Abstract
The present study aimed to investigate (1) the association between left ventricular diastolic dysfunction (LVDD), graded according to the algorithm proposed by the Cirrhotic Cardiomyopathy Consortium, and long‐term survival in patients with cirrhosis undergoing transjugular intrahepatic portosystemic shunt (TIPS) and (2) the additive prognostic value of left atrial (LA) function, as assessed by LA reservoir strain, using two‐dimensional speckle‐tracking echocardiography (2D‐STE). A total of 129 TIPS candidates (mean ± SD, 61 ± 12 years; 61% men) underwent a comprehensive preprocedural echocardiography. LA dysfunction was defined by LA reservoir strain ≤35%, based on a previously suggested cut‐off value. The outcome was all‐cause mortality after TIPS. In the current cohort, 65 (50%) patients had normal diastolic function, 26 (20%) patients had grade 1 LVDD, 21 (16%) patients had grade 2 LVDD, and 17 (13%) patients had indeterminate diastolic function. Additionally, LA dysfunction (based on LA reservoir strain ≤35%) was noted in 67 (52%) patients. After a median follow‐up of 36 months (range, 12–80), 65 (50%) patients died. All‐cause mortality rates increased along worse grades of LVDD (log‐rank p = 0.007) and with LA dysfunction (log‐rank p = 0.001). On multivariable Cox regression analysis, Model for End‐Stage Liver Disease score (hazard ratio [HR],1.06; p = 0.003), hemoglobin (HR, 0.74; p = 0.022), and LA strain, expressed as a continuous variable (HR, 0.96; p = 0.005) were independently associated with all‐cause mortality. Notably, the addition of LA strain to the model provided incremental prognostic value over the established prognostic variables (delta χ 2 = 8.27, p = 0.004). Conclusion: LA dysfunction assessed with 2D‐STE is independently associated with all‐cause mortality in patients with cirrhosis treated by TIPS.
Left ventricular diastolic dysfunction (LVDD), graded according to the algorithm proposed by the Cirrhotic Cardiomyopathy Consortium, and left atrial (LA) reservoir strain are independently associated with long‐term survival in patients with cirrhosis treated by TIPS. The assessment of LA reservoir strain provides incremental prognostic value over the severity of liver disease, assessed by the MELD score, and grading of LVDD using conventional parameters.
![]()
INTRODUCTION
Transjugular intrahepatic portosystemic shunt (TIPS) is widely used to treat portal hypertension‐related complications in patients with cirrhosis.[ 1 ] TIPS placement shifts a large amount of blood from the portal to the systemic circulation, resulting in a sudden increase in cardiac preload and output. The failure of cardiovascular adaptation to these abrupt hemodynamic changes may unmask a subclinical myocardial dysfunction, eventually leading to poor outcomes, including cardiac failure and death.[ 2 , 3 , 4 ] End‐stage liver disease is frequently associated with a specific type of myocardial dysfunction, referred to as cirrhotic cardiomyopathy (CCM).[ 5 ] Left ventricular diastolic dysfunction (LVDD) is the most pronounced feature of CCM and is reported in approximately one third of the patients with cirrhosis.[ 6 ] Of importance, LVDD has been related to several adverse outcomes in patients with end‐stage liver disease, including hepatorenal syndrome, decompensated cirrhosis, and increased morbidity or mortality following transplantation.[ 7 , 8 , 9 ] The presence of LVDD has also been associated with an increased risk of cardiac failure shortly after TIPS placement.[ 3 ] Nevertheless, the impact of LVDD on long‐term survival in patients with cirrhosis undergoing TIPS is unclear, with conflicting results being reported,[ 10 , 11 , 12 ] in the absence of a comprehensive and multiparametric assessment of left ventricular (LV) diastolic function.
The Cirrhotic Cardiomyopathy Consortium[ 13 ] has recently proposed a standardized algorithm for the assessment of LV diastolic function in patients with end‐stage liver disease that incorporates multiple echocardiographic parameters, including the mitral inflow pattern derived from pulsed‐wave Doppler imaging, annular velocities assessed by tissue Doppler imaging (TDI), left atrial (LA) enlargement, and peak velocity of the tricuspid regurgitant jet (TR velocity) evaluated by routine echocardiography. In addition, the authors suggested the integration of myocardial strain imaging[ 13 ] as an additional tool to reclassify patients for whom the algorithm fails to categorize LV diastolic function. Strain imaging, assessed by two‐dimensional speckle tracking‐echocardiography (2D‐STE), is a novel echocardiographic technique that provides objective quantification of cardiac function based on the analysis of myocardial deformation. Particularly, LV global longitudinal strain (LV GLS) enables accurate evaluation of LV systolic function,[ 14 ] whereas LA reservoir strain provides an estimate of LA function and has been reported as a sensitive marker of LVDD.[ 15 ] Despite its advantages and the suggestion of the Cirrhotic Cardiomyopathy Consortium, the prognostic value of LA reservoir strain in patients with cirrhosis has not been evaluated.
Therefore, the aim of the current study was twofold: (1) to investigate the association between LVDD, assessed according to the algorithm of the Cirrhotic Cardiomyopathy Consortium, and long‐term survival in patients with cirrhosis undergoing TIPS and (2) to assess the potential additive prognostic value of LA reservoir strain and LV GLS measured by 2D‐STE.
MATERIALS AND METHODS
Study population
All patients with cirrhosis treated by TIPS between January 2003 and June 2019 at the Leiden University Medical Center (Leiden, the Netherlands) were retrospectively evaluated. The diagnosis of cirrhosis was based on clinical, laboratory, and ultrasonographic criteria and confirmed by histologic analysis if necessary. TIPS insertion was indicated for treatment of the following portal hypertension‐related complications: uncontrolled gastrointestinal variceal bleeding, refractory ascites, and refractory hydrothorax or as preoperative treatment in patients with clinically significant portal hypertension undergoing abdominal surgery.
As currently recommended,[ 1 , 16 ] patients with severe pulmonary hypertension or congestive heart failure (therefore also overt LV dysfunction) were not considered as candidates for TIPS. Moreover, no patients with alcoholic cardiomyopathy were included.
According to the institutional protocol, all patients planned for TIPS placement underwent echocardiography a few weeks before the procedure. Patients treated with emergency TIPS or without available echocardiographic assessment were excluded. In addition, patients with suboptimal image quality for 2D‐STE analysis and patients with significant valvular heart disease were also excluded (Figure 1).
FIGURE 1.
