Abstract
An unusual property, human leukemic cell-recognizing activity, associated with parasporal inclusions of a noninsecticidal Bacillus thuringiensis soil isolate was investigated, and a protein (named parasporin in this study) responsible for the activity was cloned. The parasporin, encoded by a gene 2,169 bp long, was a polypeptide of 723 amino acid residues with a predicted molecular weight of 81,045. The sequence of parasporin contained the five conserved blocks commonly found in B. thuringiensis Cry proteins; however, only very low homologies (<25%) between parasporin and the existing classes of Cry and Cyt proteins were detected. Parasporin exhibited cytocidal activity only when degraded by proteases into smaller molecules of 40 to 60 kDa. Trypsin and proteinase K activated parasporin, while chymotrypsin did not. The activated parasporin showed strong cytocidal activity against human leukemic T cells (MOLT-4) and human uterus cervix cancer cells (HeLa) but not against normal T cells.
Bacillus thuringiensis, a gram-positive and endospore-forming bacterium, produces large crystalline parasporal inclusions during sporulation. The inclusions often contain the δ-endotoxin proteins that are highly and specifically toxic to agriculturally and medically important insect pests of several orders, including Lepidoptera, Diptera, and Coleoptera (1). The strong and rapid insecticidal activity of inclusions makes B. thuringiensis an environmentally sound biological agent for pest control (3, 13). Earlier studies, however, have reported that noninsecticidal B. thuringiensis strains are more widely distributed than insecticidal ones (7, 16, 17, 19–21, 24). This raises the question of whether such noninsecticidal inclusions have any biological activity which is as yet undiscovered (22). In a preceding study (18), we reported that strong cytocidal activities against human cancer cells are often associated with noninsecticidal inclusion proteins of B. thuringiensis. The proteins were heterogeneous in cytotoxicity spectra and in activity levels as well. Of these, the proteins of a B. thuringiensis strain, designated 84-HS-1-11, discriminated between leukemic and normal T cells and specifically killed the former cells (18).
This paper describes a further characterization of the 84-HS-1-11 inclusions and the cloning of a novel gene encoding a protein which may have clinical value. The protein constitutes a new family of B. thuringiensis endotoxin, the parasporin, functioning as a toxin which is preferential for human cancer cells.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The organism used in this study was the B. thuringiensis soil isolate 84-HS-1-11 from Hiroshima Prefecture, Japan (18). An acrystalliferous mutant strain, designated BFR1, generated from a strain of B. thuringiensis serovar kurstaki (H3abc) in this laboratory was used for expression of the toxin gene. The vector Lambda ZAP II was grown in Escherichia coli XL-1 Blue MRF′. The pBluescript SK(−) phagemid was excised from Lambda ZAP II using E. coli SOLR. The vector and E. coli strains were purchased from Stratagene, La Jolla, Calif.
The cloned gene was expressed in B. thuringiensis by using the E. coli-B. thuringiensis shuttle vector pHY300 (Takara Shuzo Co., Kyoto, Japan). The antibiotic concentrations for transformant selection were 25 μg of tetracycline per ml for B. thuringiensis and 50 μg of ampicillin per ml for E. coli.
Human and insect cells and culture condition.
The human cell lines used were MOLT-4 (leukemic T cell), HeLa (uterus cervix cancer cell), A549 (lung cancer cell), and MRC-5 (normal lung fibroblast cell). This study also examined two insect cell lines: BM-N, from silkworms (Bombyx mori), and NIAS-AeA1-2, from mosquitoes (Aedes albopictus). Cell lines were obtained from RIKEN Cell Bank (Tsukuba, Japan) and were maintained under the conditions recommended by the supplier. Normal human T cells were prepared from buffy coats supplied from Fukuoka Red Cross Blood Center (Fukuoka, Japan). They were separated from lymphocytes as described previously (18) and were cultured in RPMI 1640 medium containing 10% fetal bovine serum and kanamycin (30 μg ml−1) at 37°C. Human erythrocytes of blood group O were prepared from a volunteer.
EM.
The strain 84-HS-1-11 was cultured on nutrient agar at 28°C for 2 days. The sporulating culture was harvested and washed in distilled water by centrifugation. For transmission electron microscopy (EM), ultrathin sections were prepared by the method described previously (9).
DNA manipulations.
Restriction enzymes and alkaline phosphatase were used for gene manipulation as recommended by the manufacturer (Takara Shuzo Co.). Isolation of total DNA from the strain 84-HS-1-11 was performed as described previously (10). Plasmid DNA was extracted from E. coli by using a QIAprep spin miniprep kit (QIAGEN, Hilden, Germany).
Cloning, subcloning, and expression of the toxin gene.
For cloning of the cytocidal protein gene of the strain 84-HS-1-11, a genomic library was made from a total DNA preparation using an EcoRI-NotI adapter (Amersham Pharmacia Biotech, Uppsala, Sweden) and a Lambda ZAP II/EcoRI/CIAP cloning kit (Stratagene). In brief, a mixture of the size-selected (2- to 8-kb) AluI and AfaI fragments of the total DNA were mixed and ligated with the EcoRI-NotI adapter, and the fragments were then integrated into the EcoRI-cut Lambda ZAP II vector. The recombinant Lambda ZAP DNA was packaged with Gigapack III Gold (Stratagene) according to the manufacturer's instructions and used to infect host cells, E. coli XL-1 Blue. Selection of positive plaques was done by using a picoBlue immunoscreening kit (Stratagene) with polyclonal antibodies against whole inclusion proteins of the strain 84-HS-1-11. The antibodies were raised in rabbits according to the method of Ishii and Ohba (11). After purification of the plaques, DNA clones were cut out as pBluescript SK(−) plasmids in the bacterial host E. coli SOLR in accordance with the in vivo excision protocol of the manufacturer. One of the excised plasmids was designated pLEUK3.4, and the plasmid pLEUK3.4S was constructed by inserting a SalI-SmaI fragment from pLEUK3.4 into the SalI-SmaI site of the plasmid pHY300.
