Abstract
There is a heavy burden of liver disease in West Africa. While the role of hepatitis B virus (HBV) infection is well recognized, less is known about the contributing role of liver steatosis and how the two interact in the context of human immunodeficiency virus (HIV) infection. Adults with HIV in Ghana underwent FibroScan measurements to determine prevalence of liver steatosis (expressed as controlled attenuation parameter [CAP]) and fibrosis (expressed as liver stiffness [LS]). We explored contributing factors in linear regression models, including demographics, lifestyle characteristics, medical history, HIV and HBV status, and measurements of metabolic syndrome. Among 329 adults (72.3% women; median age, 47 years), 322 (97.9%) were on antiretroviral therapy (median duration, 8.9 years). CD4 counts were preserved (median, 619 cells/mm3); plasma HIV RNA was fully suppressed in 162 (50.3%) of the treated participants. Cigarette smoking, excessive alcohol consumption, and use of traditional or herbal remedies were uncommon (6.1%, 1.8%, 3.3%, respectively). Largely undiagnosed metabolic syndrome was detected in 87 (26.4%) participants. We obtained readings indicative of ≥S2 steatosis and ≥F2 fibrosis in 43 (13.1%) and 55 (16.7%) participants, respectively. Higher CAP values were associated with metabolic syndrome and longer prior stavudine exposure. Higher LS values were associated with male sex, higher HIV RNA, and higher CAP values. Relative to people without HBV, those with HBV (n = 90) had a similar prevalence of ≥S2 steatosis but a higher prevalence of ≥F2 fibrosis (36.7% vs. 9.2%, p < 0.0001) and concomitant ≥S2 steatosis and ≥F2 fibrosis (9.1% vs. 1.3%, p < 0.001). Conclusion: Both HBV and liver steatosis pose a threat to long‐term liver health among people with HIV in West Africa. Urgently required interventions include improving HIV suppression and diagnosing and managing determinants of the metabolic syndrome.
We determined the prevalence of both liver steatosis and liver fibrosis in a HIV cohort in Ghana using a portable FibroScan, and we interpreted the data within a broad context, including measurements that are not part of routine HIV care in Kumasi. We identified two main reasons for concern: (1) metabolic syndrome was common and a predictor of liver steatosis, but it was largely undiagnosed and untreated; (2) only about half of the population had a suppressed HIV RNA load while receiving antiretroviral therapy, and uncontrolled virus replication was a predictor of liver fibrosis regardless of HBV status.

INTRODUCTION
Multiple factors may contribute to increase the risk of liver disease in the context of human immunodeficiency virus (HIV) infection, including infectious and noninfectious comorbidities, metabolic disorders, and the possible direct effects of HIV infection and antiretroviral therapy (ART).[ 1 , 2 , 3 , 4 ] In a systematic review of 10 cross‐sectional studies of primarily men living with HIV in North America, Western Europe, China, and Japan, 35% met the definition of nonalcoholic fatty liver disease (NAFLD) based on imaging or liver histology.[ 1 ] Metabolic disorders and high CD4 cell counts were associated with NAFLD, whereas duration of HIV infection, duration of ART, HIV RNA load, and nadir CD4 cell counts were not.[ 1 ] A study from Brazil similarly reported that 35% of people with HIV had NAFLD but proposed an association with exposure to the nucleoside analogues zidovudine, stavudine, didanosine, and zalcitabine.[ 5 ]
There are scarce data on NAFLD in populations living with HIV in sub‐Saharan Africa, although rates of obesity, hypertension, dyslipidemia, and impaired glucose tolerance and diabetes are rising in the region.[ 6 , 7 ] Two studies from South Africa investigated selected patients with HIV who had undergone a liver biopsy for various care indications and reported liver steatosis in 19% and 28%, respectively.[ 8 , 9 ] In three studies from Western Africa, the prevalence of liver steatosis by liver ultrasound scan ranged between 13% and 28%.[ 10 , 11 , 12 ] While the burden of NAFLD may be significant, diagnostic resources are scarce, with limited access to liver biopsy, sophisticated liver imaging, and specialized laboratory testing.[ 6 ]
Measuring liver stiffness (LS) by FibroScan provides a feasible tool for the noninvasive assessment of liver fibrosis.[ 13 ] The controlled attenuation parameter (CAP) can be obtained at the same time to give a measure of liver steatosis.[ 14 ] A European study that evaluated performance among 420 patients with HIV found that a CAP cutoff of 280 dB/m had a sensitivity and specificity of 86% and 72%, respectively, for ≥S2 steatosis relative to liver biopsy. CAP outperformed most other noninvasive biomarkers, whereas measuring the proton density fat fraction by magnetic resonance imaging had a better performance than CAP in this study.[ 15 ] There are scarce published data on the use of CAP to assess liver steatosis in people with HIV in sub‐Saharan Africa. We measured LS and CAP in patients accessing HIV care in Ghana and analyzed the data in the context of demographic and lifestyle characteristics, medical history, HIV‐related parameters, metabolic status, and coinfection with viral hepatitis viruses. Given the high prevalence of chronic HBV coinfection in this population (approximately 16%),[ 16 ] one aim was to compare the findings in patients with and without HBV.
METHODS
Study population
The study took place in February 2018 at the Komfo Anokye Teaching Hospital (KATH) in Kumasi, Ghana, with approval from the Ethics Committee of the Kwame Nkrumah University of Science and Technology; participants provided written informed consent. Consecutive adults (≥18 years) attending the general HIV outpatient clinic were invited to participate. Additionally, targeting a research clinic allowed enrichment of people who underwent hepatitis B surface antigen (HBsAg) testing and were found to be positive. Participants were administered a structured questionnaire by local trained assistants to collect data on smoking history, alcohol intake, and use of traditional or herbal remedies. A nurse interviewed patients about concomitant morbidities or treatment other than ART. Height, weight, body mass index (BMI), and waist circumference were measured by standard methods.[ 17 ] Blood pressure readings were confirmed after the patient had rested for 20 min or longer. CAP and LS were measured using FibroScan (Echosens, France) ≥3.0 hours after the last meal. All women of child‐bearing potential underwent a pregnancy test before the FibroScan. Members of the research team with extensive clinical experience in using FibroScan (Dr Villa, Prof Geretti) performed the tests. A result was considered valid when the following three conditions were met: ≥10 captures obtained, success rate ≥60%, and interquartile range (IQR)/median ratio <0.30. Based on CAP values, liver steatosis was graded as absent/S0 (<248 dB/m), mild/S1, moderate/S2, and severe/S3 (Table 1).[ 18 ] Based on LS values, fibrosis was graded as F0–F4 using interpretative cutoffs for histologically defined METAVIR scores as previously determined for patients with HIV[ 19 ] or with HIV and HBV coinfection.[ 20 ]
TABLE 1.
