Abstract
Introduction/Objective
The patient activation measure (PAM) is considered a reliable tool for measuring patient activation. This study aimed to systematically review the scientific literature regarding the use of PAM −13 in rheumatology patients and to compare PAM scores in patients with rheumatoid arthritis (RA) following two different practices at a single institution with previously published studies.
Methods
The study consisted of a systematic review of articles reporting the PAM-13 in patients with RA, followed by a cross-sectional study evaluating PAM scores between standard rheumatology clinics and specialized rheumatology clinics (SRCs). The correlation between PAM levels and other variables, such as demographics, disease characteristics, and treatment, was assessed.
Results
Nineteen studies, published between 2012 and 2022, met the inclusion criteria. The studies in this review had inconsistent results and quality, with patient activation in RA ranging from 29 to 76. A total of 197 patients with confirmed RA diagnoses were interviewed (response rate, 88%). Most were female (n=173, 88%) and older than 40 years (n=150, 76%). The average patient activation score was 64.9 (standard deviation, 15.7). Most participants had level 3 and 4 patient activation measures (n=71 [36%] and n=72[37%], respectively). Patients who were attending SRCs also had borderline higher PAM levels. Patients with high PAM scores tended to be older, have active disease, and were taking corticosteroids.
Conclusion
Adequate activation of patients was observed from our center, which was higher than that reported in most published literature. The PAM of patients with RA was variable according to the systematic review. Longitudinal interventional studies should be considered to improve activation in patients with low scores.
Keywords: rheumatoid arthritis, patient activation measures, patient engagement, systematic review, cross-sectional study
Introduction
Patient empowerment is an essential aspect of health condition management. This enables patients to become more active in managing themselves by understanding their roles. It is also critical for patients to be knowledgeable and have skills and motivation to be proactive in their management.1,2 This approach involves patient activation, which is defined as patients’ preparedness to manage their health based on their knowledge, skills, and confidence, and understanding their role in the care process to manage their health and health care.3 The patient activation measure (PAM) is a tool that can be used to measure patient empowerment.4
Hibbard et al developed the PAM.4 It was first developed as a 22-item PAM questionnaire, and a short version was later developed with 13 items.5 The 13-item PAM questionnaire is a reliable and valid measure for different chronic illnesses.6 In addition, it is now considered one of the most frequently used questionnaires, with published evidence of its construct validity.4
However, it is important to keep in mind that patients differ in their willingness and ability to take on this role in managing their health.7 Understanding each patient’s readiness to take an active part in managing their health can help in designing customized interventions for each patient based on patient activation level, which has been reported to improve patient outcome measures.7 Studies have demonstrated that patients who are actively involved in their treatment tend to cooperate more effectively with healthcare providers and eventually experience better health outcomes.8 Those with higher activation levels reported significantly better health outcomes, with significantly lower rates of doctor’s office and emergency room visits.4 PAM levels were also observed to be a predictor for new chronic disease; patients with lower PAM levels were more likely to have a new chronic disease.9 In addition, studies have shown that higher PAM levels are associated with lower health costs.9–11 Changes in the PAM levels of patient populations might be used as an indicator of the performance of providers or healthcare delivery systems.12 In addition, the PAM could be used to segment large populations and target interventions for those who have insufficient self-management skills. The PAM can be tracked over time, used to assess individual patient progress, and monitor whole populations.5
It is also essential to consider that several factors may affect patient activation.13 A study carried out in older people with long-term conditions and multimorbidity in the United Kingdom observed that patient activation was significantly lower in older patients, those with depression, and those with poor health literacy, while it was higher in those with good quality of life and with better social support.13
To date, the PAM has been used in patients with multiple types of diseases, including cardiac diseases,14 diabetes mellitus,15 chronic kidney disease,1 depression,16 and rheumatoid arthritis (RA).13 RA affects physical activity, and may lead to disability and early mortality.17 It also affects the quality of life of patients both socially and mentally and has direct and indirect costs, including hospital and treatment costs and absence from work.18 Engaging patients in managing their RA has shown improvements in clinical, functional, and patient-reported outcomes.19
A limited number of studies have assessed the PAM in patients with RA worldwide; however, studies assessing patient engagement and activation in Saudi Arabia are lacking.20 Moreover, to the best of our knowledge, no systematic review has been conducted on the PAM in patients with RA. The present study aimed to evaluate the PAM in patients with RA in a tertiary care center in Saudi Arabia and assess the impact of multidisciplinary team care on patient scores. Moreover, the current study aimed to systematically review the literature on the PAM in patients with RA.
Materials and Methods
Study Design and Setting
Systematic Review
A systematic review was conducted using a predesigned search strategy to include all related keywords, including the patient activation measure and rheumatoid arthritis. The research question was (What is the reported prevalence of patient activation measurements by patient activation measure-13 in observational or interventional studies of adult rheumatoid arthritis patients?). A full search strategy is available (Supplementary Appendix 1). The keywords were entered into the following databases (ProQuest, Science Direct, PubMed, Cochrane Central Register of Controlled Trials, American College of Rheumatology conference proceedings, and US National Library of Medicine ClinicalTrials.gov) searching from the inception of each database until June 2022. The protocol was not registered and no language restrictions or search limits were applied. For study selection, main outcome and inclusion/exclusion criteria for all studies (observational or interventional) that reported the use of the PAM-13 in adult patients with confirmed RA diagnosis were included. No limit for number of participants per study was applied. For exclusion, books or review articles, studies not reporting the PAM or not involving adult patients with RA were excluded. This review used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting the methodology.21 Article titles were screened in duplicate, followed by abstract screening, and any discrepancies in study inclusion were resolved by consensus. Data were extracted in a predesigned and piloted form to include author, year, country, study design, study setting, population, intervention (if any), PAM level, limitations, and conclusion about the PAM. In addition, to search databases, the company website was reviewed for all publications using the PAM-13 to include published studies. A manual search of references to full-text studies was performed in duplicate. The authors were not contacted further. The included studies were assessed for quality and risk of bias using a specific quality assessment for each study design (Cochrane risk of bias tool for randomised controlled trials, National Institute of Health Quality Assessment for Cohort and Cross-sectional Studies, and the British Medical Journal quality assessment tool for qualitative research).22–24 No data synthesis or analysis was performed.
Cross-Sectional Study
The second part of this study was a cross-sectional survey assessing the level of activation of patients with RA in managing their health. The study was observational in nature and was guided using Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).25 Patients were recruited from rheumatology clinics at King Saud University Medical City (KSUMC), Riyadh, Saudi Arabia.
Ethical Approval and Consent
The study protocol and consent forms and methods were approved by the King Saud University Institutional Review Board (No. E-19-4363), and information was managed with confidentially without any patient identifiers in the data collection form. Informed written consent was obtained from a group of participants before commencement of the study. However, due to COVID 19 pandemic the clinics were changed to virtual. The consent was changed to electronic (information sheet was sent via WhatsApp for participants able to read and write and they reply with consent before start of the study). If participant was not able to read or write they were asked if they agree to participate if yes, they were included. For documentation, in the data collection sheet, a check box of consent was marked by the researchers conducting the interview. The verbal consent was also approved by King Saud University Institutional Review Board (No. E-19-4363). The study complies to all Declaration of Helsinki ethical principles for medical research involving human subjects.
