Abstract
The potential of the dense granule antigens GRA1 and GRA6 of Toxoplasma gondii to be used as diagnosis reagents in a recombinant form was evaluated. Both proteins were expressed in Escherichia coli as glutathione-S-transferase (GST) fusions. The GST-GRA1 fusion comprises the entire GRA1 sequence devoid of its N-terminal signal peptide. Separate expression of the two N- and C-terminal hydrophilic regions of GRA6 showed that only the N-terminal hydrophilic part of the protein was recognized by a pool of positive human sera in an immunoblot. One hundred T. gondii-positive and 98 negative human sera were tested in two separate immunoglobulin G (IgG)-direct enzyme-linked immunosorbent assays (ELISAs) using either GST-GRA1 or GST-GRA6-Nt recombinant protein. Whereas the sensitivity of the GST-GRA1 IgG ELISA was low (68%), the GST-GRA6-Nt IgG ELISA reached a sensitivity of 96%. The reactivity to GRA6-Nt was shown to be high even with human sera of low IgG titers. In addition, comparison of the optical density values for each serum revealed that GRA1 may complement GRA6-Nt to reach an overall sensitivity of 98%. Therefore, the GST-GRA6-Nt ELISA could be used together with another antigen like GRA1 for the development of a recombinant antigen-based test for serodiagnosis of toxoplasmosis.
Diagnosis of Toxoplasma gondii infections is of great medical importance for humans, especially pregnant women and immunosuppressed patients. During pregnancy, primary infection of women is often associated with fetal infection, which can lead to abortion or severe neonatal malformations. In immunocompromised adults (e.g., AIDS patients), toxoplasmosis (acute or, most often, reactivation of chronic infection) frequently causes a life-threatening encephalitis (for a review, see reference 12).
Among the available diagnostic tests, serology is commonly used. Despite the fact that serological tests give satisfactory results, the production of reliable reagents remains laborious and expensive. At present, the detection of specific antibodies based on the recognition of crude Toxoplasma antigens requires mass production of the parasite either from peritoneal fluids of infected mice or from tissue cultures. The use of an Escherichia coli recombinant antigen(s) would be greatly beneficial in improving standardization of the tests and reducing their production cost.
Enzyme-linked immunosorbent assay (ELISA) tests using Toxoplasma recombinant antigens have already been reported (4, 9, 10, 11, 14, 17), but compared to the current serological tests, none of these recombinant antigens has allowed detection of all serologically positive individuals. It has emerged from these studies that the use of two or several recombinant antigens could be necessary to improve the sensitivity of these ELISA tests. Our previous studies on the secreted dense granule (GRA) antigens have shown that the recombinant forms of GRA1 (1), GRA2 (8), and GRA6 (this paper) are recognized by immune human sera. Here we report expression of both the GRA1 and GRA6 proteins in fusion with glutathione-S-transferase (GST) and evaluation of these recombinant antigens to improve the sensitivity of recombinant antigen-based tests for the serodiagnosis of toxoplasmosis.
MATERIALS AND METHODS
Reagents.
Restriction enzymes and DNA-modifying enzymes were purchased from Boehringer (Mannheim, Germany); T4 DNA ligase and Taq polymerase were from Promega (Charbonnières, France). The pGEX-2T and pGEX-3X vectors were purchased from Pharmacia (Uppsala, Sweden). Glutathione agarose and reduced glutathione were from Sigma Chimie (St-Quentin, France). Alkaline phosphatase-conjugated anti-human immunoglobulin G (IgG) (heavy and light chains) were from Biosys (Compiègne, France).
Human sera.
A total of 198 serum samples provided by Sanofi Diagnostics Pasteur (Marnes la Coquette, France) were used in the ELISA. Of these, 100 samples were seropositive for T. gondii antibodies, and 98 were seronegative. They were tested for the presence of Toxoplasma-specific IgG using a direct IgG ELISA (Platelia Toxo-IgG kit; Sanofi-Diagnostics Pasteur).
