Abstract
Objectives
To quantify reader agreement for the British Society of Thoracic Imaging (BSTI) diagnostic and severity classification for COVID-19 on chest radiographs (CXR), in particular agreement for an indeterminate CXR that could instigate CT imaging, from single and paired images.
Methods
Twenty readers (four groups of five individuals)—consultant chest (CCR), general consultant (GCR), and specialist registrar (RSR) radiologists, and infectious diseases clinicians (IDR)—assigned BSTI categories and severity in addition to modified Covid-Radiographic Assessment of Lung Edema Score (Covid-RALES), to 305 CXRs (129 paired; 2 time points) from 176 guideline-defined COVID-19 patients. Percentage agreement with a consensus of two chest radiologists was calculated for (1) categorisation to those needing CT (indeterminate) versus those that did not (classic/probable, non-COVID-19); (2) severity; and (3) severity change on paired CXRs using the two scoring systems.
Results
Agreement with consensus for the indeterminate category was low across all groups (28–37%). Agreement for other BSTI categories was highest for classic/probable for the other three reader groups (66–76%) compared to GCR (49%). Agreement for normal was similar across all radiologists (54–61%) but lower for IDR (31%). Agreement for a severe CXR was lower for GCR (65%), compared to the other three reader groups (84–95%). For all groups, agreement for changes across paired CXRs was modest.
Conclusion
Agreement for the indeterminate BSTI COVID-19 CXR category is low, and generally moderate for the other BSTI categories and for severity change, suggesting that the test, rather than readers, is limited in utility for both deciding disposition and serial monitoring.
Key Points
• Across different reader groups, agreement for COVID-19 diagnostic categorisation on CXR varies widely.
• Agreement varies to a degree that may render CXR alone ineffective for triage, especially for indeterminate cases.
• Agreement for serial CXR change is moderate, limiting utility in guiding management.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00330-022-09172-w.
Keywords: Coronavirus, X-ray, Diagnosis, Observer variation
Introduction
Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 virus (SARS-CoV-2), became a global pandemic. In the UK, the pandemic caused record deaths and exerted unprecedented strain on the National Health Service (NHS). Facing such overwhelming demand, clinicians must rapidly and accurately categorise patients with suspected COVID-19 into high and low probability and severity. In March 2020, the British Society of Thoracic Imaging (BSTI) and NHS England produced a decision support algorithm to triage suspected COVID-19 patients [1]. This assumed that laboratory diagnosis might not be rapidly or widely available, emphasising clinical assessment and chest radiography (CXR).
CXR therefore assumes a pivotal role, not only in diagnosis but also in the classification and monitoring of severity, which directs clinical decision-making. This includes whether intensive treatment is required (those with “classic severe” disease), along with subsequent chest computed tomography (CT) in those with uncertain diagnosis [2–4] or whose CXR is deteriorating.
Clearly, this requires that CXR interpretation reflects both diagnosis and severity accurately. While immediate interpretation by specialist chest radiologists is desirable, this is unrealistic given demands, and interpretation falls frequently to non-chest radiologists, radiologists in training, or attending clinicians. However, we are unaware of any study that compares agreement and variation between these groups for CXR diagnosis and severity of COVID-19. We aimed to rectify this by performing a multi-case, multi-reader study comparing the interpretation of radiologists (including specialists, non-specialists, and trainees) and non-radiologists to a consensus reference standard, for the CXR diagnosis, severity, and temporal change of COVID-19.
Due to the continued admission of patients to hospital for COVID-19 as the virus becomes another seasonal coronavirus infection, this study has important ongoing relevance to clinical practice.
Materials and methods
Study design and ethical approval
We used a multi-reader, multi-case design in this single-centre study. Our institution granted ethical approval for COVID-19-related imaging studies (Integrated Research Application Service reference IRAS 282063). Informed consent was waived as part of the approval.
Study population and image acquisition
A list of patients aged ≥ 18 years of age consecutively presenting to our emergency department with suspected COVID-19 infection, as per contemporary national and international definitions [5], between 25th February 2020 and 22nd April 2020, who had undergone at least one CXR, was supplied by our infectious diseases clinical team. All CXRs were acquired as computed or digital radiographs, in the anteroposterior (AP) projection using portable X-ray units as per institutional protocol.
Readers
We recruited four groups of readers (each consisting of five individuals), required to interpret suspected COVID-19 CXR in daily practice, as follows:
Group 1: Consultant chest radiologists (CCR) (with 7 to 19 years of radiology experience)
Group 2: Consultant radiologists not specialising in chest radiology (GCR) (with 8–30 years of radiology experience)
Group 3: Radiology specialist residents in training (RSR) (with 2–5 years of radiology experience
Group 4: Infectious diseases consultants and senior trainees (IDR) (with no prior radiology experience)
ID clinicians were chosen as a non-radiologist group because, at our institution and others, their daily practice necessitated both triage and subsequent management of COVID-19 patients via their own interpretation of CXR without radiological assistance.