Patients and Recruitment
Adult patients (>18 years of age) with a confirmed RA diagnosis according to the American College of Rheumatology/European League Against Rheumatism 2010 classification criteria26 were included. Rheumatology care in KSUMC is provided through two different services:
General rheumatology clinics: Each clinic usually accommodates 15–20 patients in a standardized care facility.
Specialized rheumatology clinics (SRC): Each clinic usually accommodates 10–15 patients evaluated by a multidisciplinary team that includes a rheumatologist, clinical pharmacist, physiotherapist, clinical dietitian, and ultrasound technician. Patients rotate through these different specialties as required. During this multidisciplinary visit, each patient undergoes a complete evaluation of disease outcomes, monitoring of medical therapy safety and efficacy, comorbidity management and evaluation, patient education, and support. The clinics are divided according to commonly encountered illnesses, such as lupus, RA, vasculitis, spondylarthritis, and scleroderma spectrum disease. The SRC were launched in 2017, and approximately 80–90 patients with different rheumatological illnesses were examined and monitored each week. The SRC are novel specialized care and the first of their kind in Saudi Arabia.
Initially, all medical file numbers of patients attending clinics for appointment were reviewed to see those with a confirmed RA diagnosis. Candidate patients were approached, the study protocol was explained, and signed written informed consent was obtained if they agreed to participate. Following this, the patients who agreed to participate were interviewed to collect information using a previously designed form and answer all survey questions in person. However, owing to the coronavirus disease (COVID-19) pandemic and related restrictions, the recruitment methods and consent form process were modified as clinics were shifted to being virtual. Patients with a confirmed RA diagnosis and identified from the clinic electronic database (virtual appointment database). The patients were then contacted and interviewed via telephone and electronic consent was obtained prior to the interview. Patients were recruited for six months between November 2019 and April 2020.
Variables, Data Source, and Measurements
An electronic data collection sheet was designed using Google Forms before conducting the study. The form included baseline patient sociodemographic data, including age, sex, education, economic status, current work status, and living situation alone or with a spouse or family member. The patients were then asked questions from the PAM survey. The Arabic version of the PAM‐13. was used to evaluate the patients’ level of involvement in the self-management of their disease. Insignia Health provided permission to use the PAM-13 along with the Arabic version of the survey (license number:1570198456–1601820856). Insignia Health is the exclusive licencing body for the PAM survey on behalf of its creators and the University of Oregon. In the PAM, patients are asked to rate their statement level of agreement/disagreement using a five-point Likert scale, with the fifth option indicating “not applicable”. The measure yields a total score ranging from 0 to 100, where a greater score indicates greater activation. The score is then computed by the company to divide the patients into four possible levels of 1–4; the higher the level, the greater the activation. Other measurements were completed by researchers using medical file reviews on the day of the patients’ visit. These measurements included the disease activity score-erythrocyte sedimentation rate-28 (DAS-ESR-28). This involves examining 28 joints for 1) swelling and/or 2) tenderness by the attending physician when possible. The patient is then asked to rate their 3) global health from 0 to 10, where 0 indicates poor health status and 10 indicates excellent status. The three assessments are then fed to a mathematical formula adjusted to a fourth variable, which is either the erythrocyte sedimentation rate (ESR) or C-reactive protein level. Since the study was converted to a telephone interview, missing values in the DAS-ESR were managed by complete case analysis. Here, we used ESR, as the assessment is more prevalent in clinical settings. Other comorbidities and medications at the time of last visit were also collected from patient medical files.
Bias, Study Size, and Statistical Methods
Patients were included as they presented for a clinic visit. When contacted via telephone, all patients who answered the call and agreed to participate were included. A sample size of more than 75 was recommended by the Insignia Health licencing body for survey validity. Data for the survey were entered into a statistical package and sent to Insignia Health for response analysis. Other information was coded and entered into Statistical Package for Social Sciences (SPSS) version 26.27 The mean and standard deviation were obtained for normally distributed data; non-normally distributed data are presented as median and interquartile ranges (25th and 75th percentile values). Categorical data are presented as numbers and percentages. The baseline characteristics of patients with different levels of activation were compared using analysis of variance to compare the means of normally distributed data or the Kruskal–Wallis test for non-normally distributed data. For categorical variables, the chi-squared test was used. Multiple linear regression was performed to predict changes in PAM as a continuous variable and its interaction with different factors, considering the effects of common confounders, age, sex, and disease duration, regardless of significance.
Results
Systematic Review
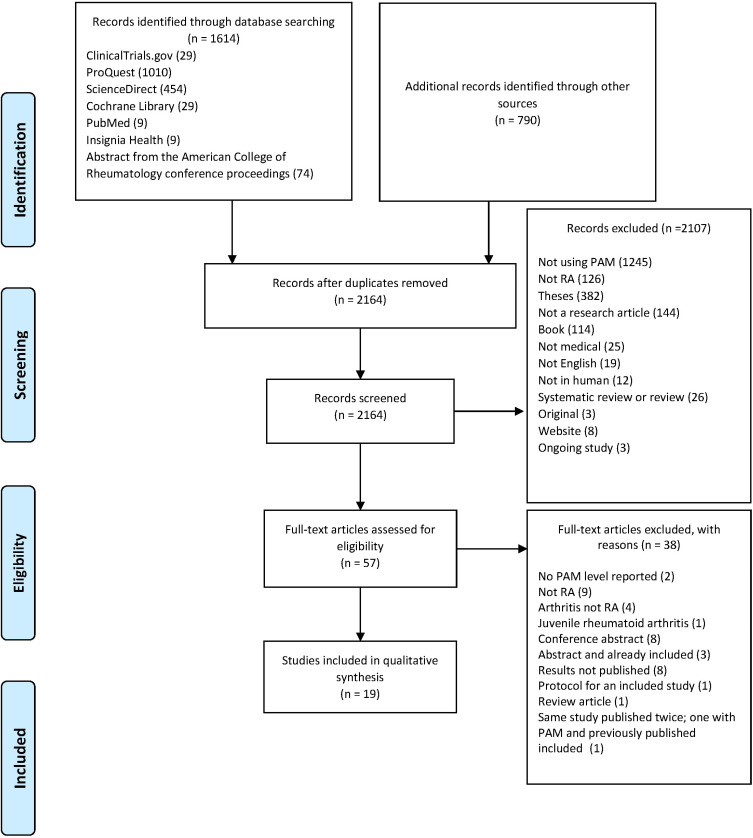
When searching the six databases, 2404 records were identified, and 2164 were screened after duplicates were removed. After removing multiple titles and abstracts, 57 titles and abstracts were eligible for full-text review. A total of 19 articles were eligible for qualitative analysis. Data on record screening and exclusion are available in the PRISMA flow diagram, as illustrated in Figure 1. Detailed systematic review records are available (Supplementary Appendix 2). Owing to the inconsistency in study designs and differences in interventions, combining the results of data synthesis and meta-analysis was not possible. The study designs were different, starting from randomized control trials, cross-sectional, mixed methods, qualitative methods, and cohorts. Some studies had an intervention and thus reported the effect of the intervention on the PAM-13 level as one of the study outcomes, while others reported the PAM in patients with RA or with comorbid RA. Most studies reported patients with RA as part of a chronic illness with the PAM-13 as a continuous variable and did not compute it into levels. The PAM-13 of patients with RA in the 19 studies ranged from 28.8 to 75.8. Quality assessment of each study design ranged from moderate to good quality (50–100% of 14 assessment items). No low-quality studies were available. Information on the results, PAM-13 levels, and quality assessment is available in Table 1. Details on quality assessment (Supplementary Appendix 3).
