Abstract
There is a wide spectrum of congenital anomalies or variations of the aortic arch, ranging from non-symptomatic variations that are mostly detected incidentally to clinically symptomatic variations that cause severe respiratory distress or esophageal compression. Some of these may be accompanied by other congenital heart diseases or chromosomal anomalies. The widespread use of multidetector computed tomography (CT) in clinical practice has resulted in incidental detection of several variations of the aortic arch in adults. Thus, radiologists and clinicians should be aware of the classification of aortic arch anomalies and carefully look for imaging features associated with a high risk of clinical symptoms. Understanding the embryological development of the aortic arch aids in the classification of various subtypes of aortic arch anomalies and variants. For accurate diagnosis and precise evaluation of aortic arch anomalies, cross-sectional imaging modalities, such as multidetector CT or magnetic resonance imaging, play an important role by providing three-dimensional reconstructed images. In this review, we describe the embryological development of the thoracic aorta and discuss variations and anomalies of the aortic arch along with their clinical implications.
Keywords: Multidetector computed tomography, Aorta, Congenital abnormalities, Technology
INTRODUCTION
Congenital anomalies and variants of the aortic arch encompass a wide range of aortic arch malformations that result from interrupted embryogenesis of the branchial arches.1) Advances in imaging modalities and increased use of cross-sectional imaging, including multidetector computed tomography (CT) and magnetic resonance imaging (MRI), have resulted in frequent incidental detection of these malformations. From a clinical perspective, aortic arch anomalies can be present without definite symptoms or may be accompanied by various symptoms, including difficulty breathing or swallowing.2) Additionally, association of arch anomalies with congenital heart disease and chromosomal/genetic abnormalities must be considered. Imaging studies are critical for detection and diagnosis of these anomalies.3) Various modalities can be used for detailed evaluation of the anatomy of the aortic arch and the hemodynamics of the arch vessels, which is necessary for planning surgery or endovascular intervention. In this article, we review the classification of aortic arch anomalies with a focus on embryologic development and clinical features.
EMBRYOLOGY AND ANATOMY OF THE AORTIC ARCH
Understanding the normal development of the aortic arch is essential to understand and classify the various subtypes of aortic arch anomalies and variants. Two distinct developmental models can explain the development of the great vessel system.
Rathke diagram
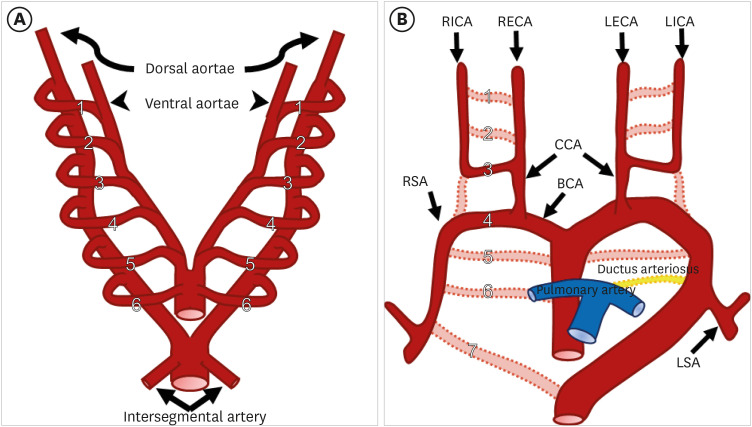
The development of the great vessel system starts at around 3 weeks of gestation and is mostly completed by 8 weeks of gestation in humans.4) The great vessel system, including the aortic arch, arises from six pairs of pharyngeal arch arteries, each connecting the dorsal and ventral primitive aortae. These primitive arches appear individually, regress, and eventually remodel and transform into portions of the great vessel system, including the aortic arch, arch branches, ductus arteriosus, and proximal segments of the pulmonary arteries. This embryologic model was originally proposed in the classic Rathke diagram (Figure 1).5) The primitive first and second arches regress first and the remnant portions become the maxillary, hyoid, and stapedial arteries. The primitive fifth aortic arch usually regresses; however, it may rarely persist as a double barrel aorta.6) After regression of some arches, the remnant third, fourth, and sixth primitive arch give rise to mature great vessels. The third arch becomes the common carotid and proximal internal carotid arteries, while the fourth arch forms the definitive adult aortic arch. The primitive sixth arch persists as a segment of the pulmonary arteries and ductus arteriosus.5)
Figure 1. (A) Schematic representation of Rathke’s diagram. Six paired branchial arches (numbered 1 to 6) connect the paired ventral aortae (arrowheads) and dorsal aortae (curved arrows). A pair of intersegmental arteries (straight arrows) arises from the aortic sac. (B) Schematic representation of the development of the normal aortic arch and branch vessels. Selective involution of the branchial arch segments results in the final adult aortic arch and is thought to be the main mechanism underlying arch and branch vessel anomalies.
BCA: brachiocephalic artery, CCA: common carotid artery, LECA: left external carotid artery, LICA: left internal carotid artery, LSA: left subclavian artery, RECA: right external carotid artery, RICA: right internal carotid artery, RSA: right subclavian artery.
Edward’s hypothetical double arch
Another important model of aortic arch embryology is the hypothetical double arch proposed by Edward.7) In this model, there is a single midline ascending and descending aorta and bilateral aortic arches and ductus arteriosus surrounding the trachea and esophagus. Each carotid and subclavian artery (SA) originates from the ipsilateral arch. This model can explain various anomalies or malformations based on the regression or persistence of various segments. For example, the normal left aortic arch can be explained by regression of the right aortic arch, right sided ductus arteriosus, and right dorsal aorta. However, because the relative size of the aortic arch branches and lateral position of the descending aorta are not considered, this model does not cover all anatomic aortic arch variants.8)
IMAGING MODALITIES FOR EVALUATION OF THE AORTIC ARCH AND ASSOCIATED STRUCTURES
Imaging techniques available for diagnosing arch anomalies include barium esophagram, echocardiography, CT angiography, MRI, and catheter angiography.9) Classical modalities such as barium esophagram and catheter angiography are not frequently used as first-line diagnostic tools because they are difficult to use in pediatric patients and provide only two-dimensional (2D) information.10),11) At present, echocardiography, MRI, and CT angiography are the main modalities used to detect and evaluate aortic arch variants and anomalies.
CT provides high spatial resolution of the anatomy of the vessels and surrounding structures. Additionally, reformation in multiple planes or three-dimensional (3D) imaging can provide detailed anatomical information, including relative positional relationships, which is advantageous, especially when assessing tracheal and esophageal compression.12),13) Technological advances in CT have markedly reduced radiation exposure and image acquisition time. Use of high pitch spiral mode can reduce cardiac pulsation artifacts and image acquisition time.14) In some cases, CT can be performed without anesthesia or breath holding in pediatric patients.15) Despite improvements in CT techniques, however, there are still concerns regarding radiation exposure and administration of iodine contrast media.
Diagnostic aortic arch imaging by CT angiography has several minimal requirements for optimal quality.16) First, the minimal scan range must include the ascending thoracic aorta, descending thoracic aorta, and large aortic arch branches (brachiocephalic artery [BCA], common carotid artery [CCA], and SA). Second, at least 200-250 HU should be used for adequate aortic enhancement.17) Computed tomography angiography (CTA) contrast enhancement depends on various factors such as contrast medium volume, injection rate, scan duration, scan timing, and patient-related factors. By adjusting these variables, appropriate contrast enhancement can be achieved.18) Third, thin slice thickness is an important factor affecting CTA spatial resolution. For adequate diagnostic aortic arch imaging, slice thickness should not be more than 2 mm. Further multi-planar reconstruction using thin slices can provide additional anatomic information for diagnosis.
Another important factor to consider is patient-related factors. For instance, body mass index-adapted protocols in which contrast agent volume and imaging timing are adjusted for can decrease noise and provide better quality images. When scanning pediatric patients, more variables should be considered including small body volume and sensitivity to radiation exposure.19) Pediatric CTA optimization aims to minimize radiation exposure while obtaining images with adequate diagnostic quality.
MRI is an alternative cross-sectional imaging modality that allows precise assessment of aortic arch anomalies without ionizing radiation. Contrast-enhanced MR angiography and non-contrast enhanced black blood and bright blood sequences can be performed to evaluate anatomical relationships between the aortic arch, esophagus, and airway.20) Like CT, 3D volumetric acquisitions provide multi-planar reformatting for detailed assessment of tracheal compression. However, the biggest drawback of MRI is the long acquisition time. To overcome this drawback, several techniques, such as compressed sensing, 3D ultra-shot echo time, and 4D flow acquisition are being used.
However, contrast-enhanced thoracic aorta CT and MRI have some disadvantages. CT scans are not widely used in pediatric patients because of radiation exposure. While there is no radiation exposure when undergoing MRI, patient cooperation is a factor to consider due to the prolonged time requirements. In addition, contrast medium injection is required for both CT and MRI, and intravenous access is required for this.
Echocardiography is often used as the initial imaging modality in pediatric patients as it is non-invasive, there is no need for intravenous contrast medium, and there is no radiation exposure.11) Echocardiography not only provides anatomic information on the vascular and surrounding structures, but functional changes can also be assessed by Doppler ultrasonography. However, limited access to the entire thoracic aorta and dependency on operator skills are limitations of echocardiography as a major imaging modality.
NORMAL VARIATIONS OF THE AORTIC ARCH AND BRANCHING PATTERNS
Aortic arch sidedness and branching patterns
Aortic arch variations are classified based on anatomical and morphologic features of the aorta, including the sidedness of the arch, course of the aorta, and branching pattern.21) Sidedness of the aortic arch is based on which bronchus is crossed by the arch. Four types of aortic arch are recognized: left-sided aortic arch (LAA), right-sided aortic arch (RAA), double aortic arch, and cervical aortic arch. In addition, the branching pattern of major neck vessels (such as the carotid or SA) is also taken into consideration in classification.22) Clinical presentation or impact of the variation, ranging from asymptomatic to airway compression or vascular flow problems, can usually be predicted based on the classification.
Normal left aortic arch and branching pattern variants
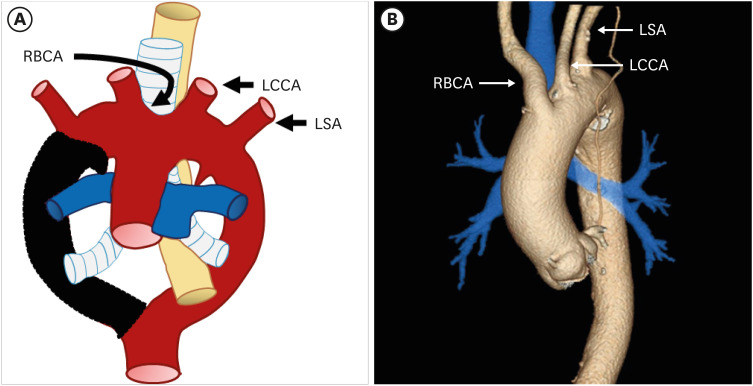
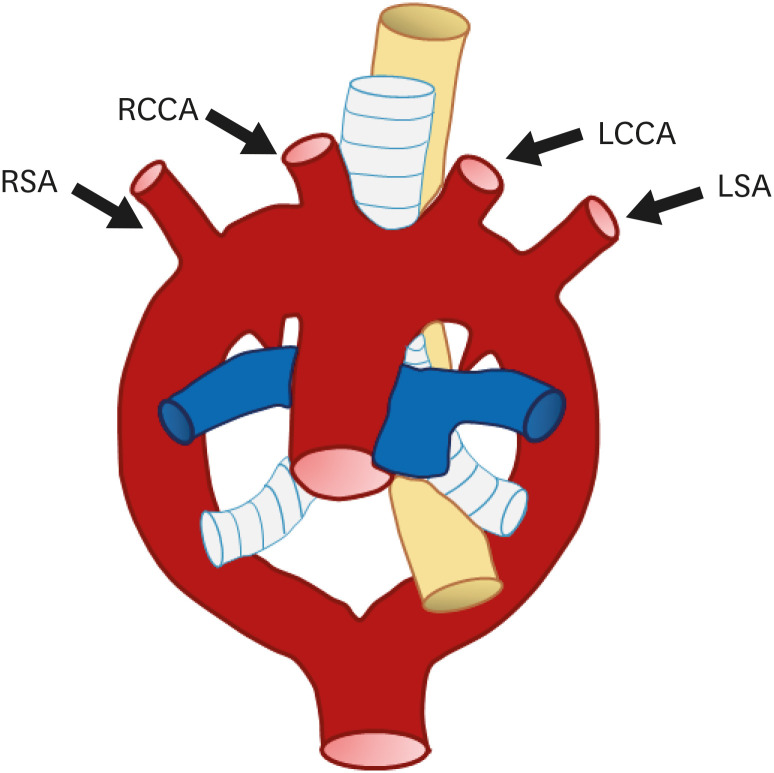
Based on Edward’s hypothetical double arch,7) the normal left aortic arch complex can be explained by regression of 1) the distal right fourth arch between the right subclavian artery (RSA) and descending aorta, 2) right ductus arteriosus, and 3) right dorsal aorta distal to the origin of the seventh intersegmental artery. The remnant proximal segment of the right fourth arch and left seventh intersegment artery persist as the RSA, while the left fourth arch becomes the definitive aortic arch. As a result, a normal aortic arch cross over the left main bronchus, and the descending aorta travels left of the midline with the left ductus arteriosus. According to the normal branching pattern, aortic branches arise in order as the right brachiocephalic artery (RBCA), left common carotid artery (LCCA), and left SA (LSA) (Figure 2). Several studies have reported that a “normal” aortic arch configuration is present in 65–75% of patients.3),22)
Figure 2. (A) Schematic figure and (B) 3D volume-rendered image of a normal left aortic arch based on Edward’s hypothetical double arch. Black-shaded area represents involuted segments in the hypothetical double arch. A normal left aortic arch results from involution of the right arch between the right subclavian artery and descending aorta.
LCCA: left common carotid artery, LSA: left subclavian artery, RBCA: right brachiocephalic artery.
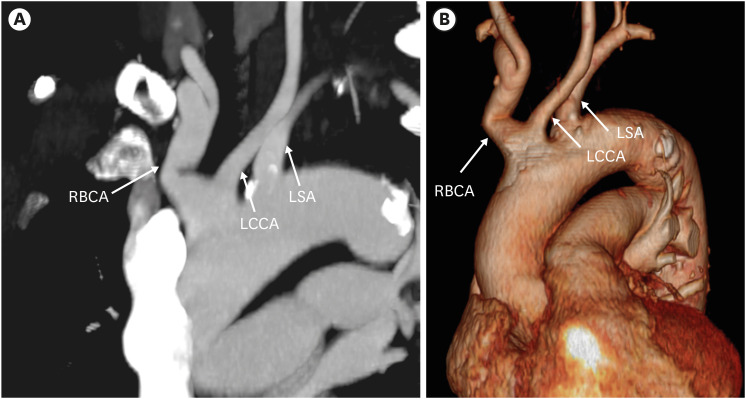
The most common variant of the arch branching pattern of the left aortic arch is the “bovine aortic arch” (10–20% of the population).3),22) The bovine aortic arch branching variant occurs when the second branch, the LCCA, has a common origin with the RBCA (13%) or originates directly from the RBCA (9%) (Figure 3). The bovine-type branching pattern has no similarity with the aortic arch pattern of cattle (who have a single brachiocephalic trunk originating from the aortic arch); therefore, the term “bovine aortic arch” is a misnomer.23) Although this branching pattern was thought to be a normal anatomic variant without symptoms, clinically significant effects such as thoracic artery aneurysm, increased risk of arch torsion, and laterality of cerebral stroke have been reported.24)
Figure 3. Common origin of the right brachiocephalic trunk and left common carotid artery in a 83-year-old man. Oblique coronal maximum intensity projection computed tomography angiographic image (A) and 3D volume-rendered image (B) show the common origin of the right brachiocephalic artery and left common carotid artery from the left aortic arch.