Plasmid DNA of pLEUK3.4S was mixed at a final concentration of 5.0 μg ml−1 with a cell suspension of the acrystalliferous BFR1 strain prepared by the method of Mahillon et al. (15). The plasmid was introduced through electroporation into strain BFR1 in a 0.2-cm cuvette by using a BTX ECM 600 apparatus set (BTX Inc., San Diego, Calif.) at 2.5 kV and 186 Ω. B. thuringiensis transformants were screened on CYS medium (29) containing erythromycin at 28°C. Inclusion formation was checked under a phase-contrast microscope.
Gene sequencing.
A dideoxy sequencing procedure, using a dye terminator cycle sequencing FS ready reaction kit (Perkin-Elmer, Foster City, Calif.), was employed to establish the nucleotide sequences. The sequences were determined by an automatic sequencer (model 373S; Perkin-Elmer) and were analyzed by the DNASIS program from Hitachi Software Engineering, Kanagawa, Japan.
Preparation of inclusion proteins, proteolytic processing, and toxin activation.
The spore-inclusion mixture was harvested from sporulated cultures and the inclusions were partially purified by a biphasic separation method (6) using polyethylene glycol 6000 (Wako Pure Chemical, Osaka, Japan) and sodium dextran sulfate 500 (Sigma, St. Louis, Mo.). Inclusions were further purified by sucrose density gradient centrifugation as previously reported (25). The purified inclusions were stored at −20°C until use.
Solubilization of purified inclusions was done in 50 mM Na2CO3 (pH 10.0) containing 1 mM EDTA and 10 mM dithiothreitol for 1 h at 37°C. After centrifugation at 20,000 × g for 5 min at 4°C to remove unsolubilized materials, the pH of the solution was adjusted to 8.0. The solubilized proteins (1.3 mg ml−1) were treated with proteinase K (final concentrations, 0.0003, 0.003, 0.03, and 0.3 mg ml−1), trypsin (0.03, 0.3, 3, and 30 mg ml−1), and chymotrypsin (0.03, 0.3, 3, and 30 mg ml−1) in 50 mM Na2CO3 (pH 10.0) for 1.5 h at 37°C. After protease treatment, phenylmethylsulfonyl fluoride (Wako Pure Chemical) was added to the solution to stop the proteolytic reaction, and the mixture was examined for both sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) profiles and cytopathic effect (CPE) on MOLT-4 cells. The CPE was monitored under a phase-contrast microscope for 24 h, and the degree of cytopathy was graded on the basis of the ratio of damaged cells as described previously (18).
One-dose assay, hemolytic assay, and dose-response study.
One-dose assays for cytotoxicity and hemolytic activity were carried out as described previously (18). Each well of a MicroTest plate received 90 μl of cell suspension containing 2 × 104 cells. After preincubation for 16 h at 37°C, 10 μl of the proteinase K-activated sample solution (1.3 mg ml−1) was added to the well. Five species of human cells and two species of insect cell lines were used for one-dose cytotoxicity assays. A hemolytic assay was done using human erythrocytes according to the method of Saitoh et al. (26).
Three human cells (MOLT-4 cells, normal T cells, and HeLa cells) were used for dose-response studies. Each well containing 90 μl of cell suspension (2 × 104 cells) received 10 μl of proteinase K-activated inclusion proteins which had been prepared in 10-fold serial dilutions in 50 mM Na2CO3 (pH 10.0) containing 10 mM DTT and 1 mM EDTA. Five wells were used for each dilution, and the test was repeated at least three times. The CPE was monitored under a phase-contrast microscope at appropriate intervals for 24 h postinoculation.
For assessment of the level of cytotoxicity, a cell proliferation test using an MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide] assay (2, 8) was conducted 24 h postinoculation by using a Premix WST-1 kit (Takara Co.). The average of absorbance in mock-inoculated negative controls was used as a blank value. The arbitrary unit was defined on the basis of the relative value of absorbance at 450 nm to the blank (1.0). The 50% effective concentrations (EC50s) were deduced from the dose-response curves using a log-probit program.
Insecticidal activity test.
Insecticidal activity against the diamondback moth (Plutella xylostella [Lepidoptera]) and the mosquito (Culex pipiens molestus [Diptera]) was tested with one-dose assays according to the method of Higuchi et al. (9).
N-terminal and internal sequencing of parasporal inclusion proteins.
The 81-kDa inclusion protein of 84-HS-1-11 and its V8 protease-digested proteins, resolved by SDS-PAGE, were transferred to a polyvinylidene difluoride membrane (Bio-Rad) for determination of their NH2-terminal sequences using an automatic sequencer model 473A (Applied Biosystems, Foster City, Calif.). V8 protease digestion of inclusion protein was carried out by the method of Cleveland et al. (4).
Protein determination and analysis.
The concentration of inclusion proteins was determined by the method of Lowry et al. (14) using bovine serum albumin as the standard. SDS-PAGE and immunoblotting were carried out as described previously by Saitoh et al. (26).
Nucleotide sequence accession number.
The nucleotide sequence obtained here has been deposited in the DDBJ, GenBank, and EMBL databases (accession no. AB031065).
RESULTS
Parasporal inclusion morphology.
