Abstract
BACKGROUND
Current guidelines indicate electrical dyssynchrony as the major criteria for selecting patients for cardiac resynchronization therapy, and 25–35% of patients exhibit unfavorable responses to cardiac resynchronization therapy (CRT). We aimed to evaluate different cardiac mechanical dyssynchrony parameters in heart failure patients using current echo-Doppler modalities and we analyzed their association with electrical dyssynchrony.
METHODS
The study included 120 heart failure with reduced ejection fraction (HFrEF) who underwent assessments for left ventricular mechanical dyssynchrony (LVMD) and interventricular mechanical dyssynchrony (IVMD).
RESULTS
Patients were classified according to QRS duration: group I with QRS < 120 ms, group II with QRS 120–149 ms, and group III with QRS ≥ 150 ms. Group III had significantly higher IVMD, LVMD indices, TS-SD speckle-tracking echocardiography (STE) 12 segments (standard deviation of time to peak longitudinal strain speckle tracking echocardiography in 12 LV-segments), and LVMD score compared with group I and group II. Group II and group III were classified according to QRS morphology into left bundle branch block (LBBB) and non-LBBB subgroups. LVMD score, TS-SD 12 TDI, and TS-SD 12 STE had good correlations with QRS duration.
CONCLUSIONS
HFrEF patients with wide QRS duration (> 150 ms) had more evident LVMD compared with patients with narrow or intermediate QRS. Those patients with intermediate QRS duration (120–150 ms) had substantial LVMD assessed by both TDI and 2D STE, regardless of QRS morphology. Subsequently, we suggest that LVMD indices might be employed as additive criteria to predict CRT response in that patient subgroup. Electrical and mechanical dyssynchrony were strongly correlated in HFrEF patients.
Keywords: Interventricuar mechanical delay, Left ventricular mechanical dyssynchrony score, Heart failure, Speckle tracking echocardiography
INTRODUCTION
Left ventricular mechanical dyssynchrony (LVMD) using different imaging modalities has been shown to be an independent predictor of poor prognosis in cardiac disease. Hence, it may have advantages in guiding cardiac resynchronization therapy (CRT), although current guidelines are still based on electrical dyssynchrony criteria.1)
A correlation between mechanical and electrical dyssynchrony has been previously described even though these two types of dyssynchrony are different. Hence, there are many reasons why echocardiographic assessment of mechanical dyssynchrony may be a valuable, yet easy, reliable, and cost-effective parameter to measure. Additionally, electrical dyssynchrony and can be important for clinical decision-making regarding treatment for patients with heart failure (HF), such as better selection of patients for CRT (given the fact that QRS duration has not entirely predicted response to CRT).2) Many single-center studies have shown encouraging results for response prediction to CRT with both traditional and deformation-derived parameters of LV dyssynchrony.3)
LVMD can be measured using several echocardiographic imaging modalities such as tissue-Doppler imaging (TDI), 2-dimension (2D) speckle-tracking echocardiography (STE), 4D echocardiography (4DE), and 4D-STE.4)
Therefore, this study aimed to evaluate cardiac mechanical dyssynchrony parameters in HF patients using current echo-Doppler modalities and their association with electrical dyssynchrony.
METHODS
This is an observational single-center study. We enrolled 120 HF patients from March 2016 to February 2020. The study included heart failure with reduced ejection fraction (HFrEF) patients who had EF ≤ 35% and sinus rhythm regardless of underlying etiology of chronic HF (ischemic on non-ischemic), QRS width, or morphology (either wide or narrow QRS duration). We excluded patients with acute decompensated HF that could be due to acute coronary syndrome, significant valvular or congenital heart disease, an implanted pacemaker or CRT, or persistent atrial fibrillation.
The study was conducted in accordance with ethical standards and was authorized by the research ethics committee of Al Azhar University’s Faculty of Medicine for Girls, and all methods were carried out in accordance with relevant guidelines and regulations. Before enrolling in this study, informed written consent was acquired from all patients after describing the purpose of the study.
Methodology
All included patients were subjected to a thorough history taking and clinical assessment including New York Heart Associations (NYHA) class and 12-lead surface ECG to evaluate QRS duration and morphology in addition to PR interval.
Trans-thoracic echocardiographic examination was performed using a Vivid E9 XD Clear Echo machine (GE Healthcare, Horton, Norway) with tissue Doppler and speckle tracking imaging, which was attached to an Echo-Pac Work Station (version 201). The following parameters were measured:
• LV dimensions by M-mode, LV volumes (2D), EF (bi-plane), TDI for evaluation of mitral annular diastolic velocities, and 2D-speckle tracking echocardiography (STE) to obtain LV longitudinal strain (LV-GLS).
• 4DE and 4D strain: 4D echocardiographic imaging six-beat full-volume 4D datasets were obtained using 4V-phased array matrix transducer. The 12-slice display was used during acquisition to ensure a complete inclusion of the LV in the data set. 4D-Auto LVQ software was used for real-time 4D volume determination of the left ventricle. For 4D strain, a large volume acquisition from the apical window was used. The following global myocardial deformation parameters were measured: longitudinal strain, circumferential strain, radial strain, and area strain. Additionally, we reported LV volume, mass, and ejection fraction.5)
A. Evaluation of LVMD parameters (defined as a difference in the timing of mechanical contraction between different segments of the left ventricle) and interventricular dyssynchrony using the following echo-Doppler modalities:
• Septal to posterior wall delay (SPWD) with cut-off > 130 ms.
• Measurement of both LV and RV pre-ejection times (LVPET and RVPET), followed by calculation of interventricular mechanical dyssynchrony (IVMD) where > 40 ms is usually considered the cut-off value.
• Color-coded TDI uses the basal segments of the apical 4-chamber view to measure time differences in peak systolic velocities (TS) between basal septal (BS) and basal lateral (BL) segments, known as the two-site method with cut-off value > 60 ms.6)
• An opposing wall delay ≥ 65 ms was assessed from 12 LV segments.