LCCA: left common carotid artery, LSA: left subclavian artery, RBCA: right brachiocephalic artery.
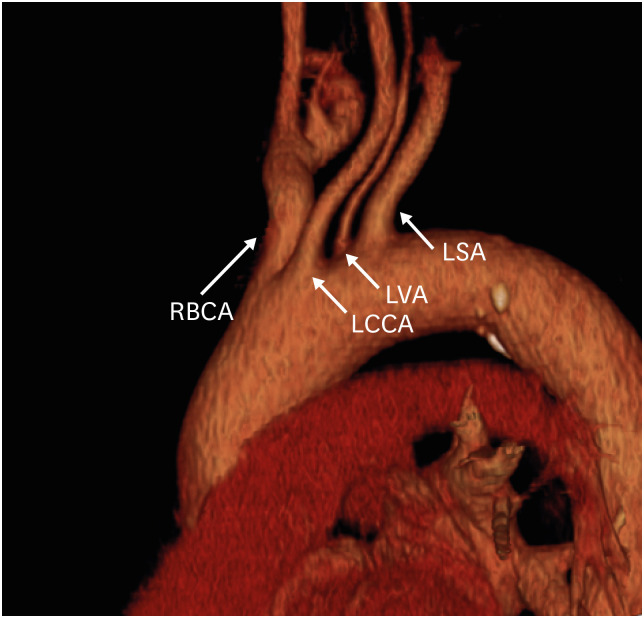
Another common variation of the left aortic arch is origination of the left vertebral artery directly from the aortic arch (Figure 4), which has been noted in 1–8% of the population.25),26) Aberrant origin of the right vertebral artery has also been observed, but this is rare.27) Although vertebral origin variants are considered asymptomatic aortic branching variants, it is important for the surgeon to be aware of these variations prior to planning and performing vascular thoracic surgery or interventional procedures.
Figure 4. Direct origin of the left vertebral artery from the aortic arch in a 53-year-old man. Oblique sagittal 3D volume-rendered computed tomography angiographic image shows the direct origin of the LVA from the left aortic arch.
LCCA: left common carotid artery, LSA: left subclavian artery, LVA: left vertebral artery, RBCA: right brachiocephalic artery.
In the adult “normal” LAA, the first great artery branch is the RBCA, which is usually located to the right of the midline. However, in most newborns, the origin of the RBCA is displaced to just left of the midline on the aortic arch (Figure 5), and there may be some degree of asymptomatic tracheal indentation on the midline crossing point of the RBCA. This anatomy may be related to immaturities of the tracheal cartilage in newborns.28) Rarely, the degree of tracheal compression may be significant, leading to clinical symptoms and resulting in innominate artery compression syndrome.29) In this condition, further assessment with cross-sectional imaging (CT or MRI) should be performed to delineate associated arch anomalies.
Figure 5. Origin of the right brachiocephalic artery in a 1-year-old boy. (A) Serial axial CT angiographic images and (B) 3D volume-rendered CT angiographic image show the origin of the brachiocephalic artery (RBCA, arrows) just left of midline on the aortic arch.
CT: computed tomography, RBCA: right brachiocephalic artery.
AORTIC ARCH ANOMALIES AND CLASSIFICATIONS
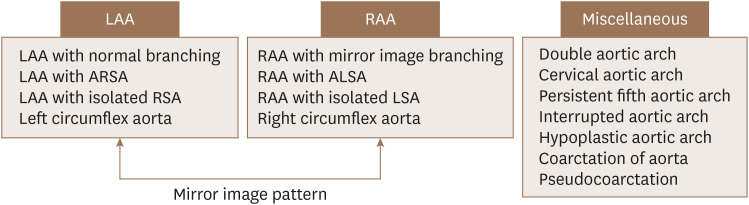
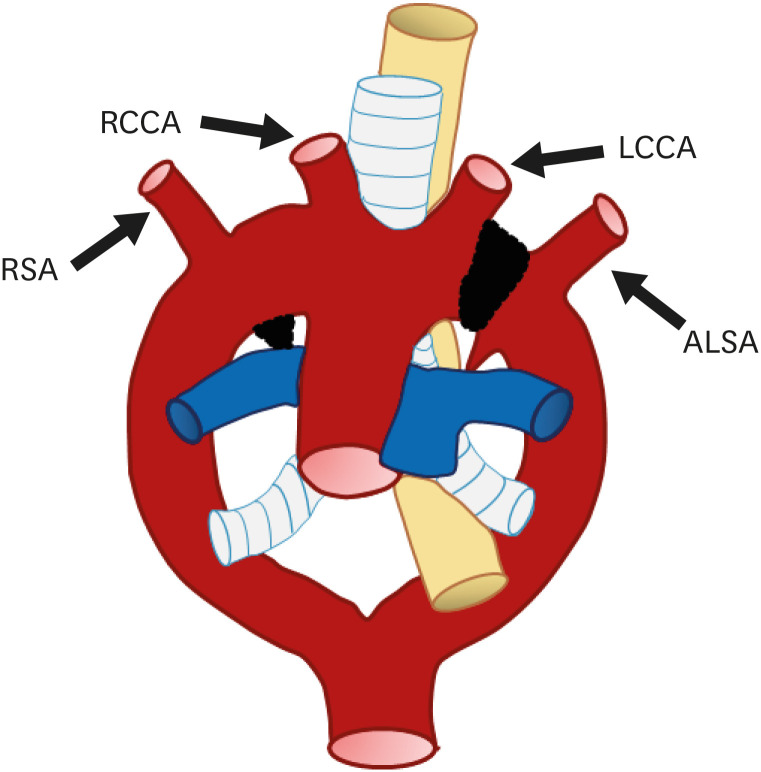
Based on previous studies, aortic arch anomalies can be classified into three main groups: left aortic arch, right aortic arch, and other arch variants. Interestingly, detailed variants and anomalies of the right arch type and left arch type show mirror imaging. Variants and anomalies from the viewpoint of mirror imaging are classified and reviewed in Figure 6. The clinical importance of each subtype and its association with other mutations are discussed below (Table 1).
Figure 6. Classification of aortic arch variants and anomalies based on arch-sidedness and mirror imaging.
ALSA: aberrant left subclavian artery, ARSA: aberrant right subclavian artery, LAA: left-sided aortic arch, LSA: left subclavian artery, RAA: right-sided aortic arch, RSA: right subclavian artery.
Table 1. Summary of aortic arch anomalies and variants.
| Vascular anomalies | Possible embryology | Arch and ductus | Clinical features | |
|---|---|---|---|---|
| Lt-sided arch | ||||
| Lt aortic arch with ARSA | Regression of the Rt 4th arch between the RCCA and RSA | Lt arch, Lt ductus | Most common congenital anomaly of the aorta (0.5–2%) | |
| Usually isolated | ||||
| Lt aortic arch with ARSA with Kommerell diverticulum | Regression of the Rt 4th arch between the RCCA and RSA and persistence of the Rt 6th arch component | Lt arch, Rt ductus, vascular ring (+) | Dysphagia lusoria (if tight vascular ring) | |
| 15–30% of ARSA | ||||
| Lt aortic arch with isolated Rt subclavian artery | Regression of the Rt 4th arch between the RCCA and RSA and regression of the segment distal to the RSA and ductus | Lt arch, no ductus | Congenital subclavian steal syndrome, vertebrobasilar insufficiency, syncope | |
| Lt circumflex aorta | Regression of the Rt 4th arch between the RCCA and RSA and persistence of the Rt 6th arch and Rt dorsal aorta | Lt arch, Rt ductus, Rt descending aorta, vascular ring (+) | Extremely rare | |
| Arch itself courses behind the esophagus | ||||
| Severe tracheobronchial compression | ||||
| Rt-sided arch | ||||
| Rt aortic arch with mirror image branching | Regression of the Lt 4th arch between the Lt ductus and dorsal aorta and persistence of the Lt 6th arch | Rt arch, Lt ductus | Usually asymptomatic | |
| Strong association with CHD (> 75%) | ||||
| Rt aortic arch with ALSA with Kommerell diverticulum | Regression of the Lt 4th arch between the LCCA and LSA and persistence of the Lt 6th arch component | Rt arch, Lt ductus, vascular ring (+) | Most common form of Rt-sided aortic arch | |
| Usually isolated (CHD in 5–10%) | ||||
| Dysphagia lusoria (if tight vascular ring) | ||||
| 60% of ALSA | ||||
| Rt aortic arch with ALSA without Kommerell diverticulum | Regression of the Lt 4th arch between the LCCA and LSA and regression of the Lt 6th arch | Rt arch, Rt ductus, or absent ductus | Usually combined with CHD | |
| Right aortic arch with isolated RSA | Regression of the Lt 4th arch between the LCCA and LSA origin and regression distal to the LSA and ductus | Rt arch, no ductus | Associated with CHD (> 50%), vertebrobasilar insufficiency, or subclavian steal syndrome | |
| Right circumflex aorta | Regression of the Lt 4th arch between the LCCA and LSA and persistence of the Lt 6th arch and Lt dorsal aorta | Rt arch, Lt ductus, Lt descending aorta, vascular ring (+) | More common than Lt circumflex aorta | |
| Miscellaneous | ||||
| Double aortic arch | Abnormal persistence of both aortic arches | Both aortic arches, vascular ring (+) | Most common cause of symptomatic vascular ring (50%) | |
| Cervical aortic arch | i) Persistence of the 2nd or 3rd branchial arches with regression of the 4th arch | Variable branching pattern | More common on the Rt-sided arch | |
| or ii) failure of caudal migration of the 4th arch | Usually asymptomatic (occasionally pulsatile neck mass) | |||
| Persistent fifth aortic arch | Persistence of the primitive 5th aortic arch | Variable branching pattern, vascular ring (+/−) | Usually associated with CHD | |
| Often misdiagnosed as ductus arteriosus or aortopulmonary window | ||||
| Interrupted aortic arch | i) Regression distal to the LSA, | Complete discontinuity between the ascending and descending aorta | Extremely rare | |
| or ii) regression between the LCCA and LSA, | Shock or severe heart failure occurs a few weeks after birth | |||
| or iii) regression between the RBCA and LCCA | ||||
| Hypoplastic aortic arch | May be associated with restriction of aortic flow | Relatively small diameter of the aortic arch | With or without other CHD (m/c with aortic coarctation) | |
| Coarctation of the aorta | i) Abnormal ductal tissue extension to the aortic isthmus or | Focal stenosis of the aortic arch adjacent to the aortic isthmus | Relatively common anomaly (0.04%) | |
| ii) altered fetal hemodynamics due to an abnormal angle between the ductus and aorta or abnormal pre-ductal flow | Commonly accompanied by CHD or an arch anomaly (44–84%, m/c with bicuspid aortic valve) | |||
| Pseudocoarctation | Failure of compression and fusion of the dorsal roots and 4th arch segments | Segmental kinking or buckling at the aortic isthmus | Incidentally found without clinical symptoms | |
ALSA: aberrant left subclavian artery, CHD: congenital heart disease, LCCA: left common carotid artery, LSA: left subclavian artery, Lt: left, m/c: most common, RBCA: right brachiocephalic artery, RCCA: right common carotid artery, RSA: right subclavian artery, Rt: right.
Left aortic arch
Branching variants of the LAA can be classified as normal, aberrant right subclavian artery (ARSA), isolated RSA, or left circumflex artery.
