Abstract
In this study, network pharmacology and molecular docking technology were used to explore the molecular mechanisms of the Duhuo Jisheng decoction in the treatment of osteoarthritis (OA). The chemical composition of the prescriptions was obtained from the traditional Chinese medicine systems pharmacology database and analysis platform (TCMSP) database and the retrieved literature. Targets for the active ingredients were obtained using TCMSP and the Swiss Target Prediction Database. Disease targets were obtained from GeneCards and DisGeNET databases. The online tool, Venny, was used to obtain common targets for drugs and diseases. Protein-protein interactions (PPI) between common targets were analyzed using the search tool for the retrieval of interacting genes/proteins (STRING) database. Common targets were analyzed for gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment using the database for annotation, visualization and integrated discovery (DAVID) database. Molecular docking of the first 10 targets and first 10 components was verified using AutoDock Tools software, and the docking diagram was visualized using PyMOL software. After screening, 210 chemical components of the Duhuo Jisheng decoction (DHJSD) were identified. The 253 common targets of drugs and diseases were combined by eliminating repeat values. Based on PPI network analysis, the top ten targets were SRC, STAT3, MAPK3, MAPK1, RELA, PIK3R1, HSP90AA1, TP53, EP300, and AKT1. KEGG analysis showed that DHJSD could regulate the HIF-1, PI3K-Akt, and JAK-STAT signaling pathways. The biological processes involved include inflammatory reactions, the negative regulation of apoptosis, and the positive regulation of cell proliferation. Molecular docking results showed that all targets, except the RELA protein, showed good binding to the compounds, indicating that the 10 components might exert therapeutic effects by binding to the above targets. DHJSD can treat OA by regulating the HIF-1, PI3K-Akt, and JAK-STAT signaling pathways. The proteins involved were SRC, STAT3, MAPK3, MAPK1, and PIK3R1. In this study, network pharmacology was used to predict the mechanism of DHJSD in OA treatment, which was verified by molecular docking to provide experimental research ideas and scientific basis for OA treatment.
Keywords: Duhuo Jisheng decoction, molecular docking, molecular mechanism, network pharmacology, osteoarthritis
1. Introduction
Osteoarthritis is a chronic, progressive, and degenerative joint disease that is the most common joint disease in the clinic.[1] The main manifestations include joint swelling, pain, and progressive sexual dysfunction.[2] It mainly manifests as joint pain and stiffness, which seriously affects the health and life of patients. Epidemiological surveys have found that osteoarthritis (OA) has become the main cause of disability worldwide[3] and causing a heavy social and economic burden.[4] Scholars estimate that by 2030, 25% of the population in the United States will have osteoarthritis,[5] which may result in an economic burden of $10 billion to $20 billion.[6] In developing countries, owing to the aggravation of population aging and the impact of production and lifestyle, the number of patients with OA may increase in the future.[7,8] Therefore, prevention and treatment of osteoarthritis are imminent.
In the theory of traditional Chinese medicine, OA falls into the category of “mpediment syndrome,” which was first recorded in the classic Internal Classic of the Yellow Emperor (Huangdi’s Internal Classic) by pre-Qin physicians,[9] stating that the internal cause of OA was the weakness of the liver, spleen, and kidney. The external cause was the invasion of external wind and cold dampness. Therefore, it was proposed that preventive treatment should first strengthen body resistance, consolidate the essence, invigorate the spleen and kidney, and be supplemented by dispelling wind, cold, and dampness.[10] Currently, OA treatment mainly includes medications (analgesics, glucocorticoids, hyaluronic acid, and glucosamine), physical exercise, surgical treatment, and other means.[11] In fact, long-term administration of chemosynthetic drugs leads to adverse reactions and drug dependence.[12] Simultaneously, the effectiveness of the exercise was slow and insignificant. Surgical treatment may damage a patient’s body, mainly at certain risks.[13] Therefore, the use of Chinese medicines with obvious curative effects and fewer side effects has become a hot topic in current research.[14,15] Duhuo Jisheng decoction (DHJSD), a classical prescription for the treatment of knee OA created by Sun Simiao, a physician of the Tang Dynasty, has been widely used in China for more than 1400 years in China. It was recorded in “Bei Ji Qian Ji Yao Fang” (备急千金要方), which had the effects of dispelling cold and removing dampness, relieving joint pain and tonifying liver and kidney.[16,17] Pharmacological studies have shown that DHJSD has immune regulation,[18] anti-inflammatory, and analgesic effects,[19] improves microcirculation,[20] and dilates blood vessels.[21]
The prescription mainly comprises 15 traditional Chinese medicines: Radix Angelicae Pubescentis (Du Huo), Radix Saposhnikoviae (Fang Feng), Rhizoma Chuanxiong (Chuan Xiong), Radix Achyranthis Bidentatae (Niu Xi), Herba Taxilli (Sang Ji Sheng), Radix Gentianae Marcrophyllae (Qin Jiao), Eucommiae Cortex (Du Zhong), Radix Angelicae Sinensis (Dang Gui), Poria (Fu Ling), Codonopsis Radix (Dang Shen), Radix Rehmanniae Preparata (Shu Di Huang), Radix Paeoniae Alba (Bai Shao), Herba Asari (Xi Xin), Cortex cinnamomi (Rou Gui), and Glycyrrhizae Radix (Gan Cao). Radix Angelicae Pubescentis is taken as a monarch drug in prescriptions and has the effects of expelling wind, removing dampness, dredging impediments, and relieving pain. Herba asari, Radix saposhnikoviae, Radix gentianae marcrophyllae, and Cortex cinnamomi are examples of ministerial medicine. Herba Asari can dispel cold winds from the yin meridian to achieve analgesia. Radix Saposhnikoviae can dispel the wind, remove dampness, and relieve pain. Radix Gentianae Marcrophyllae relaxes tendons, activates collaterals, and protects joints. Cortex Cinnamomi warms the meridian and activates the collaterals to promote blood circulation in the joint. Herba Taxilli, Eucommiae Cortex, and Radix Achyranthis Bidentatae can be used as adjuvants to nourish the liver and kidneys, and strengthen bones and muscles. Rhizoma Chuanxiong, Radix Angelicae Sinensis, Radix Rehmanniae Preparata, and Radix Paeoniae Alba can replenish blood, fully nourish the viscera and tissues, and restore the function of the viscera and tissues. Poria, Codonopsis Radix, and Glycyrrhizae Radix can invigorate the spleen and replenish qi.[22] The synergistic effect of “Jun-Chen-Zuo-Shi”[23] has confirmed the efficacy of DHJSD in OA treatment. Network pharmacology is an emerging discipline based on systems biology, pharmaceutical chemistry, pharmacology, and biochemistry.[24] The principle is to explore the mechanism of action of drugs in diseases from the perspective of drug molecules, proteins, genes, and other aspects, by establishing biological networks of active drug molecules, key targets, and diseases. Molecular docking is a theoretical simulation method that predicts the binding mode and affinity of drugs and proteins based on the recognition relationship between the key and the lock.[25] Network pharmacology and molecular docking have recently become important virtual computer technologies for drug development and verification.
In clinical applications, DHJSD is commonly prescribed for OA and its curative effect is stable and definite. Studies have shown that compared with the control group, DHJSD has statistically significant advantages in treating OA.[26–28] However, to date, experiments on the basic substances and mechanisms of DHJSD have not been sufficiently thorough and comprehensive. Therefore, this study is based on network pharmacology and molecular docking, looking for the material basis of DHJSD’s curative effect, providing theoretical support for the treatment of OA with traditional Chinese medicine at the molecular level, and providing a new foundation for the secondary development of famous prescriptions and the fine treatment of OA. Figure 1 illustrates the concepts of network pharmacology and molecular docking.
Figure 1.
Graphic abstract diagram of DHJSD against OA. DHJSD = Duhuo Jisheng decoction, OA = osteoarthritis.
