Abstract
The aim of this retrospective multicenter study was to clarify the antifibrotic effect and long‐term outcome of sodium glucose cotransporter 2 inhibitors (SGLT2‐Is) in patients with nonalcoholic fatty liver disease (NAFLD) complicated by type 2 diabetes mellitus (T2DM). Of the 1262 consecutive patients with T2DM who recently received SGLT2‐Is, 202 patients with NAFLD had been receiving SGLT2‐Is for more than 48 weeks and were subjected to this analysis. Furthermore, 109 patients who had been on SGLT2‐I therapy for more than 3 years at the time of analysis were assessed for the long‐term effects of SGLT2‐Is. Significant decreases in body weight, liver transaminases, plasma glucose, hemoglobin A1c, and Fibrosis‐4 (FIB‐4) index were found at week 48. Overall, the median value of FIB‐4 index decreased from 1.42 at baseline to 1.25 at week 48 (p < 0.001). In the low‐risk group (FIB‐4 index < 1.3), there was no significant change in the FIB‐4 index. In the intermediate‐risk (≥1.3 and <2.67) and high‐risk (≥2.67) groups, the median levels significantly decreased from 1.77 and 3.33 at baseline to 1.58 and 2.75 at week 48, respectively (p < 0.001 for both). Improvements in body weight, glucose control, liver transaminases, and FIB‐4 index were found at 3 years of SGLT2‐I treatment. In the intermediate‐risk and high‐risk groups (≥1.3 FIB‐4 index), the FIB‐4 index maintained a significant reduction from baseline throughout the 3 years of treatment. Conclusion: This study showed that SGLT2‐Is offered a favorable effect on improvement in FIB‐4 index as a surrogate marker of liver fibrosis in patient with NAFLD complicated by T2DM, especially those with intermediate and high risks of advanced fibrosis, and this antifibrotic effect is sustained for the long term.
SGLT2‐Is treatment is useful for improving liver fibrosis in patients with NAFLD complicated by T2DM, especially those with intermediate and high risks of advanced fibrosis, and this anti‐fibrotic effect is sustained for long term.

INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most frequent chronic liver disease, and its morbidity rate in adults is approximately 25% worldwide.[ 1 ] NAFLD progresses to liver cirrhosis and hepatocellular carcinoma (HCC), along with the deterioration of liver fibrosis.[ 2 ] Therefore, liver fibrosis is the most critical liver‐related prognostic factor in patients with NAFLD. It is also associated with extrahepatic complications, specifically cardiovascular disease and extrahepatic cancers. These account for most extrahepatic causes of death[ 3 , 4 , 5 , 6 , 7 , 8 ] because NAFLD is a multifactorial disease that is mutually correlated with metabolic syndrome, a common risk factor for cardiovascular disease and cancer.[ 9 ] In particular, type 2 diabetes mellitus (T2DM) facilitates the progression of liver fibrosis and the development of HCC in patients with NAFLD.[ 10 , 11 ] To date, no drug therapy has been established to improve liver fibrosis in patients with NAFLD. However, recent studies indicated the antifibrotic effect of antidiabetic drugs,[ 12 , 13 , 14 , 15 , 16 , 17 ] especially in sodium glucose cotransporter 2 inhibitors (SGLT2‐Is).[ 14 , 15 , 16 , 17 ]
SGLT2‐Is reduce blood glucose levels by inhibiting glucose resorption in the proximal kidney tubule and consequently increasing urinary glucose excretion. Several studies suggested that SGLT2‐Is improve liver enzymes by reducing hepatic steatosis in patients with NAFLD complicated by T2DM.[ 14 , 15 , 16 , 17 , 18 , 19 , 20 ] However, these studies were small in the sample size (<60 patients) and/or short in the treatment period (≤48 weeks). No large‐scale study has examined the long‐term effects of SGLT2‐Is in patients with NAFLD. Furthermore, only a few prospective studies reported their effects on liver fibrosis based on biopsy‐proven histological findings.[ 14 , 15 , 16 ]
Liver biopsy is the gold standard for NAFLD diagnosis or liver fibrosis staging, but it has some limitations, including sampling errors, being costly, invasive and time‐consuming procedures, and complication risks. As a noninvasive alternative to liver biopsy, transient elastography has been used to evaluate liver fibrosis–reducing effects; however, the obtained findings remain uncertain due to the limited number of patients and short observation periods.[ 17 ] Alternatively, the usefulness of the fibrosis‐4 index (FIB‐4 index) for the assessment of liver fibrosis has been established.[ 21 , 22 , 23 ] Moreover, several studies reported that the FIB‐4 index is associated with extrahepatic complications, such as cardiovascular events and extrahepatic cancers, as well as liver‐related complications.[ 6 , 7 , 24 ] These findings suggest that an improvement in the FIB‐4 index by treatment reflects a decrease in liver fibrosis and may lead to a reduction in the risk of complications and improvement in the prognosis of patients with NAFLD.
This study aimed to investigate the antifibrotic effect of SGLT2‐Is using the FIB‐4 index and the long‐term treatment outcomes in patients with NAFLD complicated by T2DM.