Left aortic arch with an ARSA
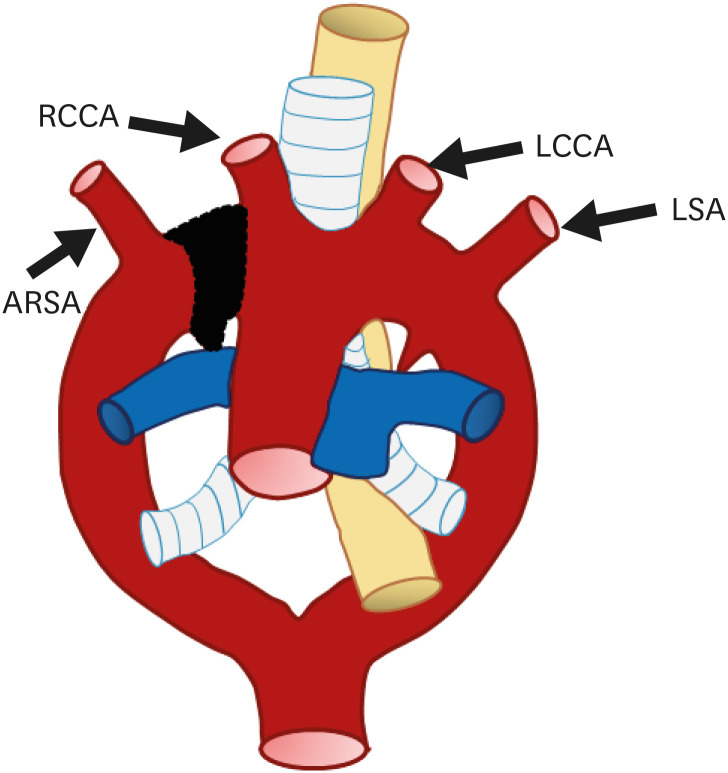
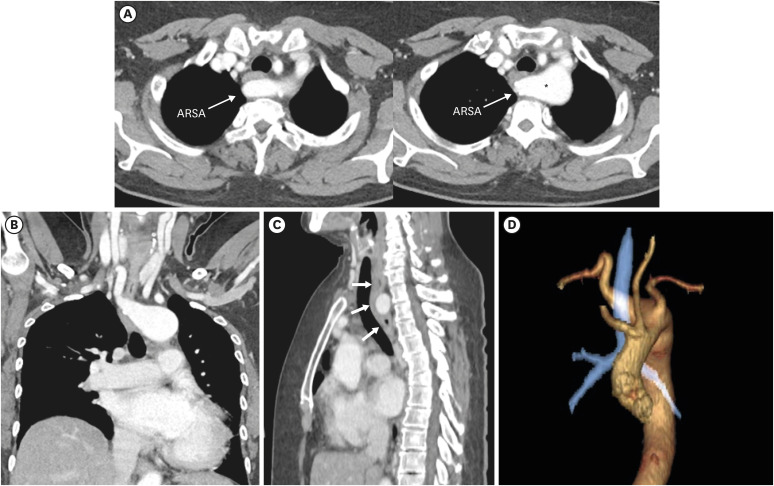
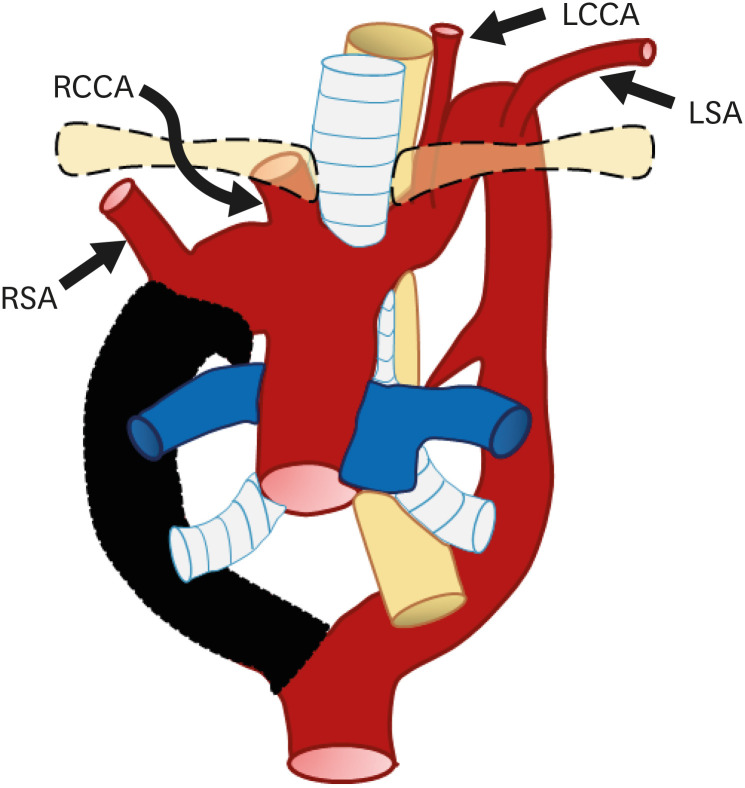
The most common type of aortic anomaly is a left aortic arch with an ARSA (Figures 7 and 8). This anomaly is found in 0.4–2% of the population with a female predominance.13) ARSA is thought to be a result of right aortic arch regression between the right CCA (RCCA) and RSA.7) After arising from the distal portion of the right dorsal aorta as the last branch, ARSA shows various courses.30) The course of the ARSA is classified based on its locational relationship with the esophagus as retroesophageal (most common), between the esophagus and trachea, or anterior to the trachea.31)
Figure 7. Schematic figure of a left aortic arch with an ARSA. Black-shaded area represents involuted segments in a hypothetical double arch. This anomaly results from regression of the right arch between the right subclavian artery and right common carotid artery.
ARSA: aberrant right subclavian artery, LCCA: left common carotid artery, LSA: left subclavian artery, RCCA: right common carotid artery.
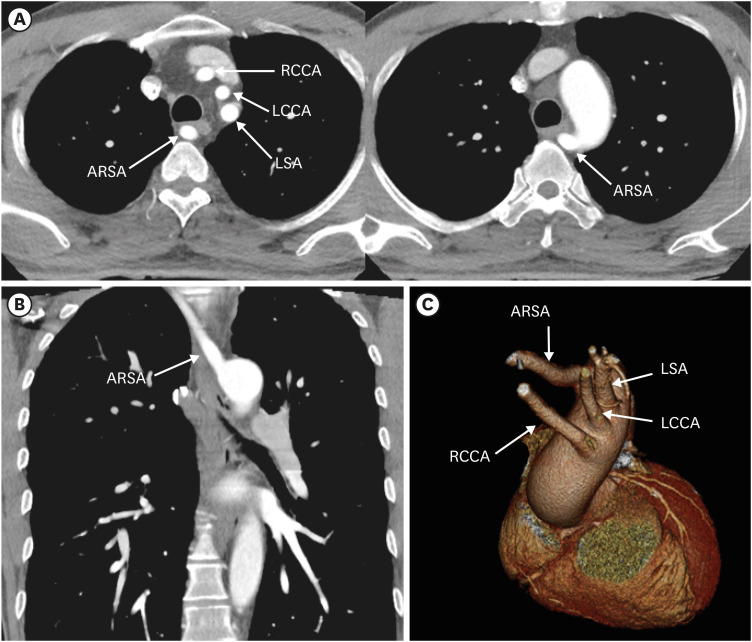
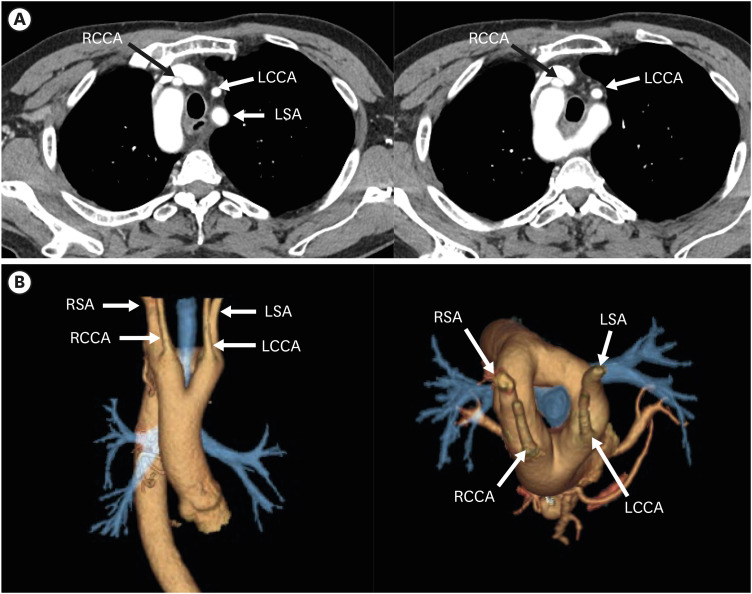
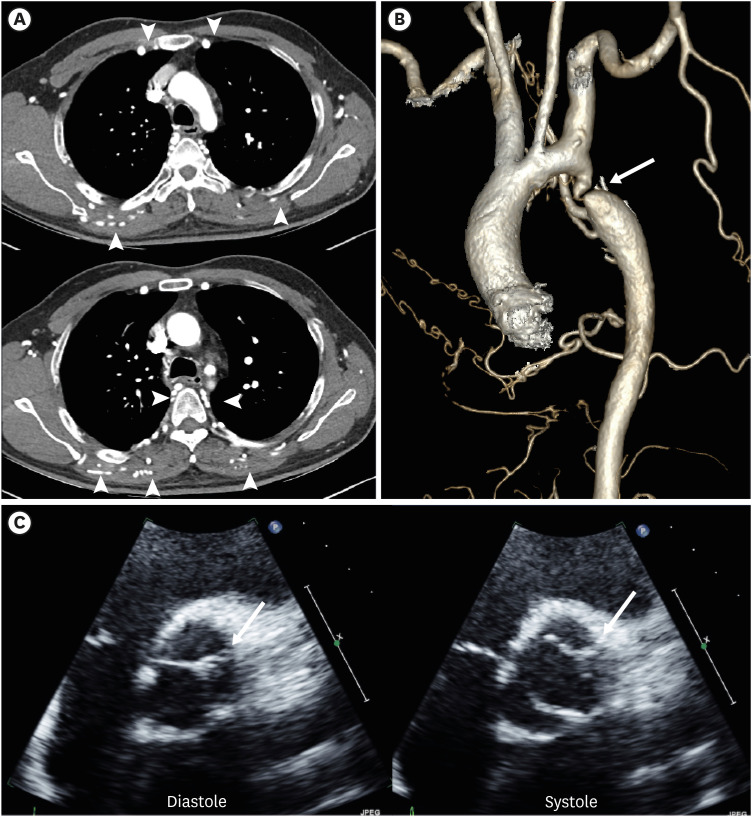
Figure 8. Left aortic arch with ARSA without Kommerell diverticulum in a 45-year-old man. (A, B) Transaxial and coronal CT angiographic images show an ARSA with a posterior mediastinal course. (C) Oblique coronal 3D volume-rendered CT angiographic image shows the branch arteries arising from the left aortic arch in order of RCCA, LCCA, left subclavian artery, and aberrant right subclavian artery.
ARSA: aberrant right subclavian artery, CT: computed tomography, LCCA: left common carotid artery, LSA: left subclavian artery, RCCA: right common carotid artery.
In this type of anomaly, the right ductus arteriosus usually undergoes regression, thus, a complete vascular ring is not formed. ARSA is therefore usually asymptomatic and incidentally detected. Rarely, tracheoesophageal compression by a retroesophageal segment of the ARSA can cause symptoms, referred to as “dysphagia lusoria,” which is usually found in infants.32) In addition, combined anomalies such as a persistent double aortic arch with right-sided ductus arteriosus can influence the occurrence of symptoms.33)
This anomaly is usually found as an isolated anomaly; however, it uncommonly occurs in conjunction with other anomalies such as tetralogy of Fallot (TOF), coarctation of the aorta, ventricular septal defect (VSD), and patent ductus arteriosus (PDA).34) Various studies have investigated the association between ARSA and trisomy 21, and Scala et al. demonstrated that ARSA was a clinically useful marker of trisomy 21.35)
As mentioned above, because a vascular ring is usually not formed, this anomaly does not result in clinical symptoms or require treatment. In cases where the ARSA is prominently dilated and there is aneurysm formation or it causes severe symptoms such as dysphagia or dyspnea, surgical treatment may be considered.36) The goal of surgery is to reposition the anomalous SA. Usually, this surgery involves carotid-SA bypass or re-implantation of the ARSA at a more proximal arch position. A two-stage hybrid procedure consisting of transcatheter occlusion and open carotid-subclavian bypass was recently performed.37)
Left aortic arch with an ARSA with Kommerell diverticulum
Another variant of the ARSA is associated with persistence of the right sixth arch component. Remnant right sixth arch component consists of a dorsal aortic arch segment and right-sided ductus arteriosus. Dorsal aortic arch segment persists as the retroesophageal diverticulum (Kommerell diverticulum), which gives rise to the ARSA (Figure 9).38) The right-sided ductus arteriosus (or ligamentum arteriosum) along with the opposite side’s left aortic arch complete a loose vascular ring surrounding the trachea and esophagus.39) Kommerell diverticulum is found in 15–30% cases of LAA with ARSA,40) and more commonly occurs in association with RAAs. If dysphagia lusoria is present due to the vascular ring or aneurysm of Kommerell is present, surgical resection should be performed.41)
Figure 9. Left aortic arch with an ARSA with Kommerell diverticulum in a 63-year-old woman. (A, B) Axial and coronal CT angiographic images show an ARSA with Kommerell diverticulum (asterisk). (C) Sagittal CT angiographic image shows the Kommerell diverticulum causing esophageal compression (arrows). (D) 3D volume-rendered CT angiographic image shows the retro-tracheal course of the ARSA with diverticulum of Kommerell.
ARSA: aberrant right subclavian artery, CT: computed tomography.
Left aortic arch with an isolated RSA
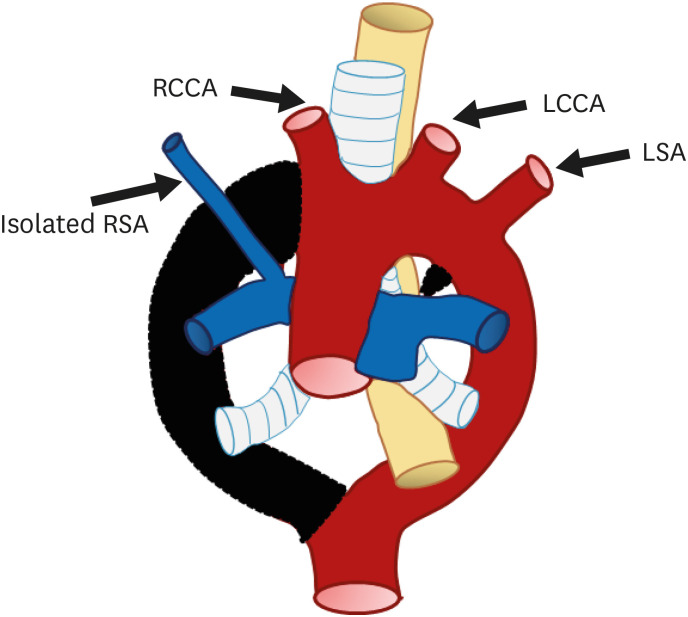
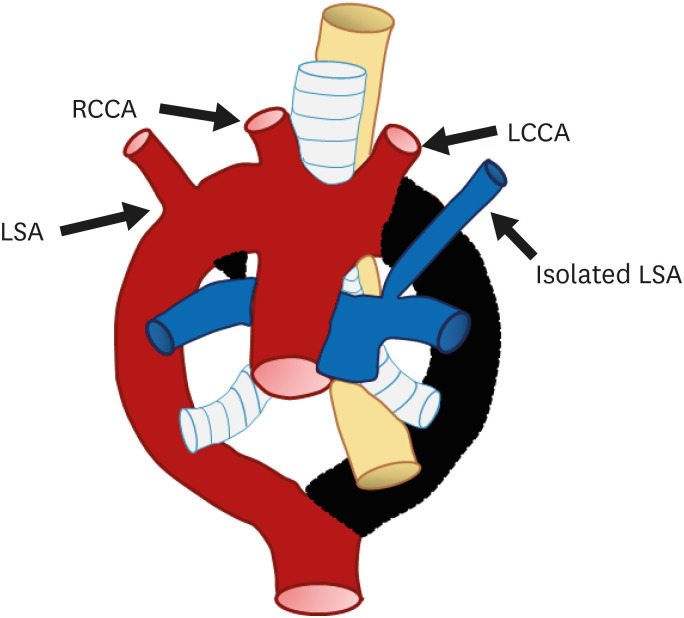
Isolation of the RSA, defined as absence of continuity between the RSA and aortic arch, is a rare great vessel anomaly; instead, there is a connection with the pulmonary artery via the ductus arteriosus42) (Figure 10). Isolated RSA with left aortic arch is extremely rare compared to isolated LSA.43) Two-point segmental regression of the right arch between the RCCA and RSA origin and distal to the origin of the right ductus and RSA is the cause of this anomaly. The vertebral artery or the ductus arteriosus collaterals, instead of the aortic arch, fills the isolated RSA.
Figure 10. Schematic figure of the left aortic arch with an isolated right subclavian artery. Black-shaded area represents involuted segments in a hypothetical double arch. This anomaly results from (i) regression of the right arch between the origin of the right common carotid artery and right subclavian artery and (ii) distal to the origin of the right ductus and right subclavian artery. Thus, the right subclavian artery is connected to the pulmonary artery instead of the aortic arch.
LCCA: left common carotid artery, LSA: left subclavian artery, RCCA: right common carotid artery, RSA: right subclavian artery.