2. Materials and Methods
2.1. Collection of chemical ingredients in DHJSD
With the help of traditional Chinese medicine systematic pharmacology analysis platform database (TCMSP) (http://tcmspw.com/tcmsp.php),[29] we listed the keywords of “Duhuo”, “Sang Jisheng”, “Duzhong”, “Niuxi”, “Xixin”, “Qinjiao”, “Fuling”, “Rougui”, “Fangfeng”, “Chuanxiong”, “Renshen”, “Gancao”, “Danggui”, “Baishao”, “Shu Dihuang” to search for the active compounds of DHJSD. According to the ADME theory, oral bioavailability (OB) refers to the rate and degree of absorption of the active ingredient or active group of an oral drug into systemic circulation.[30] A higher OB value generally indicates better drug-like properties (DL) for biologically active drug molecules. Based on the literature, eligible chemical components were screened as active components with OB ≥ 30% and DL ≥ 0.18.[31]
2.2. Acquisition of action targets of DHJSD during OA treatment and OA targets
The chemical structures of the components were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/), and the active components selected from DHJSD were searched using the TCMSP and Swiss Target Prediction (http://www.swisstargetprediction.ch/) databases to identify the corresponding targets. The UniProt database (https://www.uniprot.org/) was used to query gene names corresponding to the protein names. The genes related to OA were searched in GeneCards database (https://www.genecards.org/) and DisGeNET database (https://www.disgenet.org/) with the keyword “osteoarthritis”.
2.3. Identification for DHJSD-OA common targets
Drug and disease targets were imported into the Venn website (http://bioinformatics.psb.ugent.be/webtools/Venn/) for visual analysis, and their common targets were obtained by establishing a Venn diagram.
2.4. Protein-protein interaction (PPI) analysis
The construction of PPI networks can predict the core proteins in the interaction network. Common targets for DHJSD treatment of OA were uploaded to the protein-protein interaction network analysis search tool for the retrieval of interacting genes/proteins (STRING) database (https://string-db.org/). The species was limited to Homo sapiens, with the minimum required interaction score set to the “highest confidence of 0.9”. Simultaneously, the disconnected nodes in the network were hidden to obtain a PPI network between common targets. In the network, the targets are represented as nodes, and connectors represent the interactions between the targets. Cytoscape software (version Cytoscape 3.7.2) was used to visualize the PPI network and analyze network topology characteristics. Based on the three important topological parameters of degree, betweenness centrality (BC), and closeness centrality (CC), we obtained ten core targets of DHJSD for the treatment of OA. Common and core targets could explain the molecular mechanism of DHJSD in the treatment of OA from the perspective of targets.
2.5. Gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analysis
To further explore the role of the potential target genes of DHJSD in OA treatment and their role in signaling pathways, the obtained common target genes were imported into the database for annotation, visualization, and integrated discovery (DAVID) database (https://david.ncifcrf.gov/). The species was limited to Homo sapiens (P < .05). GO and KEGG pathway enrichment analyses were performed. Pathways with significant meanings were screened and sequenced according to the number of enrichment targets, to identify more advanced and significant pathways. GO enrichment analyses were visualized as second-order categorical histograms using the bioinformatics cloud platform (http://www.bioinformatics.com.cn/) and KEGG pathway analyses were visualized as bubble charts using the ImageGP cloud platform (http://www.ehbio.com/ImageGP/).
2.6. Construction of the “drugs-ingredients-targets-disease-pathways” network
Data on drugs, active ingredients, targets, and pathways were imported into Cytoscape 3.7.2, to construct a visual network. The degree, BC, and CC were analyzed using a network analyzer function. The above parameters were taken as the criteria for evaluating the importance of nodes in the network, and the drug-active ingredient-target-disease-pathway interaction network diagram was constructed.
2.7. Molecular docking
The top 10 targets were screened based on PPI results. Ten active ingredients were selected according to the network diagram described in Section 2.6. To evaluate the reliability of the network analysis and prediction, we used AutoDock Tools software to perform molecular docking between the main active components and core targets. First, ligands and receptors were prepared. The 2D structures were downloaded from the PubChem database and the energy of the structures was minimized using Chembio3D Ultra 14.0. And then converted them to “PDB” format file. The protein crystal structures were obtained from the PDB database (http://www.rcsb.org/). The screening principles were as follows: The scientific name of the source organism was Homo sapiens. X-ray diffraction (XRD) was used as an experimental method. The refinement resolution was less than 3 Å. Ligands and non-protein molecules were deleted using PyMOL software. AutoDock Tools was used to add hydrogen, remove water molecules, and add charges. Second, a grid file was generated by considering the original ligand as the center, and docking parameters were set. The binding energy after docking was less than 0 kcal/mol, indicating that the receptor and ligand could bind spontaneously. The binding energy was less than or equal to –5.0 kcal/mol, indicating that the 2 had good binding. The PyMOL software was used to visualize the results of the three-dimensional images and conduct a clustering heat map analysis.
3. Results
3.1. Potential active ingredients of DHJSD
The 15 drugs in the prescription were separately inputted through the retrieval of the TCMSP database, and more than 1900 components were obtained. According to a literature search, 196 components were screened with OB ≥ 30% and DL ≥ 0.18 as the index. Since the “Rougui” didn’t own the components met the requirements in the database. The index components of “Rougui” stipulated in the Chinese Pharmacopoeia were included in the network. Through a literature review, we found that osthol, gentiopicroside, and loganic acid can effectively treat OA and that osthol and gentiopicroside are the index components of DHJSD stipulated in the Chinese Pharmacopoeia. However, because these 3 values did not meet the OB and DL values, they were not used; therefore, we also combined them. Basic information on the active compounds commonly found in DHJSD is presented in Table 1. All the search strategies for the databases are listed in Tables S1–S7, http://links.lww.com/MD/H542: http://links.lww.com/MD/H543: http://links.lww.com/MD/H544: http://links.lww.com/MD/H545: http://links.lww.com/MD/H546: http://links.lww.com/MD/H547: http://links.lww.com/MD/H548.
Table 1.
Basic information of common active compounds in DHJSD.
| Molecule ID | Number | Molecule name | Source | OB (%) | DL |
|---|---|---|---|---|---|
| MOL001941 | A | Ammidin | Radix Angelicae Pubescentis (Du Huo), Radix Saposhnikoviae (Fang Feng) | 34.55 | 0.22 |
| MOL000358 | B | Beta-sitosterol | Du Huo, Fang Feng, Gentianae Marcrophyllae (Qin Jiao), Radix Achyranthis Bidentatae (Niu Xi), Eucommiae Cortex (Du Zhong), Radix Paeoniae Alba (Bai Shao), Radix Angelicae Sinensis (Dang Gui) | 36.91 | 0.75 |
| MOL001942 | C | Isoimperatorin | Du Huo, Fang Feng | 45.46 | 0.23 |
| MOL000098 | D | Quercetin | Niu Xi, Du Zhong, Herba Taxilli (Sang Ji Sheng), Glycyrrhrizae Radix (Gan Cao) | 46.43 | 0.28 |
| MOL000359 | E | Sitosterol | Sang Ji Sheng, Gan Cao, Fang Feng, Qin Jiao, Bai Shao, Rhizoma Chuanxiong (Chuan Xiong) | 36.91 | 0.75 |
| MOL000173 | F | Wogonin | Fang Feng, Niu Xi, | 30.68 | 0.23 |
| MOL000422 | G | Kaempferol | Herba Asari (Xi Xin), Niu Xi, Bai Shao, Du Zhong, Gan Cao | 41.88 | 0.24 |
| MOL001006 | H | Chondrillasterol | Niu Xi, Dang Shen | 42.98 | 0.76 |
| MOL004355 | I | Spinasterol | Niu Xi, Dang Shen | 42.98 | 0.76 |
| MOL012537 | J | Spinoside A | Niu Xi, Dang Shen | 41.75 | 0.40 |
| MOL000449 | K | Stigmasterol | Niu Xi, Codonopsis Radix (Dang Shen), Dang Gui | 43.83 | 0.76 |
| MOL001494 | L | Mandenol | Fang Feng, Chuan Xiong | 42.00 | 0.19 |
| MOL002140 | M | Perlolyrine | Chuan Xiong, Dang Shen | 65.95 | 0.27 |
| MOL000211 | N | Mairin | Bai Shao, Du Zhong, Gan Cao | 55.38 | 0.78 |
| MOL003896 | O | 7-Methoxy-2-methyl isoflavone | Gan Cao, Dang Shen | 42.56 | 0.20 |
| MOL007059 | P | 3-Beta-hydroxymethyllenetanshiquinone | Dang Shen, Du Zhong | 32.16 | 0.41 |
| MOL001944 | Q | Marmesin | Du Huo, Fang Feng | 84.77 | 0.18 |
DHJSD = Duhuo Jisheng decoction, OB = oral bioavailability.
