Abstract
Schistosome parasites cause a chronic inflammatory disease in humans, and recent studies have emphasized the importance of control programs for understanding the aquatic phases of schistosomiasis transmission. The host-seeking behavior of larval schistosomes (miracidia) for their snail intermediate hosts plays a critical role in parasite transmission. Using field-derived strains of Kenyan snails and parasites, we tested two main hypotheses: (1) Parasites prefer the most compatible host, and (2) parasites avoid hosts that are already infected. We tested preference to three Biomphalaria host snail taxa (B. pfeifferi, B. sudanica, and B. choanomphala), using allopatric and sympatric Schistosoma mansoni isolates and two different nonhost snail species that co-occur with Biomphalaria, Bulinus globosus, and Physa acuta. We also tested whether schistosomes avoid snail hosts that are already infected by another trematode species and whether competitive dominance played a role in their behavior. Preference was assessed using two-way choice chambers and by visually counting parasites that moved toward competing stimuli. In pairwise comparisons, we found that S. mansoni did not always prefer the more compatible snail taxon, but never favored an incompatible host over a compatible host. While parasites preferred B. pfeifferi to the nonhost species B. globosus, they did not significantly prefer B. pfeifferi versus P. acuta, an introduced species in Kenya. Finally, we demonstrated that parasites avoid infected snails if the resident parasite was competitively dominant (Patagifer sp.), and preferred snails infected with subordinates (xiphidiocercariae) to uninfected snails. These results provide evidence of “fine tuning” in the ability of schistosome miracidia to detect hosts; however, they did not always select hosts that would maximize fitness. Appreciating such discriminatory abilities could lead to a better understanding of how ecosystem host and parasite diversity influences disease transmission and could provide novel control mechanisms to improve human health.
Keywords: Biomphalaria, choice chamber, field-derived, Schistosoma mansoni, schistosomiasis, western Kenya
INTRODUCTION
Schistosomiasis is one of the great neglected tropical diseases of our time, caused by digenetic trematodes in the genus Schistosoma. Approximately 237 million people worldwide are infected with schistosomes, and the majority of infected individuals reside in sub-Saharan Africa (World Health Organization, 2020). Although progress has been made in limiting the spread of schistosomiasis through the use of mass drug administration campaigns, in March 2020, the World Health Organization reemphasized that snail host control will be critical in the elimination of schistosomiasis as a public health problem. Therefore, enhancing our understanding of the snail host-seeking behavior of field-derived schistosomes is of timely importance.
The snail-infective larval stages of schistosomes, miracidia, must navigate complex aquatic labyrinths, through a diverse community of freshwater snails, seeking out their compatible snail host species while avoiding similar snail species that are incompatible with infection, all within a life span of less than 24 h (Christensen, 1980; Esch et al., 2001; Rohr et al., 2015; Rynkiewicz et al., 2015). “Nonhost” gastropods could act as “decoy” hosts that exhaust or absorb miracidia, preventing the successful continuation of their life cycle (Civitello et al., 2015; Combes & Mone, 1987; Johnson et al., 2009; Keesing et al., 2010; Laracuente et al., 1979; Marszewska et al., 2020). Further, even when multiple snail species maintain infection in a transmission zone, the compatibility of those host species can be highly variable (Mutuku et al., 2021). Thus, selection is expected to fine-tune the parasites’ ability to discriminate between compatible and incompatible hosts (Johnson et al., 2019). Previous studies have shown that the recognition behaviors of a miracidium are largely mediated by snail chemical cues, such as glycoproteins, kairomones, or other peptides (Chernin, 1970; Fogarty et al., 2019; Haberl et al., 1995; Haberl & Haas, 1992; Theron et al., 1998; Wang et al., 2019). However, the degree of conservation of these chemical cues among and within host species is unknown.
In the Lake Victoria Basin, Schistosoma mansoni is transmitted by three different snail host taxa, Biomphalaria pfeifferi, B. sudanica, and B. choanomphala. Although the latter two taxa have been reported to be conspecific ecophenotypes (Standley et al., 2011; Zhang et al., 2018), we will refer to them by their original species designations for clarity. These three taxa exhibit distinct differences in habitat use: deep waters (occasionally found on the shoreline) of Lake Victoria for B. choanomphala, shallow shoreline of Lake Victoria for B. sudanica, and small rivers, dams, and impoundments surrounding Lake Victoria for B. pfeifferi. Each species also differs in its compatibility with S. mansoni. In the Lake Victoria Basin, their compatibilities range from highest compatibility (B. pfeifferi) to lowest compatibility (B. sudanica) (Laidemitt, Anderson, et al., 2019; Mutuku et al., 2014, 2017, 2019). Also, in this basin are several other species of snails that are incompatible with schistosome parasites, and thus are potential “decoys” if the parasites cannot differentiate between these species and compatible hosts (Johnson et al., 2009; Laidemitt, Anderson, et al., 2019).
Infection of snails by trematodes results in long associations (often lifelong), and the asexually proliferating progeny derived from a single miracidium come to occupy a large amount of the snail’s body for the production of cercariae (Gérard et al., 1993). Thus, multiple parasites infecting the same snail would be expected to compete for both space and nutrition. Although coinfections of trematode species and multiple genotypes of the same species occur (Eppert et al., 2002), experimental infections show that one trematode often outcompetes or preys upon the other, replacing it entirely (Fernandez & Esch, 1991; Hechinger et al., 2011; Lafferty et al., 1994; Laidemitt, Anderson, et al., 2019; Lim & Heyneman, 1972). Thus, if a snail is already infected with one or more trematodes, it would seem to be advantageous for a newly arriving miracidium (of any species) to avoid that infected snail because of the prospect of imminent competition for host space and resources, the possibility of consumption by preexisting predatory stages, or bystander immune responses triggered by the first infection (Allan et al., 2009; Sandland et al., 2007; Vannatta et al., 2020). For example, Allan et al. (2009) found that schistosome miracidia could distinguish between uninfected and already-infected hosts; likewise, Vannatta et al. (2020) found that S. mansoni will avoid Biomphalaria glabrata infected with the African echinostome, Echinostoma caproni, a competitively dominant parasite. There may also be situations where the infection of one trematode species might favor the subsequent infection of another species, where one trematode species may actually prefer a snail already infected with a different species (Southgate et al., 1989; Walker, 1979). Our previous work detailed the competitive interactions of the common trematode species infecting Biomphalaria in western Kenya (Carpenter et al., 2021; Laidemitt, Anderson, et al., 2019). In the new results we report here, we used four different species of parasites: Schistosoma mansoni, Calicophoron sukari, Patagifer sp., and a virgulate xiphidiocercariae species. Based on our previously published hierarchy, xiphidiocercariae are subordinate to S. mansoni (infection is taken over and replaced by S. mansoni), and C. sukari and Patagifer sp. are dominant to S. mansoni, with the echinostome, Patagifer sp., being the most dominant (Laidemitt, Anderson, et al., 2019).
