Abstract
Hematopoiesis is a continuous and well-regulated process requiring both the capacity for self-renewal and the potential for differentiation of hematopoietic stem cells. Multiple studies indicate that sex hormones exert significant effects on not only hematopoietic stem and progenitor cells, but also the development of hematopoietic lineages, resulting in sexual dimorphisms in normal hematopoiesis. Hematologic malignancies comprise a wide variety of cancers affecting the blood, bone marrow, and lymphatic system, such as leukemia, lymphoma, myeloma, myelodysplastic syndrome, and myeloproliferative diseases. Overall, males are at greater risk and have worse prognosis for most of these malignancies compared with females. A better understanding of the differences between male and female could be of substantial value in research as well as clinical management.
Keywords: Hematopoietic stem cells, Malignant hematopoiesis, Normal hematopoiesis, Sex hormones, Sexual dimorphism
1. INTRODUCTION
Hematopoiesis is a process that is continuous and well regulated. It requires the capacity for self-renewal and the potential for differentiation of hematopoietic stem cells (HSCs). Hematopoiesis eventually gives rise to all the various mature blood and immune cell lineages, such as mast cells which do not typically circulate in the blood.1 HSCs are located at the top of the hematopoietic hierarchy and give rise to progenitor cells that gradually differentiate into lineage-restricted cells.2 Hematopoiesis is strictly controlled by a complex network of cell-intrinsic factors and cell-extrinsic modulators to maintain the hematopoietic homeostasis.3 Dysregulation may occur at any stage of hematopoiesis and can cause hematological malignancies.4
Males and females are similar in many aspects. However, the considerable differences in biological characteristics and behaviors between them5 account for the differences in clinical incidence, manifestation, and outcome of many widespread diseases6–10 and affect the approach to health care.5 Male and female differences may be influenced by both sex and gender. “Sex differences” are based on the biological factors, such as the X and Y chromosomes, reproductive organs and sexual hormones. By contrast, “gender differences” are determined by social factors related to behavior, lifestyle, and life experience.5,11 In this review, we summarize what is known about sex differences in normal and malignant hematopoiesis, provide examples, and discuss possible mechanisms that drive the differences.
2. SEX DIFFERENCES IN NORMAL HEMATOPOIESIS
Multiple studies have identified differences involved in male and female hematopoietic systems, including HSCs, lineage-committed progenitors, and niche components.12,13
2.1. HSC self-renewal and proliferation
In 2014, Nakada et al14 reported that although males and females have similar basal numbers of HSCs and their immediate progeny, multipotent progenitor cells (MPPs), female mice exhibited increased frequency of proliferation of these cells without depletion of the stem cell pool. This indicates that female HSCs undergo more frequent self-renewing divisions. The enhanced proliferation of HSCs in females is driven by endogenous estrogens and mediated mainly by intrinsic estrogen receptor alpha (ERα), which is highly expressed in HSCs. During pregnancy, more HSCs were detected in the bone marrow and spleen relative to non-pregnant female mice. Significant increases in spleen cellularity, erythropoiesis, and myelopoiesis were also observed during pregnancy with elevated estrogen levels, highlighting the importance of sex hormones in HSC activity.14,15
Nakada et al14 detected little or no ERβ, progesterone (PG) receptor or androgen receptor expression in HSCs (CD150+CD48-Lin-Sca-1+c-kit+) and MPPs.14 However, in a murine bone marrow–derived hematopoietic stem/progenitor cell (HSPC) subset (Sca-1+Lin-CD45+)16 and a CD34+Lin-CD45+ population isolated from human umbilical cord blood,17 expressions of receptors for gonadal sex hormones including estrogens, androgen, and PG, as well as the pituitary sex hormone receptors, such as follicle-stimulating hormone (FSH), luteinizing hormone (LH), and prolactin, were detected.16–18 In vivo administration of sex hormones, including LH, enhances BrdU incorporation into Sca-1+Lin-CD45+HSPCs and accelerates hematopoietic recovery in sublethally irradiated mice.16 CD34+ cells sorted from human umbilical cord blood stimulated with the sex hormones (including estradiol and LH) in vitro exhibit an increase in the number of clonogenic BFU-E, CFU-GM, CFU-Meg, and more primitive CFU-Mix progenitors.17 Further detailed studies indicate that expression of the LH receptor is highly restricted to mouse CD150+/−CD48−Lin-Sca-1+c-Kit HSC/MPPs, is activated on HSCs after birth and peaks after sexual maturation.19 Human and mouse long-term self-renewing HSCs expanded ex vivo when stimulated with LH.20 Another pituitary sex hormone, FSH, was reported to mobilize Lin−CD235a−CD45+CD133+ HSPCs into peripheral blood.21
2.2. Hematopoietic progenitors
Sex hormones exert significant effects on not only hematopoietic stem and progenitor cells, but also on the development of hematopoietic lineages.