Isolated RSA may be associated with other cardiovascular anomalies such as PDA, TOF, or 22q11 deletion syndrome.44),45) Although this anomaly does not form a complete vascular ring, various symptoms, including syncope, absent pulses in the upper limb, and ischemic limb pain can occur and raise the suspicion of vertebrobasilar insufficiency. If such symptoms are present, surgical re-anastomosis of the isolated SA to the carotid artery should be considered.46)
Left circumflex aorta
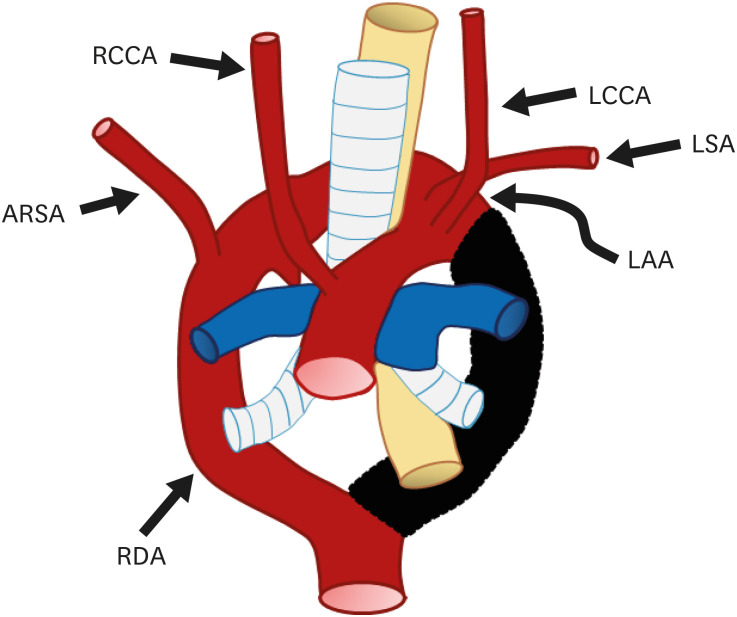
Usually, a definite aortic arch consists of the dorsal aorta on the side of the arch. However, due to regression of the right fourth arch between the RCCA and RSA, and persistence of the right-sided sixth arch component forming the ductus arteriosus, the distal left dorsal aorta forms the definite distal aortic arch; thus, the left circumflex aorta consists of the left aortic arch with midline crossing, right sided descending aorta, and right ductus arteriosus (Figure 11).46)
Figure 11. Schematic figure of the left circumflex aorta. Black-shaded area represents involuted segments in a hypothetical double arch. This anomaly results from regression of the right fourth arch between the RCCA and right subclavian artery and persistence of the right-sided sixth arch component.
ARSA: aberrant right subclavian artery, LAA: left aortic arch, LCCA: left common carotid artery, LSA: left subclavian artery, RCCA: right common carotid artery, RDA: right descending aorta.
This is an extremely rare type of aortic anomaly and usually shows a similar branching pattern to that of a left aortic arch with an ARSA. However, in this anomaly, the distal arch itself crosses the midline, rather than the ARSA crossing the midline.46) In most cases of left circumflex aorta, the right ductus arteriosus completes a vascular ring by connecting the right pulmonary artery to the descending aorta. Consequently, this anomaly may result in respiratory compromise by compressing the tracheobronchial structure; surgical repositioning is required in this case.47)
RAA
A RAA is found in 0.05% to 0.1% of the population.48) The pathological mechanism of the right aortic arch is unknown, but it is presumed to be related to embryonic blood flow.49) Based on the arrangement of the arch vessels and ligamentum arteriosum, three subtypes of right aortic arch are recognized: type I (RAA with mirror image branching), type II (RAA with aberrant left subclavian vein with or without Kommerell diverticulum), and type III (RAA with isolated SA). Each RAA subtype shows mirror imaging with the subtypes of the left aortic arch.50) RAA is frequently associated with other congenital cardiac anomalies, esophageal atresia, and tracheoesophageal fistula.51)
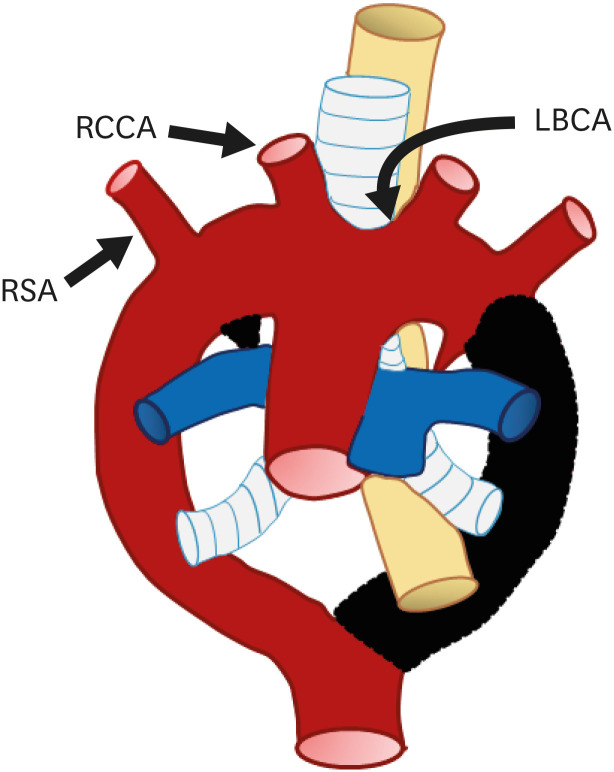
Right aortic arch with mirror image branching
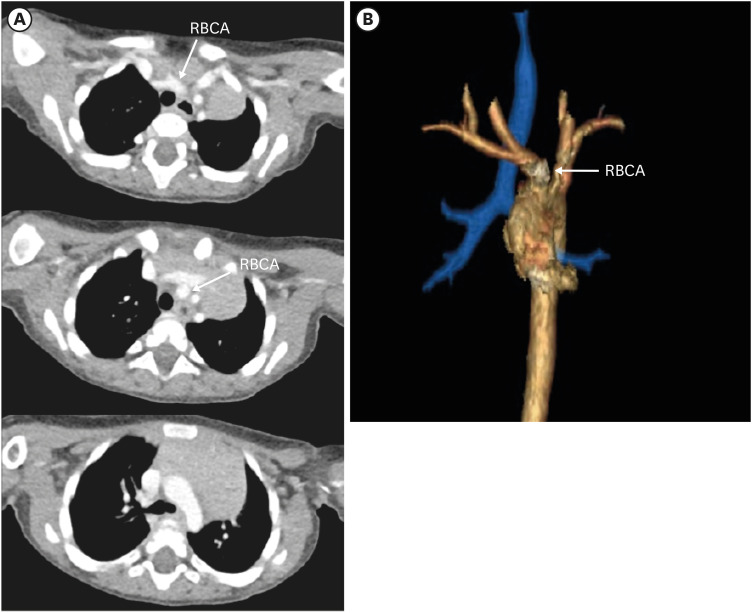
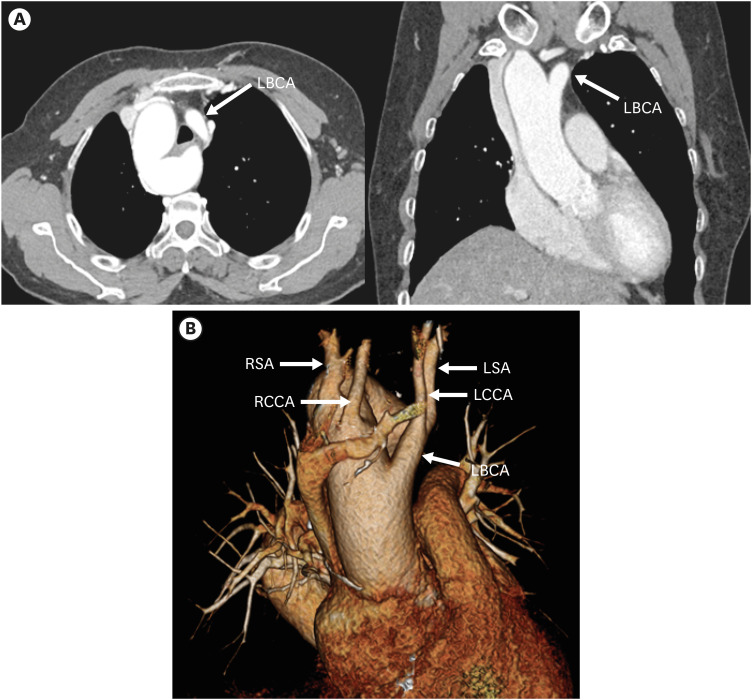
RAA with mirror image branching is the second most common subtype of RAA. Embryologically, this pathology is due to regression of the left fourth branchial arch between the left ductus and dorsal aorta, along with persistence of the left sixth arch.52) As a result, the left fourth arch remains a proximal component of the LSA and the definite aortic arch descends to the right of the trachea, crossing above the right main bronchus.53) As the name suggests, this type of RAA has a branching pattern that is a mirror image of the normal left aortic arch branching pattern in order of left BCA (LBCA), RCCA, and RSA (Figures 12 and 13).
Figure 12. Schematic figure of the right aortic arch with mirror image branching. Black-shaded area represents involuted segments in a hypothetical double arch. This anomaly results from regression of the left aortic arch between the left subclavian artery and descending aorta.
LBCA: left brachiocephalic artery, RCCA: right common carotid artery, RSA: right subclavian artery.
Figure 13. Right aortic arch with mirror image branching in a 75-year-old woman. (A) Axial and coronal CT angiographic images show the left brachiocephalic artery arising from the aortic arch and then branching into the left common carotid and subclavian arteries. (C) Coronal 3D volume-rendered CT angiographic image shows branch arteries arising from the right aortic arch in order of left brachiocephalic artery, right common carotid artery, and right subclavian artery.
CT: computed tomography, LBCA: left brachiocephalic artery, LCCA: left common carotid artery, LSA: left subclavian artery, RCCA: right common carotid artery, RSA: right subclavian artery.
In most cases, the remnant left sixth arch becomes the left ductus arteriosus; thus, it does not typically form a complete vascular ring.8) In extremely rare cases, when the left ductus arises from the descending aorta or the remnant right ductus arteriosus, a vascular ring is present.53)
Clinical importance of this subtype is that it is strongly associated with other anomalies or congenital heart diseases (> 75%) such as truncus arteriosus, TOF, tricuspid atresia, and transposition of the great arteries (TGA).54),55) Additionally, chromosomal anomalies such as 22q11 deletion syndromes have also been reported to be associated with this subtype.56)
Usually, this subtype does not cause tracheoesophageal compression. However, due to the high associated anomaly rate, whenever this type of aortic branching is detected, precise evaluation of the circulatory system, including the heart chambers, is required.
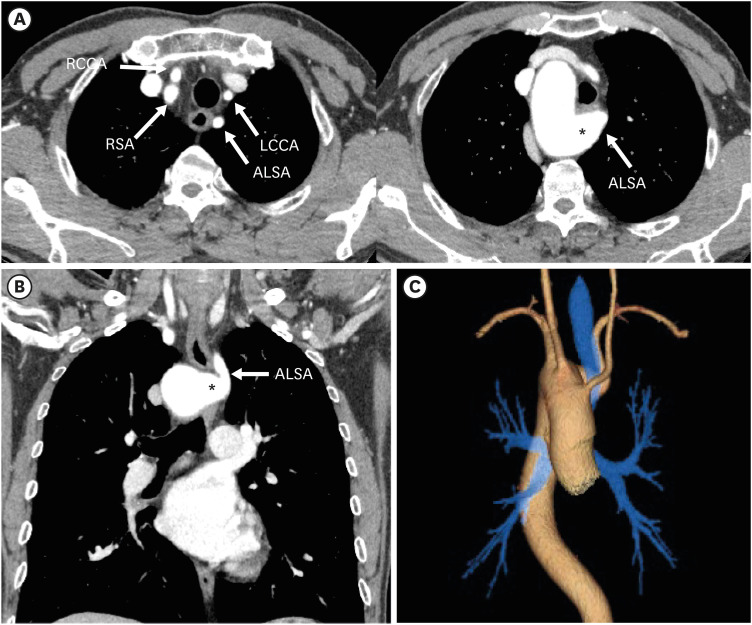
Right aortic arch with an aberrant left subclavian artery (ALSA) with Kommerell diverticulum
RAA with an ALSA is the most common variation of an RAA.13) This arch anomaly is a mirror image of an LAA with ARSA, but Kommerell diverticulum is more commonly present (approximately 60% of the cases) than with LAA (Figures 14 and 15). RAA with an ALSA with Kommerell diverticulum is the result of abnormal involution of the left fourth arch in-between the LSA and LCCA, usually accompanied by persistence of the left sixth arch. Major branching vessels of the aortic arch are in order of LCCA, right CCA, RSA, and ALSA. ALSA arises from the retroesophageal diverticulum at the junction of the right arch and descending aorta, and takes an oblique retroesophageal course, completing a vascular ring that encircles the trachea and esophagus.57),58) If the vascular ring is tight enough, tracheoesophageal compression can cause symptoms like poor feeding tolerance, recurrent aspiration, and failure to thrive, which are more commonly seen in infants and young adults. Additionally, the aneurysmal diverticulum itself can cause compression of the esophagus or spontaneously rupture.59) Patients with a symptomatic Kommerell diverticulum are therefore candidates for surgery, and early surgical treatment is recommended due to the risk of aneurysmal rupture.
Figure 14. Schematic figure of a right aortic arch with an ALSA. Black-shaded area represents involuted segments in a hypothetical double arch. This anomaly results from regression of the left arch between the left subclavian artery and left common carotid artery.
ALSA: aberrant left subclavian artery, LCCA: left common carotid artery, RCCA: right common carotid artery, RSA: right subclavian artery.
Figure 15. Right aortic arch with an ALSA with Kommerell diverticulum in a 59-year-old man. (A, B) Axial and coronal CT angiography images show an ALSA with dilated Kommerell diverticulum (asterisk). The branch arteries arise in order of LCCA, RCCA, RSA, and ALSA. (C) 3D volume-rendered CT angiographic image shows the retro-tracheal course of the ALSA with diverticulum of Kommerell.
ALSA: aberrant left subclavian artery, CT: computed tomography, LCCA: left common carotid artery, RCCA: right common carotid artery, RSA: right subclavian artery.