Case identification, allocation, and consensus standard
Two subspecialist chest radiologists (with 16 and seven years of experience, respectively) first independently assigned BSTI classifications (Table 1) to the CXRs of 266 consecutive eligible patients, unaware of the ultimate diagnosis and all clinical information. Of these, 129 had paired CXRs; that is, they had a second CXR at least 24 h after their presentation CXR. The remaining 137 patients had a single presentation CXR. We included patients with unpaired CXRs as well as paired CXRs to enable us to enrich the study cohort with potential CVCX2 cases, because a high institutional prevalence of COVID-19 during the study period meant that few consecutive cases would be designated “indeterminate” or “normal”. However, evaluating this category is central to understanding downstream management implications for patients. There were 47/137 unpaired CXRs where at least one of the two subspecialist chest radiologists classified the CXR as CVCX2 (indeterminate), and so we used these 47 CXRs to enrich the cohort with CVCX2 cases. The final study cohort comprised 176 patients with 305 CXRs: 129 paired and 47 unpaired.
Table 1.
BSTI CXR category definitions and interpretation
| Code | Category | Main findings and interpretation |
|---|---|---|
| CVCX0 | Normal | Normal CXR. COVID-19 not excluded- correlate with RT-PCR and clinical suspicion |
| CVCX1 | Classic/probable COVID-19 | Lower lobe and peripheral predominant multiple opacities that are usually bilateral |
| CVCX2 | Indeterminate for COVID-19 | Does not fit Classic or Non-COVID-19 descriptors- correlate with RT-PCR and clinical suspicion |
| CVCX3 | Non-COVID-19 | No typical findings of COVID-19, and other findings suggesting an alternative diagnosis (e.g. pneumothorax, lobar pneumonia, pleural effusion(s), pulmonary oedema) |
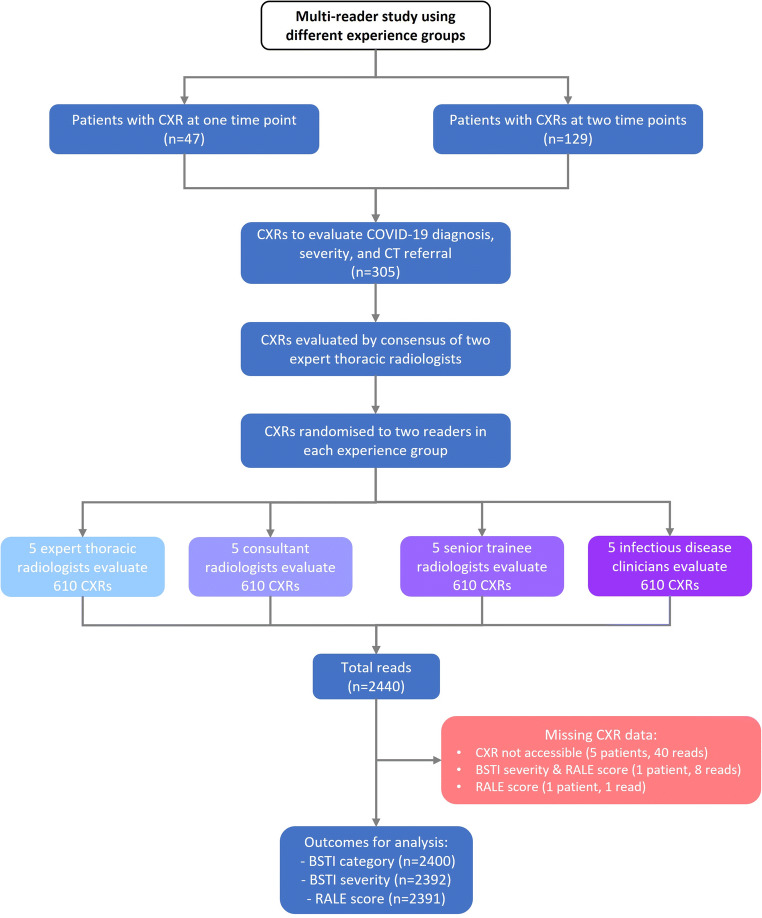
From this cohort of 305 CXRs, five random reading sets were generated, each containing approximately equal numbers of paired and unpaired CXRs (Table 2); each CXR was interpreted by 2 readers from each group. The same reader interpreted both time points for paired CXRs. Minor number variations were due to randomisation. Accordingly, individuals designated Reader 1 (CCR1, GCR1, RSR1, and IDR1) in each group would read the same cases, Reader 2 would read the same, and so on. In this way, 610 reads were generated for each reader group, resulting in 2440 reads overall (Fig. 1). The distribution of the total number of these cases paralleled cumulative COVID-19 referrals to London hospitals over the period under study (Fig. S1).
