Abstract
Purpose:
Patients with Merkel cell carcinoma (MCC) with chronic immunosuppression (IS) have worse outcomes, but the mechanisms are not well understood. We hypothesized that these differences may be mediated in part by differential response to treatment, and we evaluated whether radiation therapy (RT) efficacy is altered among IS compared with immune-competent (IC) patients with MCC.
Methods and Materials:
Among 805 patients with MCC, recurrence-free survival (RFS) and patterns of first recurrence were compared between 89 IS and 716 IC patients with stage I to III MCC treated with curative intent. We used a Fine and Gray’s competing risk multivariable analysis to estimate associations with RFS.
Results:
IS and IC patients with MCC had similar demographic and disease characteristics. Most (77% IC, 86% IS) were irradiated (median, 50.4 Gy IC, 50.3 Gy IS), although more IS patients were irradiated to the primary site (97% vs 81%). With a median follow-up of 54.4 months, IS patients had inferior RFS (2-year: 30% vs 57%; P < .0001) and higher rates of local recurrence as the first site of relapse (25% vs 12%; P = .0002). The association between RT and RFS differed by immune status (interaction P = .01). Although RT was associated with significantly improved RFS among IC patients (hazard ratio 0.56, 95% confidence interval 0.44–0.72), no difference in RFS was observed with RT among IS patients (hazard ratio 1.49, 95% confidence interval 0.70–3.17).
Conclusions:
Radiation therapy efficacy at current standard RT doses for MCC is impaired among immunosuppressed patients with MCC. Although a strong link between durability of RT response and immune function does not appear to be evident in most cancers, our results may reflect an especially dynamic interaction between immune status and RT efficacy in MCC.
Summary
The effect of chronic immunosuppression on radiation therapy (RT) efficacy was evaluated among patients with nonmetastatic Merkel cell carcinoma (MCC) treated with curative intent. Despite higher proportions of immunosuppressed patients with MCC receiving RT to the primary site compared with immune-competent patients with MCC, immunosuppressed patients experienced increased local failure as first relapse (25% vs 12%; P = .0002) and had lower recurrence-free survival (2-year: 30% vs 57%; P < .0001). The efficacy of conventional RT for tumor control may be impaired in an immunosuppressed patient.
Introduction
Merkel cell carcinoma (MCC) is a rare and aggressive neuroendocrine cancer of the skin that predominantly afflicts Caucasians, the elderly, and patients with chronic immunosuppression (1). Prolonged immunosuppression has been associated with increased risk of nonmelanoma skin cancers (2, 3), which was described as early as 1971 among kidney-allograft recipients (4). Increased risk of MCC has been observed after solid organ transplantation (5–7), among patients with lymphoproliferative disorders including chronic lymphocytic leukemia (CLL) and other non-Hodgkin lymphoma (8, 9), HIV/AIDS (6, 10), and autoimmune disorders (11, 12).
The immune system likely plays an important role in MCC. In 60% to 80% of patients, the Merkel cell polyomavirus (MCPyV) may have a causative association with MCC (13). Although most of the population is exposed to MCPyV, as a result of immune surveillance very few develop MCC (14). However, in immunosuppressed patients, a compromised immune system may permit integration of MCPyV, which may lead to mutagenesis and carcinogenesis. The interplay of MCC and immune system regulation has been highlighted by recent reports of durable responses among patients with advanced MCC treated with antibodies targeting the PD-L1/PD-1 pathway (15, 16). Although patients with MCC with immunosuppression have worse survival outcomes compared with immune-competent patients with MCC (17–20), the mechanisms mediating these differences in survival are not fully understood.
Radiation therapy (RT) plays an important role in MCC management. However, because RT tumor control may depend on immune function (21), we hypothesized that recurrence-free survival (RFS) and local control may be worse among conventionally irradiated immunosuppressed patients with MCC compared with immunocompetent patients with MCC. Using a large MCC registry, we compared patterns of first recurrence and outcomes among patients with MCC with and without chronic immunosuppression.
Methods and Materials
Patient cohort and eligibility
The Seattle MCC registry, which was approved by the Fred Hutchinson Cancer institutional review board, includes comprehensive clinical, histopathologic, treatment, and disease-status information on patients with MCC who provided informed consent for release of medical records and future contact and were enrolled between 2004 and 2016. Set protocols were followed for data entry, maintenance, and patient updates. Patients were methodically followed up at least annually by email and/or phone for any changes in disease status, recurrence/progression, and treatments. Staging with positron emission tomography was not uniformly performed for all patients and was based on clinical stage and results of sentinel lymph node biopsy (if performed). Cancer treatment was delivered at our institution or outside institutions based on patient preference or residence. Time from pathologic diagnosis to registry enrollment (ie, lag time) was also captured and dichotomized as <6 months or ≥6 months.
We queried this registry of 1272 patients for patients who fulfilled the following eligibility criteria: age ≥18 years, nonmetastatic MCC (stage I-III), and initial treatment with curative intent. Given the potential heterogeneity of treatment in the palliative-intent or metastatic setting, 467 patients were excluded from our study, which left 805 eligible patients who comprised our analyzed cohort.
Definition of immunosuppression
Patients with MCC were categorized as immunosuppressed if they had been diagnosed with a form of chronic immunosuppression before or within 1 year after diagnosis of MCC. Types of immunosuppression were categorized as autoimmune disease (eg, inflammatory bowel disease, lupus, rheumatoid arthritis), HIV/AIDS, solid organ transplant, CLL, and other hematologic malignancy. Because risk of MCC and death is higher among patients with CLL compared with other lymphoproliferative disorders (9, 22), patients with CLL were considered to comprise an immunosuppression group distinct from those with other hematologic malignancies. Duration of immunosuppression was defined as time between diagnosis of chronic immunosuppression and date of last follow-up or death.
Radiation treatment
Details regarding radiation treatment, including treatment fields, total dose, and dates of treatment, were collected from the RT electronic medical record or, for patients who received RT outside our institution, from clinic notes. In instances when details from notes were incomplete, treatment plans were requested for review when possible.