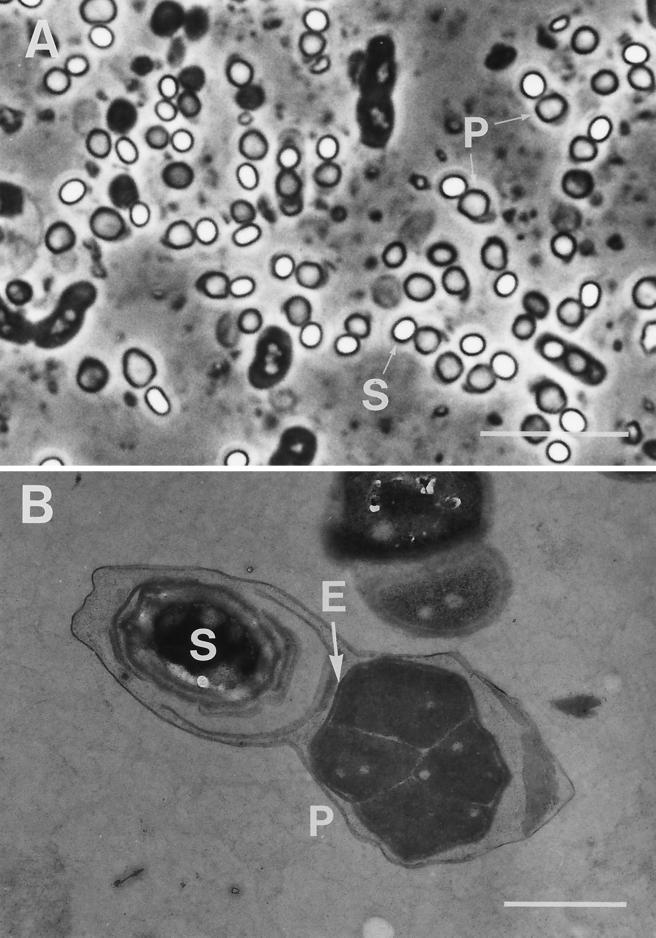
Parasporal inclusions of the strain 84-HS-1-11 were roughly spherical when observed with a phase-contrast microscope (Fig. 1A). The inclusions had diameters ranging from 0.58 to 1.66 μm and were often larger than the spores. When observed with transmission EM, the inclusion was a polygonal body with several unpointed corners, surrounded by an envelope (Fig. 1B). Homogeneous, electron-dense material was a component of the inclusion matrix, which was separated by electron-lucent narrow spaces into four to six parts. Spore and inclusion were coenveloped by a thin exosporium membrane.
FIG. 1.
Phase-contrast and electron micrographs of parasporal inclusions of B. thuringiensis isolate 84-HS-1-11. (A) Phase-contrast photograph of a sporulating culture. (B) Transmission electron micrograph of a sporangium containing a maturated spore and a parasporal inclusion. S, spore; P, parasporal inclusion; E, electron-dense envelope. Bars, 10 μm (A) and 1 μm (B).
Proteolytic processing and toxin activation.
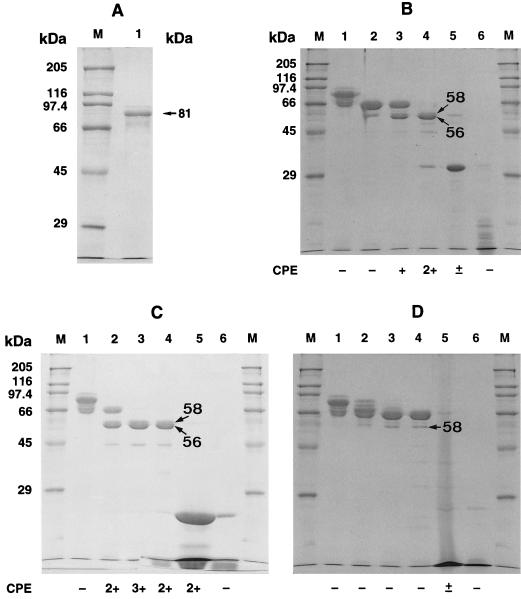
Purified parasporal inclusions of strain 84-HS-1-11 contained a single protein of 81 kDa (Fig. 2A). Figure 2B through D show the results of processing alkali-solubilized inclusion proteins with the three proteases. Upon treatment with proteinase K, the 81-kDa protein was degraded into four proteins with molecular masses of 66, 58, 56, and 44 kDa. Trypsin treatment gave a similar proteolysis profile. Chymotrypsin, however, produced a different profile lacking the 56-kDa protein.
FIG. 2.
SDS-PAGE profiles of native and protease-treated parasporal inclusion proteins of B. thuringiensis isolate 84-HS-1-11. (A) Native parasporal inclusion proteins (lane 1). (B) Proteinase K-digested inclusion proteins. Lane 1, alkali-solubilized inclusion proteins; lanes 2 through 5, alkali-solubilized and proteinase K (0.0003, 0.003, 0.03, and 0.3 mg ml−1)-treated inclusion proteins; lane 6, proteinase K (0.3 mg ml−1). (C) Trypsin-treated inclusion proteins. Lane 1, alkali-solubilized proteins: lanes 2 through 5, alkali-solubilized and trypsin (0.03, 0.3, 3, and 30 mg ml−1)-digested proteins; lane 6, trypsin (30 mg ml−1). (D) Chymotrypsin-digested inclusion proteins. Lane 1, alkali-solubilized proteins; lanes 2 through 5, alkali-solubilized and chymotrypsin (0.03, 0.3, 3, and 30 mg ml−1)-digested proteins; lane 6, chymotrypsin (30 mg ml−1). Lanes 1 through 5 in panels B, C, and D each contained 12 μg of inclusion proteins, and lane 1 in panel A contained 5 μg of inclusion proteins. Molecular masses of standard protein markers are indicated on the left. The degree of CPE on MOLT-4 cells, graded as described previously (18), is shown under each lane: 3+, high; 2+, moderate; +, low; ±, very low; −, no cell damage.
The protein exhibited cytotoxicity against MOLT-4 cells when treated with trypsin or proteinase K. No cytocidal activity was induced after treatment with chymotrypsin. The greatest activity was associated with a sample (Fig. 2C, lane 3) digested with trypsin at a concentration of 300 μg ml−1. The SDS-PAGE profile of this sample contained three major bands with molecular masses of 58, 56, and 44 kDa. Without protease digestion, inclusion proteins showed no cytocidal toxicity.
Gene cloning and sequencing.
