Background:
There is no established cryptococcal antigen (CrAg) screening guideline for people with HIV who are antiretroviral therapy experienced but have poor virologic control. We assessed factors associated with CrAg screening and describe missed opportunities for earlier testing.
Setting:
Ambulatory clinics affiliated with Montefiore Medical Center, Bronx, NY.
Methods:
This was a retrospective chart review of CrAg screening among asymptomatic people with HIV with absolute CD4 counts200 cells/mm3 and HIV viral loads (VLs) > 200 copies/mL receiving HIV care from 2015 to 2020. We used Cox proportional hazards regression to identify predictors of screening, including longitudinal CD4 count and HIV VL as time-varying covariables. Among cases of diagnosed cryptococcosis, we assessed for opportunities for earlier diagnosis.
Results:
Screening CrAg was performed in 2.9% of 2201 individuals meeting the inclusion criteria. Compared with those not screened, those who were screened had a shorter duration of HIV infection (0.09 vs. 5.1 years; P = 0.001) and lower absolute CD4 counts (12 vs. 24 cells/mm3; P < 0.0001). In a multivariable model stratified by median HIV duration, CD4 < 100 [hazard ratio (HR), 7.07; 95% confidence interval (CI): 2.43 to 20.6], VL > 10,000 (HR, 15.0; 95% CI: 4.16 to 54.0), and a shorter duration of HIV infection (HR, 0.60; 95% CI: 0.42 to 0.86) were associated with screening for those with HIV < 5 years. Among those diagnosed with cryptococcosis (n = 14), 6 individuals had an ambulatory visit in the preceding 6 months but did not undergo screening.
Conclusion:
CrAg screening was infrequently performed in this at-risk population. Those with a longer duration of HIV infection were less likely to undergo CrAg screening, highlighting potential missed opportunities for earlier diagnosis.
Key Words: cryptococcal antigen test, screening, cryptococcosis, HIV, missed opportunities, minorities
INTRODUCTION
There has been remarkable progress toward ending the HIV epidemic in the United States since the first official reporting of AIDS by the Centers for Disease Control and Prevention in 1981.1,2 However, disparities exist, and people who face barriers to obtaining health care are disproportionately affected by delayed HIV diagnoses and poor outcomes, including morbidity and mortality from opportunistic infections.3,4
The proportion of cryptococcosis cases among people with HIV has declined in the United States.5,6 However, most cryptococcosis cases at Montefiore Medical Center (MMC) in the Bronx, NY, continue to occur among people with HIV rather than HIV-negative individuals.7 The Bronx, which is among the poorest urban congressional districts in the United States, is a racially and ethnically diverse borough of 1.4 million people, ∼32% of whom are in care at MMC, the largest health care provider in the Bronx.8 In the era of antiretroviral therapy (ART) and widely available cryptococcal antigen (CrAg) screening, cryptococcal meningitis (CM) should be preventable. However, at MMC, there were 79 cases of cryptococcosis in people with HIV, among which 65% presented with CM, between 2005 and 2017, with a 1-year mortality of 13%, although mortality as high as 48% was reported in another US institution, especially among individuals with low socioeconomic status.9 These studies underscore the disproportionate burden of CM among historically marginalized populations and highlight missed opportunities for earlier diagnosis and prevention.10 This study was undertaken to understand gaps in the HIV care continuum that may lead to missed opportunities for CrAg screening and subsequent institution of preemptive antifungal therapy.
CrAg lateral flow assays can detect serum CrAg in a median of 22 days before the development of symptoms,11 providing a window of opportunity for early diagnosis. This is important because asymptomatic CrAg positivity is an independent predictor of subsequent neurocognitive deficits,12 CM, and death.13,14 In the ART era when >50% of HIV-associated cryptococcal disease was diagnosed in ART-experienced individuals,7,15,16 ART-experienced vs. ART-naive status was associated with asymptomatic cryptococcal antigenemia among people with HIV with CD4 counts <100 cells/mm3.14 In the United States, CrAg positivity was 2.5% in a prevalence study of stored sera from individuals with CD4 counts <100 cells/mm3 and no known history of cryptococcal disease conducted from 1986 to 2012.5 Although there is no US prevalence data from the past decade of the ART era, a cost-effectiveness study showed CrAg screening in the United States to be cost-saving when applied to a population with prevalence of cryptococcal antigenemia as low as 0.1%.17 The cost of treating CM is extremely high in the United States.18 By contrast, CrAg lateral flow assay strips are more than several thousand folds less expensive.
At present, there is no formal guidance on screening people with HIV who are ART experienced with poor virologic control. This is particularly problematic in areas where the HIV care continuum is challenged by barriers to health care due to high rates of adverse social and structural determinants of health (SSDoH). To better understand current CrAg screening practices in a large urban medical center at the epicenter of the ongoing HIV pandemic in NYC, we analyzed CrAg screening patterns and identified missed opportunities for earlier CrAg testing in the Bronx, NY.