Associations between sex hormones and erythropoiesis have been known for several decades. The initial observations that men have a higher blood cell mass than women and administering androgens to various animals causes an increase in hemoglobin levels call attention to the possible specific roles that androgen plays in erythropoiesis.22 Before the introduction of recombinant human erythropoietin in 1987, androgen was used as a red cell stimulant.22 On the other hand, erythrocytosis is the most common dose-limiting adverse event associated with testosterone therapy, and aged men appear more sensitive to the stimulatory effect on erythropoiesis of testosterone than young men.23 Interestingly, addition of testosterone to culture of human erythroid progenitors expanded from umbilical cord blood of male and female newborns in vitro significantly promotes proliferation of female but not male erythroid progenitors.24 In an early study, prolactin, as well as plasma from pregnant or lactating mice, was found to stimulate erythropoiesis.25 Administration of estradiol, also known as E2, which is the predominant estrogen in females, induces erythropoiesis in the spleens of both male and female mice.14 In the same study, more erythroid cells in the spleen were found in female mice during pregnancy with increased estrogen levels.14
Several lines of evidence suggest that sex hormones might participate in or contribute to the steady state regulation of B lymphopoiesis.26 Marked reduction in pre-B cells and a corresponding reduction in interleukin -7–responding precursors were found in the bone marrow of normal pregnant mice,27 and treatment of estrogen selectively inhibits the B lymphocyte precursors without affecting myeloid progenitors in male and female mice.28 Although PG alone has no influence on B lymphopoiesis, it increases the sensitivity of precursors of B lymphocytes to estrogen.28 Conversely, reduced levels of circulating sex hormones stimulate B lymphopoiesis in the bone marrow.26 For example, B lymphocyte precursors are selectively accumulated in bone marrow in ovariectomized mice and can be reversed by estrogen replacement.29 In addition, androgen receptor-deficient testicular feminization mice show selective expansion of B cell production in the bone marrow.26 Dramatic and long-lived enhancement of B lymphopoiesis was observed in castrated male mice.30,31 The castration-induced expansion of B cell populations is reversible with androgen replacement in the bone marrow, but not in the spleen.32
Multiple studies have shown that precursors of human and murine myeloid cells are responsive to estrogen.33 E2 in physiological concentrations augments the number of granulocyte/macrophage progenitor cell colonies (CFU-GM) developed from human peripheral blood or cord blood mononuclear cells in vitro.34 In an ex vivo culture system for GM-CSF–supported dendritic cells (DC) differentiation from murine bone marrow myeloid precursors, E2 preferentially promotes the differentiation of CD11c+ CD11bint DC population from BM precursor cells, suggesting that estrogen could increase the number of potent antigen-presenting cells.35 However, the production rate in murine bone marrow of another subset of DC, plasmacytoid DC, are not affected by in vivo E2 treatment.36 In a study examining DC differentiation from the same highly purified myeloid progenitor cells in both GM-CSF- and Flt3 Ligand-driven culture models, E2 promotes DC differentiation in GM-CSF cultures, but decreases viable cell numbers in Flt3 Ligand-culture with a consequent reduction in differentiated DC.37
3. SEX DIFFERENCES IN MALIGNANT HEMATOPOIESIS
A wide range of cancer types that are unrelated to reproductive functions have exhibited sexual dimorphism in incidence, prognosis and mortality.38 Overall, males are at greater risk and have worse prognosis compared with females for most cancers.7,39 Hematologic malignancies comprise a wide variety of cancers affecting the blood, bone marrow and lymphatic system, such as leukemia, lymphoma, myeloma, myelodysplastic syndrome, and myeloproliferative diseases.6
Leukemia is caused by uncontrolled clonal expansion of leukemic cells in the bone marrow and blood. The subtypes of leukemia are determined by and classified according to their cell origins (lymphocytic or myeloid) as well as their stage of maturation arrest (acute or chronic).40 According to the International Agency for Research on Cancer’s GLOBOCAN database, in 2018, leukemia represented the 15th leading cause of cancer occurrence and 11th leading cause of cancer mortality worldwide, accounting for 437,033 new cases and 309,006 deaths.40,41 Altogether, males have higher age-standardized incidence and mortality rates of leukemia (6.1 and 4.2 per 100,000) than females (4.3 and 2.8 per 100,000) in 2018.40 Another report using data from the Global Burden of Disease shows that between 1990 and 2017, the age-standardized incidence rate of leukemia was higher in males than in females, and a more pronounced decrease occurs in females compared with males.42 It is worth noting that as the most common type of pediatric cancer, leukemia accounts for about one-third of all cancers diagnosed in children under 15 years old.43 Within this population, about 3 out of 4 leukemia diagnoses are acute lymphocytic leukemia (ALL) which develops slightly more frequently in boys than in girls43,44; most of the remaining cases are acute myeloid leukemia (AML), which occurs about equally among boys and girls.43 In adults, sex-biased differences in leukemias become more evident.6,39 As we know, sex hormones remain in low concentrations in children before puberty, which indicates an important role that sex hormones play in mediating these effects.