Table 2.
Reader allocation of paired and unpaired CXRs for each group
| Reader no | No. of paired CXRs | No. of unpaired CXRs | Total |
|---|---|---|---|
| 1 | 104 | 21 | 125 |
| 2 | 102 | 21 | 123 |
| 3 | 94 | 21 | 115 |
| 4 | 102 | 15 | 117 |
| 5 | 114 | 16 | 130 |
| TOTAL reads per group | 516 | 94 | 610 |
Fig. 1.
STARD flowchart showing the derivation of CXR reading dataset per reading group
The same two subspecialist chest radiologists assigned an “expert consensus” score to all 305 CXRs at a separate sitting, two months following their original reading to avoid recall bias, blinded to any reader interpretation (including their own).
Image interpretation
Readers were provided with a refresher presentation explaining BSTI categorisation and severity scoring, with examples. Readers were asked to assume they were reading in a high prevalence “pandemic” clinical scenario, with high pre-test probability, and to categorise incidental findings (e.g. cardiomegaly or minor atelectasis) as CVCX0, and any non-COVID-19 process (e.g. cardiac failure) as CVCX3.
Irrespective of the diagnostic category, we asked readers to classify severity using two scoring systems: the subjective BSTI severity scale (normal, mild, moderate, or severe), and a semiquantitative score (“Covid-RALES”) modified by Wong et al for COVID-19 CXR interpretation from the Radiographic Assessment of Lung Edema (RALE) score [3]. This score quantifies the extent of airspace opacification in each lung (0 = no involvement; 1 = < 25%; 2 = 25–50%; 3 = 50–75%; 4 = > 75% involvement). Thus, the minimum possible score is 0 and the maximum 8. We evaluated this score because it has been assessed by others and is used to assess the severity for clinical trials at our institution.
All cases were assigned a unique anonymised identifier on our institutional Picture Archiving and Communications System (PACS). Readers viewed each CXR unaware of clinical information and any prior or subsequent imaging. Any paired CXRs were therefore read as individual studies, without direct comparison between pairs. Observers evaluated CXRs on displays replicating their normal practice. Thus, radiologists used displays conforming to standards set by the Royal College of Radiologists while ID clinicians used high-definition flat panel liquid crystal display (LCD) monitors used for ward-based clinical image review at our institution.
Sample size and power calculation
The study was powered to detect a 10% difference between experts and other reader groups for correct detection of CXR reads for CT referral based on indeterminate CXR findings (defined as CVCX2). It was estimated the most experienced group (CCR) would correctly refer 90% of patients to CT. At 80% power, 86 indeterminates would be required to detect a 10% difference in referral to CT using paired proportions, requiring 305 CXRs (176 patients) based on the prevalence of uncertain findings in pre-study reads of CXRs by 2 expert readers > 1 months prior to study reads.
Statistical analysis
The primary outcome was reader group agreement with expert consensus for an indeterminate CXR which, from the BSTI is the surrogate for CT referral. Indeterminate COVID-19 (CVCX2) is the potential surrogate for triage for CT, but an alternative clinical triage categorisation for CT referral would be to combine “indeterminate” and “normal” BSTI categories (CVCX0 and CVCX2). Therefore, we first calculated the percentage agreement between each reader and the consensus reading for each BSTI diagnostic categorisation. We then also assessed this percentage agreement when the BSTI categorisation was dichotomised to (1) CVCX0 and CVCX2 (i.e. the categories that might still warrant CT if there was sufficiently high clinical suspicion), versus (2) CVCX1 and CVCX3 (i.e. the categories that would probably not warrant CT). We assessed agreement for BSTI severity scoring. All percentage agreements are described with their means and 95% confidence intervals per reader group.
Finally, for paired CXR reads we calculated the number and percentage agreement between each group and the consensus standard for no change, decrease, or increase in (1), the BSTI severity classification and (2), the COVID-RALES.
Results
Baseline characteristics
The 176 patients had a median age of 70 years (range 18–99 years); 118 (67%) were male. Due to image processing errors, a CXR was unreadable in one patient without paired imaging and three with, leaving 301 CXRs.
The expert consensus assigned the following BSTI categories: CVCX0 in 97 (32%), CVCX1 in 119 (40%), CVCX2 in 58 (19%), and CVCX3 in 27 (9.0%). Consensus BSTI severity was normal, mild, moderate, or severe in 97 (32%), 93 (31%), 68 (23%), and 27 (14%) respectively. The median consensus COVID-RALES was 2, IQR 0 to 4, range 0 to 8).