METHODS
Study Design and Setting
This was a retrospective chart review study of serum CrAg screening among people with HIV receiving care at clinics affiliated with the MMC, the largest HIV care provider in the Bronx, NY, between January 1, 2015, and December 31, 2020. The study was approved by the Albert Einstein College of Medicine Institutional Review Board with a waiver of informed consent.
Study Population
CrAg screening was analyzed for people with HIV with absolute CD4 counts200 cells/mm3 and HIV viral loads (VLs) > 200 copies/mL at any point during the study period who sought care at an ambulatory HIV care clinic at least once during the study period. Individuals were excluded if they had a previous diagnosis of cryptococcal disease or CM identified by the International Classification of Diseases, Clinical Modification (ICD-CM), 9th (117.5, 321.0) and 10th Revision (B45.9, B45.1) diagnosis codes or positive serum or cerebrospinal fluid (CSF) CrAg. Individuals who underwent diagnostic CrAg testing were excluded from the primary analysis.
To assess for missed opportunities for earlier diagnosis of cryptococcal disease, we reviewed charts of all patients with absolute CD4 counts200 cells/mm3 and HIV VLs > 200 copies/mL who also had an ICD-9 or 10-CM diagnostic code for cryptococcosis during the study period.
Data Source
Data were extracted from the Einstein-Rockefeller-CUNY Center for AIDS Research Clinical Cohort Database, which includes data for all MMC patients with laboratory-confirmed HIV infection since 1997.19 Data collected include demographic characteristics (age, sex, race, and ethnicity), HIV transmission risk category, date of HIV diagnosis, diagnosis of cryptococcal disease or meningitis identified by ICD-9 or 10 diagnosis codes, ambulatory visit data, date of the last contact with the MMC, death date (when available), and serial laboratory studies (CD4 counts, HIV VLs, and serum and CSF CrAg tests).
Definitions
Cryptococcosis was identified by ICD-9 or 10 codes and confirmed by chart reviews as having positive serum or CSF CrAg or isolation of Cryptococcus neoformans in culture and clinical signs or symptoms of the disease involving the central nervous system, lungs, bloodstream, or skin.
A serum CrAg test was classified as either a screening or diagnostic test depending on the absence (screening) or presence (diagnostic) of clinical symptoms suggestive of cryptococcosis during the ambulatory visit when the test was performed. Suggestive symptoms included cough, shortness of breath, chest pain, fever, headache, neck pain, nausea, vomiting, neurological changes, fatigue, or skin rash. Three study team members manually reviewed medical records to classify the CrAg tests performed in the ambulatory setting as either screening or diagnostic tests based on documented symptoms on visit notes. Individuals who did not have CrAg testing performed were assumed to be asymptomatic.
For cases of cryptococcosis identified during the study period, missed opportunities for screening were assessed. A missed opportunity was defined as an ambulatory visit within the 6 months before the cryptococcosis diagnosis if CrAg screening was not performed and the latest CD4 count to which the provider had access was <200 cells/mm3. The 6-month time frame was based on evidence that CrAg may be present weeks to months before symptom onset.11
Follow-up time was defined as the time between study entry (first meeting CD4 and VL criteria) and date of the CrAg screening test, cryptococcosis diagnosis, death, last known contact with the MMC, or the end of the study period December 31, 2020, whichever occurred first. Change in CD4 to >200 cells/mm3 or VL < 200 copies/mL during the follow-up time was not a censoring criterion. The duration of HIV infection was calculated by subtracting the date of HIV diagnosis from the date of study entry.
Study Outcomes
The primary outcome was the association between hypothesized predictors (CD4 count, HIV VL, age, ethnicity, race, and length of HIV infection) and time to an incident CrAg screening test for asymptomatic people with HIV who had at least 1 ambulatory clinic visit.
In an exploratory analysis, missed opportunities for screening were assessed in people with HIV who were newly diagnosed with cryptococcosis during the study period.
Statistical Methods
Baseline characteristics by CrAg screening status were compared using Wilcoxon rank-sum tests for continuous and Fisher exact tests or χ2 tests for categorical variables. We performed Cox proportional hazards regression to estimate the hazard ratio (HR) and 95% confidence interval (CI) of the incident CrAg screening test. Serial CD4 count and HIV VL were included in the Cox model as time-varying covariables because the last available values may affect the provider's decision to perform CrAg testing. The CD4 cell count was categorized as <100, 100–200, 200–500, and >500 (reference category) cells/mm3 and the HIV VL as <200 (reference category), 200–10,000, and >10,000 copies/mL. Covariables assessed in the bivariate analysis were CD4 count, HIV VL, age, sex, race/ethnicity, and duration of HIV infection. Variables with a P value <0.10 on the bivariate analysis were included in the multivariable analysis. Testing for proportional hazards assumption was conducted by Schoenfeld residuals and inspecting log–log survival plots. P < 0.05 (2-tailed sides) was considered significant. All statistical analyses were performed in Stata/IC 16.1 software (StataCorp LLC, College Station, TX).