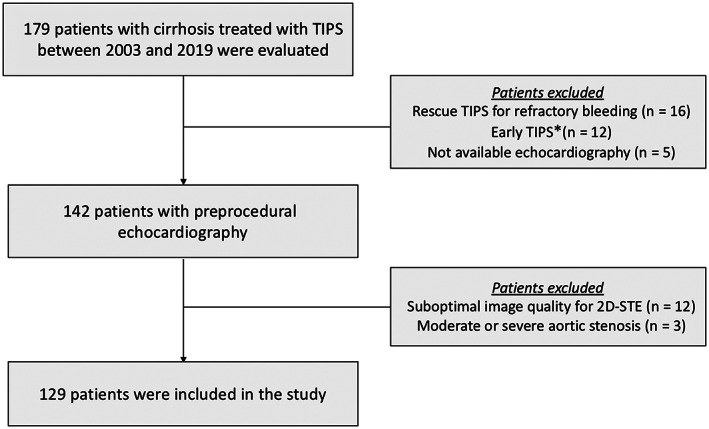
Flow chart of the study with inclusion and exclusion criteria. *Early TIPS placement (within 24 hours) in patients with bleeding from gastroesophageal varices at high risk for treatment failure after initial pharmacological and endoscopic therapy. 2D‐STE, two‐dimensional speckle‐tracking echocardiography; TIPS, transjugular intrahepatic portosystemic shunt.
Demographics and clinical data were collected from the departmental electronic medical records (HiX and EPD‐vision; Leiden University Medical Center). Information about medical history, clinical examination, and standard laboratory tests, including full blood count, international normalization ratio, and markers of kidney and liver function, was available for all patients at the time of admission.
Because this study concerned the retrospective analysis of clinically acquired data, the institutional review board of the Leiden University Medical Center waived the need for written patient informed consent. The study was conducted in accordance with the principles of the Helsinki Declaration.
Conventional echocardiographic examination
Comprehensive transthoracic echocardiography was performed using commercially available systems (VIVID7, E9 and E95 system; General Electric Vingmed, Horten, Norway). Two‐dimensional, color, spectral continuous‐ and pulsed‐wave Doppler images were acquired from the parasternal, apical, and subcostal views. All images were digitally stored for offline analysis (EchoPAC version 203; General Electric Vingmed).
LV linear dimensions were measured from the parasternal long‐axis view, and LV mass was calculated by the Devereux formula[ 17 ] and indexed for body surface area (BSA). From the apical two‐ and four‐chamber views, the LV end‐diastolic and end‐systolic volumes were measured and indexed for BSA, and the LV ejection fraction (LVEF) was calculated using the biplane Simpson method.[ 18 ] The LA volume was measured from the apical two‐ and four‐chamber views using the biplane Simpson method and indexed for BSA (LAVi).[ 18 ] In addition, the stroke volume was calculated by multiplying the LV outflow tract time‐velocity integral, obtained from the apical five‐chamber view, with LV outflow tract cross‐sectional area and indexed for BSA.[ 19 ]
The assessment of LV filling pressure and diastolic function was performed according to the recommendations provided by the Cirrhotic Cardiomyopathy Consortium[ 13 ] and revised from the American Society of Echocardiography/European Association of Cardiovascular Imaging guidelines,[ 20 ] using the combination of several parameters. First, the ratio between the trans‐mitral early (E‐wave) and late (A‐wave) diastolic filling velocities (E/A ratio) and deceleration time of the E‐wave were measured by the pulsed‐wave Doppler recording of the mitral inflow, from the apical four‐chamber view.[ 20 ] Additionally, using pulsed‐wave TDI, the early peak diastolic septal and lateral mitral annular velocities (e') were measured and averaged. The ratio of the trans‐mitral E‐wave to e' (E/e' ratio) was calculated.[ 13 , 20 ] Moreover, the TR velocity was obtained from the continuous‐wave Doppler recording of the TR. According to the algorithm proposed by the Cirrhotic Cardiomyopathy Consortium,[ 13 ] the following four criteria were evaluated for the assessment of LV filling pressure: LAVi ≤34 ml/m2, septal e' velocity ≥7 cm/second, E/e' <15, and TR velocity ≤2.8 m/second; thereafter, the diastolic function was graded on the basis of the E/A ratio. Normal LV filling pressure was defined by at least three fulfilled criteria (or at least two of three available), and in patients with normal LV filling pressure, the diastolic function was defined as normal if the E/A ratio was >0.8 while an E/A ≤0.8 indicated grade 1 LVDD. Conversely, elevated LV pressure was diagnosed if only one of the parameters met the cut‐off thresholds, and then the corresponding LVDD was classified as grade 2 in patients with E/A >0.8 but <2 and grade 3 in the presence of E/A ≥2. Evaluation of LV filling pressure and diastolic function was considered inconclusive in the presence of two fulfilled criteria (of four available).[ 13 ]
In accordance with the updated criteria of CCM,[ 13 ] the presence of (a) LV systolic dysfunction, defined by LVEF ≤50% or LV GLS <18% (see speckle‐tracking examination) and/or (b) advanced (grade 2 or 3) LVDD met the diagnosis of CCM.
Right ventricular (RV) systolic function was assessed measuring tricuspid annular plane systolic excursion (TAPSE) and RV fractional area change (RVFAC), from an RV‐focused apical four‐chamber view. Finally, the pulmonary arterial systolic pressure (PASP) was calculated from the TR velocity and estimated right atrial pressure, according to current recommendations.[ 21 ]
Speckle‐tracking echocardiographic examination
The assessment of LV GLS and LA reservoir strain by 2D‐STE was performed offline using EchoPAC version 203 software (General Electric Vingmed Ultrasound). Images from the apical four‐ and two‐chamber and long‐axis views zoomed on the LV and acquired with a frame rate of ≥50 frames/second were used for the measurement of LV GLS. After the manual definition of the LV endocardial border, the endocardium was automatically tracked through the cardiac cycle by the software. The LV GLS was obtained by averaging all segmental strain values and later by averaging values of all apical views.[ 18 ] In the present study, the values of LV GLS were reported in absolute value. Impaired LV GLS was defined by values <18%, as recommended.[ 13 , 15 ]
For the measurement of LA reservoir strain (Figure 2), the onset of the QRS wave was selected as the reference point (R–R gating). The LA endocardial border was traced manually in the apical four‐chamber view. The region of interest was then adjusted to cover the entire LA myocardium and was divided into six segments by the software. LA reservoir strain was defined as the average of the peak values during the cardiac cycle of all six segments.[ 22 ] LA strain provides an estimate of LA function (with lower values of strain indicating worse LA mechanics) and is a sensitive marker of LVDD.[ 15 ] An LA reservoir strain ≤35% has been shown to identify early (grade 1) LVDD.[ 15 , 23 ] Thereby, this cut‐off value has been adopted in the present study to define LA dysfunction. As additional analysis, validated ranges of LA reservoir strain values were used to reclassify patients in which the algorithm failed to categorize the diastolic function: >35% (normal), 24%–35% (grade 1), 19%–24% (grade 2), and < 19% (grade 3).[ 23 ] For this analysis, grade 2 and 3 LVDD were combined (advanced LVDD).