METHODS
Study design
This study was a retrospective, multicenter study to investigate the effect of SGLT2‐Is on patients with NAFLD complicated by T2DM. A flowchart of the study is shown in Figure 1. Among patients who visited Nippon Medical School Hospital, Nippon Medical School Chiba Hokusoh Hospital, Kikkoman General Hospital, and Kitasato University Hospital between May 2014 and April 2020, 1262 patients newly received SGLT2‐Is for T2DM with inadequately controlled hyperglycemia as determined by their physicians. Of the 1262 patients, 237 were diagnosed with NAFLD and did not fulfill the exclusion criteria. NAFLD was diagnosed by (1) the presence of steatosis in ≥5% of hepatocytes on liver biopsy specimens or fat deposition on imaging modalities, such as ultrasonography, computed tomography, and magnetic resonance imaging; (2) daily alcohol consumption <30 g for men and <20 g for women; (3) negative for hepatitis B surface antigen and hepatitis C virus antibody; and (4) absence of other chronic liver diseases, such as autoimmune hepatitis, primary biliary cholangitis, Wilson disease, and hemochromatosis, as determined by specific laboratory and imaging examinations, as well as the patients’ medical histories. The main exclusion criteria were as follows: (1) age < 20 years and (2) concomitant medication with pioglitazone, GLP‐1 analogues, and insulin. Of the 237 patients with NAFLD, 202 had been receiving SGLT2‐Is for more than 48 weeks and were subjected to this study analysis. Furthermore, 109 patients who had been on SGLT2‐Is therapy for more than 3 years at the time of analysis were assessed for the long‐term effects of SGLT2‐Is. This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the institutional review board of each participating institution.
FIGURE 1.
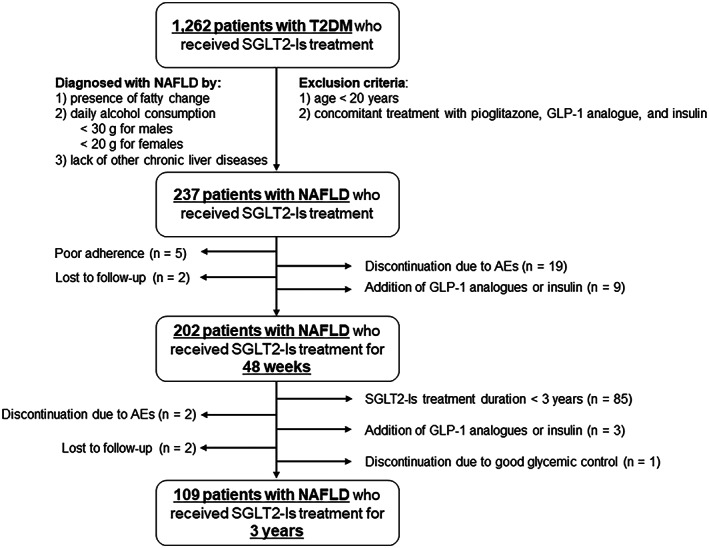
Flow chart of the study. Abbreviations: AEs, adverse events; GLP‐1, glucagon‐like peptide‐1; NAFLD, nonalcoholic fatty liver disease; SGLT2‐Is; SGLT2 inhibitors; T2DM, type 2 diabetes mellitus.
Clinical and laboratory data
Clinical and laboratory data were collected every 3 months during the study period. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Laboratory evaluation included complete blood count, routine liver biochemistry (aspartate aminotransferase [AST], alanine aminotransferase [ALT], albumin, and gamma‐glutamyl transpeptidase [GGT]), kidney biochemistry (urea nitrogen, creatinine, and estimated glomerular filtration rate), fasting lipids (triglyceride, high‐density lipoprotein cholesterol, and low‐density lipoprotein cholesterol), fasting plasma glucose, hemoglobin A1c (HbA1c), and uric acid. The FIB‐4 index was calculated to estimate the degree of liver fibrosis, as reported previously.[ 21 ] FIB‐4 index values of <1.30, between 1.30 and 2.67, and ≥2.67 corresponded to low, intermediate, and high risk of advanced fibrosis, respectively, as proposed previously.[ 23 ] The change in platelet counts was also examined in patients with platelet counts <180 × 103/μl, which corresponds to patients with advanced fibrosis according to previous reports.[ 25 , 26 ]
Statistical analyses
Continuous variables are presented as medians and interquartile ranges (IQRs) in parentheses, whereas categorical variables are presented as numbers and percentages in parentheses. The kinetics of the aforementioned factors were examined using the Wilcoxon signed‐rank test. The McNemar–Bowker test was used to analyze the changes in the proportion of the FIB‐4 index–based fibrosis risk groups from baseline to week 48. Changes in the FIB‐4 index during the 3‐year SGLT2‐I treatment period were evaluated using the Friedman test, followed by post hoc pairwise comparisons with the Bonferroni test. All statistical analyses were performed using IBM SPSS version 17.0 (IBM Japan). The level of statistical significance was set at p < 0.05.
RESULTS
Patient characteristics
The baseline characteristics of the 202 patients with NAFLD complicated by T2DM who received SGLT2‐I treatment for 48 weeks are given in Table 1. There were 120 males and 82 females, with a median age of 56 years (IQR, 48–66 years) and a median HbA1c level of 7.7% (IQR, 7.0%–8.6%). Before SGLT2‐I initiation, 59 patients (29.2%) received dietary and/or exercise therapies without oral hypoglycemic agents (OHAs), whereas the remaining 143 (70.8%) received other OHAs, including biguanides (n = 102), dipeptidyl peptidase‐4 inhibitors (n = 127), and others (n = 59). The following SGLT2‐Is were administered: canagliflozin (n = 43), ipragliflozin (n = 42), tofogliflozin (n = 9), dapagliflozin (n = 29), luseogliflozin (n = 38), and empagliflozin (n = 41). The median FIB‐4 index was 1.42 (IQR, 0.92–2.14). Among the FIB‐4‐based fibrosis risk groups, 44.5% (n = 90) were in the low‐risk group, 42.1% (n = 85) were in the intermediate‐risk group, and 13.4% (n = 27) were in the high‐risk group.