We used choice chambers to test the extent to which field-derived Kenyan S. mansoni miracidia can differentiate between potential snail hosts. We predicted (1) that schistosome parasites will favor host species (compatible hosts) over nonhost (incompatible hosts) species, and they would choose uninfected snails over infected snails, and (2) that the strength of avoidance behaviors will increase when schistosomes encounter snails infected with competitively dominant parasites. Our unique study used snails recently derived from the field rather than laboratory lines that might be significantly impacted by either artificial selection or relaxed selection from the laboratory environment.
MATERIALS AND METHODS
The behavioral assays were performed during two separate field seasons in Kenya during May 2017 and June 2019 with materials collected freshly for each set of trials. An effort was made to keep methodology as similar as possible between the two field seasons; however, minor differences are noted in the text below.
Parasite and snail sources
Schistosoma mansoni eggs were collected and pooled from fecal samples from people living near schistosome transmission sites in the Lake Victoria Basin, Kenya. See the ethical statement below. Fecal samples were pooled from two to five people from various locations from lake or stream sites (Figure 1). Stool samples were processed as described by Mutuku et al. (2014) to concentrate eggs from the fecal samples. Briefly, the stool samples were pooled and homogenized in a blender and then filtered through a series of sieves in descending order (710, 425, 212, and 45 μm). The eggs are larger than the smallest sieve size (45 μm), so are captured on that sieve and then washed into a glass Erlenmeyer flask. Eggs were hatched in the flask using bottled water and ambient light. Miracidia that displayed positive phototaxis and normal shape and swimming behavior were used in the trials.
FIGURE 1.
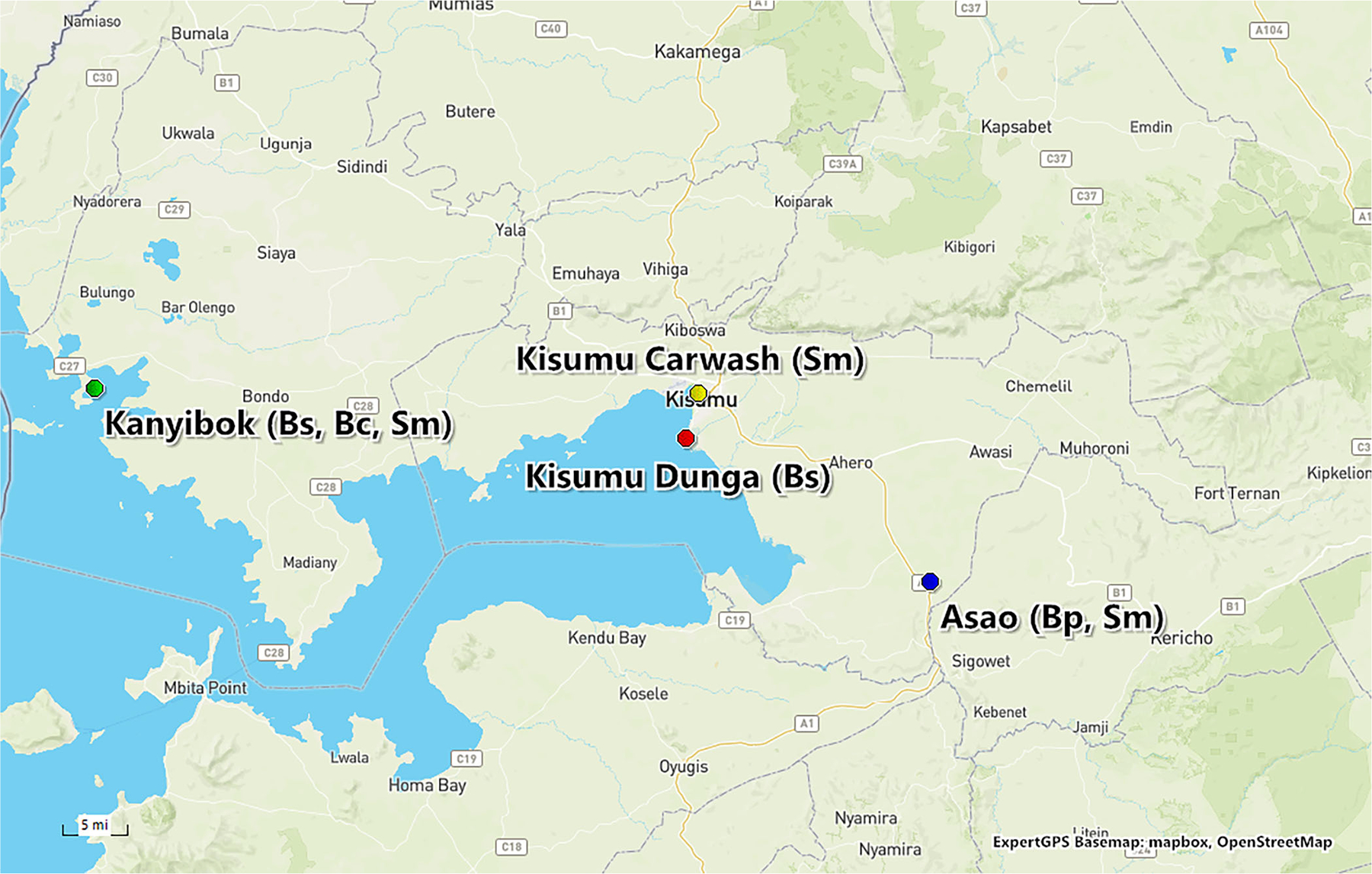
ExpertGPS BaseMap of locations in western Kenya in the Lake Victoria Basin where Schistosoma mansoni (Sm) parasites and Biomphalaria snails (B. pfeifferi [Bp], B. sudanica [Bs], B. choanomphala [Bc]) were collected. 1 mile = 1.609 km
Three taxa of Biomphalaria, B. pfeifferi (Bp), B. choanomphala (Bc), and B. sudanica (Bs), and two species of nonhost snails, Bulinus globosus and Physa acuta, were used in the trials (Table 1). Abbreviations of the host taxa are used in the tables. Snails were collected from the field via long-handled scoops or dredging (Mutuku et al., 2019) and screened for parasitic infection by individually placing each snail into cell culture wells for 2 h under ambient light. Infected snails were separated from uninfected snails. Snail and cercaria identifications were based on taxonomic keys and previously published work (Brown, 1994; Frandsen & Christensen, 1984; Laidemitt et al., 2017; Laidemitt, Brant, et al., 2019; Schell, 1985). Subsequent molecular confirmation was performed. For the 2019 trials, some snail species could not be predictably collected from the field, so F1–F3 laboratory-bred snails were used instead. For example, in comparing B. sudanica with B. choanomphala, we used B. sudanica at the F1 generation and B. choanomphala at the F3 generation. Field-derived snails were always paired with field-derived snails, and laboratory-bred snails were always paired with other laboratory-bred snails. We also tested this in our choice chamber experiments to determine whether laboratory-bred or field-derived origins mattered. In some of the 2017 trials, we used F1s versus field-derived snails to determine whether there were differences between field-derived and F1s and we found that the parasites did not prefer one to the other (F = 9.39, p = 0.51). Snails used in each trial are detailed in Table 1. In the 2017 trials, because the field-derived snails may have had prepatent infections, snails were crushed after the trial was done, and if a snail was observed with a prepatent infection, the trial was redone.
TABLE 1.
