Abstract
The routine technique for detecting antibodies specific to infectious bursal disease virus (IBDV) is a serological evaluation by enzyme-linked immunosorbent assay (ELISA) with preparations of whole virions as the antigens. To avoid using complete virus in the standard technique, we have developed two new antigens through the expression of the VPX and VP3 genes in insect cells. VPX and especially VP3 were expressed at high levels in insect cells and simple to purify. The immunogenicity of both proteins was similar to that of the native virus. VPX was able to elicit neutralizing antibodies but VP3 was not. Purified VPX and VP3 were tested in an indirect ELISA with more than 300 chicken sera. There was an excellent correlation between the results of the ELISA using VPX and those of the two commercial kits. VP3 did not perform as well as VPX, and the linear correlation was significantly lower. A comparison with the standard reference technique, seroneutralization, showed that the indirect ELISA was more sensitive. Therefore, VPX-based ELISA is a good alternative to conventional ELISAs that use whole virions.
Infectious bursal disease (IBD), also called Gumboro disease, is a highly contagious viral disease. It causes heavy economic losses to the poultry industry worldwide, either by causing a high-mortality acute condition or by leading to immunosuppression in young chickens (between 3 and 6 weeks of age) provoked by the destruction of immature B lymphocytes within the bursa of Fabricius (14). IBD virus (IBDV) is the etiological agent of IBD. It belongs to the genus Avibirnavirus of the family Birnaviridae (20). To date, two antigenically distinct serotypes (I and II) of IBDV have been identified (12). Serotype I infects chickens and comprises at least six different subtypes of IBDV, which vary considerably in virulence (10). Viruses in one of these subtypes are routinely known as variant strains, whereas viruses in the other subtypes are known as classic strains. Serotype II infects mainly turkeys and is not pathogenic for chickens (12).
The IBDV genome consists of two segments of double-stranded RNA designated A and B (3). Segment A encodes a 108-kDa polyprotein that is self-cleaved to produce VPX (48 kDa), VP3 (32 kDa), and VP4 (28 kDa). In the mature virions, VPX is processed into VP2 (41 kDa). VP2 and VP3 are the major structural proteins of the IBDV virion. VP2 has been identified as the main host-protective antigen of IBDV and carries major neutralizing epitopes (1, 2, 4, 23). VP3 is considered a group-specific antigen (2), and monoclonal antibodies directed to VP3 were able to prevent virus attachment (24) and to neutralize the virus (26). However, recombinant VP3 failed to protect chickens from challenge by virulent IBDV (21). Also, VP3 has been suggested to be the major immunogenic protein of IBDV, since the earliest antibodies that appear after infection with live or inactivated viruses are directed to VP3 (5).
IBDV infection in young chickens is controlled by the transfer via yolk sac of maternal antibodies induced by the administration of live attenuated or inactivated virus to breeder hens. To monitor the serostatus of flocks, the enzyme-linked immunosorbent assay (ELISA) is routinely used by diagnostic laboratories and poultry producers worldwide (16a, 25a). Commercial ELISA kits are available to detect antibodies for IBDV in field samples. These kits are based on the use of whole virus preparations, which are produced by conventional technology, as the antigen source. ELISAs based on the use of recombinant virus antigens have been found previously to determine the antigenic relatedness among IBDV strains (11) and to correlate VP2 ELISA titers with protection (13). However, there are no reports of studies using recombinant expression products as ELISA antigens and field samples. Since most of the neutralizing epitopes are located on VP2, it was possible to establish a correlation among the VP2-specific antibody titers, virus neutralization titers, and protection (13).
In this study, we have prepared recombinant IBDV VPX and VP3 proteins expressed in the baculovirus system. The value of the recombinant proteins for diagnostic purposes was tested by indirect ELISA using field chicken sera. The results were compared with those of commercial kits that use whole virus preparations as test antigens and with the seroneutralization assay, which constitutes the reference technique for detecting IBDV antibodies.
MATERIALS AND METHODS
Cells and viruses.
Spodoptera frugiperda clone 9 (Sf9) cells were grown and maintained in suspension or monolayer cultures at 28°C using TNM-FH media supplemented with 5% fetal calf serum (FCS) (Gibco BRL). Wild-type and recombinant strains of Autographa californica nuclear polyhedrosis virus were propagated in Sf9 cells according to standard methods (21).
Baby grivet monkey kidney (BGM70) cells, kindly provided by Y. M. Saif (Ohio State University), were maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL) supplemented with 10% FCS at 37°C in a humidified 8% CO2 incubator. The serotype I IBDV SAL strain and the serotype II IBDV OH strain, both supplied by Y. M. Saif, were grown in BGM70 cell monolayers. The Soroa strain (serotype I) of IBDV (7, 8) was used as the starting material for the cloning of VPX and VP3 antigens.
Sera.
