Objectives:
Tinnitus is highly prevalent, but only a few risk factors for developing tinnitus are known and little is known about factors associated with the degree of annoyance of new-onset tinnitus. Longitudinal analysis can reveal risk factors associated with the development of tinnitus and might lead to targeted prevention. The aim of this study is twofold. (1) To identify risk factors that are longitudinally associated with the odds of developing tinnitus 5 years later. (2) To identify factors that are cross-sectionally associated with tinnitus annoyance in adults with new-onset tinnitus.
Methods:
Baseline, 5-year, and 10-year follow-up data of participants in the Netherlands Longitudinal Study on Hearing (NL-SH) were used. The NL-SH is a web-based prospective cohort study, which started in 2006 and includes both normal hearing and hearing-impaired adults aged 18 to 70 years at baseline. The NL-SH uses an online digit-triplet speech-in-noise test to asses speech recognition ability in noise, and online questionnaires on various aspects of life. At follow-up, participants are asked (1) if they suffer from tinnitus and (2) to rate tinnitus annoyance on a 0 to 100 numeric rating scale. We investigated whether demographic (age, sex, living arrangement, educational level), lifestyle (history of tobacco smoking, alcohol use), health (asthma, severe heart disease, hypertension, history of stroke, osteoarthritis, rheumatoid arthritis, epilepsy, multiple sclerosis, and migraine), hearing (speech recognition ability in noise, hyperacusis, and occupational noise exposure), and psychological variables (distress, somatization, depression, and anxiety) were potential risk factors for new-onset tinnitus, or associated with annoyance caused by new-onset tinnitus. Generalized estimating equations were used to longitudinally analyze the association between potential risk factors and new-onset tinnitus measured 5 years later. A multivariable association model was constructed using a forward selection procedure with p < 0.05 for model entry. Linear regression analysis was used to cross-sectionally analyze the association between potential factors and tinnitus annoyance in new-onset tinnitus. For this purpose, a multivariable association model was constructed using a forward selection procedure with p <0.05 for model entry.
Results:
In total, 734 participants without tinnitus at baseline were included, from which 137 participants reported to suffer from new-onset tinnitus 5 or 10 years later. Risk factors for new-onset tinnitus were history of smoking (odds ratio 1.5, 95% confidence interval [CI] 1.0 to 2.2, p = 0.027) and higher levels of somatization (odds ratio 2.0, 95% CI 1.2 to 3.3, overall p = 0.024). Factors associated with the degree of tinnitus annoyance were increased levels of anxiety (β = 11.6, 95% CI 2.3-20.8, overall p = 0.035) and poor speech recognition ability in noise (β = 13.5, 95% CI, 4.4 to 22.6, overall p = 0.014).
Conclusions:
Higher levels of somatization and a history of smoking were found to be risk factors for new-onset tinnitus 5 years later. Anxiety and poor speech recognition ability in noise were associated with higher degrees of tinnitus annoyance in new-onset tinnitus. Somatization deserves to be addressed in future research and clinical practice as it might provide part of a model for the development of chronic tinnitus.
Keywords: Adults, Audiometry/speech recognition ability, Follow-up studies, Incidence, Longitudinal studies, Prevention, Risk factors, Tinnitus annoyance, Tinnitus/epidemiology
INTRODUCTION
Tinnitus is the subjective perception of sound in the absence of a corresponding external source. Although tinnitus is highly prevalent with estimates ranging between 5 and 43% (McCormack et al. 2016), only a few risk factors for developing tinnitus have been identified. Once present, tinnitus bothers some people more than others. Little is known about factors associated with the degree of annoyance of new-onset tinnitus. Knowledge of factors that are associated with the development of tinnitus and annoyance of tinnitus are important for prevention and treatment.
Factors associated with tinnitus have mainly been studied cross-sectionally. Tinnitus is associated with hearing loss, noise exposure, ototoxic medication, head and neck trauma, a variety of illnesses and lifestyle factors such as smoking, and reduced emotional well-being and especially depression and anxiety (Nondahl et al. 2002, 2011; Shargorodsky et al. 2010; Kim et al. 2015; Trevis et al. 2018; Dawes et al. 2020). Hereditary predisposition has also been described (Hendrickx et al. 2007) as a risk factor. Except for hereditary predisposition, cross-sectional studies do not allow to differentiate whether these factors cause tinnitus, co-occur with tinnitus, or are a sequela of tinnitus. A longitudinal study design can identify factors that are associated with developing tinnitus, which is important for preventive health care programs and for identifying causal mechanisms underlying the development of tinnitus.
Only a few studies exist that report on risk factors for developing tinnitus. Pre-existing risk factors shown to be associated with the development of tinnitus are hearing loss, temporomandibular joint disorders, dizziness, middle ear infections, history of head and neck injury, history of migraines, otosclerosis, history of smoking, high cholesterol, chronic kidney disease, arthritis, no/low caffeine intake, chemotherapeutic agents (cisplatin and carboplatin), alcohol consumption (protective), and obesity in men (Table 1; Nondahl et al. 2002, 2010; Dille et al. 2010; Gopinath et al. 2010; Bernhardt et al. 2011; Glicksman et al. 2014; Lee et al. 2016; Shih et al. 2017; Hwang et al. 2018; Dawes et al. 2020).
TABLE 1.
Longitudinal studies reporting on risk factors for the development of tinnitus
| Author and Year | Cohort | No. Patients Included (N) | Length of Follow-Up (Years) | Risk Factors |
|---|---|---|---|---|
| Bernhardt et al. 2011 | Germany, Study of Health in Pomerania | 3134 | 5 | Palpation pain in the TMJ |
| Dawes et al. 2020 | UK, UK Biobank | 3177 | 4 | None identified |
| Dille et al. 2010 | USA, Veterans Affairs Rehabilitation Research and Development Service | Case control study, cisplatin N = 98; carboplatin N = 38; controls N = 57 | Cisplatin, carboplatin | |
| Glicksman et al. 2014 | Nurses’ Health Study II | 65085 | 18 | Caffeine intake (protective) |
| Gopinath et al. 2010 | Australia, Blue Mountains Hearing Study | 1214 | 5 | Lower age, hearing loss, reported history of whiplash, symptomatic dizziness |
| Hwang et al. 2018 | Taiwan, Longitudinal Health Insurance Database | Case control study, with migraine N=1056; without migraine N=4224 | 6 | Migraine |
| Lee et al. 2016 | Taiwan, Longitudinal Health Insurance Database | Case control study, with TMJ disorder N=362, without TMJ disorder N=530 | 6 | TMJ disorder |
| Nondahl et al. 2002 | USA, Epidemiology of Hearing Loss Study | 2513 | 5 | Hearing loss, total cholesterol, history of head injury, otosclerosis |
| Nondahl et al. 2010 | USA, Epidemiology of Hearing Loss Study | 2922 | 10 | History of arthritis, history of head injury, history of ever smoking, among women hearing loss, moderate alcohol consumption (protective), lower age among women, obesity among men (protective) |
| Shih et al. 2017 | Taiwan, Longitudinal Health Insurance Database | Case control study, with CKD N=185430; without CKD N=566290 | 10 | CKD |
CKD, chronic kidney disease; TMJ, temporomandibular joint.
