Introduction:
We describe a reliable approach for double nerve transfer of the medial triceps branch and thoracodorsal nerve to the axillary nerve to increase axonal input. We present a review of outcomes for both end-to-end and reverse end-to-side nerve transfer.
Methods:
A retrospective review of patients who underwent nerve transfer for improvement of shoulder abduction at Harborview Medical Center and Northwestern Memorial Hospital between 2012 and 2021 was conducted. Patients were prospectively contacted to fill out a 30 item Disabilities of the Arm, Shoulder and Hand questionnaire, with an option to upload a video demonstrating active range of motion.
Results:
Twenty-one patients with 23 affected extremities were included in the final analysis. Fifteen patients completed the prospective arm of the study (71% response rate). Seventy-nine percent of patient limbs achieved a Medical Research Council Motor Scale (MRC-MS) of 4 or greater, and measured shoulder abduction active range of motion (AROM) was 139.2 degrees (range, 29–174 degrees) and 140.9 degrees (range, 60–180 degrees) (P = 0.95) for end-to-end and reverse end-to-side, respectively. Comparing end-to-end with reverse end-to-side neurorrhaphy, outcomes, including follow-up, mean postoperative MRC-MS, mean change in MRC-MS, Disabilities of the Arm, Shoulder and Hand, abduction AROM, and flexion AROM, were not statistically different.
Conclusions:
We showed improvements in shoulder abduction with the thoracodorsal nerve, in addition to the medial triceps branch, to increase axonal donation and power the axillary nerve without sacrificing the spinal accessory nerve. Furthermore, we demonstrated improvements with reverse end-to-side coaptation when intraoperative stimulation of the axillary nerve revealed residual function.
Takeaways
Question: What are the clinical outcomes for both end-to-end and reverse end-to-side coaptations for an alternative double nerve transfer?
Findings: 79% of patient limbs achieved an MRC-MS of 4 or greater, and measured shoulder abduction active range of motion was 139.2 degrees and 140.9 degrees for end to end and reverse end to side, respectively.
Meaning: We showed improvements in shoulder abduction by utilizing the thoracodorsal nerve, in addition to the medial triceps branch, to increase the axonal donation to power the axillary nerve, without the sacrifice of the spinal accessory nerve.
INTRODUCTION
Axillary nerve palsy can occur in isolation, after shoulder surgery or dislocation, or as part of complex brachial plexus injuries. Nerve transfer techniques for restoration of shoulder abduction have dramatically improved the function and quality of life for patients in the past 20 years. Leechavengvongs et al1 and Witoonchart et al2 first described transferring the radial nerve branch that innervates the long head of the triceps to the axillary nerve in 2003. Colbert and Mackinnon3 expanded on this 3 years later, adding the spinal accessory to suprascapular transfer through a posterior approach to improve strength.
Since that time, various nerve transfer combinations have been described using the triceps nerve branch,2,4 phrenic nerve,5 spinal accessory,6 thoracodorsal,7,8 or cervical branches.5 Utilizing multiple donor nerves has been shown to improve shoulder abduction when compared with single nerve transfer techniques.9 Several basic science studies have correlated decreased axon counts with decreased force generation of the muscle,10–13 and thus, strategies to increase the number of axons transferred to the target muscle should be used to improve function.
The thoracodorsal nerve, emerging from the C6 to C8 roots, serves as a reliable donor nerve. It has previously been described for axillary nerve reconstruction, though reports are few, and the nerve is typically used in isolation as a single donor.7,8 We describe a reliable approach for transfer of both the medial triceps nerve branch and thoracodorsal nerve to the axillary nerve to increase axonal input to the axillary nerve, which may be especially beneficial in patients with larger body mass index (BMI) that requires increased force generation to lift the weight of the arm. It has been shown in shoulder nerve transfer patients that there is a negative correlation between BMI and the ultimate degree of shoulder abduction.14 We present the surgical approach along with a retrospective review of these patients, as well as outcomes for end-to-end compared with reverse end-to-side technique.
METHODS
Surgical Technique
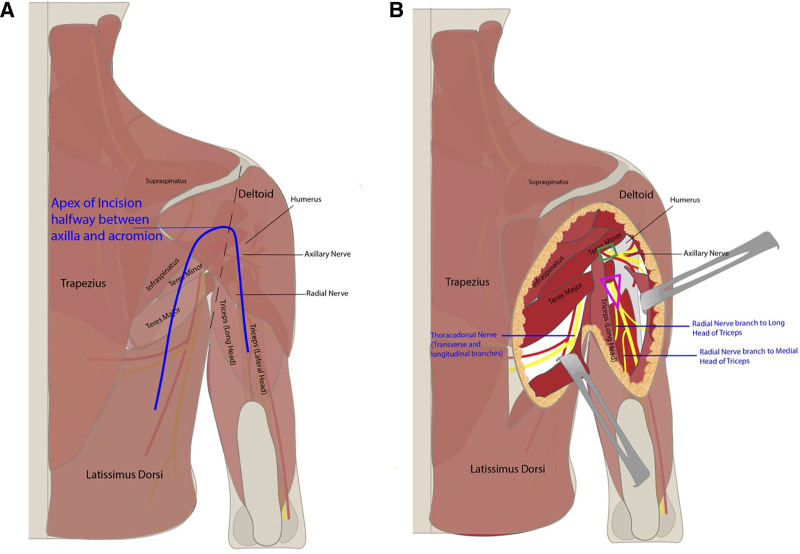
Preoperatively, the midline and tip of the scapula are marked with the patient upright. The patient is brought to the operating room and placed in the lateral decubitus position with the affected extremity up, and the hand is left uncovered to evaluate for muscle contractions. The senior author prefers the lateral decubitus position, as opposed to prone, because it allows for more manipulation of the arm and “opens” up the quadrangular space more than the prone position permits. General anesthesia is used without long-acting paralysis to allow for intraoperative nerve stimulation. A “boomerang”-shaped incision is made starting between the long and lateral heads of the triceps with the apex along the posterior aspect of the deltoid at the level of the axilla and continuing down the axis of the latissimus (Fig. 1A). If the apex of the boomerang is too high, there is more risk of inadvertently cutting the sensory branch of the axillary nerve.