Definitions and grading of parameters analyzed in the study
| Parameter[ 18 , 19 , 20 , 24 , 25 , 26 , 27 , 28 , 29 ] | Categories | ||||
|---|---|---|---|---|---|
| Central obesity based on waist circumference (cm)[ 24 , 25 ] | Men ≥94; women ≥80 | ||||
| Raised blood pressure (mm Hg)[ 25 , 26 ] | Grade 1: systolic 130–159 or diastolic 85–99, or specific therapy | Grade 2: systolic 160–179 or diastolic 100–109 | Grade 3: systolic ≥180 or diastolic ≥110 | ||
| Raised total cholesterol (mmol/L)[ 26 ] | Grade 1: 5.2 to <6.2 or specific therapy | Grade 2: 6.2 to <7.8 | Grade 3: ≥7.8 | ||
| Raised LDL (mmol/L)[ 26 ] | Grade 1: 3.4 to <4.1, or specific therapy | Grade 2: 4.1 to <4.9 | Grade 3: ≥4.9 | ||
| Low HDL (mmol/L)[ 24 , 25 ] | Men <1.0; women <1.3 | ||||
| Raised triglycerides (mmol/L)[ 27 ] | Grade 1: 1.7 to 2.3 or specific therapy | Grade 2: >2.3 to 5.6 | Grade 3: >5.6 | ||
| Raised HbA1c (mmol/mol)[ 28 ] | Hyperglycemia ≥48 or specific therapy | Impaired glucose tolerance 42–47 | |||
| Raised ALT (IU/L)[ 29 ] | Men ≥35; women ≥25 | ||||
| BMI range (kg/m2)[ 25 ] | Underweight <18.5 | Normal 18.5–24.9 | Overweight 25.0–29.9 | Obese ≥30.0 | |
| Liver steatosis by CAP value (dB/m)[ 18 ] | None (S0) <248 | Mild (S1) 248–268 | Moderate (S2) 268–280 | Severe (S3) >280 | |
| Liver fibrosis by stiffness value: | None (F0/F1) | Mild (F2) | Moderate (F3) | Severe (F4) | |
| HBsAg positive[ 20 ] (kPa) | <5.9 | ≥5.9 to 7.5 | ≥7.6 to 9.3 | ≥9.4 | |
| HBsAg negative[ 19 ] (kPa) | <7.1 | ≥7.1 to 9.3 | ≥9.4 to 13.9 | ≥14 | |
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; HbA1c, glycated hemoglobin; HBsAg, hepatitis B surface antigen; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Laboratory investigations
Full blood cell counts and CD4 cells counts were measured at the KATH diagnostic laboratory. Plasma HIV RNA was quantified on site using Xpert HIV‐1 Viral Load (Cepheid, Sunnyvale, CA, United States), with a lower limit of quantification (LLQ) of 40 copies/ml and lower limit of detection (LLD) of 20 copies/ml. Aliquots of plasma, serum, and whole blood were stored at −80°C and shipped frozen to the United Kingdom for further testing. Serum hepatits B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) were measured by Architect (Abbot Diagnostics, Ireland). Plasma HBV DNA was quantified by Xpert HBV Viral Load (Cepheid; LLQ, 20 IU/ml; LLD, 4 IU/ml). Samples with quantifiable HBV DNA underwent genotyping by population sequencing as described.[ 16 ] Total hepatitis delta virus (HDV) antibodies (anti‐HDV) were measured by using the Dia.Pro HDV antibody enzyme‐linked immunosorbent assay (Launch Diagnostics, United Kingdom). HDV RNA (LLD, 640 copies/ml) was measured at the Liver Unit of King's College Hospital, London.[ 21 ] Hepatitis C virus (HCV) RNA was tested by Xpert HCV Viral Load (Cepheid) first in plasma pools prepared with 100 μl from each of 10 samples, then in individual samples of positive pools; the assay LLQ and LLD were 10 and 4 IU/ml (100 and 40 IU/ml when testing pools), respectively. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST), total cholesterol, high‐density lipoprotein cholesterol (HDL), and triglycerides were measured on the Roche Cobas (Roche Diagnostics Limited, United Kingdom) at the accredited diagnostic laboratory of the Royal Liverpool University Hospital (Liverpool, United Kingdom). Low‐density lipoprotein cholesterol (LDL) was calculated using the Freidewald equation. Glycated hemoglobin (HbA1c) was measured in whole blood in sodium fluoride in the same laboratory using either ion exchange high‐performance liquid chromatography on the TOSOH G8 analyzer (TOSOH Bioscience, United States) or boronate affinity with fluorescence detection on the Quo‐Test (EKF Diagnostics, United Kingdom) if the first test yielded invalid results due to hemoglobin variant interference. The AST‐to‐platelet ratio index (APRI) score and the fibrosis‐4 index (Fib‐4) score were calculated as described.[ 22 , 23 ]
Definitions and grading
Definitions and grading are given in Table 1.[ 18 , 19 , 20 , 24 , 25 , 26 , 27 , 28 , 29 ] Excessive alcohol consumption was defined as drinking more than once a week in moderate to large quantities (see Supporting File). Metabolic syndrome was defined according to Alberti and colleagues,[ 25 ] modified to include HbA1c for blood glucose. Specifically, metabolic syndrome was defined by the occurrence of three or more of the following: central obesity (waist circumference >94 cm for men and >80 cm for women), blood pressure ≥130/85 mm Hg (or drug treatment for hypertension), triglycerides ≥1.7 mmol/L (or treatment with fibrates or niacin), HDL cholesterol <1.0 mmol/L in men and <1.3 mmol/L in women (or treatment with fibrates or niacin), and HbA1c ≥42 mmol/mol (or drug treatment for elevated blood glucose).[ 24 , 28 ]
Statistical analysis
Characteristics of the study population according to HBsAg status were compared with χ 2, Fisher's exact, or Mann‐Whitney U tests, as appropriate. For the calculation of median HIV RNA levels, results below the LLQ were assigned an arbitrary value of either 20 (target detected) or 5 (target not detected) copies/ml. For the calculation of median HBV DNA levels, results below the LLQ were assigned a value of either 20 (target detected) or 2 (target not detected) IU/ml. Confidence intervals (CIs) for the prevalence of HCV RNA in the whole cohort and the prevalence of anti‐HDV in patients positive for HBsAg were calculated using the binomial exact test. Correlation between AST/ALT levels and CAP values was explored by Spearman's correlation analysis. Triglycerides levels in patients receiving non‐nucleoside reverse transcriptase inhibitors (NNRTI) or boosted‐protease inhibitors (PI/b) were compared by the Mann‐Whitney U test. ALT differences between individuals with and without S2 steatosis were compared by the χ 2 test. Factors associated with CAP and LS values were explored by linear regression analyses after natural log‐transformation of the outcome variables. Factors with p < 0.1 in the univariable models were considered for inclusion in the multivariable models. Robustness of the models was evaluated by standard postestimation tests (i.e., variance inflation factors, residual vs. fitted plots). In the analysis of factors associated with CAP values, multivariable models were adjusted for CD4 cell counts and cumulative duration of exposure to stavudine plus either (i) metabolic syndrome or (ii) central obesity, hypertension, and LDL, triglyceride, and HbA1c levels in place of metabolic syndrome. Sensitivity analyses included adjustment for cumulative total ART exposure in place of cumulative stavudine exposure, for BMI in place of central obesity, and for HDL in place of LDL. In the analysis of factors associated with LS values, the multivariable model was adjusted for sex, HIV RNA load, and CAP values; CD4 cell counts were not included due to the collinearity with HIV RNA load. A sensitivity analysis replaced CAP values with central obesity. A separate model analyzed factors associated with LS values in patients positive for HBsAg. Sensitivity analyses were conducted replacing LS values with the APRI and FIB‐4 scores after their natural log‐transformation. A sensitivity analysis was conducted after exclusion of pregnant women given that FibroScan is not validated for use in pregnant women. Statistical analyses were performed with STATA software, version 16.0 (StataCorp Inc, College Station, TX, United States).
RESULTS
Study population
All 340 consecutive patients invited to participate in the study consented. Valid CAP and LS measurements were obtained in 329/340 (96.8%) patients (Table 2). Eleven women yielded invalid measurements and were excluded from the analysis; 2/238 (0.8%) had a positive pregnancy test.
TABLE 2.
