Abstract
Chronic hepatitis B virus (HBV) infection is the leading risk factor for hepatocellular carcinoma (HCC). The aim of this study was to explore the incidence of HCC in a cohort of subjects with HBV and correlate with HBV treatment current guidance. We identified 2846 subjects with HBV over the study period. HCC was diagnosed in 386 of 2846 (14%) subjects; 209 of 386 (54%) were on nucleos(t)ide analogue (NA) therapy at time of HCC diagnosis, and 177 of 386 (46%) were not on NA therapy. Of the 177 subjects not on NAs who developed HCC during follow‐up, 153 of 177 (86%) had cirrhosis. Within the 177 subjects not on NAs, 158 of 177 (89%) had undetectable HBV DNA, 10 of 177 (6%) had detectable HBV DNA < 2000 IU/L, and 9 of 177 (5%) had HBV DNA > 2000 IU/L. Of those with cirrhosis and undetectable HBV DNA, 115 of 141 had compensated cirrhosis, and 26 of 141 had decompensated cirrhosis. Significant predictors of HCC on time to event analysis included cirrhosis (hazard ratio [HR] 10, 95% confidence interval [CI] 5.8–17.5; p < 0.001), alanine aminotransferase level (HR 1.004, 95% CI 1.002–1.006; p < 0.001), age (HR 1.04, 95% CI 1.03–1.06; p < 0.001), (HR 1.9, 95% CI 1.2–3.1; p 0.007), and nonalcoholic fatty liver disease (HR 1.7, 95% CI 1.1–2.8; p 0.02). Kaplan–Meier analysis demonstrated the cumulative incidence of HCC in subjects with compensated cirrhosis receiving NA therapy was significantly lower compared to subjects with compensated cirrhosis outside current HBV treatment practice guidance (undetectable HBV DNA) (32% vs. 51%; p < 0.001). Conclusion: Those with untreated compensated cirrhosis with undetectable HBV DNA who do not meet current guidance for treatment had higher rates of HCC than those with compensated cirrhosis and suppressed HBV DNA by NA therapy. This study highlights the need for earlier diagnosis and treatment of HBV.
The aim of our study was to explore the incidence of HCC in a cohort of HBV subjects both receiving and not receiving NA therapy and correlate with corresponding HBV treatment guidelines at time of presentation. We found compensated cirrhosis patients with undetectable HBV DNA not on NAs had higher HCC rates than those receiving NA therapy.

INTRODUCTION
Chronic hepatitis B virus infection (HBV) is one of the leading risk factors worldwide for hepatocellular carcinoma (HCC) in patients with and without cirrhosis.[ 1 , 2 , 3 ] While both nucleos(t)ide analogues (NAs) and interferon are approved for the treatment of hepatitis B, most individuals are treated with NAs and therapy for HBV; NA therapy in particular reduces the risk of disease progression as well as the incidence of HCC.[ 4 ] Indeed, the risk of developing HCC is decreased by 50%–60% in patients receiving HBV antiviral therapy compared with those not on treatment, and these data in part inform our current treatment guidelines.[ 5 , 6 , 7 ] However, there are variances by both practice guidance recommendations and practitioners in the threshold at which to initiate therapy for HBV. Indications for therapy have been based primarily on HBV DNA, alanine aminotransferase (ALT) levels, and the level of fibrosis, and with emerging data, recommendations for initiation of treatment have evolved.[ 7 , 8 , 9 ] The aim of this study was to explore the incidence of HCC in a cohort of subjects with HBV both receiving and not receiving NA therapy and correlate with corresponding HBV treatment guidelines at time of presentation. This study also explored predictors of HCC in subjects with HBV who were outside HBV treatment practice guidance.
METHODS
We performed a single‐center, electronic database, retrospective analysis of all subjects 18 years and older with HBV between January 1, 2000 to December 31, 2019. Those with concurrent hepatitis C virus infection (HCV) and alcohol‐associated and fatty liver disease were also identified. We excluded all individuals who presented with an initial diagnosis of HCC. The Stanford University Institutional Review Board approved the study. Subjects were identified via the Stanford Research Repository Tools (i.e., STRIDE‐web) of Stanford University electronic data warehouse. Data were fully anonymized after data extraction. Data abstracted included demographic information (age, gender, race), cirrhosis, fibrosis level, ALT, aspartate aminotransferase, quantitative HBV‐DNA level, alpha fetoprotein, alkaline phosphatase, total bilirubin, creatinine, international normalized ratio, sodium, platelets, HBV treatment, and HCC. Serum ALT and HBV‐DNA levels were collected over time; cirrhosis (compensated and decompensated) was correlated with current societal HBV practice guidance.[ 7 ] Multiple serum HBV‐DNA assays were available during the study period from January 1, 2000, to 2004 and included branched DNA assays (Versant; Bayer Diagnostics) and real‐time polymerase chain reaction (PCR) from the National Genetics Institute (National Genetic Institute's validated, proprietary PCR methodology). From 2004, the primary HBV‐DNA assay was PCR/nucleic acid amplification by Roche COBAS automation (REFERENCE RANGE: <2.0 × 10[exp]2 copies/ml), followed by subsequent real‐time PCR assays with lower limit of linear range 60 IU/ml in 2006, Roche COBAS automation real‐time PCR assay with lower limit of linear range 40 IU/ml in 2010, and Roche COBAS automation and real‐time PCR assay with lower limit of linear range 10 IU/ml in 2019. For our cohort, we defined ALT > 35 IU/L for male and >25 IU/L for female as the upper limits of normal (ULN). Decompensated cirrhosis was defined as cirrhosis and evidence of hepatic encephalopathy, ascites, jaundice, and/or variceal bleeding. Area under the receiver operating characteristic curve (AUROC) analysis was used for the threshold values. Statistical analysis was conducted using RStudio version 1.1.463. Shapiro–Wilk test was used to test normality of continuous variables; two‐sample t‐test was used to compare normally distributed continues variables; and Wilcoxon rank‐sum test was used to compare continuous variables that were not normally distributed. Categorical variables were compared using Fisher's exact test. Multivariate analysis model for predictors of HCC was built by performing backward elimination of variables and selecting the model with the highest adjusted R‐squared value and the most significant F‐statistics p‐value. AUROC analysis was used to determine threshold values. Time‐to‐event analysis of predictors of HCC was analyzed with multivariable Cox proportional hazards model by performing backward elimination and testing LRT with analysis of variance. Cumulative incidence of HCC was analyzed by Kaplan–Meier survival analysis. p‐Value < 0.05 was considered statistically significant.