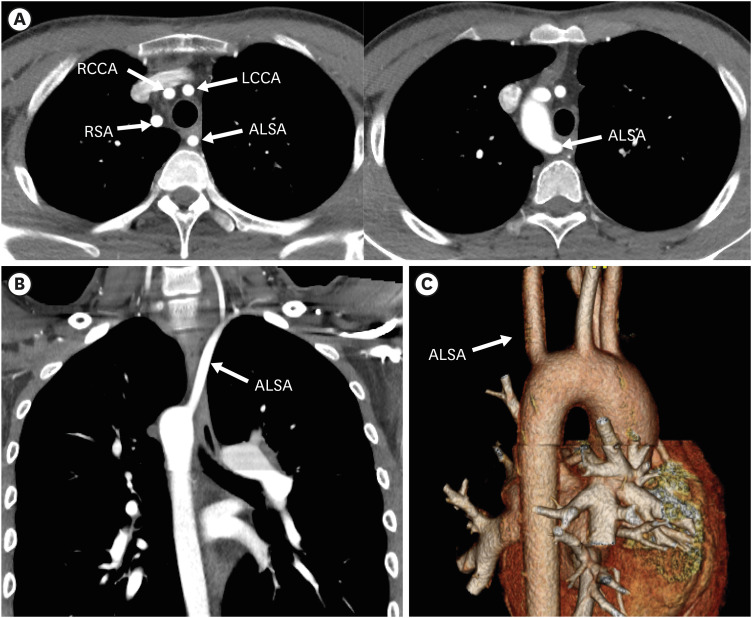
Right aortic arch with an ALSA without Kommerell diverticulum
RAA with an ALSA without Kommerell diverticulum, though rare, can be found incidentally.60) Involution of both the left fourth and sixth arches is the cause of this subtype. A vascular ring is usually absent, because the ductus arteriosus or ligamentum is usually absent or is right sided. Unlike RAA with an ALSA with Kommerell diverticulum, this subtype is usually found in individuals with congenital cardiac disease, including TOF or truncus arteriosus (Figure 16).58)
Figure 16. Right aortic arch with an ALSA without Kommerell diverticulum in a 20-year-old man who underwent total correction surgery for Tetralogy of Fallot. (A-C) Serial axial and coronal computed tomography angiographic images show a right sided aortic arch with a non-dilated ALSA. The branch arteries arise from the right aortic arch in order of LCCA, RCCA, RSA, and ALSA.
ALSA: aberrant left subclavian artery, LCCA: left common carotid artery, RCCA: right common carotid artery, RSA: right subclavian artery.
Right aortic arch with an isolated LSA
RAA with an isolated LSA is a rare subtype of RAA, observed in only 0.8% of patients with a RAA.43) RAA with an isolated LSA is the result of regression of two segments of the left arch, one between the LCCA and LSA and the other distal to the left ductus.13) As a result, the isolated LSA is connected to the pulmonary artery by the ductus arteriosus or to the left vertebral artery rather than the aortic arch (Figures 17 and 18).61) In more than half of cases, RAA with an isolated SA is associated with congenital heart diseases such as TOF, coarctation of aorta, or left pulmonary artery stenosis.43)
Figure 17. Schematic figure of a right aortic arch with an isolated LSA. Black-shaded area represents involuted segments in a hypothetical double arch. This anomaly results from regression of the left arch i) between the origin of the LCCA and LSA and ii) distal to the origin of the left ductus and LSA.
LCCA: left common carotid artery, RCCA: right common carotid artery, LSA: left subclavian artery.
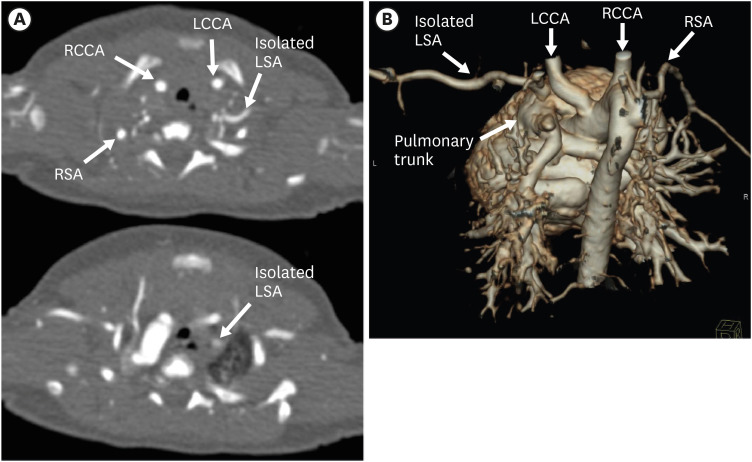
Figure 18. Isolated LSA in a newborn boy. (A) Serial axial CT angiographic images and (B) 3D volume-rendered CT angiographic image show the isolated LSA arising from the pulmonary trunk.
CT: computed tomography, LCCA: left common carotid artery, LSA: left subclavian artery, RCCA: right common carotid artery, RSA: right subclavian artery.
Although an isolated SA itself may be asymptomatic, it can cause vertebrobasilar insufficiency or subclavian steal syndrome, which manifests as arm weakness.62) Furthermore, if the LSA originates from the pulmonary artery branch, low pulmonary vascular resistance can cause pulmonary steal syndrome and heart failure.43) This flow problem is visualized as retrograde flow from the left vertebral artery or collateral vessels by conventional angiography or phase contrast MR angiography.63)
Asymptomatic patients with compensated blood flow from other systemic arteries may not require surgical or interventional treatment. If the isolated SA causes symptoms such as steal syndrome, LSA occlusion and re-implantation or stent insertion are usually performed.43)
Right circumflex aorta
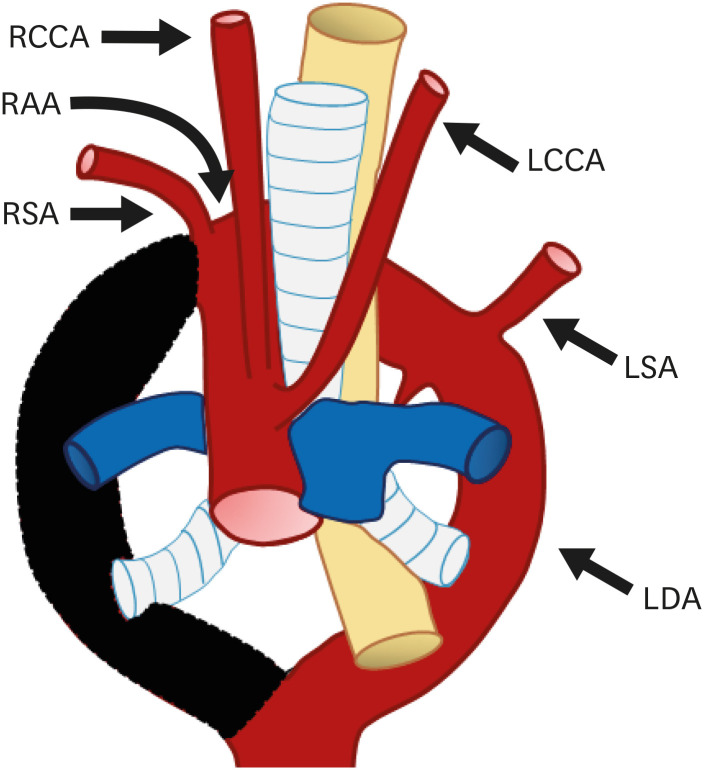
Right circumflex aorta is a rare anomaly, although it is relatively more common than left circumflex aorta (Figures 19 and 20). In this anomaly, the aortic arch courses behind the esophagus, crosses the midline, and gives rise to the left diverticulum with the left ductus arteriosus arising from it.46) Abnormal involution of the left dorsal aorta between the LCCA and LSA or regression of the left fourth arch between the LSA and left ductus may give rise to a circumflex aorta. A right circumflex aorta is therefore formed from the remnant right aortic arch, left sided dorsal aorta, and left ductus arteriosus.60)
Figure 19. Schematic figure of the right circumflex aorta. Black-shaded area represents involuted segments of a hypothetical double arch. This anomaly results from regression of the left fourth arch between the LCCA and LSA and persistence of the left-sided sixth arch component.
LCCA: left common carotid artery, LDA: left descending aorta, LSA: left subclavian artery, RAA: right aortic arch, RCCA: right common carotid artery, RSA: right subclavian artery.
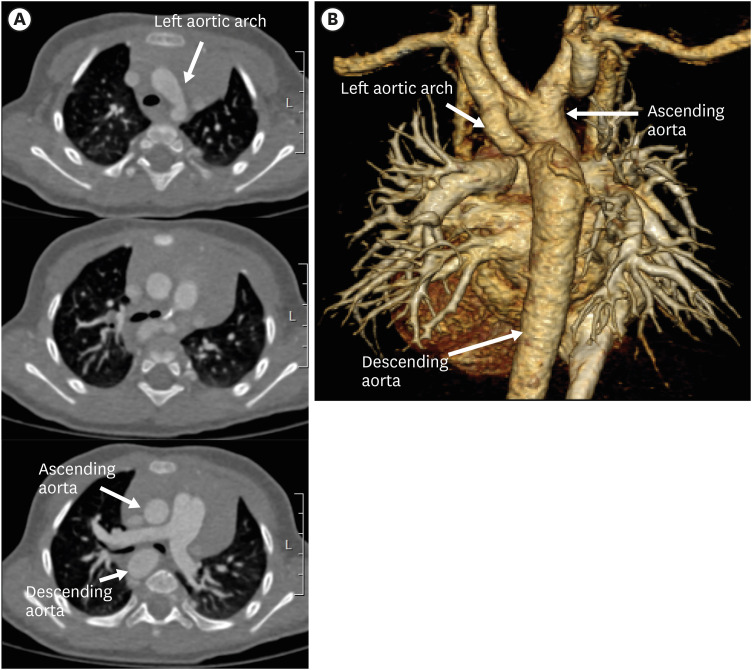
Figure 20. Left circumflex aorta in a 3-year-old boy. (A) Serial axial CT angiographic images and (B) 3D volume-rendered CT angiographic image show a left aortic arch crossing the midline posterior to the trachea and further descending on the right side of spine. Additionally, aortic arch hypoplasia and coarctation of the left aortic arch are present.
CT: computed tomography.
Two branching patterns may be seen depending on the existence of an ALSA.46) If an aberrant SA is present, the LCCA, RCCA, RSA, and ALSA arise from the aortic arch in the above order. Otherwise, the branching pattern is similar to RAA with a mirror branching pattern. Symptoms can occur if the diverticulum or vascular ring compresses the trachea or esophagus. In these cases, surgical treatment is similar to that for left circumflex aorta.47)
Miscellaneous
Among aortic arch deformities, there are also subtypes that do not correspond to the left or right aortic arch. Representative examples include double aortic arch, cervical aortic arch, persistent fifth aortic arch, interrupted aortic arch (IAA), hypoplastic aortic arch, coarctation of the aorta, and pseudocoarctation, all of which are relatively rare.8)
Double aortic arch
A double aortic arch is a rare anomaly found in only 0.05–0.3% of the population. However, this is the most common cause of a symptomatic vascular ring (symptoms occurs in 40–50% of cases).64),65) Embryologically, this anomaly results from abnormal persistence of both aortic arches with absence of physiological regression.8) As a result, after the ascending aorta divides into the left and right arches, each arch gives rise to an ipsilateral CCA and SA, and then the arches join as the descending thoracic aorta (Figures 21 and 22). Without regard to the location of the ductus, this vascular anomaly completely encircles the trachea and esophagus, and usually causes clinical symptoms.65) The size of the arches may be similar, but in up to 75% of the cases, the right arch is larger and dominant.21),66) Furthermore, there are cases of one atretic or hypoplastic arch with the other one relatively normal. The descending thoracic aorta in a double aortic arch is usually found on the contralateral side to the dominant arch.21)
Figure 21. Schematic figure of a double aortic arch with ductus arteriosus on each side. This anomaly results from abnormal persistence of both aortic arches with absence of physiological regression.
LCCA: left common carotid artery, LSA: left subclavian artery, RCCA: right common carotid artery, RSA: right subclavian artery.
Figure 22. Double aortic arch in a 68-year-old man. (A, B) Serial transaxial and 3D volume-rendered computed tomography angiographic images show a double aortic arch with symmetric bilateral common carotid arteries and subclavian arteries also referred to as the “four vessels” sign. Double aortic arch encircling the trachea forms a complete vascular ring.
CT: computed tomography, LCCA: left common carotid artery, LSA: left subclavian artery, RCCA: right common carotid artery, RSA: right subclavian artery.
If one or both arch segments show atresia or hypoplasia, visualization by CT or MR angiography is poor. Therefore, the ancillary findings mentioned below may be important for discrimination of this anomaly from an RAA with mirror imaging branching or an RAA with an ALSA.47)
1) Double aortic arch branching pattern shows bilateral and symmetric carotid and subclavian arteries arising from each ipsilateral arch.66) This is a unique feature of a double aortic arch and is called the “four vessel sign.” In comparison, in cases of an RAA with an ALSA, the LBCA originates from the right arch with mirror image branching, and asymmetry of the branch vessels at the transverse level can be noted.
2) In case of a double aortic arch with left arch atresia, the ductal diverticulum projects superiorly,67) whereas in RAA with mirror image branching, the diverticulum projects anteriorly towards the pulmonary artery.
3) In comparison to the LBCA in RAA with mirror imaging branching, the left arch with atresia in double aortic arch patients has a relatively posterior course.65)
4) The SA takes an inferior course and turns in the cephalad direction in a double aortic arch.68)
Double aortic arch is only rarely associated with congenital heart disease, but if present, various heart diseases including TOF, TGA, and VSD may be simultaneously present.60) Additionally, chromosomal abnormalities such as 22q11 deletion are present in approximately 20% of patients diagnosed with a double aortic arch.56) Common clinical symptoms in infants due to tracheoesophageal compression include a barky cough, respiratory stridor, dysphagia, and/or severe cyanosis. If associated symptoms are present, surgical correction of the vascular ring should be performed.69) The long-term prognosis of patients with a double aortic arch who have undergone surgical treatment is excellent.70) During surgery, the smaller segment or atretic portion of the aortic arch is usually resected to disrupt the complete vascular ring and resolve tracheoesophageal compression.
Cervical aortic arch
Cervical aortic arch is defined as an aortic arch positioned abnormally high at the level of the clavicle, and is present in 0.01% of the population (Figure 23).71) Two possible embryological causes have been suggested: abnormal regression of the fourth branchial arches and persistence of the second or third arch instead, or failure of caudal migration of the immature fourth arch.5) An association between cervical aortic anomaly and 22q11 microdeletion syndrome, a chromosomal abnormality related to underdevelopment of the fourth primitive aortic arches, has been reported.56)
Figure 23. Schematic figure of the left cervical aortic arch. There are two hypothetical pathophysiological causes of a cervical aortic arch based on embryology: i) abnormal involution of the fourth branchial arches and persistence of the second or third arch instead, or ii) failure of caudal migration of the immature fourth arch.
LCCA: left common carotid artery, LSA: left subclavian artery, RCCA: right common carotid artery, RSA: right subclavian artery.
