Background.
Successful intestinal transplantation is currently hindered by graft injury that occurs during procurement and storage, which contributes to postoperative sepsis and allograft rejection. Improved graft preservation may expand transplantable graft numbers and enhance posttransplant outcomes. Superior transplant outcomes have recently been demonstrated in clinical trials using machine perfusion to preserve the liver. We hypothesized that machine perfusion preservation of intestinal allografts could be achieved and allow for transplantation in a porcine model.
Methods.
Using a translational porcine model, we developed a device for intestinal perfusion. Intestinal samples were collected at the time of organ procurement, and after 6 h of machine perfusion for gross and histologic evaluation, hourly chemistry panels were performed on the perfusate and were used for protocol optimization. Following transplantation, porcine recipient physical activity, systemic blood parameters, and vital signs were monitored for 2 d before sacrifice.
Results.
In initial protocol development (generation 1, n = 8 grafts), multiple metabolic, electrolyte, and acid-base derangements were measured. These factors coincided with graft and mesenteric edema and luminal hemorrhage and were addressed with the addition of dialysis. In the subsequent protocol (generation 2, n = 9 grafts), differential jejunum and ileum perfusion were observed resulting in gross evidence of ileal ischemia. Modifications in vasodilating medications enhanced ileal perfusion (generation 3, n = 4 grafts). We report successful transplantation of 2 porcine intestinal allografts after machine perfusion with postoperative clinical and gross evidence of normal gut function.
Conclusions.
This study reports development and optimization of machine perfusion preservation of small intestine and successful transplantation of intestinal allografts in a porcine model.
INTRODUCTION
Intestinal transplantation (IT) is the current standard of care for irreversible intestinal failure when parenteral nutrition becomes unsustainable.1 Of all abdominal solid organ transplants, IT is the least commonly performed, with only 91 completed in the United States in 2020. It is well known that the intestine has an extremely limited ability to tolerate ischemia, with evidence of epithelial detachment at the villous tips with even short periods (4–6 h) of cold ischemia, extending deep into the crypts by 9 h.2-6 Reperfusion further exacerbates the mucosal injury with epithelial cell shedding into the lumen and blunting of villi.3,7,8 Therefore, it is not surprising that IT has one of the lowest 5-y patient survival rates (62%–66%) compared with other organ allografts and the highest rate of graft loss (18.4% within the first 6 mo).9,10 The mucosal barrier damage that results from ischemia-reperfusion injury (IRI) from standard methods of graft preservation (ie, cold storage) and transplantation, bacterial translocation, and inflammation has been associated with development of IT rejection.11,12 Consequently, IT recipients experience rejection rates of 35%to65% in the first year after transplant, substantially higher than other abdominal organs. Beyond the first year, however, the rate of graft loss is similar for IT recipients compared with other organ allografts (3%–5% per year).10,13 This suggests that addressing IRI and the consequences leading to early graft loss may significantly improve the long-term intestine transplant patient and graft survival.
To minimize IRI, recent preclinical studies and clinical trials have demonstrated that liver preservation with oxygenated machine perfusion results in successful liver transplantation with lower graft discard rates and superior clinical outcomes compared with cold stored hepatic allografts.14-16 Despite clear benefits of normothermic machine perfusion (NMP) for preservation of other allografts, there is a paucity of prior studies of machine perfusion preservation of the intestine, none of which have produced a clinically transplantable intestine.17-19 We hypothesized that oxygenated machine perfusion of intestinal grafts would be a feasible approach to improve outcomes after intestine transplantation, as has been demonstrated in other transplanted organs. The aim of this study was to develop a translational NMP protocol of full-length intestinal allografts and validate the feasibility of this preservation method by orthotopic allotransplantation in a porcine model.
MATERIALS AND METHODS
Animal studies were approved through the United States Army Medical Research and Development command animal care and use review office and the North Carolina State University Institutional Animal Care and Use Committee (IACUC number 21-489).
Donor Intestine Procurement
Juvenile Yorkshire crossbred pigs of both sexes, weighing 25to30 kg, served as intestinal allograft donors and recipients and autologous whole blood donors (Figure 1). General anesthesia was induced with xylazine (1.5 mg/kg IM) and ketamine (11–20 mg/kg IM). Pigs were maintained under general anesthesia with isoflurane (2%–5%) vaporized in 100% O2 until euthanasia. Intraoperatively, the small intestinal allograft was isolated on a vascular pedicle consisting of the cranial mesenteric artery (CMA) (analogous to the human superior mesenteric artery) and cranial mesenteric vein (CMV). Intravenous heparin (1000 IU/kg) anticoagulation was administered 3to5 min before blood collection and explant. The abdominal aorta and caudal vena cava were cannulated, and whole blood was collected in Citrate Phosphate Dextrose Adenine-1 blood bags. After exsanguination, the CMA and CMV were divided, and the proximal jejunum and distal ileum were ligated and transected. The graft was flushed with 1–2 L of 4 °C University of Wisconsin solution,5 marking the beginning of the 1 h of static cold storage (SCS). The infra-renal abdominal aorta and caudal vena cava were procured to serve as vascular extensions for later graft implantation.
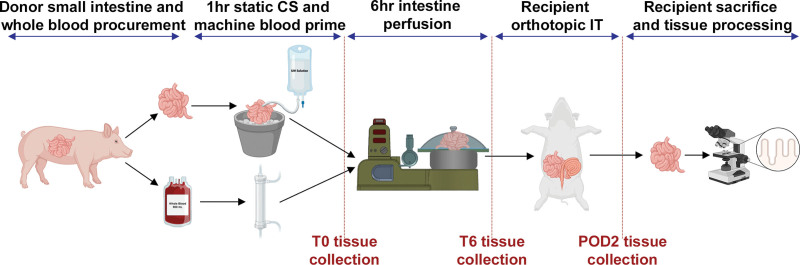
FIGURE 1.
Experimental outline of the intestinal procurement, preservation, transplantation, and tissue collection. The small intestine is procured from the donor pig, during which time approximately 900 mL of the donor’s whole blood is collected. The intestinal allograft is flushed with University of Wisconsin solution (4 °C) and stored on ice for 1 h. During this period of cold storage, the whole blood is used to prime the machine in preparation for perfusion of the intestine, and tissue samples are collected. After 6 h of machine preservation, tissue samples are again collected, the graft is flushed, and transplanted into the recipient pig. On the second day after transplantation, the recipient pig is euthanized, and the tissue is collected and processed. CS, cold storage; IT, intestinal transplantation; POD2, postoperative day 2; T0, time 0 h; T6, time 6 h.
Graft Preparation, Machine Priming, and Infusion Mixture
All grafts underwent 1 h of SCS. Baseline biopsies from jejunum and ileum were collected, and cannulas were placed in the CMA (for attachment to the machine) and in the distal ileum (for enteric evacuation). Simultaneously, the perfusion machine was primed with 200 mL of 25% human albumin (Grifols Therapeutics Inc) (target perfusate total protein level = 8 g/dL and oncotic pressure = 30 mm Hg) and 900 mL of autologous whole blood.