Characteristics of the study population, total and stratified by HBsAg status
| Parameter | Total N = 329 | HBsAg negative n = 239 | HBsAg positive n = 90 | p value | |
|---|---|---|---|---|---|
| Female, n (%) | 238 (72.3) | 179 (74.9) | 59 (65.6) | 0.09 | |
| Age | Median years (IQR) | 47 (42–53) | 48 (41–54) | 47 (42–52) | 0.90 |
| CD4 count | Median cells/mm3 (IQR) | 619 (358–830) | 602 (349–829) | 663 (390–840) | 0.32 |
| ART regimen, n (%) | Tenofovir/lamivudine | 180 (54.7) | 106 (44.4) | 74 (82.2) | <0.0001 |
| Zidovudine/lamivudine | 137 (41.6) | 123 (51.5) | 14 (15.6) | ||
| Other NRTI a | 5 (1.5) | 4 (1.7) | 1 (1.1) | ||
| NNRTI, efavirenz | 208 (63.2) | 137 (58.8) | 71 (79.8) | 0.002 | |
| NNRTI, nevirapine | 79 (24.0) | 68 (29.2) | 11 (12.4) | ||
| PI/b, lopinavir/ritonavir | 31 (9.4) | 24 (10.3) | 7 (7.9) | ||
| PI/b, atazanavir/ritonavir | 4 (1.2) | 4 (1.7) | 0 (0) | ||
| None b | 7 (2.1) | 6 (2.5) | 1 (1.1) | ||
| Cumulative ART exposure, median years (IQR) | Total | 8.9 (5.7–11.3) | 8.7 (4.9–11.2) | 9.6 (6.9–11.3) | 0.07 |
| Tenofovir | 0.7 (0–5.9) | 0 (0–1.9) | 6.1 (1.6–6.4) | <0.0001 | |
| Zidovudine | 3.0 (0–8.0) | 5.2 (0–8.4) | 2.0 (0–4.8) | 0.006 | |
| Stavudine | 0 (0–1.3) | 0 (0–0.7) | 0 (0–1.8) | 0.08 | |
| Efavirenz | 3.1 (0–8.0) | 1.7 (0.‐7.2) | 5.9 (2.4–9.1) | 0.0001 | |
| Nevirapine | 0 (0–5.6) | 0 (0–6.3) | 0 (0–4.6) | 0.61 | |
| Lopinavir/ritonavir [range] | 0 [0–7.1] | 0 [0–6.8] | 0 [0–8.1] | 0.19 | |
| HIV‐1 RNA load | Median log10 copies/ml (IQR) | 1.3 (1.3–2.6) | 1.6 (1.3–2.7) | 1.3 (1.3–2.4) | 0.67 |
| <40 copies/ml, n (%) | 162 (49.2) | 144 (48.7) | 48 (53.3) | 0.46 | |
| HBV DNA load c | Median log10 IU/ml (IQR) | – | – | 0.30 (0.30–1.30) | – |
| <40 IU/ml, n (%) | – | – | 74 (82.2) | ||
| 40–2000 IU/ml, n (%) | – | – | 9 (10.0) | ||
| >2000 IU/ml, n (%) | – | – | 2 (2.2) | ||
| >20,000 IU/ml, n (%) | – | – | 5 (5.6) | ||
| Cigarette smoking, n (%) | 20 (6.1) | 14 (5.9) | 6 (6.7) | 0.80 | |
| Excessive alcohol, n (%) | 6 (1.8) | 4 (1.7) | 2 (2.2) | 0.67 | |
| Herbal or traditional remedies, n (%) | 11 (3.3) | 7 (2.9) | 4 (4.4) | 0.50 | |
| Metabolic syndrome d , n (%) | 87 (26.4) | 68 (28.6) | 19 (21.1) | 0.17 | |
| BMI | Median kg/m2 (IQR) | 23.9 (20.8–27.1) | 23.9 (21.0–26.9) | 23.8 (20.8–27.9) | 0.96 |
| Underweight | 31 (9.4) | 22 (9.2) | 9 (10.0) | 0.90 | |
| Overweight | 92 (28.0) | 68 (28.5) | 24 (26.7) | ||
| Obese | 41 (12.5) | 28 (11.7) | 13 (14.4) | ||
| Waist circumference | Male, median cm (IQR) | 82 (76–88) | 82 (77–88) | 82 (75–88) | 0.94 |
| Female, median cm (IQR) | 88 (80–96) | 89 (80–96) | 86 (77–98) | 0.74 | |
| Central obesity, n (%) | 198 (60.2) | 150 (62.8) | 48 (53.3) | 0.12 | |
| Blood pressure | Median systolic mm Hg (IQR) | 128 (113–144) | 130 (113–147) | 125 (110–140) | 0.14 |
| Median diastolic mm Hg (IQR) | 82 (71–91) | 82 (72–92) | 82 (71–90) | 0.88 | |
| Grade 1 elevation, n (%) e | 119 (36.2) | 87 (36.4) | 32 (35.6) | 0.66 | |
| Grade 2 elevation, n (%) | 33 (10.0) | 25 (10.5) | 8 (8.9) | ||
| Grade 3 elevation, n (%) | 27 (8.2) | 22 (9.2) | 5 (5.6) | ||
| No data, n (%) | 7 (2.1) | 5 (2.1) | 2 (2.2) | ||
| Total cholesterol | Median mmol/L (IQR) | 4.7 (4.1–5.4) | 4.8 (4.1–5.5) | 4.4 (4.0–5.1) | 0.011 |
| Grade 1 elevation, n (%) | 78 (23.7) | 58 (24.3) | 20 (22.2) | 0.05 | |
| Grade 2 elevation, n (%) | 27 (8.2) | 25 (10.5) | 2 (2.2) | ||
| Grade 3 elevation, n (%) | 6 (1.8) | 5 (2.1) | 1 (1.1) | ||
| LDL | Median mmol/L (IQR) | 2.7 (2.2–3.4) | 2.8 (2.2–3.4) | 2.6 (2.2–3.2) | 0.12 |
| Grade 1 elevation, n (%) | 54 (16.4) | 39 (16.3) | 15 (16.7) | 0.09 | |
| Grade 2 elevation, n (%) | 18 (5.5) | 16 (6.7) | 2 (2.2) | ||
| Grade 3 elevation, n (%) | 9 (2.7) | 9 (3.8) | 0 (0) | ||
| No data, n (%) | 6 (1.8) | 5 (2.1) | 1 (1.1) | ||
| HDL | Median mmol/L (IQR) | 1.3 (1.1–1.6) | 1.3 (1.0–1.6) | 1.3 (1.1–1.6) | 0.74 |
| Low, n (%) | 132 (40.1) | 98 (41.0) | 34 (37.8) | 0.65 | |
| Triglycerides | Median mmol/L (IQR) | 1.2 (0.9–1.6) | 1.2 (0.9–1.7) | 1.1 (0.8–1.4) | 0.004 |
| Grade 1 elevation, n (%) | 46 (14.0) | 36 (15.1) | 10 (11.1) | 0.024 | |
| Grade 2 elevation, n (%) | 30 (9.1) | 28 (11.7) | 2 (2.2) | ||
| Grade 3 elevation, n (%) | 2 (0.6) | 2 (0.8) | 0 (0) | ||
| No data, n (%) | 1 (0.3) | 0 (0) | 1 (1.1) | ||
| HbA1c | Median mmol/mol (IQR) | 33 (29–37) | 33 (29–37) | 34 (31–37) | 0.20 |
| Impaired glucose tolerance, n (%) | 15 (4.6) | 12 (5.0) | 3 (3.3) | ||
| Hyperglycemia, n (%) | 17 (5.2) | 14 (5.9) | 3 (3.3) | ||
| No data, n (%) | 2 (0.6) | 2 (0.8) | 0 (0) | ||
| Platelet count | Median ×109/L (IQR) | 231 (195–277) | 237 (204–285) | 209 (168–255) | 0.001 |
| ALT | Median IU/L (IQR) | 18 (14–24) | 17 (13–22) | 21 (15–29) | <0.0001 |
| AST | Median IU/L (IQR) | 27 (23–34) | 26 (22–33) | 30 (25–37) | 0.001 |
| APRI score | Median (IQR) | 0.27 (0.20–0.36) | 0.25 (0.19–0.34) | 0.37 (0.25–0.47) | <0.0001 |
| FIB‐4 score | Median (IQR) | 1.35 (1.00–1.81) | 1.30 (0.97–1.69) | 1.58 (1.18–2.16) | <0.0001 |
| Liver stiffness | Median kPa (IQR) | 4.9 (4.0–6.0) | 4.9 (3.9–5.9) | 4.9 (4.2–6.4) | 0.18 |
| Fibrosis grade, n (%) | F0–F1 | 274 (83.3) | 217 (90.8) | 57 (63.3) | <0.0001 |
| F2–F4 | 55 (16.7) | 22 (9.3) | 33 (36.7) | ||
| F2 | 38 (11.6) | 17 (7.1) | 21 (23.3) | ||
| F3 | 12 (3.7) | 4 (1.7) | 8 (8.9) | ||
| F4 f | 5 (1.5) | 1 (0.4) | 4 (4.4) | ||
| CAP | Median dB/m (IQR) | 207 (175–240) | 206 (172–239) | 214 (181–242) | 0.33 |
| Steatosis grade, n (%) | S0 | 260 (79.0) | 189 (79.1) | 71 (78.9) | 0.97 |
| S1–S3 | 69 (21.0) | 50 (20.9) | 19 (21.1) | ||
| S1 | 26 (7.9) | 20 (8.4) | 6 (6.7) | ||
| S2 | 20 (6.1) | 14 (5.9) | 6 (6.7) | ||
| S3 | 23 (7.0) | 16 (6.7) | 7 (7.8) | ||
Abbreviations: ALT, alanine aminotransferase; APRI, aspartate aminotransferase to platelet ratio index; ART, antiretroviral treatment; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; FIB‐4, fibrosis‐4; HbA1c, glycated hemoglobin; HBsAg, hepatitis B virus surface antigen; HBV, hepatitis B virus; HDL, high‐density lipoprotein; HIV, human immunodeficiency virus; IQR, interquartile range; LDL, low‐density lipoprotein; NNRTI, non‐nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI/b, boosted.
Other NRTI combinations comprised one each of tenofovir + abacavir; tenofovir/lamivudine + abacavir; tenofovir + zidovudine; tenofovir + zidovudine/lamivudine; and abacavir/lamivudine.
Comprised 4/329 (1.2%) patients who were ART naive and 3/329 (0.9%) patients who had discontinued ART ≥3 months earlier.
