Abstract
Codonopsis pilosula, a traditional Chinese medicinal and edible plant, contains several bioactive components. However, the biosynthetic mechanism is unclear because of the difficulties associated with functional gene analysis. Therefore, it is important to establish an efficient genetic transformation system for gene function analysis. In this study, we established a highly efficient Agrobacterium-mediated callus genetic transformation system for C. pilosula using stems as explants. After being pre-cultured for 3 days, the explants were infected with Agrobacterium tumefaciens strain GV3101 harboring pCAMBIA1381-35S::GUS at an OD600 value of 0.3 for 15 min, followed by co-cultivation on MS induction medium for 1 day and delayed cultivation on medium supplemented with 250 mg l−1 cefotaxime sodium for 12 days. The transformed calli were selected on screening medium supplemented with 250 mg l−1 cefotaxime sodium and 2.0 mg l−1 hygromycin and further confirmed by PCR amplification of the GUS gene and histochemical GUS assay. Based on the optimal protocol, the induction and transformation efficiency of calli reached a maximum of 91.07%. The establishment of a genetic transformation system for C. pilosula calli lays the foundation for the functional analysis of genes related to bioactive components through genetic engineering technology.
Keywords: callus, Codonopsis pilosula, genetic transformation
Introduction
Codonopsis pilosula (Franch.) Nannf. (C. pilosula) is a perennial herb. The root, known as “Dangshen”, is a commonly used traditional Chinese medicine renowned for its remarkable effect on improving gastrointestinal function, nourishing the spleen and lungs, enhancing immunity, and delaying senescence (Bai et al. 2020). It is also a popular health food and is included in the daily diet (He et al. 2015). The pharmacological and health care effects of C. pilosula are attributed to its bioactive compounds, including secondary metabolites, such as polysaccharides, lobetyolin, syringin, angelicin, and atractylenolide III (Gao et al. 2019).
Several genes related to the biosynthesis of bioactive compounds have been identified in C. pilosula (Gao et al. 2015). For example, Ji et al. (2019) found that CpUGPase and CpPMK may participate in bioactive metabolite accumulation under methyl jasmonate (MeJA) induction (Ji et al. 2019). However, none has been verified by experiments due to the lack of a genetic transformation system.
An Agrobacterium-mediated genetic transformation is a suitable option. The Ti plasmid in Agrobacterium tumefaciens can stably transfer genetic material through plant wounds into the host cells (Chilton et al. 1982). In addition, the binary vector pCAMBIA1381 carries the hygromycin phosphotransferase gene. Therefore, successfully transformed tissues or plants can be rapidly screened for hygromycin resistance (Idnurm et al. 2017). Genetic transformation systems have been established for many plants. Most genetic transformation systems in medicinal plants use hairy roots. Miao et al. optimized the hairy root transformation conditions for Rubia yunnanensis (Miao et al. 2021). The hairy roots of Scutellaria spp. (Lamiaceae) were induced using wild-type Agrobacterium rhizogenes strain A4 with cotyledons as explants (Stepanova et al. 2021). In C. pilosula, the hairy root genetic transformation system has been established (Yang et al. 2020). Overexpression of the functional genes CpSPL2 and CpSPL10 could promote the growth of hairy roots and the accumulation of secondary metabolites, such as total saponins, in C. pilosula (Yang et al. 2021). However, hairy root systems have certain limitations. For example, plant traits cannot be observed in hairy roots compared with regenerated transgenic plants. Callus induction is an important process for the regeneration of intact plants (Li N et al. 2020). In addition, bioactive components, such as polysaccharides (Wang et al. 2012), can also be synthesized in the callus of C. pilosula.
In this study, the C. pilosula callus genetic transformation system was established by transforming a recombinant vector with a beta-glucuronidase (GUS) reporter gene. These results provide a technological basis for studying functional genomics and metabolic pathways of active compounds.
Materials and methods
Plant materials
Seeds were collected from Pingshun County, Shanxi Province, China, and identified as C. pilosula seeds by Professor Jian-Ping Gao. After soaking in warm water for 1 h, the seeds were disinfected with 75% ethanol for 15 s and washed three times with sterile water. The seeds were then sterilized in 1% NaClO for 20 min and rinsed five times with sterile water. Finally, the sterile seeds were placed on 1/2 MS medium (with 30 g l−1 sucrose and 7 g l−1 agar, pH 6.5±0.2) and cultivated for 7 days at 25±1°C in the dark. After germination, the sterile seedlings were cultured at 25±1°C with a 16 h light/8 h dark cycle for 3 weeks.