RESULTS
We identified 2846 subjects with HBV over the study period. Tables 1 and 2 describe baseline characteristics after excluding 213 subjects who presented with HCC and HBV infection. Median follow‐up time was 8.5 (interquartile range 3–15) years. Hepatocellular cancer was diagnosed in 386 of 2846 (14%) subjects during the study period, of whom 209 of 386 (54%) were on NA therapy at time of HCC diagnosis, and 177 of 386 (46%) not on NA therapy. Of the 209 subjects who developed HCC and were taking NAs, 90 of 209 (43%) were diagnosed with HBV infection only, and 119 of 209 (57%) had HBV with concomitant chronic liver disease (CLD) (HCV, 37; alcohol‐associated liver disease [ALD], 46; and nonalcoholic fatty liver disease [NAFLD], 36) with 163 of 209 (78%) having cirrhosis. Of the 177 subjects who developed HCC and were not taking NAs, 79 of 177 (45%) were diagnosed with HBV only, and 98 of 177 (55%) had HBV with concomitant CLD (ALD, 48; HCV, 16; and NAFLD, 34) with 153 of 177 (86%) having cirrhosis (Figure 1). Of the 153 subjects with cirrhosis not on NAs who developed HCC during follow‐up, 127 of 153 (83%) had compensated cirrhosis, and 26 of 153 (17%) had decompensated cirrhosis. With regard to HBV‐DNA levels, in the 177 untreated subjects who developed HCC, 158 of 177 (89%) had undetectable HBV DNA, 10 of 177 (6%) had detectable HBV DNA < 2000 IU/L, and 9 of 177 (5%) had HBV DNA > 2000 IU/L. Of the 141 subjects with HCC with cirrhosis and undetectable HBV DNA, 115 of 141 had compensated cirrhosis, and 26 of 141 had decompensated cirrhosis (Table 2). In the 115 subjects with compensated cirrhosis/HCC with undetectable HBV DNA, 46 of 90 (51.1%) males had ALT > 70 IU/L, and 25 of 37 (67.6%) females had ALT > 50 IU/L (Table 3).
TABLE 1.
Baseline characteristics in subjects with HBV not on NAs who did not meet the 2018 AASLD HBV treatment guidelines and developed HCC (no NA/HCC), subjects with HBV on NAs who developed HCC (NA/HCC), and subjects with HBV not on NAs who did not meet the 2018 AASLD HBV treatment guidelines and did not develop HCC (no NA/no HCC)
| Factor | No NA/HCC (n = 133) n (%) or median (IQR) | NA/HCC (n = 209) n (%) or median (IQR) | No NA/No HCC (n = 1110) n (%) or median (IQR) | p‐Values a |
|---|---|---|---|---|
| Male | 91 (68%) | 165 (79%) | 526 (47%) | 0.04, <0.001 |
| Female | 42 (32%) | 44 (21%) | 584 (53%) | 0.04, <0.001 |
| Asian | 61 (46%) | 165 (79%) | 772 (69%) | <0.001, <0.001 |
| White | 32 (24%) | 14 (7%) | 119 (11%) | <0.001, <0.001 |
| Hispanic | 5 (4%) | 2 (1%) | 4 (0.4%) | 0.1, 0.001 |
| F2/F3 fibrosis | 3 (2%) | 30 (14%) | 12 (1%) | <0.001, 0.2 |
| Cirrhosis | 115 (86%) | 163 (78%) | 111 (10%) | 0.06, <0.001 |
| Age (years) | 61 (55–69) | 58 (50–67) | 42 (33–53) | 0.03, <0.001 |
| ALT (IU/L) | 67 (48–107) | 71 (51–109) | 33 (26–41) | 0.6, <0.001 |
| HBV DNA (mIU/ml) | Undetected (undetected–undetected) | 20 (undetected–51) | Undetected (undetected–undetected) | <0.001, <0.001 |
| ALP (U/L) | 124 (87–177) | 131 (113–131) | 76 (61–86) | 0.7, <0.001 |
| AST (IU/L) | 70 (50–115) | 77 (47–128) | 26 (21–30) | 0.5, <0.001 |
| TB (mg/dl) | 1.01 (0.7–1.9) | 0.9 (0.7–1.6) | 0.7 (0.5–0.9) | 0.09, <0.001 |
| INR | 1.26 (1.1–3.04) | 1.2 (1.1–1.4) | 1.09 (1.06–1.1) | 0.3, <0.001 |
| NAs (mmol/L) | 137 (136–144) | 139 (137–144) | 139 (138–140) | <0.001, <0.001 |
| Platelets (K/ul) | 143 (91–200) | 163 (120–205) | 217 (187–249) | 0.01, <0.001 |
| Albumin (g/dl) | 3.3 (2.8–3.7) | 3.8 (3.3–4) | 4.2 (3.9–4.4) | <0.001, <0.001 |
| ALD | 48 (36%) | 46 (22%) | 10 (1%) | 0.006, <0.001 |
| HCV | 16 (15%) | 37 (18%) | 62 (5%) | 0.2, 0.007 |
| NAFLD | 34 (22%) | 36 (17%) | 92 (8%) | 0.07, <0.001 |
| HIV | 3 (2%) | 1 (1%) | 3 (0.3%) | 0.3, 0.02 |
| HDV | 0 (0%) | 1 (1%) | 0 (0%) | 1, 1 |
Abbreviations: AASLD, American Association for the Study of Liver Diseases; ALD, alcoholic‐associated liver disease; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HCV, hepatitis C virus; HDV, hepatitis D virus; HIV, human immunodeficiency virus; INR, international normalized ratio; IQR, interquartile range; NAFLD, nonalcoholic fatty liver disease; TB, total bilirubin.
p‐Values comparing NA/HCC with no NA/HCC, and no NA/no HCC with no NA/HCC, respectively.