NMP Protocol
Three generations (Gen1, Gen2, Gen3) of NMP protocol development are summarized in Table 1. Time 0 (T0) was defined as the time the intestine allograft was placed on the machine for perfusion after the 1 h of SCS, during which back table preparation was performed and biopsies of the graft obtained. The Gen1 (n = 8 grafts) protocol was adapted from a published liver NMP protocol.16 The perfusion circuit consisted of a graft chamber with open venous return by gravity into a reservoir (Functional Circulation, LLC, Northbrook, IL) (Figure 2). A roller pump circulated the perfusate from reservoir to oxygenator and into the CMA at a constant mean arterial pressure (MAP) of 50 ± 5 mm Hg for 6 h. Perfusion pressure, temperature, and arterial flow were monitored continuously in real-time using in-line sensors (Functional Circulation, LLC, Northbrook, IL). Perfusate additives are detailed in Table 2.
TABLE 1.
Normothermic machine perfusion protocol descriptions
| Protocol generation | Gen1 | Gen2 | Gen3 |
|---|---|---|---|
| Perfused grafts, n | 8 | 9 | 4 |
| Transplants, n | 0 | 1 | 4 |
| Clinical observations | Discolored; not transplantable | Ileal hypoperfusion | Homogeneous pink serosa |
| Vasodilators | Epoprostenol | Verapamil | Taurocholate + epoprostenol + verapamil |
| Perfusate temperature | 37 °C | Controlled oxygenated rewarming to 34 °C at a rate of 1 °C/10 min | |
| MAP | 75 ± 5 mm Hg | 50 ± 5 mm Hg | |
| Perfusate oxygenation | Oxygen 100% | Carbogen (oxygen 95% + CO2 5%) | |
| Perfusate dialysis | None | Hemodiafiltration at Qw = 40 mL/min | |
| Nutrition substrates | Glucose only | Glucose + amino acids + alanyl-glutamine | |
| Ileal drainage | None | Cannulated | |
| Organ chamber | 279 mm | 360 mm | |
| Pump max flow | 379 mL/min | 757 mL/min | |
| Flow meter | None | Tachometer | |
| Blood reservoir bottom | Flat | Flat | Conical |
Protocols and outcomes of the different normothermic machine perfusion generations.
Gen, generation; MAP, mean arterial pressure; Qw, dialysate waste.
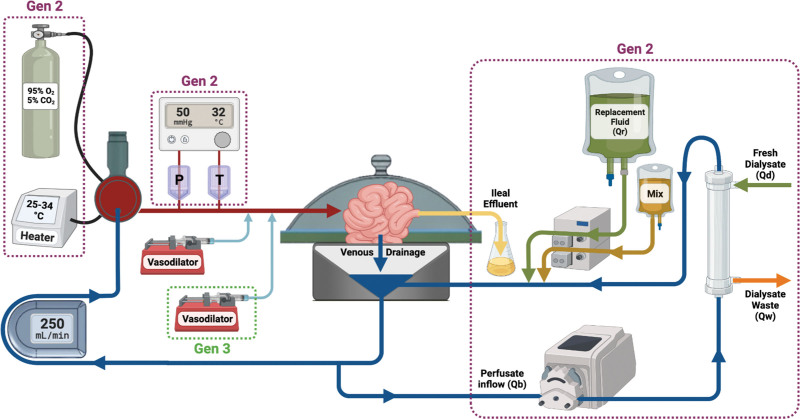
FIGURE 2.
Schematic of the normothermic machine perfusion system. Oxygenated blood leaves the oxygenator, passes through the P and T sensors, and enters the cannulated cranial mesenteric artery. Venous drainage from the graft is collected in the reservoir of the machine and either is channeled to the roller pump and back to the oxygenator or is directed as the Qb through a roller pump and into the dialysis filter. Within the filter, Qw is removed from the perfusate, and Qd is added. A Mix and Qr are added to the perfusate via infusion pumps, and the perfusate flows back into the reservoir to continue through the circulation of the machine. Arrows indicate direction of flow of the perfusate or additives. Purple boxes denote component added to system in Gen2, and these remain in Gen3. Green boxes denote components added to the system in Gen3. Gen, generation; Mix, medication mixture; P, pressure;Qb, perfusate inflow; Qd, fresh dialysate; Qr, replacement fluid; Qw, dialysate waste; T, temperature.
TABLE 2.
Perfusate composition
| Base perfusate composition | Concentration | Volume | Manufacturer/source |
|---|---|---|---|
| Autologous whole blood | 25%–30% (hematocrit) | 900 mL | Donor blood |
| Human albumin | 25% | 200 mL | Grifols |
| Piperacillin/tazobactam | 2.25 g | – | Sandoz |
| Clinimix 5/25 or E 5/25 | 10% (amino acids only) | 5 mL | Baxter |
| Solu-Medrol | 100 mg/mL | 5 mL | Pfizer |
| Fluconazole | 2 mg/mL | 5 mL | Sagent |
| 1-bag medication mix | Concentration | Volume | Manufacturer |
| Clinimix 5/25 or E 5/25 | 10% (amino acids only) | 120 | Baxter |
| Clinimix 5/25 or E 5/25 | 50% (dextrose only) | 10 | Baxter |
| Glutamax | 1 molar | 50 | Thermo Fisher |
| Solu-Medrol | 100 mg/mL | 5 | Pfizer |
| Piperacillin/tazobactam | 2.25 g | 0 | Sandoz |
| Humulin R | 100 units/mL | 0.5 | Lilly |
| Heparin | 1K units/mL | 30 | APP |
| Fluconazole | 2 mg/mL | 7.5 | Sagent |
| Vasodilator syringes | Concentration | Rate (mL/h) | Manufacturer/source |
| Verapamil | 5 mg/2 mL | 0.1 | Exela |
| Sodium epoprostenol | 1.5 mg/10 mL | 0.1 | GSK |
| Sodium taurocholate | 6 g in 50 mL Duosol 4555 | 2 | Sigma-Aldrich |
Medications, manufacturer, concentration, and volume used of the perfusate components.
h, hour.
Hemodiafiltration Circuit
The hemodiafiltration (HDF) circuit was added in Gen2. The perfusate chemistry, acid-base balance, osmotic pressure, oncotic pressure, and hematocrit were corrected to physiological levels before connecting the graft (HDF circuit outlined in Figure 2).20
Perfusate, Tissue Sampling, and Allograft Assessment
Hourly recordings and sampling of perfusate temperature, pressure, flow, graft vascular resistance, arterial inflow, and dialysate waste were obtained. Blood gas analysis, blood chemistry, and lactate measurements were performed on arterial inflow samples using an i-STAT unit (i-STAT Portable Clinical Analyzer; Abbott Laboratories, Abbott Park, IL) and handheld lactate meter (Nova Lactate Plus reader, Waltham, MA), respectively. Signs of ischemia or allograft injury were evaluated grossly by assessment of serosal appearance, intestinal and/or mesenteric edema, bowel distension, and appearance of ileal effluent. Full thickness jejunal and ileal biopsies were obtained before connecting the graft to the normothermic perfusion machine (T0h) and at the end of the perfusion (T6h).
