Abstract
Anti‐mitochondrial autoantibodies (AMAs) are highly specific for the diagnosis of primary biliary cholangitis (PBC) but are also occasionally found in other diseases. In the present study, we evaluated the incidence of and predictors for PBC development in AMA‐positive patients with other liver or non‐liver diseases at baseline. In this retrospective study, we screened patients who tested positive for AMA and/or anti‐mitochondrial M2 antibody (AMA‐M2) at Beijing Friendship Hospital, Capital Medical University, from October 2005 to January 2017. They were categorized by their diagnosis at the baseline as patients with PBC or non‐PBC cases. We followed up on the non‐PBC cases through telephone interviews and reviewing of medical records to obtain laboratory results and clinical outcomes. In total, 139 patients were AMA‐positive but did not fulfill the diagnostic criteria of PBC at baseline, including 51 patients with non‐PBC liver diseases and 88 cases with non‐liver diseases. The titers of AMA‐M2, alkaline phosphatase, gamma‐glutamyl transpeptidase, and immunoglobulin M were significantly higher in patients with PBC compared to those with non‐PBC liver diseases and non‐liver diseases. After a median follow‐up of 4.6 (interquartile range: 2.4–7.6) years, 4.3% (6 of 139) developed PBC, with an accumulative 5‐year incidence rate of 4.2%. None of the patients with non‐PBC liver diseases developed PBC, whereas the 5‐year incidence rate of PBC was 7.8% among 88 patients with non‐liver diseases. Lower alanine aminotransferase and higher immunoglobulin M were independent predictors for developing PBC. Conclusion: Our results suggest a low risk of developing PBC over time in AMA‐positive patients with other liver and non‐liver diseases.
In this study, we found that AMA‐positive patients with non‐PBC diseases had a low risk of developing PBC, especially for those with other liver diseases. Higher immunoglobulin M and lower alanine aminotransferase were independent predictors for developing PBC.
![]()
INTRODUCTION
Anti‐mitochondrial autoantibodies (AMAs) are the immunological hallmark and diagnostic modality for primary biliary cholangitis (PBC), with both sensitivity and specificity ranging from 90% to 95%.[ 1 , 2 ] Therefore, AMA has become a routine test in the work‐up of cholestatic liver diseases.[ 3 , 4 ] Of note is that most of the studies have been conducted in the setting of patients with clinical and biochemical signs of intrahepatic cholestasis, whereas the diagnostic performance of AMA in the settings of normal liver tests or non‐cholestatic profiles is less well elucidated.
One earlier study reported by Metcalf et al. in 1996[ 5 ] indicated that among 29 patients who were AMA‐positive but had normal liver function tests, 24 (83%) showed persistent elevation of alkaline phosphatase (ALP) after more than 10 years of follow‐up. Therefore, AMA positivity has been regarded as a biomarker of preclinical PBC even in subjects with no clinical or biochemical signs of cholestasis. However, a recent study[ 6 ] found that among the subjects with normal ALP and positive AMA, the 5‐year incidence rate of PBC was only 16%. Furthermore, Lazaridis et al.[ 7 ] observed that none of the first‐degree relatives of patients with PBC, who were AMA‐positive but with normal ALP at baseline, developed PBC during follow‐up. Obviously, the natural history of AMA‐positive subjects with normal liver tests needs to be further investigated, and the clinical utility of isolated AMA positivity in predicting the development of PBC remains to be defined.
Furthermore, AMA can also be positive in patients with other non‐PBC liver diseases, including acute liver failure, autoimmune hepatitis (AIH), and drug‐induced liver injury (DILI),[ 8 , 9 , 10 ] or non‐liver conditions including systemic lupus erythematosus, lymphoma, and epilepsy.[ 11 ] Whether these patients will develop PBC is not completely clear.
Therefore, in the current study, we described the characteristics and incidence of PBC in a retrospective cohort of patients with AMA positivity and other liver or non‐liver diseases.
PATIENTS AND METHODS
Patient enrollment
This was a retrospective study on patients who tested positive for AMA and/or anti‐mitochondrial M2 antibody (AMA‐M2) in Beijing Friendship Hospital, Capital Medical University, from October 2005 to January 2017. The major inclusion criteria were (1) AMA‐positive with titer equal to or higher than 1:80 and/or (2) AMA‐M2 positive with titer equal to or higher than 10 U/ml. The exclusion criteria were (1) patients with insufficient data for analysis and (2) patients with PBC‐AIH overlap syndrome.