Plasmid constructions.
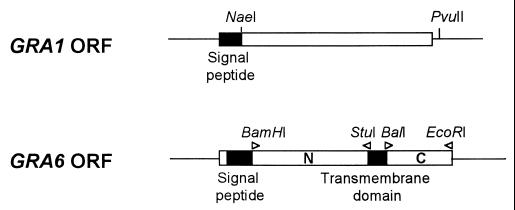
The expression vector that has been used to express soluble GRA polypeptides was pGEX (13). The constructs used in this study are as follows (Fig. 1).
FIG. 1.
pTgGRA1.2 construct expressing the GST-GRA1 fusion protein (top panel) was obtained by cloning the NaeI-PvuII cDNA fragment from the GRA1 cDNA in frame with the GST reading frame. This fragment encodes the GRA1 protein without its N-terminal hydrophobic signal peptide. To obtain the pTgGRA6-Nt and the pTgGRA6-Ct constructs expressing the GST-GRA6-Nt and GST-GRA6-Ct fusion proteins, respectively (lower panel), the DNA fragments encoding separate regions of the GRA6 protein were amplified by PCR (positions of primers and created restriction sites are shown at the top) and subcloned in frame with the GST ORF. Untranslated regions of the GRA1 and GRA6 genes are shown as black lines. ORFs are represented as boxes; hydrophobic domains are shown as solid boxes and hydrophilic regions are shown as open boxes.
(i) pTgGRA1.2.
The 648-bp NaeI-PvuII fragment encoding GRA1 without its signal peptide was isolated from the λgt11-derivative TX11 containing the GRA1 cDNA (1), blunted by treatment with T4 DNA polymerase, and ligated into the SmaI-digested and calf intestine alkaline phosphatase-dephosphorylated pGEX-3X vector.
(ii) pTgGRA6-Nt.
The DNA fragment (330 bp) encoding the N-terminal part of GRA6 was amplified by PCR from plasmid pBluescript containing the GRA6 cDNA (6) using primers G6N5′ (5′-CGTTGGGTGGATCCCGTGTCG-3′) and G6N3′ (5′-GAGTCTGAGGCCTTTCTCTC-3′) that were designed to contain BamHI and StuI sequences (underlined), respectively. The PCR product was digested with both BamHI and StuI and inserted into the BamHI and SmaI sites of pGEX2T.
(iii) pTgGRA6-Ct.
The DNA fragment (180 bp) encoding the C-terminal part of GRA6 was amplified by PCR from plasmid pBluescript containing the GRA6 cDNA using primers G6C5′ (5′-CTTCGATGGCCAGGACGACGC-3′) and G6C3′ (5′-CCCTGAATTCATCTTTAATAA-3′) that were designed to contain BalI and EcoRI sequences (underlined), respectively. The PCR product was digested with BalI and EcoRI and then ligated into the SmaI and EcoRI sites of pGEX 3X. The resulting constructs were characterized by restriction enzyme digestion and subsequent sequencing. PCR was carried out with Taq DNA polymerase (Promega) in a final volume of 50 μl containing 1 μM oligonucleotide primers and 200 μM each of the four deoxynucleoside triphosphates in 1× Taq DNA polymerase buffer. Reactions were incubated for 1 min at 94°C prior to the addition of 4 U of Taq DNA polymerase and 50 μl of mineral oil. Amplifications were carried out at 94°C for 45 s (denaturation), 55°C for 1 min (hybridization), and 72°C for 1 min (elongation) for a total of 25 cycles in a DNA thermal cycler (Perkin-Elmer Cetus). The size of the PCR products was estimated by agarose gel electrophoresis.
Production and purification of fusion proteins.