Snail source table of their collection location, habitat type, compatibility with Schistosoma mansoni, and whether they were laboratory- or field-derived
| Snail species | Location collected | Habitat | Compatibility with S. mansoni | Laboratory-reared or field-derived |
|---|---|---|---|---|
| Biomphalaria pfeifferi | Asao −0.316062, 35.006040 |
Stream | High | Field-derived |
| Bulinus globosus | Asao −0.316062, 35.006040 |
Stream | None | Field-derived |
| Physa acuta | Asao −0.316062, 35.006040 |
Stream | None | Field-derived |
| Biomphalaria choanomphala | Kanyibok Village −0.091337, 34.085053 |
Deepwater Lake Victoria | Medium-high | Laboratory (three generations of breeding in the laboratory) |
| Biomphalaria sudanica | Kanyibok Village −0.089581, 34.085928 |
Shoreline Lake Victoria | Low | Laboratory (one generation of breeding in the laboratory) |
| Biomphalaria sudanica | Dunga (Kisumu) Village −0.144784, 34.736261 |
Shoreline Lake Victoria | Low | Field-derived |
Identities of a subsample of snail specimens were confirmed by sequencing a partial portion of the cytochrome oxidase 1 gene (cox1). Snail genomic DNA was extracted using the ENZA Mollusc Kit (Omega Bio-Tek, Norcross, CA). Cox1 was amplified using the Folmer et al. (1994) primers LCO1490: 5′-GGT CAA CAA ATC ATA AAG ATA TTG G-3′ and HCO2198: 5′-TAA ACT TCA GGG TGA CCA AAA AAT CA-3′. The volume of each polymerase chain reaction (PCR) was 25 μL with 1 μL of 100 ng of DNA, 0.8 mM/L dNTPs, 2.5 mM/L MgCl2, 0.2 units of Ex Taq DNA (Clontech, Mountain View, CA), and 0.4 μM/L of each primer. PCR cycles were followed as described by Folmer et al. (1994), with the exception of an annealing temperature at 43.5°C. PCR fragments were separated by agarose gel electrophoresis and visualized with 0.5% GelRed nucleic acid gel stain and were purified using ExoSap-IT (Applied Biosystems, Foster City, CA). Both strands were sequenced using an Applied Biosystems 3130 automated sequencer and BigDye Terminator Cycle Sequencing Kit, version 3.1 (Applied Biosystems, Foster City, CA). DNA sequences were verified by aligning reads from the 5′ and 3′ directions using Sequencher 5.1 and manually corrected for ambiguous base calls (Gene Codes, Ann Arbor, MI). Not all snails could be sequenced due to permitting issues or amplification issues. Sequences generated in this study were submitted to GenBank accession number OL423116-OL423117. Snail and parasite sequences from the same or nearby locations have also been generated in other studies (Ebbs et al., 2018; Laidemitt et al., 2017; Laidemitt, Brant, et al., 2019; Zhang et al., 2018; C. Babbitt, 2021, personal communication).
Choice chambers
The chamber used in this study is described in detail by Vannatta et al. (2020). The chamber was filled with 25 mL of bottled water. A barrier made with Play-Doh wrapped in plastic was used to divide the chamber into two equal halves. The snail(s) were placed on one or both sides of the divided chamber for 10 min while being observed by the experimenter to ensure the snail remained at the end of the divided chamber (away from the barrier). Once the 10 min was completed, the snail(s) and barrier were removed, ensuring that parasites could be counted after the trial. By eliminating signals that a parasite might gain from direct interaction with, or infection attempts of, a particular host individual, this approach also isolated the type of cue the parasite can use to make a choice to secretions from the host and its mucus. Once the snails were removed, 15 S. mansoni miracidia were placed via pipette in the center. After 10 min, the barrier was placed back in the center of the chamber, and the miracidia on each side of the divided chamber were counted by pipetting them out and into a petri dish. We waited for 10 min because in pre-control trials, less than 10 min was too short a time for the miracidia to choose, and longer than 20 min the miracidia would start to shed their plates and were more challenging to collect and count. Once a trial was completed, the chamber was cleaned with bleach and water before reuse. For the 2019 trials, choice chambers were set up with a small piece of lettuce on opposite sides, and with a forceps, snails were gently waved back in forth in the water for 3 s prior to releasing them in the chamber. These actions increased mucous secretions in the chamber, which presumably ensured the presence of chemical signals upon snail removal.
Controls for the choice chambers included no snail on either side of the divided chamber, as well as a snail versus no snail setup. The latter was performed for all three taxa of host snails (Biomphalaria), using two populations of miracidia, one sympatric and one allopatric. Details of the trials, including host species versus nonhost species, allopatric versus sympatric, low compatibility versus high compatibility, and infected versus uninfected snails, are given in Table 1.
Data analysis
We used generalized linear mixed models (GLMMs) to determine whether S. mansoni miracidia choose snail hosts based on compatibility, sympatry, or infection status. GLMMs were used to ensure that the non-independence of miracidia within each trial (multiple miracidia per trial) was accounted for when determining whether miracidial choice was or was not random, given the choices presented. We used a binomial distribution in our GLMMs based on AIC (Akaike’s information criterion) comparisons. Within each trial, the number of miracidia that moved toward the predicted direction was compared with the number that moved in the opposite direction to determine whether a significant preference was shown toward one side. We used the package “glmmTMB” (Brooks et al., 2017) in the software R (version 3.6.1) for all data analyses. Preference indices (PI: number of miracidia in predicted direction – number of miracidia in opposite direction/total number of miracidia) were calculated for each treatment, treating each trial as an independent unit (Fischler et al., 2007). Positive values where the 95% confidence intervals do not overlap with zero indicate that the miracidia show a significant preference for the predicted choice (likewise, negative values that do not overlap with zero indicate significant preference in the opposite direction as predicted). Frequency distributions of PIs were examined to determine whether the behavior of miracidia appeared to be independent, or whether individuals tended to move in groups (the choice of others influences the choice of one). A normal distribution indicates independence, while a bimodal distribution suggests that other factors may influence choice. A Shapiro-Wilk test was used to assess whether PIs had a normal distribution (Royston, 1982). A bimodal distribution in the negative control treatment could potentially indicate group influences, while this distribution within a given treatment, or a positive control, may suggest that differences in snail stimuli (e.g., amount of mucus/signal) may be influencing behavior.
Ethical statement
Human subjects were enrolled in our study to provide fecal samples as a source of S. mansoni to conduct the choice chamber experiments. Samples were collected and pooled from five primary school children from Obuon Primary School near Asao Stream, Kenya (−0.304915, 35.006476), in 2017 and 2019, from five adults from Kanyibok Village (−0.090124, 34.085722) in 2019, and from two adults from the Kisumu (Carwash) site (−0.095328, 34.750264) in 2017 that were discarded clinical (fecal) samples. Informed consent was obtained from all individual participants included in the study. The KEMRI Scientific and Ethics Review Unit (SSC No. 2373) and the UNM Institution Review Board (IRB 821021–1) approved all aspects of this project involving human subjects. Individuals who were tested and found positive for S. mansoni were treated with praziquantel following standard protocols. Details of recruitment and participation of human subjects for fecal collection are described in Mutuku et al. (2014).