Monospecific rabbit antisera against VPX/VP2 and VP3 were prepared as previously described (8). Rabbit polyclonal antisera against baculovirus-expressed proteins were also produced. Recombinant proteins, expressed and purified as described below, were used to immunize rabbits by following methods described elsewhere (18).
Blood samples from 10-week-old chickens vaccinated at 3 weeks of age were collected from several farms in Spain and supplied by J. C. Abad (Cobb, Madrid, Spain). IBDV-free chicken sera were kindly provided by O. Vainio (Turku University, Turku, Finland).
Construction of recombinant baculoviruses.
The coding sequences of VPX and VP3 were obtained by PCR with Vent DNA polymerase (Biolabs), using the recombinant plasmid pFastBac/POLY as the template (Martínez-Torrecuadrada et al., submitted for publication). The PCR comprised 25 cycles of denaturation at 94°C for 1 min, primer annealing at 50°C for 1 min, and extension at 72°C for 2 min. The oligonucleotides used were IBDV1 (5′ TTCGATGATCACGATGACAAACCTGTCAGATC 3′) and IBDV2 (5′ ACTACTGATCACCCCTTGTCGGCGGCGAGAG 3′), which cover nucleotides 1 to 1548 of open reading frame A1, for VPX gene amplification and IBDV3 (5′ GTACCTGATCACCATGGCTGCATCAGAGTTC 3′) and IBDV4 (5′ GCGGCTGATCACTCAAGGTCCTCATCAGAG 3′), from nucleotides 2260 to 3039 of open reading frame A1, for VP3 gene synthesis, according to previous reports (25). BclI sites, shown in italics, were included to generate BamHI-compatible ends. The resulting PCR products were subjected to digestion with BclI and ligated into BamHI-digested baculovirus transfer vector pAcYM1 (19). The derivative plasmids, pAcYM1-VPX.IBDV and pAcYM1-VP3.IBDV, were proof sequenced. The corresponding recombinant baculoviruses, AcVPX.IBDV and AcVP3.IBDV, were obtained according to standard procedures (15).
Protein expression analysis.
Sf9 cells were infected with the corresponding recombinant baculovirus at a multiplicity of infection (MOI) of 1 PFU/cell. Cells were harvested at 72 h postinfection, washed with phosphate-buffered saline (PBS), and resuspended in 1× loading buffer (10 mM Tris-HCl [pH 6.8], 10% sodium dodecyl sulfate [SDS], 10% β-mercaptoethanol, 0.02% bromophenol blue, 25% glycerol). The mixture was heated at 100°C for 5 min, and proteins were resolved by SDS–11% polyacrylamide gel electrophoresis (PAGE) (16). Gels were stained with Coomassie brilliant blue.
For immunoblotting analyses, proteins resolved by SDS-PAGE were electroblotted onto a Hybond-C nitrocellulose membrane (Amersham Pharmacia Biotech). The membrane was incubated for 1 h in blocking solution (3% milk powder and 0.05% Tween 20 in PBS [PBST]) at room temperature. After blocking, filters were incubated with rabbit VPX/VP2- and VP3-monospecific antisera or rabbit polyclonal sera against recombinant VPX or VP3 for 2 h at room temperature and were diluted 1/500 in blocking buffer. After several washes with PBST, bound antibodies were detected by using alkaline phosphatase-conjugated protein A (Sigma), nitroblue tetrazolium chloride (Gibco BRL), and bromochloroindolyl phosphate (Pierce) as substrates.
Purification of IBDV VPX and VP3.
For VPX, Sf9 cells were infected with the recombinant baculovirus AcVPX.IBDV at an MOI of 1 PFU per cell. Cells were harvested at 72 h postinfection, washed with PBS, and lysed by osmotic shock with 25 mM bicarbonate solution at 4°C. After removal of nuclei and cellular debris by low-speed centrifugation, the supernatant was recovered and layered on top of a 25% (wt/vol) sucrose cushion in PES buffer {25 mM piperazine-N,N′-bis(2-ethanesulfonic acid) [PIPES] [pH 6.2], 150 mM NaCl, and 20 mM CaCl2}; and spun at 125,000 × g for 3 h at 4°C. The pelleted material containing the expressed recombinant protein was resuspended in PES buffer.
For VP3, Sf9 cells were infected with AcVP3.IBDV at an MOI of 1 PFU per cell. At 72 h postinfection, cells were centrifuged for 10 min at 200 × g, washed with PBS, and lysed in 25 mM bicarbonate solution by osmotic shock. Cell lysates were then spun at 10,000 rpm for 15 min in an SS34 rotor, and the resulting pellet, containing most of the VP3 protein, was resuspended in a solution of 50 mM Tris-HCl (pH 8.0), 0.5% glycerol, and 1 M NaCl. After centrifugation at 15,000 × g for 15 min, the supernatant with the fraction soluble in a high salt concentration, highly enriched in recombinant VP3, was collected and used as the antigen source.
The VPX and VP3 concentrations were estimated by Bradford assay (Bio-Rad) and by densitometry of Coomassie blue-stained gels. The purity was determined by SDS-PAGE analysis.