Competent E. coli JM109 cells were transformed with parental or recombinant pGEX-2T and pGEX-3X DNA. Fusion proteins or the GST wild-type protein was prepared from bacterial cultures of pTgGRA1.2, pTgGRA6-Nt, pTgGRA6-Ct, pGEX-3X, and pGEX-2T as described previously (13). Briefly, a mid-log-phase culture was stimulated for 1 h at 37°C with 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Cells were pelleted at 4,000 × g for 15 min and resuspended in 0.02 M phosphate-buffered saline (PBS, pH 7.4)–0.5 mM phenylmethylsulfonyl fluoride–1 mM EDTA–1% Triton X-100. Cells were lysed on ice by multiple rounds of sonication. Lysates were centrifuged at 10,000 × g for 10 min at 4°C. The recombinant polypeptides were purified from the supernatant using glutathione-agarose beads (Sigma) and eluted by resuspending the beads in 50 mM Tris-HCl (pH 8.0) containing 5 mM free reduced glutathione (Sigma).
SDS-PAGE and immunoblot analysis.
Proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 13% polyacrylamide gel (5). The concentration of the recombinant polypeptides was determined by a quantitative Coomassie blue-stained gel using bovine serum albumin as the standard. The reactivity of the recombinant proteins with human sera was tested by immunoblot. The electrophoretic transfer of recombinant proteins to a nitrocellulose membrane was carried out as described before (15). Blots of GRA1, GRA6-Nt, GRA6-Ct, and wild-type GST proteins were incubated with human sera (dilution, 1:100) as primary antibodies, followed by antispecies alkaline phosphatase conjugate (Sanofi Pasteur Diagnostics), both diluted in PBS–1% nonfat dry milk.
ELISA.
Microtiter plates (Greiner GmbH, Nürtingen, Germany) were coated overnight at 4°C with the purified fusion protein (or a matched quantity of purified wild-type GST as a control) diluted in 0.1 M carbonate-bicarbonate buffer (pH 9.6). The optimum antigen dilution corresponded to approximately 0.5 μg/ml for GRA1 and 1 μg/ml for GRA6-Nt. Plates were washed three times with 0.01 M PBS (pH 7.2)–0.5% Tween 20 and blocked with 150 μl of a 5% skim milk solution in 0.01 M PBS for 1 h at room temperature. After washing, 100 μl of human serum diluted in 0.01 M PBS (pH 7.2)–5% milk (dilution, 1:100) was added to each well for 3 h at 37°C. After washing, 100 μl of alkaline phosphatase-conjugated anti-human IgG antibodies diluted 1:1,500 in 0.01 M PBS (pH 7.2)–5% milk were added for 1 h at 37°C. Finally after three washes, the alkaline phosphatase activity was detected by using p-nitrophenyl phosphate (Sigma) for 1 h at 37°C. The absorbance was measured at 405 nm in a Titertek Multiscan MCC/340 (Flow, Les Ullis, France), and the A405 of each test was expressed as follows: optical density at 405 nm (OD405) of serum reacting with the fusion protein − OD405 of serum reacting with GST. A sample was considered positive if the calculated absorbance value was higher than the mean + 4 standard deviations for the negative individuals.
Statistical analysis.
Detection of outliers in negative sera was made by the box plot. The box plot is conceived to express the dispersion of the data around their median (16). One of its uses is the detection of possible outliers, which can influence different statistical parameters such as mean and standard deviation. Any value greater than the third quartile plus threefold the interquartile range is considered an outlier and then eliminated for the determination of mean and standard deviation of OD obtained for samples from healthy subjects. The Spearman test was used to test correlation between the Platelia Toxo-IgG ELISA and the two GRA ELISAs. These analyses were made with the SAS System for Microsoft Windows release 6.10 (SAS Institute Inc., Cary, N.C.).
RESULTS
Expression of recombinant GRA1 in E. coli.