Fig. 1.
Surgical anatomy of nerve transfer approach. Incision placement (A) and surgical approach (B).
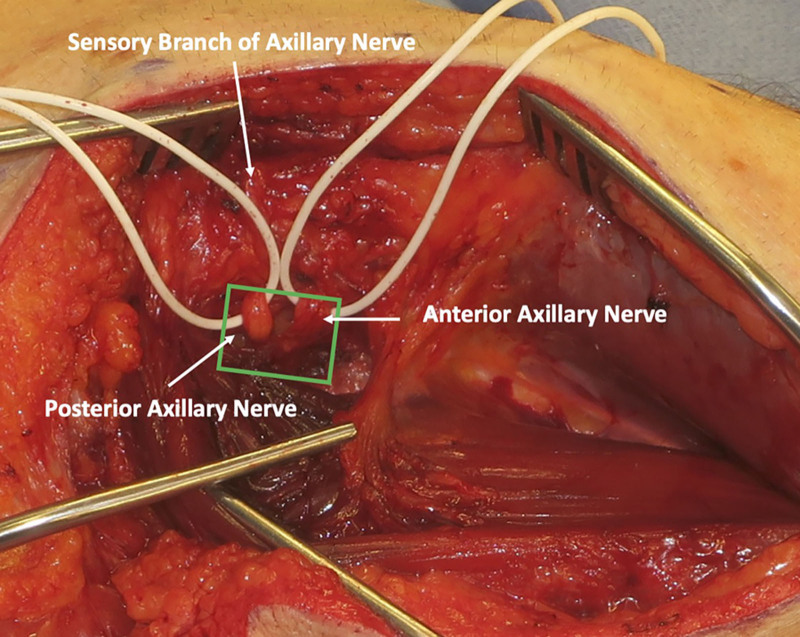
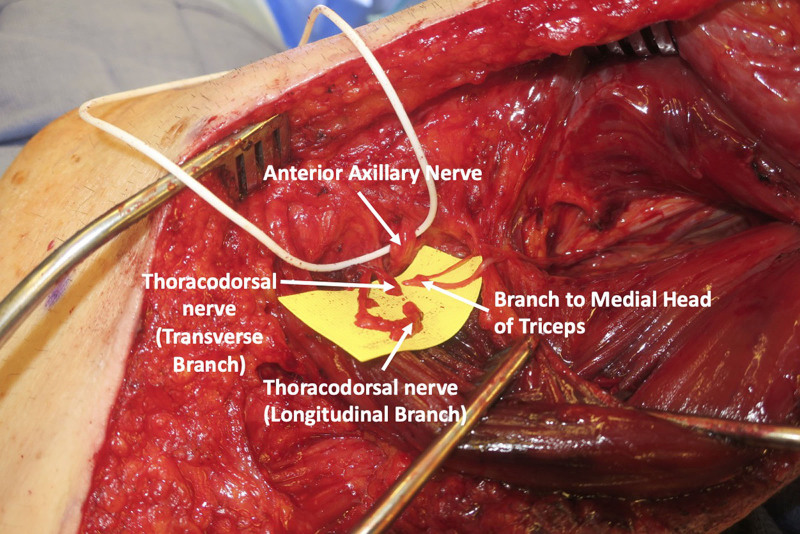
The skin and fascia are incised, taking care to preserve the sensory branch of the axillary nerve, which can be found along the posterior border of the deltoid. The sensory branch of the axillary nerve is dissected in a retrograde fashion to its branchpoint off the posterior branch of the axillary nerve. The anterior and posterior axillary nerve branches are found emerging from the quadrangular space (Fig. 2), and a handheld nerve stimulator is used to evaluate for any muscle contractions. Based on intraoperative nerve stimulation, the decision is made at this point whether to proceed with end-to-end or reverse end-to-side nerve transfers. If there are any meaningful contractions of the deltoid when stimulating the anterior branch of the axillary nerve at 2 mA, reverse end-to-side nerve transfers are performed. If no contractions at 2 mA, then end-to-end nerve transfers will be performed.
Fig. 2.
View of the axillary nerve emerging from the quadrangular space. The posterior axillary branch can be distinguished by finding the posterior sensory nerve branching off.
The quadrangular space is decompressed by releasing the tight fascia of the teres major. The branch to the medial or long head of the triceps is identified, as has been previously described, and nerve stimulation is performed to confirm that the selected triceps nerve branch is functioning (Fig. 1B).1,3 The senior author prefers the medial triceps branch, especially for reverse end-to-side nerve transfers, because it is a longer nerve branch than the long and lateral head branches.