RESULTS
Do parasites orient toward snail cues?
When no snails were introduced to the chamber (Table 2), miracidia showed no apparent preference for either side of the divided chamber (Z = −0.40, p = 0.629). When given a choice between a host snail and an empty side of the chamber, the parasites were significantly more likely to choose the side with a snail for each of three compatible host taxa (B. pfeifferi: average Z = 6.42, p < 0.001; B. sudanica: average Z = 4.37, p < 0.001; B. choanomphala: average Z = 5.34, p < 0.001). Preference indices confirmed these results and were normally distributed, indicating independence of miracidial choice. Parasites always chose the snail in control trials where the choice was no snail versus snail, and the strength of that choice did not significantly differ whether the snail was a laboratory-reared or field-derived snail. We tested this in our choice chamber experiments to determine whether laboratory-bred or field-derived origins mattered. In some of the 2017 trials, we used F1s versus field-derived snails to determine whether there were differences between field-derived and F1s and we found that the parasites did not prefer one to the other (F = 9.39, p = 0.51). Likewise, the field or laboratory status of the snail did not impact choice in our B. sudanica versus B. pfeifferi trials.
TABLE 2.
Statistical results by treatment indicating whether parasites can differentiate between different host taxa
| Treatment | Estimate | SE | Z | N | p | Direction | Percentage toward predicted |
|---|---|---|---|---|---|---|---|
| No snail versus no snail | −0.04 | 0.16 | −0.24 | 11 | 0.807 | None | 48.5 |
| Bc versus no snail | 1.06 | 0.20 | 5.34 | 28 | <0.001 | Toward snail | 73.34 |
| Bs versus no snail | 0.83 | 0.19 | 4.37 | 36 | <0.001 | Toward snail | 68.94 |
| Bp versus no snail | 1.18 | 0.18 | 6.42 | 53 | <0.001 | Toward snail | 75.54 |
| Bs versus Bc | 0.47 | 0.21 | 2.29 | 19 | 0.022 | Toward Bc | 60.54 |
| Bs versus Bp | −0.10 | 0.18 | −0.57 | 51 | 0.566 | None | 53.78 |
| Bp versus Physa acuta | 0.33 | 0.22 | 1.48 | 12 | 0.139 | None | 57.24 |
| Bp versus Bulinus globosus | −0.49 | 0.25 | −2.01 | 9 | 0.045 | Toward Bp | 63.97 |
Note: The significance, direction of the choice, and average % of miracidia (averaged across all trials in a treatment) are in bold if past a significance threshold of p < 0.05. Biomphalaria sp. host names are abbreviated, with Bc representing Biomphalaria choanomphala, Bs representing B. sudanica, and Bp representing B. pfeifferi.
Do parasites choose the most compatible host?
For these trials, we first compared compatible snail species versus incompatible snail species, and then, we compared compatible snail species that exhibited different levels of compatibility (Table 2). When given a choice between B. pfeifferi (host - compatible) and B. globosus (nonhost - incompatible), S. mansoni miracidia were found on B. pfeifferi’s side of the divided chamber more often (Z = −2.01, p = 0.045). There was no significant difference in choice between B. pfeifferi (host) and P. acuta (nonhost - incompatible) (Z = 1.48, p = 0.139). Preference indices confirmed these findings and independence of miracidia choice.
As shown in Table 2, when given a choice between B. sudanica (less compatible) and B. choanomphala (more compatible), parasites chose the more compatible host (Z = 2.29, p = 0.022). However, the choice between B. sudanica (less compatible) and B. pfeifferi (more compatible) did not differ significantly overall (Z = −5.73, p = 0.566). Two sets of trials with this snail combination were completed, each set using sympatric and allopatric snail hosts from different locations. When pooled, the results are not significant, as stated above. However, examined separately, we see location-specific effects. In trials using a full factorial design in which Asao and Kanyibok miracidia (see Figure 1) were given a choice between Asao-collected B. pfeifferi and Kanyibok-collected B. sudanica, we found that Asao miracidia significantly selected the sympatric host (Z = −2.278, p = 0.023), while Kanyibok miracidia exhibited no clear choice (Z = 0.454, p = 0.649) (Appendix S1: Table S1). In the second set of trials, miracidia from Kisumu and Asao were given a choice between B. pfeifferi (from Asao) and B. sudanica (from Kisumu). In these trials, there were no significant choices made by either Asao miracidia (Z = 1.01, p = 0.311) or Kisumu miracidia (Z = −0.50, p = 0.617) (Appendix S1: Table S2). Allopatric and sympatric results are in Appendix S1: Tables S1 and S2.
Do parasites avoid infected hosts?
As shown in Table 3, when given a choice between a snail patently infected with S. mansoni or an uninfected snail, parasites showed no significant preference (Z = −1.62, p = 0.106). This was contrary to our hypothesis that the parasites would avoid a snail already infected with conspecifics. All infected snails were patent, “shedding” cercariae.
TABLE 3.
Statistical results by treatment indicating whether parasites can differentiate between hosts of varying infection status
| Treatment | Estimate | SE | Z | p | N | Direction | Percentage toward predicted |
|---|---|---|---|---|---|---|---|
| No snail versus no snail | −0.06 | 0.16 | −0.40 | 0.692 | 11 | None | 48.5 |
| Laboratory F1 Bp uninfected versus no snail | 0.80 | 0.23 | 3.48 | <0.001 | 12 | Toward snail | 66.5 |
| Field-uninfected Bp versus no snail | 0.91 | 0.23 | 3.94 | <0.001 | 12 | Toward snail | 70.2 |
| Schistosoma mansoni-infected Bp versus uninfected Bp | −0.37 | 0.23 | −1.62 | 0.106 | 11 | None | 39.3 |
| S. mansoni-infected Bp and Bs versus Patagifer sp.-infected Bp and Bs | 0.24 | 0.24 | 0.99 | 0.321 | 9 | None | 54.2 |
| Uninfected Bp versus Bp-infected with Patagifer sp. | 0.53 | 0.19 | 2.85 | 0.004 | 34 | Toward uninfected | 61.1 |
| Uninfected Bp versus Bp-infected with Patagifer sp. Metacercariae | 0.24 | 0.22 | 1.06 | 0.291 | 12 | None | 54.8 |
| Uninfected Bp versus Bp-infected with C. sukari | −0.29 | 0.23 | −1.29 | 0.198 | 11 | None | 58.9 |
| Uninfected Bp versus Bp-infected with Xiphidiocercariae | −0.48 | 0.23 | −2.06 | 0.039 | 11 | Toward infected | 36.4 |
Note: The significance, direction of the choice, and average % of miracidia (averaged across all trials in a treatment) are in bold if past a significance threshold of p < 0.05. Biomphalaria sp. host names are abbreviated, with Bc representing Biomphalaria choanomphala, Bs representing B. sudanica, and Bp representing B. pfeifferi.