ELISAs.
Polystyrene microtiter plates (Labsystems, Helsinki, Finland) were coated with purified VPX or VP3 in 50 mM carbonate buffer (pH 9.6) overnight at 4°C. The optimal coating concentration for both antigens was 1 μg/ml, as determined by serial titration. After absorption, the plates were washed three times with PBST. All serum samples were diluted 1:100 and 1:500 in blocking buffer (350 mM NaCl and PBST) and incubated for 1 h at 37°C. After four washes with PBST, plates were treated with peroxidase-labeled anti-chicken immunoglobulin G (Sigma) diluted 1/2,000 in blocking buffer for 1 h at room temperature. The reaction was developed by using ABTS [2,2′-azinobis(3-ethylbenzthiazoline sulfonic acid)] (Sigma) as the chromogen. The color development was stopped with 2% SDS after 10 min, and the absorbance was measured at 405 nm using an ELISA reader (Bio-Tek Instruments).
For comparison, two commercial diagnostic kits, the FlockCheck IBD antibody test kit (IDEXX Laboratories, Westbrook, Maine) and the ProFlok IBD antibody test (Kirkegaard & Perry Laboratories [KPL], Gaithersburg, Md.) were used according to the manufacturers' protocols.
IBDV neutralization assay.
The IBDV neutralization capability of chicken and rabbit sera was measured by monolayer protection assay (10). The serum samples were diluted twofold, starting at a 1:25 dilution, in DMEM and mixed with 1.5 × 104 PFU of serotype I (SAL strain) or serotype II (OH strain) per well (final serum dilution, 1:50). After 3 h at 37°C, the antibody-virus mixture was added to 1.5 × 104 BGM70 cells in DMEM–10% FCS per well of a 96-well culture plate (Becton-Dickinson, Lincoln Park, N.J.). After 4 days at 37°C, cell monolayers were washed with PBS and stained for 20 min with 1.5% crystal violet in 50% ethanol. The level of protection was evaluated by visual screening of the infected monolayers. End point titration was determined as the reciprocal value of the highest serum dilution that causes a 50% reduction of the cell monolayer. All incubations were undertaken in the absence of serum complement.
Statistical analysis.
Statistical analysis was performed with the MedCalc software package (26). Regression analysis was used to determine the relationship between ELISA data.
RESULTS
Expression and purification of IBDV VPX.
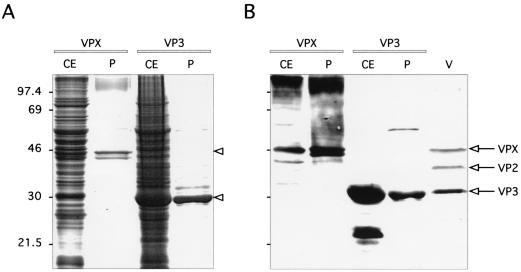
To synthesize recombinant VPX, Sf9 cells were infected with the baculovirus AcVPX.IBDV and harvested 72 h later. The protein extracts were analyzed by SDS–11% PAGE. Results are shown in Fig. 1. An extra band of approximately 48 kDa was found by Coomassie staining to be already in the cell extract (Fig. 1A). This polypeptide was specifically recognized by the VPX/VP2-monospecific serum (Fig. 1B), together with high-molecular-weight aggregates and additional smaller proteins that may represent cleavage products. To determine the correct migration rate of the viral proteins, an IBDV-infected cell extract was also included in the immunoblotting analysis as a control. The expressed VPX protein comigrated with the authentic viral VPX protein (Fig. 1B).
FIG. 1.
Expression and purification of IBDV VPX and VP3 in the baculovirus system. Crude extracts (CE) from cells infected with the corresponding recombinant baculovirus expressing VPX or VP3 and purified recombinant proteins (P) were electrophoresed in an SDS–11% polyacrylamide gel and stained with Coomassie blue (A) or transferred to nitrocellulose for immunoblotting (B). Proteins were incubated with a mixture of monospecific rabbit antisera against VPX/VP2 and VP3 and made to react with alkaline phosphatase-conjugated protein A. BGM70 cells infected with the SAL strain of IBDV (V) were included as a positive control. The numbers on the left indicate the molecular mass, in kilodaltons.
Since the expression of VPX leads to the formation of tubule-like structures (J. L. Martínez-Torrecuadrada, unpublished data), we took advantage of the particulate nature of our product and designed a sucrose cushion-based procedure to purify VPX. With this method, one ultracentrifugation step was enough to obtain a VPX preparation with a purity of about 80% (Fig. 1A). A slightly smaller band was also observed in the purified sample, which was not present in the crude extract. This protein was likely formed as a consequence of a further proteolytic processing of VPX, as demonstrated by its immunoreactivity with the VP2-specific antisera (Fig. 1B).
Expression and purification of IBDV VP3.