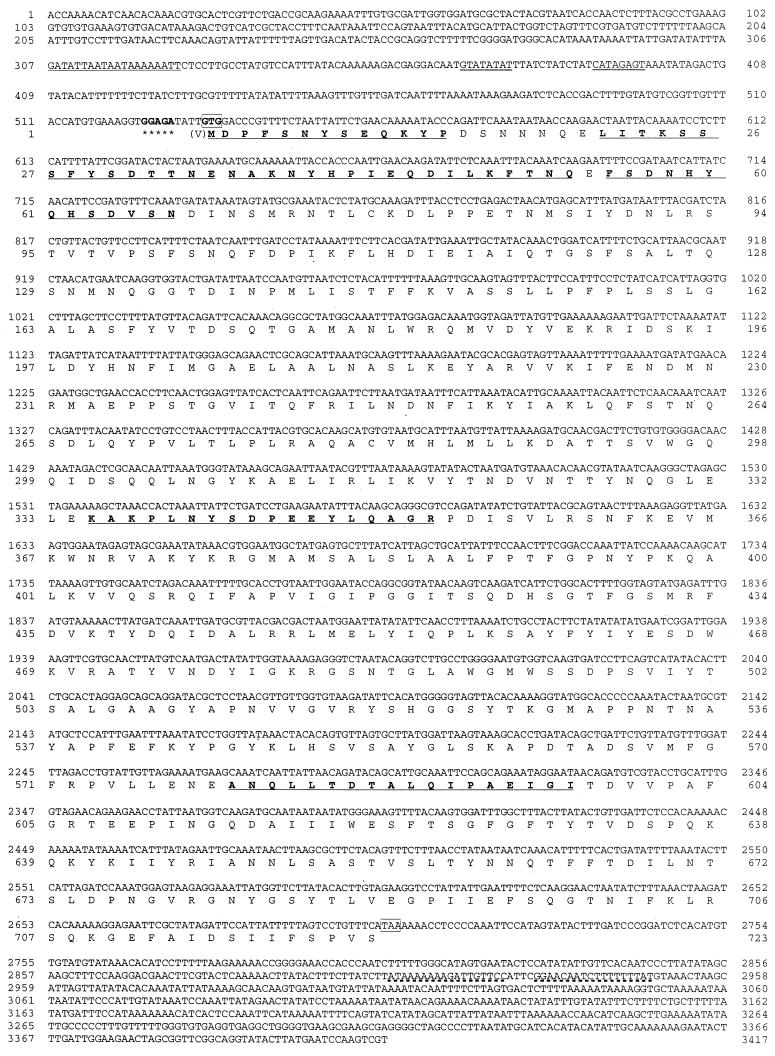
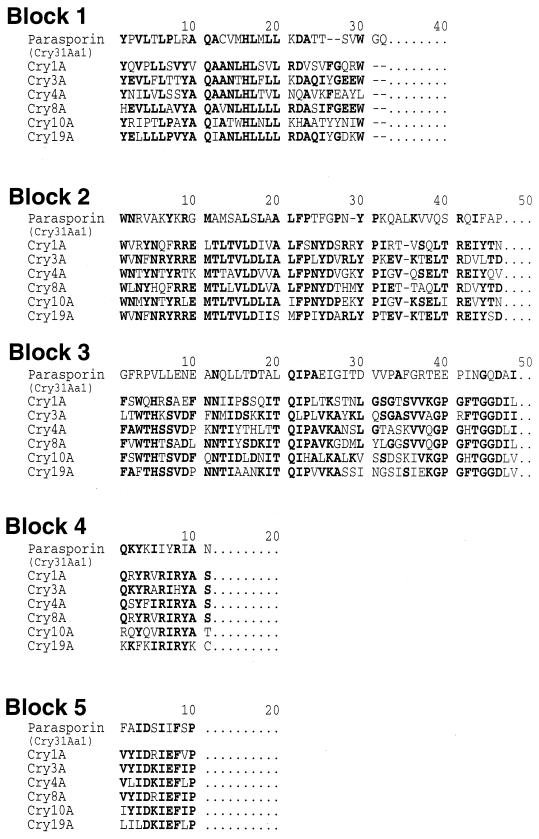
Positive plaques were selected by an immunoscreening technique from a genomic library constructed in Lambda ZAP II. A recombinant pBluescript plasmid (pLEUK3.4), harboring a 3.4-kb DNA insert, was obtained from one of the positive plaques by in vivo excision. Nucleotide sequencing showed that only one cry gene existed in this insert (Fig. 3). The gene was 2,169 bp long, encoding a polypeptide of 723 amino acid residues with a predicted molecular weight of 81,045. The start codon was not ATG but GTG. A putative ribosome binding site, GGAGA, was located four bases upstream from the start codon, and the putative promoter was located 137 bp upstream from the start codon, GTG, of the 81-kDa protein. A typical terminator sequence was identified 202 bp downstream from the stop codon. Six partial sequences, one N terminal and five internal, of a wild-type 84-HS-1-11 protein were all contained in the deduced amino acid sequence of the cloned 81-kDa protein (Fig. 3). The five block sequences conserved in the Cry proteins were also detectable in the sequence of the 81-kDa protein. As shown in Fig. 4, however, the sequence homologies between our protein and the five established Cry proteins in each block were very low. In particular, the third block of the 81-kDa protein was substantially different from those of the other Cry proteins. The overall sequence of the 81-kDa protein was dissimilar to that of the existing classes of the Cry proteins, showing a low homology (<25%).
FIG. 3.
Nucleotide sequence of the parasporin gene, cry31Aa1, and the deduced amino acid sequence. The putative promoter region is double underlined. A ribosome-binding site is marked with asterisks. The start and stop codons are boxed. A terminator sequence is underlined by a dashed line. An N-terminal and five internal amino acid sequences of a wild-type 81-kDa protein, as determined in this study, are underlined. Numbers on the sides are the numbers of nucleotide (upper) and amino acids (lower).
FIG. 4.
Comparison of the deduced amino acid sequences of the five regions in parasporin (Cry31Aa1) with those of the conserved blocks in B. thuringiensis δ-endotoxin Cry proteins. Highly conserved residues are in bold.