• The maximum difference between TS values among the 12 LV sites; ≥ 100 ms was used as the cut-off value.7)
• The mechanical dyssynchrony index: known as the Yu index, was derived from calculating the SD of TS in 12-site and a cut-off value of > 33 ms was used.6)
• 6-site standard deviation and cut-off value of > 34.5 ms was used.8)
• SD of TS segmental longitudinal strain of 12 LV segments with cut-off value > 60 ms.4)
We proposed the following scoring system for LVMD:
1. SPWD > 130 ms (one point if present and 0 if absent)
2. TS of BS-BL > 60 ms (one point if present and 0 if absent)
3. ≥ 3 TS of opposing segments > 65 ms (one point if present and 0 if absent)
4. Ts max > 100 ms (one point if present and 0 if absent)
5. TS-SD of LVTDI 12 segments > 33 ms (one point if present and 0 if absent)
6. TS-SD of LVTDI 6segments > 34.5 ms (one point if present and 0 if absent)
7. TS-SD of LV STE 12 segments > 65 ms (one point if present and 0 if absent)
LVMD was considered present if the patient had ≥ 4/7 of LVMD indices with the maximum possible 7 points.
Reproducibility of LVMD parameters
Reproducibility of evaluated LVMD echocardiographic parameters was assessed in 20 randomly selected patients from each group by two independent investigators who were blinded to each other’s evaluations and to patient data. Assessments were performed at one-month intervals.
Statistical analysis
Distribution normality of all metric data was tested with the Shapiro-Wilk test, with significance set at p < 0.05. For parametric variables, data are expressed as means with standard deviations, and as medians (interquartile range) for non-parametric variables. To estimate continuous variables, Student’s t-tests were used. In univariate analyses, χ2 analysis, Student’s unpaired t-test, one-way ANOVA, and the Mann-Whitney test were used to compare variables between groups. To assess the association between variables, Pearson and linear regression analyses were used. Interobserver variability was assessed by inter- and intraclass correlation coefficients (ICCC), and ICCC > 0.90 was considered excellent agreement. Receiver operating characteristic (ROC) curve analysis was used to determine the diagnostic power of investigated parameters. We considered p-values < 0.05 statistically significant. SPSS software, version 23.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Ethic statement
All procedures were followed in accordance with the ethical standards of the Committee on Human Experimentation (institutional and national) and with the Declaration of Helsinki (1975), as revised in 2000. Informed consent was obtained from all patients.
Written informed consent was obtained from patients for publication.
RESULTS
The HFrEF patients were classified into three groups according to QRS width in 12-lead surface ECG:
• Group I: QRS duration < 120 ms (n = 40)
• Group II: intermediate QRS duration between 121–149 ms (n = 38)
• Group III: wide QRS duration ≥ 150 ms (n = 42)
Baseline clinical data, risk factors, and ECG are summarized in Table 1 with no significant differences among the three studied groups. All compared groups were age- and sex-matched. The number of smokers was higher in group I than in group III. As expected, QRS duration was substantially higher in group III than in groups II and I. No significant differences were found among the studied groups regarding the remaining parameters (Table 1).
Table 1. Comparison among all studied groups regarding baseline clinical data, risk factors, ECG and medications.
| Parameters | GI (n = 40) | GII (n = 38) | GIII (n = 42) | p-valuea | p-valueb | p-valuec | ||
|---|---|---|---|---|---|---|---|---|
| Gender | 0.75 | 0.23 | 0.34 | |||||
| Male | 33 (82.5) | 29 (76.3) | 30 (71.4) | |||||
| Female | 7 (17.5) | 8 (21.1) | 12 (28.6) | |||||
| Age (years) | 58.2 ± 9.4 | 57.8 ± 6.4 | 54.8 ± 8.8 | 0.97 | 0.17 | 0.26 | ||
| Risk factors | ||||||||
| Hypertension | 25 (62.5) | 21 (55.3) | 27 (64.3) | 0.78 | 0.83 | 0.95 | ||
| DM | 21 (52.5) | 14 (36.8) | 12 (28.6) | 0.24 | 0.18 | 0.95 | ||
| Smoking | 28 (70) | 15 (39.5) | 12 (28.6) | 0.06 | 0.03 | 0.82 | ||
| Etiology of HF | 0.91 | 0.89 | 0.78 | |||||
| IDCM | 25 (62.5) | 25 (65.8) | 26 (61.9) | |||||
| DCM | 15 (37.5) | 13 (34.2) | 16 (38.1) | |||||
| NYHA class | 0.22 | 0.99 | 0.62 | |||||
| II | 11 (27.5) | 6 (15.8) | 11 (26.2) | |||||
| III | 27 (67.5) | 23 (60.5) | 28 (66.7) | |||||
| IV | 5 (5) | 9 (23.7) | 3 (7.1) | |||||
| HR (bpm) | 84.7 ± 24.3 | 81.2 ± 11.6 | 82.6 ± 13.7 | 0.66 | 0.85 | 0.94 | ||
| BP | ||||||||
| SBP (mmHg) | 110.5 ± 20.3 | 113.7 ± 17.6 | 116.3 ± 23.4 | 0.78 | 0.42 | 0.85 | ||
| DBP (mmHg) | 70.8 ± 12.1 | 71.7 ± 18.3 | 72.4 ± 13.1 | 0.95 | 0.87 | 0.98 | ||
| QRS duration (ms) | 88.8 ± 9.0 | 124.2 ± 8.3 | 160.0 ± 0.0 | 0.0001 | 0.0001 | 0.0001 | ||
| ECG criteria | 0.87 | 0.76 | 0.98 | |||||
| PR interval (ms) | 217.5 ± 92.6 | 227.4 ± 90.6 | 231.4 ± 81.5 | |||||
| QRS morphologies: | ||||||||
| LBBB | - | 18 (47.4) | 22 (52.4) | |||||
| Non LBBB | - | 20 (52.6) | 20 (47.6) | |||||
| ACEIs/ARBs | 23 (57.5) | 25 (65.8) | 24 (57.1) | 0.25 | 0.92 | 0.12 | ||
| BBs | 33 (82.5) | 36 (94.7) | 35 (83.3) | 0.59 | 0.72 | 0.97 | ||
| Diuretics | 32 (85) | 34 (89.5) | 36 (85.7) | 0.97 | 0.52 | 0.69 | ||
Data are presented as number (%) or mean ±SD.