TABLE 1.
Baseline characteristics of the 202 patients with NAFLD complicated by T2DM who received SGLT2‐Is for 48 weeks
| Factors | n = 202 |
|---|---|
| Age (years) | 56 (48–66) |
| Gender (male/female) | 120/82 |
| Premenopausal/postmenopausal | 18/64 |
| Body weight (kg) | 76.1 (67.2–89.0) |
| BMI (kg/m2) | 28.1 (24.9–31.7) |
| Platelets (×103/μl) | 214 (174–256) |
| AST (U/L) | 37 (25–54) |
| ALT (U/L) | 52 (33–82) |
| GGT (U/L) | 56 (36–88) |
| Uric acid (mg/dl) | 5.3 (4.4–6.1) |
| Plasma glucose (mg/dl) | 158 (137–199) |
| HbA1c (%) | 7.7 (7.0–8.6) |
| Diabetes treatment prior to SGLT2‐Is | |
| Diet/exercise only (without OHAs) | 59 (29.2%) |
| OHAs | 143 (70.8%) |
| Biguanides | 102 (50.5%) |
| DPP4 inhibitors | 127 (62.9%) |
| α‐glucosidase inhibitors | 31 (15.3%) |
| Sulfonylureas | 23 (11.4%) |
| Glinides | 5 (2.5%) |
| SGLT2‐Is | |
| Canagliflozin | 43 (21.3%) |
| Ipragliflozin | 42 (20.8%) |
| Tofogliflozin | 9 (4.5%) |
| Dapagliflozin | 29 (14.4%) |
| Luseogliflozin | 38 (18.8%) |
| Empagliflozin | 41 (20.3%) |
| FIB‐4 | 1.42 (0.92–2.14) |
| FIB‐4‐based risk groups | |
| Low risk (<1.3) | 90 (44.5%) |
| Intermediate risk (1.3≤ and <2.67) | 85 (42.1%) |
| High risk (≥2.67) | 27 (13.4%) |
Note: Data are presented as numbers (percentages) or medians (interquartile ranges).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DPP4, dipeptidyl peptidase‐4; GGT, gamma‐glutamyl transpeptidase; HbA1c, hemoglobin A1c; OHAs, oral hypoglycemic agents.
Changes in clinical characteristics of 48‐week SGLT2‐I treatment
Significant decreases in body weight, BMI, AST, ALT, GGT, uric acid, plasma glucose, HbA1c, and FIB‐4 index were found at week 48 (Table 2). Significant reductions in these parameters were observed regardless of gender (Table S1) and menopausal status (Table S2). No significant changes in overall platelet counts were observed, whereas they significantly increased in patients with levels <180 × 103/μl (146 × 103/μl to 158 × 103/μl; p < 0.05) (Table 2). Overall, the median value of FIB‐4 index decreased from 1.42 at baseline to 1.25 at week 48 (p < 0.001; Figure 2A). In the low‐risk group, the median level decreased from 0.88 at baseline to 0.81 at week 48, although the difference was not statistically significant (p = 0.25; Figure 2B). In the intermediate‐risk and high‐risk groups, the median levels significantly decreased from 1.77 and 3.33 at baseline to 1.58 and 2.75 at week 48, respectively (p < 0.001 for both; Figure 2C,D).
TABLE 2.
Changes in clinical characteristics in the 202 patients who received SGLT2‐is for 48 weeks
| SGLT2‐Is therapy | |||
|---|---|---|---|
| Baseline | 48 weeks | p‐Value | |
| Body weight (kg) | 76.1 (67.2–89.0) | 74.0 (64.0–85.9) | <0.001 |
| BMI (kg/m2) | 28.1 (24.9–31.7) | 27.4 (24.1–30.6) | <0.001 |
| Platelets (×103/μl) | 214 (174–256) | 213 (174–254) | 0.36 |
| <80 × 103/μl | 146 (134–164) | 158 (128–169) | <0.05 |
| AST (U/L) | 37 (25–54) | 24 (19–39) | <0.001 |
| ALT (U/L) | 52 (33–82) | 30 (21–51) | <0.001 |
| GGT (U/L) | 56 (36–88) | 37 (24–63) | <0.001 |
| Uric acid (mg/dl) | 5.3 (4.4–6.1) | 4.8 (4.0–5.6) | <0.001 |
| Plasma glucose (mg/dl) | 158 (137–199) | 134 (116–158) | <0.001 |
| HbA1c (%) | 7.7 (7.0–8.6) | 6.9 (6.4–7.5) | <0.001 |
| FIB‐4 | 1.42 (0.92–2.14) | 1.25 (0.82–1.93) | <0.001 |
Note: Data are expressed as medians (interquartile ranges).
FIGURE 2.