In addition to having or developing tinnitus per se, it is important to consider the degree of tinnitus annoyance. Tinnitus annoyance is associated with a broad range of psychological, cognitive, demographic, and health-related factors. Meta-analysis indicates that increased levels of anxiety and depression are associated with increased tinnitus annoyance (Trevis et al. 2018). Other psychological factors that have been associated with higher levels of tinnitus annoyance are increased pain and somatization, decreased sleep quality, maladaptive coping styles, personality traits of distressed-type, and decreased resilience (Trevis et al. 2018). Cognitive factors that have been associated with higher levels of tinnitus annoyance are poorer performance in executive functioning, general short-term memory, processing speed, and general learning and retrieval (Clarke et al. 2020). Demographic, hearing, and health-related factors found to be associated with tinnitus annoyance are: age, sex, vertigo, hearing loss, tinnitus characteristics (i.e., perceived loudness, continuous tinnitus as opposed to intermittent, pitch, location of perceived tinnitus, ability to mask the tinnitus), hyperacusis, and temporomandibular joint disorders (Hoekstra et al. 2014).
The aim of our study is twofold. (1) To identify risk factors that are longitudinally associated with the odds of developing tinnitus 5 years later (further referred to as new-onset tinnitus). (2) To identify factors that are cross-sectionally associated with tinnitus annoyance in adults with new-onset tinnitus.
Methods
Study Design and Settings
For this study, we used data of the NL-SH. This is an ongoing prospective cohort study conducted over the internet. It uses a convenience sampling method with enrolment through a publicly available online hearing screening test. The study sample includes male and female adults, aged 18 to 70 years at study entry. Baseline data collection (T0) started in 2006, the second measurement round (T1, 5-year follow-up) started in 2011, and the third measurement round (T2, 10-year follow-up) started in 2016. Baseline data collection and follow-up measurements are still ongoing. For the present study, data collected until January 2019 were used. Further details about participant recruitment, data collection, and follow-up measurement rounds have been reported by Stam et al. (2015). The survey combines a speech recognition in noise test known as the “National Hearing Test” (Smits et al. 2006), with questionnaires on different domains of life such as work status, health care use, general health, and psychosocial health. The NL-SH study protocol was approved by the Medical Ethics Committee of Amsterdam UMC, location VUmc in Amsterdam, the Netherlands.
Study Sample
Participants of the NL-SH who reported no tinnitus at baseline and who participated in T0 and T1, or in T0, T1 and T2 were included. Data from the T2 measurement were not used for participants who had new-onset tinnitus at T1. Participants who reported having a cochlear implant at baseline or during follow-up measurements were excluded because cochlear implantation significantly changes cochlear function. It is known that cochlear implantation is associated with both developing tinnitus and tinnitus resolution, which is why the (small number of) cochlear implant users were excluded (Arts et al. 2015; Dixon et al. 2020). Use of other hearing devices was not an exclusion criterion as hearing aids and bone conduction devices are no known risk factors or protective factors for developing tinnitus.
Dependent Variables: Presence of Tinnitus and Annoyance of Tinnitus
At every measurement round, participants were asked: “Do you suffer from ringing in the ears (tinnitus)” with answer categories “yes,” “no,” and “I do not know.” Participants who answered “no,” or “I do not know” at baseline or T1 and “yes” 5 years later at respectively T1 or T2 were considered having new-onset tinnitus. Participants who answered “no”, or “I do not know” at T1 and T2 (if T2 data was available) were considered having no new-onset tinnitus. At T1 and T2 annoyance of tinnitus was measured by using a numeric rating scale (NRStinnitus). Participants were asked “How bothered are you by your tinnitus in everyday life on a scale of 0 to 100, with 0 = no annoyance at all and 100 = extremely annoying” in accordance with (Meikle et al. 2008; Kim et al. 2016).
Independent Variables
Factors associated with prevalent and new-onset tinnitus and with tinnitus annoyance were identified from literature and included if these factors were available from the questionnaires of the NL-SH. These factors were age, sex, living arrangement, educational level, occupational noise exposure, hearing ability, hyperacusis, tobacco smoking, alcohol consumption, asthma, myocardial infarction, hypertension, history of stroke, diabetes, osteoarthritis, rheumatoid arthritis, epilepsy, multiple sclerosis, migraine, distress, somatization, depression, and anxiety (Baguley et al. 2013; Kim et al. 2015; Deklerck et al. 2020). These factors were collected at all measurement rounds (i.e. T0, T1, and T2) and included as independent variables in the analyses.
Age was categorized into 5 age groups: 18 to 30 years, 31 to 40 years, 41 to 50 years, 51 to 60 years, and 61 to 70 years.
Living arrangement was assessed by asking “How do you characterize your living arrangement?”; “living alone,” “with partner,” “with partner and children,” “with parents,” “single parent with children.” This was dichotomized into “living alone,” or “living with other people in a household.”
Educational level was divided into “low” (elementary school or attended high school but no degree), “mid” (high school graduate or having an associate degree), or “high” (having a bachelor’s degree, master’s degree, or doctoral degree).
Occupational noise exposure was measured by asking: “how often are you exposed to loud sounds or loud noises at work?” Answer categories were: “almost never,” “occasionally,” “frequently,” or “almost always.” Information on occupational noise exposure was only available for participants with a paid job of 12 hours or more per week at time of filling out the questionnaire.
Hearing ability was operationalized as the ability to recognize speech in noise. It was measured using the “National Hearing Test.” This is a screening test that measures the recognition of digit triplets in noise by determining the signal-to-noise ratio (SNR) that corresponds to 50% intelligibility [i.e., the speech reception threshold (SRT); Smits et al. 2006)]. Speech recognition ability in noise was categorized as good (SRT < −5.5 dB SNR), insufficient (−5.5 dB SNR ≤ SRT ≤ −2.8 dB SNR), or poor (SRT > −2.8 dB SNR) (Smits et al. 2005). The “National Hearing Test” could be completed either with headphones or speakers, but it was advised to use headphones. Participants were instructed to perform the test without a hearing aid if applicable.
The presence of hyperacusis was measured using the question “do you feel that loud noises bother you more than they bother other people (hyperacusis)?” Answer categories were “yes,” “no,” or “I do not know.” Participants who answered “no” or “I do not know” were considered having no hyperacusis.
Tobacco smoking was measured by asking participants if and how often they had smoked tobacco. Answer categories were: “yes. I smoke every day,” “yes, I smoke occasionally,” “no, but I used to smoke every day,” “no, but I used to smoke occasionally,” or “no I never smoked.” Participants who had chosen the option “no, I never smoked” were considered having no history of smoking. All others were categorized as having a history of smoking.