ACEIs: angiotensin converting enzyme inhibitors, ARBs: angiotensin receptor blockers, BB: beta blockers, DBP: diastolic blood pressure, DCM: dilated cardiomyopathy, DM: diabetes mellitus, HR: heart rate, IDCM: ischemic dilated cardiomyopathy, LBBB: left bundle branch block, NYHA: New York Heart Associations, SBP: systolic blood pressure.
aComparison between group I and group II; bcomparison between group I and group III; ccomparison between group II and group III.
Echocardiographic data of the included groups are summarized in Table 2. There were no significant differences among the three groups regarding LV dimensions, volumes, functions and strain (either by 2-D or 4D).
Table 2. Comparison among the studied groups regarding 2D and 4D-echocardiographic parameters.
| Parameters | GI (n = 40) | GII (n = 38) | GIII (n = 42) | p-valuea | p-valueb | p-valuec |
|---|---|---|---|---|---|---|
| LVEDD (mm) | 67.2 ± 8.8 | 68.2 ± 5.9 | 70.0 ± 8.2 | 0.83 | 0.24 | 0.56 |
| LVESD (mm) | 57.2 ± 5.5 | 58.3 ± 6.2 | 59.9 ± 7.5 | 0.74 | 0.14 | 0.51 |
| LVEDV4 (mL) | 150.0 ± 49.3 | 160.8 ± 41.8 | 169.5 ± 34.0 | 0.49 | 0.09 | 0.63 |
| LVESV4 (mL) | 117.2 ± 39.5 | 121.7 ± 31.8 | 129.7 ± 31.2 | 0.83 | 0.23 | 0.56 |
| LVEDV2 (mL) | 156.0 ± 47.8 | 162.7 ± 48.0 | 166.3 ± 26.4 | 0.76 | 0.51 | 0.92 |
| LVESV2 (mL) | 153.0 ± 45.5 | 161.7 ± 40.6 | 170.6 ± 27.4 | 0.56 | 0.34 | 0.94 |
| EF-biplane (%) | 28.1 ± 4.1 | 26.4 ± 3.9 | 26.2 ± 6.8 | 0.33 | 0.33 | 0.98 |
| E/EmAV | 23.2 ± 11.6 | 25.6 ± 12.2 | 26.6 ± 18.5 | 0.74 | 0.54 | 0.95 |
| 4D-EF (%) | 25.9 ± 5.9 | 24.9 ± 5.6 | 23.3 ± 8.6 | 0.81 | 0.20 | 0.54 |
| 2D-LV GLS (%) | 8.0 ± 2.6 | 7.3 ± 2.7 | 7.2 ± 3.1 | 0.58 | 0.48 | 0.90 |
| 4D-GLS (%) | 3.71 ± 1.3 | 3.6 ± 1.7 | 3.3 ± 1.4 | 0.86 | 0.36 | 0.69 |
| 4D-GCS (%) | 3.6 ± 1.6 | 3.1 ± 1.4 | 3.0 ± 1.1 | 0.19 | 0.12 | 0.98 |
| 4D-GRS (%) | 8.7 ± 3.3 | 7.6 ± 3.1 | 7.4 ± 2.4 | 0.22 | 0.12 | 0.95 |
| 4D-area strain (%) | 6.0 ± 2.0 | 5.7 ± 2.4 | 5.4 ± 1.6 | 0.83 | 0.35 | 0.72 |
Data are presented as mean ±SD.
2: 2 chamber, 4: 4 chamber, E/Em: early diastolic mitral velocity/average early diastolic velocities at 6 mitral annular sites derived from TDI, EF: ejection fraction, GCS: global circumferential strain, GLS: global longitudinal strain, GRS: global radial strain, LVEDD: left ventricular end diastolic dimensions, LVEDV: left ventricular end diastolic volume, LVESD: left ventricular end systolic dimensions, LVESV: left ventricular end systolic volume.
aComparison between group I and group II; bcomparison between group I and group III; ccomparison between group II and group III.
Interventricular and LV mechanical dyssynchrony indices using conventional, TDI and STE echocardiographic modalities were evaluated in each group, and are listed in Table 3. Group III had significantly higher LV PET, IVMD, LVMD indices, and LVMD scores compared with group I and group II. However, we found no significant differences between groups II and III regarding TS-BS to BL and number of TS-opposing segments. Only RVPET was not significantly different among all compared groups. Additionally, group II had significantly higher IVMD, LVMD indices, and LVMD scores compared with group I, while there were no significant differences between groups I and II regarding SPWD.
Table 3. Comparison among the studied groups regarding different IVMD and LVMD indices.