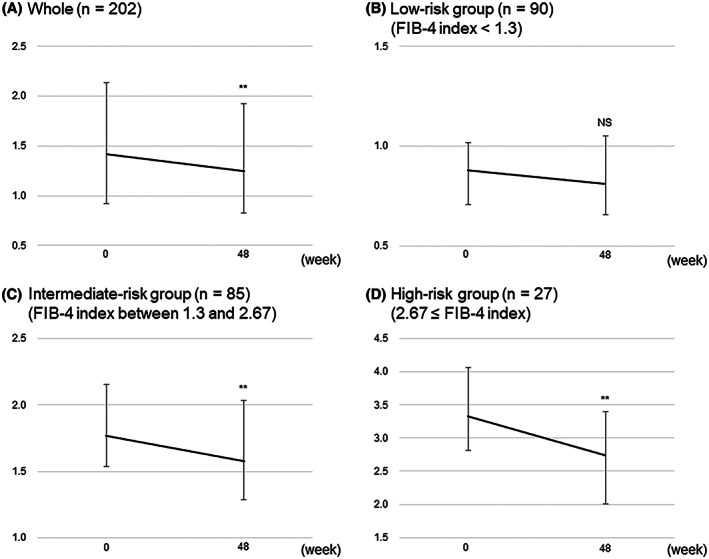
Changes in Fibrosis‐4 index (FIB‐4) from baseline to 48 weeks after administration of SGLT2‐Is: all patients (A), low‐risk group (FIB‐4 index < 1.3) (B), intermediate‐risk group (1.3 ≤ FIB‐4 index < 2.67) (C), and high‐risk group (2.67 ≤ FIB‐4 index) (D). Error bars show the interquartile ranges. **p < 0.001 versus baseline. Abbreviation: N.S., not significant.
The changes in the proportion of fibrosis risk groups from baseline to week 48 are shown in Figure 3. Overall, the proportion of patients in the low‐risk group increased from 44.5% (90 of 202) to 52.5% (106 of 202) at week 48, whereas that of the high‐risk group decreased from 13.4% (27 of 202) to 8.4% (17 of 202) (p < 0.001; Figure 3A). Among 90 patients in the low‐risk group, 83 (92.2%) remained at low risk, and 7 (7.8%) changed to intermediate risk at week 48 (Figure 3B). Among the 85 patients in the intermediate‐risk group, 23 (27.1%) improved to low risk; 59 (69.4%) remained at intermediate risk; and 3 (3.5%) changed to high risk at week 48 (Figure 3C). Among the 27 patients in the high‐risk group, 13 (48.1%) improved to intermediate risk, and 14 (51.9%) remained at high risk at week 48 (Figure 3D).
FIGURE 3.
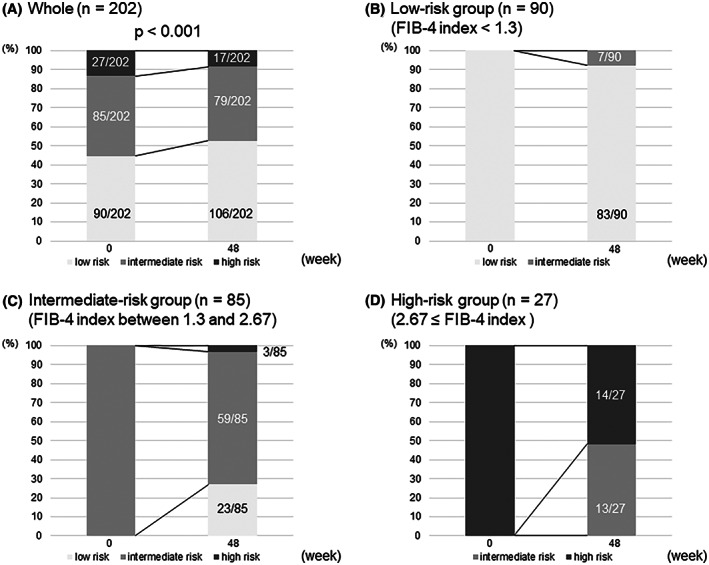
Changes in the proportion of fibrosis risk groups from baseline to week 48 in all patients (A), low‐risk group (FIB‐4 index < 1.3) (B), intermediate‐risk group (1.3 ≤ FIB‐4 index < 2.67) (C), and high‐risk group (2.67 ≤ FIB‐4 index) (D).
Long‐term outcomes of SGLT2‐I treatment
Of the 202 patients analyzed earlier, 109 had available data to evaluate the long‐term outcomes of SGLT‐I treatment (Figure 1). Significant decreases in body weight, BMI, AST, ALT, GGT, uric acid, plasma glucose, HbA1c, and FIB‐4 index were found at 3 years of SGLT‐I treatment (Table 3). Overall, the FIB‐4 index maintained a significant reduction from baseline throughout the 3 years of treatment (Figure 4A). In the low‐risk group, there was no significant change in the FIB‐4 index over the 3 years (Figure 4B). Meanwhile, in the intermediate‐risk and high‐risk group (1.3 ≤ FIB‐4 index), the FIB‐4 index significantly decreased from baseline throughout the 3 years (Figure 4C).
TABLE 3.
Changes in clinical characteristics in the 109 patients who received SGLT2‐Is for 3 years
| SGLT2‐I therapy | |||
|---|---|---|---|
| Baseline | 3 years | p‐Value | |
| Body weight (kg) | 75.0 (67.9–89.7) | 71.8 (64.0–85.7) | <0.001 |
| BMI (kg/m2) | 27.2 (24.8–31.8) | 26.2 (24.4–30.6) | <0.001 |
| Platelets (×103/μl) | 214 (182–258) | 211 (177–244) | 0.10 |
| AST (U/L) | 38 (26–54) | 28 (18–36) | <0.001 |
| ALT (U/L) | 55 (37–75) | 35 (21–57) | <0.001 |
| GGT (U/L) | 56 (35–89) | 33 (24–58) | <0.001 |
| Uric acid (mg/dl) | 5.2 (4.3–5.9) | 4.9 (4.3–5.6) | <0.01 |
| Plasma glucose (mg/dl) | 162 (144–207) | 146 (120–170) | <0.001 |
| HbA1c (%) | 7.9 (7.3–8.8) | 7.2 (6.6–7.8) | <0.001 |
| FIB‐4 index | 1.33 (0.93–2.06) | 1.29 (0.85–1.86) | <0.05 |
Note: Data are expressed as medians (interquartile ranges).