TABLE 2.
Baseline characteristics in subjects with HBV not on NAs who did not meet the 2018 AASLD HBV treatment guidelines and developed HCC (no NA/HCC), subjects with HBV on NAs who developed HCC (NA/HCC), and subjects with HBV not on NAs who did not meet 2018 AASLD HBV treatment guidelines and did not develop HCC (no NA/no HCC) compensated cirrhosis with undetectable HBV DNA only
| Factor | Compensated cirrhosis/undetectable HBV DNA/no NA/HCC (115 subjects) n (%) or median (IQR) | Compensated cirrhosis/undetectable HBV DNA/NA/HCC (112 subjects) n (%) or median (IQR) | Compensated cirrhosis/undetectable HBV DNA/no NA/no HCC (111 subjects) n (%) or median (IQR) | p‐Values a |
|---|---|---|---|---|
| ALT (IU/L) | 68 (49–106) | 67 (48–110) | 35 (28–42) | 0.8, <0.001 |
| Male | 80 (70%) | 89 (79%) | 75 (67%) | 0.09, 0.08 |
| Female | 35 (30%) | 23 (20%) | 36 (32%) | 0.09, 0.08 |
| Asian | 48 (42%) | 87 (78%) | 82 (74%) | <0.001, <0.001 |
| White | 30 (26%) | 11 (10%) | 13 (12%) | 0.002, 0.007 |
| Hispanic | 4 (3%) | 0 (0%) | 5 (4%) | 0.01, 0.7 |
| Age (years) | 62 (56–70) | 60 (50–68) | 46 (37–53) | 0.1, <0.001 |
| ALP (U/L) | 129 (90–180) | 131 (113–131) | 79 (64–95) | 0.2, <0.001 |
| AST (IU/L) | 74 (51–119) | 74 (47–122) | 26 (22–33) | 0.6, <0.001 |
| TB (mg/dl) | 1.1 (0.7–1.9) | 1.02 (0.8–1.7) | 0.9 (0.7–1.02) | 0.8, <0.001 |
| INR | 1.3 (1.1–1.4) | 1.2 (1.1–1.3) | 1.1 (1.05–1.1) | 0.08, <0.001 |
| NAs (mmol/L) | 137 (136–139) | 139 (137–143) | 139 (138–140) | <0.001, <0.001 |
| Platelets (K/ul) | 128 (88–194) | 146 (116–194) | 193 (154–226) | 0.04, <0.001 |
| Albumin (g/dl) | 3.3 (2.7–3.7) | 3.7 (3–4) | 4.1 (3.8–4.3) | 0.001, <0.001 |
| ALD | 38 (33%) | 35 (31%) | 4 (4%) | 0.08, <0.001 |
| HCV | 16 (14%) | 7 (6%) | 8 (7%) | 0.08, 0.1 |
| NAFLD | 27 (23%) | 22 (20%) | 16 (14%) | 0.5, 0.09 |
| HIV | 3 (3%) | 1 (1%) | 3 (3%) | 0.6, 1 |
| HDV | 0 (0%) | 0 (0%) | 0 (0%) | 1, 1 |
p‐Values comparing NA/HCC to no NA/HCC, and no NA/no HCC to no NA/HCC, respectively.
FIGURE 1.
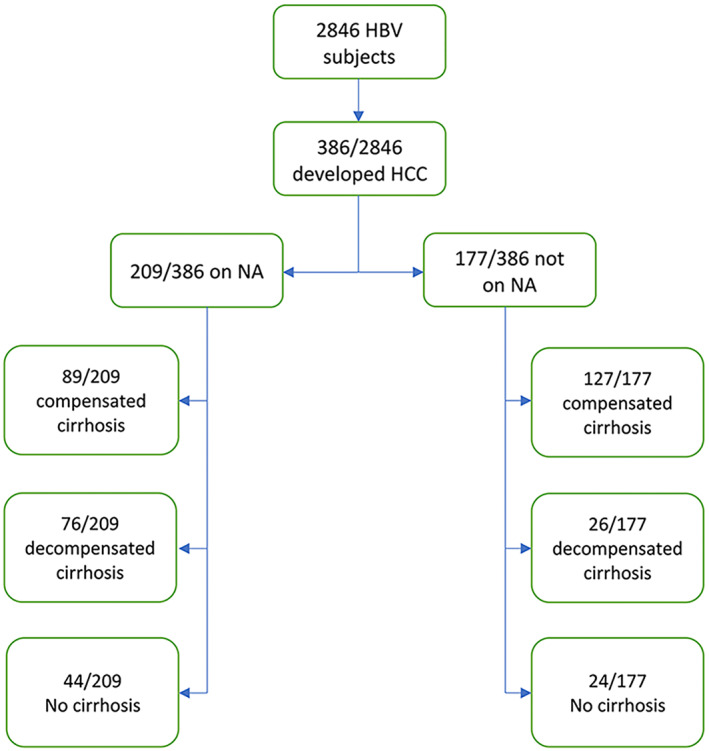
Flow chart
TABLE 3.