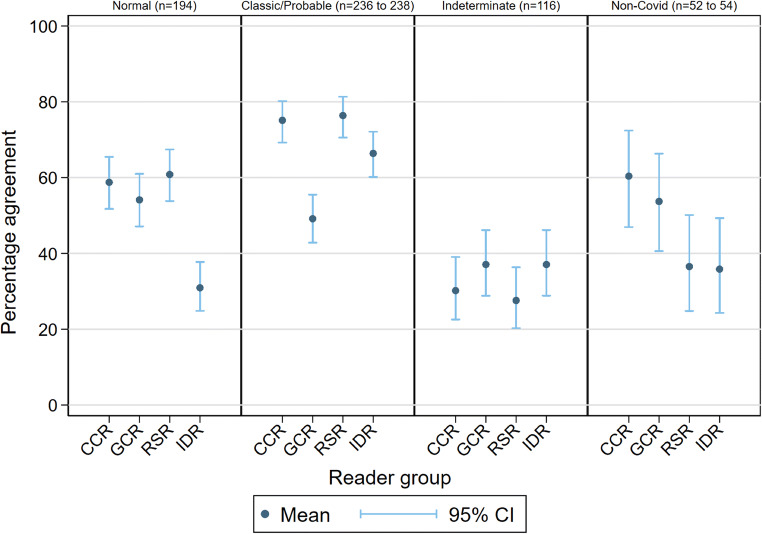
Agreement for indeterminate category (Fig. 2)
Fig. 2.
Percentage agreement with consensus for individual BSTI categories for reader groups
Our primary outcome was reader group agreement with expert consensus for indeterminate COVID-19 (CVCX2), reflecting potential triage to CT. The mean agreement for CVCX2 was generally low (28 to 37%). For all reader groups, the main alternative classification for CVCX2 was CVCX1 (“classic” COVID-19), followed by CVCX3 (not COVID-19) (Fig. S2). Even CCR1 and CCR2, who were the two subspecialist readers composing the expert consensus, demonstrated low agreement with their own consensus for CVCX2 (Fig. S3). These data suggest that basing CT referral on CXR interpretation is unreliable, even when interpreted by chest subspecialist radiologists.
An alternative clinical triage categorisation for CT referral would be to combine “indeterminate” and “normal” BSTI categories (CVCX0 and CVCX2), which resulted in higher agreement (CCR 73% (95% CI 68%, 77)% , RSR 75% (71%, 79%), GCR 58% (53%, 62%), and IDR 61% (56%, 65%)).
Agreement for BSTI categorisation (Table 3 and Fig. 2)
Table 3.
Percentage agreement with consensus for individual BSTI categories for reader groups
| Reader group | Agreement for BSTI category (%) | |||
|---|---|---|---|---|
| CVCX0 | CVCX1 | CVCX2 | CVCX3 | |
| CCR | 59 (52, 65) | 75 (69, 80) | 30 (23, 39) | 60 (47, 72) |
| GCR | 54 (47, 61) | 49 (43, 55) | 37 (29, 46) | 54 (41, 66) |
| RSR | 61 (54, 67) | 76 (71, 81) | 28 (20, 36) | 37 (25, 50) |
| IDR | 31 (25, 38) | 66 (60, 72) | 37 (29, 46) | 36 (24, 49) |
Values given are means (95% lower and upper limits)
Agreement was highest for CVCX1 (“classic/probable”) for the CCR (75% (69%, 80%)), RSR (76% (71%, 81%)), and IDR (66%(60%, 72%)) groups, but interestingly not for GCR (49% (43%, 55%)), where agreement was comparable to their agreement for CVCX0 and CVCX3 (“non-COVID-19”) (although still higher than their agreement for CVCX2 (“indeterminate”)). When disagreeing with the consensus CVCX1, GCR were most likely to assign CVCX2 (Fig. S1).
Agreement with consensus for CVCX0 (“normal”) was similar for radiologists of all types (mean agreement for CCR, GCR, and RSR of 59%, 54%, and 61% respectively), but lower for IDR (31%). For CVCX3 (not COVID-19), CCR and GCR were generally more likely than RSR and IDR readers to agree with the consensus.