3.2. Acquisition of the main targets of DHJSD and OA
Target prediction was performed for the selected compounds according to the TCMSP and Swiss Target Prediction databases and compounds without targets were deleted. In total, 210 active compounds and their related targets were identified in this study. After sorting and retaining unique terms, 1137 drug targets were obtained, including ADRB2, PIK3CG, CA2, HSD11B1, MMP1, MET, PTGS1, and F2. The results showed that different Chinese medicines had not only a single compound interacting with multiple targets but also different compounds simultaneously acting on the same targets. This indicates that the therapeutic effect of Chinese medicine has the characteristics of multi-component and multi-target combinations, which is consistent with the diversity and complexity of the compatibility of Chinese medicine. A total of 3096 OA disease genes were retrieved from the GeneCards database and 867 genes were screened, with a correlation score greater than an average of 2.59. A total of 1827 disease genes were collected from the DisGeNET database, and 379 genes were screened, with a score >0.04. After the repetitive values were deleted, 1033 related genes were identified.
3.3. Acquisition of DHJSD and OA common targets
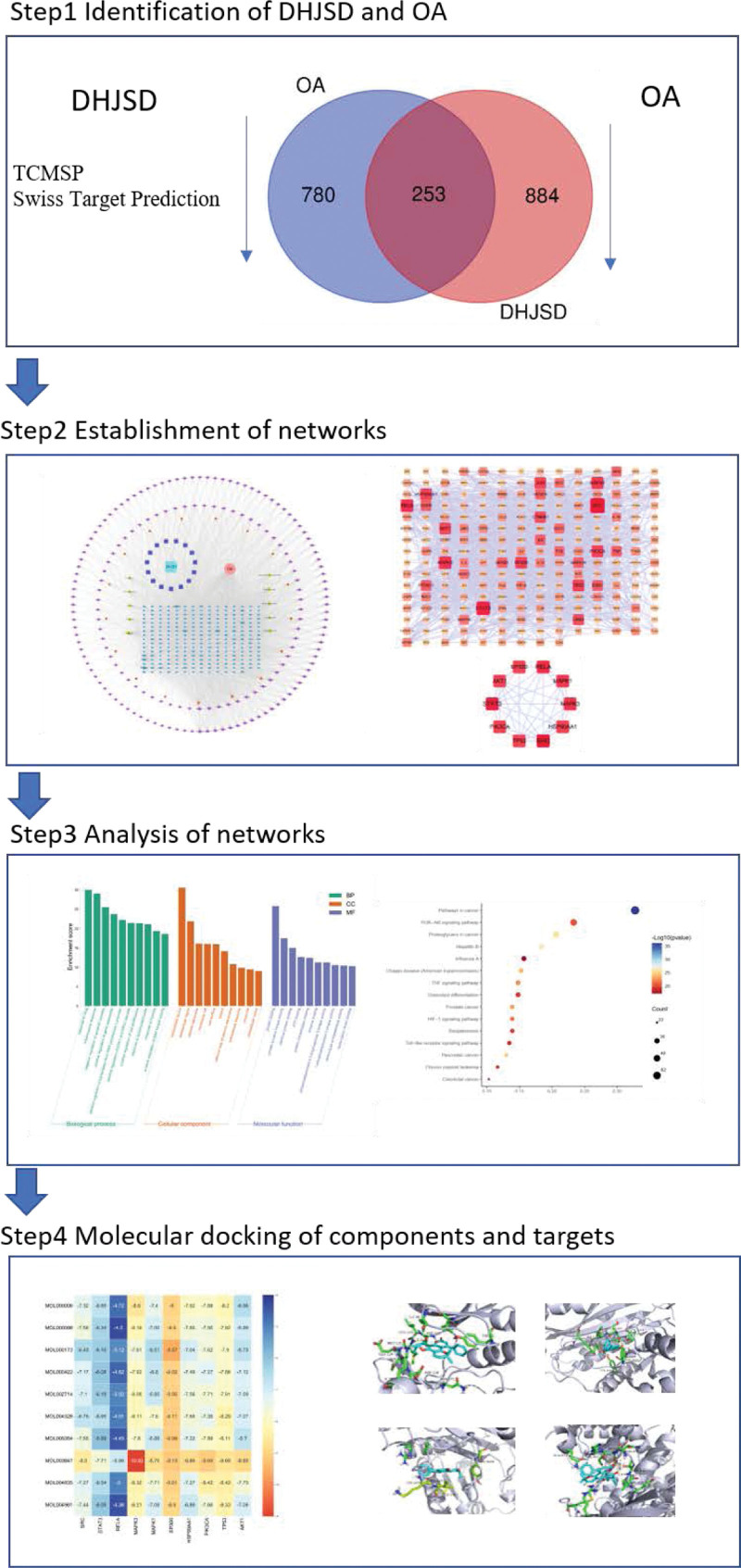
Using Venny online platform to draw Venny diagram, the regulatory targets of active pharmaceutical components were mapped and intersected with the potential targets of OA, and a total of 253 common targets were obtained, which were the potential targets of DHJSD for the treatment of OA, including PTGS2, ESR1, NOS2, ESR2, HSP90AA1 and other targets. The results are shown in Figure 2.
Figure 2.
Venn diagram of DHJSD targets and OA targets. DHJSD = Duhuo Jisheng decoction, OA = osteoarthritis.
3.4. PPI network construction
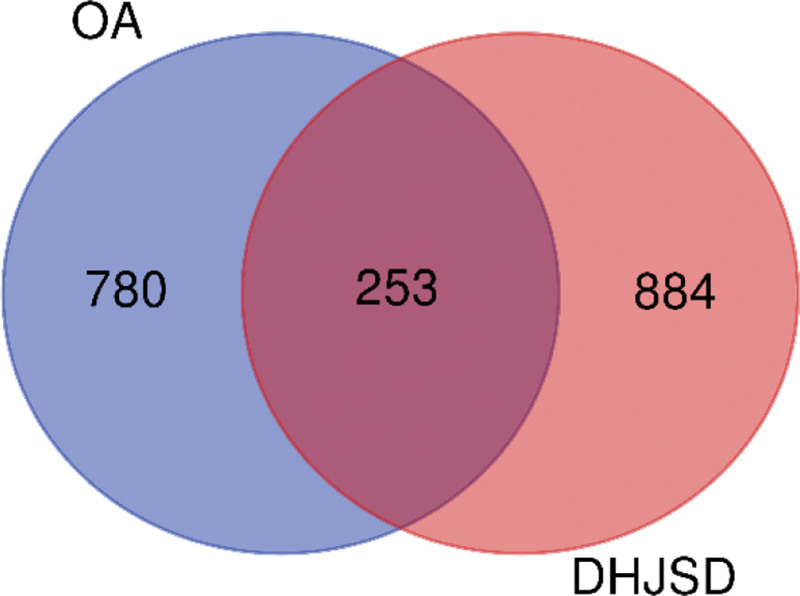
The PPI network of common targets of DHJSD and diseases is shown in Figure 3. In total, 253 effective targets of DHJSD for OA treatment were imported into the STRING database. The results were imported into Cytoscape 3.7.2 software to construct a protein-protein interaction network diagram. Figure 3(a) shows 219 nodes (34 proteins not involved in network construction) and 1272 edges. The larger the degree, BC, and CC values of a target in the network, the higher the level and importance of the target associated with other targets in the network, the larger its shape, and the redder its color. In contrast, it was smaller and shallower. The mean protein degree was 10.05, with 93 key target values being greater than the mean. The top 10 key targets in the network were screened out according to the degree values, as shown in Figure 3(b), including SRC, STAT3, MAPK3, MAPK1, RELA, PIK3R1, HSP90AA1, TP53, and EP300 (Figure 3(b)).