To express VP3, AcVP3.IBDV was used to infect Sf9 cells as described above. As shown in Fig. 1, a very strong protein band was noticed in the cell extracts with the same size as that of viral VP3 (30 kDa). The level of expression was about 40 μg/106 cells as estimated by visual comparison. The authenticity of the expressed product was confirmed by immunoblotting analysis using VP3-monospecific serum. A smaller, 24-kDa form of the VP3 protein was also detected. The origin of this protein is not known; it may have arisen by degradation or by in-frame internal initiation at a downstream methionine codon in the VP3-coding sequence.
After cellular lysis by osmotic shock, it was noticeable that the major part of the expressed protein remained insoluble in the nuclear fraction of the infected cells and only traces of VP3 were detected in the soluble cytoplasmic fraction. To increase the solubility of VP3, cells containing VP3 were treated with various denaturing agents, such as 6 M guanidine chloride or 8 M urea. However, the complete solubilization of VP3 was achieved only when the nuclear fraction was resuspended in a high-ionic-strength buffer. Using this procedure, the purity of VP3 preparations was found to be approximately 90% (Fig. 1A) and the final yield of VP3 was estimated to be 80% of the total expressed protein.
Immunogenicity of recombinant proteins.
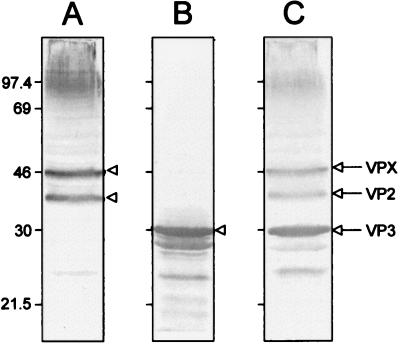
To analyze whether the immunogenicity of recombinant VPX and VP3 mimics that of the authentic viral proteins, rabbits were immunized with purified recombinant VPX or VP3. Antibodies developed against recombinant VPX reacted with IBDV as shown by indirect ELISA, reaching titers of about 106 (data not shown). Similar results were obtained with the anti-VP3 serum. By Western blot analysis, anti-VPX sera were shown to give a positive signal with viral VPX and VP2 (Fig. 2A) and anti-VP3 sera reacted strongly with VP3 and smaller forms of VP3 (Fig. 2B). As a positive control, two monospecific antisera (anti-VPX/VP2 and anti-VP3) were made to react with the same viral extracts (Fig. 2C).
FIG. 2.
Recognition of IBDV VPX, VP2, and VP3 by rabbit anti-recombinant VPX and VP3 sera. BGM70 cell extracts infected with the SAL strain of IBDV were resolved by SDS–11% PAGE and electroblotted onto a nitrocellulose sheet. The nitrocellulose was cut into strips and incubated with rabbit anti-VPX serum (A), rabbit anti-VP3 serum (B), and a mixture of rabbit monospecific sera against VPX/VP2 and VP3 (C) as a positive control. Viral proteins are indicated by arrows and arrowheads on the right of every strip. Molecular mass is given in kilodaltons on the left.
The ability of recombinant VPX and VP3 to induce IBDV-neutralizing antibodies was determined by a monolayer protection assay using the rabbit antisera and the two IBDV serotypes. The results are shown in Table 1. Rabbit serum anti-VPX tubules neutralized the serotype I virus at a titer of 12,800 and also were able to cross-neutralize the serotype II virus, albeit to a lesser extent (titer, 100). However, the rabbit serum elicited against VP3 showed no detectable neutralization activity (titer of <50).
TABLE 1.
Neutralization activity induced by recombinant VPX and VP3
| Serum | Neutralization titera
|
|
|---|---|---|
| Anti-SAL-1 | Anti-OH-2 | |
| Rabbit anti-VPX tubules | 12,800 | 100 |
| Rabbit anti-VP3 | <50 | <50 |
| Preimmune rabbit serum | <50 | <50 |
| Serum from D78-vaccinated chickens | 3,200 | <50 |
| Serum from IBDV-free chickens | <50 | <50 |
Each neutralization titer is the reciprocal value of the highest dilution of the corresponding antiserum that causes a 50% reduction of the cell monolayer in a protection assay described in Materials and Methods. Pooled serum from chickens vaccinated with the D78 strain was included as a positive control. Sera from preimmune rabbits and IBDV-free chickens were used as negative controls. Sera were inactivated for 30 min at 56°C to remove any complement activity.
Use of recombinant VPX and VP3 to detect IBDV-specific antibodies.