Gene expression.
For gene expression, a plasmid, pLEUK3.4S, was constructed from pLEUK3.4 by using the E. coli-B. thuringiensis shuttle vector pHY300. When transformed by pLEUK3.4S, the acrystalliferous B. thuringiensis strain BFR1 formed large, irregularly shaped inclusions (not shown). No inclusions were formed in the control; BFR1 contained the shuttle vector pHY300 alone. Figure 5 shows the results of SDS-PAGE and immunoblotting of the inclusion protein from the recombinant pLEUK3.4S. The inclusion contained 81-, 66-, and 57-kDa proteins. Antibodies against whole inclusion proteins of strain 84-HS-1-11 reacted to the three proteins (Fig. 5B). N-terminal sequences of the three proteins were all contained in the sequence deduced from the nucleotide sequence of the 81-kDa protein gene (data not shown).
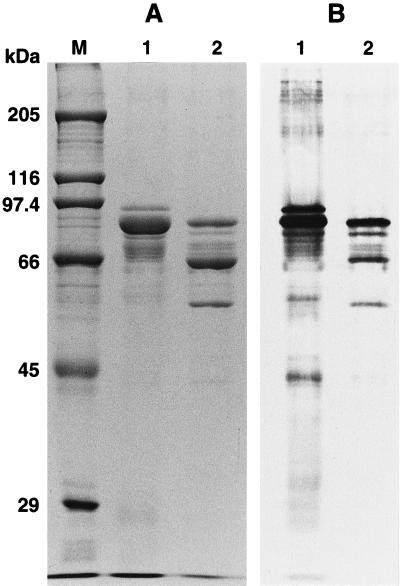
FIG. 5.
SDS-PAGE and immunoblot analysis of the cloned parasporin (Cry31Aa1) protein. (A) Purified inclusions were subjected to SDS–10% PAGE and stained with Coomassie brilliant blue. (B) Immunoblotting was done with antibodies against solubilized total inclusions of the B. thuringiensis strain 84-HS-1-11 (lane 1) and B. thuringiensis BFR1(pLEUK3.4S) (lane 2). Lane M, molecular marker. Lanes 1 and 2 (A and B) contained 5 and 0.5 μg of inclusion protein, respectively.
One-dose assay.
Table 1 shows the results of one-dose assays of proteinase K-activated proteins against several species of cultured cells. The toxicity spectrum of the protein from the recombinant BFR1(pLEUK3.4S) was similar to that of the protein of the wild strain 84-HS-1-11. Both cloned and wild-type proteins were highly or moderately cytocidal for MOLT-4 cells, HeLa cells, and normal human lung cells but were slightly toxic or nontoxic for normal T cells, lung cancer cells (A549), and two insect cell lines. Both proteins had no insecticidal activities against the lepidopteran and dipteran insects tested and no hemolytic activity against human erythrocytes.
TABLE 1.
In vitro cytocidal and in vivo insecticidal activities of purified inclusion proteins from B. thuringiensis strains
| Strain | Hemolysis on human erythrocytes | Cytocidal activitya against:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Human T cells
|
Human lung cells
|
Human uterus cervix cancer cells | Insect cells
|
Insecticidal activity againstb:
|
||||||
| Leukemic | Normal | Cancer | Normal | B. mori | A. albopictus | P. xylostella | C. pipiens molestus | |||
| 84-HS-1-11 | − | ++ (++) | − (−) | + (±) | ++ (+) | +++ (+++) | − (+) | − (+) | − | − |
| BFR1(pLEUK3.4S) | − | ++ (+++) | − (−) | + (−) | ++ (+) | +++ (+++) | − (−) | − (+) | − | − |
Alkali-solubilized, protease-treated inclusion proteins (124 μg ml−1) were examined for cytotoxic activities on MOLT-4 (human leukemic T cells), normal human T cells (primary culture), A549 (human lung cancer cells), MRC-5 (normal human lung fibroblast cells), HeLa (human uterus cervix cancer cells), BM-N (B. mori silkworm cells), and NIAS-AeAI-2 (A. albopictus mosquito cells). The results of microscopic observations are shown, and the levels of cytotoxicity, assessed by cell proliferation assay, are presented in parentheses. The degree of cell damage was graded as follows: high (+++), moderate (++), low (+), very low (±), and no damage (−).
Purified inclusions were examined for insecticidal activity against larvae of the diamonback moth, P. xylostella, and the mosquito C. pipiens molestus. Ten fourth-instar larvae of P. xylostella were fed on an artificial diet (0.5 g) contaminated with 160 μg of purified inclusion. The test with C. pipiens molestus was done by introducing ten 4-day-old larvae into wells of 12-well tissue culture plates. Each well contained 160 μg of purified inclusion and 5 mg of diet in 3 ml of deionized water.
CPE.