FIGURE 2.
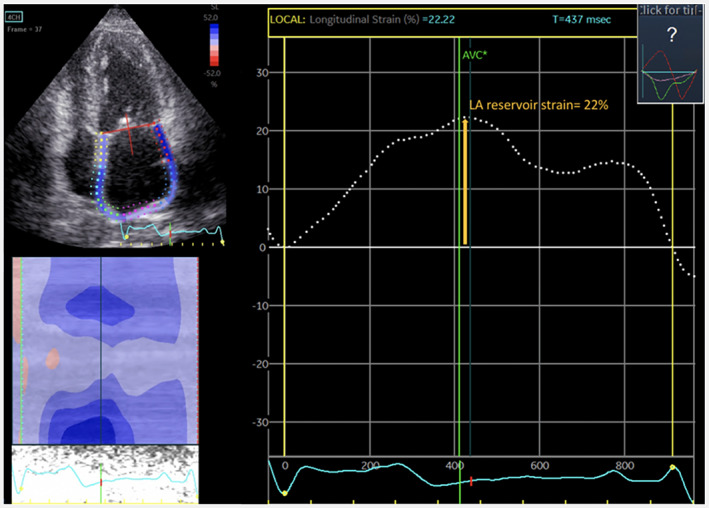
Measurement of LA reservoir strain by 2D‐STE. The region of interest is illustrated in the upper left quadrant covering the left atrium. The average LA reservoir strain from the six myocardial segments is displayed by the dotted white curve in the right of the figure; in this case, the peak was 22%. 2D‐STE, two‐dimensional speckle‐tracking echocardiography; AVC, aortic valve closure; LA, left atrial.
TIPS procedure
TIPS placement was performed as reported[ 24 ] in an interventional angio‐suite under deep sedation or general anesthesia. All procedures were performed with covered stents (Viattor TIPS endoprostheses; Gore & Associates, Flagstaff, AZ, USA). The stents were dilated to 10 mm in diameter in patients undergoing TIPS for gastrointestinal variceal bleeding and to 8 mm in patients undergoing TIPS for refractory ascites or hydrothorax. The final function of the stent was confirmed by portography and by measuring the portosystemic pressure gradient to assess if the desired reduction in the portosystemic gradient was achieved.
Clinical follow‐up
The primary endpoint of the study was all‐cause mortality after TIPS placement. Mortality data after discharge were collected through municipal civil registries or by reviewing medical records. Follow‐up data were available for all patients included in the study.
Statistical analysis
Continuous variables normally distributed are presented as mean ± SD, whereas non‐normally distributed data are presented as median and interquartile range (IQR). Categorical variables are expressed as frequencies and percentages.
The comparison of LA reservoir strain between grades of LVDD was performed using one‐way analysis of variance with Bonferroni post hoc tests. Long‐term survival rates were calculated according to Kaplan‐Meier analysis and compared among groups with a log‐rank test. The association of clinical and echocardiographic variables with all‐cause mortality was investigated using univariable and multivariable Cox proportional hazard regression models. Exposure to liver transplantation was included in the analysis as a binary time‐dependent variable. Variables with a significant correlation in a univariable model (P < 0.05) were included in the multivariable regression model. The hazard ratio (HR) and 95% confidence intervals (CIs) were calculated for each variable. A minimum tolerance level of 0.5 was established to avoid multicollinearity between covariates. Additionally, the likelihood ratio χ 2 test for nested models was used to assess if the LA reservoir strain added incremental prognostic value to the multivariable model, including the Model for End‐Stage Liver Disease (MELD) score, hemoglobin, and grades of LVDD. In a sensitivity analysis, multivariable Cox regression models were repeated after excluding patients with chronic ischemic heart disease. Finally, additional cause‐specific Cox proportional regression models were built, censoring patients who received liver transplantation at this time point. All tests were two sided, and p < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 25.0 (IBM Corporation, Armonk, NY, USA) and R version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study population: clinical and echocardiographic characteristics
Among the 179 patients who were evaluated, 129 patients were included (Figure 1). Demographic and clinical characteristics of the study population are summarized in Table 1. The most frequent etiologies of cirrhosis were alcoholic disease in 64 (50%) patients and chronic viral infection in 19 (15%) patients. The most common indication for TIPS placement was secondary prevention of variceal bleeding (51%) followed by refractory ascites (40%).
TABLE 1.
Demographic and clinical characteristics of the study population
| Clinical variables | Population (n = 129) |
|---|---|
| Male sex, n (%) | 78 (61) |
| Age (years) | 61 ± 12 |
| BMI (kg/m2) | 24.8 (22.1–27.7) |
| Etiology of liver disease, n (%) | |
| Alcoholic/viral/others | 64 (50)/19 (15)/46 (36) |
| MELD score | 11 (9–17) |
| Indication for TIPS, n (%) | |
| Variceal bleeding, secondary prevention | 66 (51) |
| Refractory ascites | 52 (40) |
| Refractory hydrothorax | 10 (8) |
| Preoperative TIPS before abdominal surgery | 1 (1) |
| Cardiovascular risk factors, n (%) | |
| Hypertension | 33 (26) |
| Diabetes | 34 (26) |
| Dyslipidemia | 23 (18) |
| Active smoker | 26 (20) |
| Comorbidities, n (%) | |
| Chronic ischemic heart disease a | 8 (6) |
| Chronic kidney disease | 6 (5) |
| Chronic obstructive pulmonary disease | 14 (11) |
| Laboratory tests | |
| Hemoglobin (mmol/L) | 6.6 ± 1.4 |
| Bilirubin (μmol/L) | 23 (16–41) |
| Creatinine (μmol/L) | 77 (61–106) |
| Sodium (mmol/L) | 138 (134–141) |
| INR (ratio) | 1.2 (1.1–1.3) |
| AST (U/L) | 47 (33–68) |
| ALT (U/L) | 28 (19–43) |
| Cardiovascular medications, n (%) | |
| Cardioaspirin | 11 (9) |
| Beta‐blockers | 70 (54) |
| ACE‐inhibitors or AT‐receptor antagonists | 7 (5) |
| Calcium‐channel blockers | 5 (4) |
| Insulin | 16 (12) |
| Oral glucose lowering drugs | 20 (16) |
| Statins | 17 (13) |
| Loop diuretics | 66 (51) |
| Mineralocorticoid receptor antagonists | 68 (53) |
Note: Values are expressed as n (%), mean ± SD, or median (interquartile range).