| Parameters | GI (n = 40) | GII (n = 38) | GIII (n = 42) | p-valuea | p-valueb | p-valuec |
|---|---|---|---|---|---|---|
| SPWD (ms) | 83.4 ± 42.6 | 99.6 ± 48.8 | 199.5 ± 111.6 | 0.61 | 0.0001 | 0.0001 |
| RVPET (ms) | 112.5 ± 15.1 | 116.0 ± 19.8 | 117.5 ± 22.4 | 0.71 | 0.45 | 0.94 |
| LVPET (ms) | 126.2 ± 16.2 | 138.0 ± 16.9 | 170.5 ± 19.7 | 0.01 | 0.0001 | 0.0001 |
| IVMD (ms) | 15.3 ± 10.9 | 33.5 ± 16.3 | 53.0 ± 16.6 | 0.0001 | 0.0001 | 0.0001 |
| TS-BS to BL (ms) | 31.8 ± 19.2 | 54.5 ± 45.8 | 69.8 ± 36.5 | 0.01 | 0.0001 | 0.48 |
| TSmax (ms) | 72.0 ± 32.8 | 108.4 ± 46.2 | 135.5 ± 31.3 | 0.0001 | 0.0001 | 0.01 |
| Number of TS-opposing segments (median, IQR) | 0.00 (0.75) | 1.0 (2) | 2.0 (2.0) | 0.0001 | 0.0001 | 0.19 |
| TS-SDTDI 12 segments (ms) | 23.7 ± 9.1 | 33.4 ± 8.3 | 43.8 ± 7.5 | 0.0001 | 0.0001 | 0.0001 |
| TS-SDTDI 6 segments (ms) | 22.0 ± 8.5 | 35.4 ± 11.0 | 41.4 ± 7.5 | 0.0001 | 0.0001 | 0.0001 |
| TS-SDSTE 12 segments (ms) | 48.7 ± 12.5 | 65.1 ± 2.5 | 81.2 ± 8.2 | 0.0001 | 0.0001 | 0.0001 |
| LVMD score (median, IQR) | 0 (1) | 4 (4) | 6 (1) | 0.0001 | 0.0001 | 0.0001 |
Data are presented as mean ±SD or number (%).
IVMD: interventricular mechanical delay, IQR: interquartile range, LVMD: LV mechanical dyssynchrony, LVPET: left ventricular pre-ejection time, RVPET: right ventricular pre-ejection time, SPWD: septal to posterior wall delay, TS-BS to BL: time to peak systolic velocity between basal septal to basal lateral, TSmax: The maximum difference between time-to-peak velocity values among the 12 LV sites, TS-SDSTE12: standard deviation of time to peak systolic segmental longitudinal strain of 12 LV sites, TS-SDTDI12: standard deviation of the time-to-peak systolic velocity in 12-site, TS-SDTDI6: standard deviation of the time-to-peak systolic velocity in 6-site.
aComparison between group I and group II; bcomparison between group I and group III; ccomparison between group II and group III.
The latest activated segments (assessed by TDI and STE) were the mid-inferior segment in 27.5% (n = 11) of patients followed by basal and mid-posterior segments in 20% (n = 8) of patients for each segment among group I. In group II, the most delayed segment was the mid-inferior segment in 10.5% (n = 4) of patients, followed by the basal inferior segment in 13.2% (n = 5) of patients. Meanwhile, in group III, the most delayed segment was the basal posterior in 16.7% (n = 7) of patients followed by the mid-lateral segment in 16.7% (n = 7) of patients.
Cut-off value for detection of mechanical dyssynchrony using LVMD score
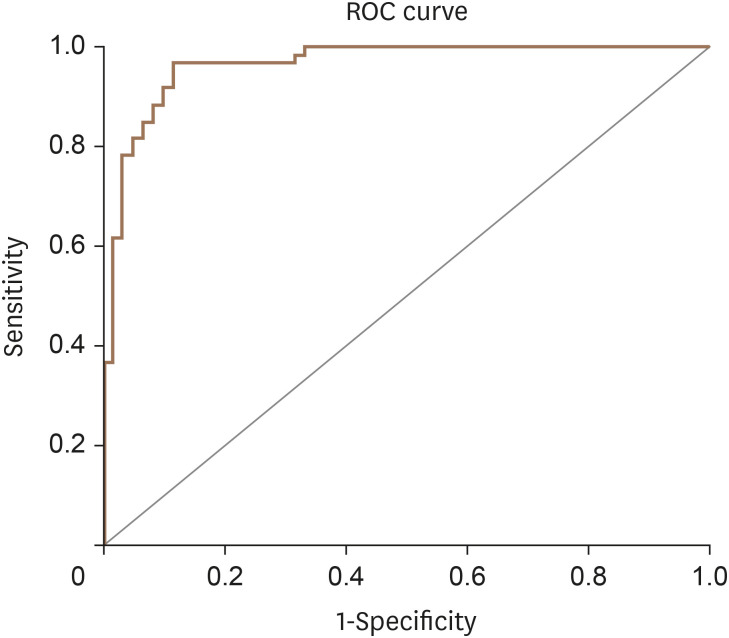
An ROC curve was plotted that revealed an LVMD score cut-off value of 4.0 with a 90% sensitivity and 100% specificity to define patients with LV mechanical dyssynchrony (Figure 1).
Figure 1. Sensitivity and specificity of suggested LVMD scores for of LVMD detection in heart failure patients (area under the curve = 0.964).
LVMD: left ventricular mechanical dyssynchrony, ROC: receiver operating characteristic.
IVMD and LVMD prevalence among the studied groups
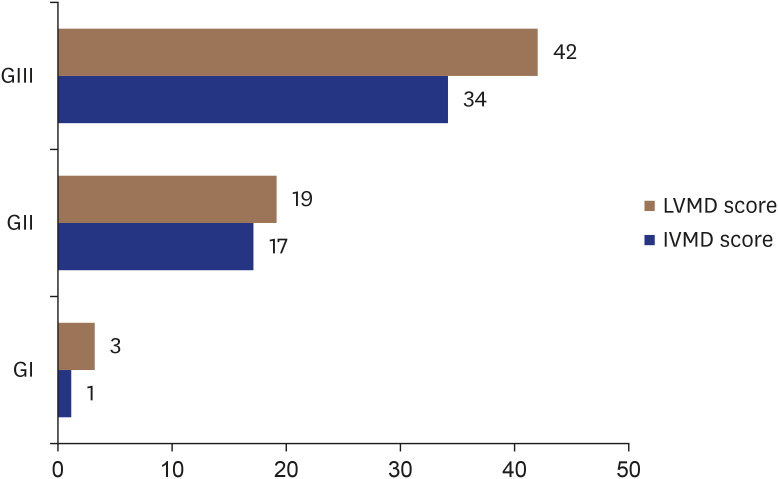
IVMD and LVMD prevalence (based on LVMD score) were distributed as follows: among group I, one patient (2.5%) had IVMD, and 3 patients (7.5%) had LVMD. Among group II, 17 patients (44.7%) had IVMD, and 19 patients (50%) had LVMD. Among group III, 34 patients (80.9%) had IVMD, and all patients (100%) had LVMD (Figure 2).