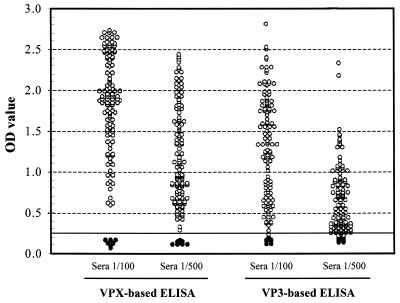
An indirect ELISA was set up to determine the effectivity of recombinant proteins VPX and VP3 as diagnostic reagents for IBDV. Both proteins were tested using an indirect ELISA (Fig. 3). To test the specificity of the assay, a collection of 150 positive chicken sera and 10 negative sera were used at two different dilutions, 1:100 and 1:500. The cutoff value was established with the 10 chicken sera, which were shown to be negative for IBDV-specific antibodies, at 0.25 absorbance units. The cutoff value represents an absorbance 2.5 times greater than that of the blank sera. Sera with absorbance values greater than the cutoff were considered positive. The VPX ELISA was more specific than the VP3 ELISA. At a 1:100 dilution, it was able to clearly discriminate the negative from all the positive sera, including weakly positive sera and doubtful sera. In contrast, although the values of the negative sera were lower with VP3, there was no clear discrimination between negative and weakly positive sera, which is more relevant at a higher dilution of the sera (1:500). Still, at a 1:100 dilution, the VP3 ELISA was specific for positive and negative sera.
FIG. 3.
Use of recombinant VPX or VP3 in the IBDV-specific ELISA. OD values were obtained with two dilutions (1/100 and 1/500) of chicken sera. ●, negative samples; ○, positive samples. The horizontal solid line corresponds to the cutoff value of 0.25.
Comparison of recombinant IBDV capsid proteins with commercial ELISAs.
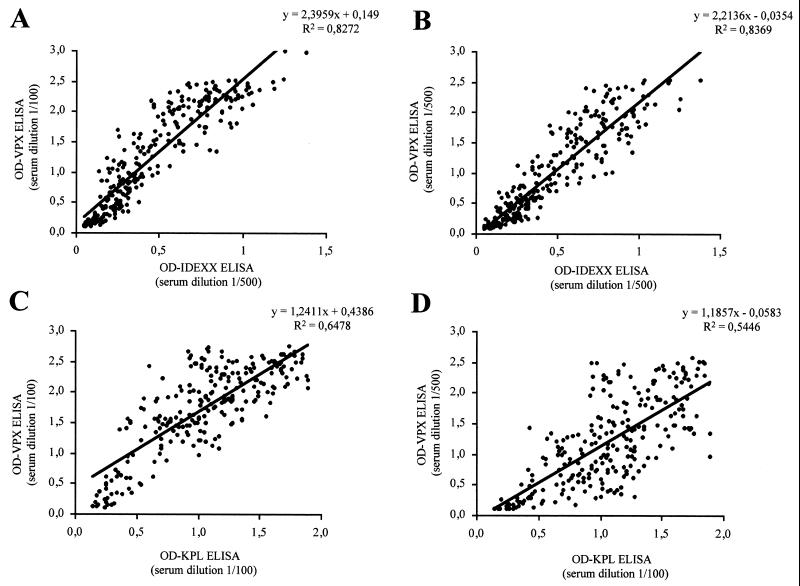
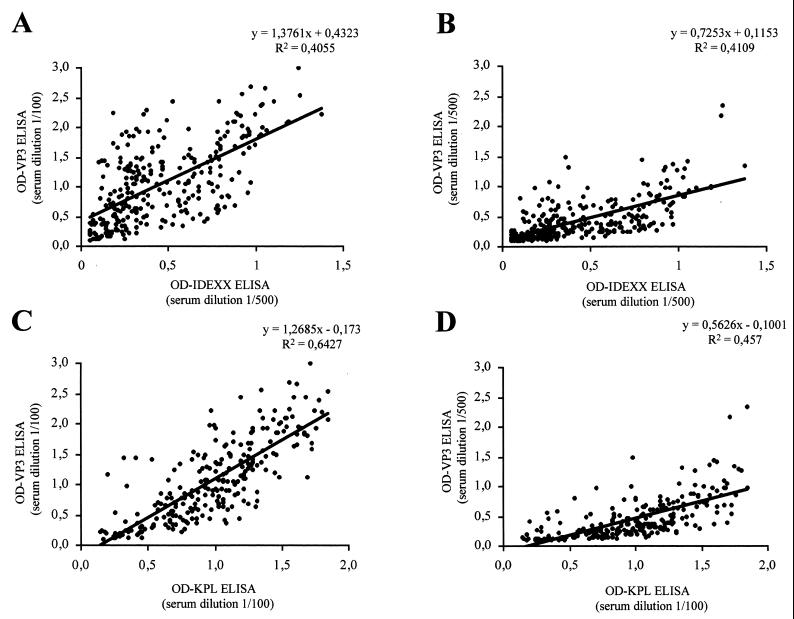
To evaluate the sensitivity and specificity of the indirect ELISA, the results obtained with a collection of 300 field chicken sera using our recombinant proteins VPX and VP3 were compared with those obtained with two commercial kits. Since recommended serum dilutions for these two kits are different (1:500 for IDEXX and 1:100 for KPL), all samples were tested at these two dilutions for comparison. Results, which are shown in Fig. 4 and 5 for VPX and VP3 ELISA, respectively, were recorded as optical density (OD) values obtained for each serum at each dilution. For VPX the best correlation was obtained with the IDEXX kit at a dilution of 1:500 (Fig. 4B), with a correlation coefficient (R2) of 0.837. At a 1:100 dilution the correlation coefficient was also excellent (R2, 0.827) (Fig. 4A). With the KPL ELISA, the correlation coefficient was lower at both dilutions (R2 between 0.54 and 0.64) and values moved in a broader range, but the results were still in good agreement (Fig. 4C and D). For VP3 there was an overall lower correlation with both kits. The best correlation (R2 = 0.643) was obtained with the KPL kit at a 1:100 dilution (Fig. 5C). In this case, the correlation of the results with those from the IDEXX kit was lower (R 2 < 0.5) (Fig. 5A and B). On the basis of these correlations, it is clear that the recombinant VPX-based ELISA is a good alternative to currently available kits, yielding similar if not superior results.