RESULTS
Baseline Characteristics of People With HIV by CrAg Screening Status
A total of 2201 people with HIV who entered the study with absolute CD4 counts200 cells/mm3 and HIV VLs > 200 copies/mL, had at least 1 ambulatory visit at an HIV care site between 2015 and 2020 and were asymptomatic at visits were eligible for the primary outcome analysis (Fig. 1A). The overall cohort had a median age of 45.7 years [interquartile range (IQR), 33.0–55.0], 64.7% men, 52.5% Black, 38.0% Hispanic, 35.5% men who have sex with men, with a median absolute CD4 count of 24 cells/mm3 (IQR, 15–33), and an HIV VL of 1816 copies/mL (IQR, 1056–2601).
FIGURE 1.
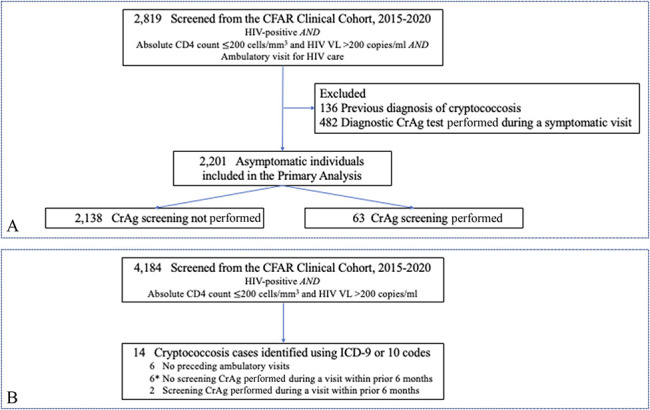
Flow diagram for individuals included in the (A) primary outcome analysis and (B) exploratory analysis of missed opportunities. Asterisk (*) indicates individuals who had missed opportunities for earlier testing. Missed opportunity was defined as an ambulatory visit within 6 months before the cryptococcosis diagnosis if CrAg screening was not performed and the latest CD4 count to which the provider had access was <200 cells/mm3. CFAR, Einstein-Rockefeller-CUNY Center for AIDS Research.
During the median follow-up period of 3.2 years (IQR, 1.4–4.8), 97.1% (2138/2201) of the eligible individuals for screening did not undergo CrAg screening and 2.9% (63/2201) underwent CrAg screening at least once. All the CrAg tests performed for screening purposes were negative. Compared with those who underwent CrAg screening, those who did not were older (45.8 vs. 40.6 years; P = 0.09), had HIV infection for a longer duration (5.1 vs. 0.09 years; P = 0.001), and a higher absolute CD4 count (24 vs. 12 cells/mm3; P < 0.0001). Among those who underwent CrAg screening, 52.4% were diagnosed with HIV within the preceding 3 months (Table 1).
TABLE 1.
Baseline Characteristics by CrAg Screening Status in Asymptomatic People With HIV
| Eligible for CrAg Screening (n = 2201) | P | ||
| Screening Not Performed (n = 2138, 97.1%) | Screening Performed (n = 63, 2.9%) | ||
| Age, median years (IQR) | 45.8 (33.1–55.1) | 40.6 (31.6–51.1) | 0.09 |
| Sex, no. (%) | |||
| Female | 753 (35.2) | 24 (38.1) | 0.68 |
| Male | 1385 (64.8) | 39 (61.9) | |
| Ethnicity and race, no. (%) | |||
| Hispanic | 809 (38.0) | 27 (43.6) | 0.69 |
| Non-Hispanic Black | 1125 (52.6) | 29 (46.8) | |
| Non-Hispanic White | 7 (0.3) | 0 | |
| Others | 196 (9.2) | 6 (9.7) | |
| Ryan White HIV/AIDS Program, transmission risk category, no. (%) | |||
| Blood product recipient | 2 (0.1) | 0 | 0.001 |
| Heterosexual contact | 945 (49.1) | 21 (44.7) | |
| IDU | 216 (11.2) | 4 (8.5) | |
| MSM | 662 (34.4) | 12 (25.5) | |
| MSM and IDU | 25 (1.3) | 1 (2.1) | |
| Perinatal transmission | 34 (1.8) | 4 (8.5) | |
| Other/Unknown | 39 (2.0) | 5 (10.6) | |
| Time from HIV diagnosis to study entry, median years (IQR) | 5.1 (0.02–12.6) | 0.09 (0.002–9.5) | 0.001 |
| Individuals with a duration of HIV infection <3 months, no. (%) | 680 (31.8) | 33 (52.4) | 0.54 |
| Absolute CD4 count, cells/mm3, median (IQR) | 24 (16–33) | 12 (6–28) | <0.0001 |
| HIV VL, copies/mL, median (IQR) | 1812 (1055–2596) | 1900 (1116–2837) | 0.40 |
| Duration of follow-up, median years (IQR)* | 3.2 (1.4–4.8) | 0.18 (0.005–1.9) | 0.19 |
| Mortality by the end of study, no. (%) | 168 (7.9) | 3 (4.8) | 0.37 |
Follow-up time was defined as the time between study entry (first meeting CD4 count and HIV VL criteria) and date of the CrAg screening test, cryptococcosis diagnosis, death, last known contact with MMC, or the end of the study period on December 31, 2020, whichever occurred first.