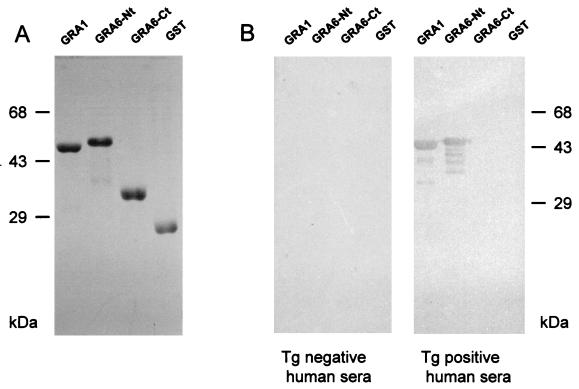
Previous studies had shown that the precursor of GRA1 is encoded by an open reading frame (ORF) of 570 bp with a predicted primary translation product of 190 amino acids (1). The sequence was found to contain a predicted signal sequence of 24 amino acids. Therefore, after processing, the mature protein was predicted to be 166 amino acids long. In order to optimize expression of GRA1 as a soluble protein, the NaeI-PvuII fragment of the TX11 cDNA encoding the GRA1 protein, which lacks the 27 N-terminal amino acids (1), was subcloned into the pGEX-2T expression system (Fig. 1). The resulting recombinant, pTgGRA1.2, expressed a soluble GST fusion protein of approximately 50 kDa, as expected, which could be readily purified by affinity to glutathione-agarose. Coomassie blue-stained gel analysis showed that the GRA1 fusion protein represents more than 95% of the stainable material (Fig. 2A).
FIG. 2.
(A) Coomassie blue staining of recombinant fusion proteins after affinity purification and resolution in SDS-PAGE. (B) Immunoblot detection of the GST recombinant proteins by a pool of human sera. The purified recombinant proteins were separated by SDS-PAGE and blotted onto nitrocellulose. Membranes were incubated either with a pool of Toxoplasma (Tg)-negative (left panel) or -positive (right panel) human sera. Only GST-GRA1 and GST-GRA6-Nt were recognized by positive sera.
Expression of recombinant polypeptides corresponding to both the N-terminal and C-terminal portions of GRA6 in E. coli.
Expression of the entire GRA6 ORF in E. coli as a fusion protein in the pGEX system and its further purification on glutathione-agarose were unsuccessful because of a low level of expression and insolubility of the fusion protein (data not shown). We thus focused on expression of fragments encoding GRA6 polypeptides that could be expressed at a high level, with minimal degradation, and would retain immunoreactivity with T. gondii-immune human sera. Based on our initial studies on the GRA6 gene (6), it was determined that GRA6 potentially encodes a 230-amino-acid polypeptide that contains two hydrophobic regions with the characteristics of transmembrane domains. The first hydrophobic domain is N-terminally located. The second domain is central and is flanked by two hydrophilic domains (Fig. 1). Since hydrophobic regions might account for insolubility and are supposed to be less exposed to the immune system, the fragments encoding the N- and the C-terminal hydrophilic regions were cloned separately in the pGEX vector for further expression in E. coli. PCR was used to amplify a 340-bp fragment which encodes the N-terminal hydrophilic region (amino acids 40 to 150) and a 180-bp fragment encoding the C-terminal hydrophilic region (amino acids 174 to 230). Oligonucleotides carrying BamHI and StuI sites were used to amplify the 340-bp fragment, which was spliced into the pGEX-2T vector, creating a GST fusion protein that was designated GRA6-Nt, whereas oligonucleotides carrying BalI and EcoRI were used to amplify the 180-bp fragment, which was spliced into the pGEX-3X vector, creating a GST fusion protein that was designated GRA6-Ct (Fig. 1). The GRA6-Nt and the GRA6-Ct proteins were approximately 53 and 35 kDa, respectively (Fig. 2A), and could be expressed as soluble proteins in large quantities. Coomassie blue-stained gel analysis revealed that the GRA6-Nt and Ct fusion proteins represent >95% of the stainable material (Fig. 2A).
Reactivity of human sera with recombinant GRA1, GRA6-Nt, and GRA6-Ct fusion proteins on immunoblots.