FIG. 4.
Correlation between the VPX-based ELISA and commercial kits. OD measurements using the IDEXX kit were obtained by diluting the chicken sera 1/500 and were compared with those obtained from serum dilutions of 1/100 (A) and 1/500 (B) in the VPX ELISA. When the KPL kit was used, chicken sera were diluted 1/100 and OD values were compared with those obtained from serum dilutions of 1/100 (C) and 1/500 (D) in the VPX ELISA. The linear regression formula and correlation coefficient are shown at the top of each plot.
FIG. 5.
Relationship between commercial kits and the VP3-based ELISA. OD measurements using the IDEXX kit were obtained as for Fig. 4 and compared with those obtained from serum dilutions of 1/100 (A) and 1/500 (B) in the VP3 ELISA. When the KPL kit was used, chicken sera were diluted 1/100 and OD values were compared with those obtained from serum dilutions of 1/100 (C) and 1/500 (D) in the VP3 ELISA. The linear regression formula and correlation coefficient are shown at the top of each plot.
Correlation between seroneutralization and ELISA titers.
Seroneutralization is the reference technique for diagnosing IBDV. Therefore, establishing a correlation with this technique is essential to standardize any diagnostic technique. In order to make this correlation, the same panel of sera was tested for neutralization. Results are shown in Table 2. In general, there was good agreement between the neutralization titers and the VPX ELISA; all the neutralization-positive sera were positive by ELISA. The same situation was found with the commercial kits. In contrast, 10 neutralizing sera were not recognized in the VP3 ELISA. However, there was a small percentage of sera in all the assays that were positive for ELISA but negative for neutralization. This happened mainly with low-titer sera. This could be explained by a major difference in sensitivity between the ELISA and seroneutralization.
TABLE 2.
Correlation between ELISA and seroneutralization results
| Antigen or kita | ELISA resultb | No. of serum samples (%) with seroneutralization resultc
|
|
|---|---|---|---|
| Positive | Negative | ||
| VPX | Positive | 254 (100) | 13 (43.3) |
| Negative | 0 (0) | 17 (56.6) | |
| IDEXX | Positive | 254 (100) | 16 (53.3) |
| Negative | 0 (0) | 14 (46.6) | |
| KPL | Positive | 254 (100) | 22 (73.3) |
| Negative | 0 (0) | 8 (26.6) | |
| VP3 | Positive | 244 (96) | 22 (73.3) |
| Negative | 10 (4) | 8 (26.6) | |
Sera were diluted 1/500 for the IDEXX kit or 1/100 for the KPL kit (manufacturers' recommended dilution) and 1/500 for the VPX- and VP3-based ELISAs.
For the IDEXX and KPL kits, a serum was considered positive or negative for IBDV according to manufacturers' calculations. For VPX- and VP3-based ELISAs, a serum was considered positive if its OD value was above the cutoff level (0.25).
A serum was positive for neutralization if its titer was >50. The percentage was calculated as the number of sera positive or negative relative to the total of sera positive or negative for neutralization, respectively.
DISCUSSION
In the poultry field, where an enormous number of samples has to be analyzed in a simple and economical way, cheap and reproducible antigens are essential. At the moment, the only kits available for the detection and monitoring of IBDV antibodies are based on ELISAs that use whole virions as the antigen. The propagation of IBDV in embryonated eggs or in embryo cells is very time-consuming and labor-intensive. In this study we investigated the two major IBDV antigens, VPX and VP3 of the viral capsid, expressed in insect cells as a feasible alternative in terms of safety and economy to these kits. There have been some previous studies on the diagnostic use of recombinant IBDV antigens (11, 13). However, none of the reported antigens was identical to those described in this report, purified VPX and VP3.