Myelodysplastic syndrome is typically considered a heterogeneous collection of clonal myeloid neoplasms characterized by ineffective hematopoiesis, progressive cytopenia, and increased risk of developing AML. A higher age-adjusted incidence rate is found in males, with a male/female ratio of 1.67.45 Male myeloid dysplastic syndrome (MDS) patients, regardless of age and race, show a higher probability of mortality compared with females in the same group, indicating sex as a prognostic factor for the overall survival of myelodysplastic syndrome.46
Non-Hodgkin lymphomas, involving heterogeneous groups of lymphoproliferative malignancies with diverse patterns of behaviors, represent 1 of the 2 major types of lymphoma. By the latest GLOBOCAN statistics, the overall non-Hodgkin lymphoma incidence is approximately 50% higher among males than females (6.7 vs 4.7 per 100,000, respectively), and the mortality rates also reflect a male predominance (3.3 vs 2.0).40 Considerable variation exists in male to female incidence rate ratios (IRRs) by non-Hodgkin lymphoma subtype. The male predominance was most pronounced for mantle cell (IRR = 3.07) and Burkitt lymphoma (IRR = 2.79), and less pronounced for marginal zone (IRR = 1.05) and follicular lymphomas (IRR = 1.18).40,47 HIV/AIDS is known as a strong risk factor for Burkitt lymphoma and may contribute to the strong male predominance, since HIV/AIDS is more prevalent in males than females.40,47
4. MECHANISMS UNDERLYING SEX DIMORPHISM IN NORMAL AND MALIGNANT HEMATOPOIESIS
4.1. Sex hormones
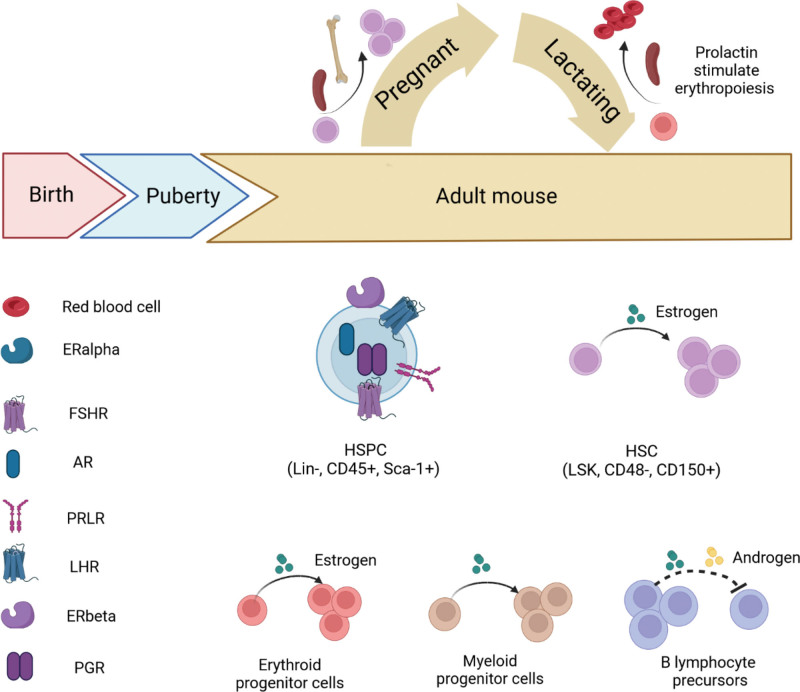
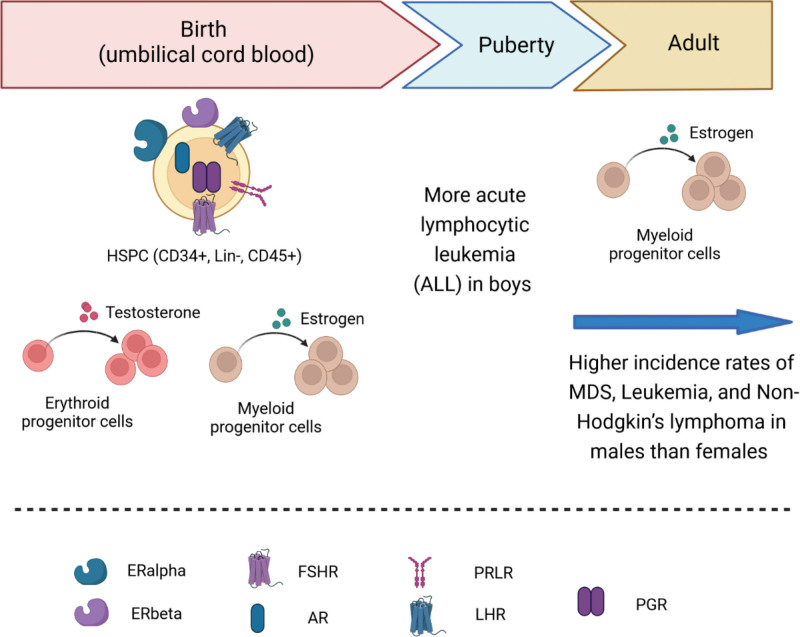
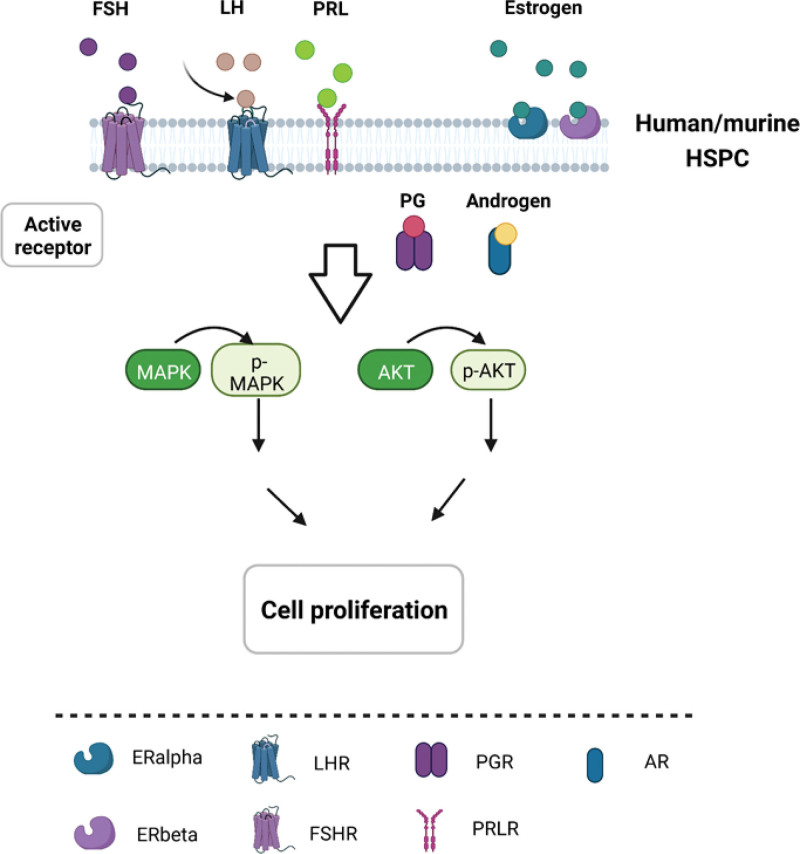
Although males and females are alike in many aspects, important differences exist between the 2 genders. In normal hematopoiesis, multiple sex hormone receptors are detected in blood cells, and more and more evidence proves that sex hormones exert significant effects on hematopoietic stem and progenitor cells as well as the development of