We also hypothesized that infected/uninfected host preference would be stronger when the snail was infected with a competitively dominant parasite (one that outcompetes S. mansoni and dominates the infection). We tested representatives from two groups of parasites that are dominant to S. mansoni, an echinostome, Patagifer sp., and an amphistome, C. sukari (Table 3). Parasites chose the noninfected snail more often than the infected snail when the snail was infected with Patagifer sp. (Z = 2.85, p = 0.004). Interestingly, when the snail was infected with a nonreplicative ontogenetic stage of Patagifer sp. (metacercariae), there was no significant difference in preference between the uninfected and infected snail (Z = 1.06, p = 0.291). Also, with the other “dominant” parasite, C. sukari, there was no significant preference between the infected and uninfected snail (Z = −1.29, p = 0.198).
Finally, when given a choice between the subordinate xiphidiocercariae-infected snail and an uninfected snail (Table 3), the parasite chose the infected snail more often (Z = −2.06, p = 0.039). Preference indices confirmed these findings and indicated that miracidia made choices independently.
DISCUSSION
After hatching from the egg, a schistosome parasite has a short time (<24 h) to locate and invade a compatible snail species (Maldonado et al., 1949; Prah & James, 1977). They must navigate complex environments with diverse communities of snails, many of which are already infected by an equally diverse array of trematode parasites. Our study supports previous findings that miracidia actively navigate toward snail cues, but it goes further to determine the extent to which field-derived miracidia can differentiate these snail-derived cues. With a series of binary choices, we asked whether parasites can select hosts that will increase their fitness. Our study is among the few studies that utilize African snails and schistosomes either directly derived or recently derived from the field in choice chamber experiments (see Allan et al., 2009). One of the limitations of this study was the testing system that allowed only binary choices and that not every combination of possible choices could be tested in the time that we had access to field material.
When given a choice between highly compatible host species (B. pfeifferi) versus incompatible nonhost species (Bulinus globosus or P. acuta), the parasites significantly oriented toward the compatible host when B. globosus was the alternative, but not when P. acuta was the alternative. In the latter case, the parasites did not show a significant preference for either snail. Previous choice experiments with S. mansoni and various snails show mixed results with regard to preference between compatible host and incompatible nonhost species (Basch, 1976; Chernin, 1970; Hassan et al., 2003; Kalbe et al., 1996). However, the pattern in previous studies appears to be that the ability to discern between compatible hosts and incompatible nonhosts is stronger with more distantly related snails, perhaps due to the conservation of biochemical signals (Allan et al., 2009; Christensen, 1980; Kalbe et al., 1996). Our results show the opposite. Bulinus and Biomphalaria belong to the family Planorbidae, while Physa belongs to a different family, the Physidae. One possible explanation for this result is that P. acuta is a relatively recent invader in Africa, sometime after the 1870s (Ebbs et al., 2018). Thus, the ability for S. mansoni miracidia to discern between Bulinus and Biomphalaria species may have evolved from the long history of co-occurrence (>1 million years ago) (DeJong et al., 2001; Neubauer et al., 2017). It has also been noted in other studies that avian schistosomes did not choose compatible host species versus incompatible alien species when given a choice in snail-conditioned choice chamber experiments (Marszewska et al., 2020). More comparisons of other planorbids and alien species against compatible host species are needed to test this hypothesis fully. It should also be noted that although the chances are low, penetrating a nontarget intermediate host is not necessarily always a dead end. It might lead to a host-switching event that may favor the parasite’s transmission in the future by broadening host or geographic range, as was the case for other schistosome species (Brant & Loker, 2013; Combes & Mone, 1987). Also, our results exemplify the possibility that an introduced species can have more subtle effects, such as diluting the impact of human parasites, than normally considered.
Within the Lake Victoria Basin, S. mansoni can infect three taxa of Biomphalaria with differing levels of success. We hypothesized that given a choice between two compatible host species, the parasite would prefer the most compatible. Results from B. choanomphala (more compatible) versus B. sudanica (less compatible) supported this hypothesis. This finding is interesting, because B. choanomphala is genetically quite similar to B. sudanica (Standley et al., 2014; Zhang et al., 2018), but shows clear differences in habitat use (Gouvras et al., 2017; Mutuku et al., 2019) and is more compatible with Kenyan S. mansoni (Mutuku et al., 2021). Our data suggest that schistosomes can distinguish between these snails (even if researchers cannot and bin them into the same species) and orient toward B. choanomphala when given a choice.
When the choice was B. pfeifferi (highly compatible) versus B. sudanica (least compatible), there was no significant preference between these species. These two species of snails are rarely found in sympatry, with B. pfeifferi in streams and small impoundments and B. sudanica in papyrus swamps associated with larger water bodies (Brown, 1994). Nested within this trial, we also tested whether the source of the parasite mattered. We predicted that parasites would show a greater preference for sympatric hosts due to local adaptation. Our trials used parasites sympatric to either the B. pfeifferi snail or the B. sudanica snail (parasites collected from humans who inhabit the same general locality). Due to logistics, the B. sudanica snail source and corresponding parasite source differed between field seasons (either Kanyibok or Kisumu), but the B. pfeifferi source and corresponding parasite source remained the same (Asao). Parasites from Asao chose B. pfeifferi (sympatric) over B. sudanica from Kanyibok (allopatric), but Kanyibok parasites showed no significant preference to either snail species. In contrast to these results, when the trials compared B. sudanica and B. pfeifferi from Kisumu and Asao, no significant preferences were found, and parasite source was not a significant variable in the model. It is possible that there were host genotypic differences that play a role and that the paired choice matters (e.g., Asao snail vs. Kisumu snail or Asao snail vs. Kanyibok snail).
Our results are similar to previous tests of different schistosome and snail systems, which show mixed results. Allan et al. (2009) assessed S. haematobium choice between two different compatible species of Bulinus, and the miracidia did not show significant preference. Kalbe et al. (1996) found that an Egyptian strain of S. mansoni miracidia preferred snail-conditioned water from Biomphalaria alexandrina (sympatric) to B. glabrata (allopatric); however, miracidia from two Brazilian strains did not show a preference between snail-conditioned water from the two snail species.
In trials comparing preference to infected or uninfected snails, S. mansoni miracidia avoided snails infected with the most dominant trematode in the hierarchy, Patagifer sp., but showed no preference when the infection was with either C. sukari (competitively dominant to S. mansoni, but subordinate to Patagifer sp.) or S. mansoni (conspecific), and favored snails infected with xiphidiocercariae (competitively subordinate). These results are generally in line with our expectations from the dominance hierarchy, but there are important differences. Because C. sukari is dominant to S. mansoni, we predicted that S. mansoni would avoid snails infected with this species. One potential explanation is the complicated interdependence of C. sukari on S. mansoni. Although C. sukari can invade and establish within B. pfeifferi, it cannot complete its development unless the snail becomes infected with S. mansoni (Laidemitt, Anderson, et al., 2019); thus, there could be selection pressure on C. sukari to hide signals of snail infection.