Figure 2. Prevalence of IVMD and LVMD among the studied groups.
IVMD: interventricular mechanical dyssynchrony, LVMD: left ventricular mechanical dyssynchrony.
Group II and III classifications according to QRS morphology
Group II patients were further classified by QRS morphology into the following: group IIa that included 20 patients (52.6%) with non-left bundle branch block (LBBB) (right bundle branch block [RBBB] and IVMD) and group IIb that included 18 patients (47.4%) with LBBB. We found no significant differences between the groups regarding any compared parameters (IVMD, LVMD indices, and LVMD score; all p > 0.5).
Group III patients were further classified into group IIIa that included 20 patients (47.6%) with non-LBBB (RBBB and IVMD) and group IIIb that included 22 patients (52.4%) with LBBB. No significant differences were detected between the groups regarding any compared parameters (IVMD, LVMD indices, and LVMD score; all p > 0.5).
Correlations between QRS duration with echocardiographic indices
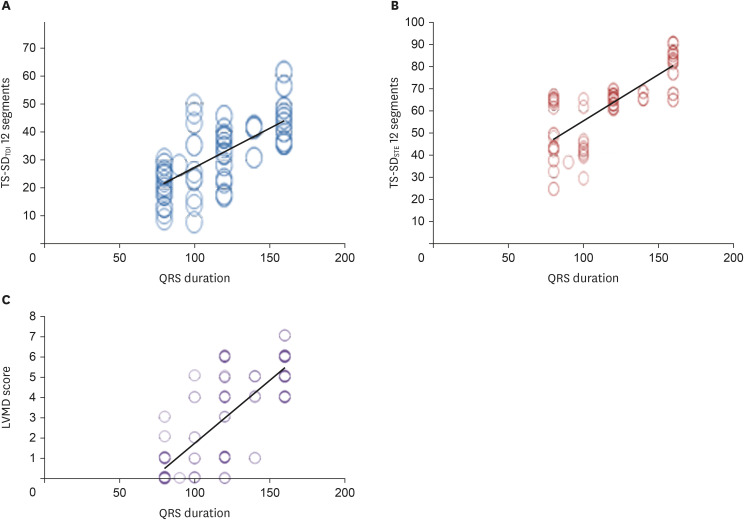
We found a significant positive correlation between QRS duration and the following: IVMD, LVMD score, and LVMD indices (SPWD, LVPET, IVMD, TS-BS to BL, TSmax, TS-SDTDI 12 segments, TS-SDTDI 6 segments, and TS-SDSTE12 segments) (Table 4, Figure 3). Using linear regression, LVMD score, TS-SD12 TDI, and TS-SD12 STE had strong correlations with QRS duration (r2 = 0.840, r2 = 0.740, and r2 = 0.808, respectively; p < 0.0001 for all). Meanwhile, no significant correlations were detected between QRS duration with LV dimensions, volumes, or LV EF (either by 2-D or 4-D) (Table 4).
Table 4. Correlation between QRS duration and different echocardiographic indices.
| Parameters | r | p-value |
|---|---|---|
| SPWD (ms) | 0.519 | 0.0001 |
| LVPET (ms) | 0.661 | 0.0001 |
| IVMD (ms) | 0.710 | 0.0001 |
| TS BS-BL (ms) | 0.411 | 0.001 |
| TSmax (ms) | 0.602 | 0.0001 |
| TS-SDTDI 12 segments (ms) | 0.740 | 0.0001 |
| TS-SDTDI 6 segments (ms) | 0.682 | 0.0001 |
| TS-SDSTE 12 segments (ms) | 0.808 | 0.0001 |
| LVMD score | 0.804 | 0.0001 |
| LVEDD (mm) | 0.134 | 0.15 |
| LVESD (mm) | 0.153 | 0.09 |
| EF (%) | −0.035 | 0.70 |
| LVEDV4 (mL) | 0.064 | 0.49 |
| LVESV4 (mL) | 0.111 | 0.23 |
| EF-biplane (%) | −0.114 | 0.22 |
| 4D-EF (%) | −0.137 | 0.12 |
EF: ejection fraction, IVMD: interventricular mechanical dyssynchrony, LVEDD4: LV end diastolic dimension, LVEDV4: LV end diastolic volume in 4 chamber, LVESD: LV end systolic dimension, LVESV4: LV end systolic volume in 4 chamber, LVPET: left ventricular pre-ejection time, SPWD: septal to posterior wall delay, TS-BS to BL: time to peak systolic velocity between basal septal to basal lateral, TSmax: The maximum difference between time-to-peak velocity values among the 12 LV sites, TS-SDSTE12: standard deviation of time to peak systolic segmental longitudinal strain of 12 LV sites, TS-SDTDI12: standard deviation of the time-to-peak systolic velocity in 12-site, TS-SDTDI6: standard deviation of the time-to-peak systolic velocity in 6-site.
Figure 3. Correlation between QRS duration with (A) TS-SDTDI 12 segments, (B) TS-SDSTE 12 segments, and (C) LVMD score among studied patients.
IVMD: interventricular mechanical dyssynchrony, LVMD: left ventricular mechanical dyssynchrony.
Interobserver reproducibility of LVMD parameters
ICCC of LVMD parameters showed good agreement (ICCC = 0.81). The mean time between repeated measurements by two investigators was 29.6 ± 4.8 days.
DISCUSSION
Selection of patients with HFrEF for CRT has recently been focused exclusively on ECG criteria of QRS width and morphology, and uncertainties remain for the candidacy of many potential CRT recipients with intermediate QRS (120–149 ms) or non-LBBB morphology. TDI-based dyssynchrony indices did not improve patients selection for CRT, but 2D-STE based dyssynchrony indices have demonstrated added value in selecting potential candidates for CRT even when QRS duration is borderline.9)
We aimed to evaluate mechanical dyssynchrony in HFrEF—particularly those with QRS duration 120–149 ms—using current echo-Doppler modalities and to estimate correlations between electrical and mechanical dyssynchrony.