hematopoietic lineages in both mouse and human. Sex hormone expression changes along development stages from birth, puberty through the adulthood. Even in adult, especially in female, sex hormone levels undergo dramatic changes during the pregnancy, lactation, and menopause. However, there is little research on the effects of sex hormones on hematopoiesis, and the majority of studies have been done in adults. In mouse (Fig. 1), receptors for estrogen, androgen, PG, FSH, LH, and prolactin (PRL) were found to be expressed in HSPC.16 Estrogen receptor alpha (ERα), but not ERβ, is highly expressed in HSCs.14 Estrogen promotes HSC proliferation in both male and female HSCs, but female HSCs experience more self-renewing divisions than male HSCs.14 Estrogen selectively inhibits B lymphopoiesis28 in females. Estrogen has been shown to increase the number of myeloid cells while suppressing the proliferation of B lymphocyte precursors.28,35 During the pregnancy and lactation in female mouse, more HSCs were found in the bone marrow and spleen during pregnancy.14 In lactating mouse, increased level of prolactin was found to stimulate erythropoiesis.25 In human (Fig. 2), cord blood–derived CD34+ cells are widely used to study sex hormone effect on hematopoiesis. It was found that receptors for sex hormones such as estrogens, androgens, PG, FSH, LH, and PRL were expressed in cord blood HSPCs.17 Testosterone increases the proliferation of female but not male erythroid progenitors.24 In human adult, estrogen increase the number of myeloid progenitor colonies.34 In malignant hematopoiesis, boys developed more ALL than girls.44 Higher incidence of MDS, leukemia and non-Hodgkin lymphoma are reported in males than females, suggesting the sex disparity in hematologic malignancy.40,42,45,46 Two signaling pathways are reported to become active when sex hormones bind their receptors in HSPCs, MAPKp42/44, and AKT-dependent signaling pathways, which results in cell proliferation (Fig. 3).16,17
Figure 1.