Among patients with detectable HBV DNA, 11 were on tenofovir/lamivudine (median HBV DNA load, 344 IU/ml; range, 60–548,000,000), four were on lamivudine without tenofovir (median HBV DNA load, 7084 IU/ml; range, 72–4,080,000), and one was off ART (HBV DNA load, 123,200 IU/ml).
One female meeting two metabolic syndrome criteria (hypertension and low HDL) but lacking HbA1C results was classified in the group without metabolic syndrome.
Included eight patients with normal blood pressure while on antihypertensive medication.
Patients with liver stiffness values indicative of F4 fibrosis/cirrhosis did not show signs of clinical decompensation, and all underwent a liver ultrasound scan to exclude ascites and focal lesions. Liver steatosis by CAP value (dB/m): none (S0) <248; mild (S1) 248–268; moderate (S2) 268–280; severe (S3) >280. Liver fibrosis by stiffness value: HBsAg positive (kPa) none (F0/F1) <5.9; mild (F2) ≥5.9 to 7.5; moderate (F3) ≥7.6 to 9.3; severe (F4) ≥9.4; HBsAg negative (kPa): F0/F1 <7.1; F2 ≥7.1 to 9.3; F3 ≥9.4 to 13.9; F4 ≥14.
HIV status
Overall, 322/329 (97.9%) participants were receiving ART, predominantly with first‐line NNRTI‐based combinations (287/322, 89.1%) (Table 2). There was a history of stavudine treatment in 114/329 (34.7%) patients, which totaled a median of 2.3 years (IQR, 1.1–3.4) and had stopped a median of 8.2 years (IQR, 6.5–8.4) previously. CD4 cell counts were generally preserved, but only 162/322 (50.3%) of those on ART showed an HIV RNA <40 copies/ml; among those with quantifiable HIV RNA, the median load was 436 copies/ml (IQR, 92–25,700).
Viral hepatitis
Among 90 participants with HBsAg, 12/90 (13.3%) tested HBeAg positive (Table 2). Relative to patients who were HBsAg negative, the HBsAg‐positive group comprised a slightly higher proportion of men and was more likely to be receiving tenofovir‐based ART (75/90, 83.3%), but there was no difference in median HIV RNA load and CD4 cell counts. HBV DNA was quantified in 16/90 (17.8%) patients who were HBsAg positive, with a median load of 1244 IU/ml (IQR, 216–71,920). Among the 16 patients with quantifiable HBV DNA, 11 (68.8%) also had quantifiable HIV‐1 RNA. Based on 11 HBV DNA sequences, HBV genotypes were E (n = 10) and A4 (n = 1) (Table S1; Figures S1 and S2); 5/11 (45.5%) patients harbored mutations conferring HBV resistance to lamivudine, but there were no mutations known to confer HBV resistance to tenofovir (Table S1). HCV RNA (18,700,000 IU/ml) was detected in 1/329 patients (0.3%; 95% CI, 0.0%–1.7%); the patient was HBsAg negative. Anti‐HDV was detected in 4/90 patients who were HBsAg positive (4.4%; 95% CI, 1.2%–11.0%); two had detectable HDV RNA (7550 and 1660 copies/ml), one tested HDV RNA negative (<640 copies/ml), and one did not yield a polymerase chain reaction (PCR) amplicon.
Metabolic status
Metabolic syndrome was detected in 87/329 (26.4%) patients (Table 2). Overall, 179/329 (54.4%) showed grade ≥1 hypertension; 57/329 (17.3%) were on antihypertensive medication (nifedipine, losartan, amlodipine, bendroflumethiazide, lisinopril, methyldopa, or valsartan); 32/329 (9.7%) had HbA1c ≥42 mmol/mol; and 7/329 (2.1%) were on antidiabetic medications (metformin, glimepiride). Dyslipidemia was common, but only one patient (0.3%) was receiving lipid‐lowering therapy (atorvastatin). Median triglyceride levels did not differ with NNRTI‐based versus PI/b‐based ART (median, 1.2; IQR, 0.8–1.6 versus median, 1.2; IQR, 1.0–1.6 mmol/L; p = 0.49). Patients who were HBsAg positive had lower total cholesterol levels and lower prevalence of hypertriglyceridemia than patients who were HBsAg negative (Table 2). Overall, a small number of participants (16/329 [4.9%]) reported any alcohol consumption, and just 6/329 (1.8%) patients met the definition of drinking in excess. Of the latter, five of six patients had F0–F1 fibrosis whereas one (who was HBsAg positive) had F3 fibrosis. Reporting of cigarette smoking and use of herbal or traditional remedies was uncommon.
Liver health status
CAP and LS data are shown in Table 2. Values indicative of ≥S2 liver steatosis and ≥F2 liver fibrosis were measured in 43/329 (13.1%) and 55/329 (16.7%) patients, respectively. Median CAP values and prevalence of ≥S2 steatosis were similar in patients who were HBsAg positive versus those who were HBsAg negative. Median LS values were also similar; however, patients who were HBsAg positive had a higher prevalence of ≥F2 fibrosis (36.7% vs. 9.2%, p < 0.001), higher transaminase levels, higher prevalence of raised ALT levels (27.3% vs. 14.1%, p = 0.006), lower platelet counts, and higher median APRI and Fib‐4 scores. The distribution of steatosis and fibrosis among the 323 patients who did not report excessive alcohol consumption and according to HBsAg status is shown in Figure 1. The prevalence of concomitant ≥S2 liver steatosis and ≥F2 liver fibrosis was 8/88 (9.1%) versus 3/235 (1.3%) in individuals who were HBsAg positive versus HBsAg negative (p < 0.001). Transaminase levels did not correlate with CAP values (Figure S3), although prevalence of raised ALT levels was 12/43 (27.9%) among those with CAP values indicative of ≥S2 liver steatosis versus 45/279 (16.1%) in those with lower values (p = 0.06).
FIGURE 1.
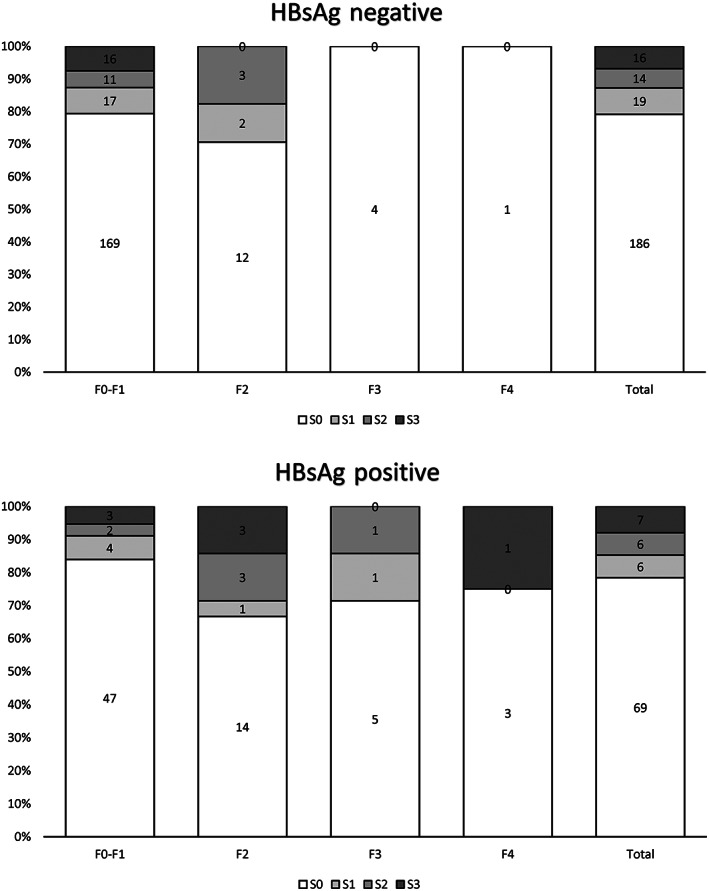
Difference in the prevalence of liver steatosis (based on controlled attenuation parameter values) according to liver fibrosis (based on liver stiffness values) and HBsAg status (n = 323, excluding six patients who reported excessive alcohol consumption). HBsAg, hepatitis B surface antigen.