Alcohol consumption was measured by asking how many units of alcohol participants consumed per day. The outcome was used to categorize each participant in one of the following categories: non-user, moderate user (≤1 unit/day), above moderate user [2 units/day (women), 2 or 3 units/day (men)], and heavy user [>2 units/day (women), >3 units/day (men)] (Reinhard et al. 1998).
Self-rated presence of chronic medical conditions was assessed by a list of Statistics Netherlands (Mootz et al. 1989). Participants were asked to tick the box for a chronic condition if this chronic condition was present now or had been present during the previous 12 months. This list comprises 28 conditions in total, but for the current study we only included “asthma, chronic bronchitis or emphysema,” “severe heart disease or myocardial infarction,” “hypertension,” “stroke or consequences of stroke,” “diabetes mellitus”, ”osteoarthritis of knees, hips or hands,” “inflammation of the joints of hands and/or feet (Rheumatoid Arthritis),” “epilepsy,” “multiple sclerosis,” and “migraine,” as these factors are associated with tinnitus (Baguley et al. 2013; Kim et al. 2015; Deklerck et al. 2020).
Distress, somatization, depression, and anxiety were assessed using the “Four Dimensional Symptom Questionnaire” (4DSQ) (Terluin et al. 2006). Response categories are divided in “no” (scored as 0), “sometimes” (scored as 1), and “regularly” or “often or constantly” (scored as 2). The somatization scale and the distress scale have 16 items (range 0 to 32). The depression scale has 6 items (range 0 to 12). The anxiety scale has 12 items (range 0 to 24).
Statistical Analysis
Descriptive Analysis
Continuous variables were described by mean (with standard deviation) or by median (with interquartile range), depending on its distribution. Percentages were calculated for independent categorical variables.
New-Onset Tinnitus
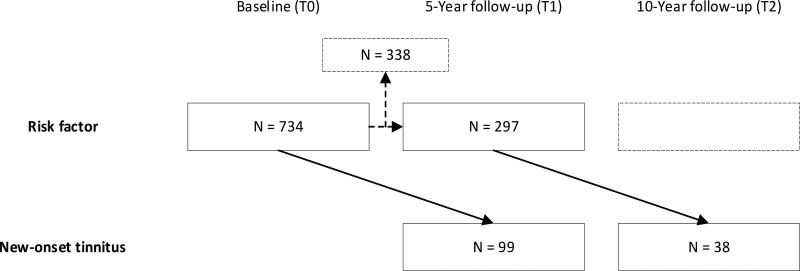
To identify potential risk factors for new-onset tinnitus in a 5-year interval, longitudinal analyses were performed using generalized estimating equations with an exchangeable correlation structure. Potential risk factors at T0 were used to predict new-onset tinnitus at T1 (T0-T1). If no tinnitus occurred at T0 and T1 the 5-year interval from T1 to T2 was included as well using potential risk factors at T1 to predict new-onset tinnitus at T2 (see Fig. 1). First, a univariable analysis was performed to explore potential risk factors. Next, a multivariable association model was built (p-entry < 0.05) using a forward selection procedure. At least 10 participants with new-onset tinnitus and 10 participants without new-onset tinnitus were required per included variable in the final multivariable model.
Fig. 1.
Illustration of time points in model used for generalized estimating equations. Note that for participants that were included twice (n = 297), baseline variables (T0) were used as risk factors for the T0-T1 interval, and T1 variables were used as risk factors for the T1-T2 interval. A total of 338 participants had no follow-up measurement at T2 and were thus not analyzed for the T1-T2 interval.
Degree of Tinnitus Annoyance
The degree of tinnitus annoyance (NRStinnitus as dependent variable) in new-onset tinnitus was analyzed cross-sectionally. Factors potentially associated with tinnitus annoyance were taken from the measurement round in which the new-onset tinnitus was reported (i.e., either T1 or T2). First, a univariable analysis was performed to explore potential risk factors. Next, a multivariable association model was built (p-entry < 0.05) using a forward selection procedure. At least 10 participants were required per included variable in the final multivariable model.
Assumptions of the models were verified. In case of nonlinearity continuous variables were categorized based on distribution (quartiles), based on previously used categories with clinical value, or based on regular intervals. All statistical analyses were performed using SPSS version 26.0 (IBM Corp, Armonk, NY, USA).
RESULTS
Participants
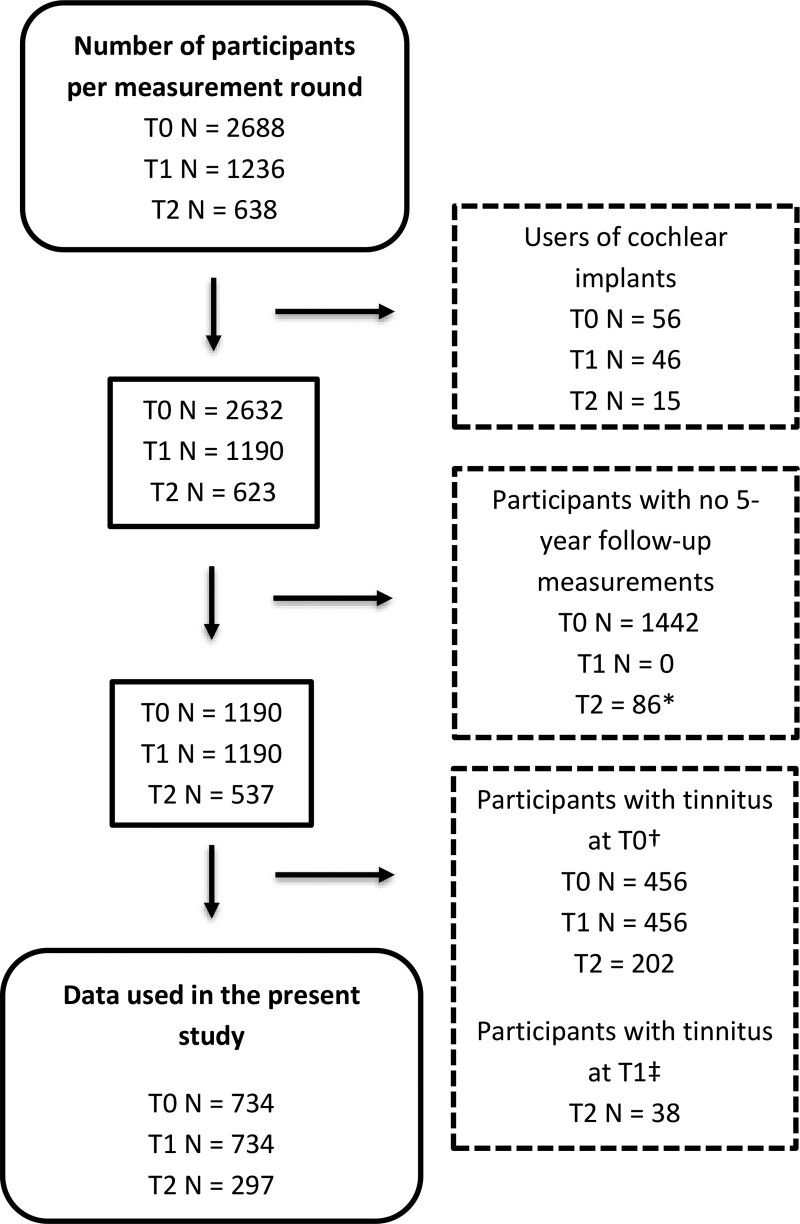
After applying exclusion criteria, data from 734 out of 2688 participants were included. Figure 2 shows the number of eligible participants according to the exclusion criteria applied in each measurement round. Participant characteristics are shown in Supplemental Digital Content, Table 1, http://links.lww.com/EANDH/B23. More women than men participated. Mean age at baseline was 46 years. Of the participants, 59% had good speech recognition ability in noise at the baseline measurement. Of the total group of 734 participants who did not have tinnitus at baseline, 99 reported new-onset tinnitus at T1. Of the total group of 297 participants who did not have tinnitus at T0 and T1, 38 reported new-onset tinnitus at T2. Thus, in total, 137 participants reported new-onset tinnitus at T1 or T2. Their characteristics at these respective time points are shown in Supplemental Digital Content, Table 1, http://links.lww.com/EANDH/B23. The median NRStinnitus in participants with new onset-tinnitus was 20 (range 0 to 100, interquartile range 10 to 40).