Role of sex hormones in the regulation of murine hematopoiesis. In the murine HSPC, estrogen, androgen, PG, FSH, LH, and PRL receptors were found to be expressed. Estrogen promotes HSC proliferation via ER alpha, which is highly expressed in HSCs. Female HSCs experience more self-renewing divisions than male HSCs. Estrogen increases the number of myeloid cells while suppressing the proliferation of B lymphocyte precursors. During the pregnancy and lactating time of female mouse, more HSCs were found in the bone marrow and spleen during pregnancy. Lactating mouse prolactin was discovered to stimulate erythropoiesis. HSPCs are defined by cell surface markers Lin-, CD45+ and Sca-1+ in flow cytometry. HSCs are defined as Lin-, Sca1+, c-kit+ (LSK), CD48, and CD150+ cells. Majority of studies are performed in adult hematopoiesis. AR = androgen receptor, ER = estrogen receptor, FSH = follicle-stimulating hormone, FSHR = follicle stimulating hormone receptor, HSC = hematopoietic stem cell, HSPC = hematopoietic stem/progenitor cell, LH = luteinizing hormone, LHR = luteinizing hormone receptor, PG = progesterone, PGR = prostaglandin receptor, PRL = prolactin, PRLR = prolactin receptor.
Figure 2.
Role of sex hormones in the regulation of human hematopoiesis. Human cord blood–derived HSPCs expressed receptors for sex hormones such as estrogens, androgens, PG, FSH, LH, and PRL. The addition of testosterone to an in vitro culture of human erythroid progenitors significantly increases the proliferation of female but not male erythroid progenitors. Boys developed more acute lymphocytic leukemia than girls in pediatric hematological malignancy. In human adult, estrogen increase the number of myeloid progenitor colonies. Higher incidence of MDS, leukemia, and non-Hodgkin lymphoma are reported in males than females. Cord blood HSPCs are defined by cell surface markers Lin-, CD45+, and CD34+ in flow cytometry. AR = androgen receptor, FSH = follicle-stimulating hormone, FSHR = follicle stimulating hormone receptor, HSPC = hematopoietic stem/progenitor cell, LH = luteinizing hormone, LHR = luteinizing hormone receptor, MDS = myeloid dysplastic syndrome, PG = progesterone, PGR = prostaglandin receptor, PRL = prolactin, PRLR = prolactin receptor.
Figure 3.
Sex hormone signaling pathways in the regulation of hematopoietic cells. Both human and murine HSPCs expressed receptors for sex hormones such as estrogens, androgens, PG, FSH, LH, and PRL. In response to these sex hormones, MAPKp42/44 and AKT-dependent signaling pathways were activated, resulting in cell proliferation. AR = androgen receptor, FSH = follicle-stimulating hormone, FSHR = follicle stimulating hormone receptor, HSPC = hematopoietic stem/progenitor cell, LH = luteinizing hormone, LHR = luteinizing hormone receptor, PG = progesterone, PRL = prolactin, PRLR = prolactin receptor.
4.2. Sex chromosome related genes in the regulation of normal and malignant hematopoiesis
The sex chromosome, in addition to sex hormone, is a significant contributor to gender differences.48 In females, there are 2 X chromosomes. The dosage disparity between males (XY) and females (XX) is balanced by the X chromosome inactivation (XCI) process.49 Twenty-five percent of human genes have been shown to escape the XCI, and expression of these genes is doubled in females compared with males.49 Hematologic malignancies originate as a result of altered gene expression and XCI escaper mutation.48,50
The X chromosome accounts for 5% of all genetic material in humans, and has about 900 genes. In contrast, the Y chromosome is one-third the size of the X chromosome. It accounts for 2% of the human genome, and has approximately 55 protein-coding genes (https://www.genome.gov/about-genomics/fact-sheets/Y-Chromosome-facts). However, very few genes were discovered to be involved in both normal and malignant hematopoiesis. Recently, it was reported that KDM6A (lysine-specific demethylase 6A) regulates hematopoiesis.51 It is an X chromosome-located histone H3K27me3 demethylase gene.52 In vitro knockdown of KDM6A reduces HSPC colony formation and suppresses the proliferation of several leukemia cell lines, suggesting its potential role as an oncogene.51 However, in vivo deletion of KDM6A in the knockout (KO) mouse model showed enhanced self-renewal of HSCs, myeloid expansion and a