Abstract
Background
Data pertaining to biologic agents used for treating psoriasis in real-world settings are lacking at present. To compare drug survival at 52 weeks for a range of biologics used to treat psoriasis under real-world conditions.
Methods
This was a retrospective, single-center, observational study of a cohort of patients diagnosed with plaque psoriasis treated using ixekizumab, secukinumab, guselkumab, or adalimumab between January 2020 and December 2021. Baseline demographic characteristics, duration of psoriasis, and prior biological treatments for all patients were recorded. Drug survival rates were analyzed in different patient groups using Kaplan–Meier curves and Log rank tests.
Results
In total, this study included 386 plaque psoriasis patients, of whom 70, 175, 36, and 105 were, respectively, treated using ixekizumab, secukinumab, guselkumab, and adalimumab. Over a 52-week period, the overall cumulative drug survival rates for ixekizumab, secukinumab, guselkumab, and adalimumab were 67.1%, 63.0%, 72.2%, and 37.1%, respectively. Lack of efficacy was the primary cause of discontinuation for these biologic therapies, followed by economic burden and adverse event incidence.
Conclusion
These results suggest that guselkumab exhibited superior drug survival, drug survival outcomes for ixekizumab and secukinumab were comparable, and significantly better than those of adalimumab in China. Preventing a loss of drug efficacy represents a primary approach to improving biologic drug survival in psoriasis patients.
Keywords: biologics, drug survival, real-world, psoriasis, ixekizumab, secukinumab, guselkumab, adalimumab
Introduction
Psoriasis is a chronic inflammatory cutaneous condition that impacts roughly 125 million people throughout the world, can occur at any age, and is characterized by the recurrent development of erythematous scales.1 Biological agents have achieved great success in the management of patients suffering from moderate-to-severe plaque psoriasis, with IL-17 or IL-23 targeting antibody inhibitors including secukinumab, ixekizumab, and guselkumab having shown marked efficacy in Phase 3 clinical trials comparing them to biologics targeting TNF-α.2–4 Despite their excellent efficacy, however, psoriasis patients undergoing biologic treatment regimens often discontinue treatment or switch to other biologics for a variety of reasons in clinical practice.
Drug survival has been shown to offer value as a means of assessing the efficacy of biologic treatments for psoriasis in real-world settings,5 and can be impacted by many factors including safety, cost, convenience, therapeutic efficacy, and the incidence of side effects. However, the available real-world data pertaining to biologic drug survival in psoriasis patients are limited, and results are sometimes inconsistent across studies.6,7 Relative to other countries, the approval of biologics for treating psoriasis is relatively recent, and there is thus a limited amount of real-world data regarding drug survival outcomes.
The present study was developed to assess drug survival among Chinese psoriasis patients undergoing treatment using ixekizumab, secukinumab, guselkumab, and adalimumab, and to assess factors with the potential to impact drug survival over a 52-week follow-up period.
Materials and Methods
Study Design
This was a retrospective, observational, and single-center analysis of a cohort of chronic plaque psoriasis patients with or without psoriatic arthritis (PsA). For this study, medical records for all individuals with moderate-to-severe psoriasis that underwent ixekizumab, secukinumab, guselkumab, or adalimumab treatment from January 2020-December 2021 at Shanghai Skin Disease Hospital were reviewed. The patients included were moderate to severe plaque psoriasis, aged ≥ 18 years old, and patients were excluded if they exhibited pustular psoriasis or erythrodermic psoriasis. Ixekizumab, secukinumab, guselkumab, and adalimumab were administered as per the indications on the drug label. All patients received a minimum of one biologic treatment during the defined study period and had undergone at least one follow-up visit were included in this study. Patient baseline demographic details, duration of psoriasis, and prior biological treatment regimens were recorded. All patients were informed and provided written informed consent. The Ethics Committee of Shanghai Skin Disease Hospital approved this study. Our study also complied with the Declaration of Helsinki.
Drug Survival
Drug survival was defined as the interval during which a patient was treated using a particular biologic regimen from the time that treatment was initiated to the discontinuation of that therapeutic approach, including both the termination of treatment and switching to other treatments.8 Drug survival rates were analyzed and compared among groups using Kaplan–Meier curves and Log rank tests. Cox regression models were used to identify potential factors associated with treatment discontinuation.
Statistical Analysis
Normally distributed categorical and continuous variables were respectively compared using Pearson’s chi-square tests and ANOVAs. P < 0.05 was significance threshold, and SPSS 22.0 was used for all statistical testing.