Fig. 2.
Flowchart of participant numbers after applying exclusion criteria per measurement round. The number of measurements excluded per measurement round are given in the dashed line rectangles. *Participants who had a T0 and T2 measurement, but who did not participate or complete the T1 measurement round. †For the analysis of new-onset tinnitus at T1 and T2, participants who reported tinnitus at T0 were excluded from the T0-T1 and the T1-T2 measurement interval. ‡For the analysis of new-onset tinnitus at T2 participants who reported new-onset tinnitus at T1 were excluded from the T1-T2 measurement interval. T0 indicates baseline measurement; T1, 5-year follow-up measurement; and T2, 10-year follow-up measurement.
Risk Factors for Developing Tinnitus
The potential risk factors age, SRT and the 4DSQ variables (i.e., distress, somatization, depression, and anxiety) were categorized because of non-linearity. Categorization of age and SRT have been described in the methods section. Somatization and distress were divided into quartiles. Because a large proportion of participants scored 0 at baseline for depression (73%) and anxiety (51%), division in quartiles was not feasible. Scores on depression and anxiety were therefore divided into three ordinal categories. Participants with a 0 score formed one category. The group with a score >0 was divided in two groups of the same size with a cut-off of ≥ 2 for anxiety and a cut-off of ≥3 for depression.
In the univariable analysis, hyperacusis, history of smoking, migraine, and higher levels of somatization were significantly associated with a higher odds ratio for reporting tinnitus after 5 years (Supplemental Digital Content, Table 2, http://links.lww.com/EANDH/B23). The final multivariable association model showed that history of smoking (odds ratio 1.5, 95% CI, 1.0 to 2.2, p = 0.027) and higher levels of somatization (overall p = 0.024, somatization score on 4DSQ of 9 to 29; odds ratio 2.0, 95% CI, 1.2 to 3.3, as compared to a somatization score on 4DSQ of 0 to 2) were associated with higher odds of reporting tinnitus 5 years later (Table 2).
TABLE 2.
Multivariable generalized estimating equations model of factors associated with new-onset tinnitus reported 5 years later
| Multivariable Analysis, Generalized Estimating Equations | |||
|---|---|---|---|
| Variables | OR | 95% CI (for OR) | p |
| History of smoking | 1.5 | 1.0 to 2.2 | 0.027 |
| Somatization (4DSQ), 1st quartile (score 0–2) | 0.024 | ||
| 2nd quartile (score 3–5) | 1.0 | 0.6 to 1.9 | |
| 3rd quartile (score 6–8) | 1.3 | 0.8 to 2.1 | |
| 4th quartile (score 9–29) | 2.0 | 1.2 to 3.3 | |
4DSQ, four-dimensional symptom questionnaire; CI, confidence interval; OR, odds ratio.
Factors Associated With the Degree of Annoyance of New-Onset Tinnitus
The variables age, SRT, depression, anxiety, and somatization were categorized because of non-linearity. Though the assumption of linearity was not violated for distress, we categorized distress to be consistent with the other 3 variables of the 4DSQ (i.e., depression, anxiety, and somatization). Somatization and distress were divided into quartiles. Because a large proportion of participants scored 0 for depression (66%) and anxiety (50%), division into quartiles was not feasible. Scores on depression and anxiety were therefore divided into three ordinal categories. Participants with a 0 score formed one category. The group with a score >0 was divided in two groups of the same size, with a cut-off of ≥ 3 for both depression and anxiety.
Age group 18 to 30 years was combined with the group aged 31 to 40 years, to have a sufficiently large reference group.
In the univariable analysis tinnitus annoyance was associated with low education, poor speech recognition ability in noise, having osteoarthritis, somatization, and anxiety (Supplemental Digital Content, Table 3, http://links.lww.com/EANDH/B23). The final multivariable model showed that tinnitus annoyance in new-onset tinnitus was associated with anxiety (overall p = 0.035, score 1 to 2; β = 11.6, 95% CI, 2.3 to 20.8, as compared to an anxiety score of 0) and poor speech recognition ability in noise (overall p = 0.014, β = 13.5, 95% CI, 4.4 to 22.6, as compared with good speech recognition ability in noise; Table 3).
TABLE 3.
Multivariable linear regression model of variables associated with tinnitus annoyance in participants with new-onset tinnitus
| Multivariable Linear Regression | |||
|---|---|---|---|
| Variables | (β) | 95% CI (for β) | p |
| Anxiety (4DSQ) (score 0) | 0.035 | ||
| Score 1–2 | 11.6 | 2.3 to 20.8 | |
| Score 3–24 | 7.4 | −1.7 to 16.4 | |
| Speech recognition ability in noise* (good) | 0.014 | ||
| Insufficient | 1.4 | −9.0 to 11.8 | |
| Poor | 13.5 | 4.4 to 22.6 | |
β is the regression coefficient of the independent variable.
*Speech recognition ability was adjusted for transducer type (i.e., speakers or headphones).
4DSQ, four dimensional symptom questionnaire; CI, confidence interval.