Figure 3.
PPI network diagram of target proteins. PPI = protein-protein interaction.
3.5. GO and KEGG pathway enrichment analysis of targets
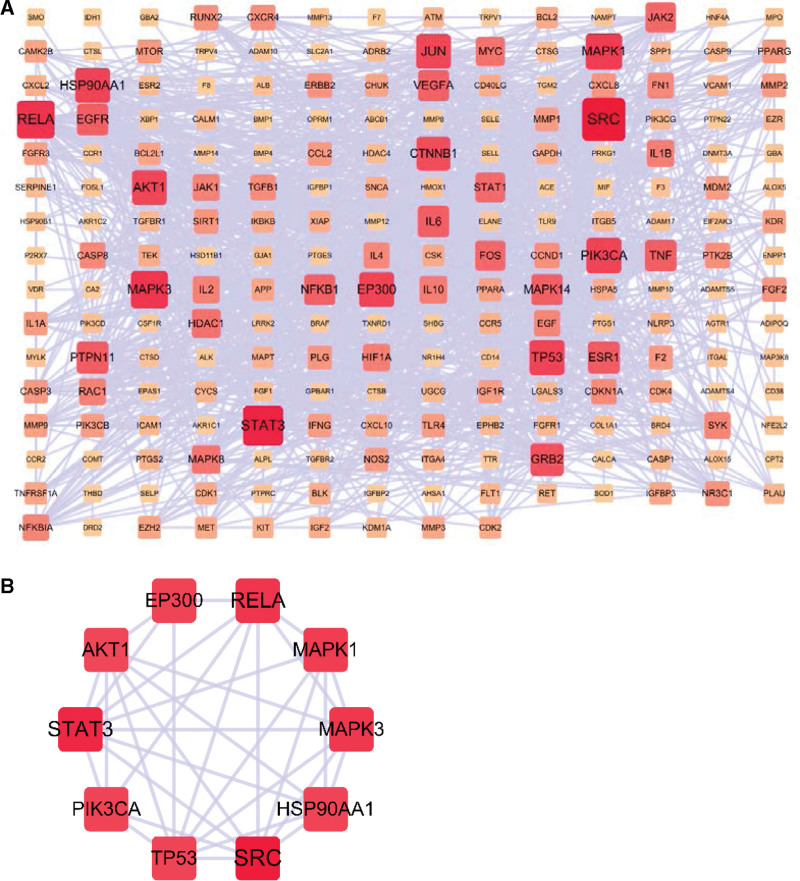
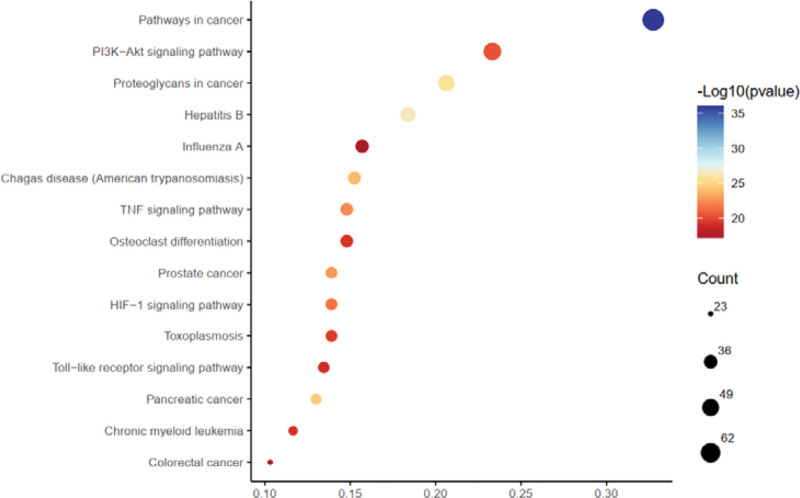
GO enrichment analysis and KEGG pathway analysis were performed on key targets using DAVID database. GO enrichment analysis included cellular component (CC), biological process (BP), and molecular function (MF). A total of 1259 GO entries and 127 KEGG entries were obtained (P < .05). The first 10 GO items enriched in the genes were selected for visualization using a classification histogram, as shown in Figure 4. The BP aspects were enriched in 903 entries. It is mainly enriched in response to drugs, inflammatory responses, negative regulation of apoptotic processes, positive regulation of gene expression, positive regulation of transcription from RNA polymerase II, positive regulation of cell proliferation, positive regulation of the ERK1 and ERK2 cascade, response to lipopolysaccharide, positive regulation of MAP kinase activity, and positive regulation of cell migration. CC was enriched with 92 entries. It was mainly enriched in the extracellular space, extracellular region, plasma membrane, membrane raft, cell surface cytosol, external side of the plasma membrane, extracellular exosomes, extracellular matrix, and lysosomes. MF aspect was enriched in 264 entries. It was mainly enriched for protein binding, protein kinase activity, identical protein binding, protein phosphatase binding, ATP binding, phosphatidylinositol-4,5-bisphosphate 3-kinase activity, enzyme binding, 1-phosphatidylinositol-3-kinase activity, serine-type endopeptidase activity, and transcription factor binding. The KEGG pathways were enriched for 127 entries. The first 15 gene enrichment pathways were selected to draw the corresponding point bubbles, as shown in Figure 5. The top 10 entries according to P values were primarily related to pathways in cancer, proteoglycans in cancer, hepatitis B, prostate cancer, pancreatic cancer, Chagas disease (American trypanosomiasis), HIF-1 signaling pathway, PI3K-Akt signaling pathway, TNF signaling pathway, and toxoplasmosis.
Figure 4.
The top 10 GO enrichment analysis of DHJSD targets in the OA. DHJSD = Duhuo Jisheng decoction, GO = gene ontology, OA = osteoarthritis.
Figure 5.
The top 15 KEGG pathway enrichment analysis of DHJSD targets the treatment of OA. DHJSD = Duhuo Jisheng decoction, KEGG = Kyoto encyclopedia of genes and genomes, OA = osteoarthritis.
3.6. Construction and analysis of the “drugs-ingredients-targets-disease-pathways” network
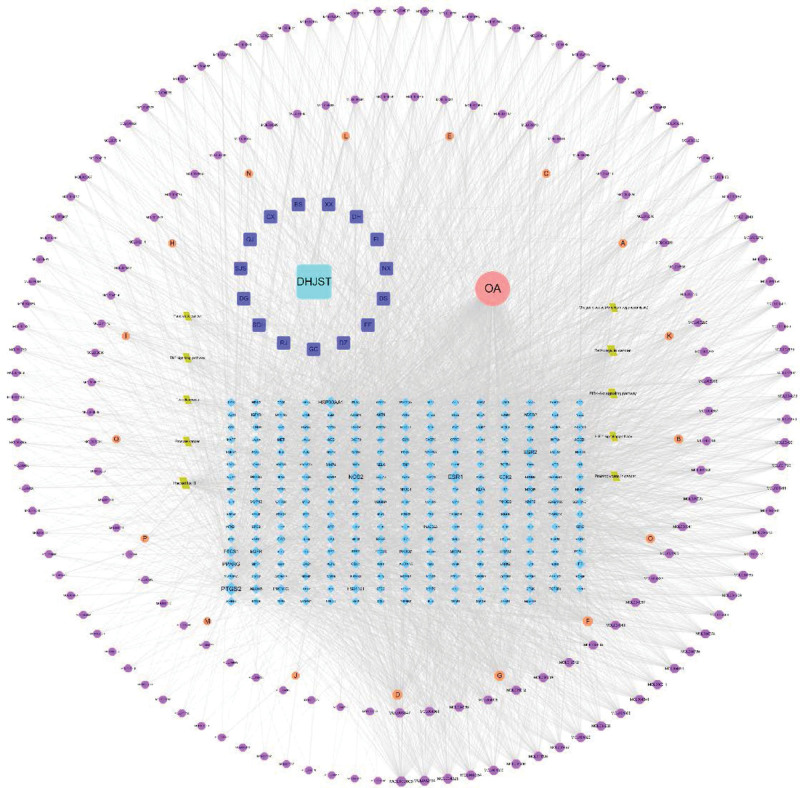
The drug-active compound-target-disease-pathway network in DHJSD was constructed using Cytoscape 3.7.2 software, as shown in Figure 6. The network consisted of 466 nodes, including 15 drug nodes, 186 active compound nodes, 253 target nodes, and 10 KEGG signaling pathways, with 4630 edges. Each edge of the network represents the active compounds contained in the drug and interactions between the active compounds and targets. The degree value of one node indicated the number of connections between the network node and other nodes, and the topological properties of the network were analyzed by means of “Network analyze” function in Cytoscape 3.7.2 software. Among these, degree and BC are 2 important parameters that measure the node key in the network.