Abbreviations: ACE, angiotensin‐converting enzyme; AT, angiotensin; BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; MELD, Model for End‐Stage Liver Disease; TIPS, transjugular intrahepatic portosystemic shunt.
Patients with a prior diagnosis of obstructive coronary artery disease, treated with percutaneous or surgical coronary revascularization.
Echocardiographic characteristics of the overall population, including strain parameters, are shown in Table 2. All patients had preserved LVEF (>50%), whereas 20 (15%) patients exhibited a mildly reduced LV GLS (with values from 15% to 18%). According to the algorithm proposed by the Cirrhotic Cardiomyopathy Consortium, the diastolic function was classified as normal in 65 (50%) patients, impaired in 47 (36%) patients, and indeterminate in 17 (13%) patients. Among patients with LVDD, 26 (20%) patients had grade 1 LVDD and 21 (16%) patients had grade 2 LVDD. A total of 33 patients (26%) had CCM (defined by the presence of LV systolic dysfunction and/or advanced LVDD). Additionally, LA dysfunction, defined by LA reservoir strain ≤35%, was observed in 67 (52%) patients. Of note, LA reservoir strain appeared to progressively worsen with increasing grades of LVDD (p < 0.001) and showed significantly lower values in patients with grade 2 LVDD and in patients with indeterminate function compared to patients with normal function (Figure 3). When the ranges of LA reservoir strain values were used to reclassify patients with indeterminate diastolic function (n = 17), five of them were categorized as normal function (LA strain >35%), seven as grade 1 LVDD (LA strain 24%–35%), and five as advanced (grade 2–3) LVDD (LA strain <24%).
TABLE 2.
Echocardiographic characteristics of the study population
| Echocardiographic variables | Population (n = 129) |
|---|---|
| Heart rate (bpm) | 72 ± 16 |
| LVEDVi a (ml/m2) | 48.8 ± 12.1 |
| LVESVi a (ml/m2) | 17.8 ± 5.6 |
| LVMi a (g/m2) | 88 (74–107) |
| LVEF (%) | 64 (60–68) |
| LV GLS (%) | 20.7 ± 2.5 |
| SVi a (ml/m2) | 50.6 (44.9–59.3) |
| LAVi a (ml/m2) | 33.3 (26.7–40.8) |
| LA reservoir strain (%) | 34.0 ± 9.7 |
| Mitral E:A ratio | 1.1 (0.9–1.5) |
| E‐wave deceleration time (milliseconds) | 223 (186–269) |
| Septal e' velocity (m/second) | 8 (6–9) |
| Mitral E/e' ratio | 9.1 (7.1–11.7) |
| Peak TR velocity b (m/second) | 2.3 (2.1–2.5) |
| Grading of LVDD (n, %) | |
| Normal | 65 (50%) |
| Grade 1 | 26 (20%) |
| Grade 2 | 21 (16%) |
| Indeterminate | 17 (13%) |
| TAPSE (mm) | 25 (21–28) |
| RVFAC (%) | 51 (45–54) |
| PASP (mm Hg) | 26 (22–30) |
Note: Values are expressed as n (%), mean ± SD, or median (interquartile range).
Abbreviations: bpm, beats per minute; BSA, body surface area; LA, left atrial; LAVi, left atrial volume indexed; LVDD, left ventricular diastolic dysfunction; LVEDVi, left ventricular end‐diastolic volume indexed; LVESVi, left ventricular end‐systolic volume indexed; LVEF, left ventricular ejection fraction; LVGLS, left ventricular global longitudinal strain; LVMi, left ventricular mass indexed; PASP, pulmonary arterial systolic pressure; RVFAC, right ventricular fractional area change; SVi, stroke volume indexed; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitant jet.
Indexed for BSA.
Peak TR velocity was measurable in 113 patients (90% of the overall population).
FIGURE 3.
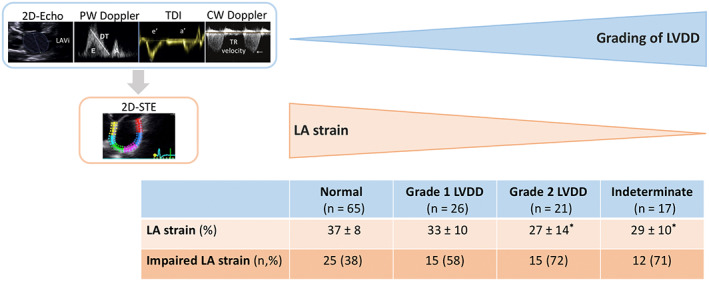
LA reservoir strain according to the grades of LVDD. Mean values (±SD) of LA reservoir strain and the proportion of impaired LA reservoir strain for increasing grades of LVDD are shown. *p < 0.05 versus the group with normal LV diastolic function. 2D‐STE, two‐dimensional speckle‐tracking echocardiography; CW, continuous wave; DT, deceleration time; E/A, E/A wave; LA, left atrial; LAVi, left atrial volume indexed for body surface area; LV, left ventricular; LVDD, left ventricular diastolic dysfunction; PW, pulsed‐waved; TDI, tissue Doppler imaging; TR, tricuspid regurgitation jet; 2D‐Echo, two‐dimensional echocardiography.
Follow‐up: outcome
After a median follow‐up of 36 months (IQR, 12–80 months), 65 (50.4%) patients died. Additionally, a total of 23 (17.8%) patients underwent liver transplantation at a median time of 8 months (IQR, 5–24 months) after TIPS. Kaplan‐Meier curves for all‐cause mortality based on LVDD and on LA reservoir strain are illustrated in Figure 4.
FIGURE 4.