Bacterial strain and recombinant DNA
The plant expression vectors pCAMBIA1381-35S and pCAMBIA1381-35S::GUS were transformed into A. tumefaciens GV3101 using the freeze-thaw method (Weigel and Glazebrook 2006).
Bacteria culture
The native GV3101 A. tumefaciens strain (negative control) and the recombinant GV3101 strain harboring pCAMBIA1381-35S and pCAMBIA1381-35S::GUS were streaked on solid LB medium containing either 50 mg l−1 rifampicin (Rif) or 50 mg l−1 rifampicin (Rif) plus 50 mg l−1 kanamycin (Kan) and incubated at 28°C in the dark for 2–3 days. After PCR identification using the GUS reporter gene, a positive single colony was inoculated into LB liquid medium containing the specified antibiotics and incubated overnight at 28°C (shaking at 180 rpm) until the OD600 value of the bacterial solution was 1.0–1.2. The culture was then diluted at a ratio of 1 : 30, and incubated at 28°C (shaking at 180 rpm) until the OD600 value of the bacterial solution was 0.3, 0.6, 0.9, and 1.2. The cultures were centrifuged for 8 min at 5,000 rpm, and the precipitated cells were resuspended in the same volume of MS liquid medium. This served as the infection solution.
Hygromycin resistance test of C. pilosula
The stems of 4-week-old C. pilosula seedlings without axillary buds were sterilized and trimmed to an average length of 0.5 cm for use as explants. The explants were placed on MS medium containing 30 g l−1 sucrose, 7 g l−1 agar, 0.5 mg l−1 6-Benzylaminopurine (6-BA), 0.2 mg l−1 2,4-Dichlorophenoxyacetic acid (2,4-D), and different concentrations of hygromycin (Hyg) (0, 0.5, 1.0, 1.5, and 2.0 mg l−1) to induce callus. The most suitable screening concentration was selected according to the induction rate of calli after being cultured for 20 days (Olhoft et al. 2003). Each treatment was repeated in triplicate.
Optimization of protocol for inducing transgenic callus
The explants were pre-cultured on the MS medium containing 30 g l−1 sucrose and 7 g l−1 agar supplemented with 0.5 mg l−1 6-BA and 0.2 mg l−1 2,4-D. Then the pre-cultured explants were immersed in the infection solution and placed on the MS medium supplemented with 0.5 mg l−1 6-BA and 0.2 mg l−1 2,4-D in the dark for co-cultivation. Following the inoculation period, the explants were transferred to the MS medium supplemented with 0.5 mg l−1 6-BA, 0.2 mg l−1 2,4-D, and 250 mg l−1 cefotaxime sodium (Cef) for delayed cultivation at 25°C in the light. Then, the explants were transferred to the MS medium supplemented with 0.5 mg l−1 6-BA, 0.2 mg l−1 2,4-D, and 250 mg l−1 Cef plus Hyg at incubated at 25°C in the light to screen the positive callus. The effect of Hyg concentration on the induction efficiency was investigated. We then optimized the pre-cultivation time, infection time, co-cultivation time, delayed cultivation time, and bacterial concentration with stem explants on the MS medium (Table 1) using an orthogonal experimental design (L16(45)). The induction rate was calculated after explants were cultured for 30 days.
Table 1. Orthogonal test design of the protocol of C. pilosula callus genetic transformation system (L16(45)).