All procedures followed were in accordance with the Declaration of Helsinki and the ethical standards for clinical studies by the Ethics Committee of the Beijing Friendship Hospital, Capital Medical University. All patients gave verbal consent that was granted by the ethical committee.
Diagnosis of PBC
PBC was diagnosed based on the presence of at least two of the following criteria[ 3 ]: (1) biochemical evidence of cholestasis based on elevated ALP levels, (2) presence of AMA or AMA‐M2, and (3) diagnostic or compatible liver biopsy. Development of PBC in AMA‐positive patients was ascertained by persistent elevation of ALP and/or gamma‐glutamyl transpeptidase (GGT) or liver biopsy.
We divided the enrolled AMA‐positive patients into patients with PBC and non‐PBC groups, with the latter being subdivided into patients with non‐PBC liver diseases and non‐liver diseases. Of note, the patients with elevated ALP levels but diagnosed with non‐PBC liver disease were categorized as the non‐PBC group if their ALP returned to normal after the underlying etiology was removed or controlled.
Collection of baseline data and follow‐up information
Demographic, baseline clinical, and laboratory data were collected, including sex, age, liver biochemistries, AMA‐M2 and/or AMA, immunoglobulin, abdominal ultrasound, and liver histology if available.
Follow‐up information was obtained from the electronic medical records and telephone interviews. The follow‐up information included the dynamic change of liver biochemistries, therapeutic regimen, and clinical outcomes. The development of persistent elevation of ALP in non‐PBC patients was recorded. The duration of follow‐up was defined as the interval between the date of AMA or AMA‐M2 first detected and the date of the last follow‐up.
Detection of AMA and AMA‐M2
AMA was detected by indirect immunofluorescent assay (Euroimmun Inc.), with titers equal to or higher than 1:80 being regarded as positive. AMA‐M2 was detected by enzyme‐linked immunosorbent assay (ORGENTEC Diagnostika GmbH), with titers equal to or higher than 10 U/ml being considered positive.
Statistical analyses
Continuous variables were expressed as median and interquartile ranges. Categorical variables were summarized by counts and percentages. The Mann–Whitney U test and the chi‐square test were used to compare the differences between patients with PBC and without.
Cumulative incidence rates of PBC were estimated using the Kaplan–Meier method, and statistical significance was determined by the log‐rank test. The Cox regression model was used to identify variables that were associated with the development of PBC.
A two‐sided p‐value of < 0.05 was used to define statistical significance. The statistical analyses were performed using SPSS statistics version 20.
RESULTS
Baseline characteristics of the study population
We reviewed 16,338 tests of AMA or AMA‐M2 and found 2320 positive ones. In total, 139 patients were AMA‐positive but did not fulfill the diagnostic criteria of PBC at baseline, including 51 patients with non‐PBC liver diseases and 88 cases with non‐liver diseases (Figure 1). These patients were primarily from the departments of hepatology, rheumatology, hematology, endocrinology, pulmonary medicine, intensive care unit, neurology, and general surgery.
FIGURE 1.