Assessment of response, disease recurrence/progression, and death
The primary endpoint was MCC-specific RFS, which was measured from date of diagnosis to first MCC recurrence/progression or MCC-specific death. Time to first recurrence was recorded and categorized as local (within 5 cm from primary site), in-transit, regional (draining lymph node basin), or distant (beyond draining lymph node basin). Of note, a local recurrence in an irradiated patient could potentially reflect an in-field, marginal miss or a complete miss because radiation-specific fields were not available for verification for all patients. Secondary endpoints include cancer-specific survival (CSS) and overall survival (OS). For patients who received RT to gross disease, the best response to radiation was categorized as complete response (CR; disappearance of gross disease), partial response (PR; ≥50% reduction), or no response. Response assessment was based on imaging (if available) or clinical evaluation. Timing and cause of death was gathered from aforementioned patient charts, public records, and, when necessary, collateral contacts who were involved in the patient’s care. For 63 patients (n = 7 immunosuppressed, n = 56 immune competent) cause of death was unknown; 31 (n = 4 immunosuppressed, n = 27 immune competent) of these 63 patients had MCC recurrence. For RFS and CSS, all 63 patients were included as deaths from MCC given the higher likelihood of death from MCC over non-MCC causes, especially in the setting of recurrent disease (23).
Statistical analysis
Outcomes (RFS, CSS, OS) were compared between immunosuppressed and immunocompetent patients with MCC. OS of immunosuppressed and immunocompetent patients were estimated by the Kaplan-Meier method and compared with a log-rank test. Because most patients with MCC are elderly and may have competing medical comorbidities, RFS and CSS were estimated using a cumulative incidence function in which non-MCC death was a competing risk. Gray’s test for competing risks was used to assess for significant differences. Rates of RFS, CSS, and OS were reported at 2 and 5 years based on a median follow-up of 54.4 months among living patients.
In less than 5% of patients with MCC, RT status was unknown. Because inclusion of these patients may bias results, we compared patients with known (n = 769) versus unknown RT status (n = 36). We found no significant difference between these 2 groups in various demographic, pathologic, and treatment characteristics (results not shown), suggesting that inclusion of patients with unknown RT status would be unlikely to bias our results.
A Fine and Gray’s competing risk multivariable analysis was used to estimate associations with RFS. A logistic regression model was created to identify those covariates associated with RT use. We evaluated the following covariates: age at diagnosis (continuous), immune status (immune suppressed versus competent), sex, and time from pathologic diagnosis to registry enrollment (lag time; <6 versus ≥6 months). Age and lag time were significantly associated (P < .05) with RT use and were included in the multivariate model for RFS, in addition to immune status, radiation (yes versus no), and pathologic stage (stage IA/IIA vs IB/IIB/IIC vs IIIA vs IIIB). We also constructed a propensity score analysis evaluating the effect of RT on RFS by presence or absence of immunosuppression. The same covariates used in the multivariable analysis were incorporated into the propensity score analysis. Because the results were very similar, only the results of the Fine and Gray model are presented here. Analyses were performed with SAS (version 9.4). P values < .05 were considered statistically significant, and all tests were 2-sided.
Results
Patient cohort
Among 805 patients with nonmetastatic MCC, 89 (11%) were immunosuppressed. Immunosuppressed patients with MCC included those with HIV/AIDS (8%), CLL (31%), other hematologic malignancies (18%), solid organ transplant (21%), and autoimmune disease (21%). Median duration of immunosuppression was 97.7 months (range, 3.5–536.6). Details regarding these patients have been reported separately (24).
The distribution of demographic and disease characteristics including stage were similar among MCC patients with and without immunosuppression (Table 1). Treatment received was also similar, with 77% and 86% of immune-competent and immunosuppressed patients receiving RT, respectively. Among those irradiated, more immunosuppressed patients received treatment to the primary site compared with immune-competent patients: 97% versus 81%.
Table 1.
Demographic, pathologic, and treatment characteristics of patients with MCC by immunosuppressed versus immune-competent status
| Characteristics | Immune competent N = 716 | Immunosuppressed N = 89 |
|---|---|---|
|
| ||
| Median age at diagnosis (range), y | 68 (21–98) | 68 (37–91) |
| Sex | ||
| Female | 275 (38%) | 25 (28%) |
| Male | 441 (62%) | 64 (72%) |
| Race | ||
| Asian | 14 (2%) | 0 |
| Black | 3 (1%) | 0 |
| White | 568 (96%) | 81 (100%) |
| Other | 8 (1%) | 0 |
| Unknown | 123 | 8 |
| Median time from diagnosis to registry enrollment (range), mo | 2.2 (0.1–242.0) | 3.0 (0.2–86.7) |
| Unknown | 92 | 6 |
| Primary site | ||
| Buttock | 37 (6%) | 5 (6%) |
| Head and neck | 249 (41%) | 31 (36%) |
| Lower limb | 128 (21%) | 13 (15%) |
| Upper limb | 157 (26%) | 26 (30%) |
| Trunk | 42 (7%) | 11 (13%) |
| Unknown | 103 | 3 |
| Stage | ||
| IA/IIA | 233 (33%) | 29 (33%) |
| IB/IIB/IIC | 161 (22%) | 23 (26%) |
| IIIA | 129 (18%) | 19 (21%) |
| IIIB | 193 (27%) | 18 (20%) |
| Imaging at diagnosis | ||
| PET | 213 (31%) | 39 (49%) |
| CT scan alone | 257 (38%) | 27 (34%) |
| None | 134 (20%) | 8 (10%) |
| Other* | 81 (12%) | 5 (6%) |
| Unknown | 31 | 10 |
| Additional surgery beyond initial biopsy† | 546 (92%) | 76 (94%) |
| Unknown | 123 | 8 |
| Received radiation | 526 (77%) | 76 (86%) |
| Unknown | 35 | 1 |
| Radiation details‡ | ||
| Local RT | 409 (81%) | 72 (97%) |
| Regional RT | 333 (66%) | 52 (70%) |
| Unknown RT target | 22 | 2 |
| Irradiation of gross disease | ||
| Yes | 60 (11%) | 9 (12%) |
| No | 463 (89%) | 65 (88%) |
| Unknown | 3 | 2 |
| Median radiation dose (range), Gy | 50.4 (39–78.8) | 50.3 (37.5–70) |
| Unknown RT dose | 99 | 8 |
| Gross disease | 58 Gy (42–66) | 63 Gy (50–69) |
| Unknown dose | 9 | 1 |
| No gross disease | 50.4 Gy (39–78.8) | 50 Gy (37.5–70) |
| Unknown dose | 88 | 7 |
| Received chemotherapy | 77 (12%) | 16 (18%) |
| Unknown | 47 | 1 |
Abbreviations: CT = computed tomography; MCC = Merkel cell carcinoma; PET = positron emission tomography; RT = radiation therapy. Those with unknown category values are not included within the denominator to calculate the percentages.