Abnormal location of the descending aorta or aortic knob and posterior indentation of the trachea or esophagus are significant signs of a cervical aortic arch in CT or MRI. Various kinds of aortic anomalies, such as aortic diverticulum, aberrant SA, or separate carotid origins may also be present. Although a cervical aortic arch is more frequently found with a RAA than with a LAA, there is a strong association between a left cervical aortic arch and an aortic diverticulum.72)
Cervical aortic arch is usually asymptomatic and incidentally detected; thus, no treatment is required.73) Rarely, a pulsatile mass can be found in the neck portion, or tracheoesophageal compression can cause clinical symptoms such as dysphagia, wheezing, and coughing. If the clinical compressive symptoms are significant or an aneurysm is present, surgical repair such as endovascular repair with graft placement and subsequent axillary-axillary bypass may be considered.74)
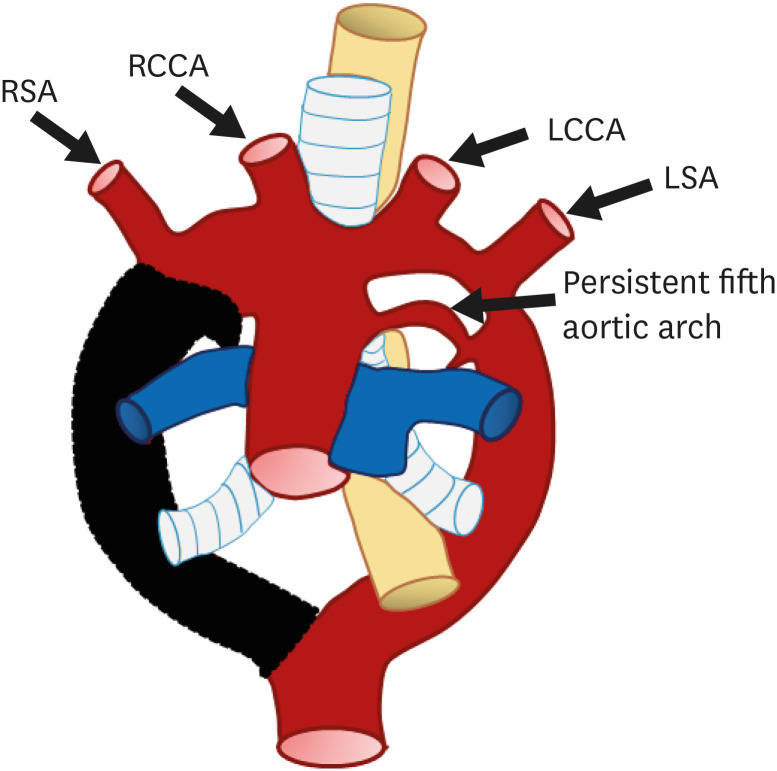
Persistent fifth aortic arch
A fifth aortic arch positioned inferior to the definite aortic arch, which is referred to as a persistent fifth aortic arch (PFAA; “double-lumen aortic arch”), may occur due to lack of physiologic regression (Figure 24).75) The remnant fifth aortic arch usually connects the ascending segment of the aortic arch to the descending aorta (systemic-to-systemic connection) or pulmonary artery (systemic-to-pulmonary connection). Because of its location and connection, a PFAA can mimic a ductus arteriosus or aorto-pulmonary window. PFAA arises proximal to the origin of the BCA, has an extra pericardial tortuous course, and terminates in the dorsal aorta or pulmonary artery.76)
Figure 24. Schematic figure of a persistent fifth aortic arch. Remnant of the aortic arch to the descending aorta or pulmonary artery.
LCCA: left common carotid artery, LSA: left subclavian artery, RCCA: right common carotid artery, RSA: right subclavian artery.
PFAA can be left-sided or right-sided. Based on anatomic variants, a PFAA can be classified into three subtypes77): i) a double-lumen aortic arch with both lamina patent (the most common subtype), ii) atresia and interruption of the superior arch with a patent inferior (persistent fifth) arch, and iii) a systemic-to-pulmonary arterial connection arising proximal to the first BCA; this subtype is associated with pulmonary atresia and VSD.
Previous studies have reported associations between a PFAA and various cardiovascular anomalies such as pulmonary atresia, tricuspid atresia, coarctation of aorta, IAA, and VSD.78),79) PFAA has various clinical presentations depending on the anatomical variations, including vascular ring formation, connection between the systemic and pulmonary vascular system, and combined anomalies.76) For example, the LSA arising from the fifth aortic arch and persistence of a large posterior aortic diverticulum can result in a complete vascular ring, which can cause symptoms.80)
IAA
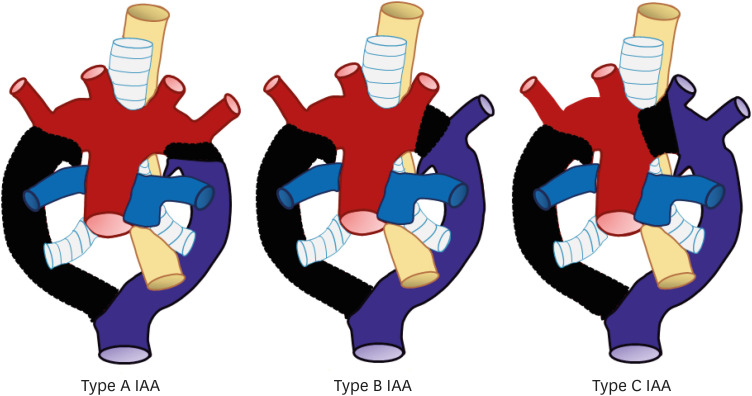
IAA is a very rare aortic arch anomaly that is characterized by complete luminal discontinuity between the ascending and descending aorta.81) IAA includes cases of aortic atresia with an anatomic fibrous remnant, no luminal connection, and presence of an aortic diverticulum on both sides. Due to luminal discontinuity, blood flows to the descending aorta through the PDA or PFAA.82) IAA can be classified into three types based on the location of the interrupting segment (Figure 25).83) Type A IAA is characterized by interruption distal to the origin of the LSA. Embryologically, this anomaly results from abnormal regression of the fourth arch segment after the SA ascends to the proper position.84) This subtype is the second common type of IAA, occurring in 30–40% of cases, and is the most common subtype in adult patients (Figure 26).85)
Figure 25. Schematic figure of the three subtypes of IAA based on the location of the interrupted segment: Type A, interruption distal to the origin of the left subclavian artery; Type B, interruption between the origins of the left common carotid artery and left subclavian artery; Type C, interruption between the origins of the right brachiocephalic artery and left common carotid artery.
IAA: interrupted aortic arch.
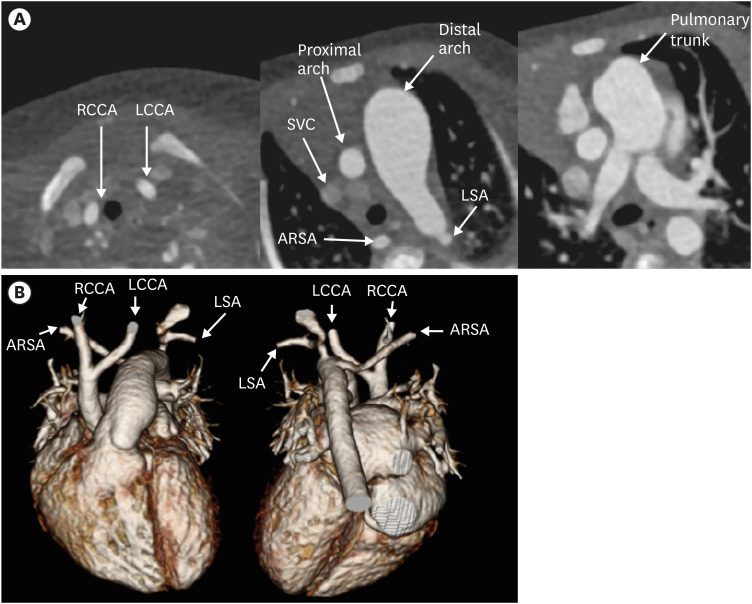
Figure 26. Type B interrupted aortic arch in a newborn girl. (A) Axial CT image shows interruption of the aortic arch distal to left CCA, and the descending aorta is continuous with the main pulmonary trunk. Additionally, an aberrant right SCA is noted in this case. (B) 3D volume-rendered CT angiographic image of another type B interrupted aortic arch patient.
ARSA: aberrant right subclavian artery, CT: computed tomography, LCCA: left common carotid artery, LSA: left subclavian artery, RCCA: right common carotid artery, RSA: right subclavian artery, SVC: superior vena cava.
Type B IAA is defined as IAA with interruption between the origins of the LCCA and LSA. Early abnormal regression of the left fourth arch segment before migration of the LSA is thought to be the cause of the anomaly. Type B is the most common subtype of IAA, accounting for 50–60% of IAA cases.86) Additionally, about 30% cases of type B IAA are accompanied by an ARSA. There is a close relationship between 22q11 deletion and type B IAA.83) In over half of cases, type B IAA is found in the context of specific syndromes such as DiGeorge syndrome, and has a high association rate (98% of cases) with various congenital heart diseases.87)
Type C IAA is defined as aortic arch interruption between the origins of the BCA and LCCA. This subtype results from regression of the ventral segment of the left third and fourth arches. This is the least common type of IAA (less than 5% of IAA cases).88)
Clinical manifestations of an IAA include the onset of shock or severe heart failure a few weeks after birth.89) The only treatment option is surgical correction of the anomaly using a bypass graft, stent, or end-to-end anastomosis.90)
Hypoplastic aortic arch
Aortic arch hypoplasia is defined as a proximal aortic arch with an external diameter smaller than 60%, a distal aortic arch with an external diameter smaller than 50%, or an isthmic segment with an external diameter smaller than 40% of the assumed diameter of the ascending aorta.91) Aortic arch hypoplasia may occur without any other anomaly but can also occur with other aortic anomalies that restrict aortic flow, such as coarctation of the aorta. It may be accompanied by ASD, VSD, or PDA.92)
Coarctation of the aorta
Coarctation of the aorta is an aortic anomaly with focal stenosis of an aortic segment in the proximal descending aorta or adjacent to the aortic isthmus.93) This is a relatively common congenital anomaly that affects approximately 4 per 10,000 live births.
The definite etiology of coarctation of the aorta is not well understood.93) One hypothesis is that abnormal ductal tissue extension to the aortic isthmus becomes a coarctation shelf and causes aortic obstruction. Another theory is that there are altered fetal hemodynamics due to an abnormal angle between the ductus arteriosus and aorta, or abnormal pre-ductal flow. As a result, there is increased right-to-left ductal flow and decreased antegrade arch flow, which may give rise to coarctation of the aorta.
In patients with coarctation, various degrees of hypoplastic aortic arch and aortic isthmus are generally noted. Hemodynamically, a blood pressure gradient higher than 20 mm Hg is a component of the classical diagnosis of coarctation.94) In the setting of a high blood pressure gradient, and to compensate for blood flow obstruction, there is recruitment and enlargement of collateral vessels (Figure 27).
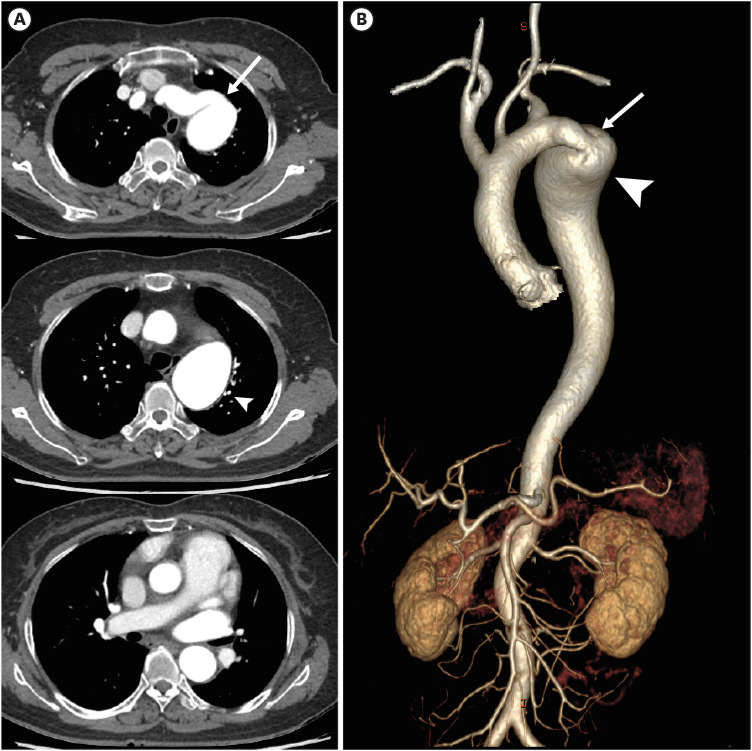
Figure 27. Coarctation of the aorta with a left aortic arch in a 52-year-old man. (A, B) Serial axial images and 3D volume-rendered CT angiographic images showing focal stenosis of the descending thoracic aorta (arrow). Additionally, hypertrophy of both internal mammary, superior epigastric, intercostal, and lateral thoracic arteries (arrowheads) is visible in the axial CT angiographic images. (C) Echocardiography shows a typical bicuspid aortic valve with small raphe (arrows) between the right and left coronary cuspid valves.
CT: computed tomography.
In neonates, transthoracic echocardiography is recommended for diagnosis of coarctation of the aorta and has high sensitivity (94–98%).95) Otherwise, CT angiography is generally used as a diagnostic modality as this can reveal the degree of narrowing, pseudo-aneurysms, and development of the collateral system. MR angiography can be used for functional evaluation of the pressure gradient or collateral flow status using phase contrast imaging.96) Coarctation of the aorta is accompanied by congenital heart diseases or arch anomalies in 44–84% of cases. It is most commonly associated with a bicuspid aortic valve, followed by VSD, ASD, and others.97) Coarctation of the aorta is a relatively common finding in individuals with genetic diseases such as Turner's syndrome, Shone complex, and PHACE syndrome.98),99),100)
Clinical manifestations of coarctation of the aorta are related to the severity of the stenosis and efficiency of the collateral pathways.96) In infants, symptoms are dependent on the persistence of the PDA to maintain systemic blood flow.101) If the ductus closes, severe left ventricular pressure overload and failure will appear in turn. Additionally, decreased systemic perfusion of the lower body can cause tissue hypoxia along with upper body hypertension due to activation of the renin-angiotensin-aldosterone system. Subsequently, renal failure and metabolic acidosis can develop in untreated patients.102) The above complications can also occur in adult patient without adequate collateral pathways.
Surgical repair must be completed as early as possible due to the high mortality rate (up to 50%) and long-term sequelae, including persistent hypertension.103),104) Open resection of the aortic segment with anastomosis is the optimal surgical plan in young patients. Methods such as subclavian flap angioplasty, synthetic patch repair, and graft interposition are alternative treatment options.
Pseudocoarctation
Pseudocoarctation is a rare anomaly of the aortic arch characterized by segmental kinking or buckling at the level of the aortic isthmus (Figure 28).105) Different from true coarctation of the aorta, hemodynamic obstruction (pressure gradient) or collateral circulation are absent.106) Although the etiology of pseudocoarctation is not clear, failure of compression and fusion of dorsal roots and fourth arch segments have been proposed as the embryologic cause.107) Almost all cases of pseudocoarctation are detected as isolated anomalies and incidentally diagnosed without any clinical symptoms. However, in rare cases, when combined with aortic aneurysm, surgical intervention is required due to the risk of rupture.105),108)
Figure 28. Pseudocoarctation of the aorta with a left aortic arch in a 69-year-old woman. (A, B) Serial axial images and 3D volume-rendered computed tomography angiographic images show focal narrowing of the proximal descending thoracic aorta (arrows) with aneurysmal dilatation (arrowheads) distal to the narrowed portion. Aortic arch shows an elongated course without abnormal collateral vessel formation.