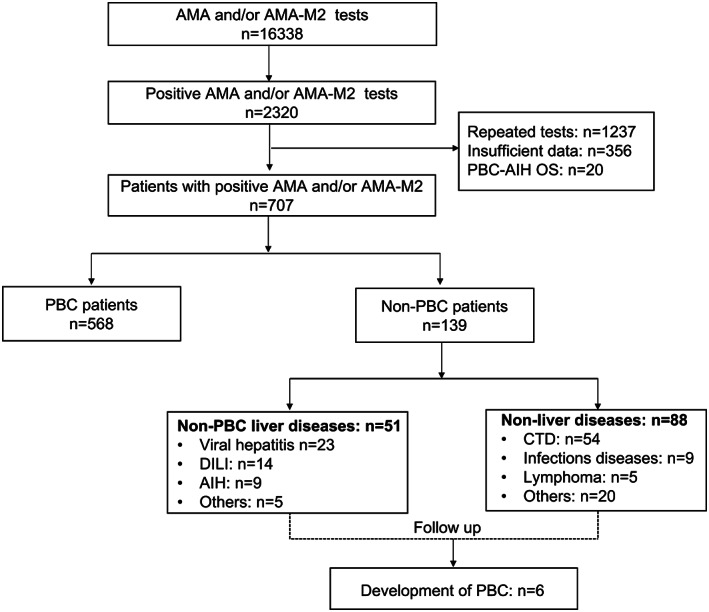
Flowchart of the study. AIH, autoimmune hepatitis; AMA, anti‐mitochondrial autoantibody; AMA‐M2, anti‐mitochondrial M2 antibody; CTD, connective tissue diseases; DILI, drug‐induced liver injury; PBC, primary biliary cholangitis; PBC‐AIH OS, PBC‐AIH overlap syndrome
Demographic and baseline characteristics of patients with PBC and non‐PBC are summarized in Table 1. The most frequent non‐PBC liver diseases were viral hepatitis (n = 23), drug‐induced liver injury (DILI; n = 14), and AIH (n = 9); the characteristics of patients with non‐PBC liver diseases are given in Table 2. The median RUCAM score of DILI patients was 7, with 4 cases being rated as highly probable, 9 probable, and 1 possible. The pattern of liver injury was hepatocellular in 12 patients, cholestatic in 1 patient, and mixed type in 1 patient. The most frequent non‐liver diseases were connective tissue diseases, including Sjogren's syndrome (n = 15), rheumatoid arthritis (n = 12), and systemic lupus erythematosus (n = 10).
TABLE 1.
Demographics and baseline characteristics of AMA‐positive patients
| Characteristics | PBC (n = 568) | Non‐PBC liver diseases (n = 51) | Non‐liver diseases (n = 88) |
| Age | 56 (49–64) | 53 (47–60) | 56 (44–72) |
| Female gender, n (%) | 481 (84.7) | 38 (74.5) | 73 (83.0) |
| AMA‐M2, U/ml | 70 (23–143) | 12 (12–20) b | 16 (4–52) b |
| ANA positivity a , n (%) | 421 (92.9) | 22 (57.9) b | 71 (92.2) |
| ALP, ULN | 1.39 (0.98–2.21) | 0.78 (0.64–1.29) b | 0.59 (0.48–0.76) b |
| GGT, ULN | 3.49 (1.76–7.24) | 1.82 (0.91–2.98) b | 0.76 (0.47–1.16) b |
| ALT, ULN | 1.25 (0.73–1.95) | 4.38 (1.28–7.23) b | 0.48 (0.30–0.76) b |
| AST, ULN | 1.57 (0.97–2.31) | 3.23 (1.63–7.17) b | 0.66 (0.49–0.83) b |
| ALB, g/L | 39.5 (34.8–42.4) | 37.6 (32.0–42.3) b | 36.8 (32.2–41.3) b |
| GLO, g/L | 35.1 (31.7–40.1) | 30.5 (27.9–38.5) b | 30.8 (27.4–36.3) b |
| TBIL, umol/L | 16.1 (11.3–26.5) | 24.5 (14.7–72.4) b | 9.8 (7.4–15.1) b |
| IgG, mg/dl | 1650 (1370–2020) | 1715 (1298–2330) | 1500 (1270–1838) b |
| IgM, mg/dl | 329 (217–510) | 142 (110–229) b | 130 (88–209) b |
Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANA, antinuclear antibodies; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transpeptidase; GLO, globulin; IgG, immunoglobulin G; IgM, immunoglobulin M; TBIL, total bilirubin; ULN, upper limit of normal.
Available in 568 patients.
Refers significantly different from the variables of patients with PBC.
TABLE 2.
Characteristics of AMA‐positive patients with non‐PBC liver diseases
| DILI (n = 14) | AIH (n = 9) | Viral hepatitis (n = 23) | Others a (n = 5) | |
| Females, n (%) | 12 (85.7) | 8 (88.9) | 13 (56.5) | 4 (80.0) |
| Age | 51 (44–59) | 55 (53–61) | 52 (47–65) | 50 (28–60) |
| Follow‐up duration (years) | 4.58 (2.46–6.68) | 5.33 (2.96–10.56) | 7.16 (4.70–7.77) | 5.84 (3.56–8.12) |
| Liver biopsy, n (%) | 5 (35.7) | 9 (100) | 0 (0) | 2 (40) |
| Suspicious drugs | ||||
| TCM | 8 | / | / | / |
| Statins | 2 | / | / | / |
| Antibiotics | 2 | / | / | / |
| Chemotherapeutic | 1 | / | / | / |
| Dietary supplement | 1 | / | / | / |
Abbreviations: DILI, drug‐induced liver injury; TCM, traditional Chinese medicine.