Figure 6.
The drug-active compound-target-disease-pathway network in DHJSD. DHJSD = Duhuo Jisheng decoction.
Each active compound interacted with an average of 19 targets and each target interacted with an average of 14 active compounds in the network. Therefore, there was a phenomenon that 1 active compound interacts with multiple targets, and multiple active compounds act on the same target at the same time, reflecting the characteristics of traditional Chinese medicine to play an overall regulatory role. Among these, 89 were found to bind to more than 20 targets. Through the degree value analysis of the compounds, the first 10 active compounds were found to be MOL000006-luteolin, MOL000098-quercetin, MOL000173-wogonin, MOL000422-kaempferol, MOL002714-baicalein, MOL004328-naringenin, MOL000354-isorhamnetin, MOL003847-InophyllumE, MOL004835-Glypallichalcone, Mol004961-Quercetin 3,3′-dimethyl ether. They interacted with 61, 56, 55, 55, 47, 46, 43, 42, 41, and 41 targets.
3.7. Validation of molecular docking
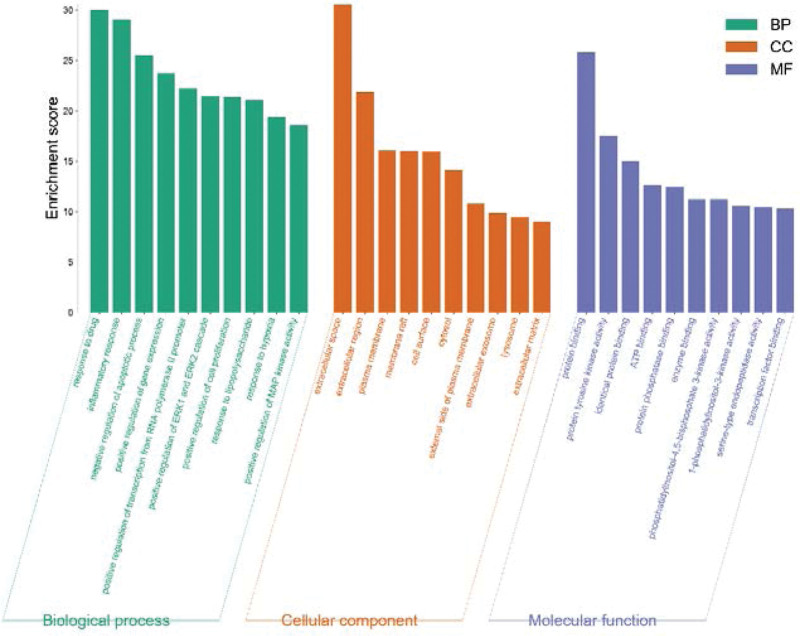
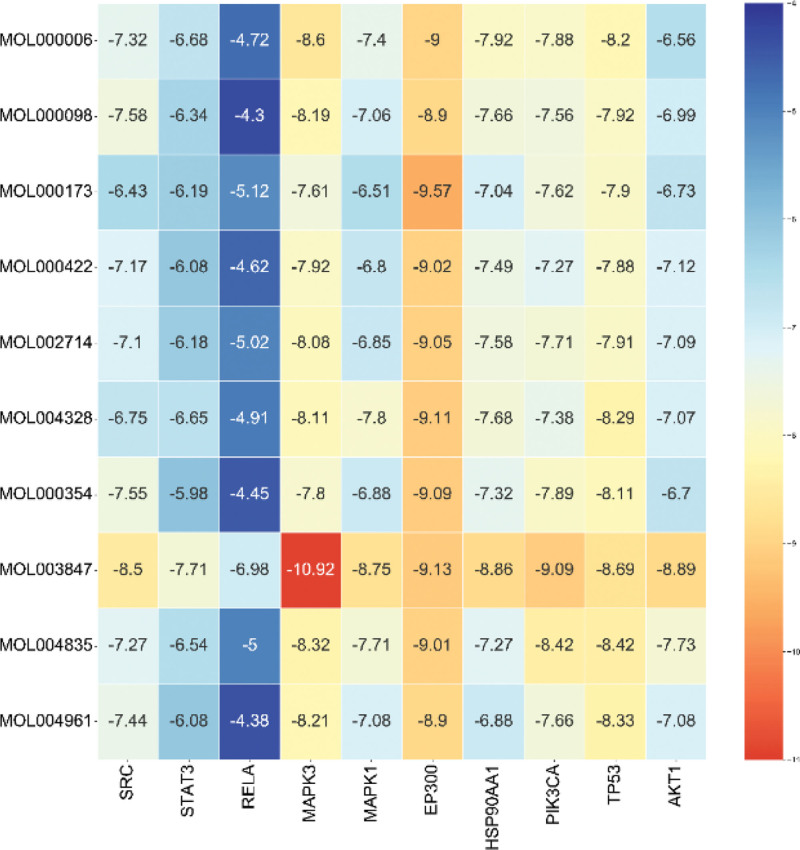
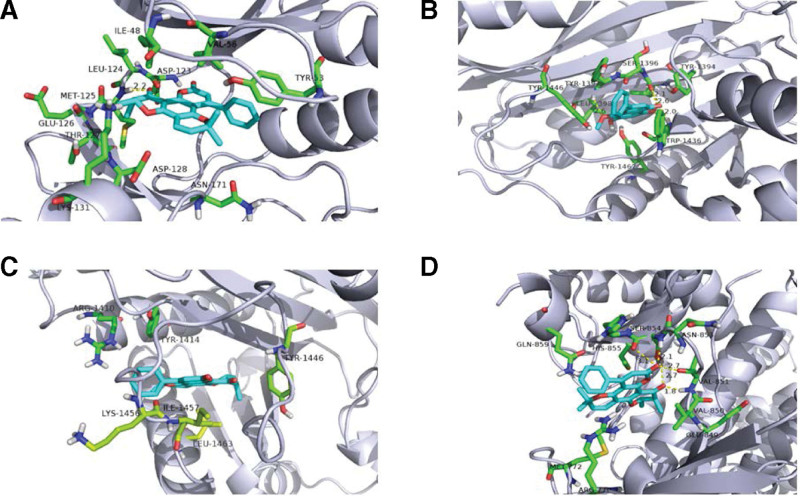
The 10 highest-degree active ingredients in the network diagram were docked with core proteins (SRC, STAT3, MAPK3, MAPK1, RELA, PIK3R1, HSP90AA1, TP53, EP300, and AKT1.) via the Autodock software. The 2D chemical structures were obtained from the PubChem database and the target structures were obtained from the PDB database. The hydrogen atoms and ligands were removed using the PyMOL software. It was then imported into AutoDock for the docking simulations. The lower the binding energy of the ligand and receptor, the more stable is the binding. The best binding energies were inophylum-MAPK3 (–10.92 kcal mol–1), wogonin-EP300 (–9.57 kcal mol–1), inophylum-EP300 (–9.13 kcal mol–1) and inophylum-PIK3R1 (–9.09 kcal mol–1). Except for the RELA protein, the other compounds could bind well to the target protein, and some compounds showed good binding ability. Docking results indicated that the active components of DHJSD exerted a therapeutic effect by binding to the targets. The docking scores of the targets and compounds were visualized using a heatmap (Fig. 7), and the 4 best combinations were visualized using PyMOL (Fig. 8).