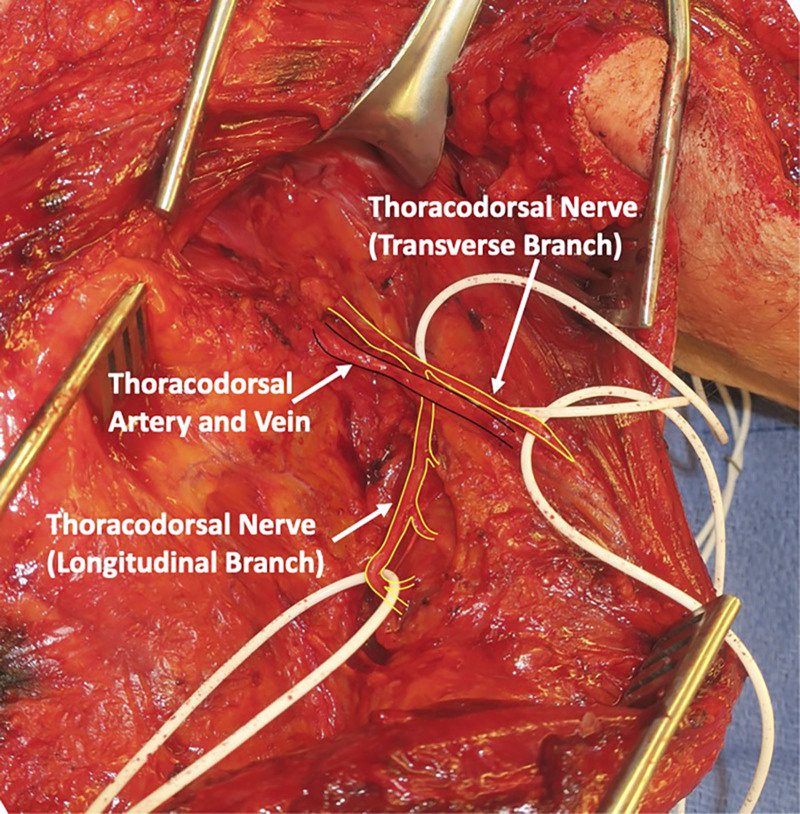
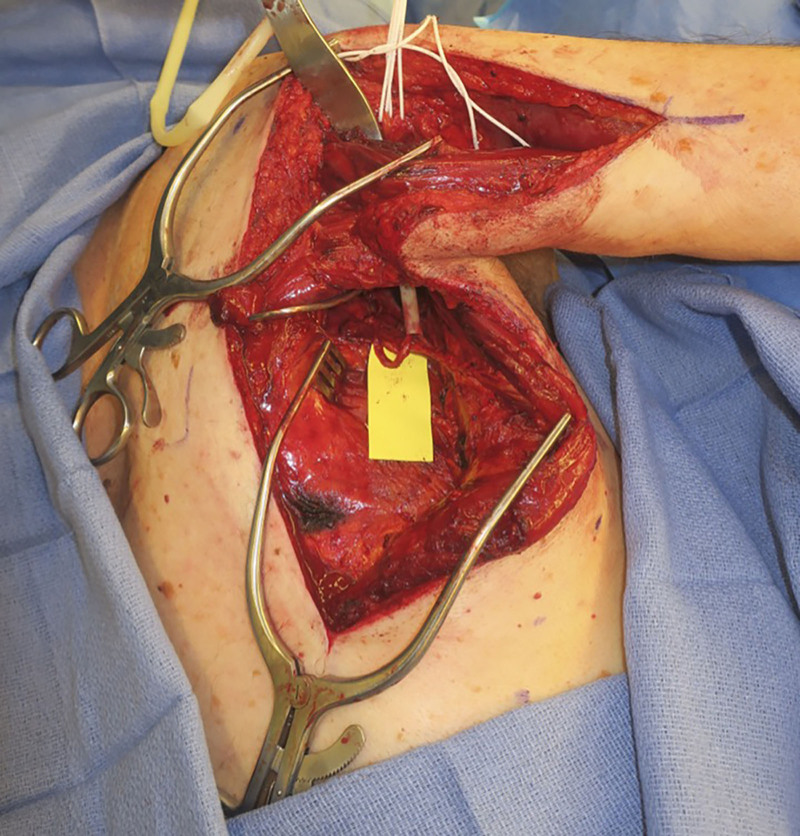
The skin overlying the latissimus is then elevated from the incision to the midline. The latissimus muscle is released along its superomedial surface and then reflected anteriorly until the thoracodorsal neurovascular bundle is encountered (Fig. 3). Nerve stimulation is performed to confirm that the thoracodorsal nerve stimulates well. Once the main thoracodorsal nerve splits into the transverse and longitudinal branches, dissection should continue to obtain additional length that will be needed for reverse end-to-side coaptation. More length is needed for reverse end-to-side than end-to-end (Fig. 3). It is important to dissect the thoracodorsal nerve in a retrograde fashion as far proximally under the teres major as possible so that the pivot point of the nerve is as close to the recipient axillary nerve as possible. A tunnel is then developed deep to teres major and the long head of the triceps, and the thoracodorsal nerve is then delivered underneath the teres major and triceps long head to reach the axillary nerve, using a one-fourth inch Penrose drain (Fig. 4).
Fig. 3.
Intraoperative view of approach to thoracodorsal neurovascular bundle. Transverse and longitudinal branches of the thoracodorsal nerve off the main trunk should be dissected to obtain additional length.
Fig. 4.
Passage of the thoracodorsal nerve underneath the teres major and triceps long head to reach the axillary nerve, using a Penrose drain.
With the aid of the operating microscope, all three nerve endings (medial head to triceps branch, transverse branch of thoracodorsal, and longitudinal branch of thoracodorsal) are then coapted to the anterior branch of the axillary nerve, end to end, or reverse end to side, depending on the absence or presence of residual axillary nerve function, respectively, based on nerve stimulation (Fig. 5).
Fig. 5.
Final nerve coaptations of the medial head of triceps, transverse, and longitudinal branches of thoracodorsal nerve to the anterior branch of the axillary nerve.
If end-to-end coaptation is performed, interrupted epineurial stitches with a 9-0 nylon are placed, and the coaptation is reinforced with fibrin glue. If reverse end-to-side transfer is performed, an epineurial window is made in the anterior axillary nerve branch, and interrupted epineurial stitches with a 9-0 nylon are placed and reinforced with fibrin sealant.