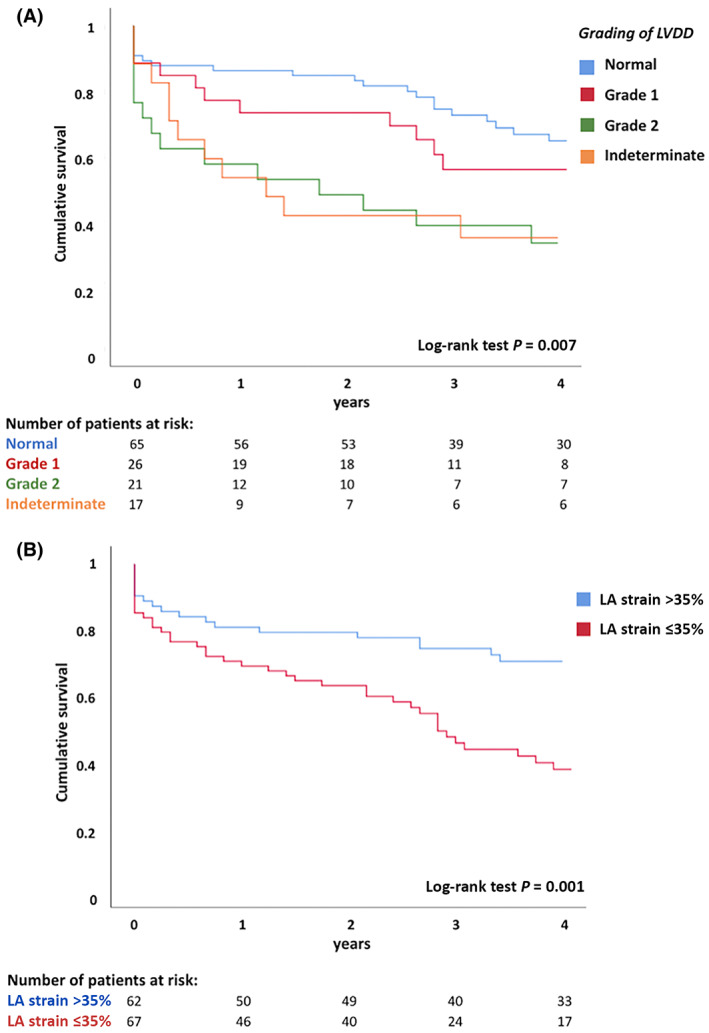
Kaplan‐Meier curves for survival. Curves are shown according to the grades of (A) LVDD and (B) LA reservoir strain. LA, left atrial; LVDD, left ventricular diastolic dysfunction.
Cumulative event rates at 4 years were significantly higher in more advanced grades of LVDD: 32%, 42%, and 67% for normal function, grade 1, and grade 2, respectively, and 65% for indeterminate function (p = 0.007). Furthermore, patients with LA dysfunction (LA reservoir strain ≤35%) had higher event rates compared to patients with preserved LA function (LA reservoir strain >35%) (58% versus 29%; log‐rank p = 0.001). Notably, even considering only patients with normal LV diastolic function, LA reservoir strain ≤35% identified a subset of 25 patients (38% of the subjects with normal LV diastolic function) with an increased rate of all‐cause mortality compared to patients with LA reservoir strain >35% (48% versus 22%; log‐rank p = 0.018) (Figure S1). In addition, more advanced grades of LA strain impairment were associated with progressively higher risk of death (log rank p = 0.004): 36%, 54%, and 66% at 4 years for LA strain higher than 35%, LA strain between 35% and 24%, and LA strain lower than 24%, respectively (Figure S2). Similarly, when LA reservoir strain was used to reclassify patients with indeterminate diastolic function, increasing event rates were observed for worse grades of LVDD (log‐rank p = 0.004): 36%, 56%, and 66% for normal function, grade 1 LVDD, and grade 2–3 LVDD, respectively (Figure S3).
Univariable and multivariable Cox regression analyses were constructed to identify the clinical and echocardiographic variables associated with long‐term mortality (Table 3). The univariable analysis showed an association between MELD score, hemoglobin, grades of LVDD, and LA reservoir strain, with the endpoint of all‐cause mortality. Conversely, LV GLS was not significantly associated with the risk of mortality after TIPS. Similarly, the overall diagnosis of CCM did not show significant association with post‐TIPS outcomes. In the multivariable Cox regression analysis incorporating the parameters with a significant association on the univariate analysis but not LA reservoir strain (model 1), MELD score and more advanced grades of LVDD (grade 2 and indeterminate function) showed a significant association with the outcome. When LA reservoir strain was included in the multivariable analysis (model 2), only MELD score, hemoglobin, and LA reservoir strain were independently associated with all‐cause mortality. Of importance, the addition of LA reservoir strain to model 1 provided incremental prognostic value on all‐cause mortality, with an increase of χ 2 from 27.95 to 36.22 (delta χ 2 = 8.27, p = 0.004). After adjustment for significant variables (MELD score, hemoglobin, grades of LVDD), LA reservoir strain expressed as a categorical variable (≤35%) was associated with an approximately double risk of mortality after TIPS (HR, 2.187; 95% CI, 1.287–3.742; p = 0.004). Additional Cox regression analyses were built incorporating all the individual parameters included in the grading of LVDD and LV GLS (using the recommended thresholds [ 13 ]), as shown in Table S1. In these multivariable models, LA dysfunction was consistently associated with an excess risk of mortality after TIPS. A sensitivity analysis performed excluding patients with chronic ischemic heart disease (n = 8) confirmed the significant prognostic value of LVDD and LA reservoir strain (Table S2). Finally, cause‐specific Cox regression models (built by censoring for liver transplantation) showed a consistent association of LVDD and LA reservoir strain with the risk of all‐cause mortality (Table S3).
TABLE 3.