Figure 1.
PRISMA flow diagram.
Notes: Adapted from: Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71. Creative Commons Attribution (CC BY 4.0) license (https://creativecommons.org/licenses/by/4.0/legalcode).52
Abbreviations: PAM, patient activation measure; RA, rheumatoid arthritis.
Table 1.
Summary of Included Articles on Patient Activation Measure and Rheumatoid Arthritis with Quality Assessment
| No. | Author | Year | Country | Study Design | Study Settings | Population | Intervention | PAM Level | Limitations with Regards to PAM | Conclusion | Quality AssessMent (Number and % out of 14 Checklist Items) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Interventional studies | |||||||||||
| 1 | Zuidema et al38 | 2019 | Netherlands | Randomized Control Trial | 2 Dutch hospitals | 157 patients with RA | Web-based self-management enhancing program | Control group mean 46.9 (±4.9), intervention 47.2 (±3.7) | PAM was reported as a continuous variable and was not computed to levels | No positive effects of the program were detected | 9 (64%) |
| 2 | Mollard et al39 | 2018 | US | Mixed methods | University hospital in Nebraska | 63 patients with RA | Live With Arthritis app | Intervention 67.4, control 71.9 | PAM was reported as a continuous variable and was not computed to levels | The PAM was negatively correlated with non-significant changes of HAQ-II (Pearson correlations: −0.33, p = 0.10) | 11 (79%) For qualitative part 3 (21%) |
| 3 | Van Den Bosch et al19 | 2017 | Europe, Israel, Mexico, Puerto Rico, and Australia | Observational study (cohort) | Australia, Belgium, Czech Republic, France, Germany, Greece, Israel, Mexico, Netherlands, Portugal, Puerto Rico, Slovakia, Switzerland, and the United Kingdom | 1025 patients with RA | Patient support programs (PSP) and instructions on how to use adalimumab | PSP users mean score 60.7 (±15.3), non-users 58.9 (±14.6) | Focusing on adalimumab treatment, PAM was reported as a continuous variable and was not computed to levels | The percentage of patients that demonstrated improvement in PAM-13 levels was significantly higher among PSP users vs PSP non-users (35.7 vs 28.1%, p = 0.01). Compared to PSP users, PSP non-users had a significantly higher percentage of patients that started at PAM-13 level 4 at baseline and remained at level 4 until week 78 of ADA treatment (64.5 vs 53.8%, p = 0.028). | 12 (86%) |
| 4 | Gronning et al42 | 2016 | Norway | Qualitative study within the published randomized control trial | University hospital | Adult RA patients last included in the randomized control trial | Nurse-led hospital-based patient education program | 26 (intervention 15, control 11) PAM of intervention group 73, of control group 70 |
A small group of which had RA | The experiences from the Patients in the nurse-led patient education program were in concordance with the pathway of self-efficacy and patient activation, which many patient education programs are based on | 10 (71%) |
| 5 | Joplin et al40 | 2014 | Australia | Cohort | Mid-North Coast Arthritis Clinic (MNCAC), a community-based rheumatology practice in Coffs Harbour | 18 patients with RA | Joint ultrasound | 60.0 (±15.5) at baseline | Very small sample size, PAM was reported as a continuous variable and was not computed to levels | PAM-13 scores did not change during the The study, with levels of activation at T1, T2, and T3 of 60.0 (±15.5), 55.7 (±12.0) and 57.8 (±14.8) (p = 0.21), respectively. |
7 (50%) |
| 6–8 | Gronning et al41,49 follow-up study after 5 years50 | 2012 2013, 2019 |
Norway | Randomized control trial | University hospital | 141 of which a group of patients had RA 101 follow-up 63 of which had RA |
Nurse-led hospital-based patient education program | Reports of the first study include intervention PAM 65.7 (±13.3), control PAM 65.6 (±16.4) | Not all patients had RA, the exact number of RA patients was not mentioned, PAM was reported as a continuous variable and was not computed to levels | Reported results at 4 and 12 months, then 5 years follow-up. A significant improvement of PAM scores among women with intervention at 12 months, mean change 4.2 (0.0, 8.3, p = 0.048). |
11 (79%) |
| 9 | Sandhu et al43 | 2012 | Canada | Qualitative Pilot before and after study |
Patients were recruited either from the primary care clinic in Toronto or via email sent from the Arthritis Society in Canada | Mentors: Adult patients with IA for more than 2 years and receiving drug therapy Mentees: adult patients with early IA |
In the mentor training program, the mentee was assessed before and after pairing with a mentor | 17 (9 mentors, 8 mentees PAM of mentees mean 75.80 (SD 73.11) p= 11.75 effect size 0.22 |
A very small number of patients as it is mainly qualitative and IA was not clear if it was RA or not | No difference between groups with regards to PAM level | 10 (71%) |
| Non-interventional studies | |||||||||||
| 10 | Jones et al32 | 2021 | UK | Qualitative Semi‐structured interviews at two timepoints | Patient were recruited from two rheumatology departments in south west England | 17 participants total, 13 of which had RA | None | Participants demonstrated high levels of patient activation Four were at level 2, four at level 3 and six at level 4 |
Did not mention the exact level and RA patient were not assessed separately | Patients’ perceptions and experiences of patient activation covered are not always captured by the PAM | 14 (100%) |
| 11 | Huang et al33 | 2021 | Singapore | Cross-sectional | Specialist outpatient clinics of a tertiary hospital in Singapore |
200 participants with chronic conditions 56 of which had other chronic conditions including RA | None | The mean activation score was 58.8 (SD = 15.0) | Small number of participants and RA patient were not assessed separately | Some factors as age, income, education and health literacy are factors contributing to change in patient activation | 9 (64%) |
| 12 | Zakeri et al35 | 2021 | Iran | Cross-sectional | Chronic patients admitted to the Cardiac Care Unit and medical wards in Ali Ibn Abitaleb Hospital of Rafsanjan |
293 patients with chronic conditions 33 had other conditions including RA | None | The mean score of PAM-13 was 56:99 ± 15:32 | Small number of participants and RA patient were not assessed separately |
The physical and psychological subscales of Quality Of Life (QOL) significantly predicted the levels of PAM | 9 (64%) |