Patients are referred to physical therapy at the 2-week postoperative appointment to initiate passive range of motion exercises. Patients are then instructed to slowly initiate targeted active motion exercises for the nerve transfers, focusing on triceps and thoracodorsal contractions at around 4 weeks postoperatively.
Retrospective Review
After institutional review board approval, a retrospective review of patients at Harborview Medical Center and Northwestern Memorial Hospital was conducted. Inclusion criteria included all patients 18 and older who underwent upper extremity nerve transfer surgery for improvement of shoulder abduction by the senior author (J.H.K.) between January 1, 2012, and January 1, 2021. Exclusion criteria included patients with spinal cord injury, traumatic brain injury, or neurodegenerative disorders, patients with less than 6 months of follow-up, and patients with inadequate preoperative or postoperative records of functional status. Data collected from the chart included demographics, mechanism of injury, time from injury to surgery, and Medical Research Council Motor Scale (MRC-MS) strength of shoulder flexion and abduction when recorded by the senior author (J.H.K.) in the physical examination of preopeartive and postoperative visits.
Prospective Survey
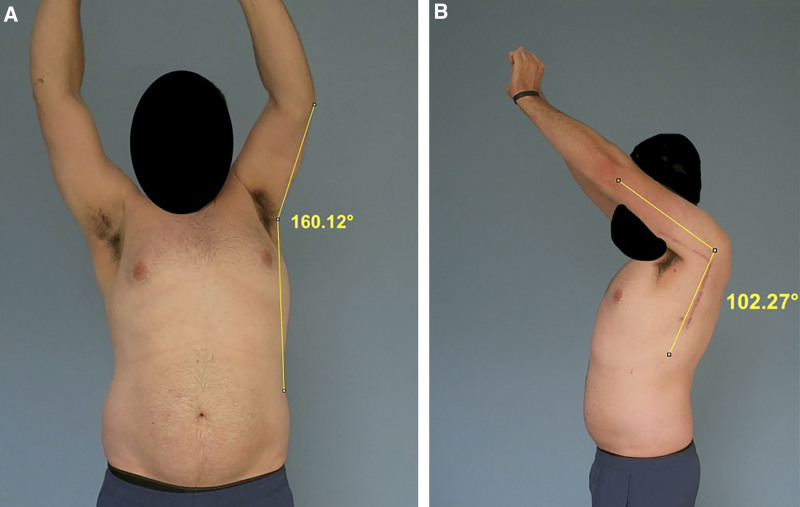
Patients identified for study inclusion during the retrospective review were then prospectively contacted for potential participation in a survey portion of the study. Subjects willing to participate were provided with a REDCap link, which contained a consent form, a 30-item Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire, and a link to upload a video of themselves demonstrating their shoulder function. The video upload was optional. For those who elected to participate in the video upload, an instructional video was provided demonstrating how patients should move their arm without momentum in the following order: facing camera, unaffected arm abduction three times; turning to the side, unaffected arm, shoulder flexion three times; facing camera, affected arm abduction three times; turning to the side, affected arm, shoulder flexion three times. A screenshot of the patient’s maximum active range of motion at each trial was captured and measured utilizing ImageJ (Fig. 6). Measurements were obtained by the first author (L.E.J.).
Fig. 6.
Video measurement of active range of motion of shoulder flexion and abduction. A, Frontal view. B, Side view.
Statistical Analysis
Statistical analysis was performed utilizing GraphPad Prism (Version 8.3.0, San Diego, CA). Comparison of age, BMI, injury to surgery, follow-up, DASH score, and active range of motion was performed utilizing Welch’s t test.
RESULTS
Retrospective Review
Twenty-two patients who underwent “alternative” double nerve transfer surgery for shoulder function between January 2012 and December 2021 were identified. Two of these patients had this surgery on bilateral extremities after complications resulting from cervical spine fusion. One patient was excluded due to inadequate follow-up or records, leaving 21 patients, with 23 extremities included in the final analysis. Mechanism of injury included motor vehicle or motorcycle collision, iatrogenic (cervical or orthopedic surgery), and falls. Seven patients had isolated axillary nerve palsies, and 14 had additional upper plexus injuries. Fifteen patients elected to participate in the survey portion of the study (71% response rate).
Among the 21 patients who underwent “alternative” double nerve transfer, three patients sustained their axillary nerve injury during reverse total shoulder arthroplasty and one during arthroscopic rotator cuff surgery. These patients were noted to have particularly poor outcomes and were excluded from subsequent analysis (Table 1). None of these patients achieved MRC-MS greater than 3—three out of four of them had no change in their postoperative MRC-MS, and average measured active range of motion was 36.9 degrees and 56.7 degrees for shoulder abduction and flexion, respectively.
Table 1.
Among the 21 Patients Who Underwent Nerve Transfer to Improve Shoulder Function, Four Patients Sustained Their Axillary Nerve Injury during Reverse Total Shoulder Arthroplasty and One during Arthroscopic Rotator Cuff Surgery
| Patient Characteristics and Outcome Measure | Double Nerve Transfer after Shoulder Surgery, Mean (SD) |
|---|---|
| Age | 64.25 (7.08) |
| n = 4 | |
| Range, 54–73 | |
| Injury to surgery (d) | 197.5 (91.18) |
| n = 4 | |
| Range, 77–327 | |
| Follow-up (d) | 463.25 (248.71) |
| n = 4 | |
| Range, 236–873 | |
| Preoperative shoulder abduction (MRC-MS) | 1.75 (1.09) |
| n = 4 | |
| Range, 0–3 | |
| Postoperative shoulder abduction (MRC-MS) | 2.25 (0.43) |
| n = 4 | |
| Range, 2–3 | |
| Shoulder abduction mean difference in MRC-MS | 0.5 (0.86) |
| n = 4 | |
| Range, 0–2 | |
| No. patients with final MRC-MS ≥4 | 0 (0/4) |
| Video measured shoulder abduction AROM | 36.5 (9.5) |
| n = 2 | |
| Range, 28–46 | |
| Video measured shoulder flexion AROM | 56.0 (12.0) |
| n = 2 | |
| Range, 45–69 | |
| DASH score | 26.3 (15.4) |
| n = 2 | |
| Range, 11–42 |
These patients were noted to have particularly poor outcomes and were excluded from subsequent analysis. AROM, active range of motion.