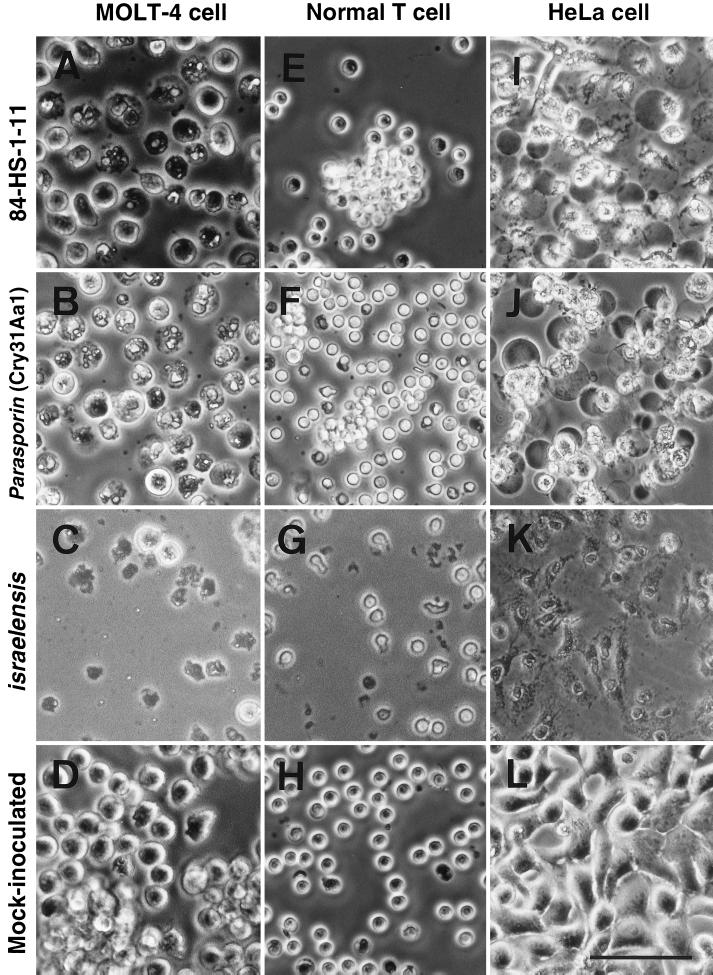
Figure 6 shows the cytopathy induced by the proteinase K-activated inclusion proteins of the three strains: the 84-HS-1-11, the recombinant BFR1(pLEUK3.4S), and the type strain of Bacillus thuringiensis serovar Israelensis. The B. thuringiensis serovar Israelensis protein exhibited strong CPE on all of the human (MOLT-4, HeLa, and normal T) cells tested as early as 15 to 30 min after inoculation of the protein. In contrast, both the 84-HS-1-11 protein and the cloned protein showed CPE on MOLT-4 and HeLa cells 8 to 10 h after administration of the protein. No detectable cytopathy was induced on normal T cells by inoculation of the 84-HS-1-11 protein and the cloned protein (Fig. 6E and F). In susceptible cell lines, these two proteins induced similar cytopathological changes characterized by the granulation of the cytoplasm and marked cell ballooning (Fig. 6A, B, I, and J). The cytopathy induced by the B. thuringiensis serovar Israelensis protein was characterized by drastic cell lysis with no cell ballooning (Fig. 6C, G, and K).
FIG. 6.
Cytopathic effect of proteinase K-treated parasporal inclusion proteins of B. thuringiensis strain 84-HS-1-11 (A, E, and I), the recombinant BFR1(pLEUK3.4S) (B, F, and J), and the type strain of B. thuringiensis serovar Israelensis (C, G, and K) on MOLT-4 (leukemic T cells), normal T cells, and HeLa cells. (D, H, and L) Mock-inoculated cells. All images were obtained by phase-contrast microscopy at 24 h postinoculation. Bar, 50 μm (A through H) and 75 μm (I through L).
Dose-response analysis.
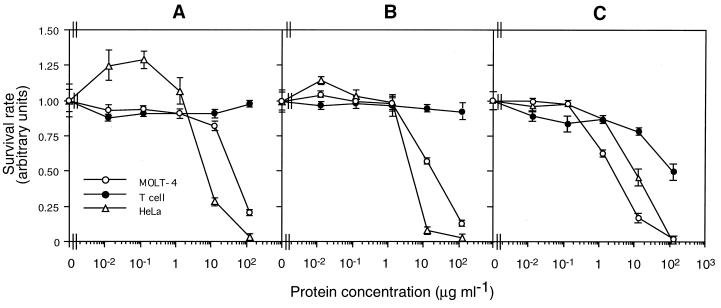
Figure 7 shows the results of the dose-response study with proteinase K-treated proteins of the three strains: strain 84-HS-1-11, the recombinant BFR1 (pLEUK3.4S), and the type strain of B. thuringiensis serovar Israelensis. Proteins from the strain 84-HS-1-11 and the recombinant strain exhibited a similar dose-dependent activity, highly toxic to cancer cells (MOLT-4 and HeLa), while they were not active on normal T cells (Fig. 7A and B). In contrast, the B. thuringiensis serovar Israelensis protein showed dose-dependent cytotoxicity against all of the three cell lines tested (Fig. 7C). EC50s of the three proteins are summarized in Table 2. The values for the cloned protein from the recombinant strain were smaller than those for the 84-HS-1-11 protein. The results obtained with these two proteins also showed that the values for HeLa cells were significantly smaller than those for MOLT-4 cells. Similar EC50s for HeLa cells were associated with the three proteins.
FIG. 7.
Dose-response curves of cytotoxicity associated with proteinase K-treated parasporal inclusion proteins of B. thuringiensis strain 84-HS-1-11 (A), the recombinant B. thuringiensis(pLEUK3.4S) (B), and the type strain of B. thuringiensis serovar Israelensis (C) against MOLT-4, normal T, and HeLa cells. The vertical bar represents the standard deviation.
TABLE 2.
Cytocidal activity of purified inclusions from B. thuringiensis strains
| Organism | Inclusion component | EC50 (μg ml−1)a
|
||
|---|---|---|---|---|
| MOLT-4 cells | Normal T cells | HeLa cells | ||
| 84-HS-1-11 | Wild type | 36.6 (32.8–40.9) | >130 | 8.7 (8.1–9.3) |
| BFR1(pLEUK3.4) | Parasporin (Cry31Aa1) | 14.6 (13.5–15.7) | >130 | 5.8 (5.4–6.1) |
| B. thuringiensis serovar Israelensis | Cyt1Ab | 2.4 (2.1–2.6) | 126.2 (101.7–160.3) | 8.6 (7.9–9.3) |
Values were calculated on the basis of 24-h cell mortalities using a log-probit program. The fiducial limit at the 95% level is given parentheses.
Dipteran-specific Cry proteins (Cry4A, Cry4B, and Cry11A) coexist in inclusions (3).
DISCUSSION
Recently, we reported the occurrence of a unique B. thuringiensis isolate, designated 84-HS-1-11, that produces parasporal inclusions consisting of an 81-kDa protein with a cytocidal activity preferential for leukemic cells (18). We report herein the results of a further characterization of this isolate and the activity and cloning of a novel gene encoding a leukemic-cell-killing protein.