| 13 | Kosar et al30 | 2019 | Turkey | Cross-sectional | University Hospital in Izmer | 130 a group of which had RA | None | PAM scores of the patients ranged from 28.8% to 83.3%. Up to 28.7% of patients were in activation level 1, 44.9% were in activation level 2, 20.2% were in activation level 3 and 6.2% were in activation level 4. |
130 patients of which patients with RA, did not mention the exact number | Validity study of PAM-13 in Turkish | 9 (64%) |
| 14 | McBain et al31 | 2018 | UK | Cross-sectional | National Rheumatoid Arthritis Society, UK | 841 (95%) had RA | None | PAM was 57.8 (±15.5). The samples were evenly split across the 4 levels of activation, 251 (28.4%) level 1, 182 (20.6%) at level 2, 204 (23.1%) at level 3 and 248 (28.0%) at level 4. | Not all patients had RA | Only a small proportion of patients attended a self-management structure support program. | 8 (57%) |
| 15 | Lofland et al37 | 2017 | US | Cross-sectional survey and cost cohort | Claims database | Adult patients receiving biologics | None | 453 responders to the cross-sectional survey to assess shared decision making. PAM scores SDM vs non-SDM: 66.9 vs 61.6; P<0.001 |
RA patients were combined with PsA patients | Patient with SDM had a significantly greater PAM score | 11 (79%) |
| 16 | Graffigna et al34 | 2017 | Italy | Cross-sectional | Online panel provided by Research Now (secondary database) | Patients over 18 years of age and with chronic conditions | None | 352 (11.1%) had RA meaning 39 patients Mean PAM of all patients was 65.3 (range 0–100) |
Reported PAM was for the entire population and limited number with RA | Mean PAM of all patients was 65.3 (range 0−100) | 10 (71%) |
| 17 | Blakemore et al13 | 2016 | UK | Cohort | Cohort study database | Patients ≥ 65 years having at least one long-term condition | None | 3390 PAM at baseline 60.8 (SD 15.4), at follow-up 60.3 (SD 20.0), no difference |
Patients with RA were combined with all other illnesses and not clear as a separate group | No difference at baseline compared to follow-up with regards to PAM level | 11 (79%) |
| 18 | Jones et al51 | 2021 | UK | Cross-sectional | Six rheumatology clinics in England | Patients> 18 with an inflammatory rheumatic condition including RA, PsA and AS or SLE | None | 166 (66%) patients had RA, PAM was 58.3 (SD 11.5) | Patients with RA were combined with all other illnesses and not clear as a separate group | Self-efficacy and health literacy are targets for patient activation interventions | 9 (64%) |
| 19 | Oliveria et al36 | 2021 | Brazil | Cross-sectional | Rheumatology outpatient facility at a high-complexity teaching hospital in Brazil | 179 patients> 18 years with RA, having at least one year of formal education and being able to read and not having neurological or psychiatric disorders that affect cognition, and having enough visual acuity to read. | None | The average PAM-13 was 65.72, with 10.1% very low activation level, 15.6% having a low activation level, 39.1% moderate activation level and 35.2% having a high activation level. | None | Activation and health literacy are very important in RA patients and improving them could increase functional capacity | 8 (57%) |
Cross-Sectional Study
Demographics
A total of 223 patients visit the clinic and were contacted, 197 were invited, and 26 did not agree to continue the interview due to lack of time, generating a response rate of 88%. Of these, 124 (63.6%) were recruited from SRC. A total of 197 (N=173, 88%) were female and seropositive (69%), with a mean (±standard deviation [SD]) age and disease duration of 50.0 (±12.2) and 13.5 (±7.8) years, respectively. Background medications included conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) (50.3%), biologics/small molecules (43.1%), and prednisolone (14.1%). The DAS28 median (interquartile range) was 1.14 (0.00–2.46) with 126 (69.6%) achieving either remission or low disease activity. A total of 119 (60%) patients had middle to high level of education. Additionally, 120 (91%) were unemployed and 109 (55%) had a low to intermediate average income. The patients lived with a minimum of one family member (187, 95%) and had other comorbidities (114, 58%; Table 2).
Table 2.
Baseline demographics of Patients with Univariate Analysis of Difference Between Activation Levels
| Patient Activation Level | Level 1 n=27 | Level 2 n=27 | Level 3 n=71 | Level 4 n=72 | Total n=197 | p value | |
|---|---|---|---|---|---|---|---|
| Sex, female (%) | 24 (13.9) | 27 (15.6) | 60 (34.7) | 62 (35.8) | 173 (87.8) | 0.195 | |
| Age, mean years, (SD) | 53.1 (11.6) | 49.6 (11.4) | 52.4 (13.2) | 46.9 (11.0) | 50.0 (12.2) | 0.024* | |
| Age (%) | |||||||
| ≤40 years | 3 (6.4) | 6 (12.8) | 15 (31.9) | 23 (48.9) | 47 (23.9) | 0.149 | |
| >40 years | 24 (16.0) | 21 (14.0) | 56 (37.3) | 49 (32.7) | 150 (76.1) | ||
| Type of clinic, (%) | |||||||
| Specialized multidisciplinary | 17 (13.7) | 14 (11.8) | 46 (37.1) | 47 (37.9) | 124 (63.6) | 0.594 | |
| Standard of care | 9 (12.7) | 13 (18.3) | 25 (35.2) | 24 (33.8) | 71 (36.4) | ||
| Education (%) | |||||||
| None to low | Uneducated, primary, and middle school | 15 (19.2) | 9 (11.5) | 30 (38.5) | 24 (30.8) | 78 (39.6) | 0.194 |
| Intermediate to high | High school, diploma, university and above | 12 (10.1) | 18 (15.1) | 41 (34.5) | 48 (40.3) | 119 (60.4) | |
| Income (%) | |||||||
| Low to intermediate | 20 (13.4) | 23 (15.4) | 50(33.6) | 56 (37.6) | 149 (75.6) | 0.458 | |
| High | 5 (12.2) | 11 (26.8) | 8 (19.5) | 17 (41.5) | 48 (24.4) | ||
| Working (%) | 5 (9.3) | 9 (16.7) | 15 (27.8) | 25 (46.3) | 54 (27.4) | 0.180 | |
| Living arrangements (%) | |||||||
| With family member | 26 (13.9) | 25 (13.4) | 67 (35.8) | 69 (36.9) | 187 (94.9) | 0.901 | |
| Alone | 1 (10.0) | 2 (20.0) | 4 (40.0) | 3 (30.0) | 10 (5.1) | ||
| Other comorbidities (%) | |||||||
| One or less | 16 (11.2) | 22 (15.4) | 50 (35.0) | 55 (38.5) | 143 (72.6) | 0.243 | |
| More than one | 11 (20.4) | 5 (9.3) | 21 (38.9) | 17 (31.5) | 54 (27.4) | ||
| Depression (%) | 3 (37.5) | 0 (0.0) | 1 (12.5) | 4 (50.0) | 8 (4.1) | 0.099 | |
| Other medication (%) | 1 (5.9) | 3 (17.6) | 7 (41.2) | 6 (35.3) | 17 (8.6) | 0.756 | |
| Supplements | 2 (5.9) | 8 (23.5) | 10 (29.4) | 14 (41.2) | 34 (17.3) | 0.140 | |
Note: *Significance level at p <0.05.