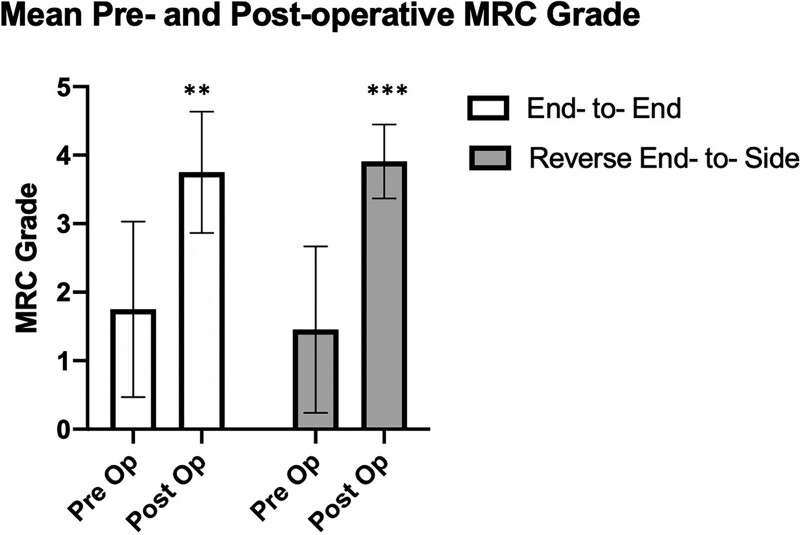
Among the 17 remaining patients (19 remaining limbs) who had “alternative” double transfer, there were eight end-to-end coaptations and 11 reverse end-to-side coaptations (Fig. 7). Age, follow-up days, and preoperative shoulder abduction MRC-MS were not statistically different between groups (Table 2). Time from injury to surgery was significantly shorter in the end-to-end group at 185 days (about 6 months) compared with 267.6 days (about 8.5 months) in the reverse end-to-side group (P = 0.04). Outcomes, including mean postoperative MRC-MS, mean change in MRC-MS, DASH, measured shoulder abduction active range of motion (AROM), and measured shoulder flexion AROM, were not statistically different between groups. Seventy-five percent of limbs with end-to-end coaptations and 82% of limbs with reverse end-to-side coaptations achieved an MRC-MS of 4 or greater. Measured shoulder abduction AROM was 139.2 degrees (range, 29–174 degrees) and 140.9 degrees (range, 60–180 degrees) (P = 0.95) for end to end and reverse end to side, respectively. Measured shoulder flexion AROM was 139.3 degrees (range, 102–174 degrees) and 135.7 degrees (range, 80–180 degrees) (P = 0.89) for end to end and reverse end to side, respectively. It should be noted, when comparing groups, that eight patients had concomitant nerve transfers for elbow flexion, which can affect the DASH, but these numbers were comparable between groups (Table 2).
Fig. 7.
Paired t-test demonstrating significant change in postoperative MRC-MS compared with preoperative in both end-to-end vs reverse end-to-side neurorrhaphy. *P < 0.05, **P < 0.005, ***P < 0.0005
Table 2.
Comparison of Outcomes in “Alternative” Double Transfer between End to End and Reverse End to Side
| Patient Characteristics and Outcome Measure | End to End, Mean (SD) | Reverse End to Side, Mean (SD) | P |
|---|---|---|---|
| Retrospective Study | |||
| Age | 45.0 (13.73) | 58.18 (13.93) | 0.06 |
| n = 8 | n = 11 | ||
| Range, 22–62 | Range, 26–73 | ||
| Injury to surgery (d) | 185.3 (55.55) | 267.6 (100.7) | 0.04 |
| n = 8 | n = 11 | ||
| Range, 117–296 | Range, 191–504 | ||
| Clinic follow-up (d) | 550.3 (242.6) | 489.9 (254.2) | 0.61 |
| n = 8 | n = 11 | ||
| Range, 228–936 | Range, 187–876 | ||
| Preoperative shoulder abduction (MRC-MS) | 1.75 (1.28) | 1.46 (1.21) | 0.62 |
| n = 8 | n = 11 | ||
| Range, 0–3 | Range, 0–4 | ||
| Postoperative shoulder abduction (MRC-MS) | 3.75 (0.89) | 3.91 (0.54) | 0.66 |
| n = 8 | n = 11 | ||
| Range, 2–5 | Range, 3–5 | ||
| Shoulder abduction mean difference in MRC-MS | 2.0 (1.31) | 2.46 (1.34) | 0.47 |
| n = 8 | n = 11 | ||
| Range, 1–4 | Range, 0–5 | ||
| No. patients with final MRC-MS ≥4 | 75% (6/8) | 82% (9/11) | |
| Prospective study | |||
| RedCap follow-up (d) | 906.7 (551.5) | 1009 (622.8) | 0.73 |
| n = 7 | n = 9 | ||
| Range, 228–1635 | Range, 320–2208 | ||
| Video measured shoulder abduction AROM | 139.2 (61.86) | 140.9 (45.12) | 0.95 |
| n = 5 | n = 8 | ||
| Range, 29 – 174 | Range, 60–180 | ||
| Video measured shoulder flexion AROM | 139.3 (33.13) | 135.7 (41.74) | 0.89 |
| n = 5 | n = 8 | ||
| Range, 102 – 174 | Range, 80–180 | ||
| DASH score | 20.7 (17.90) | 37.78 (30.36) | 0.21 |
| n = 7 | n = 9 | ||
| Range, 2–59 | Range, 0–76 | ||
| Patients with double fascicular nerve transfer for elbow flexion | 37.5% (3/8) | 45% (5/11) | |
Bolded items represent statistical significance (p < 0.05). AROM, active range of motion.