Immunoblot analysis showed that the recombinant GRA1 and GRA6-Nt GST fusion proteins were both recognized by a pool of T. gondii-immune human sera, whereas no reactivity was observed using a pool of T. gondii-negative human sera (Fig. 2B) or when the wild-type GST protein was probed with the same sera (Fig. 2B). By contrast, there was no detectable activity against the recombinant GRA6-Ct that was probed with the same pool of T. gondii-immune human sera (Fig. 2B). Together, these results indicate that a major B-cell epitope(s) is carried by the N-terminal peptide portion of the GRA6 antigen.
Reactivity of human sera with recombinant GRA1 and GRA6-Nt in IgG ELISA.
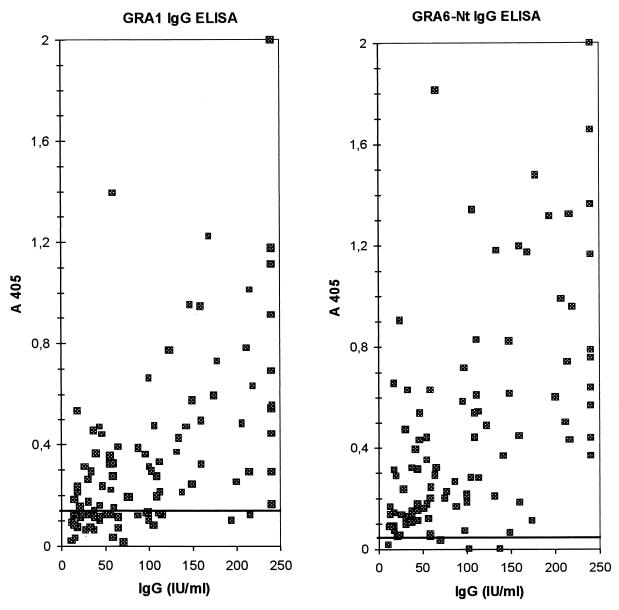
Two separate IgG ELISAs were developed using the recombinant GRA1 and GRA6-Nt fusion polypeptides as coating antigens to evaluate the potential of each of these individual recombinant GRA antigens for the serodiagnosis of toxoplasmosis. A total of 100 T. gondii-positive human sera and 98 negative human sera were tested for anti-GRA1 IgG antibodies (GRA1 IgG ELISA, Fig. 3A) and for anti-GRA6-Nt IgG antibodies (GRA6-Nt IgG ELISA, Fig. 3B). Sera were also tested against wild-type GST (produced in the corresponding pGEX system) in parallel. None of the samples had significant background reactivity against the wild-type GST. After elimination of outliers by the box plot analysis (5 and 3 of 100 negative sera in the GRA1 and GRA6-Nt tests, respectively), the cutoffs were set as the mean value of the negative samples plus 4 standard deviations, resulting in 0.13 for the GRA1 IgG ELISA and 0.05 for the GRA6 IgG ELISA. In these conditions, none of the negative serum samples was found to score above the cutoff, resulting in a specificity of 100% for both ELISAs (data not shown). Both GRA1 and Nt-GRA6 ELISAs presented a good correlation with the Platelia Toxo-IgG values (P = 0.0001, Spearman test). However, the overall sensitivity of the GRA6-Nt IgG ELISA was higher than that of the GRA1 IgG ELISA (96 versus 68%). As shown in Table 1, whereas the GRA1 ELISA sensitivity increases as the Platelia Toxo-IgG titer increases, the GRA6-Nt ELISA sensitivity is already high for low Platelia Toxo-IgG IgG titers.
FIG. 3.
IgG ELISA reactivity with 100 Toxoplasma-positive sera. The x axis indicates the IgG titers of the tested sera, as defined by a direct IgG ELISA Platelia Toxo-IgG kit. The y axis indicates the OD450. The cutoff values are indicated by a horizontal line.
TABLE 1.
