Abstract
The coronavirus disease 2019 (COVID-19) pandemic is a major challenge for global healthcare systems. Early and safe triage in the emergency department (ED) is crucial for proper therapy. However, differential diagnosis remains challenging. Rapid antigen testing (RAT) may help to improve early triage and patient safety. We performed a retrospective study of 234 consecutive patients with suspected COVID-19 who presented to our ED in November 2020. All underwent SARS-CoV-2-nasopharyngeal swab testing using both RAT and reverse transcription polymerase chain reaction (RT-PCR). The inpatient treatment was established according to an empirically developed triage algorithm. The accuracy of the suggested algorithm was analyzed based on the rate of outpatients returning within 7 days and inpatients staying for less than 48 hours. COVID-19 inpatients and outpatients were compared for symptoms, vital signs, and C-reactive protein levels. Of the 221 included patients with suspected COVID-19 infection, the diagnosis could be confirmed in 120 patients (54.3%) by a positive RT-PCR result, whereas only 72% of those had a positive antigen test. Of the 56 COVID-19 outpatients, three returned within 7 days with the need for hospital treatment due to clinical deterioration. Among the 64 COVID-19 inpatients, 4 were discharged within 48 hours, whereas 60 stayed longer (mean duration 10.2 days). The suggested triage algorithm was safe and efficient in the first 234 consecutive patients. RAT can confirm a diagnosis in 72% of PCR proven COVID-19 patients and allows early cohort isolation as an important way to save hospital capacity.
Keywords: antigen test, clinical symptoms, COVID-19, emergency department, SARS-CoV-2 testing, triage
1. Introduction
With more than 492 million cases worldwide and more than 6 million fatalities (as of 07/04/2022), coronavirus disease 2019 (COVID-19) is an unprecedented situation for society and health care.[1]
While the majority of patients present with mild symptoms[2–5] and can be treated as outpatients, approximately 5% develop a critical disease with respiratory failure, shock, or multiorgan dysfunction.[5] The overall case fatality rate is estimated to be approximately 0.7 to 2.3%.[5,6] Severely ill COVID-19 patients and those with similar symptoms cross paths in the emergency department (ED). As the variety of COVID-19 symptoms resembles many common ED diagnoses, pure clinical triage is challenging and sometimes misleading.[7] Owing to the high infectiousness of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), it is crucial to separate patients with suspected COVID-19 from other patients as soon as possible to avoid further spread of the infection.
Furthermore, the rapidly increasing number of COVID-19 inpatients and outpatients during the onset of the second wave in October 2020 required quick and safe triage between COVID-19 patients and other diagnoses, as well as the need for inpatient treatment.
Reverse transcription polymerase chain reaction (RT-PCR) is still considered the gold standard for COVID-19 diagnosis[8]; however, it is time-consuming, costly, and can even produce false-negative results.[9] Rapid antigen testing (RAT) is widely available, inexpensive, fast, and allows for point-of-care testing (POCT).
Therefore, it may be reasonable to introduce RAT into our empirically developed clinical triage algorithm to save healthcare resources without compromising safety. Our proposed fast-track triage algorithm comprises a definite rule-in/out strategy for COVID-19 as well as a triage for inpatient treatment. We included RAT in the algorithm and evaluated its efficacy and safety in 234 patients.
We, therefore, hypothesized the following:
Our triage algorithm, which was developed empirically, can safely distinguish between COVID-19 inpatients and outpatients.
COVID-19 inpatients and outpatients are two distinctly different groups.
Implementing a RAT can help save hospital resources and improve patient safety.
2. Methods
2.1. Patients
We performed a retrospective single-center case–control study that included all patients presenting to our ED with symptoms indicative of COVID-19 during the onset of the second wave between November 10 and November 30, 2020. Upon arrival, two nasopharyngeal swabs were obtained from each patient by the same previously trained ambulance staff member and tested for SARS-CoV-2: one with RT-PCR and the other with a RAT. At least one of the following symptoms upon arrival to the ED was required for inclusion: sore throat, dyspnea, cough, fever, headache, chest pain, myalgia, fatigue, nausea, diarrhea, and/or dysgeusia. Patients without any of the aforementioned symptoms and those without valid nasopharyngeal swab results for PCR were excluded. The institutional ethics committee approved our study and waived the requirement for informed consent (file number: 20-9706-BO, date: 08 December 2020, Ethik-Kommission der Medizinischen Fakultät der Universität Duisburg-Essen). This study was registered in the German Clinical Trials Registry (trial number: DRKS00023659, date 14 January 2021). Patients and the general public were not involved in this study. Anonymized raw data were uploaded as a separate Supplemental file 1, Supplemental Digital Content, http://links.lww.com/MD/H720.