CONCLUSIONS
Aortic arch anomalies and variants are relatively uncommon; however, they have a wide range of clinical symptoms ranging from life-threatening symptoms to no symptoms; regardless, their detection is extremely important before undertaking procedures or making surgical decisions. To understand these complex variations of the thoracic aorta and their accompanying clinical features, knowledge of the embryological development of the thoracic aorta is important. Cross-sectional imaging modalities, especially multidetector CT, have significant clinical advantages in terms of precise diagnosis and classification of aortic arch anomalies based on 3D information and play an important role in the differential diagnosis and detection of accompanying anomalies.
Footnotes
Funding: This study was supported by research funds from Dong-A University.
Conflict of Interest: The authors have no financial conflicts of interest.
- Data curation: Kim SH, Lim KJ, Kwon H.
- Funding acquisition: Kang EJ.
- Investigation: Kang EJ, Choo KS.
- Methodology: Kang EJ, Choo KS.
- Resources: Lee J.
- Supervision: Lee J, Kwon H.
- Writing - original draft: Bae SB.
- Writing - review & editing: Kang EJ.
References
- 1.Goldmuntz E. The epidemiology and genetics of congenital heart disease. Clin Perinatol. 2001;28:1–10. doi: 10.1016/s0095-5108(05)70067-1. [DOI] [PubMed] [Google Scholar]
- 2.Kocis KC, Midgley FM, Ruckman RN. Aortic arch complex anomalies: 20-year experience with symptoms, diagnosis, associated cardiac defects, and surgical repair. Pediatr Cardiol. 1997;18:127–132. doi: 10.1007/s002469900130. [DOI] [PubMed] [Google Scholar]
- 3.Jakanani GC, Adair W. Frequency of variations in aortic arch anatomy depicted on multidetector CT. Clin Radiol. 2010;65:481–487. doi: 10.1016/j.crad.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Schleich JM. Development of the human heart: days 15–21. Heart. 2002;87:487. doi: 10.1136/heart.87.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kau T, Sinzig M, Gasser J, et al. Aortic development and anomalies. Semin Intervent Radiol. 2007;24:141–152. doi: 10.1055/s-2007-980040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oshitani T, Kawasaki Y, Murakami Y, et al. A double-barrelled aorta with high aortic Arch. J Cardiol Cases. 2021;24:284–286. doi: 10.1016/j.jccase.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards JE. Anomalies of the derivatives of the aortic arch system. Med Clin North Am. 1948;32:925–949. doi: 10.1016/s0025-7125(16)35662-0. [DOI] [PubMed] [Google Scholar]
- 8.Hanneman K, Newman B, Chan F. Congenital variants and anomalies of the aortic arch. Radiographics. 2017;37:32–51. doi: 10.1148/rg.2017160033. [DOI] [PubMed] [Google Scholar]
- 9.Stojanovska J, Cascade PN, Chong S, Quint LE, Sundaram B. Embryology and imaging review of aortic arch anomalies. J Thorac Imaging. 2012;27:73–84. doi: 10.1097/RTI.0b013e318218923c. [DOI] [PubMed] [Google Scholar]
- 10.Leonardi B, Secinaro A, Cutrera R, et al. Imaging modalities in children with vascular ring and pulmonary artery sling. Pediatr Pulmonol. 2015;50:781–788. doi: 10.1002/ppul.23075. [DOI] [PubMed] [Google Scholar]
- 11.Mądry W, Zacharska-Kokot E, Karolczak MA. Methodology of echocardiographic analysis of morphological variations of the aortic arch and its branches in children - own experience. J Ultrason. 2019;19:24–42. doi: 10.15557/JoU.2019.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee EY, Siegel MJ, Hildebolt CF, Gutierrez FR, Bhalla S, Fallah JH. MDCT evaluation of thoracic aortic anomalies in pediatric patients and young adults: comparison of axial, multiplanar, and 3D images. AJR Am J Roentgenol. 2004;182:777–784. doi: 10.2214/ajr.182.3.1820777. [DOI] [PubMed] [Google Scholar]
- 13.Türkvatan A, Büyükbayraktar FG, Olçer T, Cumhur T. Congenital anomalies of the aortic arch: evaluation with the use of multidetector computed tomography. Korean J Radiol. 2009;10:176–184. doi: 10.3348/kjr.2009.10.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim HK, Ha HI, Hwang HJ, Lee K. Feasibility of high-pitch dual-source low-dose chest CT: reduction of radiation and cardiac artifacts. Diagn Interv Imaging. 2016;97:443–449. doi: 10.1016/j.diii.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Ramos-Duran L, Nance JW, Jr, Schoepf UJ, Henzler T, Apfaltrer P, Hlavacek AM. Developmental aortic arch anomalies in infants and children assessed with CT angiography. AJR Am J Roentgenol. 2012;198:W466-74. doi: 10.2214/AJR.11.6982. [DOI] [PubMed] [Google Scholar]
- 16.Bhalla AS, Das A, Naranje P, Irodi A, Raj V, Goyal A. Imaging protocols for CT chest: a recommendation. Indian J Radiol Imaging. 2019;29:236–246. doi: 10.4103/ijri.IJRI_34_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae KT. Optimization of contrast enhancement in thoracic MDCT. Radiol Clin North Am. 2010;48:9–29. doi: 10.1016/j.rcl.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Weininger M, Barraza JM, Kemper CA, Kalafut JF, Costello P, Schoepf UJ. Cardiothoracic CT angiography: current contrast medium delivery strategies. AJR Am J Roentgenol. 2011;196:W260-72. doi: 10.2214/AJR.10.5814. [DOI] [PubMed] [Google Scholar]
- 19.Chandra T, Podberesky DJ, Romberg EK, Tang ER, Iyer RS, Epelman M. Optimization of pediatric body CT angiography: what radiologists need to know. AJR Am J Roentgenol. 2020;215:726–735. doi: 10.2214/AJR.19.22273. [DOI] [PubMed] [Google Scholar]
- 20.Boxt LM. Magnetic resonance and computed tomographic evaluation of congenital heart disease. J Magn Reson Imaging. 2004;19:827–847. doi: 10.1002/jmri.20077. [DOI] [PubMed] [Google Scholar]
- 21.Kellenberger CJ. Aortic arch malformations. Pediatr Radiol. 2010;40:876–884. doi: 10.1007/s00247-010-1607-9. [DOI] [PubMed] [Google Scholar]
- 22.Natsis KI, Tsitouridis IA, Didagelos MV, Fillipidis AA, Vlasis KG, Tsikaras PD. Anatomical variations in the branches of the human aortic arch in 633 angiographies: clinical significance and literature review. Surg Radiol Anat. 2009;31:319–323. doi: 10.1007/s00276-008-0442-2. [DOI] [PubMed] [Google Scholar]
- 23.Layton KF, Kallmes DF, Cloft HJ, Lindell EP, Cox VS. Bovine aortic arch variant in humans: clarification of a common misnomer. AJNR Am J Neuroradiol. 2006;27:1541–1542. [PMC free article] [PubMed] [Google Scholar]
- 24.Hornick M, Moomiaie R, Mojibian H, et al. ‘Bovine’ aortic arch - a marker for thoracic aortic disease. Cardiology. 2012;123:116–124. doi: 10.1159/000342071. [DOI] [PubMed] [Google Scholar]
- 25.Nagpal P, Khandelwal A, Saboo SS, Bathla G, Steigner ML, Rybicki FJ. Modern imaging techniques: applications in the management of acute aortic pathologies. Postgrad Med J. 2015;91:449–462. doi: 10.1136/postgradmedj-2014-133178. [DOI] [PubMed] [Google Scholar]
- 26.Lacout A, Khalil A, Figl A, Liloku R, Marcy PY. Vertebral arteria lusoria: a life-threatening condition for oesophageal surgery. Surg Radiol Anat. 2012;34:381–383. doi: 10.1007/s00276-011-0867-x. [DOI] [PubMed] [Google Scholar]
- 27.Yuan SM. Aberrant origin of vertebral artery and its clinical implications. Rev Bras Cir Cardiovasc. 2016;31:52–59. doi: 10.5935/1678-9741.20150071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fawcett SL, Gomez AC, Hughes JA, Set P. Anatomical variation in the position of the brachiocephalic trunk (innominate artery) with respect to the trachea: a computed tomography-based study and literature review of innominate artery compression syndrome. Clin Anat. 2010;23:61–69. doi: 10.1002/ca.20884. [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa T, Oshima Y, Hisamatsu C, et al. Innominate artery compression of the trachea in patients with neurological or neuromuscular disorders. Eur J Cardiothorac Surg. 2014;45:305–311. doi: 10.1093/ejcts/ezt346. [DOI] [PubMed] [Google Scholar]
- 30.Abraham V, Mathew A, Cherian V, Chandran S, Mathew G. Aberrant subclavian artery: anatomical curiosity or clinical entity. Int J Surg. 2009;7:106–109. doi: 10.1016/j.ijsu.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Saeed G, Ganster G, Friedel N. Arteria lusoria aneurysm with truncus bicaroticus: surgical resection without restoring blood supply to the right arm. Tex Heart Inst J. 2010;37:602–607. [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen M, Baggen MG, Veen HF, et al. Dysphagia lusoria: clinical aspects, manometric findings, diagnosis, and therapy. Am J Gastroenterol. 2000;95:1411–1416. doi: 10.1111/j.1572-0241.2000.02071.x. [DOI] [PubMed] [Google Scholar]
- 33.Choi Y, Chung SB, Kim MS. Prevalence and Anatomy of Aberrant Right Subclavian Artery Evaluated by Computed Tomographic Angiography at a Single Institution in Korea. J Korean Neurosurg Soc. 2019;62:175–182. doi: 10.3340/jkns.2018.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai IC, Tzeng WS, Lee T, et al. Vertebral and carotid artery anomalies in patients with aberrant right subclavian arteries. Pediatr Radiol. 2007;37:1007–1012. doi: 10.1007/s00247-007-0574-2. [DOI] [PubMed] [Google Scholar]
- 35.Scala C, Leone Roberti Maggiore U, Candiani M, et al. Aberrant right subclavian artery in fetuses with Down syndrome: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46:266–276. doi: 10.1002/uog.14774. [DOI] [PubMed] [Google Scholar]
- 36.Vistarini N, Aubert S, Gandjbakhch I, Bonnet N. Aberrant subclavian artery as origin of aortic dissection. Eur J Cardiothorac Surg. 2008;34:1109. doi: 10.1016/j.ejcts.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 37.Leon M, Garibaldi M, Virgen F, Ramírez-Cerda C, Cohen-Mussali S. Hybrid treatment of aberrant right subclavian artery causing dysphagia lusoria by subclavian to carotid transposition and endovascular plug. Vasc Spec Int. 2020;36:258–262. doi: 10.5758/vsi.200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Rosendael PJ, Stöger JL, Kiès P, et al. The clinical spectrum of Kommerell’s diverticulum in adults with a right-sided aortic arch: a case series and literature overview. J Cardiovasc Dev Dis. 2021;8:25. doi: 10.3390/jcdd8030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Priya S, Thomas R, Nagpal P, Sharma A, Steigner M. Congenital anomalies of the aortic arch. Cardiovasc Diagn Ther. 2018;8(Suppl 1):S26–S44. doi: 10.21037/cdt.2017.10.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poterucha J, Anavekar N, Niaz T, et al. Incidence and clinical presentation of Kommerell diverticulum. J Am Coll Cardiol. 2015;65:A524. [Google Scholar]
- 41.Ota T, Okada K, Takanashi S, Yamamoto S, Okita Y. Surgical treatment for Kommerell’s diverticulum. J Thorac Cardiovasc Surg. 2006;131:574–578. doi: 10.1016/j.jtcvs.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Konstantinov IE, Saxena P, d’Udekem Y, Brizard CP. Isolated subclavian artery: anatomical and surgical considerations. Ann Thorac Surg. 2009;88:1685–1687. doi: 10.1016/j.athoracsur.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 43.Sen S, Mohanty S, Kulkarni S, Rao SG. Isolated subclavian artery: a rare entity revisited. World J Pediatr Congenit Heart Surg. 2016;7:744–749. doi: 10.1177/2150135116661278. [DOI] [PubMed] [Google Scholar]
- 44.Maya I, Kahana S, Yeshaya J, et al. Chromosomal microarray analysis in fetuses with aberrant right subclavian artery. Ultrasound Obstet Gynecol. 2017;49:337–341. doi: 10.1002/uog.15935. [DOI] [PubMed] [Google Scholar]
- 45.Bech AP, op den Akker J, Matthijsse PR. Isolation of the left subclavian artery from the pulmonary artery in a patient with CHARGE association. Congenit Anom (Kyoto) 2010;50:200–202. doi: 10.1111/j.1741-4520.2010.00283.x. [DOI] [PubMed] [Google Scholar]
- 46.Haranal M, Srimurugan B, Sivalingam S. Circumflex aorta: an uncharted territory. Asian Cardiovasc Thorac Ann. 2022;30:217–225. doi: 10.1177/02184923211015092. [DOI] [PubMed] [Google Scholar]
- 47.Kamran A, Friedman KG, Jennings RW, Baird CW. Aortic uncrossing and tracheobronchopexy corrects tracheal compression and tracheobronchomalacia associated with circumflex aortic arch. J Thorac Cardiovasc Surg. 2020;160:796–804. doi: 10.1016/j.jtcvs.2020.03.158. [DOI] [PubMed] [Google Scholar]
- 48.Arazińska A, Polguj M, Szymczyk K, Kaczmarska M, Trębiński Ł, Stefańczyk L. Right aortic arch analysis - Anatomical variant or serious vascular defect? BMC Cardiovasc Disord. 2017;17:102. doi: 10.1186/s12872-017-0536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin ZW, Yamada T, Kim JH, Rodríguez-Vázquez JF, Murakami G, Arakawa K. Pathogenesis of solitary right aortic arch: a mass effect hypothesis based on observations of serial human embryonic sections. Cardiol Young. 2017;27:359–368. doi: 10.1017/S1047951115002152. [DOI] [PubMed] [Google Scholar]
- 50.Shuford WH, Sybers RG, Edwards FK. The three types of right aortic arch. Am J Roentgenol Radium Ther Nucl Med. 1970;109:67–74. doi: 10.2214/ajr.109.1.67. [DOI] [PubMed] [Google Scholar]