This study evaluated 120 patients with HFrEF either due to ischemic DCM (n = 76, 63.3%) or non-ischemic DCM (n = 44, 36.7%) with different QRS durations and morphologies. The studied patients were stratified based on QRS duration into three groups: group I (QRS duration < 120 ms), group II (QRS duration 121–149 ms), and group III (QRS duration ≥ 150 ms).
All groups were sex- and age-matched. We could not find significant differences among the groups regarding risk factors, HF etiology, NYHA class, heart rate, blood pressure (systolic and diastolic), or PR interval. However, the number of smokers was higher in group I than in group III.
Our results were concordant with Haghjoo et al.10) and Bleeker et al.,11) who evaluated patients with severe HF (LVEF < 35%). Patients were classified according to QRS duration into three groups (wide, intermediate and narrow), and HF etiology, gender, and NYHA class were similar in all compared groups.10),11)
In this study, LV dimension volumes or LV-EF (either by 2-D or 4D) did not differ among the three studied groups. LV-GLS assessed by 2D/4D was similar among the three groups.
Our results were similar to Haghjoo et al.,10) who found no significant difference among the compared groups regarding LVEF and LVEDV, while there was a progressive increase in LVESV that correlated with QRS duration (p = 0.042). Ghio et al.12) reported significant differences among the three groups of HF patients with different QRS widths for 2D EF and LVEDV (p < 0.05 for both).
Our results are due to how we measured 2D-LVEF, which was based on geometric assumptions of LV volume, poor endocardial visualization, or distorted LV geometry in cases where advanced HF compromised precise estimation of global LV function and increased intra- and inter-observer variability.13) Thus, we incorporated a 4D modality for better assessment of LV volumes and functions in these patients.
In this study, we demonstrated that group III (who had the widest QRS duration) showed significantly higher LVPET, IVMD, LVMD indices, and LVMD scores compared with group I (who had the narrowest QRS duration) and group II, except for TS-BS to BL and number of TS-opposing segments that did not differ significantly between groups III and II. Only, RVPET was not significantly different among the groups. Those patients with intermediate QRS duration (group II) had significantly higher IVMD, LVMD indices, and LVMD score compared with group I, except for SPWD, which did not differ between the groups.
Regarding IVMD and LVMD prevalence (based on LVMD score), we found the following distributions: group I included 2.5% patients with IVMD and 7.5% patients with LVMD; group II included 44.7% patients with IVMD and 50% patients with LVMD; and group III included 80.9% patients with IVMD and 100% patients with LVMD.
Concordantly, Haghjoo et al.10) reported that RVPET was similar in the three QRS groups, while LVPET was longer in group 3 (> 150 ms) than groups 1 (< 120 ms) and 2 (120–150 ms), resulting in greater IVMD in group-3 patients (53 ± 21 vs. 42 ± 22 vs. 35 ± 21 ms, respectively; p < 0.001 for both). Additionally, they reported that LVMD (defined as TSmax > 100 ms) was present in 36% of group-1 patients, 58% of group-2 patients, and 79% of group 3 patients, which was consistent with our findings.
Our results are also consistent with Yu et al.,14) who reported significant differences in SD-Ts dyssynchrony index frequency between patients with wide and narrow QRS. Moreover, Bleeker et al.12) observed significant LV dyssynchrony in 70% of patients with a wide QRS complex, 60% of patients with an intermediate QRS, and 27% of patients with a narrow QRS complex.
Badano et al.15) found significant LV dyssynchrony in all QRS subgroups (LBBB, RBBB, interventricualr conduction delay, pacing and normal QRS). More importantly, although the mean LV dyssynchrony (the extent) was slightly higher in the LBBB group, significant LV dyssynchrony was equally observed in the LBBB, the RBBB, and the normal QRS groups. It was therefore concluded that echocardiographic LV dyssynchrony could be observed in all groups, regardless of QRS configuration or QRS duration.
Montazeri et al.16) stated that IVMD prevalence increased from 19.3% in patients with QRS < 120 ms to 64.9% in those with QRS ≥ 150 ms, which was consistent with previous studies that found higher IVMD prevalence in patients with wide QRS than in those with narrow QRS.10),12)
Among our patients with HFrEF, we found that the latest activated segment was the mid-inferior segment in patients with narrow and intermediate QRS. Meanwhile, in wide QRS group, the most delayed segment was the basal posterior followed by the mid-lateral segment.
Our results were inconsistent with Haghjoo et al.,10) who demonstrated that in group 1 (narrow QRS) and in group 2 (intermediate QRS) patients, the latest activated segment was the basal posterior segment in 26.4% and 24.1% of cases, respectively, while in group 3 (wide QRS) patients, the latest activated segment was the basal lateral segment in 25.0% of cases.
In this study, we could not find any significant difference in IVMD or LVMD indices between LBBB and non-LBBB patients with intermediate or wide QRS duration.
Consistent with our results, Van Bommel et al.17) observed no significant differences in LV dyssynchrony extent between the QRS subgroups (narrow QRS, LBBB, RBBB, interventricular conduction delay, and pacing). Conversely, Haghjoo et al.10) concluded that LBBB was more likely to be associated with significant LVMD than non-LBBB among all studied groups including those with QRS duration 120–150 ms. These observations tended to support use of QRS duration for patient selection. However, many CRT studies indicated that 20–30% of patients failed to respond to CRT, despite prolonged QRS duration, and 20–40% of patients with HF and QRS duration > 120 ms did not exhibit LVMD. Moreover, the inconsistent results from LVMD could be related to patient characteristics or different definitions for LVMD indices.10)
Our study revealed no significant correlations between QRS duration and both LV dimensions, volumes, or LV systolic function (either by 2-D or 4-D). Concordantly, Jiang et al.18) found no relationship between QRS duration and LV systolic function.