Patient Activation Measure Scores
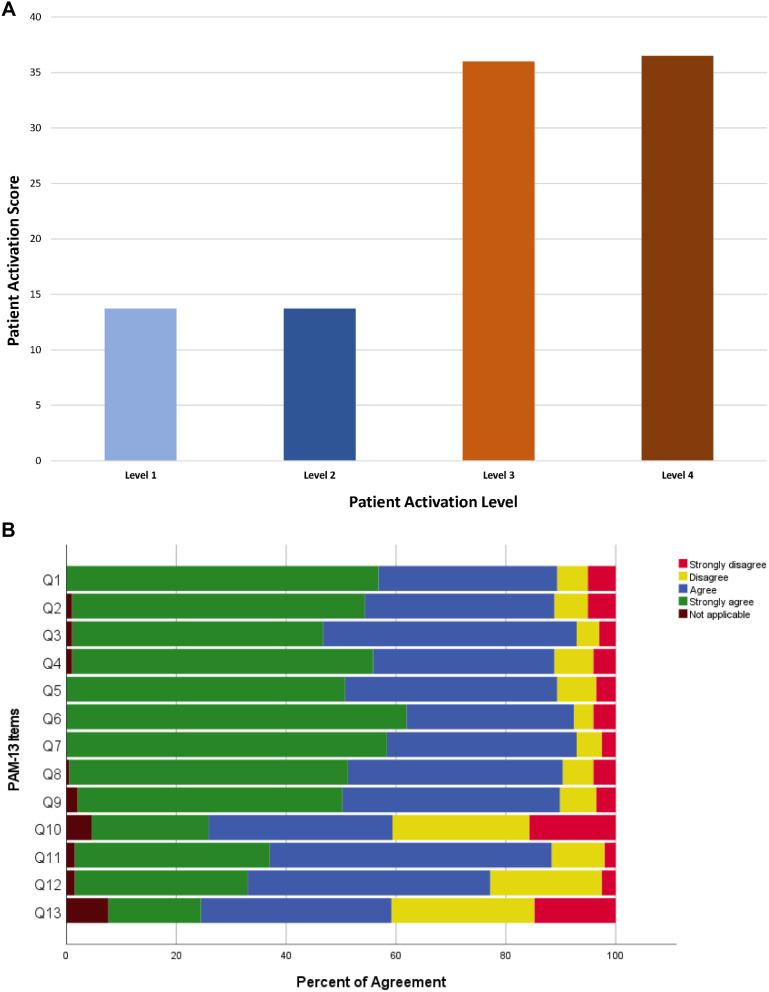
The mean PAM score was 64.9 (±15.7). Most patients were categorized as levels 3 and 4 PAM (71, 36%, 72, and 37%), with patients mostly agreeing on the 13 items of the PAM survey, as indicated in Figure 2A and B. Patients with levels 1 and 2 were in a minority, with the same number in both groups (27, 14%). The responses and level distribution of patients are illustrated in Figure 2A and B. When conducting univariate analysis between different PAM groups, all were comparable except age, which was lower in the level group with a p-value of 0.024 (Tables 2 and 3).
Figure 2.
(A) Distribution of patients by PAM level. (B) Individual responses of patients using PAM survey (green indicates strongly agree).
Table 3.
Rheumatoid Arthritis-Related Information with Univariate Analysis and Resulting p values for Differences Between Activation Levels
| Patient Activation Level | Level 1 | Level 2 | Level 3 | Level 4 | Total | p value |
|---|---|---|---|---|---|---|
| Seropositivity (%) | ||||||
| Positive | 9 (11.0) | 15 (18.3) | 32 (39.0) | 26 (31.7) | 82 (68.9) | 0.399 |
| Erythrocyte sedimentation Rate (standard deviation) | 32 (28) | 39 (30) | 31 (30) | 27 (23) | 31 (28) | 0.339 |
| Disease duration, years | 11.4 (6.6) | 13.4 (8.8) | 14.9 (7.1) | 12.8 (8.3) | 13.5 (7.8) | 0.198 |
| Disease duration (%) | ||||||
| Early (≤2 years) | 2 (33.3) | 1 (16.7) | 1 (16.7) | 2 (33.3) | 6 (3.1) | 0.466 |
| Established (>2 years) | 24 (12.7) | 26 (13.8) | 69 (36.5) | 70 (37.0) | 189 (96.9) | |
| Disease activity score - erythrocyte sedimentation rate (%) | ||||||
| DAS28-ESR median (IQR) | 1.21 (0.00–2.46) | 2.41 (0.00–3.18) | 0.68 (0.00–3.03) | 0.87 (0.00–2.64) | 1.14 (0.00–2.46) | 0.822 |
| Remission to mild | 19 (15.1) | 18 (14.3) | 42 (33.3) | 47 (37.3) | 126 (69.6) | 0.271 |
| Moderate to severe | 4 (7.3) | 9 (16.4) | 25 (45.5) | 17 (30.9) | 55 (30.4) | |
| csDMARDs | 11 (11.1) | 17 (17.2) | 33 (33.3) | 38 (38.4) | 99 (50.3) | 0.346 |
| TNFi, non-TNFi, small molecule agents | 8 (9.4) | 16 (18.8) | 33 (38.8) | 28 (32.9) | 85 (43.1) | 0.126 |
| Corticosteroids (%) | 1 (3.4) | 5 (17.2) | 12 (41.4) | 11 (37.9) | 29 (14.7) | 0.361 |
| NSAIDs (%) | 0 (0.0) | 3 (27.3) | 4 (36.4) | 4 (36.4) | 11 (5.6) | 0.367 |
Note: *Significance level according to p < 0.05.
In multiple linear regression, all assumptions were met except for the normality of the dependent variable. However, since the significance of normality in regression is uncertain and N>50 in this study, we continued with the regression.28,29 On applying simple regression to the possible confounding variables (age, sex, and disease duration), no prediction was statistically significant except for the type of clinic (p = 0.050), which may be considered borderline significant. Although most models were not significant, also keeping in mind that confidence intervals were wide, some direction of higher PAM trended with younger patients, being male, established RA, higher education, lower income, working, living alone, having one comorbidity, no depression, seropositive, more disease activity, less use of other medications, more csDMARDs, more biologics, more prednisolone, and fewer NSAIDs. Patients recruited from SRC also had borderline significantly higher PAM levels (Table 4).
Table 4.