Figure 7.
Heatmap of the docking scores of the top 10 key compounds and the key targets.
Figure 8.
Molecular docking results of “bioactive compound-hub gene”.
4. Discussion
OA is a proliferative disease characterized by articular cartilage degeneration, joint margins, and subchondral bone hyperplasia caused by aging, obesity, strain, trauma, congenital malformations, deformation, and other factors. It is one of the main causes of disabilities in adults. Currently, the main clinical drugs used for the treatment of OA are anti-inflammatory drugs, glucocorticoids, hyaluronic acid, glucosamine, and chondroitin sulfate. Compared with the single effects of chemical drugs, traditional Chinese medicine has the characteristics of multiple components and targets. It can simultaneously achieve comprehensive effects of relieving pain, diminishing inflammation, repairing damaged parts, and supplementing human nutrition. However, synthetic drugs can cause side effects after entering the human body in large amounts for a long time and can easily rebound and relapse when used for treating diseases. Traditional Chinese prescriptions can not only ensure a curative effect but also exert the advantages of small side effects and improved clinical immunity. DHJSD, which removes cold and dampness, relieves joint pain, and enhances liver and kidney function, is a classic prescription for the clinical treatment of OA. Although it has prominent clinical efficacy, it is difficult to determine the active substances and their mechanisms of action because there are as many as 15 medicinal materials and hundreds of components. Therefore, in this study, network pharmacology and molecular docking were used to clarify the mechanism of DHJSD in the treatment of OA, provide a basis for future research directions, and provide theoretical support for the treatment of OA using traditional Chinese medicine at the molecular level.
The results showed that DHJSD exerted effects on SRC, STAT3, MAPK3, MAPK1, RELA, PIK3R1, HSP90AA1, TP53, EP300, AKT1, and other targets through compounds such as luteolin, quercetin, wogonin, and kaempferol. The biological processes regulated by DHJSD include inflammatory response, negative regulation of the apoptotic process, positive regulation of cell proliferation, and positive regulation of the ERK1 and ERK2 cascade. The OA-related pathways regulated by DHJSD include HIF-1, PI3K-Akt, TNF, osteoclast differentiation, Toll-like receptor, FoxO, NF-κB, MAPK, Ras, and JAK-STAT signaling pathways. Therefore, in combination with the contents of this study, future researchers should investigate key targets, such as SRC, STAT3, MAPK3, MAPK1, and RELA, to expand our understanding of the pathological mechanism of OA and lead to the emergence of new drug targets.
Specific associations with OA were identified through a search of 10 key targets obtained using PPI. SRC is a non-receptor protein involved in controlling gene transcription, immune responses, cell adhesion, cell cycle, apoptosis, migration, and transformation. Studies have shown that MT-SYK-03, a small-molecule inhibitor of SRC, can effectively reduce chondrocyte hypertrophy, thus playing a role in protecting the bone and cartilage.[32] It can also promote energy production in osteoclasts by activating the mitochondrial cytochrome oxidase.[33] STAT3 is a signal transducer and activator of transcription (STAT). It is activated by IL-6 and regulates degradation of the extracellular matrix (ECM) and chondrocyte catabolism by affecting the expression of MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5, thus aggravating the symptoms of OA. This effect was reversed by the small-molecule inhibitor Stattic.[34] As important members of the MAPK signaling pathway, MAPK3 and MAPK1 are involved in the regulation of cell growth and adhesion, which are closely related to the inflammatory reactions of the synovium and cartilage. The MAPK signal transduction pathway regulates inflammatory cytokine and cell transduction pathways, thereby affecting joint inflammation and destruction.[35] RELA belongs to the nuclear factor-kappa B (NF-κB) family. Studies have shown that it is an effective transcriptional activator of ADAMTS5 in chondrocytes, which degrades the articular cartilage..[36] It can also regulate infection, immunity, differentiation, cell growth, tumorigenesis, and apoptosis in vivo, and is a multifunctional transcription factor present in all cells.[37]
PIK3R1 is a regulatory subunit of the phosphatidylinositol-3 kinase (including I, II, and III). In 2016, a study found that targeted PIK3R1 inhibited the phosphorylation of Akt, mTOR, and S6 proteins in OA chondrocytes, thus inhibiting their survival and matrix synthesis of chondrocytes in OA.[38] This result indicated that PIK3R1 is a key target of chondrocyte materialism. Hsp90AA1 is a biomarker associated with defective autophagy in OA.[39] Studies have shown that HSP90AA1 plays a key role in the stress response of chondrocytes and is involved in cartilage metabolism. Inhibition of the HSP90 protein can improve cartilage degeneration and prevent the development of OA.[40] TP53 is an important tumor suppressor gene. The encoded protein P53, which regulates the cell cycle, DNA repair, apoptosis, and signal transduction, is a main factor involved in cell stress reactions. In 2017, it was found that the expression of p53 in OA chondrocytes was found to be higher than that in normal chondrocytes. P53 can inhibit DNA replication, blocks the cell cycle, induces apoptosis, and accelerates cartilage degradation.[41] Therefore, the p53 signaling pathway is an important mechanism that mediates chondrocyte apoptosis, leading to OA. EP300 (E1A binding protein P300) is a protein-coding gene that plays an important regulatory role in cell proliferation and differentiation. The pathway related to EP300 is the Toll-like receptor signaling pathway, which has the potential to treat OA.[42] AKT1 plays an important role in chondrocyte apoptosis, and its level directly affects the development of OA. Studies have shown that inhibition of AKT1 can improve chondrocyte apoptosis and senescence, thus inhibiting the development of OA.[43] In summary, all 10 key targets were involved in OA progression, including regulation of ECM degradation, chondrocyte catabolism, inflammatory processes, and cartilage degeneration. Thus, they can be used as biomarkers and targets for OA drug action, thereby providing a new strategy for OA treatment. However, the regulatory effects and mechanisms of DHJSD on these targets must be verified, both in vitro and in vivo.
In the network analysis described in Section 2.6, luteolin, quercetin, wogonin, and kaempferol were the active ingredients with the highest degree values. Luteolin had the highest number of targets, reaching 61, ranking first among all components. Luteolin, a natural flavonoid with strong anti-inflammatory activity, has been found in various vegetables and medicinal plants. Studies have shown that Luteolin inhibits the expression of MMP-3, MMP-1, MMP-13, ADAMTS-4, and ADAMTS-5 in articular chondrocytes.[44] These matrix metalloproteinases and ADAMTSs degrade the ECM in large amounts, resulting in an imbalance between ECM production and degradation as well as cartilage degeneration. Therefore, luteolin has a strong pharmacological effect on inhibition of ECM degradation. GO enrichment analysis of related targets showed that the treatment of OA-related BP with DHJSD included the inflammatory response, apoptotic process, positive regulation of ERK1 and ERK2 cascades, and extracellular matrix disassembly. The ECM is not only a microenvironment for chondrocytes to survive but also a buffer for mechanical conduction. The active components and biological processes of DHJSD are strongly involved in the regulation of the ECM. The docking results of the core components and core targets showed that the docking scores of other groups, except for the RELA protein, were lower than –6 kcal mol–1, indicating that these compounds had good binding capacity with the target and were potential compounds and targets for the treatment of OA. These results suggest directions for future OA research.