2.2. COVID-19 ED triage algorithm, Essen
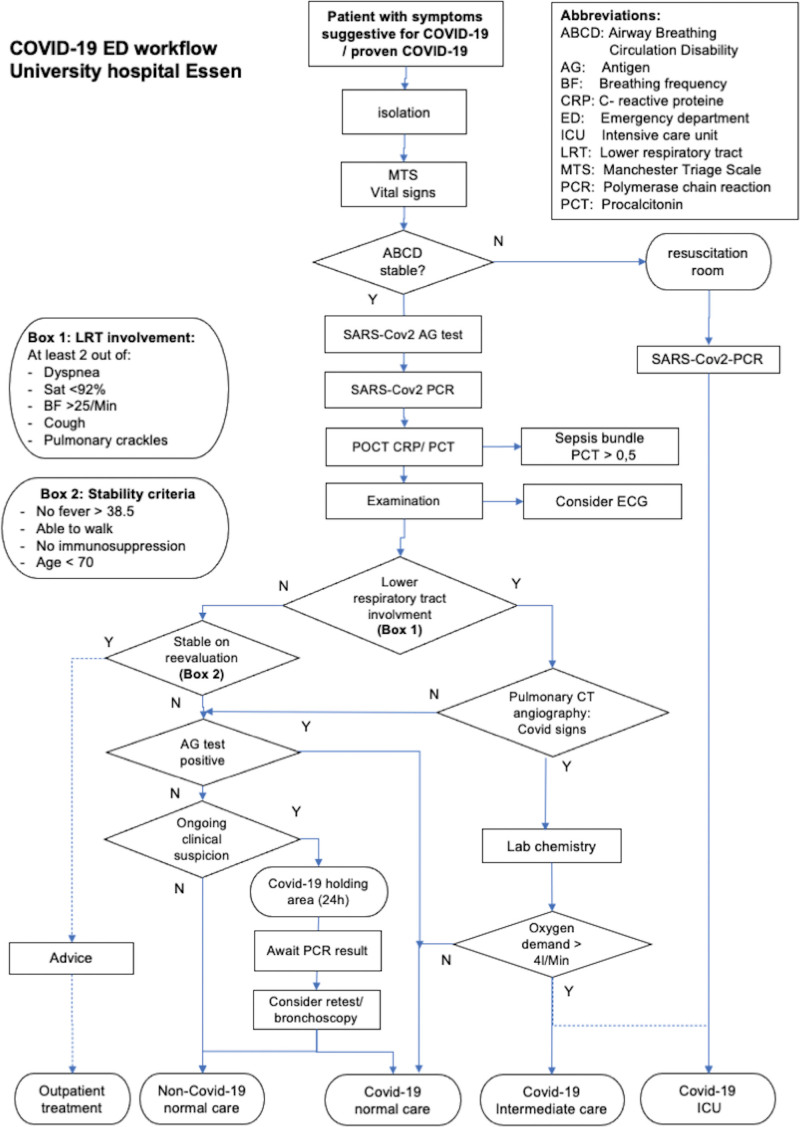
The University Hospital of Essen was the designated COVID-19 caregiver during the first and second waves of COVID-19 in Essen. Therefore, a separate COVID-19 ED was established at the beginning of November 2020, in a tent next to the non-trauma ED, for all patients with suspected COVID-19. A quick workflow (Fig. 1) was empirically developed during the first wave of COVID-19 to filter out those who needed inpatient care.
Figure 1.
Flowchart for COVID-19 Triage. COVID-19 = coronavirus disease 2019.
All patients with suspected COVID-19, either self-presenting or by ambulance service, were isolated upon arrival and received their first triage in the COVID-19 ED, consisting of the Manchester Triage Scale (MTS) rating and vital parameters. Patients with unstable ABCD were immediately transferred to a resuscitation room to receive intensive care treatment, including respiratory support with mechanical noninvasive or invasive ventilation, when required.
All stable patients received a double nasopharyngeal swab for SARS-CoV-2 rapid antigen and PCR testing by the same trained ED staff.
A SARS-CoV-2 nasopharyngeal swab (ViroCult®; Medical Wire & Equipment Co. Ltd., Corsham, Wiltshire, UK) was used for the PCR testing. To detect SARS-CoV-2, RT-PCR (SARS-CoV-2 RT-PCR Kit 1.0, Altona Diagnostics GmbH, Hamburg, Germany; Alinity m SARS-CoV-2 assay, Abbott Molecular Inc., Des Plaines, IL, USA; Xpert Xpress SARS-CoV-2, Cepheid, Sunnyvale, CA, USA) was performed. The antigen test (SARS-CoV-2 Rapid Antigen Test, Roche Diagnostics GmbH, Mannheim, Germany) was performed according to the manufacturer’s recommendations, and results were obtained after 15 min, measured using an alarm watch, and documented in the digital patient chart.