IDU, intravenous drug user; MSM, men who have sex with men.
Primary Outcome Assessment Using the Cox Regression Model
The results of bivariate and multivariable Cox regression models to assess predictors of CrAg screening are presented in Table 2 and shown in Figure 2, respectively. The proportional hazards assumption was not satisfied for the duration of HIV infection in the multivariable model, and the model was stratified by the median value of HIV duration, 5 years. Among those with a duration of HIV infection <5 years, most recent CD4 count <100 vs. >500 cells/mm3 [adjusted HR (aHR), 7.07; 95% CI: 2.43 to 20.6], most recent HIV VL > 10,000 vs. <200 copies/mL (aHR, 15.0; 95% CI: 4.16 to 54.0), and shorter duration of HIV infection (aHR, 0.60, 40% increased hazard of testing per 1 year decrease in duration; 95% CI: 0.42 to 0.86) were independent predictors of CrAg screening. In those with an HIV infection >5 years, a most recent CD4 count <100 vs. >500 cells/mm3 (aHR, 8.40; 95% CI: 1.47 to 48.1) was an independent predictor of CrAg screening.
TABLE 2.
Association Between Covariables and Time to Incident CrAg Screening Using the Cox Proportional Hazards Model
| Variable | Univariable HR (95% CI) | P |
| Age, yrs | 0.98 (0.96 to 0.99) | 0.05 |
| Sex | ||
| Female | Reference | |
| Male | 1.04 (0.61 to 1.80) | 0.88 |
| Ethnicity and race | ||
| Hispanic | Reference | |
| Non-Hispanic Black | 0.81 | 0.46 |
| Others | 1.03 | 0.95 |
| Duration of HIV infection, yrs | 0.91 | <0.0001 |
| Most recent absolute CD4 count, cells/mm3* | ||
| <100 | 21.0 (9.21 to 47.9) | <0.0001 |
| 100–200 | 1.73 (0.45 to 6.71) | 0.43 |
| 200–500 | 2.30 (0.92 to 5.77) | 0.08 |
| >500 | Reference | |
| Most recent HIV viral load, copies/mL* | ||
| <200 | Reference | |
| 200–10,000 | 6.67 (2.37 to 18.8) | <0.0001 |
| >10,000 | 26.6 (10.3 to 68.6) | <0.0001 |
Time-dependent covariable.
FIGURE 2.
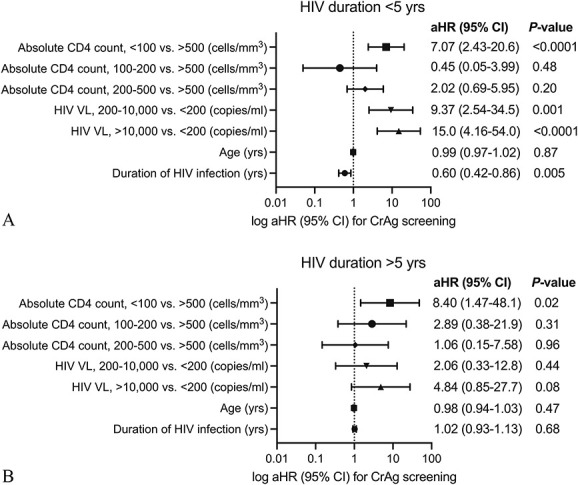
Forest plots of the multivariable Cox regression analysis in CrAg screening. Included in the multivariable Cox model were age, duration of HIV infection, and absolute CD4 count, and HIV viral load as time-varying covariables. Absolute CD4 count and HIV viral load were included as categorical variables. The model was stratified by the median value of HIV duration in the study population; HIV duration (A) <5 years and (B) >5 years.