Univariable and multivariable Cox Regression analysis for all‐cause mortality
| Variable | Univariable Analysis | Multivariable Analysis (1) | Multivariable Analysis (2) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Clinical variables | ||||||
| Age, years | 1.00 (0.98–1.02) | 0.98 | ||||
| Male sex | 0.98 (0.76–1.25) | 0.84 | ||||
| BMI (kg/m2) | 1.05 (1.00–1.10) | 0.05 | ||||
| Alcoholic liver disease | 0.68 (0.26–1.74) | 0.42 | ||||
| Indication for TIPS | ||||||
| Bleeding | Reference | – | ||||
| Ascites | 1.11 (0.66–1.86) | 0.68 | ||||
| Hydrothorax | 1.18 (0.76–4.40) | 0.18 | ||||
| Liver transplantation a | 0.86 (0.39–1.91) | 0.71 | ||||
| MELD score | 1.06 (1.02–1.10) | 0.001* | 1.06 (1.02–1.10) | 0.001* | 1.06 (1.02–1.10) | 0.003* |
| Hemoglobin (mmol/L) | 0.67 (0.53–0.85) | 0.001* | 0.78 (0.61–1.00) | 0.05 | 0.74 (0.58–0.96) | 0.022* |
| AST (U/L) | 1.00 (0.99–1.01) | 0.67 | ||||
| Hypertension | 1.50 (0.87–2.60) | 0.15 | ||||
| Diabetes | 1.40 (0.83–2.38) | 0.21 | ||||
| Chronic IHD | 0.65 (0.21–2.08) | 0.47 | ||||
| Chronic kidney disease | 2.18 (0.87–5.46) | 0.09 | ||||
| Beta‐blockers | 0.62 (0.38–1.01) | 0.06 | ||||
| Diuretics b | 1.24 (0.74–2.07) | 0.41 | ||||
| Echocardiographic variables | ||||||
| Heart rate (bpm) | 1.01 (0.99–1.02) | 0.61 | ||||
| LVMi (g/m2) | 1.01 (0.99–1.02) | 0.76 | ||||
| LVEF (%) | 0.99 (0.95–1.03) | 0.69 | ||||
| LV GLS (%) | 1.01 (0.91–1.10) | 0.98 | ||||
| Grading of LVDD | ||||||
| Normal | Reference | – | Reference | – | Reference | – |
| Grade 1 | 1.21 (0.60–2.46) | 0.59 | 1.49 (0.72–3.06) | 0.28 | 1.40 (0.67–2.90) | 0.37 |
| Grade 2 | 2.55 (1.37–4.77) | 0.003* | 2.13 (1.07–4.23) | 0.031* | 1.55 (0.76–3.14) | 0.23 |
| Indeterminate | 2.40 (1.21–4.78) | 0.012* | 2.31 (1.13–4.73) | 0.021* | 1.69 (0.80–3.57) | 0.18 |
| CCM diagnosis | 1.417 (0.84–2.40) | 0.20* | ||||
| LA reservoir strain (%) | 0.95 (0.93–0.98) | <0.001* | 0.96 (0.94–0.99) | 0.005* | ||
| TAPSE (mm) | 0.99 (0.94–1.04) | 0.77 | ||||
| RVFAC (%) | 0.98 (0.94–1.02) | 0.23 | ||||
| PASP (mm Hg) | 1.02 (0.99–1.06) | 0.10 | ||||
Note: Multivariable model (1) was constructed including variables that showed a significant correlation in univariable analysis but not LA strain. In multivariable model (2), LA strain was added to the first model.
Abbreviations: AST, aspartate aminotransferase; bpm, beats per minute; BMI, body mass index; CCM, cirrhotic cardiomyopathy; CI, confidence interval; HR, hazard ratio; IHD, ischemic heart disease; LA, left atrial; LVDD, left ventricular diastolic dysfunction; LVEF, left ventricular ejection fraction; LV GLS, left ventricular global longitudinal strain; LVMi, left ventricular mass indexed for body surface area; MELD, Model for End‐Stage Liver Disease; PASP, pulmonary arterial systolic pressure; RVFAC, right ventricular fractional area change; TAPSE, tricuspid annular plane systolic excursion; TIPS, transjugular intrahepatic portosystemic shunt.
Time‐dependent variable.
Loops diuretics or mineralocorticoid receptor antagonists.
Significant at p < 0.05.
DISCUSSION
The main findings of the present study can be summarized as follows: (1) LVDD, graded according to the algorithm of the Cirrhotic Cardiomyopathy Consortium, and LA reservoir strain were independently associated with long‐term survival in patients with cirrhosis treated by TIPS and (2) the evaluation of LA reservoir strain provided incremental prognostic value over the severity of liver disease, assessed by the MELD score, and grading of LVDD using conventional echocardiographic parameters.
Cirrhosis is frequently associated with subclinical cardiac dysfunction, characterized by a blunted contractile response to stress stimuli, LVDD, and electrophysiological abnormalities in the absence of other known causes of cardiac disease.[ 5 ] LVDD is an early manifestation of CCM and is reported in approximately one third of patients with cirrhosis.[ 6 ] From a pathophysiological point of view, LVDD has been related to the hyperdynamic circulation state, which induces the development of LV hypertrophy and interstitial fibrosis, leading to increased myocardial stiffness.[ 5 ] Of importance, parameters of LV diastolic function have been shown to correlate with poor outcomes in patients with end‐stage liver disease.[ 7 , 8 , 9 , 25 ] Particularly, LA enlargement and E/e' ratio were predictors of long‐term survival in individuals with cirrhosis.[ 7 , 9 , 25 ] Moreover, among TIPS candidates, the presence of LVDD portends an increased risk of heart failure in the first year after the procedure.[ 3 ] Nevertheless, there are limited and conflicting data regarding the predictive value of LVDD on long‐term outcomes in patients with cirrhosis undergoing TIPS.[ 10 , 11 , 12 ] The E/A ratio has been correlated with the overall survival after TIPS placement,[ 10 , 11 ] whereas another recent study failed to confirm this finding.[ 12 ] The lack of a comprehensive assessment of LV diastolic function in the abovementioned studies is most likely the reason for these differences. Moreover, the evaluation of LV diastolic function solely on the basis of E/A ratio is strongly limited by the load dependency of this parameter and the U‐shaped relationship between E/A ratio and LVDD (patients with normal LV diastolic function and patients with grade 2 LVDD show similar values of E/A ratio).
To the best of our knowledge, this is the first study to investigate the association between LV diastolic function assessed according to a multiparametric and comprehensive approach and long‐term prognosis in patients with cirrhosis treated with TIPS. In our study, the presence of grade 2 LVDD or indeterminate diastolic function identified a cohort of patients with a high risk of death at 4 years of follow‐up. Thus, our results provide evidence on the prognostic implications of the grading of LVDD proposed by the Cirrhotic Cardiomyopathy Consortium among TIPS candidates.[ 13 ] The finding of worse survival in individuals with indeterminate diastolic function may be explained by the fact that, in the majority of these patients, the algorithm likely failed to grade an existing LVDD. When LA reservoir strain was used to recategorize the diastolic function in these patients, most of them (71%) were indeed reclassified as having some degree of LVDD.