Cohort of 177 subjects with HBV who developed HCC while not on NAs
| Variable | Compensated cirrhosis | Decompensated cirrhosis | No cirrhosis | p‐Values b |
|---|---|---|---|---|
| Not on NAs | 127/177 (72%) | 26/177 (15%) | 24/177 (13%) | <0.001, <0.001 |
| HBV DNA > 2000 | 4 | 0 | 5 | 1, 0.005 |
| HBV DNA < 2000 | 8 | 0 | 2 | 0.3, 0.7 |
| HBV DNA not detected | 115 | 26 | 17 | 0.2, 0.01 |
| ALT > 2× ULN a | 46 M, 25 F | 9 M, 7 F | 10 M, 3 F | 1, 0.7, 0.4, 0.6 |
| ALT < 2× ULN a | 44 M, 12 F | 9 M, 1 F | 5 M, 6 F | 1, 0.2, 0.7, 0.04 |
| ALT < 35 M, 25 F | 4 M, 2 F | 0 M, 0 F | 4 M, 0 F | 1, 0.2, 1, 1 |
| ALT < 40 | 11 | 2 | 6 | 1, 0.03 |
| Met AASLD 2018 criteria for NA therapy | 14 | 26 | 4 | <0.001, 0.5 |
| ALD | 38 | 7 | 3 | 0.8, 0.09 |
| HCV | 16 | 3 | 1 | 1, 0.3 |
| NAFLD | 27 | 1 | 2 | 0.05, 0.2 |
| On NAs | 89 of 209 (43%) | 76 of 209 (36%) | 44 of 209 (21%) | 0.4, <0.001 |
Abbreviations: F, female; M, male; ULN, upper limit of normal.
ALT > 70 for males and >50 for females.
p‐Values comparing decompensate cirrhosis with compensated cirrhosis and no cirrhosis with compensated cirrhosis, respectively.
We correlated HBV‐DNA levels, ALT levels and the presence of cirrhosis with societal practice guidance recommendations to initiate NA therapy. The 2018 American Association for the Study of Liver Diseases (AASLD) guidance for HBV treatment included decompensated cirrhosis or ALT > 35 for men, 25 for women or ≥F2 fibrosis and HBV DNA > 20,000 for hepatitis B e antigen (HBeAg)–positive or >2000 for HBeAg‐negative, or compensated cirrhosis and low‐level viremia (HBV DNA < 2000 IU/ml). Of the 177 subjects who developed HCC and were not on treatment, 44 of 177 (25%) met the current 2018 AASLD criteria for initiation of NA therapy: 14 of 177 (8%) with compensated cirrhosis/detectable HBV DNA, 26 of 177 (15%) with decompensated cirrhosis, and 4 of 177 (2%) with HBV DNA > 2000 IU/L/ALT > ULN (Table 3). Of the 133 of 177 subjects with HCC who were outside the 2018 AASLD guidance, 115 of 177 (65%) had compensated cirrhosis with undetectable HBV DNA, and 20 of 177 (11%) were without cirrhosis and without HBV DNA > 2000 IU/L/ALT > ULN. We compared ALT levels in those not receiving NAs with compensated cirrhosis, no detectable HBV DNA, and HCC without concomitant other liver disease, with the same population but receiving NA therapy, and no difference was noted (80 IU/L vs. 64 IU/L; p = 0.14). In the HCC cohort diagnosed before 2016, 48 of 119 (40%) were outside prior AASLD treatment criteria for the period diagnosed, and 16 of 58 (28%) with HCC diagnosed after 2016 were outside the 2018 criteria.
On multivariate analysis, predictors of HCC comparing all 133 subjects with HBV with and without concomitant liver disease not on NAs who did not meet the 2018 AASLD HBV treatment guidelines and developed HCC to 1110 subjects with HBV not on NAs who did not meet 2018 AASLD HBV treatment guidelines and did not develop HCC included cirrhosis (odds ratio [OR] 17.6, 95% confidence interval [CI] 8.4–31; p < 0.001), higher ALT level (OR 1.02, 95% CI 1.01–1.03; p < 0.001), older age (OR 1.08, 95% CI 1.05–1.12; p < 0.001), ALD (OR 18.9, 95% CI 5.8–67; p < 0.001), and NAFLD (OR 2.9, 95% CI 1.05–7.7; p < 0.04). Higher serum albumin level was associated with lower odds of HCC (OR 0.18, 95% CI 0.09–0.3; p < 0.001) in this analysis (Table 4). Significant predictors of HCC on time‐to‐event analysis with multivariable Cox proportional hazards modeling included cirrhosis (hazard ratio [HR] 10, 95% CI 5.8–17.5; p < 0.001), higher ALT level (HR 1.004, 95% CI 1.002–1.006; p < 0.001), older age (HR 1.04, 95% CI 1.03–1.06; p < 0.001), ALD (HR 1.9, 95% CI 1.2–3.1; p < 0.007), and NAFLD (HR 1.7, 95% CI 1.1–2.8; p < 0.02). Again, higher serum albumin level was associated with lower odds of HCC (HR 0.4, 95% CI 0.3–0.6; p < 0.001) (Table 5).
TABLE 4.
Multivariate analysis of predictors of HCC comparing all subjects with HBV (with and without concomitant liver disease) not on NAs who did not meet the 2018 AASLD HBV treatment guidelines and developed HCC to subjects with HBV not on NAs who did not meet the 2018 AASLD HBV treatment guidelines and did not develop HCC
| Factor | HBV/no NA/HCC (n = 133) n (%) or median (IQR) | HBV/no NA/no HCC (n = 1110) n (%) or median (IQR) | OR (95% CI) | p‐Value |
|---|---|---|---|---|
| Asian race | 61 of 133 (45.9%) | 260 of 407 (63.9%) | 1.3 (0.6–2.9) | 0.4 |
| Cirrhosis | 115 of 133 (86.5%) | 111 (10%) | 17.6 (8.4–31) | <0.001 |
| ALP (U/L) | 124.1 (87.5–177.6) | 76 (61–86) | 1.005 (0.9–1.01) | 0.1 |
| Platelets (K/ul) | 142.9 (91.3–200) | 217 (187–249) | 0.997 (0.9–1.002) | 0.2 |
| Na (mmol/L) | 137.5 (136–139) | 139 (138–140) | 0.94 (0.8–1.08) | 0.4 |
| ALT > 47 IU/L | 67 (48.2–107) | 33 (26–41) | 1.02 (1.01–1.03) | <0.001 |
| Age > 54 years | 61 (55.2–69.5) | 42 (33–53) | 1.08 (1.05–1.12) | <0.001 |
| Albumin > 4 g/dl | 3.3 (2.8–3.7) | 4.2 (3.9–4.4) | 0.18 (0.09–0.3) | <0.001 |
| ALD | 48 (36%) | 10 (1%) | 18.9 (5.8–67) | <0.001 |
| NAFLD | 16 (15%) | 62 (5%) | 2.9 (1.05–7.7) | 0.04 |
| HCV | 34 (22%) | 92 (8%) | 1.6 (0.6–4.4) | 0.3 |
Note: Model F‐statistics p‐value < 0.001.