Histologic Interpretation and Scoring
Biopsies were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned (5–7 μm thickness), and stained with hematoxylin and eosin. Injury scores were evaluated by an expert pathologist blinded to time and treatment. Assessment of the villous architecture, presence of epithelial injury, epithelial cytology, immune cell infiltration, and degree of edema were determined using a scale of 0 to 4 (0, normal; 1, focal and mild; 2, diffuse/multifocal and mild; 3, diffuse and moderate; 4, diffuse and severe) as outlined in Table S1 (SDC, http://links.lww.com/TXD/A462).
Graft Implantation
Transplantation of NMP stored intestinal allograft was performed in 5 animals (Gen2, n = 1; Gen3, n = 4). A baseline activated clotting time (ACT) was measured in all animals and aspirin (10 mg/kg PO) was administered preoperatively in the last transplanted pig (P291). Native jejunoileal enterectomy was performed in the porcine recipient. The abdominal aorta and caudal vena cava were exposed, heparin was administered (75 IU/kg), and the donor arterial and venous extension grafts were anastomosed in an end-to-side fashion. Following 6 h of NMP preservation, the graft was flushed with 1–2 L UW solution (4 °C) and 500 mL 5% albumin (4 °C). End-to-end anastomosis of the CMA, and CMV, and small bowel was then performed.
Postoperative Care and Euthanasia
The recipient pigs were maintained on lidocaine-ketamine and maropitant for postoperative pain and nausea as outlined in Table 3. Other medications included ulcer prophylaxis (omeprazole, 20 mg PO) and thrombosis prophylaxis (heparin, SQ 33 IU/kg q12h, P248, P253, P267, P287 or 80 IU/kg bolus plus constant rate infusion of 18 IU/kg/h IV, P291; aspirin, 10 mg/kg PO q24h, P291) adjusted by measurement of the ACT (measured at a minimum of q12h and maintained at 1.5–2× greater than baseline, ~180–400s). Immunosuppression consisted of methylprednisolone and tacrolimus as detailed in Table 3. Blood samples were drawn daily to measure tacrolimus level, ACT, lactate, complete blood count, and blood serum chemistry. Pigs had full physical examinations performed 3 times a day, and enteral feeding and bowel movements were monitored. On day 2 postrecovery, the pigs were anesthetized with xylazine and ketamine and then euthanized using 100 mg/kg pentobarbital (Table 3).
TABLE 3.
Drugs administered to pigs
| Medication | Concentration | Dose | Route of administration | Manufacturer |
|---|---|---|---|---|
| Ceftiofur crystalline-free acid | 100 mg/mL | 5.0 mg/kg | IM | Zoetis |
| Xylazine | 100 mg/mL | 1.5 mg/kg | IM | Bimeda |
| Ketamine | 100 mg/mL | 11–20 mg/kg | IM | Zoetis |
| Isoflurane | n/a | 2%–5% | Inhalant | Phoenix |
| Lidocaine | 20 mg/mL | Loading dose: 1–2 mg/kg | IV | AgriLabs |
| Infusion dose: 20–50 µg/kg/min | ||||
| Ketamine | 100 mg/mL | Loading dose: 0.5–2.0 mg/kg | IV | Zoetis |
| Infusion dose: 5–30 µg/kg/min | ||||
| Maropitant | 10 mg/mL | 1 mg/kg | IV | Zoetis |
| Heparin | 1000 units/mL | 25–50 units/kg or loading dose: 80 IU/kg | SQ or IV | Sagent |
| Infusion dose 18 IU/kg | ||||
| Aspirin | 325 mg/tab | 10 mg/kg | PO | Generic |
| Omeprazole | 20 mg/tab | 20 mg | PO | Procter & Gamble |
| Methylprednisolone | 62.5 mg/mL | 5–10 mg/kg | IV | Pfizer |
| Tacrolimus | 5 mg/mL | 0.2 mg/kg | SQ | Astellas |
| Pentobarbital | 390 mg/mL | 100 mg/kg | IV | Vortech |
Manufacturer, concentration, dose, and route of administration used of the medications administered to recipient pigs.
IM, intramuscular; IV, intravenous; n/a, not applicable; PO, per os; SQ, subcutaneous.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (version 9.02; GraphPad Software Inc, La Jolla, CA) and SAS 9.4 (SAS Institute, Cary, NC). Arterial flow dynamics and perfusate biochemistry values were measured at baseline (T0) and at 1, 2, 3, 4, 5, and 6 h. For analyses, average of measurements for all pigs within each protocol generation were examined. The logarithm base 10 transformation was used when appropriate to improve normality and model fit. Linear regression models with repeated measures, considering correlation between time points within each pig, were fit. First-order autoregressive or compound symmetry covariance structure was assumed. Experimental condition and pig were considered as covariates. Difference of least squares means was used to compare experimental conditions pairwise; a Bonferroni adjustment was made to P values to account for the multiple comparisons. For analysis of histologic injury, Kruskal-Wallis with Dunn multiple comparison test was used to determine statistical differences between T0h, Gen1, Gen2, and Gen3 protocols. P < 0.05 was considered significant.
RESULTS
Clinical Observations and Resultant Protocol Modifications
The NMP protocol underwent 3 iterative stages of development (Table 1). Gen1 (n = 8) served as proof-of-concept perfusions without transplant. The Gen2 protocol (n = 9) addressed biochemical disturbances identified in Gen1 (described below) by adding HDF, changing epoprostenol to verapamil to enhance vasodilation and improve graft perfusion, infusing nutrient substrates, and oxygenating the perfusate with carbogen to assist in the maintenance of the perfusate pH. Controlled rewarming of the graft was also instituted in an attempt to decrease reperfusion-induced cellular oxidative damage.21,22 A variable degree of malperfusion specific to the ileum was observed by gross discoloration of the bowel in Gen2 treated bowel (Figure 3). Poor ileal perfusion was addressed in Gen3 (n = 4) by infusing a combination of verapamil, epoprostenol, and taurocholate.
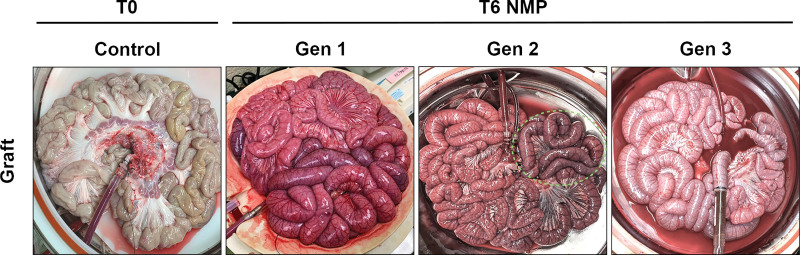
FIGURE 3.
Gross images of NMP-stored intestine. Jejunum and ileum at T0 control and T6 NMP for Gen1, Gen2, and Gen3 grafts. Grossly, Gen1 grafts had serosal discoloration and mesenteric and intestinal wall edema. Gen2 jejunum appeared grossly normal, whereas the ileal serosal appearance had variable degrees of purple discoloration (green dashed outline). Gen3 grafts had diffuse homogeneous pink serosal appearance and no mesenteric or abdominal wall edema. Gen, generation; NMP, normothermic machine perfusion; T0, before normothermic machine perfusion; T6, after 6 h of normothermic machine perfusion.