Multiple Linear Regression Predicting Factors That Influence Patient Activation Measurements Displayed as a Continuous Variable
| Factor | PAM Mean (SD) | Beta | p value | 95% Confidence Intervals | |
|---|---|---|---|---|---|
| Age | |||||
| Age, years | −0.166 | 0.072 | −0.347 to 0.015 | ||
| ≤40 | 67.8 (12.3) | ||||
| > 40 | 64.0 (16.4) | −3.823 | 0.147 | −9.00 to 1.354 | |
| Sex | |||||
| Female | 64.3 (15.3) | ||||
| Male | 69.1 (18.4) | 4.824 | 0.160 | −1.924 to 11.572 | |
| Disease duration | |||||
| Duration, years | 0.030 | 0.833 | −0.254 to 0.315 | ||
| Early | 58.8 (22.3) | ||||
| Established | 65.3 (15.4) | 6.924 | 0.287 | −5.863 to 19.710 | |
| Factors adjusted to age, sex, and disease duration regardless of significance | |||||
| Education Level | |||||
| None to low | Uneducated, primary, and middle school | 62.7 (17.5) | |||
| Middle-High | High school, diploma, university, and above | 66.3 (14.4) | 1.587 | 0.532 | −3.415 to 6.589 |
| Income | |||||
| Low | 65.1 (16.1) | ||||
| High | 64.3 (14.6) | −1.171 | 0.657 | −6.367 to 4.023 | |
| Work status | |||||
| No | 64.0 (15.9) | ||||
| Yes | 67.3 (15.3) | 1.190 | 0.678 | −4.449 to 6.830 | |
| Living | |||||
| With family | 65.0 (15.8) | ||||
| Alone | 63.0 (15.2) | 0.304 | 0.953 | −9.798 to 10.4.7 | |
| Other comorbidities | |||||
| One or more | 65.8 (16.3) | ||||
| More than one | 62.5 (16.8) | −0.942 | 0.736 | −6.450 to 4.566 | |
| Depression | |||||
| No | 65.0 (15.8) | ||||
| Yes | 62.1 (13.8) | −2.028 | 0.716 | −13.120 to 9.064 | |
| Seropositivity (rheumatoid factor or anti-citrullinated peptide positive) | |||||
| Negative | 66.3 (16.0) | ||||
| Positive | 63.8 (15.5) | −2.181 | 0.491 | −8.442 to 4.079 | |
| DAS-ESR | |||||
| DAS28-ESR continuous | 0.321 | 0.594 | −0.866 to 1.507 | ||
| Remission to mild | 64.5 (16.5) | ||||
| Moderate to sever | 65.6 (13.7) | 1.781 | 0.486 | −3.254 to 6.817 | |
| Other medication | |||||
| No | 65.1 (15.9) | ||||
| Yes | 62.9 (14.8) | −2.103 | 0.607 | −10.145 to 5.939 | |
| cDMARDs | |||||
| No | 63.9 (16.2) | ||||
| Yes | 65.9 (15.3) | 2.905 | 0.204 | −1.594 to 7.404 | |
| TNFi, non-TNFi, and small-molecule agents | |||||
| No | 65.1 (16.7) | ||||
| Yes | 64.6 (14.5) | −1.800 | 0.437 | −6.358 to 2.758 | |
| CS | |||||
| No | 64.4 (16.4) | ||||
| Yes | 67.6 (11.0) | 3.868 | 0.228 | −2.438 to 10.175 | |
| NSAIDs | |||||
| No | 65.0 (16.0) | ||||
| Yes | 63.5 (12.2) | −1.012 | 0.835 | −10.576 to 8.551 | |
| Type of clinic | |||||
| Specialised multidisciplinary | 66.5 (16.2) | ||||
| Standard of care | 62.2 (14.6) | −4.570 | 0.050 | −9.146 to 0.006 | |
Notes: *Significance level according to p < 0.05.52
Abbreviations: PAM, patient activation measure; SD, standard deviation; csDMARDs, conventional disease-modifying antirheumatic drugs, including methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, or mycophenolate; TNFi biologics, tumor necrosis factor inhibitors, including adalimumab, etanercept, certolizumab, or infliximab; non-TNFi, non-tumor necrosis factor inhibitors, including abatacept, rituximab, or tocilizumab; NSAIDs, non-steroidal anti-inflammatory drugs; IQR, interquartile range.
Discussion
The present study is the first systematic review reporting the PAM in patients with RA. In the systematic review total of 19 studies on the PAM-13 in RA were identified. Ten studies measured the level of activation without any intervention, such as the Turkish study by Kosar et al which reported a small number of patients (26.4%) achieving a level of 3 or 4.30 However, Bain et al reported higher activation in more than half of the patients at levels 3 and 4.31 Jones et al also reported high levels of patient activation in their study sample (n=17), four of which were at level 2, four at level 3, and six at level 4.32 Four other studies from the United Kingdom, Italy, Singapore, Brazil and Iran only reported mean PAM without categorization.13,32–36 Lofland et al assessed the PAM in patients who initiated biologics and the impact of adequate shared decision-making.37 A higher mean PAM was observed in patients who had adequate shared decision-making (p < 0.001). The remaining nine studies applied different interventions with variable reporting. Zuidema et al randomized 157 patients to usual care and usual care plus access to a comprehensive unguided web-based program containing nine modules with 13 objectives.38 The study showed a significant improvement, which could be partly explained by the dropout level and incomplete responses.38 Mollard and Michaud evaluated the impact of using a mobile application called LiveWith Arthritis (eTreatMD, Vancouver, BC) on self-management behaviors in a small sample of patients. The Patient-Reported Outcomes Measurement Information System Self-Efficacy Managing Symptoms score was significantly better in the intervention group (p=0.04); however, the PAM changes were not significant (p = 0.48). Similarly, the main challenge was the large dropout rate, which could have affected the study results.39 The PASSION study was a post-marketing study sponsored by ABBVIE that compared the introduction of patients to a patient support program (PSP) vs usual care. PAM levels were measured at baseline and 78 weeks. All outcome measures improved in the PSP group, including the PAM score (35.7 vs 28.1%, p=0.01).19 Joplin et al conducted a pilot study on the effect of ultrasound on patients’ perception of the need for medication change. The primary outcome was met with an improved decision; however, it did not impact the mean PAM.40 Grønning et al conducted a long-term nurse-led program on a mixed Norwegian population that included patients with RA. The intervention included group sessions moderated by two nurses, followed by individual educational sessions. After the randomization period, all patients were invited to participate in the program. At 12 months, a trend toward improved PAM was confirmed at the 5-year follow-up (p = 0.024).41 This was followed by a qualitative study of patients who participated in the trial.42 Sandhu et al paired patients with inflammatory arthritis and a disease duration of >2 years (mentors) with patients with early inflammatory arthritis and a disease duration of <1 year (mentees). Nine pairs interacted for 12 weeks with mentees experiencing improvements in the overall impact of arthritis on life, coping efficacy, and social support, with no impact on PAM scores.43
The findings of the cross-sectional study and in comparison, with reviewed literature showed that patients with RA had high activation scores in our center, with additional and possible positive impact of the multidisciplinary team on engagement. We also found that all patients were engaged equally and that no demographic, disease, and drug factors measured in this study differed statistically from these high scores except age and were adjusted for in the analysis. Patients participating in this study were provided with specialized healthcare experiences that may explain these high level scores. High activation levels were also observed in patients with atrial fibrillation treated at an academic medical center specialist clinic.44
In the general rheumatology clinic standard of care, patients visited rheumatology consultants every 3–6 months as per international guidelines, where laboratory tests for drug safety and efficacy and monitoring disease progression were performed. In addition, patients visit drug monitoring clinics to refill important medications and determine if the patient needs more frequent monitoring. However, the only team that patients encountered was medical rheumatology. The provision of specialized information tailored to patient needs has been associated with high activation levels in multidisciplinary clinic practice.45 In addition, care provided by a multidisciplinary team has been associated with high activation levels, as observed in this study and another study of patients with metabolic syndrome.46,47 In a systematic review published by Bearne et al multidisciplinary team care was not associated with improved outcomes in terms of disease activity, disability, or quality of life.48 With the acknowledgment that the p value of specialized clinics was borderline significant, no published study has evaluated the impact of multidisciplinary team care on patient activation, and we believe that our study is the first to possibly indicate a positive impact of multidisciplinary care on health outcomes. This could be explained by the design of the SRC and staff providing care. We could not precisely determine which intervention in this model played a major role in improving patient activation.