Pathway studies have shown that luteolin can downregulate the expression of JNK, p38MAPK, TNF-α, and IL-6 in OA chondrocytes by regulating the MAPK and JNK signaling pathways to protect chondrocytes and delay cartilage degeneration.[45] In target enrichment analysis related to the KEGG pathway, OA-related pathways included the HIF-1, PI3K-Akt, and JAK-STAT signaling pathways. Eight core targets were enriched in the HIF-1 signaling pathway. The HIF-1 signaling pathway is a hypoxia-stress signaling pathway that plays an important role in angiogenesis and apoptosis in cartilage tissues. Under hypoxic conditions, the high expression of HIF-1α increases the expression of VEGF, increases angiogenesis, and accelerates OA.[46] PI3K-Akt signaling pathway plays a key role in the imbalance between the proliferation and apoptosis of synovial cells. Abnormal activation leads to excessive synovial hyperplasia, infiltration of cartilage and bone tissue, and the induction of angiogenesis and pannus formation, leading to joint deformities and bone destruction.[47] JAK-STAT signal transduction pathway plays a key role in the extracellular matrix homeostasis and inflammatory reactions in OA. When IL-6 signaling molecules act on IL-6R, JAK coupled with IL-6R is phosphorylated and activated, and the STAT protein containing the SH2 domain is collected and phosphorylated. p-STATs are translocated to the nucleus for transcription and translation.[48] Studies have shown that dysfunction of the JAK/STAT signaling pathway can induce synovial hyperplasia and synovitis. Simultaneously, it can regulate the secretion of MMP3 and MMP13, and aggravate the degradation of the extracellular matrix and destruction of cartilage.[34] ECM can provide a protective barrier against the growth of chondrocytes. An imbalance between cartilage production and degradation is the major cause of cartilage degeneration. The PPI results showed that STAT3, a target of the JAK/STAT pathway, was a potential anti-OA-related target. Therefore, studying the regulation of extracellular matrix degradation by the JAK/STAT pathway may be a new direction for the treatment of OA.
In summary, the results of this study showed that 186 active compounds in DHJSD exerted anti-OA effects by regulating a variety of signaling pathways and biological processes through 253 effective targets. PPI analysis showed that the first 10 nodes of the effective targets were all related to OA, indicating that the components of DHJSD were effective in treating OA through multiple targets and pathways. In this study, the anti-OA mechanism of DHJSD was revealed from a systematic perspective for the first time, which reflects the overall concept of Chinese medicine treatment to a certain extent and lays the foundation for further elucidation of the biological connotations of DHJSD. Moreover, it also provides a direction for the treatment of OA with complicated pathogenesis and the development of new drugs. However, this study has some limitations. First, owing to limited screening conditions, only the main targets of DHJSD can be analyzed, which limits the research results to a certain extent. Second, the mechanism prediction of DHJSD does not involve dosage analysis and the results may be biased. In future, clinical trials of DHJSD for OA treatment should be conducted to further clarify the optimal effective dose. Third, the effective substances of traditional Chinese medicine may not be a simple superposition of single components. The efficacy of a disease may be related to the concentration of the active ingredients, but the content of the ingredients is neglected in network pharmacology studies. Therefore, the content should be considered early in the study. Fourth, other significant pathways, such as proteoglycans in cancer and Chagas disease, may also be important pathways for DHJSD to treat OA. However, the related literature is scarce, which needs to be further explored and may become a hot topic in future research.
5. Conclusion
Using bioinformatics and network pharmacology, we explored the possible key genes and molecular mechanisms of DHJSD in OA treatment, thereby providing a new breakthrough point for OA treatment. PPI analysis of the core targets was performed by molecular docking of the core compounds. It has been revealed that DHJSD may achieve therapeutic effects by regulating a variety of biological functions and multiple pathways in the treatment of OA, which provides support for further exploration of its mechanism of action and optimization of the experimental design. It also provides a research method for the mechanistic analysis of traditional Chinese medicine and its compounds for the treatment of certain diseases.
Authors contributions
Conceptualization: Liu Yang.
Data curation: Senwang Zheng, Ajiao Hou, Liu Yang.
Funding acquisition: Wei Lan.
Investigation: Senwang Zheng, Song Wang.
Methodology: Senwang Zheng.
Project administration: Senwang Zheng, Jiaxu Zhang.
Resources: Huan Yu.
Software: Senwang Zheng, Xuejiao Wang.
Validation: Senwang Zheng.
Writing – original draft: Senwang Zheng.
Writing – review & editing: Wei Lan, Liu Yang.
Supplementary Material
Abbreviations:
- BP =
- biological process
- DHJSD =
- Duhuo Jisheng decoction
- GO =
- gene ontology
- KEGG =
- Kyoto encyclopedia of genes and genomes
- OA =
- osteoarthritis
- PPI =
- protein-protein interaction
- TCMSP =
- traditional Chinese medicine systems pharmacology database and analysis platform
All data generated or analyzed during this study are included in this published article [and its supplementary information files] [and its supplementary information files].
Ethical approval was not required because individual patient data and privacy were not included in the study.
This study was financially supported by the National Natural Science Foundation of China (Grant Numbers 81973604 and 81960771); Heilongiiang Touyan Innovation Team Program; National Famous Old Traditional Chinese Medicine Experts Inheritance Studio Construction Program of National Administration of TCM, Grant/Award Number: [2022] No.75; The Seventh Batch of National Old Traditional Chinese Medicine Experts Academic Experience Inheritance Program (NO. [2022] No. 76); Traditional Chinese medicine Processing technology inheritance base project.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
How to cite this article: Yang L, Zheng S, Hou A, Wang S, Zhang J, Yu H, Wang X, Lan W. Discussion on the molecular mechanism of Duhuo Jisheng decoction in treating osteoarthritis based on network pharmacology and molecular docking. Medicine 2022;101:42(e31009).
Contributor Information
Senwang Zheng, Email: zsw785785@163.com.
Ajiao Hou, Email: Hou_Ajiao@163.com.
Song Wang, Email: wang18846059141@163.com.
Jiaxu Zhang, Email: zhang15765312931@163.com.
Huan Yu, Email: yu13845261571@163.com.
Xuejiao Wang, Email: wang18846059141@163.com.