All patients underwent POCT for C-reactive proteine. Electrocardiography (ECG) was performed in patients with suspected concomitant cardiac disease. Additional laboratory testing and computed tomography (CT) pulmonary angiography were performed when symptoms of lower respiratory tract involvement (at least two out of five criteria: saturation <92%, breathing frequency >25 breaths/min, pulmonary crackles, dyspnea, and/or cough) were present. All patients with lower respiratory tract involvement and/or pattern of COVID-19 pneumonia on CT scan, as well as those in an unstable clinical condition, were considered inpatients. If the RAT was positive, patients were considered to have COVID-19; otherwise, they were admitted to a holding area to await PCR results and underwent retesting/bronchoscopy in case of ongoing suspicion. Strict isolation measures were maintained until COVID-19 was definitely ruled out.
Stable patients without lower respiratory tract involvement, according to the algorithm (Fig. 1), were informed about the COVID-19 diagnosis and received isolation advice in cases of positive antigen testing before discharge. Outpatients with negative antigen testing results were advised to maintain isolation until PCR results were available.
The efficiency and safety of the algorithm were evaluated by two surrogate parameters:
1) need for hospital treatment in outpatients returning within 7 days after first ED contact as a signal for “undertriage”.
2) discharge of inpatients within 48 hours after admission as a signal of “overtriage”.
All outpatients were instructed to return to our ED in case of clinical deterioration.
2.3. Parameters
We compared and analyzed clinical parameters, MTS categories, and laboratory parameters between inpatients and outpatients with positive RT-PCR swab results for SARS-CoV-2.
Clinical parameters were symptoms upon arrival comprising dyspnea, sore throat, cough, fever, headache, fatigue, myalgia, chest pain, nausea, diarrhea, and dysgeusia.
Laboratory results included white blood cell count, lymphocytes, C-reactive protein, procalcitonin, glomerular filtration rate, creatinine, troponin, and D-dimer in inpatients and POCT C-reactive protein in outpatients.
The return of outpatients within 7 days was analyzed according to the electronic medical record and duration of stay of less than 48 hours as surrogate parameters of efficiency. Patient data were obtained from electronic medical records (ERPath, eHealth-Tec Innovations GmbH, Berlin, Germany; Medico, Cerner Health Services GmbH, Idstein, Germany). Missing data that could not be extracted from the patients’ records were excluded from statistical analysis.
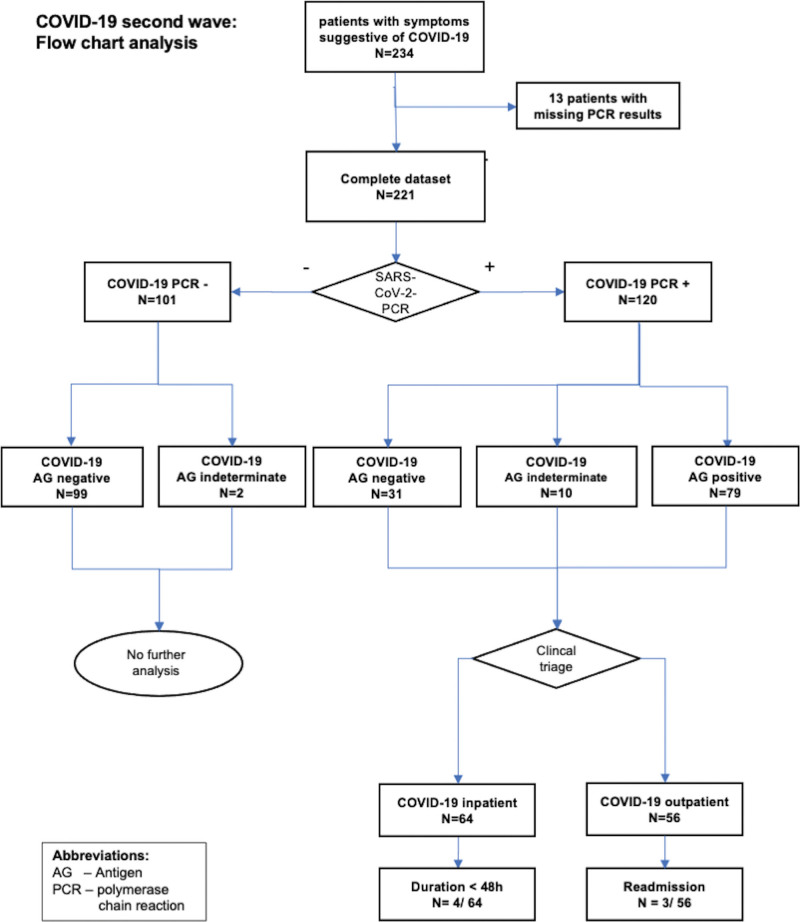
Furthermore, the results of the antigen and RT-PCR tests were compared and analyzed using the cycle threshold (Ct) values of the RT-PCR test. Missing antigen test results, most likely due to pretesting outside without available results or documentation errors, were categorized as “indeterminate” antigen test, and excluded from the analysis (Fig. 2).
Figure 2.
Flowchart analysis.