To the best of our knowledge, this is the first published study in Saudi Arabia to identify patient activation scores for chronic diseases. Although it provides important insights into the patient activation level in Saudi Arabia, there are limitations to the generalization of the results. This study was conducted analyzing a clinical setting that provided specialized, centered patient care with no representation of patients attending primary clinics. This study emphasized the impact of specialized patient-centered care on patient activation as an intermediate outcome of care, which is linked to improved clinical outcomes.3 Different patient settings are required to understand the impact of clinical services on patient activation in Saudi Arabia. Another important limitation is that this is a cross-sectional study with no longitudinal analysis to follow the changes in activation level; it only provides a snapshot of the level of patient activation in certain settings.
For future recommendations, more studies using different quantitative and qualitative methods are needed. A replicate of this study is required using multiple centers and different clinical settings to compare the impact of specialized clinics to standard care and identify this as a factor in improving patient engagement in treating patients with RA. Qualitative studies exploring the patient’s point of view are needed to gain a deeper understanding of cultural, religious, and social factors, which may affect patient activation levels. Further research should be conducted to compare patients with RA in primary care centers and other general RA clinics in Saudi Arabia. A deeper investigation should be carried out in patients who participated in this study to understand the way patients with high activation scores communicate with healthcare professionals involved in the provision of their care, identify alarming signs and report them, monitor their disease, improve medication adherence, and their involvement in appropriate health behaviors including exercise, diet, check-ups, and avoiding smoking and other unhealthy behaviors.
The limitations of the present study include the cross-sectional design and unmatched sample owing to the impact of the COVID-19 pandemic on the recruitment process. In addition, we did not assess other characteristics that might be important, such as disability and quality of life measures, which are potential subjects for future studies. The study findings cannot be generalized, as most patients were women and had established RA. Finally, the SRC model requires specific settings and cannot be easily applied to other institutions.
From the studies mentioned above, we noticed that there are limited studies that have evaluated patient activation in patients with RA. In interventional studies, small sample size, short study duration, and large dropout rate were the most important factors affecting study results. Our study provides promising results on the impact of multidisciplinary care patient activation and may be used as a model for national health transformation in Saudi Arabia.
Conclusion
In conclusion, we observed adequate activation of patients from our center compared to the published literature, with borderline higher levels in the SRC group. Longitudinal interventional studies should be considered to improve activation in patients with low scores.
Acknowledgments
We would like to acknowledge the efforts of Fatimah Alsuwayeh, Asma Alsadaawi, Sahar Abdullah Alshehri and Nawal Almutairi for the initial screening of titles and abstracts for systematic reviews.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zimbudzi E, Lo C, Ranasinha S, et al. The association between patient activation and self-care practices: a cross-sectional study of an Australian population with comorbid diabetes and chronic kidney disease. Health Expect. 2017;20(6):1375–1384. doi: 10.1111/hex.12577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilello LA, Hall A, Harman J, et al. Key attributes of patient centered medical homes associated with patient activation of diabetes patients. BMC Fam Pract. 2018;19(1):4. doi: 10.1186/s12875-017-0704-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Healthaffairs.org. 2020;32(2):207–214. doi: 10.1377/hlthaff.2012.1061 [DOI] [PubMed] [Google Scholar]
- 4.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39(4 Pt 1):1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40(6 Pt 1):1918–1930. doi: 10.1111/j.1475-6773.2005.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hibbard JH, Tusler M. Assessing activation stage and employing a “next steps” approach to supporting patient self-management. J Ambul Care Manage. 2007;30(1):2–8. doi: 10.1097/00004479-200701000-00002 [DOI] [PubMed] [Google Scholar]
- 7.Hibbard JH, Greene J, Tusler M. Improving the outcomes of disease management by tailoring care to the patient’s level of activation. Am J Manag Care. 2009;15(6):353–360. [PubMed] [Google Scholar]
- 8.Hibbard J, Gilburt H. Supporting People to Manage Their Health: An Introduction to Patient Activation. King’s Fund; 2014. [Google Scholar]
- 9.Hibbard JH, Greene J, Sacks RM, Overton V, Parrotta C. Improving population health management strategies: identifying patients who are more likely to be users of avoidable costly care and those more likely to develop a new chronic disease. Health Serv Res. 2017;52(4):1297–1309. doi: 10.1111/1475-6773.12545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jansen F, Coupé VMH, Eerenstein SEJ, Leemans CR, Verdonck-de Leeuw IM. Costs from a healthcare and societal perspective among cancer patients after total laryngectomy: are they related to patient activation? Support Cancer Ther. 2018;26(4):1221–1231. doi: 10.1007/s00520-017-3945-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindsay A, Hibbard JH, Boothroyd DB, Glaseroff A, Asch SM. Patient activation changes as a potential signal for changes in health care costs: cohort study of US high-cost patients. J Gen Intern Med. 2018;33(12):2106–2112. doi: 10.1007/s11606-018-4657-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey L, Fowles JB, Xi M, Terry P. When activation changes, what else changes? The relationship between change in patient activation measure (PAM) and employees’ health status and health behaviors. Patient Educ Couns. 2012;88(2):338–343. doi: 10.1016/j.pec.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 13.Blakemore A, Hann M, Howells K, et al. Patient activation in older people with long-term conditions and multimorbidity: correlates and change in a cohort study in the United Kingdom. BMC Health Serv Res. 2016;16(1):582. doi: 10.1186/s12913-016-1843-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia Ngooi B, Packer TL, Kephart G, et al. Validation of the Patient Activation Measure (PAM-13) among adults with cardiac conditions in Singapore. Springer. 2017;26(4):1071–1080. doi: 10.1007/s11136-016-1412-5 [DOI] [PubMed] [Google Scholar]
- 15.Aung E, Donald M, Coll JR, Williams GM, Doi SAR. Association between patient activation and patient-assessed quality of care in type 2 diabetes: results of a longitudinal study. Health Expect. 2016;19(2):356–366. doi: 10.1111/hex.12359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacks RM, Greene J, Hibbard JH, Overton V. How well do patient activation scores predict depression outcomes one year later? J Affect Disord. 2014;169:1–6. doi: 10.1016/j.jad.2014.07.030 [DOI] [PubMed] [Google Scholar]
- 17.Fatima S, Schieir O, Valois MF, et al. Health assessment questionnaire at one year predicts all-cause mortality in patients with early rheumatoid arthritis. Arthritis Rheumatol. 2021;73(2):197–202. doi: 10.1002/art.41513 [DOI] [PubMed] [Google Scholar]
- 18.Cooper NJ. Economic burden of rheumatoid arthritis: a systematic review. Rheumatology. 2000;39(1):28–33. doi: 10.1093/rheumatology/39.1.28 [DOI] [PubMed] [Google Scholar]
- 19.Van den Bosch F, Ostor AJK, Wassenberg S, et al. Impact of participation in the adalimumab (humira) patient support program on rheumatoid arthritis treatment course: results from the PASSION Study. Rheumatol Ther. 2017;4(1):85–96. doi: 10.1007/s40744-017-0061-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Tannir M, Algahtani F, Abu-Shaheen A, Al-Tannir S, Alfayyad I. Patient experiences of engagement with care plans and healthcare professionals’ perceptions of that engagement. BMC Health Serv Res. 2017;17(1):1–9. doi: 10.1186/s12913-017-2806-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data: the PRISMA-IPD Statement PRISMA Extension for Individual Patient Data PRISMA extension for individual patient data. JAMA. 2015;313(16):1657–1665. doi: 10.1001/jama.2015.3656 [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NIH. Quality assessment tools for observational cohort and cross-sectional studies. Quality assessment tools; 2019. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed September 22, 2020.