Abbreviations: CI, confidence interval; OR, odds ratio.
TABLE 5.
Cox proportional hazard model of predictors of HCC comparing all subjects with HBV (with and without concomitant liver disease) not on NAs who did not meet the 2018 AASLD HBV treatment guidelines and developed HCC to subjects with HBV not on NAs who did not meet the 2018 AASLD HBV treatment guidelines and did not develop HCC
| Factor | HBV/no NA/HCC (n = 133) n (%) or median (IQR) | HBV/no NA/no HCC (n = 1110) n (%) or median (IQR) | HR (95% CI) | p‐Value |
|---|---|---|---|---|
| Cirrhosis | 115 of 133 (86.5%) | 111 (10%) | 10 (5.8–17.5) | <0.001 |
| NAs (mmol/L) | 137.5 (136–139) | 139 (138–140) | 0.96 (0.91–1.02) | 0.18 |
| ALT > 47 IU/L | 67 (48.2–107) | 33 (26–41) | 1.004 (1.002–1.006) | <0.001 |
| Age > 54 years | 61 (55.2–69.5) | 42 (33–53) | 1.04 (1.03–1.06) | <0.001 |
| Albumin > 4 g/dl | 3.3 (2.8–3.7) | 4.2 (3.9–4.4) | 0.4 (0.3–0.6) | <0.001 |
| ALD | 48 (36%) | 10 (1%) | 1.9 (1.2–3.1) | 0.007 |
| NAFLD | 16 (15%) | 62 (5%) | 1.7 (1.1–2.8) | 0.02 |
| HCV | 34 (22%) | 92 (8%) | 1.6 (0.9–2.7) | 0.09 |
Abbreviation: HR, hazard ratio.
Kaplan–Meier analysis demonstrated that the cumulative incidence of HCC in subjects with cirrhosis who were outside HBV treatment practice guidance was significantly higher (115 of 226 [51%]) compared to subjects without cirrhosis who were outside HBV treatment practice guidance (18 of 1017 [2%]) (p < 0.01) (Figure 2A). In addition, Kaplan–Meier analysis also demonstrated that the cumulative incidence of HCC in subjects with compensated cirrhosis who were receiving NA therapy was significantly lower (112 of 348 [32%]) compared to subjects with compensated cirrhosis who were outside current HBV treatment practice guidance (115 of 226 [51%]) (p < 0.001) (Figure 2B). The cumulative incidence of HCC in all subjects with HBV/cirrhosis who were receiving NA therapy was higher (158 of 412 [38%]) compared to all subjects with HBV/cirrhosis not receiving NA therapy with undetectable HBV DNA (143 of 485 [29%]), but was not statistically significant (p < 0.07) (Figure 2C). Finally, the cumulative incidence of HCC in subjects with HBV/decompensated cirrhosis who were receiving NAs was higher at 96 of 114 (84%) compared to 26 of 230 (13%) in subjects with HBV/decompensated cirrhosis not receiving NA therapy (p < 0.001) (Figure 2D).
FIGURE 2.
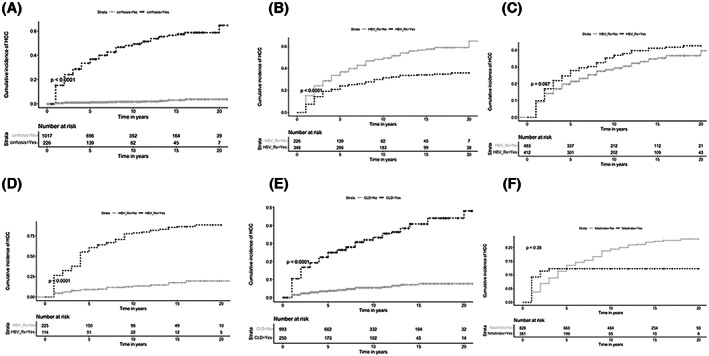
Cumulative incidence of hepatocellular carcinoma (HCC) over study period. (A) Cumulative incidence of HCC in subjects outside hepatitis B virus (HBV) treatment practice guidance with cirrhosis (115 of 226 [51%]) compared to subjects without cirrhosis who were outside HBV treatment practice guidance (18 of 1017 [2%]). (B) Cumulative incidence of HCC comparing subjects with compensated HBV/cirrhosis who were receiving nucleos(t)ide analogue (NA) therapy (112 of 348 [32%]) to subjects with compensated HBV/cirrhosis not receiving NAs therapy who were outside HBV treatment practice guidance (115 of 226 [51%]). (C) Cumulative incidence of HCC comparing all subjects with HBV/cirrhosis who were receiving NA therapy (158 of 412 [38%]) to all subjects with HBV/cirrhosis not receiving NAs therapy (143 of 485 [29%]). (D) Cumulative incidence of HCC comparing subjects with HBV/decompensated cirrhosis who were receiving NA therapy (96 of 114 [84%]) to subjects with HBV/decompensated cirrhosis not receiving NA therapy (26 of 230 [13%]). (E) Cumulative incidence of HCC in subjects with HBV and concomitant chronic liver disease (CLD) outside HBV treatment practice guidance (86 of 250 [34%]) compared to subjects with HBV and without concomitant CLD who were outside HBV treatment practice guidance (47 of 993 [5%]). (F) Cumulative incidence of HCC comparing all subjects with HBV who were receiving NA therapy at index presentation to subjects who received NAs therapy later on during the study period. *HBV_Rx indicates NA therapy.