2.4. Statistical analyses
A t-test was used to evaluate the data. To assess equality of variance, the data were tested using Levene test. Welch t-test was used to analyze the metric data for unequal variances. Results are reported as the mean ± standard deviation for metric variables. Pearson Chi-square test or Fisher exact test was used to evaluate categorical data. The results for categorical variables were reported as percentages. All data were analyzed using SPSS version 26 (IBM, Armonk, NY, USA). Statistical significance was defined as two-tailed P < .05.
3. Results
3.1. Readmission of PCR positive outpatients within 7 days and hospital discharge of inpatients within 48 hours
The 120 PCR positive patients were divided into outpatients (n = 56, 46.7%) and inpatients (n = 64, 53.3%) using the abovementioned COVID-19 ED triage algorithm of Essen (Fig. 1). Of the 56 COVID-19 outpatients, eight (14.3%) returned within 7 days with need for hospitalization in three cases (3/56, 5.4%) due to clinical deterioration, while the other five patients remained outpatients. Among the 64 COVID-19 inpatients, four (4/64, 6.25%) were discharged within 48 hours, whereas 60 (60/64, 93.75%) stayed longer (mean duration of 10.2 days).
3.2. Comparison of PCR positive inpatients and outpatients with reference to the COVID-19 ED triage algorithm of Essen
Among the 120 PCR-positive patients (59.7 ± 20 years of age), inpatients were significantly older than outpatients (69.1 years vs 49 years; P = .008) (Table 1). Of the hospitalized patients and outpatients, 51.6% and 48.2% were male, respectively.
Table 1.
Characteristics of COVID-19 patients.
| All COVID-19 + (n = 120) | Inpatients (n = 64) | Outpatients (n = 56) | P-value | |
|---|---|---|---|---|
| Age, mean (±SD, range) | 59,7 (±20,0, 18-92) | 69.1 (±18.2, 18-92) | 49.0 (±16.3, 23-91) | .008 |
| Male gender, n (%) | 60 (50.0) | 33 (51.6) | 27 (48.2) | .855 |
| Manchester triage, n (%) | ||||
| Red | 6 (5.0) | 6 (9.4) | 0 (0) | .029 |
| Orange | 8 (6.7) | 8 (12.5) | 0 (0) | .024 |
| Yellow | 30 (25.0) | 21 (32.8) | 9 (16.1) | .038 |
| Green | 60 (50.0) | 27 (42.2) | 33 (58.9) | .999 |
| Blue | 16 (13.3) | 2 (3.1) | 14 (25.0) | <.001 |
| Initial presentation, n (%) | ||||
| Self | 30 (25.0) | 7 (10.9) | 23 (41.1) | <.001 |
| Ambulance | 87 (72.5) | 56 (87.5) | 31 (55.3) | <.001 |
| Miscellaneous | 3 (2.5) | 1 (1.6) | 2 (3.6) | .598 |
COVID-19 = coronavirus disease 2019.
The 64 inpatients were categorized by MTS into five groups consisting of 6 “red” (9.4%), 8 “orange” (12.5%), 21 “yellow” (32.8%), 27 “green” (42.2%), and 2 “blue” patients (3.1%). Outpatients did not appear in “red” and “orange” triage groups, but there were 9 “yellow” (16.1%), 33 “green” (58.9%), and 14 “blue” patients (25%). Inpatients were more often admitted by ambulance (87.5% vs 55.3%) and presented less by themselves (10.9% vs 41.1%) (Table 1).
In the group of PCR positive patients, outpatients seemed to have significantly fewer comorbidities when comparing positivity for cardiac (8.9% vs 42.2%; P < .001), pulmonary (7.1% vs 20.3%; P = .039), and renal medical history (7.1% vs 21.9 %; P = .024) (Table 2).
Table 2.
Results of COVID-19 patients.