Factors associated with liver steatosis
Factors associated with higher CAP values in the univariable analysis are shown in Table 3. In the first model, after adjustment for cumulative stavudine exposure, metabolic syndrome, and CD4 cell count, metabolic syndrome was independently associated with higher CAP values. A second model adjusted for cumulative stavudine exposure, CD4 cell count, LDL levels, and individual components of the metabolic syndrome (central obesity, hypertension, triglyceride, and HbA1c levels) and showed that CAP values were higher with longer stavudine exposure, central obesity, hypertension, and higher LDL and HbA1c levels (Table 3). With both models, a sensitivity analysis that adjusted for cumulative total ART exposure in place of cumulative stavudine exposure found no independent association with CAP values and no significant changes to other findings (not shown). In the second model, adjusting for BMI in place of central obesity found an independent association between BMI and CAP values (coefficient, +0.01 per 1 kg/m2 higher; 95% CI, +0.01 to +0.02; p < 0.001) without significant changes to other findings (not shown). Replacing LDL with HDL in the second model did not find an independent association of HDL with CAP values (data not shown).
TABLE 3.
Univariate and multivariable linear regression analysis of factors associated with controlled attenuation parameter values (loge dB/m)
| Characteristics | Univariable analysis | Multivariable analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p | Model 1 | Model 2 | ||||||
| Coefficient | 95% CI | p | Coefficient | 95% CI | p | |||||
| Sex | Female vs. male | +0.05 | −0.01 to +0.11 | 0.12 | ||||||
| Age | Per 5‐year older | +0.01 | −0.01 to +0.03 | 0.23 | ||||||
| Cigarette smoking | Yes vs. No | −0.04 | −0.15 to +0.08 | 0.54 | ||||||
| Excessive alcohol | Yes vs. No | +0.07 | −0.14 to +0.28 | 0.51 | ||||||
| Herbal or traditional remedies | Yes vs. No | +0.10 | −0.06 to +0.25 | 0.21 | ||||||
| Time on ART | Per 1 year longer | +0.01 | −0.00 to +0.01 | 0.09 | ||||||
| Stavudine exposure | Per 1 year longer | +0.02 | +0.00 to +0.04 | 0.017 | +0.01 | −0.00 to +0.03 | 0.11 | +0.02 | +0.00 to +0.04 | 0.044 |
| Zidovudine exposure | Per 1 year longer | +0.00 | −0.01 to +0.01 | 0.92 | ||||||
| Tenofovir DF exposure | Per 1 year longer | +0.00 | −0.01 to +0.01 | 0.68 | ||||||
| Efavirenz exposure | Per 1 year longer | +0.00 | −0.00 to +0.01 | 0.54 | ||||||
| Nevirapine exposure | Per 1 year longer | +0.00 | −0.00 to +0.01 | 0.40 | ||||||
| Lopinavir/ritonavir exposure | Per 1 year longer | −0.01 | −0.03 to +0.01 | 0.34 | ||||||
| HIV‐1 RNA load a | Per 1 log10 copies/ml higher | −0.01 | −0.03 to +0.01 | 0.23 | ||||||
| CD4 count | Per 100 cells/mm3 higher | +0.01 | −0.00 to +0.02 | 0.06 | +0.01 | −0.00 to +0.01 | 0.15 | 0.00 | −0.01 to +0.01 | 0.47 |
| HBsAg | Positive vs. negative | +0.02 | −0.04 to +0.09 | 0.44 | ||||||
| HBV DNA (n = 90) | Per 1 log10 IU/ml higher | −0.00 | −0.04 to +0.03 | 0.90 | ||||||
| Metabolic syndrome | Yes vs. no | +0.16 | +0.10 to +0.22 | <0.001 | +0.15 | +0.08 to +0.21 | <0.001 | |||
| BMI | Per 1 kg/m2 higher | +0.02 | +0.01 to +0.02 | <0.001 | ||||||
| Central obesity | Yes vs. no | +0.13 | +0.07 to +0.18 | <0.001 | +0.09 | +0.03 to +0.15 | 0.002 | |||
| Hypertension | Per grade higher | +0.06 | +0.03 to +0.09 | <0.001 | +0.03 | +0.00 to +0.06 | 0.05 | |||
| Total cholesterol | Per 1 mmol/L higher | +0.05 | +0.02 to +0.07 | <0.001 | ||||||
| LDL | Per 1 mmol/L higher | +0.05 | +0.02 to +0.08 | 0.001 | +0.03 | +0.00 to +0.06 | 0.036 | |||
| HDL | Per 1 mmol/L higher | −0.04 | −0.11 to +0.03 | 0.26 | ||||||
| Triglycerides | Per 1 mmol/L higher | +0.06 | +0.02 to +0.09 | 0.001 | +0.02 | −0.02 to +0.07 | 0.30 | |||
| HbA1c | Per 1 mmol/mol higher | +0.01 | +0.00 to +0.01 | <0.001 | +0.01 | +0.00 to +0.01 | 0.005 | |||
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ART, antiretroviral treatment; BMI, body mass index; CI, confidence interval; DF, disoproxil fumarate; HBsAg, hepatitis B surface antigen; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Excludes four patients who were ART naive and one off ART. Model 1 adjusted for cumulative stavudine exposure, metabolic syndrome, and CD4 cell count. Model 2 adjusted for cumulative stavudine exposure, CD4 cell count, LDL levels, and individual components of the metabolic syndrome (central obesity, hypertension, triglyceride, and HbA1c levels). Excessive alcohol use was defined as drinking more than once a week in moderate to large quantities.
Factors associated with liver fibrosis
Factors associated with higher LS values in the univariable analysis are shown in Table 4. After adjustment for sex, HIV RNA load, and CAP values, higher LS values were independently associated with male sex, higher HIV RNA load, and higher CAP values. A sensitivity analysis was adjusted for central obesity in place of CAP values and found no independent association with LS values and no significant changes to other findings (data not shown). In the HBsAg‐positive population, LS values were associated with cumulative exposure to tenofovir, HIV RNA load, LDL levels, and CAP values in the univariable analysis (Table 5). After adjustment for cumulative tenofovir exposure, HIV RNA load, and CAP values, higher HIV RNA load was independently associated with higher LS values whereas cumulative tenofovir exposure and CAP values retained a marginal association (Table 5). A sensitivity analysis was adjusted for LDL levels in place of CAP values and found no independent association with LS values and no significant changes to other findings (not shown). Sensitivity analyses where the outcome variable LS was replaced with the APRI and Fib‐4 scores confirmed the independent association with higher HIV RNA and higher APRI and Fib‐4 scores. No association was found between CAP values and APRI and FIB‐4 scores (Tables S2 and S3).
TABLE 4.
Univariate and multivariable linear regression analysis of factors associated with liver stiffness values (loge kPa)
| Characteristics | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p | Coefficient | 95% CI | p | ||
| Sex | Female vs. male | −0.15 | −0.22 to −0.07 | <0.001 | −0.15 | −0.22 to −0.07 | <0.001 |
| Age | Per 5‐year older | +0.01 | −0.01 to +0.03 | 0.26 | |||
| Cigarette smoking | Yes vs. no | +0.11 | −0.03 to +0.25 | 0.11 | |||
| Herbal or traditional remedies | Yes vs. no | +0.13 | −0.06 to +0.31 | 0.18 | |||
| Excess alcohol | Yes vs. no | +0.06 | −0.19 to +0.31 | 0.63 | |||
| Time on ART | Per 1 year longer | +0.00 | −0.01 to +0.01 | 0.91 | |||
| Stavudine exposure | Per 1 year longer | −0.01 | −0.03 to +0.02 | 0.63 | |||
| Zidovudine exposure | Per 1 year longer | +0.00 | −0.01 to +0.01 | 0.88 | |||
| Tenofovir DF exposure all | Per 1 year longer | −0.01 | −0.02 to +0.00 | 0.18 | |||
| Efavirenz exposure | Per 1 year longer | −0.00 | −0.01 to +0.01 | 0.57 | |||
| Nevirapine exposure | Per 1 year longer | +0.00 | −0.01 to +0.01 | 0.93 | |||
| Lopinavir/ritonavir exposure | Per 1 year longer | −0.00 | −0.03 to +0.02 | 0.69 | |||
| HIV‐1 RNA load | Per 1 log10 copies/ml higher | +0.03 | +0.01 to +0.05 | 0.013 | +0.03 | +0.00 to +0.05 | 0.019 |
| CD4 count | Per 1 cells/mm3 higher | −0.01 | −0.02 to −0.00 | 0.024 | |||
| HBsAg | Positive vs. negative | +0.06 | −0.01 to +0.14 | 0.11 | |||
| HBV DNA (n = 90) | Per 1 log10 higher | +0.02 | −0.03 to +0.06 | 0.53 | |||
| Metabolic syndrome | Yes vs. no | +0.01 | −0.07 to +0.09 | 0.83 | |||
| BMI | Per 1 kg/m2 higher | −0.00 | −0.01 to +0.01 | 0.90 | |||
| Central obesity | Yes vs. No | −0.09 | −0.16 to −0.02 | 0.010 | |||
| Hypertension | Per grade higher | +0.03 | −0.01 to +0.06 | 0.14 | |||
| Total cholesterol | Per 1 mmol/L higher | −0.01 | −0.04 to +0.02 | 0.34 | |||
| LDL | Per 1 mmol/L higher | −0.03 | −0.07 to +0.01 | 0.11 | |||
| HDL | Per 1 mmol/L higher | −0.02 | −0.11 to +0.06 | 0.59 | |||
| Triglycerides | Per 1 mmol/L higher | +0.02 | −0.02 to +0.06 | 0.33 | |||
| HbA1c | Per 1 mmol/mol higher | +0.00 | −0.00 to +0.01 | 0.32 | |||
| CAP values | Per 10 db/m higher | +0.01 | −0.00 to +0.12 | 0.09 | +0.01 | +0.00 to +0.02 | 0.020 |
Abbreviations: ALT, alanine aminotransferase; ART, antiretroviral treatment; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; CI, confidence interval; DF, disoproxil fumarate; HBsAg, hepatitis B surface antigen; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
TABLE 5.