DISCUSSION
It is commonly stated that supraspinatus initiates shoulder abduction before deltoid activation, thus restoring input to this muscle through nerve transfer to the suprascapular nerve has been prioritized. However, several studies have demonstrated that paralyzing supraspinatus does not impede the ability to abduct the shoulder15,16 and that the supraspinatus does not activate on electromyography (EMG) any earlier than the deltoid during normal shoulder abduction.17
Biomechanical computer simulations of shoulder abduction after single axillary nerve transfer compared with nerve transfer to both axillary nerve and suprascapular nerves demonstrated similar force-generating capacity.18 Furthermore, sacrifice of the spinal accessory nerve as a donor nerve cannot be overlooked. Both the upper and lower trapezius muscles adjust the movement of the scapula and play an important role in both the stability and movement of the shoulder joint. Patients with isolated spinal accessory nerve resection or palsies report shoulder pain and weakness and have limitations in active shoulder abduction and flexion.19–23 Much of these data come from the head and neck literature, which caused a movement to spare the spinal accessory nerve during neck dissection due to the associated morbidity with limitations in shoulder abduction above 75–90 degrees.21–23 Additionally, utilizing the spinal accessory nerve will eradicate the option of trapezius to infraspinatus tendon transfer, which has been shown to improve external rotation to a greater degree than nerve transfer.24
Comparably, the use of the thoracodorsal nerve has minimal donor morbidity. Functional impairment after latissimus dorsi muscle transfer has no remarkable effect on range of motion, strength, or function of the shoulder.25,26 Lee et al27 performed a review of 60 patients who underwent functional latissimus dorsi muscle transfer with both descending and transverse thoracodorsal nerve branches harvested for smile reanimation. Fifty patients responded to the Quick-DASH questionnaire at a median follow-up of 51 months. The average score was 2.64, and all but three patients scored less than 10.
We advocate prioritizing transfer to the axillary nerve for restoration of shoulder abduction, specifically the anterior axillary nerve. Leechavengvongs et al28 have advocated transferring to the anterior branch alone to avoid sensory fascicles of the posterior branch, and because anatomic study has demonstrated that the anterior branch contributes to all three heads of the deltoid, whereas the posterior branch typically only contributes to the posterior deltoid. Additionally, biomechanical studies have demonstrated that the anterior and middle deltoids were greater contributors to maximum isometric shoulder strength and to joint moments generated during submaximal movements than the posterior deltoid.29
Transfer of multiple nerves has repeatedly demonstrated improved outcomes compared with single nerve transfer,9 likely due to the increased number of axons added with each additional nerve transfer. Several basic science studies have correlated decreased axon counts with decreased force generation of the muscle.10–13 Khair et al30 determined that the main axillary nerve has 7887 axons, and the anterior branch alone has 4052 axons. A single triceps branch (long head has 2302 axons, and medial head has 2198 axons) is not adequate to supply enough axons for the anterior branch of the axillary nerve. Thus, if you transfer both the medial head of the triceps with an axon count of 2198 and the thoracodorsal nerve with an axon count of 2409,31 one could overwhelm the axon count of the recipient nerve, leading to greater strength for shoulder motion, which is especially important for patients with larger BMIs.
When comparing our findings to those in the literature, one can appreciate the potential benefits of our “alternative” double nerve transfer. Suzuki et al32 reported a series of 12 patients with single nerve transfer of spinal accessory to suprascapular nerve, reporting average shoulder flexion was 70.4 degrees and abduction was 77.1 degrees. Bertelli and Ghizoni6 published a series of 110 patients with single nerve transfer of spinal accessory to suprascapular nerve, reporting an average shoulder abduction of 58.5 degrees. Looking at case series with “classic” double nerve transfer—both spinal accessory to suprascapular nerve and nerve to long head of triceps to anterior branch of axillary nerve—Leechavengvongs et al1 reported a series of seven cases in which all patients recovered M4 deltoid strength with an average shoulder abduction of 124 degrees (range, 70–160 degrees). Bertelli and Ghizoni6 published a series of seven patients with average shoulder abduction of 122 degrees (range, 80–170 degrees). Looking at single nerve transfer with long head triceps motor branch to axillary nerve, Lee et al27 published a series of 21 patients reporting a mean deltoid MRC-MS of 3.5 ± 1.1 and mean shoulder abduction of 119 degrees (range, 20–170 degrees). While it is difficult to compare these studies due to the large number of variables present with nerve injury, our mean shoulder abduction was higher than all these studies at 139.2 degrees (range, 29–174 degrees) and 140.9 degrees (range, 60–180 degrees) for end to end and reverse end to side, respectively.
Reverse end-to-side neurorrhaphy for motor innervation remains controversial with some studies demonstrating only minimal in-growth of the motor branches,33–35 while others have described good motor reinnervation.36–38 In our study, we demonstrated that 82% of patients who underwent reverse end-to-side double nerve transfer achieved deltoid MRC-MS of 4 or greater with a mean change in MRC-MS of 2.46 compared with preoperative MRC-MS. Furthermore, between end-to-end and reverse end-to-side groups, mean final MRC-MS grade, DASH score, and shoulder active range of motion were not statistically different. Given that the reverse end-to-side coaptation was performed due to the presence of intraoperative nerve stimulation, we cannot truly attribute the strength improvements to the reverse end-to-side nerve transfer alone, since inherent nerve recovery most likely contributed to improved functional outcomes. However, the average number of days from injury to surgery in this group was 267.6 days (8.8 months), and thus, we can infer the patient elected to undergo surgery at this time due to inadequate strength improvements with observation and physical therapy alone. The judgment to pursue surgery in these patients was made at least 6 months after injury with an extensive joint decision-making conversation. If strength improvements with therapy had plateaued with insufficient strength to meet functional needs, compound muscle action potential amplitudes remained low, and/or no EMG evidence of reinnervation was present, surgery was encouraged.