DISCUSSION
The aim of our study was to identify risk factors that are longitudinally associated with the odds of developing tinnitus 5 years later and to identify factors that are cross-sectionally associated with tinnitus annoyance in adults with new-onset tinnitus. Longitudinal studies on tinnitus are rare, although they are crucial to disentangle causes and consequences of tinnitus.
In our study, we found that increased levels of somatization and history of tobacco smoking were risk factors for developing tinnitus as reported 5 years later. Higher levels of anxiety and poor speech recognition ability in noise were associated with a higher degree of tinnitus annoyance in participants with new-onset tinnitus.
Finding risk factors for new-onset tinnitus is important to identify causal relations that can lead to the development of tinnitus. This knowledge can be used for targeted prevention. Identifying people that develop bothersome tinnitus can be of interest, as this group might benefit most from (early) intervention.
Our study showed that somatization is a risk factor for developing tinnitus. Somatization is defined as “a tendency to experience and communicate somatic distress and symptoms unaccounted for by pathological findings, to attribute them to physical illness, and to seek medical help for them” (Lipowski 1988). To predict development of tinnitus, we used levels of somatization in people 5 years before reporting tinnitus and excluded people who reported tinnitus at baseline. This means that the association between somatization and reporting tinnitus has a lag time. This was supported by the cross-sectional analysis of tinnitus annoyance, in which we found no relationship with somatization. Somatization therefore appears to precede tinnitus and has no immediate relation with tinnitus annoyance. The finding that somatization is a risk factor for developing tinnitus is in line with the earlier reported notion that tinnitus related activity changes in the central nervous system are not restricted to auditory pathways but also involve non-auditory areas such as emotional areas (Langguth et al. 2013; Roberts et al. 2013; Elgoyhen et al. 2015). It is conceivable that people with higher levels of somatization are more aware of bodily sensations and have a lower threshold of experiencing tinnitus. Roberts et al. (2013) hypothesized that mechanisms for auditory attention are involved in the development and maintenance of the neural changes that underlie tinnitus. Alternatively, acute tinnitus might become chronic more easily in people who somaticize because of emotional reinforcements. People can habituate to tinnitus, resulting in decreased notion to tinnitus or complete tinnitus resolution (Jastreboff et al. 1996). Directing emotional resources to tinnitus might prevent habituation to tinnitus and cause tinnitus to be chronic (Jastreboff et al. 1996). Trevis et al. (2018) suggested that impaired attention processing and memory impairments may be associated with the ongoing awareness of chronic tinnitus. Cortical plasticity is a mechanism that could be involved in the development into chronic tinnitus (Georgiewa et al. 2006) and somatization could be a modifier of this cortical plasticity. Interactions between central auditory processing and neural networks of (non-auditory) emotional and somatosensory processes are relevant for the development of tinnitus (Georgiewa et al. 2006; Rauschecker et al. 2010). Because of the heterogeneous nature of tinnitus, it is likely that multiple factors are involved in developing tinnitus and they could co-exist or interact.
It should be noted that the levels of somatization were relatively low in our sample. For somatoform disorder (as classified by “Diagnostic and Statistical Manual of Mental Disorders IV”) the optimum cut-off point of the somatization sub-score of the 4DSQ is 9 (de Vroege et al. 2015). This cut-off point yields a moderate sensitivity and specificity when the questionnaire is used as a screening tool for somatoform disorder (de Vroege et al. 2015). In our study, the fourth quartile of somatization in the analysis for tinnitus occurrence had a scoring range of 9 to 29. Scores in the other quartiles were lower. No subgroups could be made for scores of ≥20 [which is considered “highly elevated” somatization (Terluin et al. 2016)] because these high scores were rare in the NL-SH. It would be of interest to see if people with more elevated somatization scores have a higher risk of developing tinnitus. However, because we found an increased odds for developing tinnitus using a cut-off score of 9, elevated levels of somatization should be considered as a risk factor for tinnitus. This association between the development of tinnitus and elevated levels of somatization might be important information when counseling or treating patients with tinnitus.
A history of tobacco smoking was associated with an increased 5-year odds for developing tinnitus. This is in accordance with Nondahl et al. (2010) who found an increased 10-year risk of developing tinnitus in people with a history of tobacco smoking. A meta-analysis of cross-sectional studies also showed an association between tobacco smoking and concurrent tinnitus (Veile et al. 2018). This leaves the question how tobacco smoking affects onset of tinnitus. Nondahl et al. (2010) suggested that higher levels of anxiety, associated with tobacco smoking, may increase awareness of tinnitus. However, in our study, anxiety was not a risk factor for developing tinnitus. Alternatively, nicotine may play a role in the development of tinnitus. The auditory pathways are cholinergic, incorporating nicotinic receptors. Harkrider et al. (2001) found that transdermal nicotine administration affected neural transmission of acoustic information while the cochlea remained unaffected. Alternatively, the degenerative effects of smoking on neurocognition (Durazzo et al. 2010) might play a role in the development of tinnitus. Using the NL-SH, we previously found a longitudinal association between tobacco smoking and SRTs (Goderie et al. 2020). SRTs deteriorated faster in participants with a history of tobacco smoking. However, poorer SRTs were no risk factor for developing tinnitus in this study and will therefore not significantly affect the association between a history of smoking and the increased risk of developing tinnitus.
The present study showed that anxiety was associated with tinnitus annoyance, which is consistent with previous findings (Holgers et al. 2005; Zöger et al. 2006; Hoekstra et al. 2014; Hu et al. 2015; Trevis et al. 2018). It should be noted that anxiety scores were relatively low in our study. A cutoff score of ≥4 in the 4DSQ indicates that an anxiety disorder should be considered (Terluin et al. 2014). Nevertheless, the result that anxiety is consistently found to be associated with tinnitus annoyance highlights the importance for health care-workers to address emotional well-being in patients with tinnitus. It also highlights the importance of taking anxiety into account when researching tinnitus.
We found that participants with poor speech recognition abilities in noise, reflected by a high SRT, had a higher degree of tinnitus annoyance as compared with participants with good hearing. This is in accordance with several publications (Hiller et al. 2006; Moon et al. 2018; Mahafza et al. 2020), but not with others (Holgers et al. 2005; Hoekstra et al. 2014). Several explanations might play a role in this association. People with poor speech recognition ability will have more cochlear damage leading to higher tinnitus annoyance scores than people with good hearing. Due to poor speech recognition ability people might not benefit from the masking noise of ambient sound which could increase tinnitus annoyance. People with poor speech recognition abilities in noise are confronted with their hearing loss on a daily basis and might consequently be more aware of their tinnitus. Alternatively, the pathogenesis of tinnitus and the central processes involved in developing tinnitus might differ between people with good and poor hearing ability. People with tinnitus experience more listening effort compared with people without tinnitus (Degeest et al. 2017). Tinnitus and hearing loss pose an increased overall acoustic challenge requiring an increased cognitive demand, which is a key contributor to listening effort (Peelle 2018). The increased listening effort in people with a higher SRT (worse hearing ability) may add to the perceived annoyance of tinnitus.