Univariable and multivariable linear regression analysis of factors associated with liver stiffness (loge kPa) in the population with HBsAg
| Characteristics | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p | Coefficient | 95% CI | p | ||
| Sex | Female vs. male | −0.10 | −0.26 to +0.05 | 0.19 | |||
| Age | Per 5‐year older | −0.01 | −0.06 to +0.04 | 0.60 | |||
| Cigarette smoking | Yes vs. no | +0.24 | −0.05 to +0.53 | 0.11 | |||
| Herbal or traditional remedies | Yes vs. no | +0.22 | −0.14 to +0.57 | 0.23 | |||
| Excess alcohol | Yes vs. no | +0.12 | −0.38 to +0.62 | 0.62 | |||
| Time on ART | Per 1 year longer | −0.00 | −0.02 to +0.02 | 0.94 | |||
| Stavudine exposure | Per 1 year longer | +0.01 | −0.04 to +0.06 | 0.69 | |||
| Zidovudine exposure | Per 1 year longer | +0.00 | −0.02 to +0.03 | 0.87 | |||
| Tenofovir DF exposure | Per 1 year longer | −0.03 | −0.06 to −0.00 | 0.031 | −0.02 | −0.05 to +0.00 | 0.08 |
| Efavirenz exposure | Per 1 year longer | −0.00 | −0.02 to +0.01 | 0.70 | |||
| Nevirapine exposure | Per 1 year longer | −0.00 | −0.02 to +0.02 | 0.97 | |||
| Lopinavir/ritonavir exposure | Per 1 year longer | −0.04 | −0.09 to +0.02 | 0.16 | |||
| HIV‐1 RNA load | Per 1 log10 copies/ml higher | +0.06 | +0.01 to +0.11 | 0.012 | +0.06 | +0.02 to +0.11 | 0.008 |
| CD4 count | Per 1 cells/mm3 higher | −0.02 | −0.04 to +0.00 | 0.11 | |||
| HBV DNA | Per 1 log10 higher | +0.02 | −0.03 to +0.06 | 0.53 | |||
| Metabolic syndrome | Yes vs. no | +0.05 | −0.13 to +0.23 | 0.58 | |||
| BMI | Per 1 kg/m2 higher | +0.01 | −0.01 to +0.02 | 0.35 | |||
| Central obesity | Yes vs. no | −0.08 | −0.22 to +0.07 | 0.31 | |||
| Hypertension | Per grade higher | +0.07 | −0.02 to +0.15 | 0.12 | |||
| Total cholesterol | Per 1 mmol/L higher | −0.05 | −0.14 to +0.04 | 0.29 | |||
| LDL | Per 1 mmol/L higher | −0.10 | −0.20 to +0.01 | 0.07 | |||
| HDL | Per 1 mmol/L higher | +0.03 | −0.16 to +0.22 | 0.73 | |||
| Triglycerides | Per 1 mmol/L higher | +0.07 | −0.07 to +0.21 | 0.32 | |||
| HbA1c | Per 1 mmol/mol higher | −0.00 | −0.02 to +0.02 | 0.92 | |||
| CAP values | Per 10 db/m higher | +0.01 | −0.00 to +0.03 | 0.07 | +0.01 | 0.00 to +0.03 | 0.06 |
Abbreviations: ALT, alanine aminotransferase; ART, antiretroviral treatment; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; CI, confidence interval; DF, disoproxil fumarate; HBsAg, hepatitis B surface antigen; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Exclusion of the two pregnant women from the analysis of factors associated with liver steatosis and liver fibrosis did not alter the findings (data not shown).
DISCUSSION
This cross‐sectional study assessed liver steatosis and liver fibrosis, as indicated by CAP and LS values, respectively, in unselected (albeit enriched for HBsAg‐positive status) consecutive adults with HIV who were receiving long‐term ART in Ghana. The prevalence of at least mild liver steatosis (≥S1) was 21.0% and lower than the prevalence reported from cohorts living with HIV in North America, Western Europe, China, Japan and Brazil (32%–48%).[ 1 ] Published data from African populations with HIV are difficult to compile due to heterogeneous patient selection and variable methods of diagnosis, with estimates of 28% in Cameroon, 19%–21% in South Africa, 19% in Togo, and 13% in Nigeria.[ 9 , 10 , 11 , 12 ] In our study, 13.1% had CAP values consistent with ≥S2 steatosis and 16.7% had LS values consistent with ≥F2 fibrosis; we found concomitant ≥S2 steatosis and ≥F2 fibrosis in 3.3% overall but more commonly in people with HBV coinfection (9.1%) than in those without (1.3%).
In our study, higher CAP values were independently associated with a diagnosis of metabolic syndrome, which affected 26.4% of participants. The prevalence of metabolic syndrome was in agreement with findings from a systematic review of 18 African studies, including 11 from West Africa, which showed a pooled prevalence of 21% in populations living with HIV.[ 30 ] Considering the individual components of the metabolic syndrome,[ 25 ] 60% of participants had central obesity, although only a small subset were obese based on BMI. More than half had grade ≥1 hypertension, which is consistent with previous data from the same cohort[ 31 ] and from a global meta‐analysis of people with HIV.[ 32 ] Of note, hypertension was largely undiagnosed, and only a subset was receiving antihypertensive drugs. Dyslipidemia was also highly prevalent and largely undiagnosed and untreated. Impaired glucose tolerance and diabetes were less common and were diagnosed and treated in a subset. Central obesity (or a higher BMI), hypertension, and raised HbA1c levels as well as LDL levels were each independently predictive of higher CAP values. However, there was no apparent independent effect of two other components of the metabolic syndrome definition—HDL and triglycerides levels. African and European studies show interesting differences. In Africa studies, HDL and total cholesterol levels are lower, there is an attenuated relationship between HDL levels and adiposity, and low HDL levels are associated with lower non‐HDL cholesterol and are more common among women.[ 33 ] Further studies are needed to confirm these differences and develop a definition of metabolic syndrome that is most relevant for African populations.
Of note, we detected no association between CAP values, age, sex, and alcohol consumption; however, our cohort comprised predominantly women, had a narrow distribution of ages, and only a few patients reported excessive alcohol consumption. While there was also no independent association between CAP values and HIV‐related parameters, including CD4 cell count and HIV RNA load, one interesting observation was the small but significant effect of cumulative stavudine exposure on CAP values, despite the exposure dating back several years. Stavudine is a major cause of mitochondrial toxicity, with hyperproduction of lactates and defective fatty acid oxidation, leading to the onset of microvesicular and macrovesicular steatosis.[ 34 ] Limited data support reversibility of the mitochondrial effects of stavudine following discontinuation.[ 35 ]
HBV coinfection is highly prevalent in the Kumasi cohort.[ 16 ] We detected no association between CAP values and HBsAg status or HBV DNA load among patients who were HBsAg positive, and this is in line with findings showing that HBV infection does not increase the risk of liver steatosis in HIV‐negative populations.[ 36 ] In fact, in Asian cohorts, HBV infection was associated with significantly lower grades of liver steatosis (but higher risk of fibrosis) after adjusting for age, sex, and metabolic parameters.[ 37 , 38 ] Nonetheless, liver biopsy studies have documented coexisting steatosis in nearly a third of adults with chronic HBV infection in North America and found it to increase the risk of ALT elevation and liver fibrosis.[ 39 , 40 ] Prevalence of concomitant ≥S2 liver steatosis and ≥F2 liver fibrosis was 9.1% among people with HBV in our study and significantly higher than in individuals negative for HBsAg, highlighting the additional risk posed by liver steatosis in this population.