Vannatta et al. (2020) also found a preference for uninfected B. glabrata versus B. glabrata infected with Echinostoma caproni, also a competitively dominant echinostome parasite to S. mansoni. Echinostomes are known to engage in direct (predatory) and indirect antagonism against schistosomes (Hechinger et al., 2011; Lim & Heyneman, 1972; Sandland et al., 2007). These data suggest that either echinostome ESPs (excretory–secretory products) are being detected in the snail-conditioned water, or snail ESPs are being modified from the infection and then being detected in the snail-conditioned water that may act as miracidial deterrents. For example, it has been shown in the B. glabrata–S. mansoni system that a peptide, called P12, is secreted from B. glabrata, to which S. mansoni is significantly attracted (Fogarty et al., 2019), and there are likely other peptides or metabolites that are being excreted or secreted that S. mansoni miracidia actively avoid. Further liquid chromatography–mass spectrometry studies are needed to characterize ESPs released by the larval stages of echinostomes within their snail. Patagifer species are of interest because they commonly infect Bulinus and Biomphalaria, both of which transmit human schistosomes (Laidemitt, Brant, et al., 2019). One possibility for future testing is that the echinostome-infected snail might release less of a particular signal simply as a by-product of the infection by reduction in mucous secretions.
In regard to Patagifer sp. (echinostome) avoidance, there was also a trend (although not significant) that S. mansoni miracidia preferred uninfected snails to those infected with Patagifer sp. metacercariae. Metacercariae are encysted stages that are nonpredatory and nonreplicative within the snail host. They also tend to be found in tissues of the snail different from those occupied by sporocysts, rediae, or cercariae, and metacercariae are typically common in wild snail populations. We found it difficult to find field snails that were not infected with metacercariae of one species or another.
An interesting result was that S. mansoni miracidia preferred B. pfeifferi infected with virgulate xiphidiocercariae to uninfected B. pfeifferi. It is possible the sporocysts of xiphidiocercariae may suppress or interfere with the anti-trematode immune system of the snail, improving the chances that S. mansoni can establish an infection (Adema & Loker, 2015; Hanington et al., 2012; Lie, 1982). Alternatively, schistosome parasites may derive nutrients from the xiphidiocercariae within the snails, and these extra nutrients might boost its fitness. Similarly, Vannatta et al. (2020) found that E. caproni miracidia significantly chooses S. mansoni (subordinate)-infected B. glabrata over uninfected B. glabrata for likely similar reasons.
SUMMARY AND CONCLUSIONS
Our findings support previous results that miracidia are attracted to cues produced by snails and/or from the parasites within snails. Further, miracidia sensory receptors may be able to differentiate among compatible host species and avoid coinfection within a snail if the resident parasite is competitively dominant. However, our results demonstrate that predictions concerning host preference are not necessarily generalizable, indicating complexity in the system. Further work is needed to identify the signals involved in sensing hosts and how these signals vary within field-derived snail populations and between snail species. The advent of dramatically improved detection technology coupled with an increasing amount of information about snail and parasite genomes will enable further elucidation of the chemical language of trematodes, including within their snail hosts. This is especially important when the snails involved transmit parasites that cause infections in millions of people. We emphasize the importance of investigating this chemical language as closely as possible as it is revealed under conditions of natural transmission.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ibrahim Mwangi, Dr Eric Lelo, Joseph Kinuthia, Geoffrey Maina, and Boaz Oduor for their assistance with the collection of field samples and snail maintenance. We also thank Dr Stephen Munga, the Deputy Director of the Center for Global Health Research, Kenya Medical Research Institute (KEMRI), for providing the laboratory space to conduct these experiments. This study was undertaken with the approvals of the National Commission for Science, Technology and Innovation (permit number P/16/9609/12754), National Environmental Management Authority (permit number NEMA/AGR/46/2014), and Kenya Wildlife Service (permit number KWS/BRM/5001 and export permit number 0004754). Technical assistance at the University of New Mexico Molecular Biology Facility was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P30GM110907. We gratefully acknowledge the National Institute of Health (NIH) grants R37AI101438 and R01AI141862 for their financial support. This work was also supported, in part, by the Bill & Melinda Gates Foundation [OPP1098449]. We lastly acknowledge the Western University of Health Sciences Summer Student Fellowship award. The content for this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This paper was published with the approval of the Director-General, KEMRI.
Funding information
Bill and Melinda Gates Foundation, Seattle, WA, Grant/Award Number: OPP1098449; National Institute of General Medical Sciences of the National Institutes of Health, Grant/Award Number: P30GM110907; National Institutes of Health (NIH), Grant/Award Numbers: R01AI141862, R37AI101438; Western University of Health Sciences Summer Student Fellowship
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
Data (Gleichsner, 2022) are available from Zenodo: https://doi.org/10.5281/zenodo.6028760.
REFERENCES
- Adema CM, and Loker ES. 2015. “Digenean-Gastropod Host Associations Inform on Aspects of Specific Immunity in Snails.” Developmental and Comparative Immunology 48: 275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan F, Rollinson D, Smith JE, and Dunn AM. 2009. “Host Choice and Penetration by Schistosoma haematobium Miracidia.” Journal of Helminthology 83: 33–8. [DOI] [PubMed] [Google Scholar]
- Basch PF 1976. “Intermediate Host Specificity in Schistosoma mansoni.” Experimental Parasitology 39: 150–69. [DOI] [PubMed] [Google Scholar]
- Brant SV, and Loker ES. 2013. “Discovery-Based Studies of Schistosome Diversity Stimulate New Hypotheses about Parasite Biology.” Trends in Parasitology 29: 449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks M, Kristensen Kasper, van Benthem Koen J., Magnusson Arni, Berg Casper W., Nielsen Anders, Skaug Hans J., Mächler Martin, and Bolker Benjamin M.. 2017. “glmmTMB Balances Speed and Flexibility among Packages for Zero-Inflated Generalized Linear Mixed Modeling.” R Journal 9: 378–400. [Google Scholar]
- Brown DS 1994. Freshwater Snails of Africa and Their Medical Importance, 2nd ed. London and Bistol, PA: Taylor & Francis. https://www.taylorfrancis.com/books/mono/10.1201/9781482295184/freshwater-snails-africa-medical-importance-david-brown [Google Scholar]