Strengths and Limitations
Our web-based longitudinal study has several strengths. (1) A longitudinal study design is the only way to establish determinants of new-onset tinnitus. (2) Because the included participants all started without tinnitus at baseline, the group can be considered more representative of the general population who develop tinnitus as compared to research based on populations who present themselves with tinnitus at their clinician or a specialized tinnitus clinic. (3) Web-based studies have an increased geographic and demographic reach and recruit more effectively participants who are in good health at baseline as compared with studies using traditional methods to recruit participants and collect data (Gosling et al. 2004; Mathieu et al. 2013). (4) In the cross-sectional analysis on tinnitus annoyance, only participants who developed tinnitus in the previous 5 years were included. It might be that factors that affect tinnitus annoyance change over time. Identifying factors associated with tinnitus annoyance in people who have developed tinnitus relatively recently can help in treating patients with newly developed tinnitus. When interpreting our findings, we consider several limitations. (1) The question asked to measure new-onset tinnitus was: “Do you suffer from ringing in the ears (tinnitus)” with yes/no response. It does not involve temporal characteristics such as a minimum time span for an episode of tinnitus. Spontaneous brief unilateral tapering tinnitus lasting no more than a minute is a sensation that many people hear occasionally (Oron et al. 2011). It is possible that some participants have reported this as suffering from ringing in the ears, although they do not represent the group of people who typically suffer from tinnitus. No clear distinction has been made between any tinnitus and bothersome tinnitus. Any tinnitus does not consider burden, but bothersome tinnitus does. (2) Measuring tinnitus annoyance with an NRS provides a simple and straight forward measuring tool. It has been used before (Kim et al. 2016), but it has not been validated against the more extensively used and more elaborate Tinnitus Handicap Inventory and Tinnitus Questionnaire (Hallam et al. 1988; Newman et al. 1996). Interpreting the effect size of a variable based on the regression coefficient is therefore not possible in the analysis on tinnitus annoyance. (3) We used baseline and 5-year follow-up variables to study the development of new-onset tinnitus in a 5-year interval (i.e., between baseline and 5 years, or 5 and 10 years follow-up, respectively). When using this study design, a lag time is assumed between the exposure of a risk factor and the occurrence of tinnitus. Acute causes of tinnitus such as a noise trauma cannot be analyzed in such a study. (4) Exposure was low for specific variables (e.g., history of stroke or diabetes mellitus) and associations might be missed because of lack of power. (5) Tinnitus as a side effect of drugs was not taken into account. Cianfrone et al. (2011) describes 83 separate drugs associated with tinnitus for which ototoxicity was not mentioned as a side effect. Another 48 drugs were found to be associated with ototoxicity (Cianfrone et al. 2011). However, disentangling the effect of specific drugs from effects of the indication for which they were used is often impossible in a prospective cohort study such as ours. An experimental study set up or case-control study would be better suited to this purpose (Wung et al. 2021). (6) Hearing aids may have a positive effect on tinnitus perception and annoyance (Kikidis et al. 2021), but were not included in our analysis on risk factors for developing tinnitus and tinnitus annoyance as we focused on participants with and without a hearing loss. Hearing devices are no known protective (or risk) factor for developing tinnitus and therefore do not have to be taken into account in the longitudinal analysis. (7) The participants mainly had low scores for all subscales of the 4DSQ, indicating no or low levels of depression, anxiety, distress, and somatisation. It would be of interest to see if people with higher scores on these subscales would be more prone to develop tinnitus, by studying tinnitus in populations with a higher prevalence of psychological illnesses. (8) In our linear regression model, some variables showed a non-normal distribution of the residuals. A logistic transformation of the outcome variable did not result in normally distributed residuals in all variables. Because of non-linear relationships, the effect size of the reported coefficients should be interpreted with caution.
This study is an exploratory study on the development of tinnitus within a 5-year time interval. Other longitudinal studies using stricter definitions for tinnitus in populations that are more affected by somatization are needed to confirm somatization as a risk factor for tinnitus. Establishing this association could lead to a better understanding of the development of tinnitus and might lead to targeted intervention and better prevention of the development of tinnitus. Psychological factors such as somatization and anxiety are a central problem in the development and severity of new-onset tinnitus. We recommend that tinnitus is researched in the context of a variety of psychological functions (such as anxiety, somatization, depression, coping styles, personality traits and obsessive compulsiveness) and should possibly be added as an outcome to studies on the long-term effects of psychological disorders.
CONCLUSIONS
A higher level of somatization and a history of smoking were found to be risk factors for developing new-onset tinnitus 5 years later. Anxiety and poor speech recognition ability in noise were associated with higher degrees of tinnitus annoyance of new-onset tinnitus. Somatization as a risk factor for developing tinnitus is a novel finding. Longitudinal data can reveal risk factors associated with the development of tinnitus and might lead to targeted prevention. Somatization deserves to be addressed in future research and clinical practice as it might provide a model for the development of chronic tinnitus.
ACKNOWLEDGMENTS
The authors thank the participants on the Netherlands Longitudinal Study on Hearing. We also thank the assistance of Merel van Zutphen in performing a literature review on tinnitus incidence.
Supplementary Material
Abbreviations:
- 4DSQ
- four dimensional symptom questionnaire
- CI
- confidence interval
- NL-SH
- Netherlands Longitudinal Study on Hearing
- NRS
- numeric rating scale
- OR
- odds ratio
- SNR
- signal-to-noise ratio
- SRT
- speech reception threshold
- T0
- baseline data collection
- T1
- 5-year follow-up data collection
- T2
- 10-year follow-up data collection.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and text of this article on the journal’s Web site (www.ear-hearing.com)
The first measurement round of the Netherlands Longitudinal Study on Hearing (2006-2010) was financially supported by the Heinsius Houbolt Foundation, The Netherlands. Sonova AG, Switzerland supported the data collection of the second measurement round (since 2011). Funding for data collection of the third measurement round (since 2016) came from the EMGO Institute for Health and Care Research, The Netherlands, and Sonova AG, Switzerland.
T.G., M.v.W., B.I.L.-W., P.M., C.S., C.R.L., and S.E.K. were involved in formulating the research questions and in designing the study. T.G. performed the analysis and M.F.v.W. and B.I.L.-W. verified the analytical methods. T.G. took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis, and article.
The authors have no conflicts of interest to disclose.