block in lymphoid and erythroid/megakaryocytic differentiation. At the age of 22 months, around 63% of the mice spontaneously developed AML. With a secondary oncogenic hit of AML-ETO, KDM6A KO mice had a significantly accelerated AML development and death, suggesting that KDM6A deletion confers HSC a pre-leukemic state. KDM6D is the Y chromosome homolog of KDM6A.53 It rescues KDM6A deficiency-induced leukemia development, further supporting the tumor suppressor function of KDM6A/KDM6D genes in vivo.53 Another sex chromosome located gene is CRLF2 (cytokine receptor-like factor 2). It is located on the Xp22.3/Yp11.3 chromosome, and its mutation has been identified as an oncogene in more than 7% of B-ALL cases.54 CSF2RA encodes the granulocyte-macrophage colony-stimulating factor receptor subunit alpha (GM-CSFR), which is found on the X chromosome in humans. GM-CSFR protects chronic lymphocytic leukemia (CLL) cells from apoptosis, and high CSF2RA expression was also found in AML patients, indicating its potential role in hematologic malignancy.55,56 X-linked tumor suppressor gene PHF6 is found mutated and deleted almost exclusively in males in T-ALL, and genetic alterations in PHF6 are seven times more prevalent in males than in females with AML.57
Furthermore, mosaic loss of chromosome Y (mLOY), which is common in hematopoietic cells, causes DNA damages in HSPCs and accelerates leukemogenesis.58 The frequency and level of mLOY increase significantly with age.59 In both human and mouse AML with mLOY, 29 genes are downregulated. KDM5D (lysine-specific demethylase 5D) is one of 29 H3K4 demethylase genes on the Y chromosome. KDM5D deficiency promotes DNA damages in HSPCs. Overexpression of KDM5D, on the other hand, prevents DNA damage and leukemogenesis60 (Table 1).
Table 1.
Genes found on both sexes and their roles in normal and malignant hematopoiesis.
| Gene name | Sex chromosome | Normal hematopoiesis | Malignant hematopoiesis | References |
|---|---|---|---|---|
| KDM6A | X | Kdm6a deletion enhances self-renewal of HSCs, myeloid expansion and a block in lymphoid and erythroid/megakaryocytic differentiation | Kdm6a deletion induces spontaneous leukemia, accelerates leukemia development in combination with other oncogenic hit | 51–53 |
| KDM5D | Y | Loss of KDM5D promotes the DNA damage in HSPCs | Overexpression of KDM5D prevents the DNA damages and suppresses leukemogenesis | 60 |
| CRLF2 | X | Mutation occurs in over 7% of B-ALL | 54 | |
| CSF2RA | X | High CSF2RA expression was detected in AML patients | 55,56 | |
| PHF6 | X | Mutation is more prevalent in male T-ALL and AML | 57 |
ALL = acute lymphocytic leukemia, AML = acute myeloid leukemia, HSC = hematopoietic stem cell, HSPC = hematopoietic stem/progenitor cell.
5. CONCLUSIONS
In both healthy and malignant hematopoiesis, there is sex dimorphism. Through receptors that are selectively expressed in various populations of stem/progenitor cells, sex hormone controls their self-renewal, differentiation, and proliferation in steady state. Males are more susceptible to and have worse prognoses in the majority of malignant hematopoiesis than females. Genes associated with the sex chromosomes are significant contributors to the sex discrepancy in hematologic malignancy. Both research and clinical management could benefit greatly from a greater knowledge of the variations between men and women.
ACKNOWLEDGMENTS
The authors are supported by the National Institutes of Health under awards R01HL124015(YL) and R01DK130478-01 (YL) and the Markey Cancer Center’s Flow Cytometry and Immune Monitoring Core Shared Resource Facility (P30CA177558).
We thank the Markey Cancer Center’s Research Communications Office for editing and graphics support.
Footnotes
X.C. and X.Z. contributed equally to this study.
Conflict of interest: The authors declare that they have no conflict of interest.
The authors are supported by the National Institutes of Health under awards R01HL124015(YL) and R01DK130478-01 (YL) and the Markey Cancer Center’s Flow Cytometry and Immune Monitoring Core Shared Resource Facility (P30CA177558). We thank the Markey Cancer Center's Research Communications Office for editing and graphics support.
X.C. and X.Z. wrote the manuscript with equal contribution. Y.L. guided the overall outline of manuscript, wrote and edited the manuscript.