Other: MRI, OctreoScan, ultrasound, x-ray.
Includes Mohs or primary re-excision.
Reported RT dose represents prescribed dose and therefore includes different fractionation schemes. One or more regions (eg, local alone, regional alone, and local/regional RT) may have been irradiated. The denominator is among all RT treatments; therefore, the proportions do not add up to 100%.
RFS and patterns of first recurrence
With a median follow-up of 48.1 and 54.6 months among living MCC patients with and without immunosuppression, respectively, RFS was significantly worse among immunosuppressed versus immune-competent patients (2-year RFS: 30% vs 57%, P < .0001; Fig. 1, Table 2). Differences in RFS were primarily driven by increased rates of local recurrence among immunosuppressed patients with MCC compared with immune-competent patients (2-year: 25% vs 12%, P = .0002; Fig. 2, Table 2). There was no significant difference in rates of in-transit (P = .70), regional (P = .40), or distant first recurrence (P = .43). Among a subset of 54 immune-competent and 27 immunosuppressed patients with MCC who developed a local and/or regional recurrence and had RT records for review, a higher proportion of immunosuppressed patients had recurrences within the radiation treatment field compared with immune-competent patients: 50% versus 25% (P = .03). Median radiation dose among this subset of patients was 55.6 Gy (range, 42–70) for immune-competent patients with MCC and 50.4 Gy (range, 37.5–70) for immunosuppressed patients.
Fig. 1.
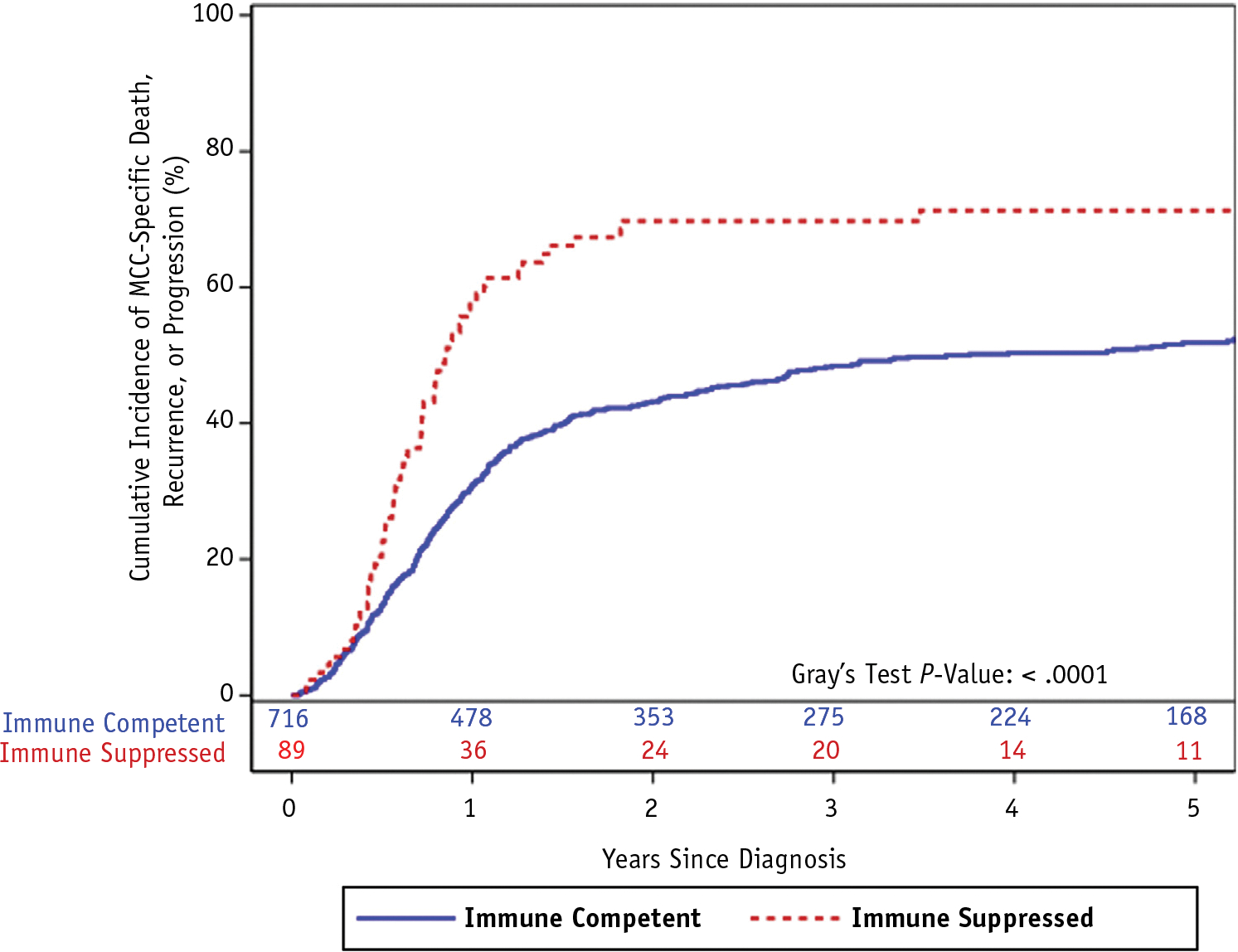
Cumulative incidence function of Merkel cell carcinoma (MCC) recurrence, progression, or death from MCC (ie, recurrence-free survival) among patients with MCC by presence or absence of immunosuppression.