We also compared the characteristics of those who initiated NA therapy after the initial study observation period compared with those who were on NAs at time of presentation (Table 6). We noted no significant difference in the cumulative incidence of HCC comparing all subjects with HBV who were receiving NA therapy at index presentation (n = 46) to subjects who received NA therapy later on during the study period (n = 163) (Figure 2F).
TABLE 6.
Baseline characteristics of patients on NA therapy at index presentation and developed HCC and patient who began on NA therapy later during the study period and developed HCC
| Factor | On NAs at index presentation (n = 46), median (IQR) | Began NA therapy later (n = 163), median (IQR) | p‐Value |
|---|---|---|---|
| ALT (IU/L) | 88 (55–116) | 66 (48–105) | 0.1 |
| HBV DNA (mIU/ml) | Undetected (undetected–undetected) | 20 (undetected–51) | <0.001 |
| ALP (U/L) | 131 (131–137) | 131 (113–131) | 0.02 |
| AST (IU/L) | 100 (60–159) | 72 (46–117) | 0.01 |
| TB (mg/dl) | 1.4 (0.8–2.6) | 0.9 (0.7–1.5) | 0.01 |
| INR | 1.3 (1.1–1.5) | 1.2 (1.1–1.3) | 0.06 |
| NAs (mmol/L) | 137 (133–139) | 139 (137–146) | <0.001 |
| Platelets (K/ul) | 170 (122–215) | 154 (120–204) | 0.5 |
| Albumin (g/dl) | 3.8 (2.9–3.9) | 3.8 (3.3–4) | 0.5 |
In the subgroup analysis examining a cohort of 146 subjects with HBV and known concomitant CLD (HCV, ALD, and/or NAFLD) who developed HCC while not on NA therapy, 48 of 146 (32.9%) subjects presented initially with HBV and HCC, whereas 98 of 146 (67.1%) developed HCC subsequently on follow‐up. Only 12 of 98 (12.2%) met the 2018 AASLD HBV treatment criteria, and 86 of 98 (87.7%) were outside the criteria. Significantly more subjects with HBV and concomitant CLD presented initially with HBV and HCC (48 of 146 [32.9%] compared to 15 of 94 [15.9%] subjects with HBV only [p < 0.004]). Significantly more subjects with HBV and concomitant CLD were outside the 2018 AASLD HBV treatment criteria (86 of 98 [87.7%] compared to 47 of 79 [59%] subjects with HBV alone [p < 0.001]). The cumulative incidence of HCC in subjects with HBV and concomitant CLD outside HBV treatment practice guidance was higher at 86 of 250 (34%) compared to subjects with HBV and without concomitant CLD who were outside HBV treatment practice guidance (47 of 993 [5%]) (Figure 2E; Table 7).
TABLE 7.
Baseline characteristics of subjects with HBV not on NAs who did not meet the 2018 AASLD HBV treatment guidelines and developed HCC (no NA/HCC), and subjects with HBV on NAs who developed HCC (NA/HCC) at time of HCC diagnosis
| Factor | No NA/HCC (n = 133) Median (IQR) | NA/HCC (n = 209) Median (IQR) | p‐Value |
|---|---|---|---|
| ALT (IU/L) | 60 (42–117) | 56 (39–99) | 0.5 |
| HBV DNA (mIU/ml) | Undetected (undetected–undetected) | 20 (undetected–51) | <0.001 |
| ALP (U/L) | 129 (93–194) | 108 (81–147) | 0.001 |
| AST (IU/L) | 64 (40–115) | 50 (31–128) | 0.1 |
| TB (mg/dl) | 1.2 (0.7–1.8) | 0.9 (0.6–1.4) | 0.02 |
| INR | 1.2 (1.1–1.4) | 1.2 (1.1–1.3) | 0.02 |
| NAs (mmol/L) | 137 (135–139) | 138 (136–140) | 0.1 |
| Platelets (K/ul) | 117 (88–170) | 139 (101–198) | 0.003 |
| Albumin (g/dl) | 3.4 (2.8–3.7) | 3.6 (3–4.1) | <0.001 |
DISCUSSION
Hepatitis B remains the most common cause of liver cancer worldwide with treatment of chronic hepatitis B.[ 1 ] Moreover, the risk of HCC development has been correlated across a biologic gradient of viral levels that has in part guided our approach to the treatment of those with chronic hepatitis B.[ 2 ] NA therapy with viral suppression has been well‐reported to reduce—though not eliminate—the risk of HCC in those with HBV with more pronounced benefit in those with cirrhosis and is the most impactful intervention available to clinicians to reduce—though not eliminate—HCC risk.[ 6 ] Our study evaluated a cohort of individuals infected with HBV over 2 decades and assessed the incidence and risk factors associated with the development of HCC. In our cohort, the overall rate of new HCC diagnosis was 386 of 2846 (14%), with most of these individuals receiving NA treatment at the time of diagnosis (209 of 386 [54%]).