Consistent with the promoting effects of steatosis on liver fibrosis, the linear regression analysis in our cohort found that higher CAP values independently predicted higher LS values. Male sex and a higher HIV RNA load were also independently associated with higher LS values. Furthermore, LS values consistent with ≥F2 fibrosis were more prevalent in patients who were HBsAg positive. An independent association between liver fibrosis and male sex has been observed in people with HIV in Zambia after adjusting for HBsAg status, age, and HIV RNA load.[ 41 ] A potential protective effect of female hormones against hepatic fibrogenesis has been proposed.[ 42 ] One key observation, however, was that HIV suppression was suboptimal in our cohort; viremia was detected in over half of participants, which reflects poor access to virologic monitoring, limited treatment options, and high rates of drug resistance, as described in other research.[ 43 ] HIV can infect Kupffer cells and activate hepatic stellate cells, promoting release of proinflammatory cytokines, apoptosis of hepatocytes, and synthesis of collagen in the hepatic tissue.[ 44 ] Conversely, starting ART has been shown to reduce indices of liver inflammation.[ 41 ]
Reassuringly, most patients who were HBsAg positive were established on tenofovir, and there was no evidence of HBV resistance to tenofovir among those with detectable HBV DNA. We have previously reported on the early beneficial impact of tenofovir on HBV DNA suppression and LS values in this cohort.[ 13 ] Here, after a median of 6.1 years of tenofovir, we observed a marginal relationship between cumulative tenofovir exposure and lower LS values among people with HBV. We explored the relationship between ALT levels and CAP values but found a poor overall correlation. However, ALT levels were more often raised among those with CAP values indicative of ≥S2 liver steatosis, with about one in four patients showing levels above the reference upper limit of normal (men ≥35 IU/L; women ≥25 IU/L). ALT levels were also poorly correlated with LS values but were higher in people with HBV. As HIV infection may modulate these parameters, whether transaminase levels provide a reliable biomarker of hepatitis in this population remains to be determined.
There are limitations to this study. We did not obtain liver biopsies to corroborate the diagnosis of liver steatosis or liver fibrosis; estimating CAP along with LS with portable FibroScan equipment makes such assessments possible in settings where there is limited access to liver biopsy and histopathology examinations.[ 14 ] The study design was cross‐sectional, and prospective studies are needed in Kumasi and other African cohorts to measure evolution of liver disease and identify individuals who would benefit from interventions to reduce progression of fibrosis and steatosis. There was a predominance of women in our cohort, which reflects the lower levels of diagnosis and engagement with care among men living with HIV in the region.[ 45 ] Compared to women living with HIV, there are more men living with HIV who do not know their HIV status, more men who know their status but are not on treatment, and more men who are not virally suppressed. While it can be proposed that multiple factors would interact to increase prevalence of liver disease in the male population, more data are needed to confirm how sex modulates the risk of liver fibrosis. Prospective studies are also needed to confirm the prevalence of hypertension in the cohort. Meanwhile, we identify coexistence of poor HIV control with highly prevalent liver steatosis and HBV coinfection as alarming indicators of a significant risk of progressive liver disease and hepatocellular carcinoma. Key interventions include (i) optimized HIV and HBV virologic control to reduce the promoting effect of uncontrolled virus replication on liver fibrosis, and (ii) identification and management of the key components of the metabolic syndrome, including central obesity, hypertension, dyslipidemia, and impaired glucose tolerance, to reduce the contributing role of hepatitis steatosis in people with and without HBV coinfection.
AUTHOR CONTRIBUTIONS
Anna Maria Geretti designed the study; Giovanni Villa, Dorcas Owusu, Marilyn Azumah, David Chadwick, Richard Odame Phillips, and Anna Maria Geretti were involved in the study management; Giovanni Villa, Dorcas Owusu, Marilyn Azumah, Adam Abdullahi, Suzannah Phillips, Laila Sayeed, Harrison Austin, David Chadwick, Richard Odame Phillips, and Anna Maria Geretti collected the data; Giovanni Villa, Adam Abdullahi, Richard Odame Phillips, and Anna Maria Geretti analyzed the data; Colette Smith performed the statistical analysis; Giovanni Villa, Richard Odame Phillips, and Anna Maria Geretti interpreted the data; Giovanni Villa and Anna Maria Geretti wrote the manuscript, with the critical input of all coauthors. All authors had full access to all the data in the study and accept responsibility to submit for publication.
CONFLICT OF INTEREST
Giovanni Villa has received a research grant from ViiV Healthcare, outside the submitted work. Dorcas Owusu has received research grants from Gilead Sciences, outside the submitted work. Anna Maria Geretti has received research grants, consulting fees, and personal fees from Gilead Sciences and Roche Pharma Research and Early Discovery, outside the submitted work. The other authors have nothing to disclose.
Supporting information
Data S1
Data S2
ACKNOWLEDGMENTS
We gratefully acknowledge the support received by the KATH personnel that made this research possible, namely interpreters, doctors, nurses, laboratory technicians, and administrative staff. Above all, we are very thankful to the patients who participated in the study.
Villa G, Owusu D, Smith C, Azumah M, Abdullahi A, Phillips S, Liver steatosis and fibrosis in people with human immunodeficiency virus in West Africa and the relationship with hepatitis B virus coinfection. Hepatol Commun. 2022;6:3036–3051. 10.1002/hep4.2000
REFERENCES
- 1. Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV‐monoinfection. AIDS. 2017;31:1621–32. [DOI] [PubMed] [Google Scholar]
- 2. Wood S, Won SH, Hsieh H‐C, Lalani T, Kronmann K, Maves RC, et al. Risk factors associated with chronic liver enzyme elevation in persons with HIV without hepatitis B or C coinfection in the combination antiretroviral therapy era. Open Forum Infect Dis. 2021;8:ofab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oikonomou KG, Tsai E, Sarpel D, Dieterich DT. Liver disease in human immunodeficiency virus infection. Clin Liver Dis. 2019;23:309–29. [DOI] [PubMed] [Google Scholar]
- 4. Squillace N, Soria A, Bozzi G, Gori A, Bandera A. Nonalcoholic fatty liver disease and steatohepatitis in people living with HIV. Expert Rev Gastroenterol Hepatol. 2019;13:643–50. [DOI] [PubMed] [Google Scholar]
- 5. Perazzo H, Cardoso SW, Yanavich C, Nunes EP, Morata M, Gorni N, et al. Predictive factors associated with liver fibrosis and steatosis by transient elastography in patients with HIV mono‐infection under long‐term combined antiretroviral therapy. J Int AIDS Soc 2018;21:e25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spearman CW, Abdo A, Ambali A, Awuku YA, Kassianides C, Lesi OA, et al.; Gastroenterology and Hepatology Association of sub‐Saharan Africa (GHASSA) . Health‐care provision and policy for non‐alcoholic fatty liver disease in sub‐Saharan Africa. Lancet Gastroenterol Hepatol. 2021;6:1047–56. [DOI] [PubMed] [Google Scholar]
- 7. Spearman CW, Afihene M, Betiku O, Bobat B, Cunha L, Kassianides C, et al.; Gastroenterology and Hepatology Association of sub‐Saharan Africa (GHASSA) . Epidemiology, risk factors, social determinants of health, and current management for non‐alcoholic fatty liver disease in sub‐Saharan Africa. Lancet Gastroenterol Hepatol. 2021;6:1036–46. [DOI] [PubMed] [Google Scholar]
- 8. Sonderup MW, Wainwright H, Hall P, Hairwadzi H, Spearman CW. A clinicopathological cohort study of liver pathology in 301 patients with human immunodeficiency virus/acquired immune deficiency syndrome. Hepatology. 2015;61:1721–9. [DOI] [PubMed] [Google Scholar]
- 9. Hoffmann CJ, Hoffmann JD, Kensler C, van der Watt M, Omar T, Chaisson RE, et al. Tuberculosis and hepatic steatosis are prevalent liver pathology findings among HIV‐infected patients in South Africa. PLoS ONE. 2015;10:e0117813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lesi OA, Soyebi KS, Eboh CN. Fatty liver and hyperlipidemia in a cohort of HIV‐positive Africans on highly active antiretroviral therapy. J Natl Med Assoc. 2009;101:151–5. [DOI] [PubMed] [Google Scholar]