Our retrospective review was subject to multiple limitations, including the small number of patients available for review. Additionally, outcomes after nerve transfer surgery are often difficult to interpret due to the varying degrees of nerve injury at various and sometimes multiple levels, with the possibility of improved strength due to spontaneous recovery rather than surgical reconstruction. Specifically, outcomes for the four patients who sustained axillary nerve injury during shoulder surgery were noted to be particularly poor. The reason for this is unclear, potentially related to overall traction injury of the nerve that extends distal to the quadrangular space where the coaptation is performed. We felt that these outcomes were more reflective of the injury pattern rather than the surgical technique, and thus, these patients were excluded from further analysis. An additional limitation of this alternative nerve transfer compared with the classic double nerve transfer that transfers spinal accessory nerve to suprascapular nerve is that it does not address external rotation, and thus that outcome was not analyzed in this study. We feel that the main priority for these patients is to achieve the greatest strength possible in shoulder flexion and abduction and to leave the spinal accessory nerve and trapezius muscle to stabilize the shoulder or for future tendon transfers for external rotation.
However, even in this small sample, we showed substantial improvements in shoulder abduction by utilizing the thoracodorsal nerve, in addition to the medial triceps branch, to increase the axonal donation to power the anterior branch of the axillary nerve, without the sacrifice of the spinal accessory nerve. Furthermore, we demonstrated functional improvements with reverse end-to-side coaptation when intraoperative stimulation of the axillary nerve revealed some residual function.
Footnotes
Published online 24 October 2022.
Disclosure: Dr. Ko is on the Scientific Advisory Boards of Checkpoint Surgical, Inc., and Mesh Suture, Inc., and is a consultant for Integra LifeSciences Corporation and Neuraptive Therapeutics, Inc. The other authors have no financial interest to declare.
REFERENCES
- 1.Leechavengvongs S, Witoonchart K, Uerpairojkit C, et al. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part II: a report of 7 cases. J Hand Surg Am. 2003;28:633–638. [DOI] [PubMed] [Google Scholar]
- 2.Witoonchart K, Leechavengvongs S, Uerpairojkit C, et al. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part I: an anatomic feasibility study. J Hand Surg Am. 2003;28:628–632. [DOI] [PubMed] [Google Scholar]
- 3.Colbert SH, Mackinnon S. Posterior approach for double nerve transfer for restoration of shoulder function in upper brachial plexus palsy. Hand (N Y). 2006;1:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khair MM, Schreiber JJ, Rosenblatt L, et al. Axon counts yield multiple options for triceps fascicular nerve to axillary nerve transfer. J Hand Surg Am. 2016;41:e405–e410. [DOI] [PubMed] [Google Scholar]
- 5.Chuang DC, Lee GW, Hashem F, et al. Restoration of shoulder abduction by nerve transfer in avulsed brachial plexus injury: evaluation of 99 patients with various nerve transfers. Plast Reconstr Surg. 1995;96:122–128. [DOI] [PubMed] [Google Scholar]
- 6.Bertelli JA, Ghizoni MF. Transfer of the accessory nerve to the suprascapular nerve in brachial plexus reconstruction. J Hand Surg Am. 2007;32:989–998. [DOI] [PubMed] [Google Scholar]
- 7.Samardzic MM, Grujicic DM, Rasulic LG, et al. The use of thoracodorsal nerve transfer in restoration of irreparable C5 and C6 spinal nerve lesions. Br J Plast Surg. 2005;58:541–546. [DOI] [PubMed] [Google Scholar]
- 8.Dai SY, Lin DX, Han Z, et al. Transference of thoracodorsal nerve to musculocutaneous or axillary nerve in old traumatic injury. J Hand Surg Am. 1990;15:36–37. [DOI] [PubMed] [Google Scholar]
- 9.Cardenas-Mejia A, O’Boyle CP, Chen KT, et al. Evaluation of single-, double-, and triple-nerve transfers for shoulder abduction in 90 patients with supraclavicular brachial plexus injury. Plast Reconstr Surg. 2008;122:1470–1478. [DOI] [PubMed] [Google Scholar]
- 10.Cederna PS, Youssef MK, Asato H, et al. Skeletal muscle reinnervation by reduced axonal numbers results in whole muscle force deficits. Plast Reconstr Surg. 2000;105:2003–2010. [DOI] [PubMed] [Google Scholar]
- 11.Terzis JK, Wang W, Zhao Y. Effect of axonal load on the functional and aesthetic outcomes of the cross-facial nerve graft procedure for facial reanimation. Plast Reconstr Surg. 2009;124:1499–1512. [DOI] [PubMed] [Google Scholar]
- 12.Thanos PK, Terzis JK. A histomorphometric analysis of the cross-facial nerve graft in the treatment of facial paralysis. J Reconstr Microsurg. 1996;12:375–382. [DOI] [PubMed] [Google Scholar]
- 13.MacQuillan AHF, Grobbelaar AO. Functional muscle transfer and the variance of reinnervating axonal load: part I. The facial nerve. Plast Reconstr Surg. 2008;121:1570–1577. [DOI] [PubMed] [Google Scholar]