Table 2.
Patterns of first recurrence, cause of death, and rates of survival, cancer-specific survival, and recurrence-free survival for patients with MCC by immunosuppressed versus immune-competent status
| Outcome | Immune competent N = 716 | Immunosuppressed N = 89 | P value |
|---|---|---|---|
|
| |||
| Dead | 274 (38%) | 53 (60%) | |
| Cause of death | |||
| MCC death | 162 (59%) | 42 (79%) | |
| Non-MCC death | 56 (20%) | 4 (8%) | |
| Unknown | 56 (20%) | 7 (13%) | |
| Recurrence | 330 (46%) | 60 (67%) | |
| Location of first recurrence | |||
| Local | 91 (27%) | 24 (38%) | |
| In-transit | 20 (6%) | 3 (5%) | |
| Regional | 92 (28%) | 15 (24%) | |
| Distant | 131 (39%) | 21 (33%) | |
| 2- and 5-year rates for survival | |||
| Relapse-free survival | 57%/48% | 30%/29% | <.0001 |
| Local-first recurrence* | 12%/13% | 25%/27% | .0002 |
| In-transit-first recurrence* | 3%/3% | 3%/3% | .70 |
| Regional-first recurrence* | 12%/13% | 16%/16% | .40 |
| Distant-first recurrence* | 15%/20% | 21%/21% | .43 |
| Cancer-specific survival | 85%/70% | 56%/43% | <.0001 |
| Overall survival | 82%/63% | 53%/38% | <.0001 |
Abbreviation: MCC = Merkel cell carcinoma.
Represents incidence of recurrence type.
Fig. 2.
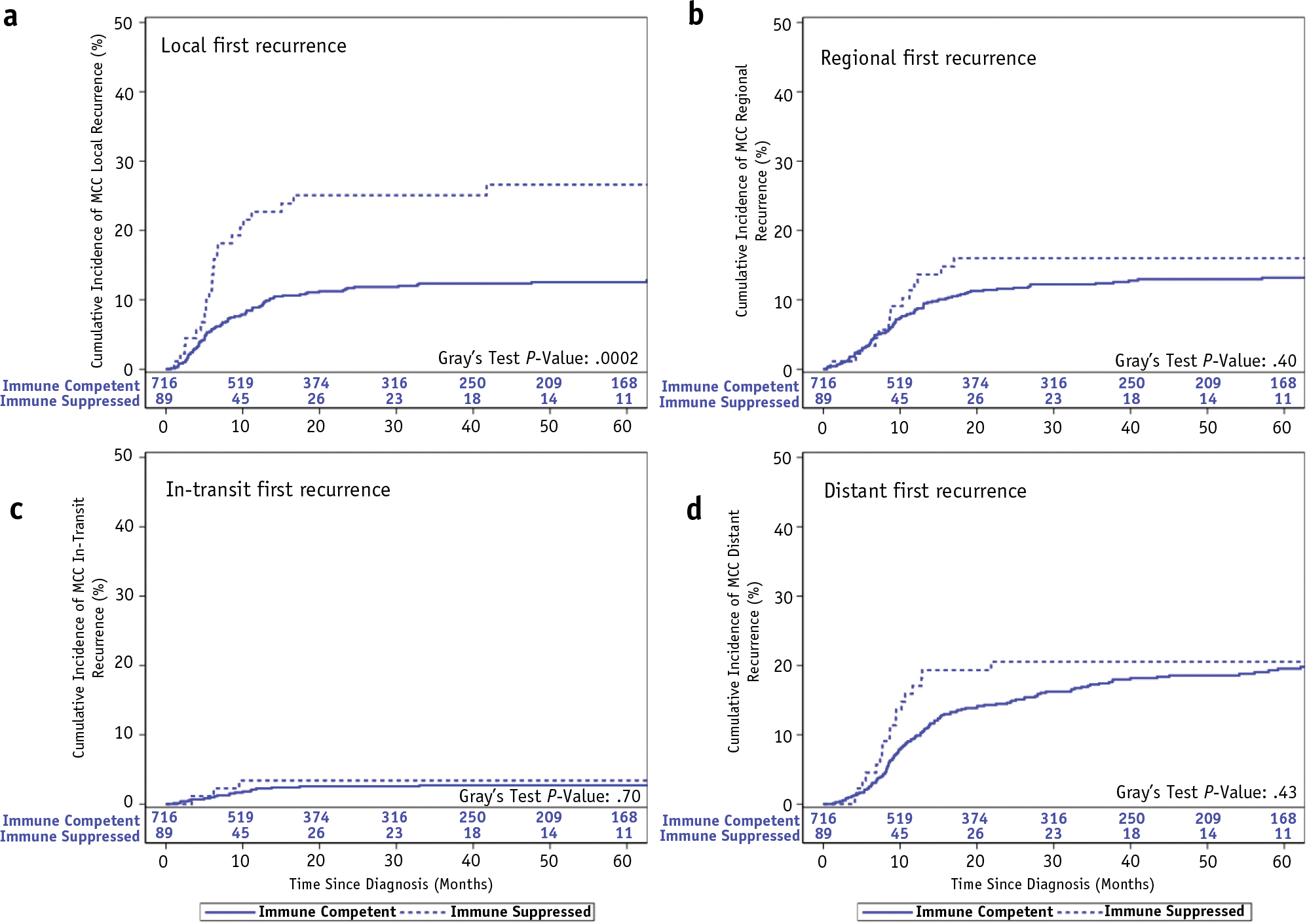
Cumulative incidence function of first recurrence among immune-competent and immune-suppressed patients with Merkel cell carcinoma by location of recurrence: (a) local, (b) regional, (c) in-transit, and (d) distant.