- 11. Ongolo‐Zogo P, Nkodo Mbia N, Mvogo Minkala TL, Biwole Sida M, Kouanfack C, Nko Amvene S. Lipodystrophy and echographic hepatic steatosis in HIV‐positive patients under highly active antiretroviral therapy (HAART) in Yaounde (Cameroon). [in French] Bull Soc Pathol Exot. 2012;105:353–60. [DOI] [PubMed] [Google Scholar]
- 12. Sonhaye L, Tchaou M, Amadou A, Gbande P, Assih K, Djibril M, et al. Abdominal ultrasound abnormalities incidentally discovered in patients with asymptomatic HIV in Lome (Togo). [in French] Med Sante Trop. 2014;24:279–82. [DOI] [PubMed] [Google Scholar]
- 13. Stockdale AJ, Phillips RO, Beloukas A, Appiah LT, Chadwick D, Bhagani S, et al.; Hepatitis B Infection in Kumasi (HEPIK) Study Group . Liver fibrosis by transient elastography and virologic outcomes after introduction of tenofovir in lamivudine‐experienced adults with HIV and hepatitis B virus coinfection in Ghana. Clin Infect Dis. 2015;61:883–91. [DOI] [PubMed] [Google Scholar]
- 14. Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with non‐alcoholic fatty liver disease. Gastroenterology. 2019;156:1717–30. [DOI] [PubMed] [Google Scholar]
- 15. Lemoine M, Assoumou L, De Wit S, Girard P‐M, Valantin MA, Katlama C, et al. Diagnostic accuracy of noninvasive markers of steatosis, NASH and liver fibrosis in HIV‐monoinfected individuals at‐risk of non‐alcoholic fatty liver disease (NAFLD): results from the ECHAM study. J Acquir Immune Defic Syndr. 2019;80:e86–94. [DOI] [PubMed] [Google Scholar]
- 16. Geretti AM, Patel M, Sarfo FS, Chadwick D, Verheyen J, Fraune M, et al. Detection of highly prevalent hepatitis B virus coinfection among HIV‐seropositive persons in Ghana. J Clin Microbiol. 2010;48:3223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization . Waist circumference and waist‐hip ratio: report of a WHO expert consultation; 2008. Geneva, Switzerland: World Health Organization. [cited 2022 June 3]. Available from: https://apps.who.int/iris/handle/10665/44583 [Google Scholar]
- 18. Karlas T, Petroff D, Sasso M, Fan J‐G, Mi Y‐Q, de Lédinghen V, et al. Individual patient data meta‐analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–30. [DOI] [PubMed] [Google Scholar]
- 19. Matthews GV, Neuhaus J, Bhagani S, Mehta SH, Vlahakis E, Doroana M, et al.; International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) START Study Group . Baseline prevalence and predictors of liver fibrosis among HIV‐positive individuals: a substudy of the INSIGHT strategic timing of AntiRetroviral treatment (START) trial. HIV Med. 2015;16(Suppl 1):129–36. [DOI] [PubMed] [Google Scholar]
- 20. Miailhes P, Pradat P, Chevallier M, Lacombe K, Bailly F, Cotte L, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV‐coinfected patients. J Viral Hepat. 2011;18:61–9. [DOI] [PubMed] [Google Scholar]
- 21. Shang D, Hughes SA, Horner M, Bruce MJ, Dong Y, Carey I, et al. Development and validation of an efficient in‐house real‐time reverse transcription polymerase chain reaction assay for the quantitative detection of serum hepatitis delta virus RNA in a diverse South London population. J Virol Methods. 2012;184:55–62. [DOI] [PubMed] [Google Scholar]
- 22. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. [DOI] [PubMed] [Google Scholar]
- 23. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al.; APRICOT Clinical Investigators . Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 24. International Diabetes Federation . The IDF consensus worldwide definition of the metabolic syndrome; 2006. Brussels, Belgium: The International Diabetes Federation. [cited 2022 June 3]. Available from: https://www.idf.org/e‐library/consensus‐statements/60‐idfconsensus‐worldwide‐definitionof‐the‐metabolic‐syndrome.html#:~:text=IDF%20Consensus%20Worldwide%20Definition%20of%20the%20Metabolic%20Syndrome&text=The%20metabolic%20syndrome%20is%20a,cholesterol%20and%20high%20blood%20pressure. [Google Scholar]
- 25. Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al.; International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- 26. National Institute of Allergy and Infectious Diseases; Division of AIDS, Regulatory Support Center . Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, corrected version 2.1; 2017. Bethesda, MD, USA: National Institute of Allergy and Infectious Diseases; Division of AIDS, Regulatory Support Center. [cited 2022 June 3]. Available from: https://rsc.niaid.nih.gov/clinical‐research‐sites/grading‐severity‐adult‐pediatric‐adverse‐events‐corrected‐version‐two‐one [Google Scholar]
- 27. Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, et al.; Endocrine Society . Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969–89. Erratum in: J Clin Endocrinol Metab. 2015;100:4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization . Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation; 2011. Geneva, Switzerland: World Health Organization. [cited 2022 June 03]. Available from: https://apps.who.int/iris/bitstream/handle/10665/70523/WHO_NMH_CHP_CPM_11.1_eng.pdf [PubMed] [Google Scholar]
- 29. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Todowede OO, Mianda SZ, Sartorius B. Prevalence of metabolic syndrome among HIV‐positive and HIV‐negative populations in sub‐Saharan Africa—a systematic review and meta‐analysis. Syst Rev. 2019;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sarfo FS, Nichols M, Singh A, Hardy Y, Norman B, Mensah G, et al. Characteristics of hypertension among people living with HIV in Ghana: impact of new hypertension guideline. J Clin Hypertens (Greenwich). 2019;21:838–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta‐analysis. J Am Soc Hypertens. 2017;11:530–40. [DOI] [PubMed] [Google Scholar]
- 33. Greiner R, Nyirenda M, Rodgers L, Asiki G, Banda L, Shields B, et al. Associations between low HDL, sex and cardiovascular risk markers are substantially different in sub‐Saharan Africa and the UK: analysis of four population studies. BMJ Glob Health. 2021;6:e005222. Erratum in: BMJ Glob Health. 2021;6:e005222corr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gervasoni C, Cattaneo D, Filice C, Galli M, Gruppo Italiano Studio‐NASH in Malattie Infettive . Drug‐induced liver steatosis in patients with HIV infection. Pharmacol Res. 2019;145:104267. [DOI] [PubMed] [Google Scholar]
- 35. McComsey G, Lonergan JT. Mitochondrial dysfunction: patient monitoring and toxicity management. J Acquir Immune Defic Syndr. 2004;37:S30–5. [DOI] [PubMed] [Google Scholar]
- 36. Machado MV, Oliveira AG, Cortez‐Pinto H. Hepatic steatosis in hepatitis B virus infected patients: meta‐analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol. 2011;26:1361–7. [DOI] [PubMed] [Google Scholar]
- 37. Wang MF, Wan B, Wu YL, Huang JF, Zhu YY, Li YB. Clinic‐pathological features of metabolic associated fatty liver disease with hepatitis B virus infection. World J Gastroenterol. 2021;27:336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng YL, Wang YJ, Kao WY, Chen PH, Huo TI, Huang YH, et al. Inverse association between hepatitis B virus infection and fatty liver disease: a large‐scale study in populations seeking for check‐up. PLoS ONE. 2013;8:e72049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khalili M, Kleiner DE, King WC, Sterling RK, Ghany MG, Chung RT, et al.; Hepatitis B Research Network (HBRN) . Hepatic steatosis and steatohepatitis in a large north American cohort of adults with chronic hepatitis B. Am J Gastroenterol. 2021;116:1686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khalili M, King WC, Kleiner DE, Jain MK, Chung RT, Sulkowski M, et al. Fatty liver disease in a prospective North American cohort of adults with human immunodeficiency virus and hepatitis B virus coinfection. Clin Infect Dis. 2021;73:e3275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vinikoor MJ, Sinkala E, Chilengi R, Mulenga LB, Chi BH, Zyambo Z, et al.; IeDEA‐ Southern Africa . Impact of antiretroviral therapy on liver fibrosis among human immunodeficiency virus‐infected adults with and without HBV coinfection in Zambia. Clin Infect Dis. 2017;64:1343–9. Erratum in: Clin Infect Dis. 2017;65:1431–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:1406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Villa G, Abdullahi A, Owusu D, Smith C, Azumah M, Sayeed L, et al. Determining virological suppression and resuppression by point‐of‐care viral load testing in a HIV care setting in sub‐Saharan Africa. EClinicalMedicine. 2020;18:100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chamroonkul N, Bansal MB. HIV and the liver. Nat Rev Gastroenterol Hepatol. 2019;16:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. UNAIDS Joint United Nations Programme on HIV/AIDS . UNAIDS data; 2021. Geneva, Switzerland: The Joint United Nations Programme on HIV/AIDS. [cited 2022 June 3]. Available from: https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data S2