Receiving NA therapy was assessed in correlation with published HBV practice guidance at the time subjects were seen. Hepatitis B treatment practice guidance has evolved over the years, particularly in the treatment of those with cirrhosis, with changes in 2016 recommending antiviral therapy for subjects with HBV with compensated cirrhosis and low‐level viremia (HBV DNA < 2000 IU/ml).[ 8 ] More recently, an additional recommendation was added for initiation of antiviral therapy in those with decompensated cirrhosis.[ 7 ] The European Guidance on hepatitis B infection recommends treatment with any detectable HBV‐DNA level in those with compensated or decompensated cirrhosis, regardless of ALT level; however, it does not make a specific recommendation regarding those with undetectable HBV DNA and compensated cirrhosis in addition to recommending treatment in those with decompensated cirrhosis, regardless of HBV‐DNA level.[ 10 ] Finally, Asian‐Pacific clinical practice guidelines recommend treatment in compensated cirrhosis with HBV‐DNA levels > 2000 IU/ml with any ALT level, and treatment is recommended for those with decompensated cirrhosis with any HBV DNA level.[ 11 ]
In our cohort, 133 of 177 (75%) subjects with HBV who developed HCC would not receive antiviral therapy based on current guidance for HBV treatment, including 115 of 177 (65%) subjects with compensated cirrhosis and undetectable HBV DNA. The other individuals had noncirrhotic HBV with viral levels above 2000 IU/L but ALT less than 2 times the ULN and would have been treated using the US treatment algorithm guidance or 2018 AASLD guidance.[ 7 , 9 ] As expected, when compared with the 1110 subjects who were also untreated and did not develop HCC, significant predictors of HCC in these 133 subjects included cirrhosis, older age, higher ALT presence of concomitant ALD, and NAFLD (Tables 4 and 5), all of which are well‐reported as predictors of HCC in HBV infection.[ 12 ] Higher albumin was associated with lower odds of HCC (Tables 4 and 5). In the cohort of 115 subjects with compensated cirrhosis, we noted that 110 of 115 (96%) subjects had ALT > ULN (35 IU/L in males, 25 IU/L in females) (Table 3). Current practice guidance does not make specific recommendations for this cohort; however, in those with cirrhosis and detectable HBV DNA < 2000 IU/L, therapy is now recommended in this group. It is not known whether antiviral therapies will reduce the risk of HCC in those with compensated cirrhosis and undetectable HBV DNA.
The cumulative incidence of HCC in subjects with cirrhosis who were outside HBV treatment practice guidance was significantly higher at 51% compared to 2% in subjects without cirrhosis and was associated with the highest risk of HCC in our cohort, consistent with previous reports noting cirrhosis as the dominant risk factor for HCC.[ 2 ] We also observed that the cumulative incidence of HCC was significantly higher in subjects with HBV and compensated cirrhosis who were not receiving NA therapy compared to subjects with HBV and cirrhosis who were receiving NA therapy who also had undetectable HBV DNA (Figure 2B). The reason for the higher rate of HCC in those not on NA therapy with undetectable HBV DNA is not clear but likely reflects prior or ongoing hepatocellular injury contributing to changes in cell signaling pathways and cell cycle regulation.[ 13 ] On the other hand, while we observed no significant differences in incidence between all patients with HBV and cirrhosis receiving NA therapy and no therapy, we also noted that the cumulative incidence of HCC was higher in subjects with HBV/decompensated cirrhosis who were receiving NA therapy compared to subjects with HBV/decompensated cirrhosis not receiving NA therapy (Figure 2C,D). The incidence of HCC in our decompensated cohort who received NA therapy was consistent with a recent report noting a 31.8% incidence rate of HCC in individuals infected with HBV with decompensated cirrhosis who were taking NA therapy.[ 14 ] However, the incidence of HCC in the decompensated cirrhosis cohort with undetectable HBV DNA not on NAs was lower than expected. Many individuals in our cohort had concomitant liver disease, and it is possible that the decompensation episodes were related to diseases other than hepatitis B. The cumulative incidence of HCC in subjects with HBV and concomitant CLD outside HBV treatment practice guidance was higher compared to subjects with HBV and without concomitant CLD who were outside HBV treatment practice guidance.
We noted elevated ALT levels in most individuals who developed HCC who were not taking NAs. An elevated ALT has been reported to be an independent risk factor for cirrhosis and HCC in those with hepatitis B.[ 15 ] In addition, multiple reports have noted that lack of ALT normalization was associated with higher risk of HCC in those receiving NA therapy.[ 16 , 17 , 18 ] In our cohort, most individuals who developed HCC had ALT levels above the ULN as defined by AASLD criteria, regardless of whether concomitant liver disease was present, and regardless of whether they were receiving NAs therapy. Individuals in this group may have intermittent low‐level viral replication, replication beneath the level of detection, or other etiologies contributing to their elevated ALT level such as steatosis. We compared ALT levels in this compensated cirrhosis untreated group who developed HCC, to those who developed HCC on NAs therapy without concomitant liver disease, and while the median ALT levels were elevated in both groups, this difference did not reach significance. A prior report noted a higher risk of HCC in those who were fully suppressed on NA therapy who had elevated baseline ALT levels compared to those with inactive hepatitis B with ALT levels < 40 IU/L, we did not have access to pretreatment baseline ALT levels in all subjects in our cohort.[ 19 ] To date, no studies have assessed prospectively the introduction of NA therapy in those with compensated cirrhosis with HBV infection with undetectable viremia.
A recent report assessed the incidence of HCC in untreated subjects with HBV, without and with cirrhosis, outside of multiple treatment guidelines (AASLD, European Association for the Study of the Liver, and Asian Pacific Association for the Study of the Liver), and noted that 46% of individuals who developed HCC were outside the AASLD guidance.[ 20 ] They noted an 8.2% incidence rate of HCC over 5 years in untreated subjects with cirrhosis with undetectable HBV‐DNA levels. In their coverall cohort consisting of those with and without cirrhosis, a higher incidence of HCC in those with undetectable HBV DNA was also noted in those with ALT > 40 IU/L, although the ALT levels were not reported separately in those with cirrhosis and undetectable HBV DNA. A risk model was proposed consisting of age, sex, hepatitis B e antigen, cirrhosis, ALT, and platelet levels, which were independent factors associated with HCC development.
Limitations to our study included reporting single‐center, retrospective data. Prior assays for HBV DNA were not as sensitive as current assays, and some subjects would likely be classified differently with the standardized use of more sensitive assays. It is possible that there was intermittent viremia not captured during the study observation period. It is also possible that concomitant liver disease was present, although not identified, in those with elevated ALT levels. It is possible that there were errors in diagnoses in this database. We were unable to assess treatment durations or indications for initiation of NAs treatment and could not determine the reasons why individuals were or were not on treatment.