We evaluated whether increased local first recurrence among immunosuppressed patients with MCC was secondary to differences in RT dose or presence of gross disease before RT. Only 11% of irradiated patients with MCC had gross disease, a rate that was similar among immune-competent and immunosuppressed patients: 11% versus 12% (Table 1). Median RT dose was similar among immune-competent and immunosuppressed patients with MCC treated for microscopic disease: 50.4 Gy versus 50 Gy. In contrast, median RT dose was higher among immunosuppressed patients with MCC treated to gross disease compared with immune-competent patients with MCC at 63 Gy versus 58 Gy (Table 1), suggesting that increased rates of local first recurrence were unlikely to be secondary to inadequate dosing of microscopic or gross disease.
We also performed a subset analysis of those patients with MCC who were irradiated to ≥50 Gy. Of note, only 26 irradiated patients with MCC received a dose <50 Gy. Similar to our findings for the entire cohort, among those patients with MCC treated to ≥50 Gy, immunosuppressed patients had significantly worse RFS compared with immune-competent patients (2-year RFS: 29% vs 62%; P < .0001), primarily driven by increased rates of local first recurrence (2-year: 29% vs 7%; P < .0001).
To address the possibility that patients may have received inadequate RT that could not be assessed because details were missing, we performed a subgroup analysis of patients who received definitive RT at our institution. Similar to our larger cohort, we found significantly worse RFS among immunosuppressed patients with MCC (P = .03) that was driven by increased rates of local first recurrence (P = .0496) but not regional (P = .28) or distant first recurrences (P = .84).
Association of radiation and other covariates on RFS
Given higher rates of local first recurrence among immunosuppressed patients with MCC, most of whom had received local irradiation, we tested the hypothesis of whether the effect of radiation on RFS differed by patient immune status. A significant interaction was noted between immune status and RT (P = .01). Although RT was associated with a statistically significant improvement in RFS among immune-competent patients with MCC (adjusted hazard ratio [HR] 0.56; 95% confidence interval [CI] 0.44–0.72), no significant association was seen between RT and RFS among immunosuppressed patients with MCC (adjusted HR 1.49; 95% CI 0.70–3.17). Similar results were noted with a propensity score analysis (results not shown).
We separately evaluated whether rates of response differed among immunosuppressed and immune-competent patients with MCC in whom radiation was used to treat gross disease. In 37 immune-competent and 9 immunosuppressed patients with MCC, gross disease was present before RT and data on response were available. Nearly all immune-competent (73% CR, 22% PR) and immunosuppressed patients with MCC (CR 78%, PR 22%) achieved a response to RT.
Other significant and independent prognostic factors for RFS included age at diagnosis (HR 1.01; 95% CI 1.00–1.02; P = .01), pathologic stage (reference IA/IIA: IB/IIB/IIC HR 1.58, 95% CI 1.19–2.10; IIIA HR 1.93, 95% CI 1.43–2.60; IIIB HR 2.26, 95% CI 1.73–2.95; P < .0001) and time between MCC diagnosis and registry enrollment (≥6 versus <6 months; HR 2.11; 95% CI 1.72–2.58; P < .0001). The latter is consistent with clinical observations that many patients presenting to our institution seek a second opinion and treatment options in the setting of recurrent or refractory disease (ie, after initial diagnosis).
CSS and OS outcomes
CSS and OS were significantly lower among immunosuppressed patients with MCC (Figs. 3 and 4; P < .0001 for both), primarily driven by MCC-related death. More than half (59%) of immune-competent and 79% of immunosuppressed patient deaths were from MCC (Table 2).
Fig. 3.
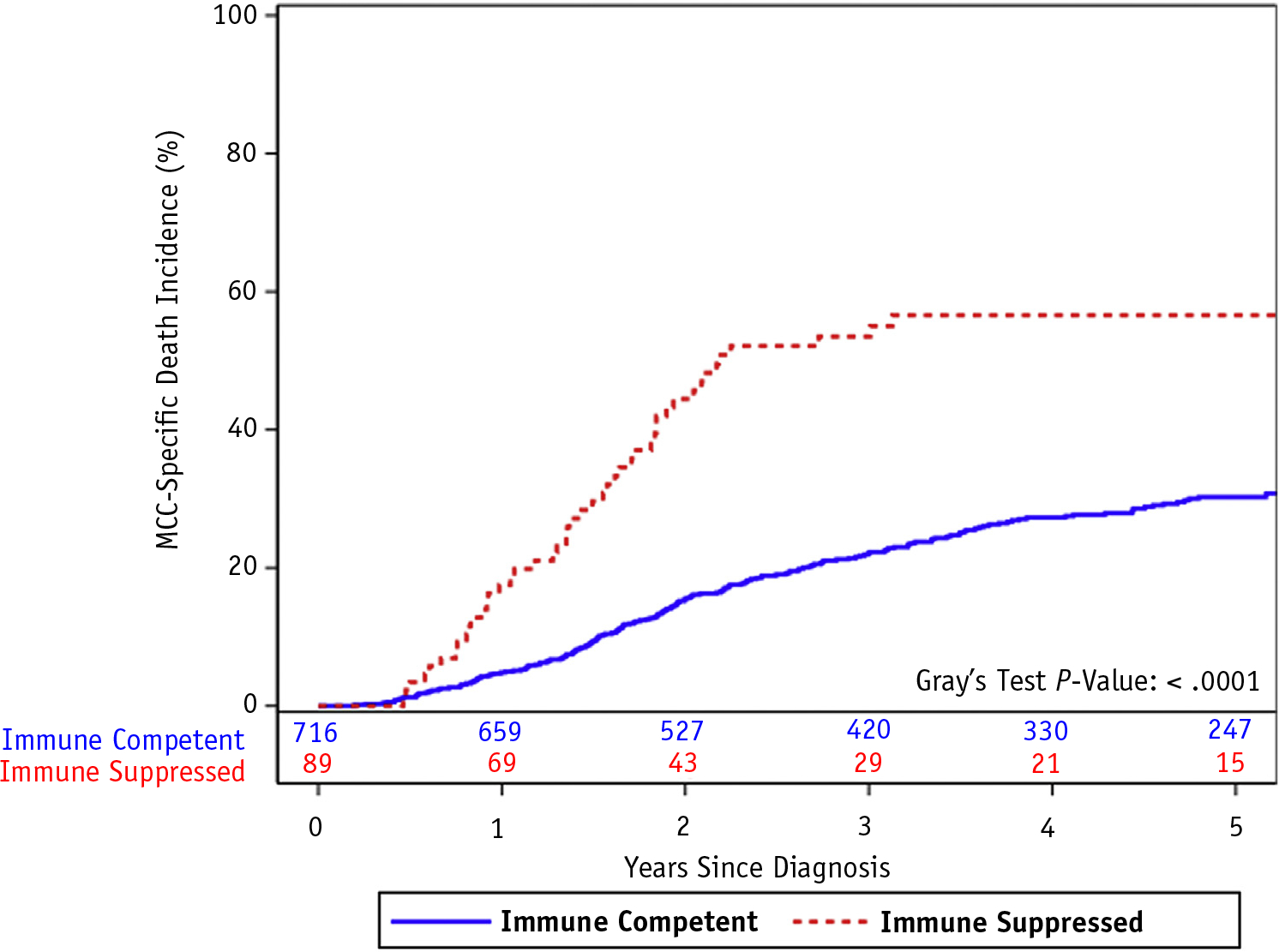
Cumulative incidence function of cancer-specific death among patients with Merkel cell carcinoma by presence or absence of immunosuppression.