- Carpenter SA, Vannatta JT, and Minchella DJ. 2021. “Host Exposure History and Priority Effects Impact the Development and Reproduction of a Dominant Parasite.” International Journal for Parasitology 51: 935–43. [DOI] [PubMed] [Google Scholar]
- Chernin E 1970. “Behavioral Responses of Miracidia of Schistosoma mansoni and Other Trematodes to Substances Emitted by Snails.” Journal of Parasitology 56: 287–96. [PubMed] [Google Scholar]
- Christensen NO 1980. “A Review of the Influence of Host- and Parasite-Related Factors and Environmental Conditions on the Host-Finding Capacity of the Trematode Miracidium.” Acta Tropica 37: 303–18. [PubMed] [Google Scholar]
- Civitello DJ, Cohen Jeremy, Fatima Hiba, Halstead Neal T., Liriano Josue, McMahon Taegan A., Ortega C. Nicole, et al. 2015. “Biodiversity Inhibits Parasites: Broad Evidence for the Dilution Effect.” Proceedings of the National Academy of Sciences of the United States of America 112: 8667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes C, and Mone H. 1987. “Possible Mechanisms of the Decoy Effect in Schistosoma mansoni Transmission.” International Journal for Parasitology 17: 971–5. [DOI] [PubMed] [Google Scholar]
- DeJong RJ, Morgan JA, Paraense WL, Pointier JP, Amarista M, Ayeh-Kumi PF, Babiker A, et al. 2001. “Evolutionary Relationships and Biogeography of Biomphalaria (Gastropoda: Planorbidae) with Implications Regarding its Role as Host of the Human Bloodfluke, Schistosoma mansoni.” Molecular Biology and Evolution 18: 2225–39. [DOI] [PubMed] [Google Scholar]
- Ebbs ET, Loker ES, and Brant SV. 2018. “Phylogeography and Genetics of the Globally Invasive Snail Physa acuta Draparnaud 1805, and Its Potential to Serve as an Intermediate Host to Larval Digenetic Trematodes.” BMC Evolutionary Biology 18: 103. 10.1186/s12862-018-1208-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppert A, Lewis FA, Grzywacz C, Coura-Filho P, Caldas I, and Minchella DJ. 2002. “Distribution of Schistosome Infections in Molluscan Hosts at Different Levels of Parasite Prevalence.” The Journal of Parasitology 88: 232–6. [DOI] [PubMed] [Google Scholar]
- Esch GW, Curtis LA, and Barger MA. 2001. “A Perspective on the Ecology of Trematode Communities in Snails.” Parasitology 123(Suppl): S57–75. [DOI] [PubMed] [Google Scholar]
- Fernandez J, and Esch GW. 1991. “Guild Structure of Larval Trematodes in the Snail Helisoma anceps: Patterns and Processes at the Individual Host Level.” The Journal of Parasitology 77: 528–39. [PubMed] [Google Scholar]
- Fischler W, Kong P, Marella S, and Scott K. 2007. “The Detection of Carbonation by the Drosophila Gustatory System.” Nature 448: 1054–7. [DOI] [PubMed] [Google Scholar]
- Fogarty CE, Zhao Min, McManus Donald P., Duke Mary G., Cummins Scott F., and Wang Tianfang. 2019. “Comparative Study of Excretory-Secretory Proteins Released by Schistosoma mansoni-Resistant, Susceptible and Naive Biomphalaria glabrata.” Parasites & Vectors 12: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, and Vrijenhoek R. 1994. “DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates.” Molecular Marine Biology and Biotechnology 3: 294–9. [PubMed] [Google Scholar]
- Frandsen F, and Christensen NO. 1984. “An Introductory Guide to the Identification of Cercariae from African Freshwater Snails with Special Reference to Cercariae of Trematode Species of Medical and Veterinary Importance.” Acta Tropica 41: 181–202. [PubMed] [Google Scholar]
- Gérard C, Moné H, and Théron A. 1993. “Schistosoma mansoni–Biomphalaria glabrata: Dynamics of the Sporocyst Population in Relation to the Miracidial Dose and the Host Size.” Canadian Journal of Zoology 71: 1880–5. [Google Scholar]
- Gleichsner A 2022. “ParasiticProf/Laidemitt_et_al._Ecosphere: Laidemitt_et_al._Ecosphere (v1.1).” Zenodo. Data Set. 10.5281/zenodo.6028760. [DOI] [Google Scholar]
- Gouvras AN, Allan Fiona, Kinung’hi Safari, Rabone Muriel, Emery Aidan, Angelo Teckla, Pennance Tom, Webster Bonnie, Nagai Honest, and Rollinson David. 2017. “Longitudinal Survey on the Distribution of Biomphalaria sudanica and B. choanomophala in Mwanza Region, on the Shores of Lake Victoria, Tanzania: Implications for Schistosomiasis Transmission and Control.” Parasites & Vectors 10: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberl B, and Haas W. 1992. “Miracidium of Schistosoma mansoni: A Macromolecular Glycoconjugate as Signal for the Behaviour after Contact with the Snail Host.” Comparative Biochemistry and Physiology 101: 329–33. [DOI] [PubMed] [Google Scholar]
- Haberl B, Kalbe M, Fuchs H, Ströbel M, Schmalfuss G, and Haas W. 1995. “Schistosoma mansoni and S. haematobium: Miracidial Host-Finding Behaviour Is Stimulated by Macromolecules.” International Journal for Parasitology 25: 551–60. [DOI] [PubMed] [Google Scholar]
- Hanington PC, Forys MA, and Loker ES. 2012. “A Somatically Diversified Defense Factor, FREP3, Is a Determinant of Snail Resistance to Schistosome Infection.” PLoS Neglected Tropical Diseases 6: e1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AH, Haberl B, Hertel J, and Haas W. 2003. “Miracidia of an Egyptian Strain of Schistosoma mansoni Differentiate between Sympatric Snail Species.” Journal of Parasitology 89: 1248–50. [DOI] [PubMed] [Google Scholar]
- Hechinger RF, Wood AC, and Kuris AM. 2011. “Social Organization in a Flatworm: Trematode Parasites Form Soldier and Reproductive Castes.” Proceedings of the Royal Society 278: 656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PT, Lund PJ, Hartson RB, and Yoshino TP. 2009. “Community Diversity Reduces Schistosoma mansoni Transmission, Host Pathology and Human Infection Risk.” Proceedings of the Royal Society 276: 1657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Calhoun DM, Riepe TB, and Koprivnikar J. 2019. “Chance or Choice? Understanding Parasite Selection and Infection in Multi-Host Communities.” International Journal for Parasitology 49: 407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbe M, Haberl B, and Haas W. 1996. “Schistosoma mansoni Miracidial Host-Finding: Species Specificity of an Egyptian Strain.” Parasitology Research 82: 8–13. [DOI] [PubMed] [Google Scholar]
- Keesing F, Belden Lisa K., Daszak Peter, Dobson Andrew, Harvell C. Drew, Holt Robert D., Hudson Peter, et al. 2010. “Impacts of Biodiversity on the Emergence and Transmission of Infectious Diseases.” Nature 468: 647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty KD, Sammond DT, and Kuris AM. 1994. “Analysis of Larval Trematode Communities.” Ecology 75: 2275–85. [Google Scholar]
- Laidemitt MR, Zawadzki Eva T., Brant Sara V., Mutuku Martin W., Mkoji Gerald M., and Loker Eric S.. 2017. “Loads of Trematodes: Discovering Hidden Diversity of Paramphistomoids in Kenyan Ruminants.” Parasitology 144: 131–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidemitt MR, Anderson Larissa C., Wearing Helen J., Mutuku Martin W., Mkoji Gerald M., and Loker Eric S.. 2019. “Antagonism between Parasites within Snail Hosts Impacts the Transmission of Human Schistosomiasis.” eLife 8: e50095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidemitt MR, Brant SV, Mutuku MW, Mkoji GM, and Loker ES. 2019. “The Diverse Echinostomes from East Africa: With a Focus on Species that Use Biomphalaria and Bulinus as Intermediate Hosts.” Acta Tropica 193: 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laracuente A, Brown RA, and Jobin W. 1979. “Comparison of Four Species of Snails as Potential Decoys to Intercept Schistosome Miracidia.” American Journal of Tropical Medicine and Hygiene 28: 99–105. [DOI] [PubMed] [Google Scholar]