| All (n = 120) | Inpatients (n = 64) | Outpatients (n = 56) | P-value | |
|---|---|---|---|---|
| Medical history, positive for, n (%) | ||||
| Cardiac | 32 (26.7) | 27 (42.2) | 5 (8.9) | <.001 |
| Pulmonary | 17 (14.2) | 13 (20.3) | 4 (7.1) | .039 |
| PE/thrombosis | 1 (0.8) | 1 (1.6) | 0 (0.0) | .348 |
| Renal | 18 (15.0) | 14 (21.9) | 4 (7.1) | .024 |
| Cancer | 13 (10.8) | 10 (15.6) | 3 (5.4) | .071 |
| Symptom onset (days),mean (±SD) | 5.2 (4.5) | 6.3 (5.6) | 4.1 (2.6) | .044 |
| Symptoms, n (%) | ||||
| Dyspnea | 56 (50.0) | 40 (62.5) | 16 (28.6) | <.001 |
| Sore throat | 19 (15.8) | 8 (12.5) | 11 (19.6) | .285 |
| Cough | 51 (42.5) | 33 (51.6) | 18 (32.1) | .070 |
| Fever | 58 (48.3) | 33 (51.6) | 25 (44.6) | .449 |
| Headache | 37 (30.8) | 13 (20.3) | 24 (42.9) | .008 |
| Fatigue | 55 (45.8) | 25 (39.1) | 30 (53.6) | .112 |
| Myalgia | 42 (35.0) | 20 (31.3) | 22 (39.3) | .357 |
| Chest pain | 8 (6.7) | 1 (1.6) | 7 (12.5) | .017 |
| Nausea | 26 (21.7) | 16 (25.0) | 10 (17.9) | .343 |
| Diarrhea | 17 (14.2) | 8 (12.5) | 9 (16.1) | .576 |
| Dysgeusia | 24 (20.0) | 11 (17.2) | 13 (23.2) | .410 |
| Vital parameters, mean (±SD) | ||||
| Respiratory rate/min (±SD) | 19.7 (±7.9) | 22.0 (±8.4) | 15.0 (±3.7) | <.001 |
| Heart rate/ min (±SD) | 93.2 (±19.9) | 94.0 (±22.8) | 92.2 (±16.0) | .039 |
| Saturation, O2 in % (±SD) | 93.3 (±7.6) | 89.8 (±8.3) | 97.5 (±3.2) | <.001 |
| Temperature in °C,(±SD) | 37.1 (±1.1) | 37.3 (±1.1) | 36.8 (±1.1) | .640 |
| BP systolic in mm Hg (±SD) | 130.4 (±22.0) | 133.4 (±25.0) | 126.8 (±17.4) | .225 |
| BP diastolic in mm Hg (±SD) | 80.9 (±18.9) | 77.7 (±22.2) | 84.6 (±13.5) | .339 |
| Pulmonary embolism, n (%) | 5 (4.2) | 5 (7.8) | 0 (0.0) | .033 |
| Death (in hospital), n (%) | 23 (35.9) | |||
| Duration of stay, days, mean (±SD) | 10.2 (6.9) | |||
| Treatment (in hospital), n (%) | ||||
| O2-therapy | 39 (60.9) | |||
| Mechanical ventilation | 9 (14.1) | |||
| Non invasive ventilation | 17 (26.6) | |||
| Intensive care | 8 (12.5) | |||
| Intermediate care | 2 (3.1) | |||
| Time of admission (days) | 10.2 (±6.9) | |||
| Laboratory values | ||||
| C-reactive proteine, mg/dL | 12.06 (±8.56) | 3.80 (±4.11) | .013 | |
| Procalcitonine, µg/L (±SD) | 0.30 (±0.68) | |||
| LDH, U/L (±SD) | 444.5 (±215.1) | |||
| Creatinine, mg/dL (±SD) | 1.40 (±1.28) | |||
| GFR, mL/min (±SD) | 46.9 (±16.56) | |||
| D-dimer, mg/L (±SD) | 2.55 (±3.53) | |||
| WBC/ mm³ (±SD) | 7.06 (±3.80) | |||
| Lymphocytes/mm³ (±SD) | 1.09 (±1.46) | |||
COVID-19 = coronavirus disease 2019.
Bold significant results (P < 0.05).
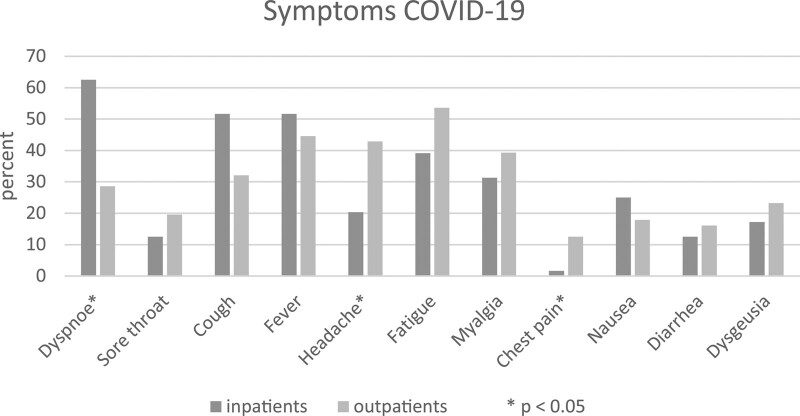
The mean onset of symptoms before ED presentation was 6.3 days in inpatients and 4.1 days in outpatients. Prospective COVID-19 inpatients reported dyspnea significantly more often (62.5% vs 28.6%; P < .001) but less headache (20.3% vs 42.9%; P = .008) and chest pain (1.6% vs 12.5%; P = .017). No significant differences were found in sore throat, cough, fever, fatigue, myalgia, nausea, diarrhea, or dysgeusia (Fig. 3).
Figure 3.
Symptoms of COVID-19 patients. COVID-19 = coronavirus disease 2019.