Exploratory Analysis of Missed Opportunities for Earlier CrAg Testing
Fourteen people with HIV were newly diagnosed with cryptococcosis during the study period (Fig. 1B). At the time of diagnosis, these individuals had a median age of 35.6 years (IQR, 27.7–48.2), median duration of HIV infection of 5.7 years (IQR, 1.24–15.4), and 78.6% had a CD4 count <100 cells/mm3. Six patients with cryptococcosis did not have an ambulatory visit in the preceding 6 months and therefore did not have a testing opportunity. Of the 8 patients who had an ambulatory visit in the preceding 6 months, 6 (75%) had a missed opportunity for CrAg screening as the provider had access to a CD4 count of <200 cells/mm3 during the visit. In 4 of these visits, the provider was not an infectious disease specialist. Notably, 14.3% (2/14) of the individuals diagnosed with cryptococcosis had absolute CD4 T-cell counts between 200 and 300 cells/mm3 at the time of diagnosis, and 64.3% (9/14) of the individuals were not consistently taking ART at the time of their diagnosis based on documentation.
DISCUSSION
We report a CrAg screening rate of 2.9% among 2201 people with HIV with absolute CD4 counts200 cells/mm3 and HIV VLs > 200 copies/mL accessing outpatient care at MMC in the Bronx, NY, from 2015 to 2020. Individuals with a CD4 count <100 cells/mm3, HIV VL > 10,000 copies/mL, and shorter duration of HIV infection were more likely to undergo CrAg screening, over 50% of which was performed within 3 months after HIV diagnosis. This suggests screening declined beyond the period of initial HIV diagnosis even in ART-experienced individuals with poor virologic control. For those with cryptococcosis, the diagnosis was made a median of 5.7 years after their HIV diagnosis, 64% reported suboptimal adherence to ART, and there were missed opportunities to screen during a preceding ambulatory visit.
The World Health Organization recommends CrAg screening in individuals with a CD4 count <100 cells/mm3 strongly or <200 cells/mm3 conditionally before initiation or reinitiation of ART,20 and 2019 South African guidelines recommend CrAg screening in ART-experienced individuals who have not followed up in care for >3 months.21 Despite improving HIV care and declining rates of cryptococcosis among people with HIV in the United States,22 there is a need for CrAg screening of people with HIV who are intermittently adherent to ART with poor virologic control. However, there are no such guidelines, which may in part explain the low screening rate in this study and contribute to the unknown prevalence of cryptococcal antigenemia among those who are at risk due to barriers to access health care and/or socioeconomic factors. The Bronx has a poverty rate of 28%,23 a population of approximately 28,078 people with HIV, among whom 42.1% are Black, 49.5% Hispanic, 63.1% men who have sex with men, and 65.9% with viral suppression in 2019.24,25 In a previous report, 59% and 32% of HIV-associated cryptococcosis occurred among Black and Hispanic Bronx residents, respectively,7 suggesting gaps along the HIV care continuum. Communities disproportionately affected by poverty and barriers to health care face challenges in retention in care, particularly when adverse SSDoH, such as substance use, mental health disorders, and food insecurity, further limit access to care.26,27 A targeted CrAg screening approach incorporating SSDoH in addition to immunological markers warrants consideration for screening beyond the initial HIV diagnosis period.
We found lower screening rates and missed opportunities for CrAg screening among those with a longer duration of HIV infection. This suggests that providers caring for people with a longer duration of HIV infection are less likely to perform CrAg testing unless an individual presents with prompting symptoms. Studies to establish an association between the interval or frequency of screening and prevention of cryptococcosis may be difficult to conduct due to the low case incidence. Nonetheless, our data and other reports of missed opportunities for CrAg screening in the United States28 and Africa29,30 suggest a potential benefit from integrating CrAg screening into the routine care of ART-experienced individuals in conjunction with efforts to enhance ART adherence. Unlike countries with higher prevalence of cryptococcosis and processes to promote screening for cryptococcal disease,31 the United States relies on provider awareness and discretion to screen individuals at-risk. In fact, in those with a CD4 count <100 cells/mm3, ART-experienced compared with ART-naive status associated with asymptomatic cryptococcal antigenemia14 and CM.32 Thus, patients who are virologically uncontrolled for a longer duration may be at higher risk for cryptococcosis.
Possible strategies to improve CrAg screening after the initial period of HIV diagnosis include focused education for providers caring for people with HIV,33 particularly those who are not infectious disease specialists. In fact, 67% (4/6) of the “missed opportunities” for screening in our study occurred during visits with a nonspecialist. In addition, quality improvement initiatives promoting periodic testing in at-risk patients may standardize the procedure with outcome measures. Missed or delayed diagnosis due to cognitive errors such as cognitive overload or overconfidence bias of the provider34 have been reported in non–HIV-associated cryptococcosis35,36 and other infectious diseases.37,38 Such bias may be applicable to CrAg screening when the providers’ long-term familiarity with the patient or the label of “noncompliant patient”39 results in failure to consider screening, whereas those biases may be less salient when providers are seeing patients recently diagnosed with HIV. Time constraints and other pressing health and social priorities may deprioritize screening procedures when a more explicit algorithm-based approach may help.40 Finally, lack of screening underscores the need for biomarkers that may better identify individuals who would benefit from periodic screening.