Phase-contrast microscopy showed that the inclusions of the isolate 84-HS-1-11 are spherical bodies which are often far bigger than the spores. This is consistent with our previous observation (18). However, EM studies revealed that at the ultrastructural level, the inclusions are roughly polygonal. It is interesting that the homogeneous electron-dense matrix is divided into several parts by the narrow spaces. To date, no similar feature has been reported in parasporal inclusions of the other known B. thuringiensis strains. SDS-PAGE analysis clearly showed that the 81-kDa protein is the only component in inclusions of the isolate 84-HS-1-11. This is in good agreement with the result of EM observation, which showed that the inclusion matrix was homogeneous in appearance.
There was no cytocidal activity in alkali-solubilized protein. The activity was observed only when alkali-solubilized protein was treated with proteases. It is clear from the results that the proteolytic processing is indispensable for activation of the 84-HS-1-11 proteins. This is analogous to the activation of insecticidal Cry and Cyt proteins of B. thuringiensis by proteases (12). Proteinase K and trypsin were effective for activation of the toxin. Interestingly, however, chymotrypsin treatment produced no cytotoxic proteins. Proteolytic activation by proteinase K or trypsin generated four proteins with molecular masses of 66, 58, 56, and 44 kDa. No data, however, are presently available for cell-killing activity of the individual proteins, and the toxic moiety awaits definitive clarification.
It is clear that the 81-kDa protein cloned in this study is identical to the single major protein contained in the 84-HS-1-11 inclusion. This is supported by the following evidence: (i) the two proteins have the same electrophoretic mobility, (ii) polyclonal antibodies against the 84-HS-1-11 protein strongly reacted with cloned protein, (iii) a 100% homology exists between the proteins in the N-terminal and five internal sequences, and (iv) the cloned protein and the 84-HS-1-11 protein have the same cytotoxicity spectrum. When inclusions of the cloned protein were subjected to SDS-PAGE, three bands of 81, 66, and 57 kDa were detected. It appeared from the results of immunoblotting and N-terminal sequencing that the 66- and 57-kDa proteins are the partially degraded forms of the 81-kDa protein.
The 81-kDa protein showed very low amino acid sequence homology (<25%) to the existing Cry and Cyt proteins of B. thuringiensis (5). Obviously, this protein is not allied to the known classes of insecticidal Cry and Cyt proteins and constitutes a new class of Cry protein, designated Cry31Aa1 by the Bacillus thuringiensis Pesticide Crystal Protein Nomenclature Committee (see N. Crickmore's nomenclature website at http://epunix.biols.susx.ac.uk./Home/Neil_Crickmore/Bt/index.html).
The overall sequencing data, coupled with unusual biological activity of the protein, support the creation of a new category of protein, the parasporin, defined as the bacterial parasporal protein that is capable of discriminately killing cancer cells. In a preceding study (18), we found that the parasporal protein(s) from another B. thuringiensis isolate, 89-T-26-17, had a similar cytocidal spectrum, active on leukemic T cells but nontoxic to normal T cells. Thus, the protein of this isolate is likely related to the parasporin.
Natural killer cells produce a cytotoxic factor known as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which induces apoptosis specifically in cancer cells (28). The high specificity of TRAIL in causing apoptosis in cancer cells is attributable to specific binding of the ligand to receptors that reside on the cell membrane (23, 27), but it is presently uncertain whether apoptosis is involved in the mechanism of cell killing by parasporin. However, it is of particular interest that the two genealogically unrelated proteins, TRAIL and parasporin, from human and prokaryote sources, respectively, are similar in their specificity for killing cancer cells.
The B. thuringiensis serovar Israelensis inclusion proteins killed not only cancer cells (leukemic T cells and HeLa cells) but normal T cells. This is consistent with the results obtained in our preceding study (18). It is well established that the Cyt protein is responsible for broad-spectrum cytolytic activity in parasporal inclusions of B. thuringiensis serovar Israelensis (12). Of particular interest is that the cytopathological changes caused by parasporin are substantially different from that induced by the B. thuringiensis serovar Israelensis Cyt protein. Apparently, these two proteins have different modes of action, leading to the different pathological events in a given susceptible cell species. Experiments to elucidate the mechanism of discrimination between leukemic and normal T cells by parasporin are under way. Identification of the cell receptor, if any, may provide insights into the specificity of this unique protein.
ACKNOWLEDGMENTS
We thank D. R. Zeigler of the Bacillus thuringiensis Pesticide Crystal Protein Nomenclature Committee for invaluable advice on the classification and numbering of the Cry31Aa1 protein, parasporin. We also thank T. Kawarabata, Kyushu University, and Y. Sasaguri, University of Occupation and Environmental Health, for invaluable advice.