References
- Arts R. A., Netz T., Janssen A. M., George E. L., Stokroos R. J. (2015). The occurrence of tinnitus after CI surgery in patients with severe hearing loss: A retrospective study. Int J Audiol, 54, 910–917. [DOI] [PubMed] [Google Scholar]
- Baguley D., McFerran D., Hall D. (2013). Tinnitus. Lancet, 382, 1600–1607. [DOI] [PubMed] [Google Scholar]
- Bernhardt O., Mundt T., Welk A., Köppl N., Kocher T., Meyer G., Schwahn C. (2011). Signs and symptoms of temporomandibular disorders and the incidence of tinnitus. J Oral Rehabil, 38, 891–901. [DOI] [PubMed] [Google Scholar]
- Cianfrone G., Pentangelo D., Cianfrone F., Mazzei F., Turchetta R., Orlando M. P., Altissimi G. (2011). Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: A reasoned and updated guide. Eur Rev Med Pharmacol Sci, 15, 601–636. [PubMed] [Google Scholar]
- Clarke N. A., Henshaw H., Akeroyd M. A., Adams B., Hoare D. J. (2020). Associations between subjective tinnitus and cognitive performance: Systematic review and meta-analyses. Trends Hear, 24, 2331216520918416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes P., Newall J., Stockdale D., Baguley D. M. (2020). Natural history of tinnitus in adults: A cross-sectional and longitudinal analysis. BMJ Open, 10, e041290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vroege L., Emons W. H., Sijtsma K., Hoedeman R., van der Feltz-Cornelis C. M. (2015). Validation of the 4DSQ somatization subscale in the occupational health care setting as a screener. J Occup Rehabil, 25, 105–115. [DOI] [PubMed] [Google Scholar]
- Degeest S., Keppler H., Corthals P. (2017). The effect of tinnitus on listening effort in normal-hearing young adults: A Preliminary Study. J Speech Lang Hear Res, 60, 1036–1045. [DOI] [PubMed] [Google Scholar]
- Deklerck A. N., Debacker J. M., Keppler H., Dhooge I. J. M. (2020). Identifying non-otologic risk factors for tinnitus: A systematic review. Clin Otolaryngol, 45, 775–787. [DOI] [PubMed] [Google Scholar]
- Dille M. F., Konrad-Martin D., Gallun F., Helt W. J., Gordon J. S., Reavis K. M., Bratt G. W., Fausti S. A. (2010). Tinnitus onset rates from chemotherapeutic agents and ototoxic antibiotics: Results of a large prospective study. J Am Acad Audiol, 21, 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon P. R., Crowson M., Shipp D., Smilsky K., Lin V. Y., Le T., Chen J. M. (2020). Predicting reduced tinnitus burden after cochlear implantation in adults. Otol Neurotol, 41, 196–201. [DOI] [PubMed] [Google Scholar]
- Durazzo T. C., Meyerhoff D. J., Nixon S. J. (2010). Chronic cigarette smoking: Implications for neurocognition and brain neurobiology. Int J Environ Res Public Health, 7, 3760–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgoyhen A. B., Langguth B., De Ridder D., Vanneste S. (2015). Tinnitus: Perspectives from human neuroimaging. Nat Rev Neurosci, 16, 632–642. [DOI] [PubMed] [Google Scholar]
- Georgiewa P., Klapp B. F., Fischer F., Reisshauer A., Juckel G., Frommer J., Mazurek B. (2006). An integrative model of developing tinnitus based on recent neurobiological findings. Med Hypotheses, 66, 592–600. [DOI] [PubMed] [Google Scholar]
- Glicksman J. T., Curhan S. G., Curhan G. C. (2014). A prospective study of caffeine intake and risk of incident tinnitus. Am J Med, 127, 739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goderie T. P. M., Stam M., Lissenberg-Witte B. I., Merkus P., Lemke U., Smits C., Kramer S. E. (2020). 10-year follow-up results of The Netherlands Longitudinal Study on hearing: Trends of longitudinal change in speech recognition in noise. Ear Hear, 41, 491–499. [DOI] [PubMed] [Google Scholar]
- Gopinath B., McMahon C. M., Rochtchina E., Karpa M. J., Mitchell P. (2010). Risk factors and impacts of incident tinnitus in older adults. Ann Epidemiol, 20, 129–135. [DOI] [PubMed] [Google Scholar]
- Gosling S. D., Vazire S., Srivastava S., John O. P. (2004). Should we trust web-based studies? A comparative analysis of six preconceptions about internet questionnaires. Am Psychol, 59, 93–104. [DOI] [PubMed] [Google Scholar]
- Hallam R. S., Jakes S. C., Hinchcliffe R. (1988). Cognitive variables in tinnitus annoyance. Br J Clin Psychol, 27, 213–222. [DOI] [PubMed] [Google Scholar]
- Hendrickx J. J., Huyghe J. R., Demeester K., Topsakal V., Van Eyken E., Fransen E., Mäki-Torkko E., Hannula S., Jensen M., Tropitzsch A., Bonaconsa A., Mazzoli M., Espeso A., Verbruggen K., Huyghe J., Huygen P. L., Kremer H., Kunst S. J., Manninen M., Diaz-Lacava A. N., et al. (2007). Familial aggregation of tinnitus: A European multicentre study. B-ENT, 3 Suppl 7, 51–60. [PubMed] [Google Scholar]
- Hiller W., & Goebel G. (2006). Factors influencing tinnitus loudness and annoyance. Arch Otolaryngol Head Neck Surg, 132, 1323–1330. [DOI] [PubMed] [Google Scholar]
- Hoekstra C. E., Wesdorp F. M., van Zanten G. A. (2014). Socio-demographic, health, and tinnitus related variables affecting tinnitus severity. Ear Hear, 35, 544–554. [DOI] [PubMed] [Google Scholar]
- Holgers K. M., Zöger S., Svedlund K. (2005). Predictive factors for development of severe tinnitus suffering-further characterisation. Int J Audiol, 44, 584–592. [DOI] [PubMed] [Google Scholar]
- Hu J., Xu J., Streelman M., Xu H., Guthrie O. (2015). The correlation of the tinnitus handicap inventory with depression and anxiety in veterans with tinnitus. Int J Otolaryngol, 2015, 689375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J. H., Tsai S. J., Liu T. C., Chen Y. C., Lai J. T. (2018). Association of tinnitus and other cochlear disorders with a history of migraines. JAMA Otolaryngol Head Neck Surg, 144, 712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff P. J., Gray W. C., Gold S. L. (1996). Neurophysiological approach to tinnitus patients. Am J Otol, 17, 236–240. [PubMed] [Google Scholar]
- Kikidis D., Vassou E., Markatos N., Schlee W., Iliadou E. (2021). Hearing aid fitting in tinnitus: A scoping review of methodological aspects and effect on tinnitus distress and perception. J Clin Med, 10, 2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Lee H. J., An S. Y., Sim S., Park B., Kim S. W., Lee J. S., Hong S. K., Choi H. G. (2015). Analysis of the prevalence and associated risk factors of tinnitus in adults. PLoS One, 10, e0127578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Jang J. H., Lee S. Y., Han J. J., Koo J. W., Vanneste S., De Ridder D., Song J. J. (2016). Neural substrates predicting short-term improvement of tinnitus loudness and distress after modified tinnitus retraining therapy. Sci Rep, 6, 29140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langguth B., Kreuzer P. M., Kleinjung T., De Ridder D. (2013). Tinnitus: Causes and clinical management. Lancet Neurol, 12, 920–930. [DOI] [PubMed] [Google Scholar]