Appearance of Intestine Allografts on the NMP Device
All grafts initially perfused well grossly in the first hours of NMP; however, by 3 to 4 h, Gen1 grafts began to display diffuse patchy darkening and mottled purple discoloration of the serosa in addition to mesenteric and intestinal wall edema (Figure 3). At the end of the Gen1 NMP perfusions, examination of the lumen revealed bloody effluent throughout the length of the intestine grafts and upon examination of the mucosa, gross parenchymal mottling, and hemorrhage. In Gen2 grafts, the jejunal serosal color remained homogeneously pink with minimal to no mesenteric edema (Figure 3). Furthermore, the jejunal luminal contents were considered normal in appearance, yellow and mucoid, and the mucosa appeared pink. In contrast, by T6h, Gen2 ileum serosal appearance displayed variable degrees of purple discoloration ranging from significant patchy areas to substantial confluent necrosis (Figure 3). There was also evidence of blood within the luminal contents in the ileum, and the ileal mucosa appeared hemorrhagic. In the Gen3 NMP allografts, the serosa of the jejunum and ileum remained homogeneously pink with no gross evidence of intestinal wall edema, mesenteric edema, or luminal or mucosal hemorrhage.
Impact of Dialysis on Perfusate Biochemistry During NMP
Lactate Clearance
Gen1 perfusate demonstrated a progressive rise in lactate levels to the upper limits of detection. However, in Gen2 and Gen3, the addition of continuous dialysis led to stable low lactate levels (Figure 4A).
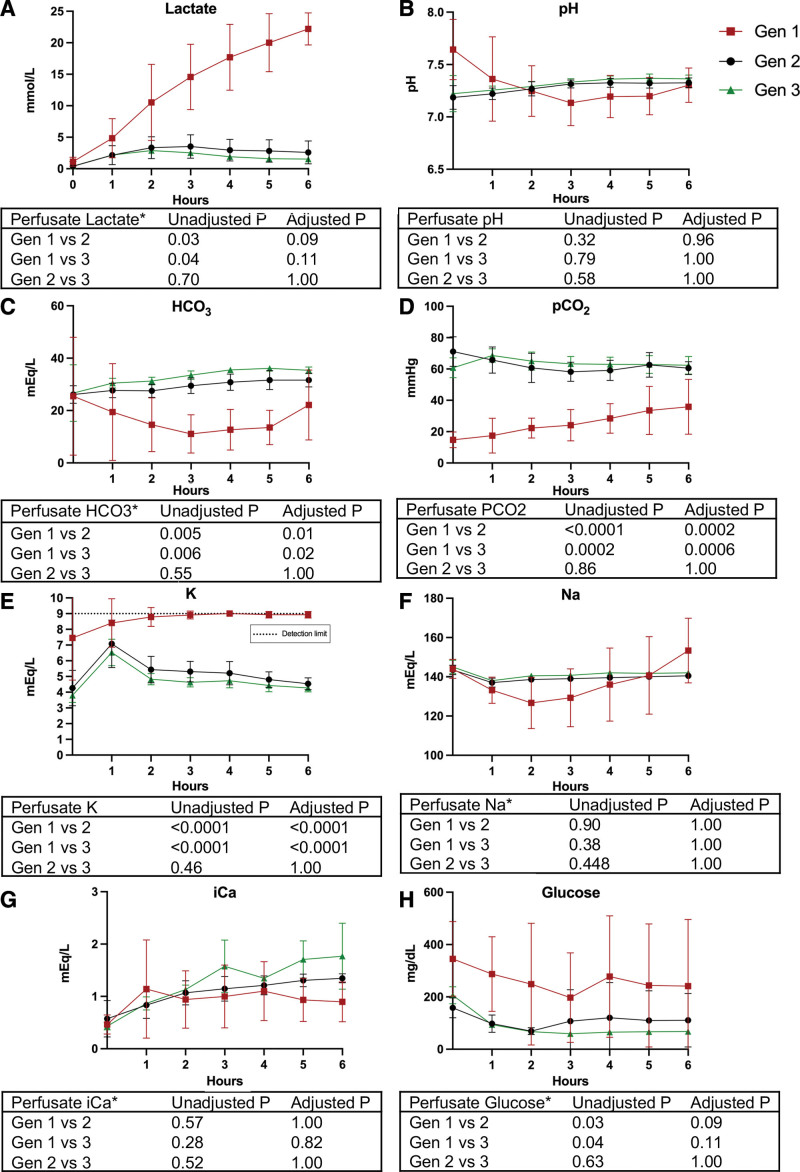
FIGURE 4.
Perfusate biochemistry values measured from cranial mesenteric artery inflow. A, Lactate concentration. B, pH. C, HCO3 concentration. D, Pco2. E, K concentration; detection limit of biochemistry assay is depicted by the dotted line at 9 mEq/L. F, Na concentration. G, iCa concentration. H, Glucose concentration. All values presented as mean ± SD. Gen1 (n = 8) is represented by red squares, Gen2 (n = 9) is represented by black circles, and Gen3 (n = 4) is represented by green triangles. *Analysis was conducted on log10 transformed data. Gen, generation; HCO3, bicarbonate; iCa, ionized calcium; K, potassium; Na, sodium.
Acid-base Balance
Gen1 grafts experienced a gradual decline in pH over the first 3 h despite intermittent supplementation of bicarbonate directly into the perfusate. Gen2 and Gen3 grafts demonstrated stable pH within a physiological range by 1–2 h of perfusion initiation (Figure 4B). Bicarbonate levels were statistically decreased in Gen1, despite bicarbonate supplementation, compared with Gen2 and Gen3 with no supplementation (Figure 4C). The Pco2 was also statistically decreased over the 6 h perfusion period in Gen1 compared with Gen2 and Gen3 (Figure 4D).
Electrolytes and Glucose
Perfusate electrolyte composition, most notably potassium and glucose levels, were maintained within physiologic levels in Gen2 and Gen3 in contrast to Gen1 (100 ± 20 mg/dL) (Figure 4E–H).