REFERENCES
- [1].Laurenti E, Göttgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature. 2018;553(7689):418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang Y, Gao S, Xia J, Liu F. Hematopoietic hierarchy – an updated roadmap. Trends Cell Biol. 2018;28(12):976–986. [DOI] [PubMed] [Google Scholar]
- [3].Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20(5):303–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hu D, Shilatifard A. Epigenetics of hematopoiesis and hematological malignancies. Genes Dev. 2016;30(18):2021–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Regitz-Zagrosek V. Sex and gender differences in health. Science & Society Series on Sex and Science. EMBO Rep. 2012;13(7):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ben-Batalla I, Vargas-Delgado ME, Meier L, Loges S. Sexual dimorphism in solid and hematological malignancies. Semin Immunopathol. 2018;41(2):251–263. [DOI] [PubMed] [Google Scholar]
- [7].Clocchiatti A, Cora E, Zhang Y, Paolo Dotto G. Sexual dimorphism in cancer. Nat Rev Cancer. 2016;16(5):330–339. [DOI] [PubMed] [Google Scholar]
- [8].Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. [DOI] [PubMed] [Google Scholar]
- [9].Sampathkumar NK, Bravo JI, Chen Y, et al. Widespread sex dimorphism in aging and age-related diseases. Hum Genet. 2019;139(3):333–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology. Circulation. 2018;138(2):198–205. [DOI] [PubMed] [Google Scholar]
- [11].Goldstein JM, Rich-Edwards JW, Kaiser UB, Chen GL, Manson JE. Sex and gender differences research design for basic, clinical, and population studies: essentials for investigators. Endocr Rev. 2018;39(4):424–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Heo HR, Chen L, An B, Kim KS, Ji J, Hong SH. Hormonal regulation of hematopoietic stem cells and their niche: a focus on estrogen. Int J Stem Cells. 2015;8(1):18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nakada D, Oguro H, Levi BP, et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505(7484):555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Leeman DS, Brunet A. Stem cells: sex specificity in the blood. Nature. 2014;505(7484):488–490. [DOI] [PubMed] [Google Scholar]
- [16].Mierzejewska K, Borkowska S, Suszynska E, et al. Hematopoietic stem/progenitor cells express several functional sex hormone receptors—novel evidence for a potential developmental link between hematopoiesis and primordial germ cells. Stem Cells Dev. 2015;24(8):927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Abdelbaset-Ismail A, Suszynska M, Borkowska S, et al. Human haematopoietic stem/progenitor cells express several functional sex hormone receptors. J Cell Mol Med. 2015;20(1):134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ratajczak MZ. Why are hematopoietic stem cells so “sexy”? on a search for developmental explanation. Leukemia. 2017;31(8):1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Peng YJ, Yu H, Hao X, et al. Luteinizing hormone signaling restricts hematopoietic stem cell expansion during puberty. EMBO J. 2018;37(17):e98984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Velardi E, Tsai JJ, Radtke S, et al. Suppression of luteinizing hormone enhances HSC recovery after hematopoietic injury. Nat Med. 2018;24(2):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zbucka-Kretowska M, Eljaszewicz A, Lipinska D, et al. Effective mobilization of very small embryonic-like stem cells and hematopoietic stem/progenitor cells but not endothelial progenitor cells by follicle-stimulating hormone therapy. Stem Cells International. 2016;2016:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shahani S, Braga-Basaria M, Maggio M, Basaria S. Androgens and erythropoiesis: past and present. J Endocrinol Invest. 2009;32(8):704–716. [DOI] [PubMed] [Google Scholar]
- [23].Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab. 2008;93(3):914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Leberbauer C, Boulmé F, Unfried G, Huber J, Beug H, Müllner EW. Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood. 2005;105(1):85–94. [DOI] [PubMed] [Google Scholar]
- [25].Jepson JH, Lowenstein L. Effect of prolactin on erythropoiesis in the mouse. Blood. 1964;24:726–738. [PubMed] [Google Scholar]
- [26].Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J Immunol. 1998;161(1):27–34. [PubMed] [Google Scholar]
- [27].Medina KL, Smithson G, Kincade PW. Suppression of B lymphopoiesis during normal pregnancy. J Exp Med. 1993;178(5):1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Medina KL, Kincade PW. Pregnancy-related steroids are potential negative regulators of B lymphopoiesis. Proc Natl Acad Sci USA. 1994;91(12):5382–5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Masuzawa T, Miyaura C, Onoe Y, et al. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J Clin Invest. 1994;94(3):1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wilson CA, Mrose SA, Thomas DW. Enhanced production of B lymphocytes after castration. Blood. 1995;85(6):1535–1539. [PubMed] [Google Scholar]
- [31].Olsen NJ, Kovacs WJ. Effects of androgens on T and B lymphocyte development. Immunol Res. 2001;23(2-3):281–288. [DOI] [PubMed] [Google Scholar]