FIGURE 4.
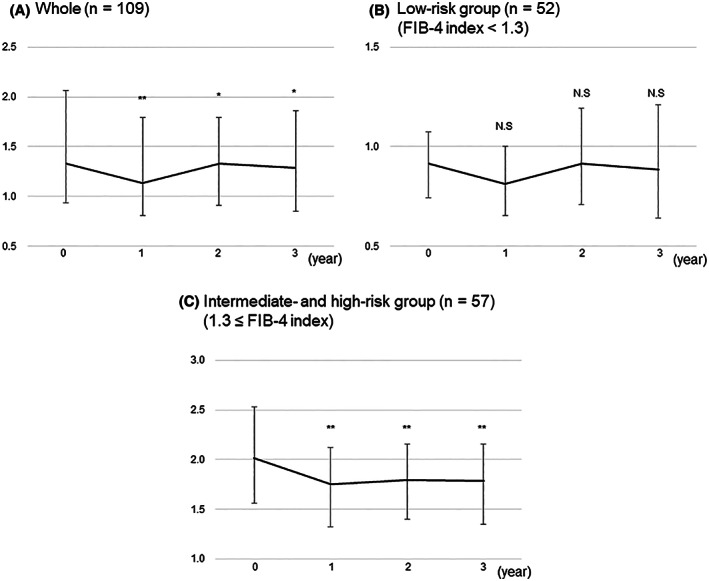
Changes in FIB‐4 from baseline to 3 years after administration of SGLT2‐Is in all patients (A), the low‐risk group (FIB‐4 index < 1.3) (B), and the intermediate‐risk and high‐risk group (1.3 ≤ FIB‐4 index) (C). Error bars show the interquartile ranges. **p < 0.001 versus baseline; *p < 0.05 versus baseline.
Adverse events
Of the 237 patients with NAFLD complicated by T2DM who received SGLT2‐I treatment, 21 (8.9%) discontinued treatment due to adverse events during the observation period (Figure 1). Of the 21 patients, 19 (90.5%) discontinued treatment within 48 weeks of treatment. Among the adverse events leading to treatment discontinuation, genitourinary tract infection was the most frequently observed (23.8%) (Table S3).
DISCUSSION
This study revealed that SGLT2‐I administration for 48 weeks not only improved glycemic control but also reduced body weight, uric acid and transaminase levels, and the FIB‐4 index in 202 patients with NAFLD complicated by T2DM. Notably, these effects were sustained during the 3 years of SGLT2‐I treatment. Recently, SGLT2‐Is have been reported to improve the severity of NAFLD complicated by T2DM.[ 14 , 15 , 16 , 17 , 18 , 19 , 20 ] The Japan Society of Hepatology guidelines suggest that SGLT2‐Is are one of the effective treatments for NAFLD complicated by T2DM.[ 27 ] However, previous studies had a small number of patients and/or a relatively short treatment study. The strength of this study is that it examined the effect of 48‐week SGLT‐I treatment in more than 200 patients, as well as 3 years of treatment in more than 100 patients. In addition to improving glycemic control, the preventive effects of SGLT2‐Is on cardiovascular disease and chronic kidney disease, well‐known extrahepatic complications in patients with NAFLD,[ 28 , 29 ] have been reported at high evidence level.[ 30 ] Thus, when combined with their sustained lowering effects on liver enzymes and FIB‐4 index, SGLT2‐Is might be one of the promising options for patients with NAFLD with T2DM.
It is well known that gender and menopausal status influence the onset and progression of NAFLD.[ 31 , 32 ] According to a systematic review and meta‐analysis, although women have a lower risk of NAFLD, women with NAFLD have a higher risk of developing liver fibrosis compared with men, especially for patients over 50 years old.[ 31 ] In this study, significant improvements in body weight, glycemic control, uric acid, liver enzymes, and FIB‐4 index were found regardless of gender and menopausal status. Because of the small number of premenopausal women in this study, further validation is needed to determine whether there are differences in the influence of SGLT2‐Is on the liver across gender and menopausal status.
As already established, ALT and GGT, routine clinical biochemical markers, reflect liver injury and inflammation.[ 33 ] Furthermore, it has recently been reported that dynamic changes in ALT and GGT well reflect changes in histopathological severity of NAFLD.[ 34 ] In particular, GGT is known to be a marker of insulin resistance,[ 35 ] which plays a key role in the progression of NAFLD. Moreover, elevated GGT activity is associated with increased risk of cardiovascular disease, a common extrahepatic complication of NAFLD.[ 36 ] Thus, the decrease in GGT with SGLT2‐I treatment might lead to prevention of disease progression, improvement in histopathological severity of NAFLD, and a reduction in the risk of developing cardiovascular disease.