Other non‐PBC liver diseases including nonalcoholic fatty liver disease (n = 3), primary sclerosing cholangitis (n = 1), and Caroli disease (n = 1).
Not surprisingly, the titers of AMA‐M2, ALP, GGT, and immunoglobulin M (IgM) were all significantly higher in patients with PBC compared to patients with non‐PBC liver diseases and non‐liver diseases (all p < 0.05). The median AMA‐M2 titers were 41 (15–105), 28 (15–72), 23 (14–44), 16 (13–61), and 15 (10–30) U/ml in patients with connective tissue diseases, miscellaneous diseases, AIH, DILI, and viral hepatitis, respectively, which were significantly lower than that in patients with PBC (70 [23–143] U/ml) (all p‐value < 0.05; Figure 2).
FIGURE 2.
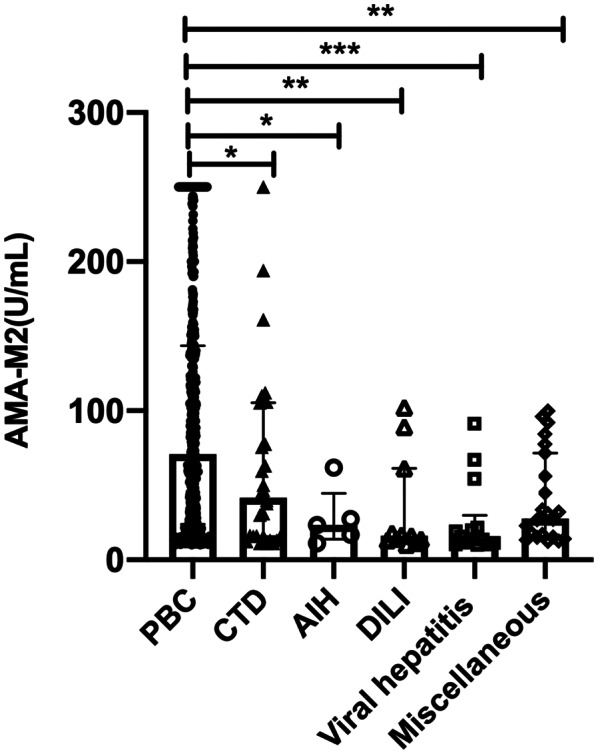
Titers of AMA‐M2 in different diseases. The titers of AMA‐M2 were significantly lower in patients with non‐PBC diseases compared to patients with PBC. Data are presented as medians with interquartile ranges. *p < 0.05, **p < 0.01, ***p < 0.001; n.s., p > 0.05
Development of PBC in non‐PBC patients during follow‐up
In total, we followed up the 139 non‐PBC patients for a median duration of 4.6 (interquartile range: 2.4–7.6) years, with 20 (14.4%) of them lost to follow‐up. A total of 15 (10.8%) patients died during follow‐up, and most of them (n = 12) were non‐liver‐related deaths. The median follow‐up duration for 15 patients before death and 20 patients before being lost to follow‐up was 3.2 (interquartile range: 2.0–4.7) and 1.2 (interquartile range: 0.2–2.2) years, respectively. The etiology of liver disease in 3 liver‐related deaths were all viral hepatitis, including 2 patients with chronic hepatitis C and 1 patient with chronic hepatitis B.
During follow‐up, only 6 of 139 non‐PBC (4.3%) patients developed PBC. They were all females and diagnosed with PBC for the persisted elevation of ALP, with one of them confirmed by liver biopsy, and 2 of them had a PBC family history. The overall cumulative incidence rates of PBC development at 3, 5, and 10 years were 1.63% (95% confidence interval [CI]: 0.48–2.81), 4.2% (95% CI: 2.1–8.58), and 7.5% (95% CI: 4.32–17.20), respectively (Figure 3A).