Perfusion Parameters
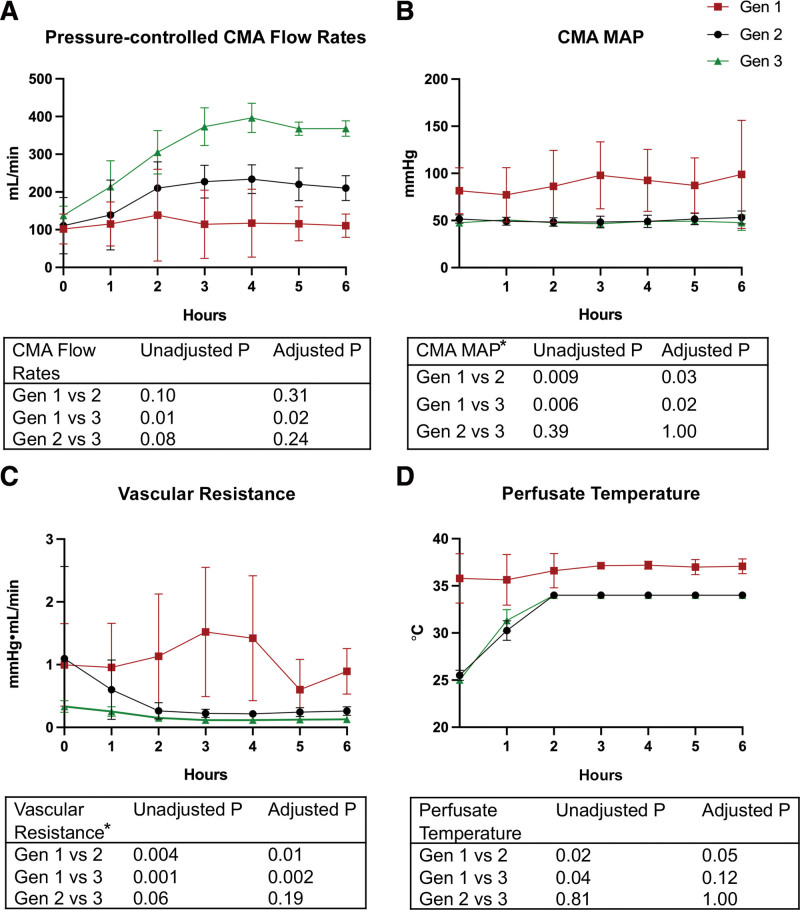
Each generation displayed iterative improvements in the pressure-controlled arterial flow rate of the perfusate through the graft (Figure 5A). Gen3 allografts demonstrated a twofold to threefold increase in pressure-controlled average arterial flow rates at T6h, compared with Gen2 and Gen1 (382 mL/min; 210 mL/min; 110 mL/min, P < 0.001, respectively) and the difference was significant between Gen1 and Gen3 (Figure 5A). The vascular resistance over the 6 h of machine preservation was significantly decreased in Gen3 and Gen2 compared with Gen1 (0.127, 0.260, 0.893 mm Hg/min/mL; P < 0.05) (Figure 5B and C).
FIGURE 5.
Arterial flow dynamics measured from CMA inflow. A, Measured CMA flow rates with manual control of perfusion pressure at 50 ± 5 mm Hg. B, MAP of the CMA. C, Vascular resistance. D, Perfusate temperature. All values presented as mean ± SD. Gen1 (n = 8) is represented by red squares, Gen2 (n = 9) is represented by black circles, and Gen3 (n = 4) is represented by green triangles. *Analysis was conducted on log10 transformed data. CMA, cranial mesenteric artery; Gen, generation; MAP, mean arterial pressure.
Synchronized Aboral Peristalsis
Upon rewarming, grafts resumed peristalsis when perfusate temperature reached 30 °C (Gen2 and 3; Figure 5D). During NMP with ileal cannulation (Gen2 and 3), we observed forward, synchronized intestinal motility, starting at the jejunum, with peristalsis pushing jejunal contents aboral resulting in ileal effluent (Video S1, SDC, http://links.lww.com/TXD/A460 and Video S2, SDC, http://links.lww.com/TXD/A461 peristalsis & ileal effluent).
Transplantation of the NMP Intestine Allografts
Five grafts were transplanted with postoperative recovery and despite some technical challenges with thrombosis in this porcine model, all 5 survived to the 48-h endpoint including 1 allograft from the Gen2 protocol, and 2 allograft recipients from the Gen3 protocol (see summary of postoperative information for all 5 transplanted grafts in Table S2, SDC, http://links.lww.com/TXD/A462). In the transplanted allograft from Gen2 NMP, there was segmental discoloration of a portion of the ileum after implantation and reperfusion and a partial ileal allograft resection was performed. In the Gen3 protocol, we noted improved gross appearance of the intestine grafts throughout the entirety of the graft after reperfusion and the bowel appeared healthy in both Gen3 viable grafts at the time of euthanasia.
Allograft Histology: Baseline and Post-NMP
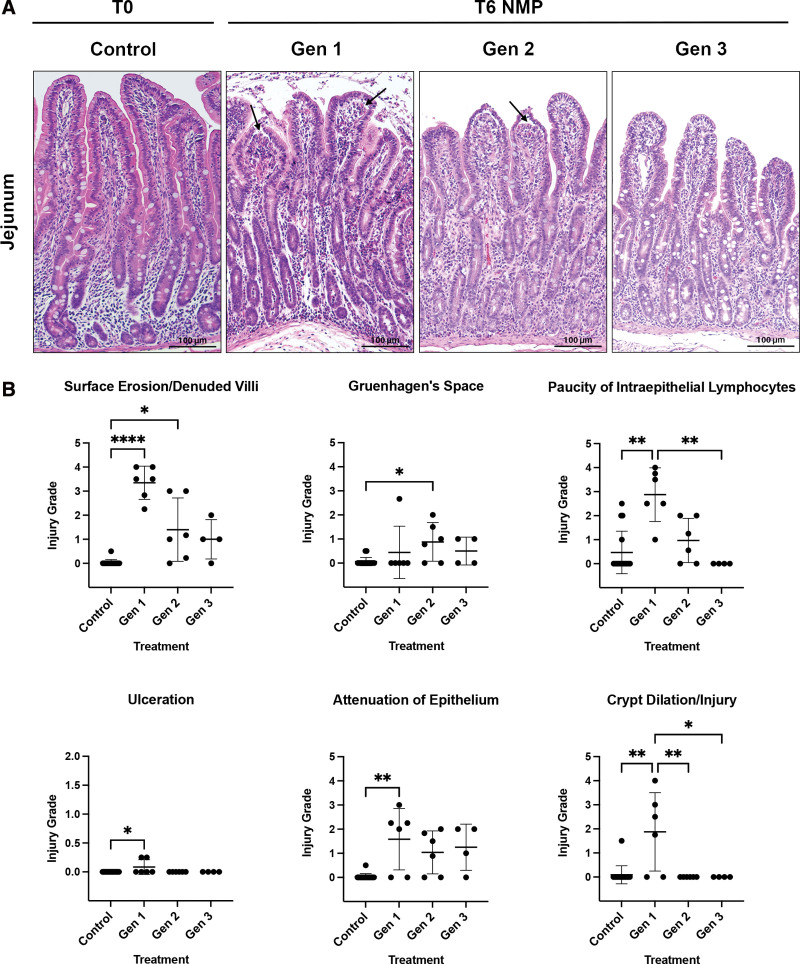
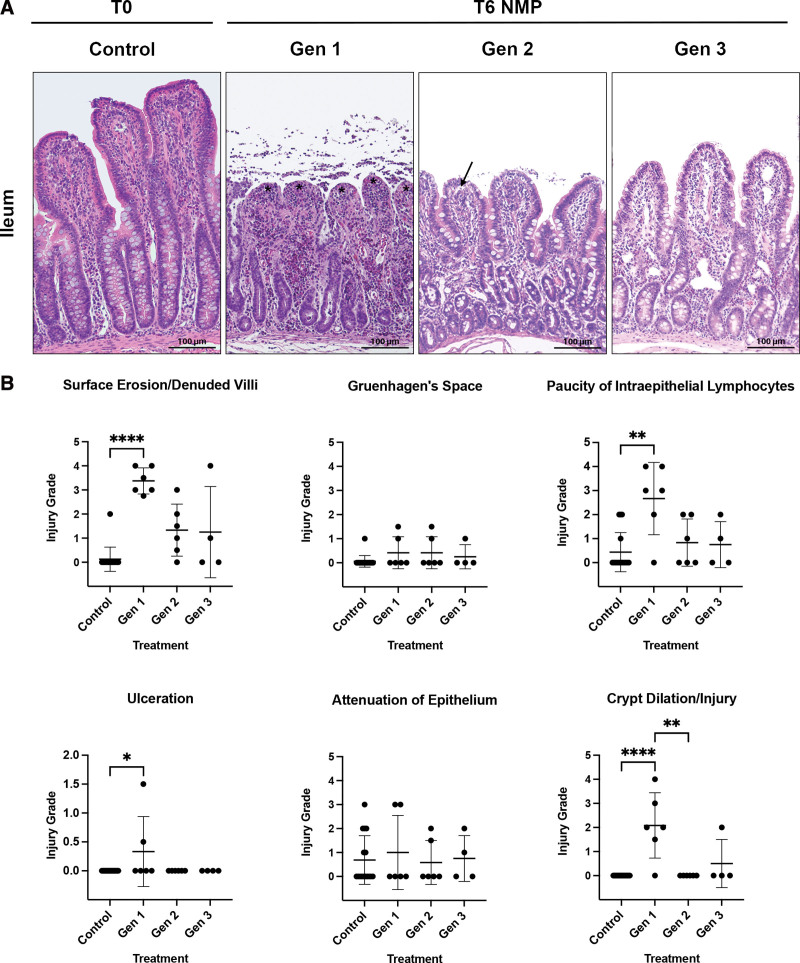
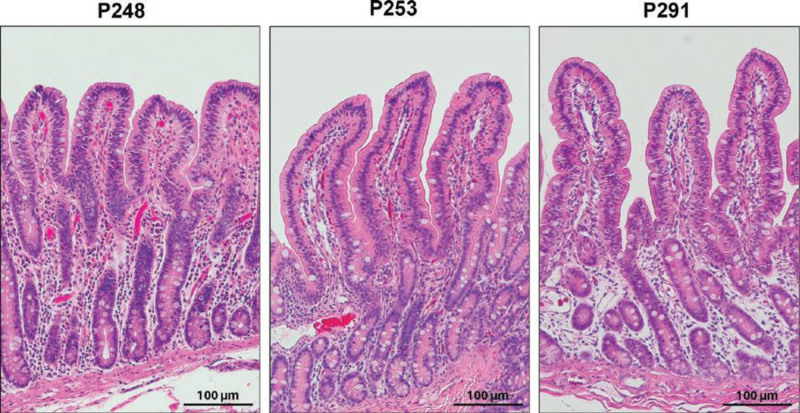
Tissues obtained at T0h demonstrated no obvious histologic injury in the jejunum and ileum (Figures 6A and 7A). After 6 h of NMP, Gen1 grafts demonstrated severe epithelial surface erosion/denuded villi of both the jejunum (Figure 6B) and ileum (Figure 7B). Although not as severe, increased surface erosion/villi denudation was also identified in Gen2 grafts compared with control (Figure 6B). Other evidence of epithelial injury in Gen1 jejunum was confirmed by a loss of intraepithelial lymphocytes, increased ulceration, attenuation of epithelium, and crypt dilation/injury (Figure 6B). In the ileum, evidence of epithelial injury in Gen1 was indicated by more pronounced loss of intraepithelial lymphocytes, increased ulceration, and crypt dilation/injury (Figure 7B). In Gen3, no histologic parameters of injury were statistically elevated compared with control in either the jejunum or the ileum (Figures 6A, 6B, 7A, and 7B). On postoperative day 2, at euthanasia, the histology of the allografts from the 3 viable intestines (Gen2, n = 1 and Gen3, n = 2) appeared healthy (Figure 8). No injury was noted in the muscular or serosal layers of any of the grafts.