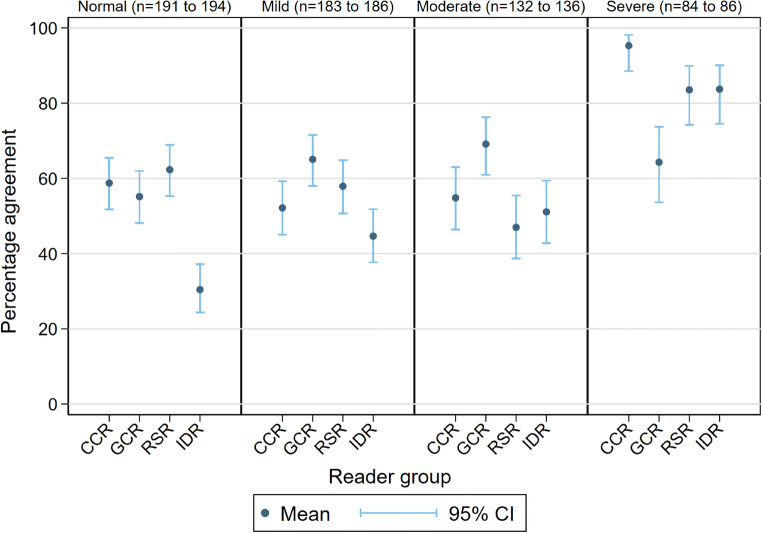
Agreement for BSTI severity classification (Table 4 and Fig. 3)
Table 4.
Percentage agreement with consensus for BSTI severity classification for reader groups
| Reader group | Agreement for BSTI Severity (%) | |||
|---|---|---|---|---|
| Normal | Mild | Moderate | Severe | |
| CCR | 59 (52, 65) | 52 (45, 59) | 55 (46, 63) | (95 (89, 98) |
| GCR | 55 (48, 62) | 65 (58, 72) | 69 (61, 76) | 64 (54, 74) |
| RSR | 62 (55, 69) | 58 (51, 65) | 47 (39, 55) | 47 (39, 55) |
| IDR | 30 (24, 37) | 45 (38, 52) | 51 (43, 59) | 84 (75, 90) |
Values given are means (95% lower and upper limits)
Fig. 3.
Percentage agreement with consensus for BSTI severity classification for reader groups
Agreement that classification was “severe” was highest for all groups, but lower for GCR (65% (54%, 74%)) than other groups (means of 95% (89%, 98%), 84% (74%, 90%), and 84% (75%, 90%) for CCR, RSR, and IDR respectively). The majority of consensus-graded normal cases were likely to be designated “mild” (Fig. S4).
Agreement for change on CXRs (Table 5 and Fig. 4)
Table 5.
Percentage agreement for change in BSTI severity classification and Covid-RALES for reader groups
| Reader Group | Serial CXR change | |||||
|---|---|---|---|---|---|---|
| BSTI severity | Covid-RALES | |||||
| No change | Decrease | Increase | No change | Decrease | Increase | |
| CCR | 85 (66) | 1 (6) | 31 (29) | 42 (50) | 9 (28) | 79 (57) |
| GCR | 77 (61) | 3 (17) | 36 (33) | 37 (44) | 9 (28) | 80 (59) |
| RSR | 56 (44) | 4 (25) | 41 (38) | 29 (35) | 11 (37) | 81 (59) |
| IDR | 62 (48) | 7 (39) | 31 (29) | 29 (35) | 13 (41) | 64 (47) |
Figures given are numbers (percentages)
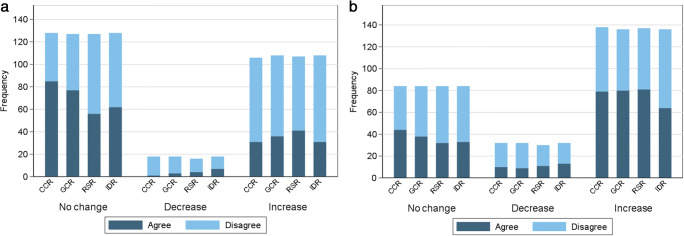
Fig. 4.
Frequency charts showing agreement with consensus for score change using the BSTI severity classification (a) and the Covid-RALES for reader groups (b)
The expert consensus reference found that the majority of BSTI severity scores did not change where paired CXR examinations were separated by just one or two days. Using the BSTI severity classification, the highest agreement with consensus across all groups was for “no change”, with percentage agreement of 66%, 61%, 44%, and 48% for CCR, GCR, RSR, and IDR respectively.
In contrast, when using Covid-RALES, the highest agreement with consensus across all groups was for an “increased score”, with percentage agreement of 57%, 59%, 59%, and 47% for CCR, GCR, RSR, and IDR respectively. This most likely reflects the larger number of individual categories assigned by Covid-RALES.