The left atrium manifests adaptive changes in its structure and mechanics in the setting of abnormal patterns of LV filling, known as LVDD. The analysis of LA reservoir strain by 2D‐STE provides a higher accuracy and reproducibility in the quantification of LA function compared to conventional echocardiography.[ 15 ] LA reservoir strain directly reflects increased LA stiffness, which is related to LA fibrosis, but LV diastolic (or systolic) dysfunction is usually the primary determinant.[ 15 ] Indeed, LA reservoir strain has emerged as sensitive marker of LVDD, with prognostic implications in several cardiovascular conditions.[ 15 ] In contrast to conventional diastolic parameters, LA reservoir strain decreases in a linear fashion with increasing severity of LVDD and as such may allow a more accurate judgment of LVDD grading.[ 15 , 23 ] In addition, LA function is less affected by loading conditions than LA dimensions.[ 15 ] LAVi may increase despite normal LV diastolic function in patients with high‐output states, including those with cirrhosis. Thereby, LA reservoir strain appears particularly promising for the evaluation of LV filling pressure and diastolic function in patients with end‐stage liver disease, overcoming the load dependency of other parameters (such as E/A ratio and LA enlargement).
Some recent studies[ 26 , 27 ] showed impaired values of LA reservoir strain in patients with cirrhosis compared to healthy controls. Notably, in patients with cirrhosis, LA reservoir strain demonstrated a better agreement with LV filling pressure, evaluated by E/E' ratio, in comparison to LAVi.[ 25 ] The only study investigating the prognostic value of LA reservoir function in cirrhosis was conducted in a small‐size population (n = 80) and described a trend toward worse posttransplantation survival in patients with cirrhosis with reduced LA reservoir strain (defined as <37%).[ 27 ]
In the present study, we demonstrated that LA dysfunction is independently associated with worse survival in patients with cirrhosis treated by TIPS. Of importance, LA reservoir strain provided incremental prognostic value over a model including significant clinical variables (MELD score and hemoglobin) and the grading of LVDD. In our population, LA reservoir strain appeared to progressively decrease with increasing grades of LVDD. However, the presence of impaired LA reservoir strain (≤35%) was still associated with a worse outcome in the subset of patients with normal LV diastolic function, highlighting the prognostic importance of this marker above conventional echocardiographic parameters.
Our results support the implementation of LA reservoir strain in the echocardiographic evaluation of TIPS candidates. In this context, LA reservoir strain may be a valuable diagnostic tool for the assessment of LV diastolic function, especially in patients with discrepant values of traditional diastolic parameters, and for patients' risk stratification.
Conversely, we did not observe a significant correlation between LV GLS and patient outcome. LV GLS measured with 2D‐STE is an early and accurate marker of LV systolic dysfunction and its superiority in comparison to conventional parameters of LV function, such as LVEF, has been demonstrated in several clinical settings.[ 14 ] However, few studies investigating the prognostic value of LV GLS in patients with end‐stage liver disease led to discordant results.[ 28 , 29 ] These inconsistences may be related to the fact that patients with end‐stage liver disease are primarily characterized by an impairment of LV diastolic function with preserved LV systolic function at rest. Accordingly, in our study, LV GLS values were mostly within the normal range, with a small proportion of patients (15%) with mildly impaired LV GLS, thus making LV GLS a suboptimal risk stratification tool in this population.
This study is subject to the limitations of its retrospective, single‐center, observational design, and further prospective studies should confirm our findings. In particular, patients with a history of ischemic heart disease or risk factors for cardiovascular disease were not excluded in the current study in order to represent a “real‐world” setting. This pragmatic approach implies that we could not discriminate between the effects of CCM or comorbidities on cardiac function. However, this was not the primary aim of our investigation. In addition, the Cox regression analysis did not reveal a significant correlation between these variables and outcomes, and a sensitivity analysis performed excluding patients with a history of ischemic heart disease confirmed the main results of the study.
The current cohort did not include patients with grade 3 LVDD. Therefore, our results cannot be extended to this subset of patients with the more advanced form of LV diastolic impairment. Finally, because the mortality data were retrieved mostly by municipal civil registries, we could not discriminate between cardiac and no‐cardiac causes of death.
LA dysfunction, evaluated by LA reservoir strain, is significantly associated with all‐cause mortality in patients with cirrhosis treated by TIPS and demonstrated an incremental prognostic value above the grading of LVDD using conventional parameters. Thereby, the assessment of LA function by 2D‐STE may help characterizing LV diastolic function and improving risk stratification in TIPS candidates.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST
Victoria Delgado received speaker's fees from Abbott Vascular, Medtronic, Edwards Lifesciences, MSD, and GE Healthcare. Jeroen Bax received speaker's fees from Abbott Vascular. Nina Ajmone Marsan received speaker's fees from Abbott Vascular and GE Healthcare, a research grant from Alnylam, and has been on the Medical Advisory Board of Philips Ultrasound. Steele Butcher received a research grant from the European Society of Cardiology. The other authors have nothing to disclose.
Supporting information
Table S1 Univariable and multivariable Cox regression analyses for all‐cause mortality including individual parameters of diastolic function and strain measurements.
Table S2. Sensitivity analysis: multivariable Cox regression analyses for all‐cause mortality performed excluding patients with history of ischemic heart disease
Table S3. Univariable and multivariable cause‐specific Cox regression models for all‐cause mortality performed by censoring for liver transplantation.
Figure S1. Kaplan–Meier curves for survival according to LA reservoir strain in the subset of patients with normal LV diastolic function.
Figure S2. Kaplan–Meier curves for survival according to increasing grades of LA strain impairment.
Figure S3. Kaplan–Meier curve for survival according to the revised grading of LVDD using LA reservoir strain.
Meucci MC, Hoogerduijn Strating MM, Butcher SC, van Rijswijk CSP, Van Hoek B, Delgado V, et al. Left atrial dysfunction is an independent predictor of mortality in patients with cirrhosis treated by transjugular intrahepatic portosystemic shunt. Hepatol Commun. 2022;6:3163–3174. 10.1002/hep4.2062
Maarten E. Tushuizen and Nina Ajmone Marsan contributed equally to this work as senior authors.