FIGURE 6.
Histologic images and pathologic scoring of NMP-stored jejunum. A, Histologically, Gen1 and Gen2 jejunum had areas of surface erosion and denuded villi (indicated by black asterisks) and Gruenhagen’s space formation (black arrows). Sections for histology were stained with hematoxylin and eosin. Original magnification ×20, scale bar 100 μm. B, The scale of 0 to 4 was used to grade the extent of abnormalities observed within each category evaluated. (0 = normal; 1 = focal and mild; 2 = diffuse/multifocal and mild; 3 = diffuse and moderate; 4 = diffuse and severe; scores are reported as mean ± SD). After 6 h of NMP, severe epithelial surface erosion/denuded villi injury was identified in Gen1 jejunum compared with T0 control (Gen1, 3.35 ± 0.69; control 0.03 ± 0.12; P < 0.0001). Paucity of intraepithelial lymphocytes (Gen1, 2.88 ± 1.11; control, 0.47 ± 0.88; P = 0.001) was significantly more pronounced in Gen 1 than control tissue and Gen1 was significantly different than Gen3 grafts, which appeared more like controls. Gen1 grafts had significantly more villus ulceration (Gen1, 0.83 ± 0.13; control, 0.00 ± 0.00; P = 0.03) and attenuation of the epithelium (Gen 1, 1.58 ± 1.27; control, 0.03 ± 0.12; P = 0.009) than control jejunum. Gen1 grafts were also found to have significantly more crypt dilation/injury than control, Gen2, or Gen3 tissue (Gen1, 2.08 ± 1.36; control, 0.00 ± 0.00; P < 0.0001). (B, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001). Scale bar 100 μm. Gen, generation; NMP, normothermic machine perfusion; T0, before normothermic machine perfusion; T6, after 6 h of normothermic machine perfusion.
FIGURE 7.
Histologic images and pathologic scoring of NMP-stored ileum. A, Histologically, Gen1 and Gen2 ileum had areas of surface erosion and denuded villi (indicated by black asterisks) and Gruenhagen’s space formation (black arrows), whereas Gen3 grafts resembled controls. Sections for histology were stained with hematoxylin and eosin. Original magnification ×20, scale bar 100 μm. B, The scale of 0 to 4 was used to grade the extend of abnormalities observed within each category evaluated. (0 = normal; 1 = focal and mild; 2 = diffuse/multifocal and mild; 3 = diffuse and moderate; 4 = diffuse and severe; scores are reported as mean ± SD). After 6 h of NMP, Gen1 ileum demonstrated severe epithelial injury (Gen1, 3.38 ± 0.54; control, 0.12 ± 0.5; P < 0.0001). Gen 1 grafts also demonstrated significant paucity of intraepithelial lymphocytes (Gen1, 2.67 ± 1.51; control, 0.44 ± 0.81; P = 0.008) and villus ulceration (Gen1, 0.33 ± 0.60; control, 0.00 ± 0.00; P = 0.03) compared with control ileum. Significant crypt dilation/injury was present in both Gen1 (Gen1, 2.08 ± 1.36; control, 0.00 ± 0.00; P < 0.0001) and Gen2 grafts. (B, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001). Scale bar 100 μm. Gen, generation; NMP, normothermic machine perfusion; T0, before normothermic machine perfusion; T6, after 6 h of normothermic machine perfusion.
FIGURE 8.