The risk of HIV-associated cryptococcosis increases when the CD4 counts <100 cells/mm3.41 However, 14% of the cryptococcosis cases in our study occurred in those with CD4 counts that improved to 200–300 cells/mm3 but with poor ART adherence. Cryptococcosis has been reported in patients with higher CD4 counts,42,43 some of whom have unmasking immune reconstitution inflammatory syndrome.7,32 These cases are missed by current screening practices because the focus is on patients with CD4 < 100 cells/mm3. Along the same lines, a study in Guatemala found that 20% of HIV-associated opportunistic infections were missed by screening only those with a CD4 count <200 cells/mm3.44 The same group showed that implementation of a screening program in people with HIV irrespective of clinical suspicion or CD4 count led to a doubling of diagnoses of histoplasmosis compared with a previous estimate and identification of a 33.8% rate of co-infection with cryptococcosis.45 Mpoza and colleagues reported that CrAg positivity was 4% in those with HIV VLs > 5000 copies/mL, irrespective of CD4 counts.15 Although it was beyond the scope of this study to examine screening in individuals with higher CD4 counts, a higher CD4 count cutoff and/or additional factors may be needed to guide screening in ART-experienced people living with HIV.
Limitations and Strengths
We may have an underestimated screening rate for several reasons. We did not include CrAg testing performed in hospitalized patients, which is sometimes conducted for screening purposes if there is a concern for poor outpatient follow-up. We set a conservative criterion to categorize a CrAg test as a diagnostic test based on the presence of relatively nonspecific symptoms. Some people with HIV may have followed up at another institution and been screened there. CD4 count200 cells/mm3 and HIV VL > 200 copies/mL were used as proxy markers of poor ART adherence because adherence data were not available, except in 14 cases of cryptococcosis for whom patient-reported adherence data were collected by a manual review of the charts. The follow-up period was relatively short, a median of 3.2 years, and in the time to event analysis using CD4 count as a time-varying covariable, 43% had CD4 counts >500 cells/mm3 during follow-up. Thus, many patients had reconstituted their CD4 counts during the study period, which may partially explain the low screening rate. Although the generalizability of a single-center study is limited, our findings may have relevance in regions such as the Bronx, NY, where health inequities faced by historically marginalized populations continue to fuel the HIV pandemic, such as in the southern United States.46
Our study also has multiple strengths. We present CrAg screening patterns longitudinally beyond the initial HIV diagnosis period in the modern ART era which may be challenging to be performed on a larger scale given the declining incidence of disease and research funding.47 To the best of our knowledge, this is the first study to examine the association between the duration of HIV infection and screening. This provides a basis for implementing preventive measures. We included longitudinal CD4 count and HIV VL as time-varying covariables in the Cox model to account for the effect of changing laboratory values on a provider's decision to test.
CONCLUSION
With widely available ART and CrAg screening tests in the United States, CM should be completely preventable. We identified low rates of CrAg screening and missed opportunities to screen ART-experienced individuals with poor virologic control. Given the cost-effectiveness of CrAg screening and the substantial cost, morbidity, and mortality associated with cryptococcal disease, there is a critical need for focused CrAg screening guidelines beyond the period of initial HIV diagnosis and in those with relatively higher CD4 count. This study provides evidence to shape such guidelines.
ACKNOWLEDGMENTS
The authors thank Nataliya Tappen at the Albert Einstein College of Medicine for data support.
Footnotes
Supported by the Einstein-Rockefeller-CUNY Center for AIDS Research (P30-AI124414) which is supported by the following National Institutes of Health (NIH) Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, FIC, NIMHD, NIGMS, NIDDK, and OAR, and NIH/National Center for Advancing Translational Service (NCATS) Einstein-Montefiore CTSA Grant Number UL1TH001073 to H.Y., NIH K23MH106386 to U.R.F., NIH R01-AI143453 to L.-a.P., and Merck Research Award (MISP 60528) to V.S.H.
The authors have no conflicts of interest to disclose.
Concept and design: H.Y., V.S.H., L.-a.P., U.R.F. Acquisition and analysis of data: H.Y., V.S.H., A.L., L.S.C.-P., L.-a.P., U.R.F. Interpretation of data: H.Y., V.S.H., L.-a.P., U.R.F. Drafting of original manuscript: H.Y. Critical revision/editing of the manuscript: all authors. Creation of tables and figures: H.Y. Supervision: H.Y., L.-a.P., U.R.F.
L.-a.P. and U.R.F. contributed equally to this work as joint senior authors.