In terms of vital parameters, the inpatients demonstrated a distinctly higher respiratory rate (mean respiratory rate of 22/minute vs 15/minute; P < .001) and a slightly higher heart rate (94/minute vs 92.2/minute; P = .039). Saturation was lower as well in the prospective COVID-19 inpatients (mean SaO2 89.8% vs 97.5%; P < .001). We did not identify any significant differences in blood pressure and temperature. Nevertheless, a difference was observed in the laboratory parameters of C-reactive protein, with a mean value of 12.06 mg/dL in inpatients and 3.80 mg/dL in outpatients (P = .013).
Among the prospective COVID-19 inpatients, 60.9% needed oxygen therapy, 26.6% needed noninvasive ventilation, and 14.1% needed invasive mechanical ventilation. Five of the 64 COVID-19 inpatients (7.8%) presented with pulmonary embolism on the CT pulmonary angiography. In total, 23/64 inpatients (35.9%) died during hospital stay.
3.3. SARS-CoV-2 PCR and RAT results
Regarding the 234 consecutive patients entering our ED with symptoms suggestive of COVID-19, we had to exclude 13 patients (13/234, 5.6%) due to missing PCR results (Fig. 3). The remaining 221 patients were all tested for SARS-CoV-2 PCR, and 101/221 (45.7%) demonstrated a negative result. On the contrary, 120/221 patients (54.3%) were diagnosed as COVID-19 PCR positive. Of the 120 PCR-positive patients, 110/120 was tested for SARS-CoV-2 RAT in our ED. The results showed that 79/110 (71.8%) were antigen positive, but 31/110 (28.2%) were a negative antigen test. For the PCR negative patients, RAT was performed in 99/101 cases, of which all were antigen negative (99/99, 100%). Combining patients with positive and negative PCR tests and matched RATs, 209 double tests (110 PCR-positive and 99 PCR-negative patients) were performed. The sensitivity of antigen testing with respect to PCR as the gold standard was 71.8% (positive predictive value of 100%), while the specificity was 100% (negative predictive value of 76.2%) (Table 3).
Table 3.
Sensitivity/specificity of AG test.
| PCR + | PCR- | Total | |
|---|---|---|---|
| Antigen+ | 79 | 0 | 79 |
| Antigen- | 31 | 99 | 130 |
| Total | 110 | 99 | 209* |
| *Indeterminate antigen tests (N = 2 in the PCR- group and N = 10 in the PCR+ group) are not included in the analysis | |||
| Sensitivity | 79/110 = 71.82% | ||
| Specificity | 99/99 = 100% | ||
| Positive predictive value (PPV) | 79/79 = 100% | ||
| Negative predictive value (NPV) | 99/130 = 76.15% | ||
AG = antigen, PCR = reverse transcription polymerase chain reaction.
3.4. Comparison of Ct-values for groups of PCR positive/AG positive and PCR positive/AG negative patients
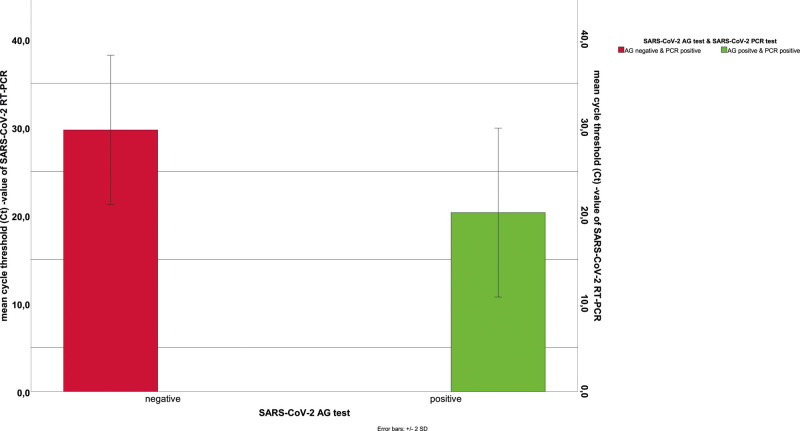
For further analysis, the cycle threshold (Ct) values of the 110 PCR-positive patients were compared between the PCR-positive/AG-positive (79 COVID-19 patients) and PCR-positive/AG-negative patients (31 COVID-19 patients). In the case of the double positive tests, the mean Ct value of SARS-CoV-2 PCR was 20.3 ± 4.8. With high significance (P < .001), the PCR-positive/AG negative group presented mean Ct-values of 29.7 ± 4.2 (Fig. 4). The mean time to symptom onset in this group was 6.5 days. Double-positive patients reported approximately 4.4 days of ongoing infection.
Figure 4.
Comparison of Ct-values in the AG/PCR test. AG = antigen, Ct value = cycle threshold value, PCR = reverse transcription polymerase chain reaction.
4. Discussion
4.1. The efficacy and safety of the triage algorithm
Our established triage algorithm (Fig. 1) proved to be safe and efficient. With a low rate of return (14.3%), a hospital admission rate of only 5.4%, and, to our knowledge, no deaths among the 56 COVID-19 patients initially triaged as “outpatients”, the safety of our outpatient criteria is given. Considering the unpredictable course of the disease in individual patients with lower respiratory tract involvement often occurring later in the course of a complicated disease, the aforementioned rate is acceptable. Most patients without respiratory compromise or signs of clinical instability, such as persistent high fever, can be treated safely as outpatients as long as they are properly instructed about “red flags” for return.