Fig. 4.
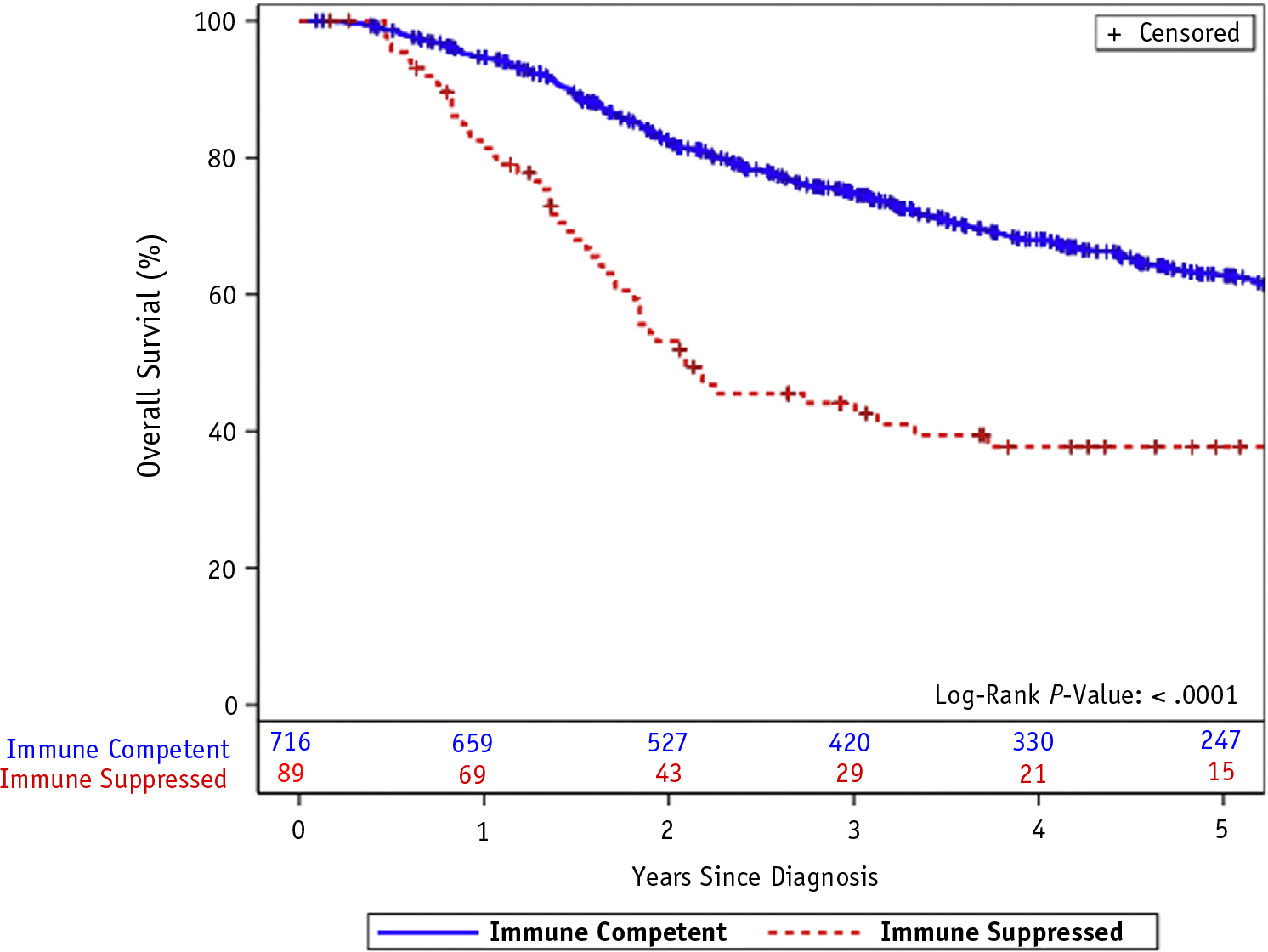
Kaplan-Meier plot of overall survival among patients with Merkel cell carcinoma by presence or absence of immunosuppression.
Discussion
Within this retrospective registry study, patients with MCC with chronic immunosuppression had significantly lower RFS, CSS, and OS despite similar demographic, pathologic, and treatment factors, which is consistent with prior retrospective single-institution (17–20, 25) and population-based registry studies (22). Indeed, as implied in the curves for OS and cancer-specific mortality, nearly all deaths among immunosuppressed patients with MCC was from MCC. This is consistent with prior findings from University of California, San Francisco, in which CSS and OS were very similar among transplant recipients with MCC (25). Although a higher proportion of immunosuppressed patients with MCC were irradiated to the primary site, the predominant pattern of first recurrence among these patients was local.