Our study revealed that QRS duration was positively correlated with IVMD, LVMD score, and LVMD indices (SPWD, LVPET, IVMD, TS-BS to BL, TSmax, TS-SDTDI 12 segments, TS-SDTDI 6 segments, and TS-SDSTE12 segments). Furthermore, LVMD score, TS-SD12 TDI, and TS-SD12 STE had good correlations for electrical dyssynchrony (evaluated by QRS duration). Contrarily, we could not find significant correlations between QRS duration with LV dimensions, volumes, and LV EF.
Our results are consistent with Haghjoo et al.,10) who demonstrated a significant relationship between QRS duration and IVMD (n = 123, r = 0.56, p < 0.0001). Similarly, Tournoux et al.19) confirmed a correlation between electrical and mechanical dyssynchrony for IVMD (r = 0.33, p < 0.002) and LVMD (r = 0.31, p < 0.003 for TS-SDTDI 12 segments, and r = 0.26, p < 0.02 for TSmax). Also, Ahmed20) found significant associations between broad QRS complex and both M-mode-derived dyssynchrony and LV dyssynchrony on TDI-based and TS-BS to BL velocity difference.
In contrast, Bleeker et al.11) failed to show a significant relationship between QRS duration and septal-to-lateral delay by TDI with linear regression. Also, Lumens et al.21) did not find a linear correlation between QRS width and dyssynchrony by SPWMD on M-mode echocardiography.
These discrepant correlation results between electrical and mechanical dyssynchrony could be because QRS width and, more broadly, electrical dyssynchrony may have been calculated as the sum of several inputs that included, in particular, morphological and mechanical factors, the interaction between left and right ventricles, and histological changes, such as fibrosis.22) We suggested a scoring system to define LVMD, and we used ROC curve analysis to estimate the LVMD cut-off value of 4.0, which had 90% sensitivity and 100% specificity to define patients with LV mechanical dyssynchrony.
Brunet-Bernard et al.23) described a seven-point CRT response score, L2ANDS2, that includes: LBBB (2 points), age > 70 years (one point), non-ischemic origin (one point), LV end diastolic diameter < 40 mm/m2 (one point), and septal flash (2 points) that was calculated for HF patients to predict CRT response. Currently, a multivariable approach and scores to identify CRT responders have only been applied in small observational retrospective studies with no or a very small control group. Because of the complexity of LV mechanics, a diverse pattern has been described for dyssynchrony expression in different components or directions.24) A more comprehensive predictive algorithm that includes other non-dyssynchrony-related factors may provide a better balance between sensitivity and specificity.25)
The PREDICT-CRT study explored the association between CRT outcome and mechanical dyssynchrony parameters, which were found to be strongly associated with improved survival and CRT response.26),27)
Beela et al.28) concluded that baseline assessment of mechanical dyssynchrony parameters could be a beneficial addition to current guidelines, offering in improved survival and response rates. This clearly suggests that specific mechanical dyssynchrony factors should be investigated further in larger prospective investigations. They concluded that taking mechanical dyssynchrony into account in both the European and American guidelines enhanced the number of correctly classified volume responders. Mechanical dyssynchrony was found to have an additional prognostic value for evaluating patients with a relatively wide QRS complex (120–150 ms).29)
Limitations: 1) The number of overall enrolled patients and number of patients within classified groups were relatively small. 2) This was a single-center, observational study that perform off-line analyses with using different echo-parameters. (3) Multiple peaks during TDI examinations were frequently observed and could have created inconsistent choices for which peak in the TDI signal should be chosen as the peak systolic velocity even by experienced operators. 4) STE is dependent on frame rates and image resolution, and this represents another limitation to the technique. 5) Finally, a prospective validation survey, with a large sample size and regular follow-up is needed to support routine application after CRT implantation.
We concluded that HFrEF patients with wide QRS duration (> 150 ms) had more pronounced LVMD compared with patients with narrow or intermediate QRS. Patients with intermediate QRS duration (120–150 ms) had significant LVMD assessed with both TDI and 2D STE, regardless of QRS morphology. Consequently, we proposed that LVMD indices might be included as additive criteria to predict CRT response in that patient subgroup. LVMD score ≥ 4.0 had 90% sensitivity and 100% specificity in identifying patients with LV mechanical dyssynchrony. Electrical and mechanical dyssynchrony had considerable correlation in HFrEF patients.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Hamdy RM.
- Data curation: Osama H.
- Investigation: Osama H.
- Methodology: Hamdy RM, Osama H.
- Project administration: Hamdy RM.
- Supervision: Fereig HM.
- Visualization: Fereig HM.
- Writing - original draft: Hamdy RM, Osama H.
- Writing - review & editing: Hamdy RM.