Recombinant baculovirus VPX presents the advantage of being a particulate antigen, forming tubule-like structures, where the capsomer structure is very similar if not identical to the virions (J. L. Martínez-Torrecuadrada et al., unpublished data). VPX as the precursor of VP2 contains all the neutralizing domains and is probably the critical protein for IBDV protection. VPX is expressed at higher levels and is more easily purified than the polyprotein (13). On the other hand, recombinant VP3 is overexpressed at very high levels in the insect cells and can be easily purified. VP3 forms the scaffolding of the virion and is not accessible to antibodies (Martínez-Torrecuadrada et al., submitted for publication). Thus, it is well conserved in all the IBDV isolates and could be of interest for the detection of invariant antibodies.
In both proteins, immunogenicity and antigenicity were well preserved after expression, as the recombinant proteins were able to satisfactorily immunize rabbits, eliciting a strong and specific immune response. The polyclonal antisera allowed us to confirm the ability of VPX to induce neutralizing antibodies in contrast to VP3, which was completely inefficient. These results confirm previous observations of the ability of VPX and VP2 to neutralize IBDV (1, 2, 4, 9, 24) and also show that the conformational neutralizing epitopes are present in recombinant VPX, as was expected due to the similar structure of the capsomers. However, they disagree with some previous data on the neutralizing ability of VP3 (6, 21, 26). Regarding the antigenicity, the dose required for coating the ELISA plates is similar to or lower than the antigen concentration using whole virus, confirming recombinant VPX's good antigenicity and suitability as a diagnostic tool.
The correlation with the seroneutralization assay was similar to that found with the IDEXX kit, which can be considered good and supports even more the use of VPX as an antigen. The reasons some sera are positive by ELISA and negative by neutralization are not completely understood yet. Moreover, it is necessary to stress that most of the sera with discrepant results gave low ELISA titers, suggesting that these animals were weakly immunized or had low titers of antibody against IBDV. Since the ELISA in general is more sensitive than other serological techniques, it is not surprising that a few positive sera are not identifiable by seroneutralization. Other possible reasons, like the presence of variant strains, do not provide a consistent explanation, as variability occurs even within animals from the same farm, vaccinated according to the same protocols, and exposed to the same environment.
In summary, the results of the present study regarding specificity, sensitivity, and correlation with other diagnostic systems demonstrated that recombinant VPX can be expressed in and purified from insect cells cheaply and reproducibly. This recombinant protein, as well as recombinant VP3, closely resembles the native proteins in size and antigenicity. The ELISA developed with recombinant VPX was as sensitive and specific as the conventional commercial kits, which use whole virion as the antigen. On the basis of these results, the baculovirus expression system is an excellent alternative for producing IBDV VPX at high levels, which can be applied economically to detect and monitor IBDV-specific antibodies in chickens and other avian species.
ACKNOWLEDGMENTS
We thank Y. Saif, J. C. Abad, and O. Vainio for kind supply of materials and samples.
This project was partially funded by grant 09/038/1997 of the Comunidad de Madrid.
REFERENCES
- 1.Azad A A, Jagadish M N, Brown M A, Hudson P J. Deletion mapping and expression in Escherichia coli of the large genomic segment of a birnavirus. Virology. 1987;161:145–152. doi: 10.1016/0042-6822(87)90180-2. [DOI] [PubMed] [Google Scholar]
- 2.Becht H, Muller H, Muller H K. Comparative studies on structural and antigenic properties of two serotypes of infectious bursal disease virus. J Gen Virol. 1988;69:631–640. doi: 10.1099/0022-1317-69-3-631. [DOI] [PubMed] [Google Scholar]
- 3.Dobos P, Hill B J, Hallett R, Kells D T C, Becht H, Teninges D. Biophysical and biochemical characterization of five animal viruses with bisegmented double-stranded RNA genomes. J Virol. 1979;32:593–605. doi: 10.1128/jvi.32.2.593-605.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahey K J, Erny K, Crooks J. A conformational immunogen on VP-2 of infectious bursal disease virus that induces virus-neutralizing antibodies that passively protect chickens. J Gen Virol. 1989;70:1473–1481. doi: 10.1099/0022-1317-70-6-1473. [DOI] [PubMed] [Google Scholar]
- 5.Fahey K J, O'Donnell I J, Azad A A. Characterization by Western blotting of the immunogens of infectious bursal disease virus. J Gen Virol. 1985;66:1479–1488. doi: 10.1099/0022-1317-66-7-1479. [DOI] [PubMed] [Google Scholar]