Discussion
Thus far, studies of CXR for COVID-19 have either reported its diagnostic accuracy [6, 7], implications of CXR severity assessment using various scores [4, 8–10], or quantification using computer vision techniques [11–13]. Inter-observer agreement for categorisation of COVID-19 CXRs, including for the BSTI classification (but not BSTI severity) has been assessed amongst consultant radiologists [14], while inter-observer differences according to radiologist experience have been described [15, 16]. Notably, in a case-control study, Hare et al compared the agreement for the BSTI classification amongst seven consultant radiologists, including two fellowship-trained chest radiologists (with the latter providing the reference standard). They found that only fair agreement was obtained for the CVCX2 category κ = 0.23), and “non-COVID-19” (κ = 0.37) categories, but that combining the scores of the CVCX2 and CVCX3 scores improved inter-observer agreement (κ = 0.58) [14]. A recent study compared the sensitivity and specificity (but not agreement) of using the “classic/probable” BSTI category for COVID-19 diagnosis between Emergency Department clinicians and radiologists (both of various grades), based on a retrospective review of their classifications [17].
Our study differs in that it pivots around three potential clinical scenarios that use the CXR to manage suspected COVID-19. Using a prospective multi-reader, multi-case design, we determined reader agreement for four clinical groups who are tasked with CXR interpretation in daily practice and compared these to a consensus reference standard. Firstly, we evaluated reader agreement when using CXR to triage patients for CT when CXR imaging is insufficient to diagnose COVID-19. Secondly, we examined agreement for disease severity using two scores (BSTI and RALES). Thirdly, we investigated whether paired CXRs could monitor any change in severity.
When CXR was used to identify which patients need CT, based on our pre-specified BSTI category of an indeterminate interpretation, agreement with our consensus was low (28 to 37%) or moderate (58 to 75%) respectively. All four reader groups had a similar agreement to the consensus for identifying indeterminates, indicating that the level or specialism or radiologist expertise did not enhance agreement. When combining indeterminates with normal, GCR and IDR groups had lower agreement because the GCR group assigned more indeterminates as non-COVID-19, whereas the IDR group assigned more to classic/probable COVID-19.
Similar (albeit modest) agreement for the “normal” category amongst radiologists of all grades and types suggests that these factors are not influential when assigning this category. Radiologists seemed willing to consider many CXRs normal despite assuming a high prevalence setting. Reassuringly, this suggests that patient disposition, if based on normal CXR interpretation, is unlikely to vary much depending on the category of radiologist. Conversely, the lower agreement of ID clinicians for a normal CXR suggests an inclination to overall abnormal, since they classified normal CXRs as mostly “indeterminate” but also “classic/probable” COVID-19. We speculate that the contemporary pandemic clinical experience of ID clinicians made it difficult for them to consider a CXR normal, even when deprived of supporting clinical information.
In contrast, general consultant radiologists were less inclined to assign the “classic/probable” category, predominantly favouring the indeterminate category. Our results are somewhat at odds with Hare et al [14], who found substantial agreement for the CVCX1 category amongst seven consultant radiologists. Reasons underpinning the reticence to assign this category (even in a high prevalence setting) are difficult to intuit but may be partly attributable to a desire to adhere to strict definitions for this category, and thus maintain specificity.
Severity scores can quantify disease fluctuations that influence patient management, have prognostic implications [8–10], and may also be employed in clinical trials. However, this is only possible if scores are reliable, which is reflected by reader agreement regarding both value and change. For our second and third clinical scenarios for CXR, we also found that assessment of severity and change, and therefore of CXR severity itself, varied between reader groups and readers using either severity scoring system, but in different ways. It is probably unsurprising that agreement for no change in BSTI severity was highest for all reader groups, given that the four-grade nature of that classification is less likely to detect subtle change. In contrast, the finer gradation of Covid-RALES allows smaller severity increments to be captured more readily. A higher number of categories also encourages disagreement; despite this, agreement was modest.
We wished to examine CXR utility in a real-world clinical setting using consecutive patients presenting to our emergency department with suspected COVID-19 infection. Our findings are important because they examine clear clinical roles for CXR beyond a purely binary diagnosis of COVID-19 versus non-COVID-19. Rather, we examined the CXR as an aid to clinical decision-making and as an adjunct to clinical and molecular testing. CXR has moderate pooled sensitivity and specificity for COVID-19 (81% and 72% respectively) [18] and, in the context of other clinical and diagnostic tests [19], such diagnostic accuracy could be considered favourable. Although thoracic CT has a higher sensitivity for diagnosing COVID-19 [18], CXR has been used and investigated in this triage role both in the UK and internationally [20]. However, our results do have important implications when using CXR for diagnosis because interpretation appears susceptible to substantial inter-reader variation. Investigating reader variability will also be crucial for development, training, and evaluation of artificial intelligence algorithms to diagnose COVID-19, such as that now underway using the National COVID-19 Chest Imaging Database (NCCID) [21].