- [32].Viselli SM, Reese KR, Fan J, Kovacs WJ, Olsen NJ. Androgens alter B cell development in normal male mice. Cell Immunol. 1997;182(2):99–104. [DOI] [PubMed] [Google Scholar]
- [33].Nalbandian G, Kovats S. Understanding sex biases in immunity: effects of estrogen on the differentiation and function of antigen-presenting cells. Immunol Res. 2005;31(2):91–106. [DOI] [PubMed] [Google Scholar]
- [34].Maoz H, Kaiser N, Halimi M, et al. The effect of estradiol on human myelomonocytic cells. 1. Enhancement of colony formation. J Reprod Immunol. 1985;7(4):325–335. [DOI] [PubMed] [Google Scholar]
- [35].Paharkova-Vatchkova V, Maldonado R, Kovats S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J Immunol. 2004;172(3):1426–1436. [DOI] [PubMed] [Google Scholar]
- [36].Harman BC, Miller JP, Nikbakht N, Gerstein R, Allman D. Mouse plasmacytoid dendritic cells derive exclusively from estrogen-resistant myeloid progenitors. Blood. 2006;108(3):878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Carreras E. Turner S, Paharkova-Vatchkova V, Mao A, Dascher C, Kovats S. Estradiol acts directly on bone marrow myeloid progenitors to differentially regulate GM-CSF or Flt3 ligand-mediated dendritic cell differentiation. J Immunol. 2008;180(2):727–738. [DOI] [PubMed] [Google Scholar]
- [38].Rubin JB, Lagas JS, Broestl L, et al. Sex differences in cancer mechanisms. Biology of Sex Differences. 2020;11(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- [40].Bispo JAB, Pinheiro PS, Kobetz EK. Epidemiology and etiology of leukemia and lymphoma. Cold Spring Harb Perspect Med. 2020;10(6):a034819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- [42].Dong Y, Shi O, Zeng Q, et al. Leukemia incidence trends at the global, regional, and national level between 1990 and 2017. Exp Hematol Oncol. 2020;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Belson M, Kingsley B, Holmes A. Risk factors for acute leukemia in children: a review. Environ Health Perspect. 2007;115(1):138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541–1552. [DOI] [PubMed] [Google Scholar]
- [45].Zeidan AM, Shallis RM, Wang R, Davidoff A, Ma X. Epidemiology of myelodysplastic syndromes: why characterizing the beast is a prerequisite to taming it. Blood Rev. 2019;34:1–15. [DOI] [PubMed] [Google Scholar]
- [46].Wang F, Ni J, Wu L, Wang Y, He B, Yu D. Gender disparity in the survival of patients with primary myelodysplastic syndrome. J Cancer. 2019;10(5):1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443–459. [DOI] [PubMed] [Google Scholar]
- [48].Ben-Batalla I, Vargas-Delgado ME, Meier L, Loges S. Sexual dimorphism in solid and hematological malignancies. Semin Immunopathol. 2019;41(2):251–263. [DOI] [PubMed] [Google Scholar]
- [49].Slavney A, Arbiza L, Clark AG, Keinan A. Strong constraint on human genes escaping X-inactivation is modulated by their expression level and breadth in both sexes. Mol Biol Evol. 2016;33(2):384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kim HI, Lim H, Moon A. Sex differences in cancer: epidemiology, genetics and therapy. Biomol Ther (Seoul). 2018;26(4):335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liu J, Mercher T, Scholl C, Brumme K, Gary Gilliland D, Zhu N. A functional role for the histone demethylase UTX in normal and malignant hematopoietic cells. Exp Hematol. 2012;40(6):487–98.e3. [DOI] [PubMed] [Google Scholar]
- [52].Hübner MR, Spector DL. Role of H3K27 demethylases Jmjd3 and UTX in transcriptional regulation. Cold Spring Harb Symp Quant Biol. 2010;75:43–49. [DOI] [PubMed] [Google Scholar]
- [53].Gozdecka M, Meduri E, Mazan M, et al. UTX-mediated enhancer and chromatin remodeling suppresses myeloid leukemogenesis through noncatalytic inverse regulation of ETS and GATA programs. Nat Genet. 2018;50(6):883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Russell LJ, Capasso M, Vater I, et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood. 2009;114(13):2688–2698. [DOI] [PubMed] [Google Scholar]
- [55].Matsuura S, Yan M, Lo MC, et al. Negative effects of GM-CSF signaling in a murine model of t(8;21)-induced leukemia. Blood. 2012;119(13):3155–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Li P, Harris D, Liu Z, et al. STAT3-activated GM-CSFRα translocates to the nucleus and protects CLL cells from apoptosis. Mol Cancer Res. 2014;12(9):1267–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Van Vlierberghe P, Patel J, Abdel-Wahab O, et al. PHF6 mutations in adult acute myeloid leukemia. Leukemia. 2010;25(1):130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Danielsson M, Halvardson J, Davies H, et al. Longitudinal changes in the frequency of mosaic chromosome Y loss in peripheral blood cells of aging men varies profoundly between individuals. Eur J Hum Genet. 2020;28(3):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Thompson DJ, Genovese G, Halvardson J, et al. Genetic predisposition to mosaic Y chromosome loss in blood. Nature. 2019;575(7784):652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang Q, Zhao L, Yang Y, Li S, Liu Y, Chen C. Mosaic loss of chromosome Y promotes leukemogenesis and clonal hematopoiesis. JCI Insight. 2022;7(3):e153768. [DOI] [PMC free article] [PubMed] [Google Scholar]