Results
Patient Characteristics
In total, this study incorporated 386 plaque psoriasis patients that underwent biologic therapy, including 70 treated with ixekizumab, 175 with secukinumab, 36 with guselkumab, and 105 with adalimumab. The characteristics of these patients at the time of initial treatment are shown in Table 1. In general, the patients treated with these four biologic agents exhibited comparable gender distributions, sex ratios, and body mass index (BMI) values among groups. PsA was more common among patients treated using adalimumab (26.7%) relative to patients treated using secukinumab (11.4%), ixekizumab (8.6%), and guselkumab (5.6%). Prior to the start of treatment, the numbers of biologic-naïve patients in the ixekizumab, secukinumab, guselkumab, and adalimumab groups were 58 (82.9%), 143 (81.7%), 20 (55.6%), and 75 (71.4%), respectively (P < 0.05).
Table 1.
Baseline Characteristics of the Study Population at Initiation of Therapy (n=386)
| Characteristics | Ixekizumab (n=70) | Secukinumab (n=175) | Guselkumab (n=36) | Adalimumab (n=105) | P |
|---|---|---|---|---|---|
| Male, n (%) | 57(81.4) | 119(68) | 28(77.8) | 83(79) | 0.07 |
| Female, n (%) | 13(18.6) | 56(32) | 8(22.2) | 22(21) | |
| Mean age, y (SD) | 42.9(13.4) | 43.1(13.6) | 41.0(9.8) | 44.8(11.9) | 0.43 |
| Mean duration, y (SD) | 14.7(8.3) | 15.9(8.7) | 16.0(10.3) | 15.1(8.9) | 0.77 |
| Mean weight, kg (SD) | 72.7(13.6) | 69.9(13.3) | 75.0(12.6) | 71.8(12.8) | 0.13 |
| Mean BMI, kg/m2 (SD) | 24.9(4.0) | 24.2(3.9) | 25.8(3.9) | 24.6(3.9) | 0.16 |
| Concurrent PsA, n (%) | 6(8.6) | 20(11.4) | 2(5.6) | 28(26.7) | <0.001 |
| Bio-naïve, n (%) | 58(82.9) | 143(81.7) | 20(55.6) | 75(71.4) | 0.002 |
| Previous biological agent | 12 | 32 | 16 | 30 | 0.002 |
| Prior biologic:1 | 8 | 27 | 10 | 26 | |
| Prior biologic:2 | 4 | 3 | 5 | 3 | |
| Prior biologic:3 | 0 | 2 | 1 | 1 | |
| Mean PASI (SD) at baseline | 16.0(6.2) | 16.9(7.0) | 17.6(4.8) | 17.7(5.3) | 0.29 |
| Mean DLQI (SD) at baseline | 12.3(4.8) | 12.7(5.7) | 13.9(4.3) | 14.1(5.5) | 0.07 |
Abbreviations: SD, standard deviation; BMI, body mass index; PsA, psoriatic arthritis; DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area Severity Index.
Drug Survival
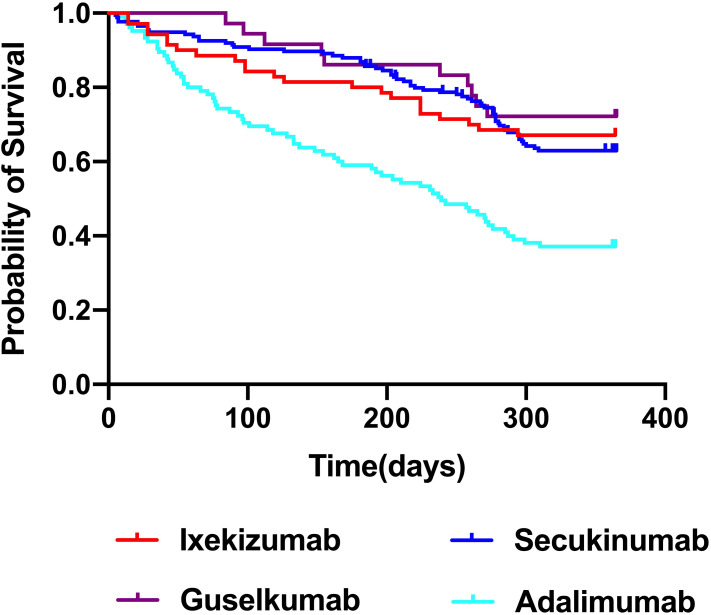
Drug survival outcomes for these four biologic agents over a 52-week follow-up period are summarized in Figure 1. Guselkumab was associated with the best observed drug survival, followed by ixekizumab and secukinumab, both of which exhibited significantly higher drug survival than that observed for adalimumab (log rank = 12.786, p < 0.001; log rank = 13.935, p < 0.001; log rank = 24.754, p <0.001, respectively). While guselkumab was associated with a trend toward increased drug survival relative to ixekizumab and secukinumab, these differences were not significant (log rank = 0.47, p = 0.493; log rank = 0.903, p = 0.342, respectively). There were similarly no differences in drug survival outcomes when comparing patients treated with secukinumab and ixekizumab (log rank = 0.088, p = 0.77).