Our study has limitations. ID clinicians, as the first clinicians to assess potential COVID-19 cases, were the only group of non-radiologist clinicians evaluated. While we would wish to evaluate the emergency department and general medical colleagues also, this proved impractical. However, we have no a priori expectation that these would perform any differently. Our reference standard interpretation used two subspecialist chest radiologists; like any subjective standard, ours is imperfect, but with precedent [14]. We point out that our data around variability of reader classifications are robust regardless of the reference standard (see data in supplementary Figs. S2 and S4). Arguably, we disadvantaged ID clinicians by requiring them to interpret CXRs using LCD monitors, but this reflects normal clinical practice. It is possible that readers may have focussed on BSTI diagnostic categories in isolation, rather than considering the implications of how their categorisation would be used to decide patient management but, again, this reflects normal practice (since radiologists do not determine management). Readers did not compare serial CXRs directly, but read them in isolation: We note a potential role for monitoring disease progression when serial CXRs are viewed simultaneously, but this outcome would require assessment by other studies.
In conclusion, across a diverse group of clinicians, agreement for BSTI diagnostic categorisation for COVID-19 CXR classification varies widely for many categories, and to such a degree that may render CXR ineffective for triage using such categories. Agreement for serial change over time was also moderate, underscoring the need for cautious interpretation of changes in severity scores if using these to guide management and predict outcome, when these scores have been assigned to serial CXRs read in isolation.
Supplementary information
(DOCX 1216 kb)
Abbreviations
- BSTI
British Society of Thoracic Imaging
- CCR
Consultant chest radiologists
- Covid-RALES
Radiographic Assessment of Lung Edema (RALE) score, modified for COVID-19 interpretation
- CVCX
BSTI COVID-19 chest radiograph category
- CXR
Chest radiograph
- GCR
General consultant radiologists
- IDR
Infectious diseases consultants and senior trainees
- NHS
National Health Service
- RSR
Radiology specialist residents in training
Funding
The authors state that this work has not received any funding.
Declarations
Guarantor
The scientific guarantor of this publication is Arjun Nair (arjun.nair1@nhs.net)
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
AN reports a medical advisory role with Aidence BV, an artificial intelligence company; AN reports a consultation role with Faculty Science Limited, an artificial intelligence company.
SH and SM report grants from the National Institute for Health Research (NIHR) outside the submitted work.
JJ reports Consultancy fees from Boehringer Ingelheim, F. Hoffmann-La Roche, GlaxoSmithKline, NHSX; is on the Advisory Boards of Boehringer Ingelheim, F. Hoffmann-La Roche; has received lecture fees from Boehringer Ingelheim, F. Hoffmann-La Roche, Takeda; receives grant funding from GlaxoSmithKline; holds a UK patent (application number 2113765.8).
The other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise- Professor Sue Mallet is a Professor in diagnostic and prognostic medical statistics, specialising in clinical trial design, methodology, and systematic reviews. She is a senior author of the paper.
Informed consent
Written informed consent was waived by the Institutional Review Board, as per our ethics approval.
Ethical approval
Institutional Review Board approval was obtained- Our institution granted ethical approval for COVID-19-related imaging studies (Integrated Research Application Service reference IRAS 282063).
Methodology
• retrospective
• observational
• performed at one institution
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Arjun Nair and Alexander Procter contributed equally to this work.