The degree of liver fibrosis is a histological index that accurately reflects the prognosis of patients with NAFLD.[ 3 , 4 ] The important goal of NAFLD treatment is to improve liver fibrosis and prevent the disease progression. Two pilot studies (24‐week canagliflozin for 5 patients[ 14 ] and 24‐week empagliflozin for 9 patients[ 15 ]) demonstrated improvement of liver fibrosis by SGLT2‐Is on liver biopsy specimens. Recently, a randomized controlled trial reported that liver fibrosis significantly improved in the ipragliflozin‐treated group (n = 21) compared with that in the control group.[ 16 ] Although liver biopsy is the gold standard for the assessment of liver fibrosis, it is invasive and carries a risk of complications; therefore, it is difficult to perform this procedure simply and repeatedly. The drawbacks of liver biopsy may have markedly restricted patient enrollment or study period in previous studies.
This study evaluated the antifibrotic effect of SGLT2‐Is using the FIB‐4 index, which is a noninvasive alternative to liver biopsy for assessing liver fibrosis,[ 21 , 22 , 23 ] in a relatively large number of patients. Of note, SGLT2‐Is significantly reduced this index in the intermediate‐risk and high‐risk groups with an FIB‐4 index of ≥ 1.3, suggesting that SGLT2‐Is may have a favorable effect on liver fibrosis in patients at risk for liver fibrosis progression. Meanwhile, no significant change in the FIB‐4 index was found in the low‐risk group with an FIB‐4 index of < 1.3. This finding may be interpreted as SGLT2‐Is preventing the disease progression. However, it is unclear whether SGLT2‐Is are beneficial or required for patients with a low FIB‐4 index. Such ameliorating and preventive effects should be further investigated in a prospective, controlled study involving a large number of patients and a long study period.
Changes in the FIB‐4 index should be interpreted with caution, as the decrease in the FIB‐4 index is not only due to improvement in liver fibrosis, but also to normalization of AST and ALT (i.e., improvement in liver inflammation). Patients with NAFLD had a slower decline in platelet counts with progressive fibrosis than patients with other chronic liver diseases,[ 25 , 26 ] which may explain why there was no change in overall platelet counts in this study. On the other hand, the significant increase in platelet counts after SGLT2‐I administration in patients with low platelet counts supported the possibility of improved liver fibrosis in patients with advanced fibrosis. Moreover, considering that liver fibrosis is caused by a protracted wound‐healing process in response to repetitive injury induced by inflammation, it is assumed that improving this inflammation might lead to prevent subsequent fibrosis development and improve existing fibrosis.[ 37 ]
The mechanism by which SGLT2‐Is prevent/ameliorate liver fibrosis is complex and may involve various factors, not all of which are fully understood.[ 38 ] It is conceivable that SGLT2‐Is may have an indirect antifibrotic effect by lowering blood glucose and insulin levels, thereby improving insulin resistance, which is a cause of steatosis and fibrosis progression in patients with NAFLD.[ 39 ] SGLT2‐Is may also contribute to the improvement of liver fibrosis by inhibiting inflammatory cytokines such as interleukin‐6, tumor necrosis factor‐α and monocyte chemoattractant protein 1, which are drivers of more advanced liver disease.[ 40 , 41 ] Reportedly, SGLT2‐Is reduce the activity of NLR family pyrin domain‐containing 3 inflammasome,[ 42 ] which plays an important role in liver fibrosis.[ 43 ] Interestingly, anti‐inflammatory and antifibrotic properties of SGLT2‐Is have been suggested as a common mechanism for its protective effects on the heart[ 44 ] and kidney,[ 45 ] as well as the liver. In addition, the potential impact of SGLT2‐Is on the development of HCC via the hepatoprotective effects described above and its direct anti‐HCC activity have attracted attention.[ 46 , 47 , 48 ] These findings are based on animal experiments, and further studies are needed to elucidate the inhibitory effect of SGLT2‐Is on the development and progression of NAFLD and HCC in clinical practice.
This study has several limitations. First, given that this study was retrospective and lacked a control group without SGLT2‐Is, the results must be interpreted carefully. The criteria for treatment decision and discontinuation were not standardized and were left to each physician. Second, liver biopsy was not performed to diagnose NAFLD or assess liver fibrosis. In this study, the FIB‐4 index was used as an evaluation of the degree of liver fibrosis as a substitute for the diagnosis of liver biopsy. However, it should be noted that the diagnostic performance of FIB‐4 index is suggested to be relatively less accurate in patients with T2DM.[ 49 ] According to the NAFLD practice guidelines in Japan and the United States,[ 27 , 50 ] the FIB‐4 index is a simple, accurate, and inexpensive method of assessing liver fibrosis, and its measurement is recommended.
CONCLUSIONS
This study suggested that SGLT2‐I treatment offered a favorable effect on improvement in the FIB‐4 index as a surrogate marker of liver fibrosis in patients with NAFLD complicated by T2DM, especially those with intermediate and high risks of advanced fibrosis and that the antifibrotic effect is sustained with long‐term SGLT2‐I treatment. Further studies are required to investigate whether long‐term SGLT2‐I treatment improves the prognosis and prevents liver‐related and extrahepatic complications in patients with NAFLD complicated by T2DM.
CONFLICT OF INTEREST
Nothing to report.
Supporting information
Table S1 Changes in clinical characteristics with 48‐week sodium glucose cotransporter 2 inhibitor (SGLT‐I) treatment with nonalcoholic fatty liver disease (NAFLD) complicated by type 2 diabetes mellitus (T2DM) according to gender
Table S2 Changes in clinical characteristics with 48‐week SGLT‐I treatment in patients with NAFLD with T2DM by menopausal status
Table S3 Adverse events leading to discontinuation of SGLT2‐Is
ACKNOWLEDGMENT
The authors thank all medical doctors from all institutions who were involved in this study.