- 14.Socolovsky M, Di Masi G, Bonilla G, et al. Spinal to accessory nerve transfer in traumatic brachial plexus palsy: is body mass index a predictor of outcome? Acta Neurochir (Wien). 2014;156:159–163. [DOI] [PubMed] [Google Scholar]
- 15.Howell SM, Imobersteg AM, Seger DH, et al. Clarification of the role of the supraspinatus muscle in shoulder function. J Bone Joint Surg Am. 1986;68:398–404. [PubMed] [Google Scholar]
- 16.McCully SP, Suprak DN, Kosek P, et al. Suprascapular nerve block results in a compensatory increase in deltoid muscle activity. J Biomech. 2007;40:1839–1846. [DOI] [PubMed] [Google Scholar]
- 17.Reed D, Cathers I, Halaki M, et al. Does supraspinatus initiate shoulder abduction? J Electromyogr Kinesiol. 2013;23:425–429. [DOI] [PubMed] [Google Scholar]
- 18.Crouch DL, Li Z, Barnwell JC, et al. Computer simulation of nerve transfer strategies for restoring shoulder function after adult C5 and C6 root avulsion injuries. J Hand Surg Am. 2011;36:1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teboul F, Bizot P, Kakkar R, et al. Surgical management of trapezius palsy. J Bone Joint Surg Am. 2004;86:1884–1890. [DOI] [PubMed] [Google Scholar]
- 20.Wiater JM, Bigliani LU. Spinal accessory nerve injury. Clin Ortho Related Res. 1999;5–16. [PubMed] [Google Scholar]
- 21.El Ghani F, Van Den Brekel MW, De Goede CJ, et al. Shoulder function and patient well-being after various types of neck dissections. Clin Otolaryngol Allied Sci. 2002;27:403–408. [DOI] [PubMed] [Google Scholar]
- 22.Erisen L, Basel B, Irdesel J, et al. Shoulder function after accessory nerve-sparing neck dissections. Head Neck. 2004;26:967–971. [DOI] [PubMed] [Google Scholar]
- 23.Kelley MJ, Kane TE, Leggin BG. Spinal accessory nerve palsy: associated signs and symptoms. J Orthop Sports Phys Ther. 2008;38:78–86. [DOI] [PubMed] [Google Scholar]
- 24.Elhassan B. Lower trapezius transfer for shoulder external rotation in patients with paralytic shoulder. J Hand Surg Am. 2014;39:556–562. [DOI] [PubMed] [Google Scholar]
- 25.Brumback RJ, McBride MS, Ortolani NC. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg Am. 1992;74:377–382. [PubMed] [Google Scholar]
- 26.Glassey N, Perks GB, McCulley SJ. A prospective assessment of shoulder morbidity and recovery time scales following latissimus dorsi breast reconstruction. Plast Reconstr Surg. 2008;122:1334–1340. [DOI] [PubMed] [Google Scholar]
- 27.Lee KT, Lee YJ, Kim A, et al. Evaluation of donor morbidity following single-stage latissimus dorsi neuromuscular transfer for facial reanimation. Plast Reconstr Surg. 2019;143:152e–164e. [DOI] [PubMed] [Google Scholar]
- 28.Leechavengvongs S, Teerawutthichaikit T, Witoonchart K, et al. Surgical anatomy of the axillary nerve branches to the deltoid muscle. Clin Anat. 2015;28:118–122. [DOI] [PubMed] [Google Scholar]
- 29.Crouch DL, Plate JF, Li Z, et al. Biomechanical contributions of posterior deltoid and teres minor in the context of axillary nerve injury: a computational study. J Hand Surg Am. 2013;38:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khair MM, Schreiber JJ, Rosenblatt L, et al. Axon counts yield multiple options for triceps fascicular nerve to axillary nerve transfer. J Hand Surg Am. 2016;41:e405–e410. [DOI] [PubMed] [Google Scholar]
- 31.Schreiber JJ, Byun DJ, Khair MM, et al. Optimal axon counts for brachial plexus nerve transfers to restore elbow flexion. Plast Reconstr Surg. 2015;135:135e–141e. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K, Doi K, Hattori Y, et al. Long-term results of spinal accessory nerve transfer to the suprascapular nerve in upper-type paralysis of brachial plexus injury. J Reconstr Microsurg. 2007;23:295–299. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto M, Hirata H, Nishiyama M, et al. Schwann cells can induce collateral sprouting from intact axons: experimental study of end-to-side neurorrhaphy using a Y-chamber model. J Reconstr Microsurg. 1999;15:281–286. [DOI] [PubMed] [Google Scholar]
- 34.Tarasidis G, Watanabe O, Mackinnon SE, et al. End-to-side neurorrhaphy resulting in limited sensory axonal regeneration in a rat model. Ann Otol Rhinol Laryngol. 1997;106:506–512. [DOI] [PubMed] [Google Scholar]
- 35.Tarasidis G, Watanabe O, Mackinnon SE, et al. End-to-side neurorraphy: a long-term study of neural regeneration in a rat model. Otolaryngol Head Neck Surg. 1998;119:337–341. [DOI] [PubMed] [Google Scholar]
- 36.Viterbo F, Trindade JC, Hoshino K, et al. Two end-to-side neurorrhaphies and nerve graft with removal of the epineural sheath: experimental study in rats. Br J Plast Surg. 1994;47:75–80. [DOI] [PubMed] [Google Scholar]
- 37.Lundborg G, Zhao Q, Kanje M, et al. Can sensory and motor collateral sprouting be induced from intact peripheral nerve by end-to-side anastomosis? J Hand Surg Br. 1994;19:277–282. [DOI] [PubMed] [Google Scholar]
- 38.Tham SK, Morrison WA. Motor collateral sprouting through an end-to-side nerve repair. J Hand Surg Am. 1998;23:844–851. [DOI] [PubMed] [Google Scholar]