- 6.Fahey K J, O'Donnell I J, Bagust T J. Antibody to the 32K structural protein of infectious bursal disease virus neutralizes viral infectivity in vitro and confers protection on young chickens. J Gen Virol. 1985;66:2693–2702. doi: 10.1099/0022-1317-66-12-2693. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Arias A, Martínez S, Rodríguez J F. The major antigenic protein of infectious bursal disease virus, VP2, is an apoptotic inducer. J Virol. 1997;71:8014–8018. doi: 10.1128/jvi.71.10.8014-8018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Arias A, Risco C, Martinez S, Albar J P, Rodriguez J F. Expression of ORF A1 of infectious bursal disease virus results in the formation of virus-like particles. J Gen Virol. 1998;79:1047–1054. doi: 10.1099/0022-1317-79-5-1047. [DOI] [PubMed] [Google Scholar]
- 9.Heine H G, Haritou M, Failla P, Fahey K, Azad A. Sequence analysis and expression of the host-protective immunogen VP2 of a variant strain of infectious bursal disease virus which can circumvent vaccination with standard type I strains. J Gen Virol. 1991;72:1835–1843. doi: 10.1099/0022-1317-72-8-1835. [DOI] [PubMed] [Google Scholar]
- 10.Jackwood D H, Saif Y M. Antigenic diversity of infectious bursal disease viruses. Avian Dis. 1987;31:766–770. [PubMed] [Google Scholar]
- 11.Jackwood D J, Henderson K S, Jackwood R J. Enzyme-linked immunosorbent assay-based detection of antibodies to antigenic subtypes of infectious bursal disease viruses of chickens. Clin Diagn Lab Immunol. 1996;3:456–463. doi: 10.1128/cdli.3.4.456-463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackwood D J, Saif Y M, Hughes J H. Characteristics and serologic studies of two serotypes of infectious bursal disease virus in turkeys. Avian Dis. 1982;26:871–882. [PubMed] [Google Scholar]
- 13.Jackwood D J, Sommer S E, Odor E. Correlation of enzyme-linked immunosorbent assay titers with protection against infectious bursal disease virus. Avian Dis. 1999;43:189–197. [PubMed] [Google Scholar]
- 14.Kibenge F S, Dhillon A S, Russell R G. Biochemistry and immunology of infectious bursal disease virus. J Gen Virol. 1988;69:1757–1775. doi: 10.1099/0022-1317-69-8-1757. [DOI] [PubMed] [Google Scholar]
- 15.Kitts P A, Possee R D. A method for producing recombinant baculovirus expression vectors at high frequency. BioTechniques. 1993;14:810–817. [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16a.Marquardt W W, Johnson R B, Odenwald W F, Schlotthober B A. An indirect enzyme-linked immunosorbent assay (ELISA) for measuring antibodies in chickens infected with infectious bursal disease virus. Avian Dis. 1980;24:375–385. [PubMed] [Google Scholar]
- 17.Martinez-Torrecuadrada J L, Iwata H, Venteo A, Casal I, Roy P. Expression and characterization of the two outer capsid proteins of African horsesickness virus: the role of VP2 in virus neutralization. Virology. 1994;202:348–359. doi: 10.1006/viro.1994.1351. [DOI] [PubMed] [Google Scholar]
- 18.Matsuura Y, Possee R D, Overton H A, Bishop D H L. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J Gen Virol. 1987;68:1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- 19.Mayo M A, Pringle C R. Virus taxonomy—1997. J Gen Virol. 1998;79:649–657. doi: 10.1099/0022-1317-79-4-649. [DOI] [PubMed] [Google Scholar]
- 20.Pitcovski J, Levi B Z, Maray T, Di-Castro D, Safadi A, Krispel S, Azriel A, Gutter B, Michael A. Failure of viral protein 3 of infectious bursal disease virus produced in prokaryotic and eukaryotic expression systems to protect chickens against the disease. Avian Dis. 1999;43:8–15. [PubMed] [Google Scholar]
- 21.Possee R D, Howard S C. Analysis of the polyhedrin gene promoter of the Autographa californica nuclear polyhedrosis virus. Nucleic Acids Res. 1987;15:10233–10248. doi: 10.1093/nar/15.24.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy S K, Silim A. Comparison of neutralizing antigens of recent isolates of infectious bursal disease virus. Arch Virol. 1991;117:287–296. doi: 10.1007/BF01310772. [DOI] [PubMed] [Google Scholar]
- 23.Reddy S K, Silim A, Ratcliffe M J. Biological roles of the major capsid proteins and relationships between the two existing serotypes of infectious bursal disease virus. Arch Virol. 1992;127:209–222. doi: 10.1007/BF01309585. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez A B, Rodríguez J F. Proteolytic processing in infectious bursal disease virus: identification of the polyprotein cleavage sites by site-directed mutagenesis. Virology. 1999;262:190–199. doi: 10.1006/viro.1999.9910. [DOI] [PubMed] [Google Scholar]
- 25.Schoonjans F, Zalata A, Depuydt C E, Comhaire F H. MedCalc: a new computer program for medical statistics. Comput Methods Programs Biomed. 1995;48:257–262. doi: 10.1016/0169-2607(95)01703-8. [DOI] [PubMed] [Google Scholar]
- 25a.Thayer S G, Villegas P, Fletcher O J. Comparison of two commercial enzyme-linked immunosorbent assays and conventional methods for avian serology. Avian Dis. 1987;31:120–124. [PubMed] [Google Scholar]
- 26.Whetzel P L, Jackwood D J. Comparison of neutralizing epitopes among infectious bursal disease viruses using radioimmunoprecipitation. Avian Dis. 1995;39:499–506. [PubMed] [Google Scholar]