Our series highlights a novel finding—differences in patterns of failure among immunosuppressed patients with MCC—and strongly suggests an association between immune status and efficacy of RT-mediated local control. Similar findings have been observed after palliative, single-fraction RT (8 Gy) for metastatic MCC tumors. Local control was worse among irradiated MCC tumors in patients with a history of immunosuppression or chemotherapy (70%) compared with MCC tumors in patients without these risk factors (91%) (26), suggesting that with current standard RT doses, RT may be less effective in an immunosuppressed patient.
The mechanism(s) of apparent MCC radioresistance in an immunosuppressed patient is not known. Gross MCC disease is eradicated by standard RT doses, as evidenced by similar response rates between immune-competent and immunosuppressed patients with MCC. However, immunosuppressed patients with MCC have notably faster kinetics of local and distant recurrences compared with immune-competent patients with MCC, suggesting that tumor repopulation may be a component. One hypothesis is that the patient immune system and, by extension, the tumor microenvironment may play a role in controlling the residual microscopic tumor cells after RT. In an intact immune system, microscopic tumor cell growth is checked or slowed, but immune surveillance is suboptimal in an immune-suppressed individual, permitting unchecked growth of microscopic tumor cells.
The immune system is thought to play an important role in the control and eradication of MCC. Patients with MCC who present with regional disease of unknown primary (stage IIIB) have improved outcomes compared with other stage IIIB patients with known primary site (18, 27), presumably reflecting an effective immune system that has already eradicated the primary. RT plays an important role in MCC treatment and has been associated with improved outcomes (28–31). Preclinical and clinical observations suggest that the effects of RT may be mediated through the immune system. In mouse models, higher radiation dose is required to control 50% of transplanted immunogenic fibrosarcoma tumors in immunosuppressed mice (21). In addition, in patients with indolent non-Hodgkin lymphoma treated with palliative, low-dose RT (4 Gy in 2 fractions), patients with CLL/small lymphocytic lymphoma had lower response rates to radiation (odds ratio 0.2; P = .2) and shorter time to additional treatment for local recurrence (HR 3.63; P = .01) (32) compared with patients with other indolent non-Hodgkin lymphoma.
Although the association between immunosuppression and worse oncologic outcomes is established for other tumor types, including nonmelanoma skin cancers (2, 3), this is not uniformly seen for other cancers associated with immunosuppression, including anal squamous cell carcinoma (33). Even fewer data, if any, are available on the interaction between immunosuppression and RT efficacy in other cancers, likely in part because of the small proportion of patients with cancer with chronic immunosuppression and the differential effect of immune suppression on outcomes across cancer types. Despite the paucity of data on RT efficacy and immunosuppression in other cancers, our findings are not wholly unexpected given that the importance of the immune system in the pathogenesis and control of MCC is well established. However, these results may not be generalizable to other cancer types given the differential effect of immune suppression on outcomes. MCC therefore represents a unique cancer in which to study the interplay between the immune system and RT.
Despite long follow-up and large patient numbers, several limitations should be noted in this retrospective study, including selection bias for treatment. However, the treatments received among patients with MCC with and without immunosuppression were similar, if not slightly skewed toward greater utilization of radiation and more comprehensive radiation fields (ie, primary and regional) among immunosuppressed patients with MCC. A small minority of patients had missing data, including relapse date and/or causes of death. Death from unknown causes was combined with death from MCC. Although this may bias results, nearly half of patients with MCC with unknown cause of death experienced relapse before death. In these patients, there is a higher likelihood of death from MCC over non-MCC causes, especially in the setting of recurrent disease (23). Categorizing these unknown deaths as non-MCC deaths would likely increase the observed significant difference in CSS between patients with MCC with and without immunosuppression.
RT status and RT details were missing for 4.5% and 7.3% of patients, respectively, and potentially could bias our results given missing data. We found that there were no significant differences in demographic, pathologic, and other treatment characteristics in patients with MCC with known versus unknown RT status. Last, given that many patients with MCC travel to our center for a second opinion and/or additional care, our cohort may be enriched for patients with relapsed/refractory (R/R) MCC disease. This may in part explain why the local recurrence rates observed among immune-competent patients with MCC are similar to or slightly higher than rates in other series (31). However, we do not anticipate that our findings are altered by a higher proportion of R/R patients because both immune-competent and immune-suppressed groups had a similar proportion of R/R patients, using lag time as a surrogate for R/R disease: 44% versus 39%, respectively.
Although our results are hypothesis generating and require validation in other MCC cohorts, they suggest a potential role of intensifying treatment for immunosuppressed patients with MCC, who have worse local control with current standard RT doses. Strategies may include radiation dose escalation through a variety of treatment modalities (eg, electrons, high-dose-rate brachytherapy, orthovoltage photons, MV photons), altered fractionation (eg, accelerated hyperfractionation), and/or concurrent systemic therapies, although effectiveness of dose escalation was not evaluated in our analysis and remains to be confirmed in other cohorts. Alternatively, other local therapies (ie, surgery) may be considered, although nearly all (94%) immunosuppressed patients in our cohort had additional surgical resection beyond their initial biopsy.
Conclusion
Presence of chronic immunosuppression is associated with decreased RFS, CSS, and OS among curatively treated patients with MCC. Although the mechanisms for these observations are not wholly understood, our data suggest that RT-mediated local control is impaired among immunosuppressed patients with MCC. These findings, which remain to be confirmed in other MCC cohorts, highlight the consideration of RT intensification and/or the unmet need for novel therapies for immunosuppressed patients with MCC.
Footnotes
Conflicts of interest: None.
Presented in part at the 59th Annual Meeting of the American Society for Radiation Oncology, September 24–27, 2017, San Diego, CA.