- Lie KJ 1982. “Swellengrebel Lecture: Survival of Schistosoma mansoni and Other Trematode Larvae in the Snail Biomphalaria glabrata. A Discussion of the Interference Theory.” Tropical and Geographical Medicine 34: 111–22. [PubMed] [Google Scholar]
- Lim HK, and Heyneman D. 1972. “Intramolluscan Inter-Trematode Antagonism: A Review of Factors Influencing the Host-Parasite System and its Possible Role in Biological Control.” Advances in Parasitology 10: 191–268. [DOI] [PubMed] [Google Scholar]
- Maldonado JF, Acosta Matienzo J, and Thillet CJ. 1949. “Biological Studies on the Miracidium of Schistosoma mansoni; Behavior of the Unhatched Miracidium in Undiluted Stools under Diverse Environmental Conditions.” Puerto Rico Journal of Public Health Tropical Medicine 25: 153–74. Also Spanish transl, 175–199. [PubMed] [Google Scholar]
- Marszewska A, Cichy A, Bulantová J, Horák P, and Żbikowska E. 2020. “The Chemotactic Swimming Behavior of Bird Schistosome Miracidia in the Presence of Compatible and Incompatible Snail Hosts.” PeerJ 8: e9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutuku MW, Dweni Celestine K., Mwangi Moses, Kinuthia Joseph M., Mwangi Ibrahim N., Maina Geoffrey M., Agola Lelo E., et al. 2014. “Field-Derived Schistosoma mansoni and Biomphalaria pfeifferi in Kenya: A Compatible Association Characterized by Lack of Strong Local Adaptation, and Presence of some Snails Able to Persistently Produce Cercariae for over a Year.” Parasites & Vectors 7: 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutuku MW, Lu Lijun, Otiato Fredrick O., Mwangi Ibrahim N., Kinuthia Joseph M., Maina Geoffrey M., Laidemitt Martina R., et al. 2017. “A Comparison of Kenyan Biomphalaria pfeifferi and B. sudanica as Vectors for Schistosoma mansoni, Including a Discussion of the Need to Better Understand the Effects of Snail Breeding Systems on Transmission.” Journal of Parasitology 103: 669–76. [DOI] [PubMed] [Google Scholar]
- Mutuku MW, Laidemitt Martina R., Beechler Brianna R., Mwangi Ibrahim N., Otiato Fredrick O., Agola Eric L., Ochanda Horace, et al. 2019. “A Search for Snail-Related Answers to Explain Differences in Response of Schistosoma mansoni to Praziquantel Treatment among Responding and Persistent Hotspot Villages along the Kenyan Shore of Lake Victoria.” American Journal of Tropical Medicine and Hygiene 101: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutuku MW, Laidemitt Martina R., Spaan Johannie M., Mwangi Ibrahim N., Ochanda Horace, Steinauer Michelle L., Loker Eric S., and Mkoji Gerald M.. 2021. “Comparative Vectorial Competence of Biomphalaria sudanica and Biomphalaria choanomphala, Snail Hosts of Schistosoma mansoni, from Transmission Hotspots in Lake Victoria, Western Kenya.” Journal of Parasitology 107: 349–57. [DOI] [PubMed] [Google Scholar]
- Neubauer TA, Mandic O, Harzhauser M, and Jovanovi c G. 2017. “The Discovery of Bulinus (Pulmonata: Planorbidae) in a Miocene Palaeolake in the Balkan Peninsula.” Journal of Molluscan Studies 83: 295–303. [Google Scholar]
- Prah SK, and James C. 1977. “The Influence of Physical Factors on the Survival and Infectivity of Miracidia of Schistosoma mansoni and S. haematobium. Effect of Temperature and Ultra-Violet Light.” Journal of Helminthology 51: 73–85. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Civitello David J., Crumrine Patrick W., Halstead Neal T., Miller Andrew D., Schotthoefer Anna M., Stenoien Carl, Johnson Lucinda B., and Beasley Val R.. 2015. “Predator Diversity, Intraguild Predation, and Indirect Effects Drive Parasite Transmission.” Proceedings of the National Academy of Sciences of the United States of America 112: 3008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston JP 1982. “Algorithm AS 181: The W Test for Normality.” Journal of the Royal Statistical Society. Series C (Applied Statistics) 31: 176–80. [Google Scholar]
- Rynkiewicz EC, Pedersen AB, and Fenton A. 2015. “An Ecosystem Approach to Understanding and Managing within-Host Parasite Community Dynamics.” Trends in Parasitology 31: 212–21. [DOI] [PubMed] [Google Scholar]
- Sandland GJ, Rodgers JK, and Minchella DJ. 2007. “Interspecific Antagonism and Virulence in Hosts Exposed to Two Parasite Species.” Journal of Invertebrate Pathology 96: 43–7. [DOI] [PubMed] [Google Scholar]
- Schell SC 1985. Handbook of Trematodes of North America North of Mexico. Moscow, ID: University of Idaho Press. [Google Scholar]
- Southgate VR, Brown DS, Warlow A, Knowles RJ, and Jones A. 1989. “The Influence of Calicophoron microbothrium on the Susceptibility of Bulinus tropicus to Schistosoma bovis.” Parasitology Research 75: 381–91. [DOI] [PubMed] [Google Scholar]
- Standley CJ, Wade CM, and Stothard JR. 2011. “A Fresh Insight into Transmission of Schistosomiasis: A Misleading Tale of Biomphalaria in Lake Victoria.” PLoS One 6: e26563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley CJ, Goodacre SL, Wade CM, and Stothard JR. 2014. “The Population Genetic Structure of Biomphalaria choanomphala in Lake Victoria, East Africa: Implications for Schistosomiasis Transmission.” Parasites & Vectors 7: 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theron A, Rognon A, and Pages JR. 1998. “Host Choice by Larval Parasites: A Study of Biomphalaria glabrata Snails and Schistosoma mansoni Miracidia Related to Host Size.” Parasitology Research 84: 727–32. [DOI] [PubMed] [Google Scholar]
- Vannatta JT, Knowles T, Minchella DJ, and Gleichsner AM. 2020. “The Road Not Taken: Host Infection Status Influences Parasite Host-Choice.” Journal of Parasitology 106: 1–8. [PubMed] [Google Scholar]
- Walker JC 1979. “Austrobilharzia terrigalensis: A Schistosome Dominant in Interspecific Interactions in the Molluscan Host.” International Journal for Parasitology 9: 137–40. [Google Scholar]
- Wang T, Wyeth Russell C., Di Liang Utpal Bose, Ni Guoying, McManus Donald P., and Cummins Scott F.. 2019. “A Biomphalaria glabrata Peptide that Stimulates Significant Behaviour Modifications in Aquatic Free-Living Schistosoma mansoni Miracidia.” PLoS Neglected Tropical Diseases 13: e0006948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2020. Weekly Epidemiological Record, Vol 95. Geneva: WHO. [Google Scholar]
- Zhang SM, Bu Lijing, Laidemitt Martina R., Lu Lijun, Mutuku Martin W., Mkoji Gerald M., and Loker Eric S.. 2018. “Complete Mitochondrial and rDNA Complex Sequences of Important Vector Species of Biomphalaria, Obligatory Hosts of the Human-Infecting Blood Fluke, Schistosoma mansoni.” Scientific Reports 8: 7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data (Gleichsner, 2022) are available from Zenodo: https://doi.org/10.5281/zenodo.6028760.