Relationship between Platelia Toxo-IgG titer and GRA ELISA sensitivity
| Platelia Toxo-IgG ELISA IgG, IU/ml (no. of samples) | Sensitivity (%)
|
|
|---|---|---|
| GRA1 ELISA | GRA6-Nt ELISA | |
| <50 (34) | 47 | 97 |
| 50–100 (21) | 62 | 95 |
| 100–150 (20) | 80 | 90 |
| >150 (25) | 92 | 100 |
Combination of GRA1 and GRA6 IgG ELISAs.
The possibility of improving the GRA6-Nt ELISA sensitivity was examined by combining the GRA6-Nt ELISA results with those obtained using the GRA1 ELISA. Of the four sera that were negative in the GRA6-Nt ELISA, two were found to be positive in the GRA1 ELISA, with an OD largely above the cutoff value (0.201 and 0.297), and the other two sera were negative. One of these was found to be borderline in the Platelia Toxo-IgG kit, with an IgG titer of 11 IU/ml, whereas the other corresponded to a serum with a higher IgG titer of 70 IU/ml. Together, these observations indicate that an overall sensitivity of 98% could be reached by combining the two ELISA tests.
DISCUSSION
In the search to develop a recombinant antigen-based ELISA for the serological diagnosis of Toxoplasma infection, the potential of using several GRA antigens expressed in E. coli as GST fusion proteins was investigated. The almost complete sequence of GRA1 (lacking the N-terminal signal peptide) was expressed as a soluble fusion protein easily purified on glutathione-agarose. The IgG ELISA developed using this recombinant material gave a sensitivity of 68%. Another GRA antigen, GRA6, was found to be insoluble when expressed using the pGEX system, while separate expression of the N-terminal and C-terminal hydrophilic regions that flank the central putative transmembrane domain was found to give high expression yields. The GRA6 C-terminal fusion protein reacted poorly with human sera. This fusion protein was not detected on a Western blot probed with a pool of T. gondii-positive human sera and, when used in ELISA, gave about 10% sensitivity (data not shown). By contrast, the GRA6 N-terminal fusion protein was found to be highly reactive with positive human sera, leading to the development of an IgG ELISA test that reached 96% sensitivity.
Various recombinant antigens have already been evaluated in ELISA for detection of anti-T. gondii IgG. An ELISA based on two recombinant truncated fragments of T. gondii antigens (H4 and H11) expressed in fusion with GST has been shown to detect 68% of patients with acute toxoplasmosis and only 14% of those with chronic infection (14). SAG2, the p22 surface antigen of T. gondii, has also been expressed in E. coli as a GST fusion protein (10). Using a cutoff set at the mean + 1 standard deviation, rSAG2 was also shown to be very sensitive in its ability to detect IgG from patients with acute toxoplasmosis (100%) but less sensitive for sera from those with chronic disease (76%), giving the test an overall sensitivity of 87%. Van Gelder and coworkers reported the expression of the 330-residue carboxy-terminal antigenic fragment of the 54-kDa rhoptry protein (ROP2) in fusion with a hexahistidyl tag and have developed an IgG ELISA that reached a sensitivity of 89% (17). The diagnostic potential of several GRA antigens has also been reported. Hence, the 50 carboxy-terminal residues of GRA2 expressed in fusion with GST were shown to contain two B-cell epitopes that, when used in an IgG ELISA, gave a test that reached 75% sensitivity (8). Recently, an IgG ELISA based on the detection of GRA7 expressed in E. coli as a polyhistidine fusion protein has been reported to reach 81% sensitivity (4).