FIGURE 3.
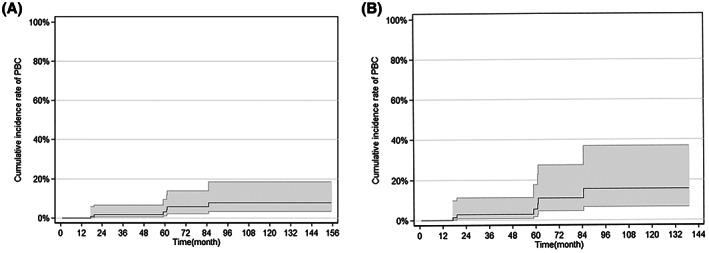
Cumulative incidence of PBC with 95% confidence interval (CI) boundaries in the AMA‐positive patients. (A) All patients. (B) Eighty‐eight patients with non‐liver diseases
Notably, none of the 51 patients with non‐PBC liver diseases developed PBC at the end of the follow‐up or censors. A liver biopsy was performed in 16 (31.4%) of the 51 non‐PBC liver patients, with none of them having histological evidence of PBC.
In contrast, 6 of the 88 non‐liver disease cases developed PBC, with 4 of them having pre‐existing autoimmune diseases, including 2 with rheumatoid arthritis, 1 with Sjogren's syndrome, and 1 with hypothyroidism. The cumulative incidence rates of PBC development in 88 patients with non‐liver disease at 3, 5, and 10 years were 2.76% (95% CI, 0.48–4.89), 7.81% (95% CI, 3.48–17.51), and 14.52% (95% CI, 9.87–39.41), respectively (Figure 3B).
Risk factors associated with PBC development
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, and IgM were significantly different between those who developed PBC (n = 6) and those who did not (n = 133) (Table S1). Multivariate Cox regression analysis showed that only ALT and IgM remained statistically significant (Table 3). Sex, age, the titer of AMA‐M2, and the levels of ALP, GGT, albumin, globulin, immunoglobulin G, or immunoglobulin A were not significantly associated with a higher risk of developing PBC.
TABLE 3.
Multivariate Cox regression analysis for development of PBC
| HR | 95% CI | p | |
|---|---|---|---|
| TBIL, μmol/L | 1.010 | 0.776–1.314 | 0.940 |
| ALT, U/L | 0.773 | 0.616–0.971 | 0.027 |
| AST, U/L | 0.932 | 0.722–1.180 | 0.522 |
| IgM, mg/dl | 1.009 | 1.003–1.016 | 0.004 |
Abbreviation: HR, hazard ratio.
DISCUSSION
In this study, we found that AMA can be positive in patients with various diseases, including non‐PBC liver diseases and non‐liver diseases; the AMA‐M2 level in non‐PBC patients was significantly lower than that in patients with PBC. More importantly, AMA‐positive patients with non‐PBC diseases had a low risk of developing PBC, especially for those with other liver diseases. Lower ALT and higher IgM were independent predictors for developing PBC.
One key observation in the study is that AMA‐positive patients with other diseases have a low risk of developing PBC, giving a 5‐year incidence rate of PBC of 4.2%. Our finding is in line with other reports. One prospective nationwide study from French[ 6 ] found that only 9 of 92 (9.8%) AMA‐positive patients with non‐established PBC at baseline developed PBC during 4.0 ± 1.8 years of follow‐up. Another study showed that none of 26 AMA‐positive first‐degree relatives of PBC with normal ALP at baseline developed PBC during 8.9 years of follow‐up.[ 7 ] A most recent study reported that 10.2% of 59 AMA‐positive patients developed PBC during 5.8 ± 5.6 years of follow‐up.[ 11 ] All of the evidence indicates that not all AMA‐positive subjects will inevitably evolve into PBC.