- Lee C. F., Lin M. C., Lin H. T., Lin C. L., Wang T. C., Kao C. H. (2016). Increased risk of tinnitus in patients with temporomandibular disorder: A retrospective population-based cohort study. Eur Arch Otorhinolaryngol, 273, 203–208. [DOI] [PubMed] [Google Scholar]
- Lipowski Z. J. (1988). Somatization: The concept and its clinical application. Am J Psychiatry, 145, 1358–1368. [DOI] [PubMed] [Google Scholar]
- Mahafza N., Zhao F., El Refaie A., Chen F. (2021). A comparison of the severity of tinnitus in patients with and without hearing loss using the tinnitus functional index (TFI). Int J Audiol, 60, 220–226. [DOI] [PubMed] [Google Scholar]
- Mathieu E., McGeechan K., Barratt A., Herbert R. (2013). Internet-based randomized controlled trials: A systematic review. J Am Med Inform Assoc, 20, 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack A., Edmondson-Jones M., Somerset S., Hall D. (2016). A systematic review of the reporting of tinnitus prevalence and severity. Hear Res, 337, 70–79. [DOI] [PubMed] [Google Scholar]
- Meikle M. B., Stewart B. J., Griest S. E., Henry J. A. (2008). Tinnitus outcomes assessment. Trends Amplif, 12, 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K. R., Park S., Jung Y., Lee A., Lee J. H. (2018). Effects of anxiety sensitivity and hearing loss on tinnitus symptom severity. Psychiatry Investig, 15, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootz M., & Van Den Berg J. (1989). Indicators of health status in the CBS-Health Interview Survey. Mndber Gezondheid (CBS), 2, 4–10.. [Google Scholar]
- Newman C. W., Jacobson G. P., Spitzer J. B. (1996). Development of the tinnitus handicap inventory. Arch Otolaryngol Head Neck Surg, 122, 143–148. [DOI] [PubMed] [Google Scholar]
- Nondahl D. M., Cruickshanks K. J., Huang G. H., Klein B. E., Klein R., Nieto F. J., Tweed T. S. (2011). Tinnitus and its risk factors in the Beaver Dam offspring study. Int J Audiol, 50, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nondahl D. M., Cruickshanks K. J., Wiley T. L., Klein B. E., Klein R., Chappell R., Tweed T. S. (2010). The ten-year incidence of tinnitus among older adults. Int J Audiol, 49, 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nondahl D. M., Cruickshanks K. J., Wiley T. L., Klein R., Klein B. E., Tweed T. S. (2002). Prevalence and 5-year incidence of tinnitus among older adults: The epidemiology of hearing loss study. J Am Acad Audiol, 13, 323–331. [PubMed] [Google Scholar]
- Oron Y., Roth Y., Levine R. A. (2011). Sudden brief unilateral tapering tinnitus: Prevalence and properties. Otol Neurotol, 32, 1409–1414. [DOI] [PubMed] [Google Scholar]
- Peelle J. E. (2018). Listening effort: How the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear Hear, 39, 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker J. P., Leaver A. M., Mühlau M. (2010). Tuning out the noise: Limbic-auditory interactions in tinnitus. Neuron, 66, 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard O. P. M., & Rood-Bakker D. S. (1998). Alcohol Gebruik in beeld. Standaard Meetlat. Nederlands Economisch Instituut. [Google Scholar]
- Roberts L. E., Husain F. T., Eggermont J. J. (2013). Role of attention in the generation and modulation of tinnitus. Neurosci Biobehav Rev, 37, 1754–1773. [DOI] [PubMed] [Google Scholar]
- Shargorodsky J., Curhan G. C., Farwell W. R. (2010). Prevalence and characteristics of tinnitus among US adults. Am J Med, 123, 711–718. [DOI] [PubMed] [Google Scholar]
- Shih C. P., Lin H. C., Chung C. H., Hsiao P. J., Wang C. H., Lee J. C., Chien W. C. (2017). Increased risk of tinnitus in patients with chronic kidney disease: A nationwide, population-based cohort study. PLoS One, 12, e0183192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits C., & Houtgast T. (2005). Results from the Dutch speech-in-noise screening test by telephone. Ear Hear, 26, 89–95. [DOI] [PubMed] [Google Scholar]
- Smits C., Merkus P., Houtgast T. (2006). How we do it: The Dutch functional hearing-screening tests by telephone and internet. Clin Otolaryngol, 31, 436–440. [DOI] [PubMed] [Google Scholar]
- Stam M., Smits C., Twisk J. W., Lemke U., Festen J. M., Kramer S. E. (2015). Deterioration of speech recognition ability over a period of 5 years in adults ages 18 to 70 years: results of the Dutch online speech-in-noise test. Ear Hear, 36, e129–e137. [DOI] [PubMed] [Google Scholar]
- Terluin B., Oosterbaan D. B., Brouwers E. P., van Straten A., van de Ven P. M., Langerak W., van Marwijk H. W. (2014). To what extent does the anxiety scale of the Four-Dimensional Symptom Questionnaire (4DSQ) detect specific types of anxiety disorder in primary care? A psychometric study. BMC Psychiatry, 14, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terluin B., Smits N., Brouwers E. P., de Vet H. C. (2016). The Four-Dimensional Symptom Questionnaire (4DSQ) in the general population: Scale structure, reliability, measurement invariance and normative data: A cross-sectional survey. Health Qual Life Outcomes, 14, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terluin B., van Marwijk H. W., Adèr H. J., de Vet H. C., Penninx B. W., Hermens M. L., van Boeijen C. A., van Balkom A. J., van der Klink J. J., Stalman W. A. (2006). The Four-Dimensional Symptom Questionnaire (4DSQ): A validation study of a multidimensional self-report questionnaire to assess distress, depression, anxiety and somatization. BMC Psychiatry, 6, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevis K. J., McLachlan N. M., Wilson S. J. (2018). A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin Psychol Rev, 60, 62–86. [DOI] [PubMed] [Google Scholar]
- Veile A., Zimmermann H., Lorenz E., Becher H. (2018). Is smoking a risk factor for tinnitus? A systematic review, meta-analysis and estimation of the population attributable risk in Germany. BMJ Open, 8, e016589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wung D., Goderie T., van Wier M. F., Stam M., Kramer S. E. (2022). Association of beta blocker use and hearing ability in adults: A cross-sectional study. Int J Audiol, 61, 102–107. [DOI] [PubMed] [Google Scholar]
- Zöger S., Svedlund J., Holgers K. M. (2006). Relationship between tinnitus severity and psychiatric disorders. Psychosomatics, 47, 282–288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.