References
- [1].Loeser RF, Goldring SR, Scanzello CR, et al. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brandt KD, Radin EL, Dieppe PA, et al. Yet more evidence that osteoarthritis is not a cartilage disease. Ann Rheum Dis. 2006;65:1261–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barbour KE, Helmick CG, Boring M, et al. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2017;66:246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10:437–41. [DOI] [PubMed] [Google Scholar]
- [5].Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–9. [DOI] [PubMed] [Google Scholar]
- [6].Kotlarz H, Gunnarsson CL, Fang H, et al. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60:3546–53. [DOI] [PubMed] [Google Scholar]
- [7].Bitton R. The economic burden of osteoarthritis. Am J Managed Care. 2009;15:S230–5. [PubMed] [Google Scholar]
- [8].Losina E, Paltiel AD, Weinstein AM, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res. 2015;67:203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yuhao S, Yong M, Yang G, et al. Efficacy and safety of Shaoyang Xibi decoction in patients with knee osteoarthritis: a multi-center, single-blind, randomized controlled trial. J Tradit Chin Med. 2018;38:733–9. [PubMed] [Google Scholar]
- [10].Yu WZ, Huang CM, Ng HP, et al. Distal acupoints outperform proximal acupoints in treating knee osteoarthritis: a randomized controlled trial. Evid Based Compl Alt. 2021;2021:4827123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Thorlund JB, Roos EM, Goro P, et al. Patients use fewer analgesics following supervised exercise therapy and patient education: an observational study of 16 499 patients with knee or hip osteoarthritis. Br J Sports Med. 2021;55:670–5. [DOI] [PubMed] [Google Scholar]
- [12].Vandeputte MM, Krotulski AJ, Papsun DM, et al. The rise and fall of isotonitazene and brorphine: two recent stars in the synthetic opioid firmament. J Anal Toxicol. 2022;46:115–21. [DOI] [PubMed] [Google Scholar]
- [13].Surges R, Shmuely S, Dietze C, et al. Identifying patients with epilepsy at high risk of cardiac death: signs, risk factors and initial management of high risk of cardiac death. Epileptic Disord. 2021;23:17–39. [DOI] [PubMed] [Google Scholar]
- [14].Ormel J, Hollon SD, Kessler RC, et al. More treatment but no less depression: the treatment-prevalence paradox. Clin Psychol Rev. 2021;9:1102111. [DOI] [PubMed] [Google Scholar]
- [15].Yang J, Zhu X, Yuan P, et al. Efficacy of traditional Chinese Medicine combined with chemotherapy in patients with non-small cell lung cancer (NSCLC): a meta-analysis of randomized clinical trials. Supportive Care Cancer. 2020;28:3571–9. [DOI] [PubMed] [Google Scholar]
- [16].Sun K, Huang F, Qi B, et al. A systematic review and meta-analysis for Chinese herbal medicine Duhuo Jisheng decoction in treatment of lumbar disc herniation: a protocol for a systematic review. Med. 2020;99:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xiong Z, Zheng C, Chang Y, et al. Exploring the pharmacological mechanism of Duhuo Jisheng decoction in treating osteoporosis based on network pharmacology. Evid Based Compl Alt. 2021;2021:5510290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu J, Sun Y. How does Chinese medicine target cytokine imbalance in rheumatoid arthritis? Chin J Integr Med. 2013;19:874–80. [DOI] [PubMed] [Google Scholar]
- [19].Liu ZC, Wang ZL, Huang CY, et al. Duhuo Jisheng decoction inhibits SDF-1-induced inflammation and matrix degradation in human degenerative nucleus pulposus cells in vitro through the CXCR4/NF-κB pathway. Acta Pharmacol Sin. 2018;39:912–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yuan HY, Zhang XH, Zhang XL, et al. Analysis of patents on anti-gout therapies issued in China. Expert Opin Ther Pat. 2014;24:555–72. [DOI] [PubMed] [Google Scholar]
- [21].Yu C, Zhang X, Yu Z, et al. The treatment of intervertebral disc degeneration using traditional Chinese medicine. J Ethnopharmacol. 2020;263:113–117. [DOI] [PubMed] [Google Scholar]
- [22].Zhang DY, Liu FF. Analysis on the theoretical basis of Duhuo Jisheng decoction in treating arthralgia syndrome in Neijing. Cap Food Med. 2016;23:46. [Google Scholar]
- [23].Zhou Z, Chen B, Chen S, et al. Applications of network pharmacology in traditional Chinese medicine research. Evid-Based Compl Alt. 2020;2020:1646905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tao X, Huang Y, Wang C, et al. Recent developments in molecular docking technology applied in food science: a review. Int J Food Sci Technol. 2020;55:33–45. [Google Scholar]
- [25].Cao JH, Feng DG, Wang YZ, et al. Chinese herbal medicine Du-Huo-Ji-Sheng-decoction for knee osteoarthritis: a protocol for systematic review and meta-analysis. Medicine. 2021;100:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu W, Jin S, Huang M, et al. Duhuo Jisheng decoction suppresses matrix degradation and apoptosis in human nucleus pulposus cells and ameliorates disc degeneration in a rat model. J Ethnopharmacol. 2020;250:112494. [DOI] [PubMed] [Google Scholar]
- [27].Wu G, Fan H, Huang Y, et al. Duhuo Jisheng decoction-containing serum promotes proliferation of interleukin-1β-induced chondrocytes through the p16-cyclin D1/CDK4-Rb pathway. Mol Med Rep. 2014;10:2525–34. [DOI] [PubMed] [Google Scholar]
- [28].Ru JL, Li P, Wang JN, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminformatics. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jin Q, Hao XF, Xie LK, et al. A network pharmacology to explore the mechanism of Astragalus Membranaceus in the treatment of diabetic retinopathy. Evid Based Compl Alt. 2020;2020:8878569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shao T, Huang K. Network pharmacology-based analysis on Lonicera japonica for chronic osteomyelitis treatment. J Oncol. 2022;2022:1706716. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [31.Novikov FN, Panova MV, Titov IY, et al. Inhibition of SYK and cSrc kinases can protect bone and cartilage in preclinical models of osteoarthritis and rheumatoid arthritis. Sci Rep. 2021;11:23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Miyazaki T, Tanaka S, Sanjay A, et al. The role of c-Src kinase in the regulation of osteoclast function. Mod Rheumatol. 2006;16:68–74. [DOI] [PubMed] [Google Scholar]
- [33].Latourte A, Cherifi C, Maillet J, et al. Systemic inhibition of IL-6/Stat3 signaling protects against experimental osteoarthritis. Ann Rheum Dis. 2017;76:748–55. [DOI] [PubMed] [Google Scholar]
- [34].Liu F, Feng XX, Zhu SL, et al. Sonic Hedgehog signaling pathway mediates proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis via MAPK/ERK signaling pathway. Front Immunol. 2018;9:2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kobayashi H, Hirata M, Saito T, et al. Transcriptional induction of ADAMTS5 protein by nuclear factor-κB (NF-κB) family member RelA/p65 in chondrocytes during osteoarthritis development. J Biol Chem. 2013;288:28620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xie W, Meng X, Zhai Y, et al. Panax notoginseng saponins: a review of its mechanisms of antidepressant or anxiolytic effects and network analysis on phytochemistry and pharmacology. Mol. 2018;23:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cui X, Wang S, Cai H, et al. Overexpression of microRNA-634 suppresses survival and matrix synthesis of human osteoarthritis chondrocytes by targeting PIK3R1. Sci Rep. 2016;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lorenzo-Gómez I, Nogueira-Recalde U, Oreiro N, et al. HSP90AA1 is a biomarker associated with defective autophagy in osteoarhritis. Osteoarthr Cartilage. 2020;28:S319. [Google Scholar]
- [39].Siebelt M, Jahr H, Groen HC, et al. Hsp90 inhibition protects against biomechanically induced osteoarthritis in rats. Arthritis Rheum. 2013;65:2102–12. [DOI] [PubMed] [Google Scholar]
- [40].Lin M, Lin Y, Li X, et al. Warm sparse-dense wave inhibits cartilage degradation in papain-induced osteoarthritis through the mitogen-activated protein kinase signaling pathway. Exp Ther Med. 2017;14:3674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Garcia-Carpizo V, Ruiz-Llorente S, Sarmentero J, et al. CREBBP/EP300 bromodomain inhibition affects the proliferation of AR-positive breast cancer cell lines. Mol Cancer Res. 2019;17:720–30. [DOI] [PubMed] [Google Scholar]
- [42].Zhao X, Wang T, Cai B, et al. MicroRNA-495 enhances chondrocyte apoptosis, senescence and promotes the progression of osteoarthritis by targeting AKT1. Am J Transl Res. 2019;11:2232. [PMC free article] [PubMed] [Google Scholar]
- [43].Kang BJ, Ryu J, Lee CJ, et al. Luteolin inhibits the activity, secretion and gene expression of MMP-3 in cultured articular chondrocytes and production of MMP-3 in the rat knee. Biomol Ther. 2014;22:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xue J, Ye J, Xia Z, et al. Effect of luteolin on apoptosis, MAPK and JNK signaling pathways in guinea pig chondrocyte with osteoarthritis. Cell Mol Biol. 2019;65:91–5. [PubMed] [Google Scholar]
- [45].Chen Y, Zhao B, Zhu Y, et al. HIF-1-VEGF-Notch mediates angiogenesis in temporomandibular joint osteoarthritis. Am J Transl Res. 2019;11:2969. [PMC free article] [PubMed] [Google Scholar]
- [46].Zou L, Zhang G, Liu L, et al. Relationship between PI3K pathway and angiogenesis in CIA rat synovium. Am J Transl Res. 2016;8:3141. [PMC free article] [PubMed] [Google Scholar]
- [47].Tang M, Huang LL, Du QQ, et al. Ginsenoside 3β-O-Glc-DM (C3DM) enhances the antitumor activity of Taxol on Lewis lung cancer by targeting the interleukin-6/Jak2/STAT3 and interleukin-6/AKT signaling pathways. World J Tradit Chin Med. 2020;6:432. [Google Scholar]
- [48].Liu YT, Situ YL, Zhao TT, et al. Mechanism research of chonglou as a pain killer by network pharmacology. World J Tradit Chin Med. 2021;7:419. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.