However, our results are inconsistent with an early study[ 5 ] that showed how 83% of AMA‐positive patients with normal liver biochemistries developed persistent ALP elevation during long‐term follow‐up. One of the explanations would be that most of their patients actually already had histological evidence of PBC (82.7%) at baseline, although their liver tests were still normal. Additionally, a recent study from China also found that more than 80% of AMA‐positive subjects with normal ALP had histological evidence of PBC, which was consistent with another Swiss study.[ 12 , 13 ] Of note, the latter two studies enrolled AMA‐positive subjects with normal ALP but elevated GGT at baseline (with a median GGT level of 1.69 upper limits of normal [ULN] and 1.46 ULN, respectively). Taken together, these data indicate that the risk of developing PBC would be very low if their ALP and GGT are both normal.
The second key observation of our current study is that the development of PBC was observed in none of the patients with non‐PBC liver diseases. We noticed that 23% of these patients would have fulfilled the conventional diagnostic criteria of PBC at baseline. However, their liver biochemistries returned to normal after the etiology was removed or controlled. Therefore, for AMA‐positive patients already diagnosed with other liver diseases, it is reasonable to observe the dynamic change of ALP and AMA level rather than rush to the diagnosis of PBC.
Interestingly, in line with previous reports,[ 8 , 14 ] we noticed that AMA could also be detected in DILI patients. However, it remains unclear whether DILI can induce PBC, although it is well known that DILI can induce AIH. One study showed AMA positivity was more frequently found in patients with DILI caused by non‐steroidal anti‐inflammatory drugs or anesthetics.[ 15 ] However, in our study, medicinal herbs were implicated in most of the 14 DILI patients who were AMA‐positive. More importantly, the liver biochemistries returned to normal, and none of them developed PBC during follow‐up. One possible explanation would be that severe acute injury caused by drugs or other noxious insults leads to the emergence of AMA by transient exposure of mitochondrial antigens.[ 10 ] The dynamic change of AMA titers needs to be investigated with longer‐term observation in a larger number of patients.
In our cohort, we found that in 9 patients with AIH with AMA positivity, all of them had a good response to immunosuppressive therapy, and none of them had biochemical features of PBC during follow‐up. This is also in line with previous reports that 5% to 35% of patients with AIH were positive for AMA,[ 9 , 16 , 17 , 18 ] but they had similar clinical, biochemical, and histological features to patients with AIH without AMA positivity.[ 19 ] Only in rare cases did patients with AIH with AMA positivity eventually develop clinical and histologic evidence of PBC.[ 20 ] It was also reported that AMA may be a transient presence in acute AIH and usually disappears over time.[ 10 ] In line with these findings, among 6 patients who underwent a recheck of AMA during follow‐up in our study, 4 patients were persistently AMA‐positive, and 2 patients subsequently became AMA‐negative.
Finally, we found that AMA was also detectable in patients with other autoimmune diseases, which was consistent with previous reports.[ 21 , 22 , 23 , 24 , 25 ] Of note, like in many medical centers in China, in our institute AMA is included in the panel of ANA testing and routinely ordered for patients with suspected rheumatologic disorders, or patients with fever of unknown origin from various departments, especially from rheumatology department. This may partially explain the relatively high proportion of patients with autoimmune diseases in AMA‐positive patients in the current study. Furthermore, studies showed that a low prevalence of AMA can even be detected in the general population, which varies between 0.16% and 3.8%.[ 26 , 27 ] All of these pieces of evidence emphasize that a caveat in interpreting even a highly sensitive and specific test like AMA for PBC is to take into account the clinical setting and appropriate scenario.
Our study had several limitations. First, this is a single‐center study with a retrospective design. However, we hope the fair number of patients included and the relatively long duration of follow‐up (a median of 4.6 years) may offset some of this weakness. Second, because of the retrospective nature, we could not assess the persistence of AMA positivity in most of the cases. We noticed that some patients subsequently changed to AMA‐negative, so we may underestimate the rate of developing PBC in patients with persistent AMA positivity. On the other hand, AMA and AMA‐M2 testing used in the current study might include some false positives or miss some true positives, which also underlines the importance of repeated testing. Future prospective studies with repeated AMA and/or AMA‐M2 testing would be justified. Third, not all of the patients received liver biopsy, so our study may underestimate the incidence of histological PBC. Obviously, further prospective studies that enrolled a larger number of patients with histological information are warranted. Finally, considering that 20 (14.4%) patients were lost to follow‐up and 9 of them with a follow‐up duration less than 1 year, we may underestimate the rate of PBC development.