References
- 1.Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:3427–3520. doi: 10.1093/eurheartj/ehab699. [DOI] [PubMed] [Google Scholar]
- 2.Bank AJ, Burns KV, Gage RM. Echocardiographic measurement of mechanical dyssynchrony in heart failure and cardiac resynchronization therapy. US Cardiol Rev. 2010;7:24–32. [Google Scholar]
- 3.Kleijn SA, Aly MF, Knol DL, et al. A meta-analysis of left ventricular dyssynchrony assessment and prediction of response to cardiac resynchronization therapy by three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. 2012;13:763–775. doi: 10.1093/ehjci/jes041. [DOI] [PubMed] [Google Scholar]
- 4.Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. doi: 10.1016/j.echo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Dillikar MV, Venkateshvaran A, Barooah B, Varyani R, Kini P. Three-dimensional versus two - dimensional strain for the assessment of myocardial function: a case series. J Ind Acad Echocardiogr Cardiovasc Imaging. 2017;1:18–23. [Google Scholar]
- 6.Yu CM, Fung JW, Zhang Q, et al. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation. 2004;110:66–73. doi: 10.1161/01.CIR.0000133276.45198.A5. [DOI] [PubMed] [Google Scholar]
- 7.Bax JJ, Bleeker GB, Marwick TH, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834–1840. doi: 10.1016/j.jacc.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Yu CM, Wing-Hong Fung J, Zhang Q, Sanderson JE. Understanding nonresponders of cardiac resynchronization therapy--current and future perspectives. J Cardiovasc Electrophysiol. 2005;16:1117–1124. doi: 10.1111/j.1540-8167.2005.40829.x. [DOI] [PubMed] [Google Scholar]
- 9.Oyenuga O, Hara H, Tanaka H, et al. Usefulness of echocardiographic dyssynchrony in patients with borderline QRS duration to assist with selection for cardiac resynchronization therapy. JACC Cardiovasc Imaging. 2010;3:132–140. doi: 10.1016/j.jcmg.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Haghjoo M, Bagherzadeh A, Fazelifar AF, et al. Prevalence of mechanical dyssynchrony in heart failure patients with different QRS durations. Pacing Clin Electrophysiol. 2007;30:616–622. doi: 10.1111/j.1540-8159.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 11.Bleeker GB, Schalij MJ, Molhoek SG, et al. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004;15:544–549. doi: 10.1046/j.1540-8167.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- 12.Ghio S, Constantin C, Klersy C, et al. Interventricular and intraventricular dyssynchrony are common in heart failure patients, regardless of QRS duration. Eur Heart J. 2004;25:571–578. doi: 10.1016/j.ehj.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Yu CM, Lin H, Zhang Q, Sanderson JE. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart. 2003;89:54–60. doi: 10.1136/heart.89.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badano LP, Gaddi O, Peraldo C, et al. Left ventricular electromechanical delay in patients with heart failure and normal QRS duration and in patients with right and left bundle branch block. Europace. 2007;9:41–47. doi: 10.1093/europace/eul144. [DOI] [PubMed] [Google Scholar]
- 16.Montazeri M, Rezvanfard M, Kazemisaeid A, et al. Assessment of left ventricular dyssynchrony in heart failure patients regarding underlying etiology and QRS duration. J Tehran Heart Cent. 2011;6:193–201. [PMC free article] [PubMed] [Google Scholar]
- 17.van Bommel RJ, Ypenburg C, Mollema SA, et al. Site of latest activation in patients eligible for cardiac resynchronization therapy: patterns of dyssynchrony among different QRS configurations and impact of heart failure etiology. Am Heart J. 2011;161:1060–1066. doi: 10.1016/j.ahj.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Jiang FX, Guo RQ, Chen JL. Evaluation of left ventricular mechanical dyssynchrony in chronic heart failure patients by two-dimensional speckle tracking imaging. Kaohsiung J Med Sci. 2013;29:374–378. doi: 10.1016/j.kjms.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tournoux F, Donal E, Leclercq C, et al. Concordance between mechanical and electrical dyssynchrony in heart failure patients: a function of the underlying cardiomyopathy? J Cardiovasc Electrophysiol. 2007;18:1022–1027. doi: 10.1111/j.1540-8167.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed H, Tai JM, Khan SA, Yousuf M. QRS duration and echocardiographic evidence of left ventricular dyssynchrony in patients with left ventricular systolic dysfunction. J Coll Physicians Surg Pak. 2010;20:146–149. [PubMed] [Google Scholar]
- 21.Lumens J, Leenders GE, Cramer MJ, et al. Mechanistic evaluation of echocardiographic dyssynchrony indices: patient data combined with multiscale computer simulations. Circ Cardiovasc Imaging. 2012;5:491–499. doi: 10.1161/CIRCIMAGING.112.973446. [DOI] [PubMed] [Google Scholar]
- 22.Lumens J, Ploux S, Strik M, et al. Comparative electromechanical and hemodynamic effects of left ventricular and biventricular pacing in dyssynchronous heart failure: electrical resynchronization versus left-right ventricular interaction. J Am Coll Cardiol. 2013;62:2395–2403. doi: 10.1016/j.jacc.2013.08.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunet-Bernard A, Maréchaux S, Fauchier L, et al. Combined score using clinical, electrocardiographic, and echocardiographic parameters to predict left ventricular remodeling in patients having had cardiac resynchronization therapy six months earlier. Am J Cardiol. 2014;113:2045–2051. doi: 10.1016/j.amjcard.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, van Bommel RJ, Chan YS, et al. Diverse patterns of longitudinal and radial dyssynchrony in patients with advanced systolic heart failure. Heart. 2011;97:574–578. doi: 10.1136/hrt.2010.198572. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Yu CM. Is mechanical dyssynchrony still a major determinant for responses after cardiac resynchronization therapy? J Cardiol. 2011;57:239–248. doi: 10.1016/j.jjcc.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Stankovic I, Prinz C, Ciarka A, et al. Relationship of visually assessed apical rocking and septal flash to response and long-term survival following cardiac resynchronization therapy (PREDICT-CRT) Eur Heart J Cardiovasc Imaging. 2016;17:262–269. doi: 10.1093/ehjci/jev288. [DOI] [PubMed] [Google Scholar]
- 27.Galli E, Leclercq C, Donal E. Mechanical dyssynchrony in heart failure: Still a valid concept for optimizing treatment? Arch Cardiovasc Dis. 2017;110:60–68. doi: 10.1016/j.acvd.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Behaghel A, Brunet-Bernard A, Oger E, et al. Electrocardiographic correlates of mechanical dyssynchrony in recipients of cardiac resynchronization therapy devices. Arch Cardiovasc Dis. 2015;108:617–625. doi: 10.1016/j.acvd.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Beela AS, Ünlü S, Duchenne J, et al. Assessment of mechanical dyssynchrony can improve the prognostic value of guideline-based patient selection for cardiac resynchronization therapy. Eur Heart J Cardiovasc Imaging. 2019;20:66–74. doi: 10.1093/ehjci/jey029. [DOI] [PubMed] [Google Scholar]
- 30.Risum N, Williams ES, Khouri MG, et al. Mechanical dyssynchrony evaluated by tissue Doppler cross-correlation analysis is associated with long-term survival in patients after cardiac resynchronization therapy. Eur Heart J. 2013;34:48–56. doi: 10.1093/eurheartj/ehs035. [DOI] [PubMed] [Google Scholar]