Figure 1.
Drug survival rate for four biological agents. The overall drug survival rate of guselkumab, ixekizumab, secukinumab and adalimumab was 72.2%, 67.1%, 63.0%, and 37.1%, respectively.
Drug survival at different time points for these four biologic agents is shown in Table 2. Cumulative drug survival rates for ixekizumab at 12, 24, and 52 weeks were 88.6%, 81.4%, and 67.1%, respectively, while for secukinumab these respective values were 92%, 88%, and 63%, for guselkumab they were 97.2%, 86.1%, and 72.2%, and for adalimumab they were 73.3%, 59%, and 37.1%, respectively.
Table 2.
Drug Survival Rate at 12 Week, 24 Week and 52 Week
| Ixekizumab (n=70) | Secukinumab (n=175) | Guselkumab (n=36) | Adalimumab (n=105) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | n | Estimate | SE | n | Estimate | SE | n | Estimate | SE | n | |
| 12 week | 0.886 | 0.038 | 62 | 0.92 | 0.021 | 162 | 0.972 | 0.027 | 36 | 0.733 | 0.043 | 76 |
| 24 week | 0.814 | 0.046 | 57 | 0.88 | 0.025 | 155 | 0.861 | 0.058 | 31 | 0.59 | 0.048 | 61 |
| 52 week | 0.671 | 0.056 | 47 | 0.63 | 0.037 | 102 | 0.722 | 0.075 | 24 | 0.371 | 0.047 | 39 |
Abbreviation: SE, standard error.
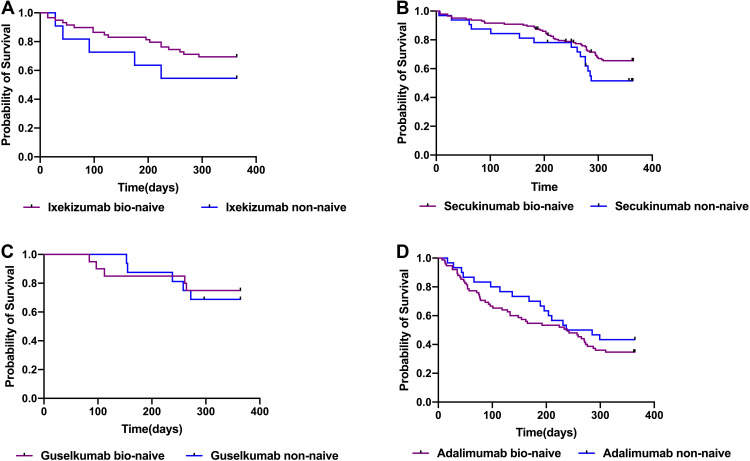
Drug survival rates were also compared between biologic-naïve and non-naïve patients (Figure 2). While drug survival rates tended to be higher among bio-naïve patients relative to non-naïve patients in the ixekizumab, secukinumab, and guselkumab groups, these differences were not significant. No trend towards increased drug survival among bio-naïve adalimumab patients was observed.
Figure 2.
Comparison of drug survival bio-naïve and non-naive among four biological agents. Bio-naïve patients had a higher drug survival trend than non-naïve patients in the ixekizumab (A), secukinumab (B), and guselkumab (C) groups, these differences were not significant. (D) No trend towards increased drug survival among bio-naïve adalimumab patients was observed.