CONCLUSIONS
Our study suggests that AMA can be present in patients with non‐PBC liver disease or even non‐liver diseases; the accumulative incidence of PBC in these AMA‐positive patients is low during long‐term follow‐up.
CONFLICT OF INTEREST
Nothing to report.
Supporting information
Table S1 Demographics and baseline characteristics of patients with developed primary biliary cholangitis (PBC) and non‐PBC after follow‐up
Duan W, Chen S, Li S, Lv T, Li B, Wang X, et al. The future risk of primary biliary cholangitis (PBC) is low among patients with incidental anti‐mitochondrial antibodies but without baseline PBC. Hepatol Commun. 2022;6:3112–3119. 10.1002/hep4.2067
Weijia Duan and Sha Chen contributed equally to this work.
REFERENCES
- 1. Leung PS, Choi J, Yang G, Woo E, Kenny TP, Gershwin ME. A contemporary perspective on the molecular characteristics of mitochondrial autoantigens and diagnosis in primary biliary cholangitis. Expert Rev Mol Diagn. 2016;16:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walker JG, Doniach D, Roitt IM, Sherlock S. Serological tests in diagnosis of primary biliary cirrhosis. Lancet. 1965;1:827–31. [DOI] [PubMed] [Google Scholar]
- 3. You H, Ma X, Efe C, Wang G, Jeong SH, Abe K, et al. APASL clinical practice guidance: the diagnosis and management of patients with primary biliary cholangitis. Hepatol Int. 2022;16:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394–419. [DOI] [PubMed] [Google Scholar]
- 5. Metcalf JV, Mitchison HC, Palmer JM, Jones DE, Bassendine MF, James OF. Natural history of early primary biliary cirrhosis. Lancet. 1996;348:1399–402. [DOI] [PubMed] [Google Scholar]
- 6. Dahlqvist G, Gaouar F, Carrat F, Meurisse S, Chazouilleres O, Poupon R, et al. Large‐scale characterization study of patients with antimitochondrial antibodies but nonestablished primary biliary cholangitis. Hepatology. 2017;65:152–63. [DOI] [PubMed] [Google Scholar]
- 7. Gulamhusein AF, Juran BD, Atkinson EJ, McCauley B, Schlicht E, Lazaridis KN. Low incidence of primary biliary cirrhosis (PBC) in the first‐degree relatives of PBC probands after 8 years of follow‐up. Liver Int. 2016;36:1378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stephens C, Castiella A, Gomez‐Moreno EM, Otazua P, Lopez‐Nevot MA, Zapata E, et al. Autoantibody presentation in drug‐induced liver injury and idiopathic autoimmune hepatitis: the influence of human leucocyte antigen alleles. Pharmacogenet Genomics. 2016;26:414–22. [DOI] [PubMed] [Google Scholar]
- 9. O'Brien C, Joshi S, Feld JJ, Guindi M, Dienes HP, Heathcote EJ. Long‐term follow‐up of antimitochondrial antibody‐positive autoimmune hepatitis. Hepatology. 2008;48:550–6. [DOI] [PubMed] [Google Scholar]
- 10. Leung PS, Rossaro L, Davis PA, Park O, Tanaka A, Kikuchi K, et al. Antimitochondrial antibodies in acute liver failure: implications for primary biliary cirrhosis. Hepatology. 2007;46:1436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zandanell S, Strasser M, Feldman A, Tevini J, Strebinger G, Niederseer D, et al. Low rate of new‐onset primary biliary cholangitis in a cohort of anti‐mitochondrial antibody‐positive subjects over six years of follow‐up. J Intern Med. 2020;287:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun C, Xiao X, Yan L, Sheng L, Wang Q, Jiang P, et al. Histologically proven AMA positive primary biliary cholangitis but normal serum alkaline phosphatase: is alkaline phosphatase truly a surrogate marker? J Autoimmun. 2019;99:33–8. [DOI] [PubMed] [Google Scholar]
- 13. Terziroli Beretta‐Piccoli B, Stirnimann G, Mertens J, Semela D, Zen Y, Mazzucchelli L, et al. Primary biliary cholangitis with normal alkaline phosphatase: a neglected clinical entity challenging current guidelines. J Autoimmun. 2021;116:102578. [DOI] [PubMed] [Google Scholar]