References
- 1.The Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: Recent progress and current priorities on etiology, pathogenesis, and clinical management. J Clin Oncol 2009;27:4021–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerlini G, Romagnoli P, Pimpinelli N. Skin cancer and immunosuppression. Crit Rev Oncol Hematol 2005;56:127–136. [DOI] [PubMed] [Google Scholar]
- 3.Scott FI, Mamtani R, Brensinger CM, et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol 2016;152:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walder B, Robertson M, Jeremy D. Skin cancer and immunosuppression. Lancet 1971;298:1282–1283. [DOI] [PubMed] [Google Scholar]
- 5.Koljonen V, Kukko H, Tukiainen E, et al. Incidence of Merkel cell carcinoma in renal transplant recipients. Nephrol Dial Transplant 2009;24:3231–3235. [DOI] [PubMed] [Google Scholar]
- 6.Lanoy E, Costagliola D, Engels EA. Skin cancers associated with HIV infection and solid-organ transplantation among elderly adults. Int J Cancer 2010;126:1724–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Nat Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard RA, Dores GM, Curtis RE, et al. Merkel cell carcinoma and multiple primary cancers. Cancer Epidemiol Biomarkers Prev 2006;15:1545. [DOI] [PubMed] [Google Scholar]
- 9.Kaae J, Hansen AV, Biggar RJ, et al. Merkel cell carcinoma: Incidence, mortality, and risk of other cancers. J Natl Cancer Inst 2010;102:793–801. [DOI] [PubMed] [Google Scholar]
- 10.Engels EA, Frisch M, Goedert JJ, et al. Merkel cell carcinoma and HIV infection. Lancet 2002;359:497–498. [DOI] [PubMed] [Google Scholar]
- 11.Hemminki K, Liu X, Ji J, et al. Kaposi sarcoma and Merkel cell carcinoma after autoimmune disease. Int J Cancer 2012;131:E326–E328. [DOI] [PubMed] [Google Scholar]
- 12.Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer 2010;103:112–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008;319:1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coursaget P, Samimi M, Nicol JTJ, et al. Human Merkel cell polyomavirus: Virological background and clinical implications. APMIS 2013;121:755–769. [DOI] [PubMed] [Google Scholar]
- 15.Nghiem PT, Bhatia S, Lipson EJ, et al. Pd-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Eng J Med 2016;374:2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multi-centre, single-group, open-label, phase 2 trial. Lancet Oncol 2016;17:1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jouary T, Kubica E, Dalle S, et al. Sentinel node status and immunosuppression: Recurrence factors in localized Merkel cell carcinoma. Acta Derm Venereol 2015;95:835–840. [DOI] [PubMed] [Google Scholar]
- 18.Asgari MM, Sokil MM, Warton E, et al. Effect of host, tumor, diagnostic, and treatment variables on outcomes in a large cohort with Merkel cell carcinoma. JAMA Dermatol 2014;150:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarantola TI, Vallow LA, Halyard MY, et al. Prognostic factors in Merkel cell carcinoma: Analysis of 240 cases. J Am Acad Dermatol 2013;68:425–432. [DOI] [PubMed] [Google Scholar]
- 20.Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression as a stage-independent predictor of diminished Merkel cell carcinoma-specific survival. J Invest Dermatol 2013;133:642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone H, Peters L, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst 1979;63:1229–1235. [PubMed] [Google Scholar]
- 22.Brewer JD, Shanafelt TD, Otley CC, et al. Chronic lymphocytic leukemia is associated with decreased survival of patients with malignant melanoma and Merkel cell carcinoma in a seer population-based study. J Clin Oncol 2012;30:843–849. [DOI] [PubMed] [Google Scholar]
- 23.Grotz TE, Tarantola TI, Otley CC, et al. Natural history of Merkel cell carcinoma following locoregional recurrence. Ann Surg Oncol 2012;19:2556–2562. [DOI] [PubMed] [Google Scholar]
- 24.Cook M, Baker K, Redman M, et al. Differential outcomes among immunosuppressed patients with Merkel cell carcinoma: Impact of immunosuppression type on cancer-specific and overall survival. Am J Clin Oncol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arron ST, Canavan T, Yu SS. Organ transplant recipients with Merkel cell carcinoma have reduced progression-free, overall, and disease-specific survival independent of stage at presentation. J Am Acad Dermatol 2014;71:684–690. [DOI] [PubMed] [Google Scholar]
- 26.Iyer JG, Parvathaneni U, Gooley T, et al. Single-fraction radiation therapy in patients with metastatic Merkel cell carcinoma. Cancer Med 2015;4:1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen KT, Papavasiliou P, Edwards K, et al. A better prognosis for Merkel cell carcinoma of unknown primary origin. Am J Surgery 2013;206:752–757. [DOI] [PubMed] [Google Scholar]
- 28.Bhatia S, Storer BE, Iyer JG, et al. Adjuvant radiation therapy and chemotherapy in Merkel cell carcinoma: Survival analyses of 6908 cases from the national cancer data base. J Natl Cancer Inst 2016;108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghadjar P, Kaanders JH, Poortmans P, et al. The essential role of radiotherapy in the treatment of Merkel cell carcinoma: A study from the rare cancer network. Int J Radiat Oncol Biol Phys 2011;81:e583–e591. [DOI] [PubMed] [Google Scholar]
- 30.Servy A, Maubec E, Sugier PE, et al. Merkel cell carcinoma: Value of sentinel lymph-node status and adjuvant radiation therapy. Ann Oncol 2016;27:914–919. [DOI] [PubMed] [Google Scholar]
- 31.Strom T, Carr M, Zager JS, et al. Radiation therapy is associated with improved outcomes in Merkel cell carcinoma. Ann Surg Oncol 2016;1–7. [DOI] [PubMed] [Google Scholar]
- 32.Russo AL, Chen Y-H, Martin NE, et al. Low-dose involved-field radiation in the treatment of non-hodgkin lymphoma: Predictors of response and treatment failure. Int J Radiat Oncol Biol Phys 2013;86:121–127. [DOI] [PubMed] [Google Scholar]
- 33.Bryant AK, Mudgway R, Huynh-Le M-P, et al. Effect of CD4 count on treatment toxicity and tumor recurrence in human immunodeficiency virus—positive patients with anal cancer. Int J Radiat Oncol Biol Phys 2018;100:478–485. [DOI] [PubMed] [Google Scholar]