Histologic images of 3 viable intestine allografts on postoperative day 2 after normothermic machine perfusion preservation and intestine transplantation. The histologic appearance of the graft mucosa from the 3 pigs with grossly normal appearing bowel at the time of euthanasia (postoperative day 2) was completely normal. See Table S2 (SDC, http://links.lww.com/TXD/A462) for detailed clinical outcome. Scale bar 100 μm.
Recipient Recovery and GI Function Following Transplantation of NMP Allograft
The recipient pigs average baseline ACTs were 112 s (range: 82–148 s) before surgery. Postoperatively, all recipients had essentially normal vital signs and normal lactate levels at 24 and 48 h. The average ACT was 138 s (range: 74–163 s). The pig that received the aspirin and heparin by constant rate infusion (P291) had a postoperative average ACT of 137 s (range: 113–160 s). The 3 pigs without thrombosis demonstrated normal alertness and responsiveness, ate normally, and passed normal stools during the 48 h after transplant until euthanasia. Thrombosis of the CMA and CMV occurred in 2 animals resulting in ischemic bowel identified at postmortem.
DISCUSSION
This study presents the first transplant-validated protocol for successful preservation of intestinal allografts by machine perfusion in a porcine model. Our goal in this study was to develop an optimized protocol for NMP preservation of the intestine suitable for rapid translation toward future clinical use. We describe here the protocol development, including the unexpected pitfalls encountered before we successfully reached the desired endpoint where full-length intestinal allografts maintained a healthy gross appearance during ex vivo machine perfusion, clinically appropriate hemodynamic perfusion parameters, normal physiologic biochemistries in the perfusate and both gross and histologic evidence of viable intestinal mucosa before allograft transplantation. In addition, we further demonstrate the clinical feasibility of the machine perfusion protocol described here for preservation of intestinal grafts with successful postoperative recipient recovery and normal short-term gut function after transplantation.
The initial intestine protocol (Gen1) was based on previously published machine perfusion studies including a human clinical trial of liver transplantation that showed evidence of improved graft function compared with SCS.16 Autologous blood was selected as the perfusate to avoid potential confounding effects of immune reactivity as well as serve as a source of red blood cells with an oxygen-carrying capacity expected to meet the metabolic needs of the full-length intestine allograft at normothermia. Finally, flow rates and pressures were selected to mimic normal porcine physiology. Despite this, there were several severe and unexpected challenges identified in ex vivo NMP of the full-length porcine intestine that differ from those previously reported of NMP of the liver allograft.16
In the Gen1 protocol, rapid and profound lactate accumulation, acidosis, hyperkalemia, and progressive anemia were observed despite what appeared to be pink viable intestine over the first 3 h of perfusion. The maintenance of a grossly normal intestinal appearance for a short-term (1 or 4 h) perfusion has been similarly described in a segment of intestine, although other metrics of intestinal health were not evaluated.18 In the present study, gross evidence of intestinal compromise including a dusky serosal appearance, patchy mucosal hemorrhage, and bloody luminal effluent were observed by 6 h of NMP, which suggested poor perfusion and intestinal compromise, later confirmed histologically. Although likely multifactorial, the acidosis and resultant hyperkalemia were largely attributed to the lactate accumulation. Lactate is the product of anaerobic metabolism, and although it is the only means for energy production in red blood cells, elevated levels are associated with cellular stress and hypoxia of intestinal tissues that depend predominantly on aerobic glycolysis to maintain cellular function. In health, the normal lactate levels in the blood remain low (<2.3 mmol/L) due to the balance of production and hepatic clearance. In this study we observed normal lactate levels at the initiation of perfusion that progressively increased to the upper limits detectable on our lactate meter (25 mmol/L). Other studies have noted elevated lactate levels (10 mmol/L) at the initiation of intestinal perfusion that climbed to >40 mmol/L,17; however, in a clinical hepatic NMP protocol, a lactate clearance to <2–2.5 mmol/L by 2 to 6 h of perfusion was used as a marker of allograft viability.23,24
Modifications in the Gen2 protocol were introduced to address biochemical derangements and inadequate perfusion and oxygen delivery. To address the biochemical derangements, we added continuous dialysis.15,20 The addition of HDF was associated with clearance of lactate to normal physiologic levels and resolution of abnormal potassium, bicarbonate, and pH levels. The improvement in metabolic parameters suggested that the absence of hepatic clearance accounts for some of the abnormalities initially observed. Furthermore, because the HDF was adjusted for volume based on the perfusate hematocrit, there was a stable hematocrit of 25% that likely contributed to resolution of mesenteric edema observed grossly.
The villus epithelial loss and crypt injury in histopathologic assessment of T6h tissues suggested some degree of ischemic injury and matched our clinical suspicion of hypoperfusion based on gross appearance. In addressing the hypoperfusion and inadequate oxygen delivery, modifications in perfusion pressure and flow rate were considered. In Gen1, a perfusion pressure of 75 mm Hg was initially selected to mimic in vivo MAPs, although other studies had described pressure ranges of 80 to 140 mm Hg with resultant flow rates of 40–400 mL/min.17-19 Since hypertensive perfusion pressures have been associated with mucosal injury including congested capillaries, hemorrhage, and ulceration, we sought to improve tissue perfusion through vasodilation, while maintaining normal physiologic mean blood pressure.17 Multiple vasodilatory drugs have been described for use including the originally selected drug epoprostenol. Verapamil was introduced in Gen2 in light of published kidney perfusion experiments demonstrating decreased vascular resistance with higher flow rates (from 100 mL/min to 200 mL/min on average) with lower perfusion pressures.25 Following these modifications in Gen2, increased flow rates and decreased vascular resistance, improved gross appearance and decreased histologic evidence of injury were achieved in the jejunum, however, a variable degree of abnormal dusky appearing ileal serosa and mucosal hemorrhage at T6h raised concerns of segmental intestinal tissue ischemia. Further modification of the vasodilatory regimen was introduced in Gen3 to combine epoprostenol, verapamil, and taurocholate. Taurocholate was added considering evidence of an ileum-specific vasodilatory effect of bile salts.26,27 The additional vasodilatory medications lowered vascular resistance, permitting the delivery of higher flows at a normal physiologic MAP. Further studies are necessary to discern the independent effects and optimal drug doses on jejunal and ileal vasculature and the degree to which perfusion parameters might be utilized in graft assessment. With this vasodilatory regimen, however, the Gen3 protocol was consistently associated with a bowel graft that looked healthy and transplantable. Finally, the ultimate metric for success, transplantation, was achieved.
Limitations of the study include the need to further verify reproducibility in a larger cohort. In addition, the study was designed with a focus on tissue preservation and prevention of IRI but not designed to assess allograft rejection. In fact, we did not perform swine leukocyte antigen or blood typing and made no attempt to match donors and recipients. A longer duration of posttransplant clinical observation of the recipient pigs is required for assessment of the impact of NMP on the occurrence of bacterial translocation and rejection that plague early clinical intestine transplantation. Although we performed a total of 5 transplants, 2 were technical failure with the development of arterial thrombosis, likely related to pigs naturally hypercoagulable state, although immunologic or technical causes of thrombosis cannot be excluded.28 Finally, further direct comparisons to intestine allografts transplanted after undergoing SCS preservation and with longer preservation times will be required to assess whether NMP decreases preservation injury and/or improves intestine allograft function over current standard cold storage preservation.