REFERENCES
- 1.Centers for Disease Control (CDC). Pneumocystis pneumonia—Los Angeles. MMWR Morb Mortal Wkly Rep. 1981;30:250–252. [PubMed] [Google Scholar]
- 2.Beyrer C, Adimora AA, Hodder SL, et al. Call to action: how can the US Ending the HIV Epidemic initiative succeed? Lancet. 2021;397:1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Social determinants of health among adults with diagnosed HIV infection, 2018. HIV Surveill Suppl Rep. 2020;25:1–111. [Google Scholar]
- 4.Sullivan PS, Satcher Johnson A, Pembleton ES, et al. Epidemiology of HIV in the USA: epidemic burden, inequities, contexts, and responses. Lancet. 2021;397:1095–1106. [DOI] [PubMed] [Google Scholar]
- 5.McKenney J, Bauman S, Neary B, et al. Prevalence, correlates, and outcomes of cryptococcal antigen positivity among patients with AIDS, United States, 1986-2012. Clin Infect Dis. 2015;60:959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchacz K, Lau B, Jing Y, et al. Incidence of AIDS-defining opportunistic infections in a multicohort analysis of HIV-infected persons in the United States and Canada, 2000-2010. J Infect Dis. 2016;214:862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon HA, Felsen U, Wang T, et al. Cryptococcus neoformans infection in human immunodeficiency virus (HIV)-infected and HIV-uninfected patients at an inner-city tertiary care hospital in the Bronx. Med Mycol. 2020;58:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montefiore Medical Center, Office of Community & Population Health. Community Health Needs Assessment and Implementation Strategy Report-MMC. 2019:1–140. Available at: https://www.montefiore.org/documents/communityservices/MMC-Community-Health-Needs-Report-2019-2021.pdf [Google Scholar]
- 9.Hevey MA, Presti RM, OʼHalloran JA, et al. Mortality after cryptococcal infection in the modern antiretroviral therapy era. J Acquir Immune Defic Syndr. 2019;82:81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellowski JA, Kalichman SC, Matthews KA, et al. A pandemic of the poor: social disadvantage and the U.S. HIV epidemic. Am Psychol. 2013;68:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French N, Gray K, Watera C, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16:1031–1038. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery MP, Nakasujja N, Morawski BM, et al. Neurocognitive function in HIV-infected persons with asymptomatic cryptococcal antigenemia: a comparison of three prospective cohorts. BMC Neurol. 2017;17:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liechty CA, Solberg P, Were W, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007;12:929–935. [DOI] [PubMed] [Google Scholar]
- 14.Wake RM, Govender NP, Omar T, et al. Cryptococcal-related mortality despite fluconazole preemptive treatment in a cryptococcal antigen screen-and-treat program. Clin Infect Dis. 2019;70:1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mpoza E, Rajasingham R, Tugume L, et al. Cryptococcal antigenemia in human immunodeficiency virus antiretroviral therapy-experienced Ugandans with virologic failure. Clin Infect Dis. 2019;71:1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okwir M, Link A, Rhein J, et al. High burden of cryptococcal meningitis among antiretroviral therapy–experienced human immunodeficiency virus–infected patients in Northern Uganda in the era of “test and treat”: implications for cryptococcal screening programs. Open Forum Infect Dis. 2022;9:ofac004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajasingham R, Boulware DR. Reconsidering cryptococcal antigen screening in the U.S. among persons with CD4 <100 cells/mcL. Clin Infect Dis. 2012;55:1742–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benedict K, Jackson BR, Chiller T, et al. Estimation of direct healthcare costs of fungal diseases in the United States. Clin Infect Dis. 2019;68:1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felsen UR, Bellin EY, Cunningham CO, et al. Development of an electronic medical record-based algorithm to identify patients with unknown HIV status. AIDS Care. 2014;26:1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Guidelines for Diagnosing, Preventing and Managing Cryptococcal Disease Among Adults, Adolescents and Children Living With HIV. June 27, 2022 Guideline. Licence: CC BY-NC-SA 3.0 IGO. 1‐64. Available at: https://www.who.int/publications/i/item/9789240052178. Last accessed July 18, 2022. [PubMed] [Google Scholar]
- 21.Govender NP, Meintjes G, Mangena P, et al. Southern African HIV Clinicians Society guideline for the prevention, diagnosis and management of cryptococcal disease among HIV-infected persons: 2019 update. South Afr J HIV Med. 2019;20:1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pyrgos V, Seitz AE, Steiner CA, et al. Epidemiology of cryptococcal meningitis in the US: 1997-2009. PLoS One. 2013;8:e56269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Census Bureau. Poverty status in the past 12 months, Bronx borough, Bronx County, New York. Available at: https://data.census.gov/cedsci/table?g=0600000US3600508510=ACSST1Y2019.S1701. Accessed July 18, 2022. [Google Scholar]
- 24.Sullivan PS, Woodyatt C, Koski C, et al. A data visualization and dissemination resource to support HIV prevention and care at the local level: analysis and uses of the AIDSVu Public Data Resource. J Med Internet Res. 2020;22:e23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bureau of HIV/AIDS Epidemiology, AIDS Institute, New York State Department of Health. New York State HIV/AIDS Annual Surveillance Report, for Persons Diagnosed Through December 2019, 1–125. Available at: https://www.health.ny.gov/diseases/aids/general/statistics/annual/2019/2019_annual_surveillance_report.pdf. Accessed July 18, 2022.