- 51.Ha GJ, Sung MJ, Lee YS, et al. A case of right sided aortic arch combined with atrial septal defect. J Cardiovasc Ultrasound. 2011;19:32–34. doi: 10.4250/jcu.2011.19.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garti IJ, Aygen MM, Vidne B, Levy MJ. Right aortic arch with mirror-image branching causing vascular ring. A new classification of the right aortic arch patterns. Br J Radiol. 1973;46:115–119. doi: 10.1259/0007-1285-46-542-115. [DOI] [PubMed] [Google Scholar]
- 53.McElhinney DB, Hoydu AK, Gaynor JW, Spray TL, Goldmuntz E, Weinberg PM. Patterns of right aortic arch and mirror-image branching of the brachiocephalic vessels without associated anomalies. Pediatr Cardiol. 2001;22:285–291. doi: 10.1007/s002460010231. [DOI] [PubMed] [Google Scholar]
- 54.Kanne JP, Godwin JD. Right aortic arch and its variants. J Cardiovasc Comput Tomogr. 2010;4:293–300. doi: 10.1016/j.jcct.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Cantinotti M, Hegde S, Bell A, Razavi R. Diagnostic role of magnetic resonance imaging in identifying aortic arch anomalies. Congenit Heart Dis. 2008;3:117–123. doi: 10.1111/j.1747-0803.2008.00174.x. [DOI] [PubMed] [Google Scholar]
- 56.McElhinney DB, Clark BJ, 3rd, Weinberg PM, et al. Association of chromosome 22q11 deletion with isolated anomalies of aortic arch laterality and branching. J Am Coll Cardiol. 2001;37:2114–2119. doi: 10.1016/s0735-1097(01)01286-4. [DOI] [PubMed] [Google Scholar]
- 57.van Son JA, Konstantinov IE. Burckhard F. Kommerell and Kommerell’s diverticulum. Tex Heart Inst J. 2002;29:109–112. [PMC free article] [PubMed] [Google Scholar]
- 58.Backer CL, Mavroudis C. Congenital Heart Surgery Nomenclature and Database Project: patent ductus arteriosus, coarctation of the aorta, interrupted aortic arch. Ann Thorac Surg. 2000;69(Suppl):S298–S307. doi: 10.1016/s0003-4975(99)01280-1. [DOI] [PubMed] [Google Scholar]
- 59.Faistauer Â, Torres FS, Faccin CS. Right aortic arch with aberrant left innominate artery arising from Kommerell’s diverticulum. Radiol Bras. 2016;49:264–266. doi: 10.1590/0100-3984.2013.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karangelis D, Loggos S, Tzifa A, Mitropoulos FA. The aberrant subclavian artery: approach to management. Curr Opin Cardiol. 2020;35:636–642. doi: 10.1097/HCO.0000000000000793. [DOI] [PubMed] [Google Scholar]
- 61.Sun AM, Alhabshan F, Branson H, Freedom RM, Yoo SJ. MRI diagnosis of isolated origin of the left subclavian artery from the left pulmonary artery. Pediatr Radiol. 2005;35:1259–1262. doi: 10.1007/s00247-005-1569-5. [DOI] [PubMed] [Google Scholar]
- 62.Shuford WH, Sybers RG, Schlant RC. Right aortic arch with isolation of the left subclavian artery. Am J Roentgenol Radium Ther Nucl Med. 1970;109:75–83. doi: 10.2214/ajr.109.1.75. [DOI] [PubMed] [Google Scholar]
- 63.Arnoult AC, Blaise S. Late discovery of a rare anomaly of the right aortic arch and an isolated left subclavian artery. J Vasc Surg. 2016;64:1853–1854. doi: 10.1016/j.jvs.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 64.Fenández-Tena A, Martínez-González C. Double aortic arch diagnosed in a 44-year-old woman with recurring respiratory infections. Respir Med Case Rep. 2017;20:176–178. doi: 10.1016/j.rmcr.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schlesinger AE, Krishnamurthy R, Sena LM, et al. Incomplete double aortic arch with atresia of the distal left arch: distinctive imaging appearance. AJR Am J Roentgenol. 2005;184:1634–1639. doi: 10.2214/ajr.184.5.01841634. [DOI] [PubMed] [Google Scholar]
- 66.Dillman JR, Attili AK, Agarwal PP, Dorfman AL, Hernandez RJ, Strouse PJ. Common and uncommon vascular rings and slings: a multi-modality review. Pediatr Radiol. 2011;41:1440–1454. doi: 10.1007/s00247-011-2131-2. [DOI] [PubMed] [Google Scholar]
- 67.Newman B, Schlesinger AE. MR of right aortic arch. Pediatr Radiol. 1996;26:367–369. doi: 10.1007/BF01395719. [DOI] [PubMed] [Google Scholar]
- 68.Holmes KW, Bluemke DA, Vricella LA, Ravekes WJ, Kling KM, Spevak PJ. Magnetic resonance imaging of a distorted left subclavian artery course: an important clue to an unusual type of double aortic arch. Pediatr Cardiol. 2006;27:316–320. doi: 10.1007/s00246-005-1118-x. [DOI] [PubMed] [Google Scholar]
- 69.Alsenaidi K, Gurofsky R, Karamlou T, Williams WG, McCrindle BW. Management and outcomes of double aortic arch in 81 patients. Pediatrics. 2006;118:e1336–e1341. doi: 10.1542/peds.2006-1097. [DOI] [PubMed] [Google Scholar]
- 70.Yang Y, Jin X, Pan Z, Li Y, Wu C. Diagnosis and surgical repair of congenital double aortic arch in infants. J Cardiothorac Surg. 2019;14:160. doi: 10.1186/s13019-019-0976-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poellinger A, Lembcke AE, Elgeti T, Filimonov S, Enzweiler CN. Images in cardiovascular medicine. The cervical aortic arch: a rare vascular anomaly. Circulation. 2008;117:2716–2717. doi: 10.1161/CIRCULATIONAHA.107.755264. [DOI] [PubMed] [Google Scholar]
- 72.Pearson GD, Kan JS, Neill CA, Midgley FM, Gardner TJ, Hougen TJ. Cervical aortic arch with aneurysm formation. Am J Cardiol. 1997;79:112–114. doi: 10.1016/s0002-9149(96)00695-9. [DOI] [PubMed] [Google Scholar]
- 73.Ilyas M, Shah SA, Gojwari T, Sheikh WA. Cervical aortic arch-when the aorta gets high. Indian J Thorac Cardiovasc Surg. 2018;34:521–524. doi: 10.1007/s12055-017-0641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhong YL, Ma WG, Zhu JM, et al. Surgical repair of cervical aortic arch: an alternative classification scheme based on experience in 35 patients. J Thorac Cardiovasc Surg. 2020;159:2202–2213.e4. doi: 10.1016/j.jtcvs.2019.03.143. [DOI] [PubMed] [Google Scholar]
- 75.Van Praagh R, Van Praagh S. Persistent fifth arterial arch in man. Congenital double-lumen aortic arch. Am J Cardiol. 1969;24:279–282. doi: 10.1016/0002-9149(69)90417-2. [DOI] [PubMed] [Google Scholar]
- 76.Liu Y, Zhang H, Ren J, et al. Persistent fifth aortic arch: a single-center experience, case series. Transl Pediatr. 2021;10:1566–1572. doi: 10.21037/tp-20-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al Akhfash AA, Al Mutairi MB, Al Habshan FM. Persistent fifth aortic arch diagnosed by echocardiography and confirmed by angiography: case report and literature review. J Saudi Heart Assoc. 2009;21:245–248. doi: 10.1016/j.jsha.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hwang MS, Chang YS, Chu JJ, Su WJ. Isolated persistent fifth aortic arch with systemic-to-pulmonary arterial connection. J Thorac Cardiovasc Surg. 2003;126:1643–1644. doi: 10.1016/s0022-5223(03)00954-1. [DOI] [PubMed] [Google Scholar]
- 79.Kim SH, Choi ES, Cho S, Kim WH. Persistent fifth aortic arch with coarctation. Korean J Thorac Cardiovasc Surg. 2016;49:39–41. doi: 10.5090/kjtcs.2016.49.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Newman B, Hanneman K, Chan F. Persistent fifth arch anomalies - broadening the spectrum to include a variation of double aortic arch vascular ring. Pediatr Radiol. 2016;46:1866–1872. doi: 10.1007/s00247-016-3693-9. [DOI] [PubMed] [Google Scholar]
- 81.Varghese A, Gatzoulis M, Mohiaddin RH. Images in cardiovascular medicine: Magnetic resonance angiography of a congenitally interrupted aortic arch. Circulation. 2002;106:E9–10. doi: 10.1161/01.cir.0000023882.32439.28. [DOI] [PubMed] [Google Scholar]
- 82.Dillman JR, Yarram SG, D’Amico AR, Hernandez RJ. Interrupted aortic arch: spectrum of MRI findings. AJR Am J Roentgenol. 2008;190:1467–1474. doi: 10.2214/AJR.07.3408. [DOI] [PubMed] [Google Scholar]
- 83.Schreiber C, Mazzitelli D, Haehnel JC, Lorenz HP, Meisner H. The interrupted aortic arch: an overview after 20 years of surgical treatment. Eur J Cardiothorac Surg. 1997;12:466–469. doi: 10.1016/s1010-7940(97)00194-2. [DOI] [PubMed] [Google Scholar]
- 84.Chan FP, Hanneman K. Computed tomography and magnetic resonance imaging in neonates with congenital cardiovascular disease. Semin Ultrasound CT MR. 2015;36:146–160. doi: 10.1053/j.sult.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 85.Westaby S, Agarwal A, Kadlec J, Flynn F. Repair of type B interrupted aortic arch using aberrant right subclavian artery. Interact Cardiovasc Thorac Surg. 2009;9:528–529. doi: 10.1510/icvts.2009.203901. [DOI] [PubMed] [Google Scholar]
- 86.Abdoli S, Tatum JM, Fratello AL, Bowdish ME, Baker CJ. Type B aortic arch interruption in an adult. Ann Thorac Surg. 2016;102:e431–e432. doi: 10.1016/j.athoracsur.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 87.Patel DM, Maldjian PD, Lovoulos C. Interrupted aortic arch with post-interruption aneurysm and bicuspid aortic valve in an adult: a case report and literature review. Radiol Case Rep. 2015;10:5–8. doi: 10.1016/j.radcr.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Volpe P, Marasini M, Caruso G, Gentile M. Prenatal diagnosis of interruption of the aortic arch and its association with deletion of chromosome 22q11. Ultrasound Obstet Gynecol. 2002;20:327–331. doi: 10.1046/j.1469-0705.2002.00818.x. [DOI] [PubMed] [Google Scholar]
- 89.Gruber PJ, Epstein JA. Development gone awry: congenital heart disease. Circ Res. 2004;94:273–283. doi: 10.1161/01.RES.0000116144.43797.3B. [DOI] [PubMed] [Google Scholar]
- 90.Jonas RA. Management of interrupted aortic arch. Semin Thorac Cardiovasc Surg. 2015;27:177–188. doi: 10.1053/j.semtcvs.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 91.Brown JW, Rodefeld MD, Ruzmetov M. Transverse aortic arch obstruction: when to go from the front. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2009;12:66–69. doi: 10.1053/j.pcsu.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 92.Onalan MA, Temur B, Aydın S, et al. Management of aortic arch hypoplasia in neonates and infants. J Card Surg. 2021;36:124–133. doi: 10.1111/jocs.15212. [DOI] [PubMed] [Google Scholar]
- 93.Kenny D, Hijazi ZM. Coarctation of the aorta: from fetal life to adulthood. Cardiol J. 2011;18:487–495. doi: 10.5603/cj.2011.0003. [DOI] [PubMed] [Google Scholar]
- 94.Agasthi P, Pujari SH, Tseng A, et al. Management of adults with coarctation of aorta. World J Cardiol. 2020;12:167–191. doi: 10.4330/wjc.v12.i5.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dodge-Khatami A, Ott S, Di Bernardo S, Berger F. Carotid-subclavian artery index: new echocardiographic index to detect coarctation in neonates and infants. Ann Thorac Surg. 2005;80:1652–1657. doi: 10.1016/j.athoracsur.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 96.Karaosmanoglu AD, Khawaja RD, Onur MR, Kalra MK. CT and MRI of aortic coarctation: pre- and postsurgical findings. AJR Am J Roentgenol. 2015;204:W224-33. doi: 10.2214/AJR.14.12529. [DOI] [PubMed] [Google Scholar]
- 97.Dijkema EJ, Leiner T, Grotenhuis HB. Diagnosis, imaging and clinical management of aortic coarctation. Heart. 2017;103:1148–1155. doi: 10.1136/heartjnl-2017-311173. [DOI] [PubMed] [Google Scholar]
- 98.Eckhauser A, South ST, Meyers L, Bleyl SB, Botto LD. Turner syndrome in girls presenting with coarctation of the aorta. J Pediatr. 2015;167:1062–1066. doi: 10.1016/j.jpeds.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Prada F, Mortera C, Bartrons J, et al. Complex aortic coarctation and PHACE syndrome. Rev Esp Cardiol. 2010;63:1367–1370. doi: 10.1016/s1885-5857(10)70261-9. [DOI] [PubMed] [Google Scholar]
- 100.Aslam S, Khairy P, Shohoudi A, et al. Shone complex: an under-recognized congenital heart disease with substantial morbidity in adulthood. Can J Cardiol. 2017;33:253–259. doi: 10.1016/j.cjca.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 101.Doshi AR, Chikkabyrappa S. Coarctation of aorta in children. Cureus. 2018;10:e3690. doi: 10.7759/cureus.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alkashkari W, Albugami S, Hijazi ZM. Management of coarctation of the aorta in adult patients: state of the art. Korean Circ J. 2019;49:298–313. doi: 10.4070/kcj.2018.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suradi H, Hijazi ZM. Current management of coarctation of the aorta. Glob Cardiol Sci Pract. 2015;2015:44. doi: 10.5339/gcsp.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaya U, Colak A, Becit N, Ceviz M, Kocak H. Surgical management of aortic coarctation from infant to adult. Eurasian J Med. 2018;50:14–18. doi: 10.5152/eurasianjmed.2017.17273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kimura K, Ohtake H, Kato H, Yashiki N, Tomita S, Watanabe G. Pseudocoarctation of the aorta complicated by thoracic aortic aneurysm. Asian Cardiovasc Thorac Ann. 2011;19:265–267. doi: 10.1177/0218492311407782. [DOI] [PubMed] [Google Scholar]
- 106.Adaletli I, Kurugoglu S, Davutoglu V, Ozer H, Besirli K, Sayin AG. Pseudocoarctation. Can J Cardiol. 2007;23:675–676. doi: 10.1016/s0828-282x(07)70232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shindo S, Katsu M, Kojima A, Kobayashi M, Tada Y. Thoracic aortic aneurysm associated with pseudocoarctation of the aorta. Jpn J Thorac Cardiovasc Surg. 2002;50:520–522. doi: 10.1007/BF02913166. [DOI] [PubMed] [Google Scholar]
- 108.Grigsby JL, Galbraith T, Shurmur S, Deligonul U. Pseudocoarctation of the aorta complicated by saccular aneurysm: treatment by aortic arch replacement. Am Heart J. 1996;131:200–202. doi: 10.1016/s0002-8703(96)90073-3. [DOI] [PubMed] [Google Scholar]