- 14. Yang J, Yu YL, Jin Y, Zhang Y, Zheng CQ. Clinical characteristics of drug‐induced liver injury and primary biliary cirrhosis. World J Gastroenterol. 2016;22:7579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weber S, Benesic A, Buchholtz ML, Rotter I, Gerbes AL. Antimitochondrial rather than antinuclear antibodies correlate with severe drug‐induced liver injury. Dig Dis. 2021;39:275–82. [DOI] [PubMed] [Google Scholar]
- 16. Mishima S, Omagari K, Ohba K, Kadokawa Y, Masuda J, Mishima R, et al. Clinical implications of antimitochondrial antibodies in type 1 autoimmune hepatitis: a longitudinal study. Hepatogastroenterology. 2008;55:221–7. [PubMed] [Google Scholar]
- 17. Nezu S, Tanaka A, Yasui H, Imamura M, Nakajima H, Ishida H, et al. Presence of antimitochondrial autoantibodies in patients with autoimmune hepatitis. J Gastroenterol Hepatol. 2006;21:1448–54. [DOI] [PubMed] [Google Scholar]
- 18. Farias AQ, Goncalves LL, Bittencourt PL, De Melo ES, Abrantes‐Lemos CP, Porta G, et al. Applicability of the IAIHG scoring system to the diagnosis of antimitochondrial/anti‐M2 seropositive variant form of autoimmune hepatitis. J Gastroenterol Hepatol. 2006;21:887–93. [DOI] [PubMed] [Google Scholar]
- 19. Muratori P, Efe C, Muratori L, Ozaslan E, Schiano T, Yoshida EM, et al. Clinical implications of antimitochondrial antibody seropositivity in autoimmune hepatitis: a multicentre study. Eur J Gastroenterol Hepatol. 2017;29:777–80. [DOI] [PubMed] [Google Scholar]
- 20. Dinani AM, Fischer SE, Mosko J, Guindi M, Hirschfield GM. Patients with autoimmune hepatitis who have antimitochondrial antibodies need long‐term follow‐up to detect late development of primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2012;10:682–4. [DOI] [PubMed] [Google Scholar]
- 21. Gui H, Wang W, Li Q, Li Z, Lu J, Xie Q. Autoimmune liver disease‐associated serologic profiling in Chinese patients with acute hepatitis E virus infection. Immunol Res. 2021;69:81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cotler SJ, Kanji K, Keshavarzian A, Jensen DM, Jakate S. Prevalence and significance of autoantibodies in patients with non‐alcoholic steatohepatitis. J Clin Gastroenterol. 2004;38:801–4. [DOI] [PubMed] [Google Scholar]
- 23. Imura‐Kumada S, Hasegawa M, Matsushita T, Hamaguchi Y, Encabo S, Shums Z, et al. High prevalence of primary biliary cirrhosis and disease‐associated autoantibodies in Japanese patients with systemic sclerosis. Mod Rheumatol. 2012;22:892–8. [DOI] [PubMed] [Google Scholar]
- 24. Hatzis GS, Fragoulis GE, Karatzaferis A, Delladetsima I, Barbatis C, Moutsopoulos HM. Prevalence and longterm course of primary biliary cirrhosis in primary Sjogren's syndrome. J Rheumatol. 2008;35:2012–6. [PubMed] [Google Scholar]
- 25. Ahmad A, Heijke R, Eriksson P, Wirestam L, Kechagias S, Dahle C, et al. Autoantibodies associated with primary biliary cholangitis are common among patients with systemic lupus erythematosus even in the absence of elevated liver enzymes. Clin Exp Immunol. 2021;203:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen BH, Wang QQ, Zhang W, Zhao LY, Wang GQ. Screening of anti‐mitochondrial antibody subtype M2 in residents at least 18 years of age in an urban district of Shanghai. China Eur Rev Med Pharmacol Sci. 2016;20:2052–60. [PubMed] [Google Scholar]
- 27. Colapietro F, Lleo A, Generali E. Antimitochondrial antibodies: from bench to bedside. Clin Rev Allergy Immunol. 2021;1–12. 10.1007/s12016-021-08904-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Demographics and baseline characteristics of patients with developed primary biliary cholangitis (PBC) and non‐PBC after follow‐up