- 26.Remien RH, Bauman LJ, Mantell JE, et al. Barriers and facilitators to engagement of vulnerable populations in HIV primary care in New York City. J Acquir Immune Defic Syndr. 2015;69(suppl 1):S16–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall HI, Frazier EL, Rhodes P, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013;173:1337–1344. [DOI] [PubMed] [Google Scholar]
- 28.Salazar AS, Keller MR, Olsen MA, et al. Potential missed opportunities for diagnosis of cryptococcosis and the association with mortality: a cohort study. EClinicalMedicine. 2020;27:100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meya DB, Tugume L, Nabitaka V, et al. Establishing targets for advanced HIV disease: a call to action. South Afr J HIV Med. 2021;22:1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baluku JB, Mugabe P, Mwebaza S, et al. Cryptococcal antigen screening among antiretroviral therapy–experienced people with HIV with viral load nonsuppression in rural Uganda. Open Forum Infect Dis. 2021;8:ofab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faini D, Kalinjuma AV, Katende A, et al. Laboratory-reflex cryptococcal antigen screening is associated with a survival benefit in Tanzania. J Acquir Immune Defic Syndr. 2019;80:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhein J, Hullsiek KH, Evans EE, et al. Detrimental outcomes of unmasking cryptococcal meningitis with recent ART initiation. Open Forum Infect Dis. 2018;5:ofy122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz BS, Chin-Hong PV. A call to action: infectious diseases medical educators needed. J Infect Dis. 2017;216(suppl 5):S600–S605. [DOI] [PubMed] [Google Scholar]
- 34.Vick A, Estrada CA, Rodriguez JM. Clinical reasoning for the infectious disease specialist: a primer to recognize cognitive biases. Clin Infect Dis. 2013;57:573–578. [DOI] [PubMed] [Google Scholar]
- 35.Deming M, Mark A, Nyemba V, et al. Cognitive biases and knowledge deficits leading to delayed recognition of cryptococcal meningitis. IDCases. 2019;18:e00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chastain DB, Henao-Martínez AF, Dykes AC, et al. Missed opportunities to identify cryptococcosis in COVID-19 patients: a case report and literature review. Ther Adv Infect Dis. 2022;9:20499361211066363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller AC, Arakkal AT, Koeneman SH, et al. Frequency and duration of, and risk factors for, diagnostic delays associated with histoplasmosis. J Fungi (Basel). 2022;8:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bull-Otterson L, Huang Y-LA, Zhu W, et al. Human immunodeficiency virus and hepatitis C virus infection testing among commercially insured persons who inject drugs, United States, 2010–2017. J Infect Dis. 2020;222:940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goddu AP, O'Conor KJ, Lanzkron S, et al. Correction to: do words matter? Stigmatizing language and the transmission of bias in the medical record. J Gen Intern Med. 2019;34:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White BL, Walsh J, Rayasam S, et al. What makes me screen for HIV? Perceived barriers and facilitators to conducting recommended routine HIV testing among primary care physicians in the southeastern United States. J Int Assoc Provid AIDS Care. 2015;14:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ford N, Shubber Z, Jarvis JN, et al. CD4 cell count threshold for cryptococcal antigen screening of HIV-infected individuals: a systematic review and meta-analysis. Clin Infect Dis. 2018;66(suppl 2):S152–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tugume L, Rhein J, Hullsiek KH, et al. HIV-associated cryptococcal meningitis occurring at relatively higher CD4 counts. J Infect Dis. 2019;219:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoon Ha, Riska PF, Jain R, et al. Unexpected case of cryptococcal meningoencephalitis in a patient with long-standing well-controlled HIV infection. Med Mycol Case Rep. 2021;32:14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samayoa B, Aguirre L, Bonilla O, et al. The diagnostic laboratory hub: a new health care system reveals the incidence and mortality of tuberculosis, histoplasmosis, and cryptococcosis of PWH in Guatemala. Open Forum Infect Dis. 2020;7:ofz534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medina N, Rodriguez-Tudela JL, Aguirre L, et al. Incidence of histoplasmosis in a cohort of people with HIV: from estimations to reality. Microorganisms. 2021;9:2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reif S, Safley D, McAllaster C, et al. State of HIV in the US Deep South. J Community Health. 2017;42:844–853. [DOI] [PubMed] [Google Scholar]
- 47.Molloy SF, Chiller T, Greene GS, et al. Cryptococcal meningitis: a neglected NTD? PLoS Negl Trop Dis. 2017;11:e0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]