Arai T, Atsukawa M, Tsubota A, Mikami S, Haruki U, Yoshikata K, et al. Antifibrotic effect and long‐term outcome of SGLT2 inhibitors in patients with NAFLD complicated by diabetes mellitus. Hepatol Commun. 2022;6:3073–3082. 10.1002/hep4.2069
Taeang Arai, Masanori Atsukawa, and Akihito Tsubota contributed equally to this work.
REFERENCES
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. [DOI] [PubMed] [Google Scholar]
- 3. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy‐proven NAFLD. J Hepatol. 2017;67:1265–73. [DOI] [PubMed] [Google Scholar]
- 5. Kogiso T, Sagawa T, Kodama K, Taniai M, Hashimoto E, Tokushige K. Long‐term outcomes of non‐alcoholic fatty liver disease and the risk factors for mortality and hepatocellular carcinoma in a Japanese population. J Gastroenterol Hepatol. 2020;35:1579–89. [DOI] [PubMed] [Google Scholar]
- 6. Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tada T, Kumada T, Toyoda H, Mizuno K, Sone Y, Akita T, et al. Progression of liver fibrosis is associated with non‐liver‐related mortality in patients with nonalcoholic fatty liver disease. Hepatol Commun. 2017;1:899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arai T, Atsukawa M, Tsubota A, Kato K, Abe H, Ono H, et al. Liver fibrosis is associated with carotid atherosclerosis in patients with liver biopsy‐proven nonalcoholic fatty liver disease. Sci Rep. 2021;11:15938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Italian Association for the Study of the Liver (AISF) . AISF position paper on nonalcoholic fatty liver disease (NAFLD): updates and future directions. Dig Liver Dis. 2017;49:471–83. [DOI] [PubMed] [Google Scholar]
- 10. Nakahara T, Hyogo H, Yoneda M, Sumida Y, Eguchi Y, Fujii H, et al. Type 2 diabetes mellitus is associated with the fibrosis severity in patients with nonalcoholic fatty liver disease in a large retrospective cohort of Japanese patients. J Gastroenterol. 2014;49:1477–84. [DOI] [PubMed] [Google Scholar]
- 11. Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharawy MH, El‐Kashef DH, Shaaban AA, El‐Agamy DS. Anti‐fibrotic activity of sitagliptin against concanavalin A‐induced hepatic fibrosis. Role of Nrf2 activation/NF‐κB inhibition. Int Immunopharmacol. 2021;100:108088. [DOI] [PubMed] [Google Scholar]
- 13. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non‐alcoholic steatohepatitis (LEAN): a multicentre, double‐blind, randomised, placebo‐controlled phase 2 study. Lancet. 2016;387:679–90. [DOI] [PubMed] [Google Scholar]
- 14. Akuta N, Watanabe C, Kawamura Y, Arase Y, Saitoh S, Fujiyama S, et al. Effects of a sodium‐glucose cotransporter 2 inhibitor in nonalcoholic fatty liver disease complicated by diabetes mellitus: preliminary prospective study based on serial liver biopsies. Hepatol Commun. 2017;1:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lai LL, Vethakkan SR, Nik Mustapha NR, Mahadeva S, Chan WK. Empagliflozin for the treatment of nonalcoholic steatohepatitis in patients with type 2 diabetes mellitus. Dig Dis Sci. 2020;65:623–31. [DOI] [PubMed] [Google Scholar]
- 16. Takahashi H, Kessoku T, Kawanaka M, Nonaka M, Hyogo H, Fujii H, et al. Ipragliflozin improves the hepatic outcomes of patients with diabetes with NAFLD. Hepatol Commun. 2022;6:120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arai T, Atsukawa M, Tsubota A, Mikami S, Ono H, Kawano T, et al. Effect of sodium‐glucose cotransporter 2 inhibitor in patients with non‐alcoholic fatty liver disease and type 2 diabetes mellitus: a propensity score‐matched analysis of real‐world data. Ther Adv Endocrinol Metab. 2021;12:20420188211000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, et al. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non‐alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res. 2017;47:1072–8. [DOI] [PubMed] [Google Scholar]
- 19. Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E‐LIFT trial). Diabetes Care. 2018;41:1801–8. [DOI] [PubMed] [Google Scholar]
- 20. Sumida Y, Murotani K, Saito M, Tamasawa A, Osonoi Y, Yoneda M, et al. Effect of luseogliflozin on hepatic fat content in type 2 diabetes patients with non‐alcoholic fatty liver disease: a prospective, single‐arm trial (LEAD trial). Hepatol Res. 2019;49:64–71. [DOI] [PubMed] [Google Scholar]
- 21. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 22. Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim GA, Lee HC, Choe J, Kim MJ, Lee MJ, Chang HS, et al. Association between non‐alcoholic fatty liver disease and cancer incidence rate. J Hepatol. 2018;68:140–6. [DOI] [PubMed] [Google Scholar]
- 25. Ikarashi Y, Kodama K, Taniai M, Hashimoto E, Tokushige K. The clinical difference in the platelet counts between liver cirrhosis with nonalcoholic fatty liver disease and hepatitis C virus. Intern Med. 2018;57:1065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoneda M, Fujii H, Sumida Y, Hyogo H, Itoh Y, Ono M, et al. Platelet count for predicting fibrosis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:1300–6. [DOI] [PubMed] [Google Scholar]
- 27. Tokushige K, Ikejima K, Ono M, Eguchi Y, Kamada Y, Itoh Y, et al. Evidence‐based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. Hepatol Res. 2021;51:1013–25. [DOI] [PubMed] [Google Scholar]