References
- 1.Nair A, Rodrigues JCL, Hare S, Edey A, Devaraj A, Jacob J, et al. A British Society of Thoracic Imaging statement: considerations in designing local imaging diagnostic algorithms for the COVID-19 pandemic. Clin Radiol. 2020;75(5):329–334. doi: 10.1016/j.crad.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Zhong NS. Clinical characteristics of Covid-19 in China. Reply N Engl J Med. 2020;382(19):1861–1862. doi: 10.1056/NEJMc2005203. [DOI] [PubMed] [Google Scholar]
- 3.Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, Lo CSY, et al. Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology. 2020;296(2):E72–EE8. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang W, Liang H, Ou L, Chen B, Chen A, Li C, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Public Health England(2020) COVID-19: investigation and initial clinical management of possible cases [updated 12/14/2020. Available from: https://www.gov.uk/government/publications/wuhan-novel-coronavirus-initial-investigation-of-possible-cases/investigation-and-initial-clinical-management-of-possible-cases-of-wuhan-novel-coronavirus-wn-cov-infection. Accessed 23 Dec 2021
- 6.Schiaffino S, Tritella S, Cozzi A, Carriero S, Blandi L, Ferraris L, et al. Diagnostic performance of chest X-ray for COVID-19 pneumonia during the SARS-CoV-2 pandemic in Lombardy, Italy. J Thorac Imaging. 2020;35(4):W105–W1W6. doi: 10.1097/RTI.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 7.Gatti M, Calandri M, Barba M, Biondo A, Geninatti C, Gentile S, et al. Baseline chest X-ray in coronavirus disease 19 (COVID-19) patients: association with clinical and laboratory data. Radiol Med. 2020;125(12):1271–1279. doi: 10.1007/s11547-020-01272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orsi MA, Oliva G, Toluian T, Valenti PC, Panzeri M, Cellina M. Feasibility, reproducibility, and clinical validity of a quantitative Chest X-ray assessment for COVID-19. Am J Trop Med Hyg. 2020;103(2):822–827. doi: 10.4269/ajtmh.20-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toussie D, Voutsinas N, Finkelstein M, Cedillo MA, Manna S, Maron SZ, et al. Clinical and chest radiography features determine patient outcomes in young and middle-aged adults with COVID-19. Radiology. 2020;297(1):E197–E206. doi: 10.1148/radiol.2020201754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balbi M, Caroli A, Corsi A, Milanese G, Surace A, Di MF, et al. Chest X-ray for predicting mortality and the need for ventilatory support in COVID-19 patients presenting to the emergency department. Eur Radiol. 2021;31(4):1999–2012. doi: 10.1007/s00330-020-07270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy K, Smits H, Knoops AJG, Korst MBJM, Samson T, Scholten ET, et al. COVID-19 on chest radiographs: a multireader evaluation of an artificial intelligence system. Radiology. 2020;296(3):E166–EE72. doi: 10.1148/radiol.2020201874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebrahimian S, Homayounieh F, Rockenbach MABC, Putha P, Raj T, Dayan I, et al. Artificial intelligence matches subjective severity assessment of pneumonia for prediction of patient outcome and need for mechanical ventilation: a cohort study. Sci Rep. 2021;11(1):858. doi: 10.1038/s41598-020-79470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang SB, Lee SH, Lee DE, Park SY, Kim JK, Cho JW, et al. Deep-learning algorithms for the interpretation of chest radiographs to aid in the triage of COVID-19 patients: A multicenter retrospective study. PLoS One. 2020;15(11):e0242759. doi: 10.1371/journal.pone.0242759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hare SS, Tavare AN, Dattani V, Musaddaq B, Beal I, Cleverley J, et al. Validation of the British Society of Thoracic Imaging guidelines for COVID-19 chest radiograph reporting. Clin Radiol. 2020;75(9):710–7e9. doi: 10.1016/j.crad.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cozzi A, Schiaffino S, Arpaia F, Della PG, Tritella S, Bertolotti P, et al. Chest x-ray in the COVID-19 pandemic: radiologists' real-world reader performance. Eur J Radiol. 2020;132:109272. doi: 10.1016/j.ejrad.2020.109272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeves RA, Pomeranz C, Gomella AA et al (2021) Performance of a severity score on admission chest radiography in predicting clinical outcomes in hospitalized patients with coronavirus disease (COVID-19). Am J Roentgenol 217(3):623–632. 10.2214/AJR.20.24801 [DOI] [PubMed]
- 17.Kemp OJ, Watson DJ, Swanson-Low CL, Cameron JA, Von Vopelius-Feldt J. Comparison of chest X-ray interpretation by Emergency Department clinicians and radiologists in suspected COVID-19 infection: a retrospective cohort study. BJR Open. 2020;2(1):20200020. doi: 10.1259/bjro.20200020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islam N, Ebrahimzadeh S, Salameh J-P et al (2021) Thoracic imaging tests for the diagnosis of COVID‐19. Cochrane Database Syst Rev 3(3):CD013639. 10.1002/14651858.CD013639.pub4 [DOI] [PMC free article] [PubMed]
- 19.Mallett S, Allen AJ, Graziadio S, Taylor SA, Sakai NS, Green K, et al. At what times during infection is SARS-CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data. BMC Med. 2020;18(1):346. doi: 10.1186/s12916-020-01810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Çinkooğlu A, Bayraktaroğlu S, Ceylan N, Savaş R. Efficacy of chest X-ray in the diagnosis of COVID-19 pneumonia: comparison with computed tomography through a simplified scoring system designed for triage. Egypt J Radiol Nucl Med. 2021;52(1):1–9. doi: 10.1186/s43055-021-00541-x. [DOI] [Google Scholar]
- 21.Jacob J, Alexander D, Baillie JK, Berka R, Bertolli O, Blackwood J, et al. Using imaging to combat a pandemic: rationale for developing the UK National COVID-19 Chest Imaging Database. Eur Respir J. 2020;56(2):2001809. doi: 10.1183/13993003.01809-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1216 kb)