REFERENCES
- 1.Beegle C C, Yamamoto T. History of Bacillus thuringiensis Berliner research and development. Can Entomol. 1992;124:587–616. [Google Scholar]
- 2.Behl C, Davis J, Cole G M, Schubert D. Vitamin E protects nerve cells from amyloid β protein toxicity. Biochem Biophys Res Commun. 1992;186:944–950. doi: 10.1016/0006-291x(92)90837-b. [DOI] [PubMed] [Google Scholar]
- 3.Cannon R J C. Bacillus thuringiensis use in agriculture: a molecular perspective. Biol Rev. 1996;71:561–636. [Google Scholar]
- 4.Cleveland D W, Fischer S G, Kirschner M W, Laemmli U K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by electrophoresis. J Biol Chem. 1977;252:1102–1106. [PubMed] [Google Scholar]
- 5.Crickmore N, Zeigler D R, Feitelson J, Schnepf E, Van Rie J, Lereclus D, Baum J, Dean D H. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62:807–813. doi: 10.1128/mmbr.62.3.807-813.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman N S, Gottfried R J, Rogoff M H. Biphasic system for separation of spores and crystals of Bacillus thuringiensis. J Bacteriol. 1967;94:485. doi: 10.1128/jb.94.2.485-.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hastowo S, Lay B W, Ohba M. Naturally occurring Bacillus thuringiensis in Indonesia. J Appl Bacteriol. 1992;73:108–113. [Google Scholar]
- 8.Heiss P, Bernatz S, Bruchelt G, Senekowitsch-Schmidtke R. Cytotoxic effect of immunoconjugate composed of glucose-oxidase coupled to an anti-ganglioside (GD2) antibody on spheroids. Anticancer Res. 1997;17:3177–3178. [PubMed] [Google Scholar]
- 9.Higuchi K, Saitoh H, Mizuki E, Hwang S-H, Ohba M. A novel isolate of Bacillus thuringiensis serovar leesis that specifically exhibits larvicidal activity against the moth-fly, Telmatoscopus albipunctatus. Syst Appl Microbiol. 1998;21:144–150. doi: 10.1016/S0723-2020(98)80018-0. [DOI] [PubMed] [Google Scholar]
- 10.Hwang S-H, Saitoh H, Mizuki E, Higuchi K, Ohba M. A novel class of mosquitocidal δ-endotoxin, Cry19B, encoded by a Bacillus thuringiensis serovar higo gene. Syst Appl Microbiol. 1998;21:179–184. doi: 10.1016/s0723-2020(98)80022-2. [DOI] [PubMed] [Google Scholar]
- 11.Ishii T, Ohba M. Haemolytic activity associated with parasporal inclusion proteins of mosquito-specific Bacillus thuringiensis soil isolates: a comparative neutralization study. FEMS Microbiol Lett. 1994;116:195–200. doi: 10.1111/j.1574-6968.1994.tb06700.x. [DOI] [PubMed] [Google Scholar]
- 12.Knowles B H. Mechanism of action of Bacillus thuringiensis insecticidal δ-endotoxins. Adv Insect Physiol. 1994;24:275–308. [Google Scholar]
- 13.Lambert B, Peferoen M. Insecticidal promise of Bacillus thuringiensis. BioScience. 1992;42:112–122. [Google Scholar]
- 14.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Mahillon J, Chungjatupornchai W, Decock J, Dierickx S, Michiels F, Peferoen M, Joos H. Transformation of Bacillus thuringiensis by electroporation. FEMS Microbiol Lett. 1989;60:205–210. [Google Scholar]
- 16.Meadows M P, Ellis D J, Butt J, Jarrett P, Burges H D. Distribution, frequency, and diversity of Bacillus thuringiensis in an animal feed mill. Appl Environ Microbiol. 1992;58:1344–1350. doi: 10.1128/aem.58.4.1344-1350.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizuki E, Ichimatsu T, Hwang S-H, Park Y S, Saitoh H, Higuchi K, Ohba M. Ubiquity of Bacillus thuringiensis on phylloplanes of arboreous and herbaceous plants in Japan. J Appl Microbiol. 1999;86:979–984. [Google Scholar]
- 18.Mizuki E, Ohba M, Akao T, Yamashita S, Saitoh H, Park Y S. Unique activity associated with non-insecticidal Bacillus thuringiensis parasporal inclusions: in vitro cell-killing action on human cancer cells. J Appl Microbiol. 1999;86:477–486. doi: 10.1046/j.1365-2672.1999.00692.x. [DOI] [PubMed] [Google Scholar]
- 19.Ohba M. Bacillus thuringiensis populations naturally occurring on mulberry leaves: a possible source of the populations associated with silkworm-rearing insectaries. J Appl Bacteriol. 1996;80:56–64. [Google Scholar]
- 20.Ohba M. Three Bacillus thuringiensis flagellar serovars widely occurring in natural environments of Japan. J Basic Microbiol. 1997;37:71–76. [Google Scholar]
- 21.Ohba M, Aizawa K. Insect toxicity of Bacillus thuringiensis isolated from soils of Japan. J Invertebr Pathol. 1986;47:12–20. [Google Scholar]
- 22.Ohba M, Yu Y M, Aizawa K. Occurrence of noninsecticidal Bacillus thuringiensis flagellar serotype 14 in the soil of Japan. Syst Appl Microbiol. 1988;11:85–89. [Google Scholar]
- 23.Pan G, O'Rourke K, Chinnaiyan A M, Gentz R, Ebner R, Ni J, Dixit V M. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 24.Roh J Y, Park H W, Jin B R, Kim H S, Yu Y M, Kang S K. Characterization of novel non-toxic Bacillus thuringiensis isolated from Korea. Lett Appl Microbiol. 1996;23:249–252. doi: 10.1111/j.1472-765x.1996.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 25.Saitoh H, Higuchi K, Mizuki E, Ohba M. Larvicidal toxicity of Japanese Bacillus thuringiensis against the mosquito Anopheles stephensi. Med Vet Entomol. 1998;12:98–102. doi: 10.1046/j.1365-2915.1998.00090.x. [DOI] [PubMed] [Google Scholar]
- 26.Saitoh H, Higuchi K, Mizuki E, Hwang S-H, Ohba M. Characterization of mosquito larvicidal parasporal inclusions of a Bacillus thuringiensis serovar higo strain. J Appl Microbiol. 1998;84:883–888. doi: 10.1046/j.1365-2672.1998.00426.x. [DOI] [PubMed] [Google Scholar]
- 27.Walczak H, Degli-Esposti M A, Johnson R S, Smolak P J, Waugh J Y, Boiani N, Timour M S, Gerhart M J, Schooley K A, Smith C A, Goodwin R G, Rauch C T. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiley S R, Schooley K, Smolak P J, Din W S, Huang C P, Nicholl J K, Sutherland G R, Smith T D, Rauch C, Smith C A. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto T. Identification of entomocidal toxins of Bacillus thuringiensis by high-performance liquid chromatography. ACS Symp Ser. 1990;432:46–60. [Google Scholar]