CONCLUSIONS
We found that those with untreated compensated cirrhosis with HBV and undetectable HBV DNA who do not meet our current guidance for treatment had higher rates of HCC than those with compensated cirrhosis and suppressed HBV DNA by NA therapy. There were high rates of ALT elevations above the ULN, regardless of whether subjects were on NA therapy. This study highlights the need for earlier diagnosis and treatment of hepatitis B before the development of cirrhosis as well as close monitoring of this cohort for the development of HCC. Although current NA therapy is directed toward suppressing HBV‐DNA levels, there are ongoing efforts underway to develop therapies to achieve functional cure with hepatitis B surface antigen clearance.[ 21 ] These results, if validated, could help reshape practice guidance to incorporate HBV treatment for all subjects with compensated or decompensated cirrhosis, regardless of ALT or HBV‐DNA levels.
CONFLICT OF INTEREST
WRK consults for and advises for Gilead. He received grants from Roche. MN consults for and advises Intercept, GSK, Eli Lilly, and Laboratory of Advanced Medicine. She received grants from Pfizer, Enanta, Vir Biotech, Helio Health, National Cancer Institute, Glycotest, B.K. Kee Foundation, and CurveBio. She also consults for, advises, and received grants from Gilead and Exact Sciences. PK advises Antios and Enanta. He advises and received grants from Gilead. He received grants from Assembly, Bristol‐Myers Squibb, TARGET registries, and Altimmune.
Alshuwaykh O, Daugherty T, Cheung A, Goel A, Dhanasekaran R & Ghaziani TT et al. Incidence of hepatocellular carcinoma in chronic hepatitis B virus infection in those not meeting criteria for antiviral therapy. Hepatol Commun. 2022;6:3052–3061. 10.1002/hep4.2064
REFERENCES
- 1. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen C, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. [DOI] [PubMed] [Google Scholar]
- 3. Yang H‐I, Lu S‐N, Liaw Y‐F, You S‐L, Sun C‐A, Wang L‐Y, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168–74. [DOI] [PubMed] [Google Scholar]
- 4. Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–31. [DOI] [PubMed] [Google Scholar]
- 5. Sung J, Tsoi K, Wong V, Li K, Chan H. Meta‐analysis: treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2008;28:1067–77. [DOI] [PubMed] [Google Scholar]
- 6. Papatheodoridis GV, Chan HL‐Y, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62:956–67. [DOI] [PubMed] [Google Scholar]
- 7. Terrault NA, Lok AS, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martin P, Nguyen MH, Dieterich DT, Lau DTY, Janssen HLA, Peters MG, et al. Treatment algorithm for managing chronic hepatitis B virus infection in the United States: 2021 update. Clin Gastroenterol Hepatol. 2022;28:1766–75. [DOI] [PubMed] [Google Scholar]
- 10. Liver EAFTSOT . EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98. [DOI] [PubMed] [Google Scholar]
- 11. Sarin S, Kumar M, Lau G, Abbas Z, Chan H, Chen C, et al. Asian‐Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raffetti E, Fattovich G, Donato F. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: a systematic review and meta‐analysis. Liver Int. 2016;36:1239–51. [DOI] [PubMed] [Google Scholar]
- 13. Zhang W, Wang X, Wang Y, Zhao X, Duan W, Wang Q, et al. Effective viral suppression is necessary to reduce hepatocellular carcinoma development in cirrhotic patients with chronic hepatitis B: Results of a 10‐year follow up. Medicine. 2017;96:e8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu D‐M, Xie J, Ye C‐Y, Qian M‐Y, Xue Y. Risk of hepatocellular carcinoma remains high in patients with HBV‐related decompensated cirrhosis and long‐term antiviral therapy. Can J Gastroenterol Hepatol. 2020;2020:8871024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen C‐J, Yang H‐I. Natural history of chronic hepatitis B REVEALed. J Gastroenterol Hepatol. 2011;26:628–38. [DOI] [PubMed] [Google Scholar]
- 16. Choi J, Kim G‐A, Han S, Lim Y‐S. Earlier alanine aminotransferase normalization during antiviral treatment is independently associated with lower risk of hepatocellular carcinoma in chronic hepatitis B. Am J Gastroenterol. 2020;115:406–14. [DOI] [PubMed] [Google Scholar]
- 17. Kim EJ, Yeon JE, Kwon OS, Lee HN, Shin SK, Kang SH, et al. Rapid alanine aminotransferase normalization with entecavir and hepatocellular carcinoma in hepatitis B virus–associated cirrhosis. Dig Dis Sci. 2017;62:808–16. [DOI] [PubMed] [Google Scholar]
- 18. Wong GL‐H, Chan HL‐Y, Tse Y‐K, Yip TC‐F, Lam KL‐Y, Lui GC‐Y, et al. Normal on‐treatment ALT during antiviral treatment is associated with a lower risk of hepatic events in patients with chronic hepatitis B. J Hepatol. 2018;69:793–802. [DOI] [PubMed] [Google Scholar]
- 19. Cho J‐Y, Paik Y‐H, Sohn W, Cho HC, Gwak G‐Y, Choi MS, et al. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut. 2014;63:1943–50. [DOI] [PubMed] [Google Scholar]
- 20. Sinn DH, Kim SE, Kim BK, Kim JH, Choi MS. The risk of hepatocellular carcinoma among chronic hepatitis B virus‐infected patients outside current treatment criteria. J Viral Hepat. 2019;26:1465–72. [DOI] [PubMed] [Google Scholar]
- 21. Cornberg M, Lok AS‐F, Terrault NA, Zoulim F, Berg T, Brunetto MR, et al. Guidance for design and endpoints of clinical trials in chronic hepatitis B—report from the 2019 EASL‐AASLD HBV Treatment Endpoints Conference. J Hepatol. 2020;72:539–57. [DOI] [PubMed] [Google Scholar]