Reasons for Discontinuation
Reasons for patient discontinuation of biologic treatment are summarized in Table 3. Over the analyzed study period, 32.9%, 36%, 27.8%, and 62.9% of the patients treated with ixekizumab, secukinumab, guselkumab, and adalimumab discontinued the use of these agents, respectively. A lack of efficacy was the most common reason for treatment discontinuation, followed by economic burden, and adverse events. The incidence of lack of efficacy was highest for adalimumab (31.4%) and lowest for guselkumab (8.3%). Rates of economic burden resulting in the discontinuation of treatment were highest and lowest for adalimumab (19.0%) and guselkumab (5.6%), respectively.
Table 3.
Reasons for Discontinuation of Four Biological Agents, n(%)
| Ixekizumab (n=70) | Secukinumab (n=175) | Guselkumab (n=36) | Adalimumab (n=105) | |
|---|---|---|---|---|
| Continued, n (%) | 47(67.1) | 112(64) | 26(72.2) | 39(37.1) |
| Discontinued, n (%) | 23(32.9) | 63(36) | 10(27.8) | 66(62.9) |
| Reason for discontinuation | ||||
| Lack of efficacy, n (%) | 12(17.1) | 36(20.5) | 3(8.3) | 33(31.4) |
| Economic burden, n (%) | 8(11.4) | 16(9.1) | 2(5.6) | 20(19.0) |
| Adverse events, n (%) | 2(2.9) | 4(2.3) | 1(2.8) | 5(4.8) |
| Other, n (%) | 1(1.4) | 7(4.0) | 4(11.1) | 8(7.6) |
Factors Associated with Drug Survival
A Cox regression analysis revealed no predictive relationship between patient age, sex, duration of psoriasis, PsA status, BMI, or prior biologic therapy regimens and the duration of biologic exposure (Table 4).
Table 4.
Hazard Ratios for Risk of Drug Discontinuation
| Covariates | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Age | 0.01 | 1.00–1.03 | 0.12 |
| Gender | 0.29 | 0.89–2.00 | 0.162 |
| Duration | 0.02 | 0.96–1.00 | 0.11 |
| BMI | 0.06 | 0.99–1.14 | 0.09 |
| Concomitant PsA | 0.159 | 0.55–1.32 | 0.47 |
| Previous biological agents | 1.00 | 0.63–1.32 | 0.61 |
Abbreviations: CI, confidence interval; PsA, psoriatic arthritis; BMI, body mass index.
Discussion
Here, drug survival outcomes were compared over a 52-week period for patients with plaque psoriasis undergoing treatment using ixekizumab, secukinumab, guselkumab, or adalimumab. Guselkumab is the only IL-23 inhibitor that has been approved to treat plaque psoriasis in China, and real-world data pertaining to its use are lacking at present. The results of this present study are consistent with those from the multinational cohort study reported by Torres et al, who observed superior drug survival outcomes for guselkumab.7
In a prospective, non-interventional and multi-center German PERSIST study analyzing 303 psoriasis patients undergoing guselkumab treatment, the overall drug survival at week 52 was 92.4%.9 While guselkumab trended towards increased drug survival relative to ixekizumab and secukinumab, there were no significant differences in these three drug survival rates. However, other studies have alternatively reported that secukinumab and guselkumab exhibit higher rates of drug survival (90% vs 88%) relative to ixekizumab (82%).10 In this study, the observed drug survival rates for these biologics in China were significantly lower than those reported in prior international studies.7,10,11 This may be attributable to differences in national conditions, insurance coverage, and concern regarding side effects.
Real-world studies have yielded inconsistent data pertaining to drug survival rates for IL-17 inhibitors and TNF-α inhibitors. For example, Ohata et al did not detect any differences in 365-day drug survival when comparing ixekizumab and secukinumab.12 Consistently, ixekizumab and secukinumab exhibited comparable drug survival rates superior to those of adalimumab in the present study. In contrast, in a 12-month nationwide Danish study of psoriasis patients conducted by Egeberg et al, secukinumab exhibited lower drug survival than ixekizumab.13 A UK prospective cohort study in BADBIR showed secukinumab has higher drug survival than adalimumab.14 At 1-year, secukinumab exhibited increased drug survival relative to ixekizumab, while no significant differences were evident when comparing secukinumab and TNF-α inhibitors.15 In a large 2-year cohort study of psoriasis patients in North America, ixekizumab was found to exhibit superior drug survival outcomes relative to TNF-α inhibitors and non-ixekizumab IL-17 inhibitors.16
In the present report, drug survival rates tended to be higher in bio-naïve patients treated with ixekizumab, secukinumab, and guselkumab relative to non-naïve patients, although these differences were not significant. Such a trend was not observed in the adalimumab group. And most patients treated with adalimumab switched from undergoing treatment with etanercept-biosimilar Yisaipu (YSP). Adalimumab is a fully human monoclonal antibody, while YSP is a recombinant TNF-α receptor II: IgG Fc fusion protein that targets soluble TNF-α and thereby prevents it from binding to receptors on the surfaces of target cells.17 Given the greater affinity of adalimumab and its lower rate of anti-drug antibody production, a history of treatment with YSP may thus not have adversely impacted overall drug survival rates for individuals undergoing treatment with adalimumab.