- 28. Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69:1691–705. [DOI] [PubMed] [Google Scholar]
- 29. Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Schattenberg JM, et al. Non‐alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta‐analysis. Gut. 2022;71:156–62. [DOI] [PubMed] [Google Scholar]
- 30. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57. [DOI] [PubMed] [Google Scholar]
- 31. Balakrishnan M, Patel P, Dunn‐Valadez S, Dao C, Khan V, Ali H, et al. Women have a lower risk of nonalcoholic fatty liver disease but a higher risk of progression vs men: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2021;19:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wegermann K, Garrett ME, Zheng J, Coviello A, Moylan CA, Abdelmalek MF, et al. Sex and menopause modify the effect of single nucleotide polymorphism genotypes on fibrosis in NAFLD. Hepatol Commun. 2021;5:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112:18–35. [DOI] [PubMed] [Google Scholar]
- 34. Newton KP, Lavine JE, Wilson L, Behling C, Vos MB, Molleston JP, et al. Alanine aminotransferase and gamma‐glutamyl transpeptidase predict histologic improvement in pediatric nonalcoholic steatohepatitis. Hepatology. 2021;73:937–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lonardo A, Lombardini S, Scaglioni F, Carulli L, Ricchi M, Ganazzi D, et al. Hepatic steatosis and insulin resistance: does etiology make a difference? J Hepatol. 2006;44:190–6. [DOI] [PubMed] [Google Scholar]
- 36. Ndrepepa G, Kastrati A. Gamma‐glutamyl transferase and cardiovascular disease. Ann Transl Med. 2016;4:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schuppan D, Surabattula R, Wang XY. Determinants of fibrosis progression and regression in NASH. J Hepatol. 2018;68:238–50. [DOI] [PubMed] [Google Scholar]
- 38. Zhang E, Zhao Y, Hu H. Impact of sodium glucose cotransporter 2 inhibitors on nonalcoholic fatty liver disease complicated by diabetes mellitus. Hepatol Commun. 2021;5:736–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nishimura N, Kitade M, Noguchi R, Namisaki T, Moriya K, Takeda K, et al. Ipragliflozin, a sodium‐glucose cotransporter 2 inhibitor, ameliorates the development of liver fibrosis in diabetic Otsuka long‐Evans Tokushima fatty rats. J Gastroenterol. 2016;51:1141–9. [DOI] [PubMed] [Google Scholar]
- 40. Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, et al. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715:246–55. [DOI] [PubMed] [Google Scholar]
- 41. Jojima T, Tomotsune T, Iijima T, Akimoto K, Suzuki K, Aso Y. Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP‐4 inhibitor), prevents steatohepatitis in a novel mouse model of non‐alcoholic steatohepatitis and diabetes. Diabetol Metab Syndr. 2016;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim SR, Lee SG, Kim SH, Kim JH, Choi E, Cho W, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11:2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Inzaugarat ME, Johnson CD, Holtmann TM, McGeough MD, Trautwein C, Papouchado BG, et al. NLR family pyrin domain‐containing 3 inflammasome activation in hepatic stellate cells induces liver fibrosis in mice. Hepatology. 2019;69:845–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. García‐Ropero Á, Vargas‐Delgado AP, Santos‐Gallego CG, Badimon JJ. Inhibition of sodium glucose cotransporters improves cardiac performance. Int J Mol Sci. 2019;20:3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heerspink HJL, Perco P, Mulder S, Leierer J, Hansen MK, Heinzel A, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62:1154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shiba K, Tsuchiya K, Komiya C, Miyachi Y, Mori K, Shimazu N, et al. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci Rep. 2018;8:2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jojima T, Wakamatsu S, Kase M, Iijima T, Maejima Y, Shimomura K, et al. The SGLT2 inhibitor canagliflozin prevents carcinogenesis in a mouse model of diabetes and non‐alcoholic steatohepatitis‐related hepatocarcinogenesis: association with SGLT2 expression in hepatocellular carcinoma. Int J Mol Sci. 2019;20:5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nakano D, Kawaguchi T, Iwamoto H, Hayakawa M, Koga H, Torimura T. Effects of canagliflozin on growth and metabolic reprograming in hepatocellular carcinoma cells: multi‐omics analysis of metabolomics and absolute quantification proteomics (iMPAQT). PLoS One. 2020;15:e0232283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boursier J, Canivet CM, Costentin C, Lannes A, Delamarre A, Sturm N, et al. Impact of type 2 diabetes on the accuracy of noninvasive tests of liver fibrosis with resulting clinical implications. Clin Gastroenterol Hepatol. 2022:S1542–3565(22)00248–8. [DOI] [PubMed] [Google Scholar]
- 50. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Changes in clinical characteristics with 48‐week sodium glucose cotransporter 2 inhibitor (SGLT‐I) treatment with nonalcoholic fatty liver disease (NAFLD) complicated by type 2 diabetes mellitus (T2DM) according to gender
Table S2 Changes in clinical characteristics with 48‐week SGLT‐I treatment in patients with NAFLD with T2DM by menopausal status
Table S3 Adverse events leading to discontinuation of SGLT2‐Is