- 24.Mays N, Pope C. Assessing quality in qualitative research. BMJ. 2000;320(7226):50LP–52 . doi: 10.1136/bmj.320.7226.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies*. Bull World Health Organ. 2007;85(11):867–872. doi: 10.2471/BLT.07.045120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 27.IBM Corp. IBM SPSS statisticaics for windows; 2021.
- 28.Li X, Wong W, Lamoureux EL, Wong TY. Are linear regression techniques appropriate for analysis when the dependent (outcome) variable is not normally distributed? Invest Ophthalmol Vis Sci. 2012;53(6):3082–3083. doi: 10.1167/iovs.12-9967 [DOI] [PubMed] [Google Scholar]
- 29.Pek J, Wong O, Wong ACM. How to address non-normality: a taxonomy of approaches, reviewed, and illustrated. Front Psychol. 2018;9:2104. doi: 10.3389/fpsyg.2018.02104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosar CBDB, Besen DB. Adaptation of a patient activatıon measure (PAM) into Turkish: reliability and validity test. Afri Health Sci. 2019;19(1):1811–1820. doi: 10.4314/ahs.v19i1.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBain H, Shipley M, Newman S. Clinician and patient views about self-management support in arthritis: a cross-sectional UK survey. Arthritis Care Res. 2018;70(11):1607–1613. doi: 10.1002/acr.23540 [DOI] [PubMed] [Google Scholar]
- 32.Jones B, Hunt A, Hewlett S, Harcourt D, Dures E. Rheumatology patients’ perceptions of patient activation and the patient activation measure: a qualitative interview study. Musculoskelet Care. 2021;20:74–85. doi: 10.1002/msc.1555 [DOI] [PubMed] [Google Scholar]
- 33.Huang LY, Lin YP, Glass GFJ, Chan EY. Health literacy and patient activation among adults with chronic diseases in Singapore: a cross-sectional study. Nurs Open. 2021;8(5):2857–2865. doi: 10.1002/nop2.873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graffigna G, Barello S, Bonanomi A, Riva G. Factors affecting patients’ online health information-seeking behaviours: the role of the Patient Health Engagement (PHE) Model. Patient Educ Couns. 2017;100(10):1918–1927. doi: 10.1016/j.pec.2017.05.033 [DOI] [PubMed] [Google Scholar]
- 35.Zakeri MA, Dehghan M, Ghaedi-Heidari F, Zakeri M, Bazmandegan G. Chronic patients’ activation and its association with stress, anxiety, depression, and quality of life: a Survey in Southeast Iran. Biomed Res Int. 2021;2021:6614566. doi: 10.1155/2021/6614566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira I, Nascimento M, Kakehasi A, et al. Association between health literacy, patient activation, and functional capacity in individuals with rheumatoid arthritis. Open Rheumatol J. 2021;15:1–8. doi: 10.2174/1874312902115010001 [DOI] [Google Scholar]
- 37.Lofland JH, Johnson PT, Ingham MP, Rosemas SC, White JC, Ellis L. Shared decision-making for biologic treatment of autoimmune disease: influence on adherence, persistence, satisfaction, and health care costs. Patient Prefer Adherence. 2017;11:947–958. doi: 10.2147/PPA.S133222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuidema R, van Dulmen S, Nijhuis-van der Sanden M, et al. Efficacy of a web-based self-management enhancing program for patients with rheumatoid arthritis: explorative randomized controlled trial. J Med Internet Res. 2019;21(4):e12463. doi: 10.2196/12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mollard E, Michaud K. A mobile app with optical imaging for the self-management of hand rheumatoid arthritis: pilot study. JMIR Mhealth Uhealth. 2018;6(10):e12221. doi: 10.2196/12221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joplin SK, van der Zwan R, Bagga H, Joshua F, Wong PKK. Pilot study assessing the novel use of musculoskeletal ultrasound in patients with rheumatoid arthritis to improve patient attitudes and adherence to medication. Int J Rheum Dis. 2016;19(7):658–664. doi: 10.1111/1756-185X.12402 [DOI] [PubMed] [Google Scholar]
- 41.Grønning K, Rannestad T, Skomsvoll JF, Rygg LØ, Steinsbekk A. Long-term effects of a nurse-led group and individual patient education programme for patients with chronic inflammatory polyarthritis - a randomised controlled trial. J Clin Nurs. 2014;23(7–8):1005–1017. doi: 10.1111/jocn.12353 [DOI] [PubMed] [Google Scholar]
- 42.Grønning K, Midttun L, Steinsbekk A. Patients’ confidence in coping with arthritis after nurse-led education; a qualitative study. BMC Nurs. 2016;15(1):28. doi: 10.1186/s12912-016-0150-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandhu S, Veinot P, Embuldeniya G, et al. Peer-to-peer mentoring for individuals with early inflammatory arthritis: feasibility pilot. BMJ Open. 2013;3(3):e002267. doi: 10.1136/bmjopen-2012-002267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCabe PJ, Stuart-Mullen LG, McLeod CJ, et al. Patient activation for self-management is associated with health status in patients with atrial fibrillation. Patient Prefer Adherence. 2018;12:1907–1916. doi: 10.2147/PPA.S172970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hibbard JH. Patient activation and the use of information to support informed health decisions. Patient Educ Couns. 2017;100(1):5–7. doi: 10.1016/j.pec.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 46.Bahrom NH, Ramli AS, Isa MR, et al. Factors associated with high patient activation level among individuals with metabolic syndrome at a primary care teaching clinic. J Prim Care Community Health. 2020;11:215013272093130. doi: 10.1177/2150132720931301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaleshgar Kordasiabi M, Akhlaghi M, Baghianimoghadam MH, et al. Self management behaviors in rheumatoid arthritis patients and associated factors in Tehran 2013. Glob J Health Sci. 2015;8(3):156–167. doi: 10.5539/gjhs.v8n3p156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bearne LM, Byrne AM, Segrave H, White CM. Multidisciplinary team care for people with rheumatoid arthritis: a systematic review and meta-analysis. Rheumatol Int. 2016;36:311–324. doi: 10.1007/s00296-015-3380-4 [DOI] [PubMed] [Google Scholar]
- 49.Grønning K, Skomsvoll JF, Rannestad T, Steinsbekk A. The effect of an educational programme consisting of group and individual arthritis education for patients with polyarthritis—A randomised controlled trial. Patient Educ Couns. 2012;88(1):113–120. doi: 10.1016/j.pec.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 50.Grønning K, Lim S, Bratås O. Health status and self-management in patients with inflammatory arthritis-A five-year follow-up study after nurse-led patient education. Nurs Open. 2019;7(1):326–333. doi: 10.1002/nop2.394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones B, Ndosi M, Hunt A, Harcourt D, Dures E. Factors associated with patient activation in inflammatory arthritis: a multisite cross-sectional study. Rheumatol Adv Pract. 2021;5(Suppl2):ii35–ii44. doi: 10.1093/rap/rkab053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]