: 10.1083/jcb.58.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]