Among the inpatients, only 6% were discharged within 48 hours, suggesting no “real” need for inpatient treatment among those. The remaining 94% had a mean duration of stay of 10.2 days, underlining the need for elaborate clinical treatment. A similar duration of hospital stay was reported by Wang et al[4] In addition to the need for hospital treatment in cases of viral pneumonia with respiratory impairment, we extended our admission criteria to high fever, frailty, and extreme fatigue to avoid focusing purely on pneumonia.
4.2. Differences between COVID-19 inpatients and outpatients
With a significantly higher rate of cardiac, pneumological, and renal preconditions and a higher mean age (69.1 years vs. 49.0 years), our COVID-19 inpatient group demonstrates the more severe clinical course of COVID-19 in elderly and chronically ill patients.[5,10]
In accordance with the earlier symptom onset, the inpatient group had dyspnea, lower oxygen saturation, and a higher respiratory rate significantly more often. A higher C-reactive protein level is considered a marker of severe disease and can also be documented here.[11] The need for oxygen treatment in 61% of inpatients, noninvasive ventilation in 27%, mechanical ventilation in 14%, and an overall mortality of 36% underlines the severity of the disease and is in good accordance with the high mortality of hospitalized COVID-19 patients reported by other groups.[10] Our tertiary care center, which offers all therapeutic options, including extracorporeal membrane oxygenation (ECMO), is even more likely to host more severely ill COVID-19 patients with a poorer prognosis.
Headache and chest pain were reported significantly more often in the outpatient group, suggesting flu-like disease course. Interestingly, there was no significant difference in sex between the two groups, whereas male sex was considered a main risk factor for an unfavorable course.[12]
Our data illustrate that COVID-19 inpatients and outpatients are two distinctly different patient groups, divided by the involvement of the lower respiratory tract, viral pneumonia, and compromised by fatigue and fever in mostly elderly patients.
4.3. The value of SARS-CoV-2 RAT in the setting of our ED at the University Hospital of Essen
With a positive predictive value of 100%, the RAT is demonstrated as a cheap and reliable tool for early cohort isolation measures in prospective hospitalized COVID-19 patients. Sparse resources in terms of rooms can be saved with quick results after 15 minutes. In addition, outpatients can be directly informed in the ED about a secure positive test result and advice for quarantine can be provided.
Regarding the negative predictive value of 76.2%, we conclude that one cannot rule out COVID-19 in a single SARS-CoV-2 RAT in ED. RT-PCR remains the gold standard for this purpose. With respect to outpatients with negative RAT results, education about possible existing COVID-19 infection is a top priority. RT-PCR test results must be awaited in home isolation, and the rules of local health authorities should be followed. Negative RAT results in prospective COVID-19 inpatients should be questioned in cases of suitable anamnesis and clinical presentation. Thus, our triage algorithm is effective.
Analysis of PCR Ct values in PCR-positive/AG-negative and PCR-positive/AG-positive patients might explain the unsatisfactory sensitivity and negative predictive value of RAT. The Ct values in PCR-positive/AG-negative patients were significantly higher, suggesting that RAT loses sensitivity when there is a lower nasopharyngeal viral load (Fig. 4). Similar findings have also been reported in other studies, showing a loss of RAT sensitivity with increasing Ct-values.[13–15]
This assumption is supported by the longer duration of ongoing infection in patients with a negative RAT. The nasopharyngeal viral load may have been lower because of the spread of infection in the lower respiratory tract.[16,17]
4.4. Limitations
Our study has a few limitations. Data collection was retrospective. Therefore, selection bias and data entry errors cannot be completely excluded. This study is a single center study, and for this reason, the data should not be generalized. Case numbers were limited because the study was an early “proof of concept” when patient numbers started to rise during the onset of the second wave.
We cannot completely exclude the possibility that deteriorating outpatients presented to other caregivers but strongly believe that it was very unlikely, as the University Hospital of Essen is the designated COVID-19 caregiver for the city, and all patients were instructed to return to our ED in case of worsening symptoms.
5. Conclusions
Our proposed triage algorithm allows rapid and safe triage between COVID-19 inpatients and outpatients, as well as a reliable diagnosis of the disease.
We demonstrated that COVID-19 inpatients and outpatients are two distinct groups in terms of the clinical picture and severity of the disease.
RAT as point-of-care tool has a high positive predictive value in ruling in the diagnosis and can therefore save hospital capacities by early cohort isolation. Additionally, outpatients awaiting definitive PCR results can be considered positive and undergo strict home isolation, thereby avoiding further spread of the virus.
As the rate of false-negative results was nearly 30% in our study, RAT cannot be recommended as the diagnostic tool of choice, and PCR remains the gold standard.