CONCLUSIONS
NMP is shown here as a technically feasible method for allograft preservation and transplantation of full-length intestine. In comparison to liver perfusion, intestinal NMP appears to require a more sophisticated approach that includes continuous dialysis support and additional vasodilator therapy due to the lack of intrinsic mechanisms for lactate clearance, lack of electrolyte and acid-base physiologic management and vascular shunting of blood preferentially to the jejunum.
Supplementary Material
Footnotes
N.A. and E.K.L. share first authorship; L.M.G. and D.L.S share senior authorship.
This work was supported by Department of Defense W81XWH-19-1-0676 W81XWH-19-1-0677 (L.M.G., D.L.S.); National Institutes of Health SERCA, K01OD01991-01A1 (L.M.G.); National Institutes of Health, K08AI150990 (A.S.B.); and National Institutes of Health, P30 DK034987.
The authors declare no conflicts of interest.
D.L.S., L.M.G., A.S.B., N.A., E.K.L., and K.S.G. conceived and planned the experiments. All authors performed the experiments and contributed to the interpretation of the results. N.A. drafted the article; all authors provided critical feedback and helped shape the research, analysis, and writing of the article.
D.N. provided guidance in planning and oversight in performance and critical review of the statistical analysis. D.M.C. performed histologic assessment and grading of bowel injury and produced representative images for figures.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Rege A, Sudan D. Intestinal transplantation. Best Pract Res Clin Gastroenterol. 2016;30:319–335. [DOI] [PubMed] [Google Scholar]
- 2.Oltean M, Jiga L, Hellström M, et al. A sequential assessment of the preservation injury in porcine intestines. J Surg Res. 2017;216:149–157. [DOI] [PubMed] [Google Scholar]
- 3.Grootjans J, Lenaerts K, Derikx JP, et al. Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model. Am J Pathol. 2010;176:2283–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez LM, Stewart AS, Freund J, et al. Preservation of reserve intestinal epithelial stem cells following severe ischemic injury. Am J Physiol Gastrointest Liver Physiol. 2019;316:G482–G494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roskott AM, Nieuwenhuijs VB, Dijkstra G, et al. Small bowel preservation for intestinal transplantation: a review. Transpl Int. 2011;24:107–131. [DOI] [PubMed] [Google Scholar]
- 6.Quaedackers JS, Beuk RJ, Bennet L, et al. An evaluation of methods for grading histologic injury following ischemia/reperfusion of the small bowel. Transplant Proc. 2000;32:1307–1310. [DOI] [PubMed] [Google Scholar]
- 7.Braun F, Hosseini M, Wieland E, et al. Expression of E-selectin and its transcripts during intestinal ischemia-reperfusion injury in pigs. Transplant Proc. 2004;36:265–266. [DOI] [PubMed] [Google Scholar]
- 8.Takeyoshi I, Zhang S, Nomoto M, et al. Mucosal damage and recovery of the intestine after prolonged preservation and transplantation in dogs. Transplantation. 2001;71:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyer K, Moon J. Adult intestinal transplantation in the United States. Curr Opin Organ Transplant. 2020;25:196–200. [DOI] [PubMed] [Google Scholar]
- 10.Horslen SP, Smith JM, Ahn Y, et al. OPTN/SRTR 2019 annual data report: intestine. Am J Transplant. 2021;21(Suppl 2):316–355. [DOI] [PubMed] [Google Scholar]
- 11.Kawai M, Kitade H, Koshiba T, et al. Intestinal ischemia reperfusion and lipopolysaccharide transform a tolerogenic signal into a sensitizing signal and trigger rejection. Transplantation. 2009;87:1464–1467. [DOI] [PubMed] [Google Scholar]
- 12.Cucchetti A, Siniscalchi A, Bagni A, et al. Bacterial translocation in adult small bowel transplantation. Transplant Proc. 2009;41:1325–1330. [DOI] [PubMed] [Google Scholar]
- 13.Horslen SP, Smith JM, Weaver T, et al. OPTN/SRTR 2020 annual data report: intestine. Am J Transplant. 2022;22(Suppl 2):310–349. [DOI] [PubMed] [Google Scholar]
- 14.Clavien PA, Dutkowski P, Mueller M, et al. Transplantation of a human liver following 3 days of ex situ normothermic preservation. Nat Biotechnol. [Online ahead of print. May 31, 2022]. doi:10.1038/s41587-022-01354-7 [DOI] [PubMed] [Google Scholar]
- 15.Eshmuminov D, Becker D, Bautista Borrego L, et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat Biotechnol. 2020;38:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasralla D, Coussios CC, Mergental H, et al. ; Consortium for Organ Preservation in Europe. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50–56. [DOI] [PubMed] [Google Scholar]
- 17.Braun F, Schütz E, Laabs S, et al. Development of a porcine small bowel ex vivo perfusion model. Transplant Proc. 1998;30:2613–2615. [DOI] [PubMed] [Google Scholar]
- 18.Hamed MO, Barlow AD, Dolezalova N, et al. Ex vivo normothermic perfusion of isolated segmental porcine bowel: a novel functional model of the small intestine. BJS Open. 2021;5:zrab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muñoz-Abraham AS, Patrón-Lozano R, Narayan RR, et al. Extracorporeal hypothermic perfusion device for intestinal graft preservation to decrease ischemic injury during transportation. J Gastrointest Surg. 2016;20:313–321. [DOI] [PubMed] [Google Scholar]
- 20.Hackbarth RM, Eding D, Gianoli Smith C, et al. Zero balance ultrafiltration (Z-BUF) in blood-primed CRRT circuits achieves electrolyte and acid-base homeostasis prior to patient connection. Pediatr Nephrol. 2005;20:1328–1333. [DOI] [PubMed] [Google Scholar]
- 21.Hoyer DP, Mathé Z, Gallinat A, et al. Controlled oxygenated rewarming of cold stored livers prior to transplantation: first clinical application of a new concept. Transplantation. 2016;100:147–152. [DOI] [PubMed] [Google Scholar]
- 22.Minor T, Efferz P, Fox M, et al. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am J Transplant. 2013;13:1450–1460. [DOI] [PubMed] [Google Scholar]
- 23.Mergental H, Laing RW, Kirkham AJ, et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat Commun. 2020;11:2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutton ME, op den Dries S, Karimian N, et al. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS One. 2014;9:e110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaths JM, Echeverri J, Chun YM, et al. Continuous normothermic ex vivo kidney perfusion improves graft function in donation after circulatory death pig kidney transplantation. Transplantation. 2017;101:754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matheson PJ, Wilson MA, Garrison RN. Regulation of intestinal blood flow. J Surg Res. 2000;93:182–196. [DOI] [PubMed] [Google Scholar]
- 27.Kvietys PR, McLendon JM, Granger DN. Postprandial intestinal hyperemia: role of bile salts in the ileum. Am J Physiol. 1981;241:G469–G477. [DOI] [PubMed] [Google Scholar]
- 28.Siller-Matula JM, Plasenzotti R, Spiel A, et al. Interspecies differences in coagulation profile. Thromb Haemost. 2008;100:397–404. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.