REFERENCES
- 1. Boyer TD, Haskal ZJ, American Association for the Study of Liver Diseases . The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41:386–400. [DOI] [PubMed] [Google Scholar]
- 2. Huonker M, Schumacher YO, Ochs A, Sorichter S, Keul J, Rössle M. Cardiac function and haemodynamics in alcoholic cirrhosis and effects of the transjugular intrahepatic portosystemic stent shunt. Gut. 1999;44:743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Billey C, Billet S, Robic MA, Cognet T, Guillaume M, Vinel JP, et al. A prospective study identifying predictive factors of cardiac decompensation after transjugular intrahepatic portosystemic shunt: the Toulouse algorithm. Hepatology. 2019;70:1928–41. [DOI] [PubMed] [Google Scholar]
- 4. Lee SS, Liu H. Cardiovascular determinants of survival in cirrhosis. Gut. 2007;56:746–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wiese S, Hove JD, Bendtsen F, Møller S. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol. 2014;11:177–86. [DOI] [PubMed] [Google Scholar]
- 6. Ruíz‐del‐Árbol L, Achécar L, Serradilla R, Rodríguez‐Gandía MÁ, Rivero M, Garrido E, et al. Diastolic dysfunction is a predictor of poor outcomes in patients with cirrhosis, portal hypertension, and a normal creatinine. Hepatology. 2013;58:1732–41. [DOI] [PubMed] [Google Scholar]
- 7. Izzy M, Soldatova A, Sun X, Angirekula M, Mara K, Lin G, et al. Cirrhotic cardiomyopathy predicts posttransplant cardiovascular disease: revelations of the new diagnostic criteria. Liver Transpl. 2021;27:876–86. [DOI] [PubMed] [Google Scholar]
- 8. Dowsley TF, Bayne DB, Langnas AN, Dumitru I, Windle JR, Porter TR, et al. Diastolic dysfunction in patients with end‐stage liver disease is associated with development of heart failure early after liver transplantation. Transplantation. 2012;94:646–51. [DOI] [PubMed] [Google Scholar]
- 9. Cesari M, Frigo AC, Tonon M, Angeli P. Cardiovascular predictors of death in patients with cirrhosis. Hepatology. 2017;68:215–23. [DOI] [PubMed] [Google Scholar]
- 10. Cazzaniga M, Salerno F, Pagnozzi G, Dionigi E, Visentin S, Cirello I, et al. Diastolic dysfunction is associated with poor survival in patients with cirrhosis with transjugular intrahepatic portosystemicshunt. Gut. 2007;56:869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rabie RN, Cazzaniga M, Salerno F, Wong F. The use of E/A ratio as a predictor of outcome in cirrhotic patients treated with transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 2009;104:2458–66. Erratum in: Am J Gastroenterol. 2009;104:2128. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong MJ, Gohar F, Dhaliwal A, Nightingale P, Baker G, Greaves D, et al. Diastolic dysfunction on echocardiography does not predict survival after transjugular intrahepatic portosystemic stent‐shunt in patients with cirrhosis. Aliment Pharmacol Ther. 2019;49:797–806. [DOI] [PubMed] [Google Scholar]
- 13. Izzy M, VanWagner LB, Lin G, Altieri M, Findlay JY, Oh JK, et al.; Cirrhotic Cardiomyopathy Consortium. Redefining cirrhotic cardiomyopathy for the modern era. Hepatology. 2020;71:334–45. Erratum in: Hepatology 2020:72:1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abou R, van der Bijl P, Bax JJ, Delgado V. Global longitudinal strain: clinical use and prognostic implications in contemporary practice. Heart. 2020;106:1438–44. [DOI] [PubMed] [Google Scholar]
- 15. Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019;73:1961–77. [DOI] [PubMed] [Google Scholar]
- 16. Dariushnia SR, Haskal ZJ, Midia M, Martin LG, Walker TG, Kalva SP, et al. Quality improvement guidelines for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2016;27:1–7. [DOI] [PubMed] [Google Scholar]
- 17. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. [DOI] [PubMed] [Google Scholar]
- 19. Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–92. [DOI] [PubMed] [Google Scholar]
- 20. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–60. [DOI] [PubMed] [Google Scholar]
- 21. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. [DOI] [PubMed] [Google Scholar]
- 22. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two‐dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19:591–600. Erratum in: Eur Heart J Cardiovasc Imaging. 2018;19:830‐833. [DOI] [PubMed] [Google Scholar]
- 23. Singh A, Addetia K, Maffessanti F, Mor‐Avi V, Lang RM. LA strain for categorization of LV diastolic dysfunction. JACC Cardiovasc Imaging. 2017;10:735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhogal HK, Sanyal AJ. Using transjugular intrahepatic portosystemic shunts for complications of cirrhosis. Clin Gastroenterol Hepatol. 2011;9:936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Merli M, Torromeo C, Giusto M, Iacovone G, Riggio O, Puddu PE. Survival at 2 years among liver cirrhotic patients is influenced by left atrial volume and left ventricular mass. Liver Int. 2017;37:700–6. [DOI] [PubMed] [Google Scholar]
- 26. Sampaio F, Pimenta J, Bettencourt N, Fontes‐Carvalho R, Silva AP, Valente J, et al. Left atrial function is impaired in cirrhosis: a speckle tracking echocardiographic study. Hepatol Int. 2014;8:146–53. [DOI] [PubMed] [Google Scholar]
- 27. von Köckritz F, Braun A, Schmuck RB, Dobrindt EM, Eurich D, Heinzel FR, et al. Speckle tracking analysis reveals altered left atrial and ventricular myocardial deformation in patients with end‐stage liver disease. J Clin Med. 2021;10:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mechelinck M, Hartmann B, Hamada S, Becker M, Andert A, Ulmer TF, et al. Global longitudinal strain at rest as an independent predictor of mortality in liver transplant candidates: a retrospective clinical study. J Clin Med. 2020;9:2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rimbas RC, Baldea SM, Guerra R, Visolu SI, Rimbas M, Pop CS, et al. New definition criteria of myocardial dysfunction in patients with liver cirrhosis: a speckle tracking and tissue Doppler imaging study. Ultrasound Med Biol. 2018;44:562–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Univariable and multivariable Cox regression analyses for all‐cause mortality including individual parameters of diastolic function and strain measurements.
Table S2. Sensitivity analysis: multivariable Cox regression analyses for all‐cause mortality performed excluding patients with history of ischemic heart disease
Table S3. Univariable and multivariable cause‐specific Cox regression models for all‐cause mortality performed by censoring for liver transplantation.
Figure S1. Kaplan–Meier curves for survival according to LA reservoir strain in the subset of patients with normal LV diastolic function.
Figure S2. Kaplan–Meier curves for survival according to increasing grades of LA strain impairment.
Figure S3. Kaplan–Meier curve for survival according to the revised grading of LVDD using LA reservoir strain.