In line with prior reports, loss of efficacy was the most common reason that patients reported discontinuing biologic therapy in this study, with economic factors also influencing treatment decision-making. Over this observation period, biologics including ixekizumab and guselkumab were not covered by medical insurance in China, and many patients are unable to afford these expensive medications. Side effects were also a common reason for treatment discontinuation within this study cohort. Following treatment termination, a majority of patients switched to undergoing treatment using biologics with distinct targets, while some elected to undergo traditional treatment.
Given the growing variety of biologic drugs available for the treatment of psoriasis, the identification of predictors of drug survival for these different agents may aid clinicians in selecting the most appropriate therapeutic regimens in an effort to maximize efficacy and to minimize treatment failure. Gniadeckie et al previously reported that drug survival was negatively associated with weight, female sex, and prior biologics utilization.18 A BADBIR prospective cohort study conducted in the United Kingdom suggested that both PsA status and prior biologic exposure were associated with different drug survival outcomes.14 Moreover, two multi-center retrospective cohort studies found female sex, higher BMI, and prior biologic exposure to be predictive of drug discontinuation.7,11 Consistently, a systematic review and meta-analysis of 16 different cohort studies determined that female sex and obesity were both predictive of biologic discontinuation, whereas concomitant PsA was linked to biologic persistence.19 Other studies have also suggested age and PsA to be related to switching between biologic agents in psoriasis patients.20
While several variables with the potential to influence the duration of biologic exposure were assessed in this study, no relationships between such exposure and patient age, sex, psoriasis duration, PsA, BMI, or previous biologic treatment were observed. These inconsistent findings may be related to the ethnicity of the patient population, the relatively short follow-up period, and the small sample size.
There are certain limitations to this analysis. For one, this was a retrospective study rather than a randomized controlled trial, and it may thus be susceptible to several forms of bias. Moreover, this was a single-center analysis of a small number of patients, particularly in the guselkumab group, and the follow-up duration was relatively short. Future multi-center, large-scale studies are necessary to validate and expand on these results.
Conclusion
In the four evaluated biologics, guselkumab exhibited superior drug survival outcomes, while drug survival for ixekizumab and secukinumab was comparable, and significantly greater than that observed for adalimumab. Lack of efficacy was the most common reason that patients reported discontinuing the use of these biologics. These results will provide a foundation for patients and physicians to enable better clinical decision-making pertaining to the initiation of biologic therapy.
Funding Statement
This work was sponsored by grants from National Natural Science Foundation of China (No. 81872522, 82073429, 82203913), Innovation Program of Shanghai Municipal Education Commission (No.2019-01-07-00-07-E000460), Clinical Research Plan of SHDC (No. SHDC2020CR1014B) and Program of Shanghai Academic Research Leader (No. 20XD1403300).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors state that there is no conflict of interest.