Several factors may explain why, compared to the other recombinant antigens already described and more particularly to the recombinant GRA1, the GRA6 N-terminal fusion protein appears to be a much more sensitive marker for the detection of Toxoplasma infection. It could be argued that the GRA6 antigen is exposed to the immune system for a longer time during the course of the infection. However, to our knowledge, both GRA1 and GRA6 are being expressed and secreted by both the tachyzoite and the bradyzoite stages of the parasite and therefore during both the acute and chronic phases of the infection. The most probable explanation involves the heterogeneity of the immune response. According to this hypothesis, the N-terminal region of GRA6 could contain at least one immunodominant B-cell epitope recognized by the majority of individuals, whereas responses to GRA1 would be much more variable. Such individual immune response could also explain why two sera found negative in the GRA6-Nt ELISA were found to be positive in the GRA1 ELISA. Another possible source of difference in the sensitivities of the ELISAs that should also be considered is antigenic diversity, which is known to occur among T. gondii strains (18). Combined analyses of isoenzyme patterns and restriction fragment length polymorphisms have recently revealed that T. gondii strains can be subdivided into three predominant clonal lineages (2, 3). Although the prevalence of these genotypes in Toxoplasma infection in humans is not known, strains belonging to the three lineages were isolated from humans, with the majority of human toxoplasmosis cases associated with type II strains (3). Such genetic diversity has to be taken into account, since the majority of genes encoding antigens with potential interest in diagnosis were cloned from type I genomes. Two distinct antigenic forms have already been demonstrated for GRA4 (7), another dense granule antigen, one form being specific to type I strains, the other form to type II and type III strains. While GRA1 polymorphism has not been examined yet, we have recently shown that the amino acid sequence of GRA6 is also polymorphic (B. Rausher, L. Lecordier, D. Deslée, U. Gross, and M.-F. Cesbron-Delauw, unpublished data). However, the observed mutations are mainly concentrated on the C-terminal region of GRA6, the N-terminal region being more highly conserved among the three genotypes. These observations may account for a possible lack of antigenic diversity in the N-terminal region and therefore may correlate with the high sensitivity of the GRA6-Nt ELISA.
Such possible heterogeneity in the immune response and/or antigenicity supports the idea that improvement of the ELISA will probably require the use of several Toxoplasma recombinant antigens to reach a sensitivity similar to that obtained with the current diagnostic tests. Although the N-terminal portion of GRA6 appeared to contain an immunodominant epitope(s), 4 of the 100 Toxoplasma-positive sera tested were under the cutoff value. Two of them were also negative in the GRA1 ELISA. However, one of these two sera was borderline in the Platelia Toxo-IgG kit. By contrast, the two other GRA6-Nt-negative sera were found to be positive in the GRA1 ELISA, with an OD value largely above the cutoff value, so that the GRA6 IgG ELISA together with GRA1 may reach an overall sensitivity of 98%. Therefore, despite a weak sensitivity of the GRA1 ELISA (68%), it may be valuable to complement the GRA6-Nt ELISA. Another interesting combination based on recombinant GRA7 together with Tg34AR (ROP2 C-terminal fragment) was recently reported (4). In this particular case, whereas each ELISA test had a sensitivity of 81 and 88%, respectively, the combination of the two increased the sensitivity to 96%. Together, these data demonstrate the potential of using two or three complementary recombinant antigens to reach a sensitivity comparable to that obtained with a crude antigen preparation.
In conclusion, our results showed that GRA6-Nt is one of the most promising recombinant antigens that, together with one (i.e., GRA1) or two other recombinant antigens, might be incorporated into a serological test for routine screening for toxoplasmosis. The recent finding that high levels of anti-recombinant GRA6-GST IgG antibodies correlate with the acute phase of toxoplasmosis with a sensitivity of 89% (11) supports the diagnostic value of the GRA6 antigen.
ACKNOWLEDGMENTS
We acknowledge D. Deslée for immunizations.
This work was supported in part by Institut Pasteur de Lille, INSERM, and CNRS.