Author contributions
DF, TH, JR, OA, and RM contributed to data acquisition.
DF, DP, TH, OA, and JR contributed to data analysis.
DF, TH, and JR interpreted the data.
DF, DP, TH, CK, OA, JR, RM, and SD drafted and substantially revised the manuscript.
All authors read and approved the final manuscript.
Conceptualization: David Fistera, Sebastian Dolff.
Data curation: David Fistera, Randi Manegold, Tobias Hoelscher.
Formal analysis: David Fistera, Dirk Pabst.
Methodology: Clemens Kill, Olympia E. Anastasiou, Randi Manegold.
Supervision: Clemens Kill, Joachim Risse.
Validation: Olympia E. Anastasiou.
Writing – original draft: David Fistera, Tobias Hoelscher.
Writing – review & editing: Clemens Kill, David Fistera, Dirk Pabst, Olympia E. Anastasiou, Randi Manegold, Sebastian Dolff, Tobias Hoelscher.
Acknowledgements
We thank all the emergency department employees for their support during difficult times.
We thank Mr. Henrik Braitsch for his excellent support in extracting patient data from the medical records.
Supplementary Material
Abbreviations:
- AG =
- antigen
- COVID-19 =
- coronavirus disease 2019
- CT =
- computed tomography
- Ct value =
- cycle threshold value
- ED =
- emergency department
- MTS =
- Manchester Triage Scale
- POCT =
- point-of-care testing
- RAT =
- rapid antigen testing
- RT-PCR =
- reverse transcription polymerase chain reaction
- SARS-CoV-2 =
- severe acute respiratory syndrome coronavirus type 2.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].Informed consent was waived by Ethik-Kommission der Medizinischen Fakultät der Universität Duisburg-Essen because only anonymized data was used (File number: 20-9706-BO, Date: 08 December 2020).
This study was registered in the German Clinical Trials Registry (trial number: DRKS00023659, date 14 January 2021). The study was conducted in accordance with current guidelines and the Declaration of Helsinki.
The authors have no funding and conflicts of interest to disclose.
Our study was approved by the institutional ethics committee (Ethik-Kommission der Medizinischen Fakultät der Universität Duisburg-Essen).
Supplemental Digital Content is available for this article.
How to cite this article: Fistera D, Hoelscher T, Pabst D, Manegold R, Anastasiou OE, Dolff S, Kill C, Risse J. Evaluation of a new COVID-19 triage algorithm in the emergency department including combined antigen and PCR-testing: A case–control study. Medicine 2022;101:42(e31278).
Contributor Information
Dirk Pabst, Email: dirk.pabst@uk-essen.de.
Randi Manegold, Email: randikatrin.manegold@uk-essen.de.
Olympia E. Anastasiou, Email: olympiaevdoxia.anastasiou@uk-essen.de.
Sebastian Dolff, Email: sebastian.dolff@uk-essen.de.
Clemens Kill, Email: clemens.kill@uk-essen.de.
Joachim Risse, Email: joachim.risse@uk-essen.de.
References
- [1].WHO. Coronavirus disease (COVID-2019) situation reports 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200607-covid-19-sitrep-139.pdf?sfvrsn=79dc6d08_2 [access date April, 07, 2022].
- [2].Bajema KL, Oster AM, McGovern OL, et al. Persons evaluated for 2019 novel coronavirus ‐ United States, January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42. [DOI] [PubMed] [Google Scholar]
- [6].Guan W-j, Ni Z-y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fistera D, Pabst D, Härtl A, et al. Separating the wheat from the chaff ‐ COVID-19 in a German emergency department: a case–control study K1 ‐ Medizin. Int J Emerg Med. 2020;13:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fang FC, Naccache SN, Greninger AL. The laboratory diagnosis of coronavirus disease 2019- frequently asked questions. Clin Infect Dis. 2020;71:2996–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Long DR, Gombar S, Hogan CA, et al. Occurrence and timing of subsequent SARS-CoV-2 RT-PCR positivity among initially negative patients. Clin Infect Dis. 2020;72:323–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of covid-19 in New York City. N Engl J Med. 2020;382:2372–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Krüttgen A, Cornelissen CG, Dreher M, et al. Comparison of the SARS-CoV-2 Rapid antigen test to the real star Sars-CoV-2 RT PCR kit. J Virol Methods. 2021;288:114024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Paul G, Plecko T, Sethi S, et al. Klinische Performance eines neuen SARS-CoV-2-Antigen-Tests in der Notaufnahme eines Maximalversorgers. Epid Bull. 2020;3:13–8. [Google Scholar]
- [15].Holzner C, Pabst D, Anastasiou OE, et al. SARS-CoV-2 rapid antigen test: Fast-safe or dangerous? An analysis in the emergency department of an university hospital. J Med Virol. 2021;93:5323–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–9. [DOI] [PubMed] [Google Scholar]
- [17].Cevik M, Tate M, Lloyd O, et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2:e13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.