References
- 1.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960. doi: 10.1001/jama.2020.4006 [DOI] [PubMed] [Google Scholar]
- 2.Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258 [DOI] [PubMed] [Google Scholar]
- 3.Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356. doi: 10.1056/NEJMoa1512711 [DOI] [PubMed] [Google Scholar]
- 4.Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with Adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the Phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417. doi: 10.1016/j.jaad.2016.11.041 [DOI] [PubMed] [Google Scholar]
- 5.Costanzo A, Malara G, Pelucchi C, et al. Effectiveness end points in real-world studies on biological therapies in psoriasis: systematic review with focus on drug survival. Dermatology. 2018;234(1–2):1–12. doi: 10.1159/000488586 [DOI] [PubMed] [Google Scholar]
- 6.Kishimoto M, Komine M, Kamiya K, Sugai J, Mieno M, Ohtsuki M. Drug survival of biologic agents for psoriatic patients in a real-world setting in Japan. J Dermatol. 2020;47(1):33–40. doi: 10.1111/1346-8138.15146 [DOI] [PubMed] [Google Scholar]
- 7.Torres T, Puig L, Vender R, et al. Drug Survival of IL-12/23, IL-17 and IL-23 Inhibitors for Psoriasis Treatment: a Retrospective Multi-Country, Multicentric Cohort Study. Am J Clin Dermatol. 2021;22(4):567–579. doi: 10.1007/s40257-021-00598-4 [DOI] [PubMed] [Google Scholar]
- 8.Menter A, Papp KA, Gooderham M, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Eur Acad Dermatol Venereol. 2016;30(7):1148–1158. doi: 10.1111/jdv.13611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerdes S, Asadullah K, Hoffmann M, et al. Real-world evidence from the non-interventional, prospective, German multicentre PERSIST study of patients with psoriasis after 1 year of treatment with guselkumab. J Eur Acad Dermatol Venereol. 2022;36(9):1568–1577. doi: 10.1111/jdv.18218 [DOI] [PubMed] [Google Scholar]
- 10.Dapavo P, Siliquini N, Mastorino L, et al. Efficacy, safety, and drug survival of IL-23, IL-17, and TNF-alpha inhibitors for psoriasis treatment: a retrospective study. J Dermatolog Treat. 2021;32:1–6. doi: 10.1080/09546634.2020.1830930 [DOI] [PubMed] [Google Scholar]
- 11.Graier T, Salmhofer W, Jonak C, et al. Biologic drug survival rates in the era of anti-interleukin-17 antibodies: a time-period-adjusted registry analysis. Br J Dermatol. 2021;184(6):1094–1105. doi: 10.1111/bjd.19701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohata C, Ohyama B, Katayama E, Nakama T. Real-world efficacy and safety of interleukin-17 inhibitors for psoriasis: a single-center experience. J Dermatol. 2020;47(4):405–408. doi: 10.1111/1346-8138.15247 [DOI] [PubMed] [Google Scholar]
- 13.Egeberg A, Bryld LE, Skov L. Drug survival of secukinumab and ixekizumab for moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2019;81(1):173–178. doi: 10.1016/j.jaad.2019.03.048 [DOI] [PubMed] [Google Scholar]
- 14.Yiu ZZN, Mason KJ, Hampton PJ, et al. Drug survival of Adalimumab, ustekinumab and secukinumab in patients with psoriasis: a prospective cohort study from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR). Br J Dermatol. 2020;183(2):294–302. doi: 10.1111/bjd.18981 [DOI] [PubMed] [Google Scholar]
- 15.Mourad AI, Gniadecki R. Biologic Drug Survival in Psoriasis: a Systematic Review & Comparative Meta-Analysis. Front Med. 2020;7:625755. doi: 10.3389/fmed.2020.625755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockshin B, Cronin A, Harrison RW, et al. Drug survival of ixekizumab, TNF inhibitors, and other IL-17 inhibitors in real-world patients with psoriasis: the Corrona Psoriasis Registry. Dermatol Ther. 2021;34(2):e14808. doi: 10.1111/dth.14808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu LF, Chen JS, Gu J, et al. Etanercept biosimilar (recombinant human tumor necrosis factor-alpha receptor II: igG Fc fusion protein) and methotrexate combination therapy in Chinese patients with moderate-to-severe plaque psoriasis: a multicentre, randomized, double-blind, placebo-controlled trial. Arch Dermatol Res. 2020;312(6):437–445. doi: 10.1007/s00403-019-02024-6 [DOI] [PubMed] [Google Scholar]
- 18.Gniadecki R, Bang B, Bryld LE, Iversen L, Lasthein S, Skov L. Comparison of long-term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol. 2015;172(1):244–252. doi: 10.1111/bjd.13343 [DOI] [PubMed] [Google Scholar]
- 19.Mourad A, Straube S, Armijo-Olivo S, Gniadecki R. Factors predicting persistence of biologic drugs in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2019;181(3):450–458. doi: 10.1111/bjd.17738 [DOI] [PubMed] [Google Scholar]
- 20.Akdogan N, Dogan S, Bostan E, et al. Age and psoriatic arthritis are important predictors of biologic agent switch in psoriasis. Expert Rev Clin Pharmacol. 2021;14(12):1535–1541. doi: 10.1080/17512433.2021.1979394 [DOI] [PubMed] [Google Scholar]