REFERENCES
- 1.Cesbron-Delauw M F, Guy B, Torpier G, Pierce R J, Lenzen G, Cesbron J Y, Charif H, Lepage P, Darcy F, Lecocq J P, Capron A. Molecular characterization of a 23 K major antigen secreted by Toxoplasma gondii. Proc Natl Acad Sci USA. 1989;86:7537–7541. doi: 10.1073/pnas.86.19.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darde M L, Bouteille B, Pestre-Alexandre M. Isoenzyme analysis of 35 Toxoplasma gondii isolates and the biological and epidemiological implications. J Parasitol. 1992;78:786–794. [PubMed] [Google Scholar]
- 3.Howe D K, Sibley L D. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs D, Vercammen M, Saman E. Evaluation of recombinant dense granule antigen 7 (GRA7) of Toxoplasma gondii for detection of immunoglobulin G antibodies and analysis of a major antigenic domain. Clin Diagn Lab Immunol. 1999;6:24–29. doi: 10.1128/cdli.6.1.24-29.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 6.Lecordier L, Moleon-Borodowski I, Dubremetz J F, Tourvieille B, Mercier C, Deslée D, Capron A, Cesbron-Delauw M F. Characterization of a dense granule antigen of Toxoplasma gondii (GRA6) associated to the network of the parasitophorous vacuole. Mol Biochem Parasitol. 1995;70:85–94. doi: 10.1016/0166-6851(95)00010-x. [DOI] [PubMed] [Google Scholar]
- 7.Meisel R, Stachelhaus S, Mevelec M N, Reichmann G, Dubremetz J F, Fischer H G. Identification of two alleles in the GRA4 locus of Toxoplasma gondii determining a differential epitope which allows discrimination of type I versus type II and III strains. Mol Biochem Parasitol. 1996;81:259–263. doi: 10.1016/0166-6851(96)02719-3. [DOI] [PubMed] [Google Scholar]
- 8.Murray A, Mercier C, Decoster A, Lecordier L, Capron A, Cesbron-Delauw M F. Multiple B-cell epitopes in a recombinant GRA2 secreted antigen of Toxoplasma gondii. Appl Parasitol. 1993;34:235–244. [PubMed] [Google Scholar]
- 9.Nockemann S, Dlugonska H, Henrich B, Kitzerow A, Daubener W. Expression, characterization and serological reactivity of a 41 kDa excreted-secreted antigen (ESA) from Toxoplasma gondii. Mol Biochem Parasitol. 1998;97:109–121. doi: 10.1016/s0166-6851(98)00138-8. [DOI] [PubMed] [Google Scholar]
- 10.Parmley S F, Sgarlato G D, Mark J, Prince J B, Remington J S. Expression, characterization, and serologic reactivity of recombinant surface antigen P22 of Toxoplasma gondii. J Clin Microbiol. 1992;30:1127–1133. doi: 10.1128/jcm.30.5.1127-1133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redlich A, Müller A W. Serodiagnosis of acute toxoplasmosis using a recombinant form of the dense granule antigen GRA6 in an enzyme-linked immunosorbent assay. Parasitol Res. 1999;84:700–706. doi: 10.1007/s004360050473. [DOI] [PubMed] [Google Scholar]
- 12.Remington J S, Krahenbul J L. Immunology of Toxoplasma gondii. In: Nahmias A J, O'Reilly R J, editors. Immunology of human infection, part 2. New York, N.Y: Plenum Publishing Corp.; 1982. pp. 327–371. [Google Scholar]
- 13.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione-S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 14.Tenter A M, Johnson A M. Recognition of recombinant Toxoplasma gondii antigens by human sera in an ELISA. Parasitol Res. 1991;77:197–203. doi: 10.1007/BF00930858. [DOI] [PubMed] [Google Scholar]
- 15.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from acrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tukey J W. Some graphicals and semigraphic displays. In: Bancroft T A, editor. Statistical papers in honor of Georges W. Snedecor. Ames, Iowa: Iowa State University Press; 1972. pp. 293–316. [Google Scholar]
- 17.Van Gelder P, Bosman F, De Meuter F, Van Heuverswyn H, Hérion P. Serodiagnosis of toxoplasmosis by using a recombinant form of the 54-kilodalton rhoptry antigen expressed in Escherichia coli. J Clin Microbiol. 1993;31:9–15. doi: 10.1128/jcm.31.1.9-15.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware P L, Kasper L H. Strain-specific antigens of Toxoplasma gondii. Infect Immun. 1987;55:778–783. doi: 10.1128/iai.55.3.778-783.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]