| Group | Pre-incubation (d) | Infection (min) | Co-cultivation (d) | Delayed incubation (d) | Bacteria concentration | Number of calli | Callus induction rate (%)* |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 5 | 0 | 0 | 1 | 4.67±0.58 | 57.41±8.49c |
| 2 | 0 | 10 | 1 | 4 | 2 | 2.50±0.71 | 18.46±0.40e |
| 3 | 0 | 15 | 2 | 8 | 3 | 0.67±0.58 | 5.34±4.64fg |
| 4 | 0 | 20 | 3 | 12 | 4 | 0.00±0.00 | 0.00±0.00g |
| 5 | 1 | 5 | 1 | 8 | 4 | 1.33±0.58 | 11.49±3.40ef |
| 6 | 1 | 10 | 0 | 12 | 3 | 7.00±0.00 | 70.00±10.00b |
| 7 | 1 | 15 | 3 | 0 | 2 | 0.00±0.00 | 0.00±0.00g |
| 8 | 1 | 20 | 2 | 4 | 1 | 1.33±0.58 | 13.70±5.48ef |
| 9 | 2 | 5 | 2 | 12 | 2 | 3.67±0.58 | 37.78±3.85d |
| 10 | 2 | 10 | 3 | 8 | 1 | 6.00±1.00 | 56.11±5.36c |
| 11 | 2 | 15 | 0 | 4 | 4 | 0.00±0.00 | 0.00±0.00g |
| 12 | 2 | 20 | 1 | 0 | 3 | 0.00±0.00 | 0.00±0.00g |
| 13 | 3 | 5 | 3 | 4 | 3 | 2.00±0.00 | 19.39±1.05e |
| 14 | 3 | 10 | 2 | 0 | 4 | 1.67±0.58 | 17.04±5.13e |
| 15 | 3 | 15 | 1 | 12 | 1 | 14.00±1.41 | 90.55±3.27a |
| 16 | 3 | 20 | 0 | 8 | 2 | 7.67±1.15 | 65.50±3.23b |
The bacterial concentration 1, 2, 3, and 4 represent OD600 values of 0.3, 0.6, 0.9, 1.2. *Different letters indicated the significant differences (p<0.05).
Ten explants were placed on each plate, and all experiments were conducted in triplicate. All data are expressed as mean±standard error and were compared using Duncan’s multiple range tests at a 5% significance level.
Molecular analysis of callus
Genomic DNA was extracted from transgenic and control calli using the cetyltrimethylammonium bromide (CTAB) method (Ghosh et al. 2009). Each PCR-amplification was conducted in a 25 µl reaction volume containing 2 µl of template, 17 µl sterile water (ddH2O), 2.5 µl 10× buffer, 2 µl dNTPs (2.5 mM), 0.5 µl of each primer, and 0.5 µl of Easy Taq DNA Polymerase (Takara Bio, Beijing, China). Primers were designed for the GUS reporter gene. The sequences of the forward and reverse gene-specific primers were GUS-F (5′-GTCAGTGGCAGTGAAGGG-3′) and GUS-R (5′-CGAGGTACGGTAGGAGTT-3′), respectively. The PCR reactions were performed as follows: initial denaturation at 94°C for 5 min; then 34 cycles of 94°C for 30 s, 53°C for 30 s, and 72°C for 1 min; and a final extension at 72°C for 10 min. PCR products were detected using 1% agarose gel electrophoresis. In addition, specific primer pairs (pc1381-F/R 5′-CAAAAGCACAAACACGCTAAGT-3′ and 5′-AGAGCATCGGAACGAAAAA-3′) were designed according to the partial DNA fragment (656 bp) outside the T-DNA region in the pCAMBIA1381-35S::GUS binary vector to exclude the possibility of Agrobacterium contamination of regenerated plants.
GUS staining of callus
GUS assays were conducted using the GUS Blue KIT (Huayueyang Biotech Co., Ltd., Beijing, China) according to the instructions. Tissues-stained blue were regarded as positive transgenic explants (Jefferson et al. 1987; Liu et al. 2020).
Results
Effect of different hygromycin concentrations on the differentiation of C. pilosula callus
The effect of hygromycin on C. pilosula calli was analyzed to ascertain the suitable screening pressure for hygromycin. When a hygromycin concentration of 2.0 mg l−1 was used, most of the explants did not expand (Figure 1), and the rate of callus induction decreased distinctly by 27.98% (Table 2). However, the callus induction rate was greater than 75% when the hygromycin concentration was lower than 1.5 mg l−1. Therefore, the adequate concentration was 2.0 mg l−1 to screen for positive transgenic calli.
Figure 1. Effect of different hygromycin concentrations on the induction of callus in C. pilosula. (A) 0 mg l−1. (B) 1.0 mg l−1. (C) 1.5 mg l−1. (D) 2.0 mg l−1.
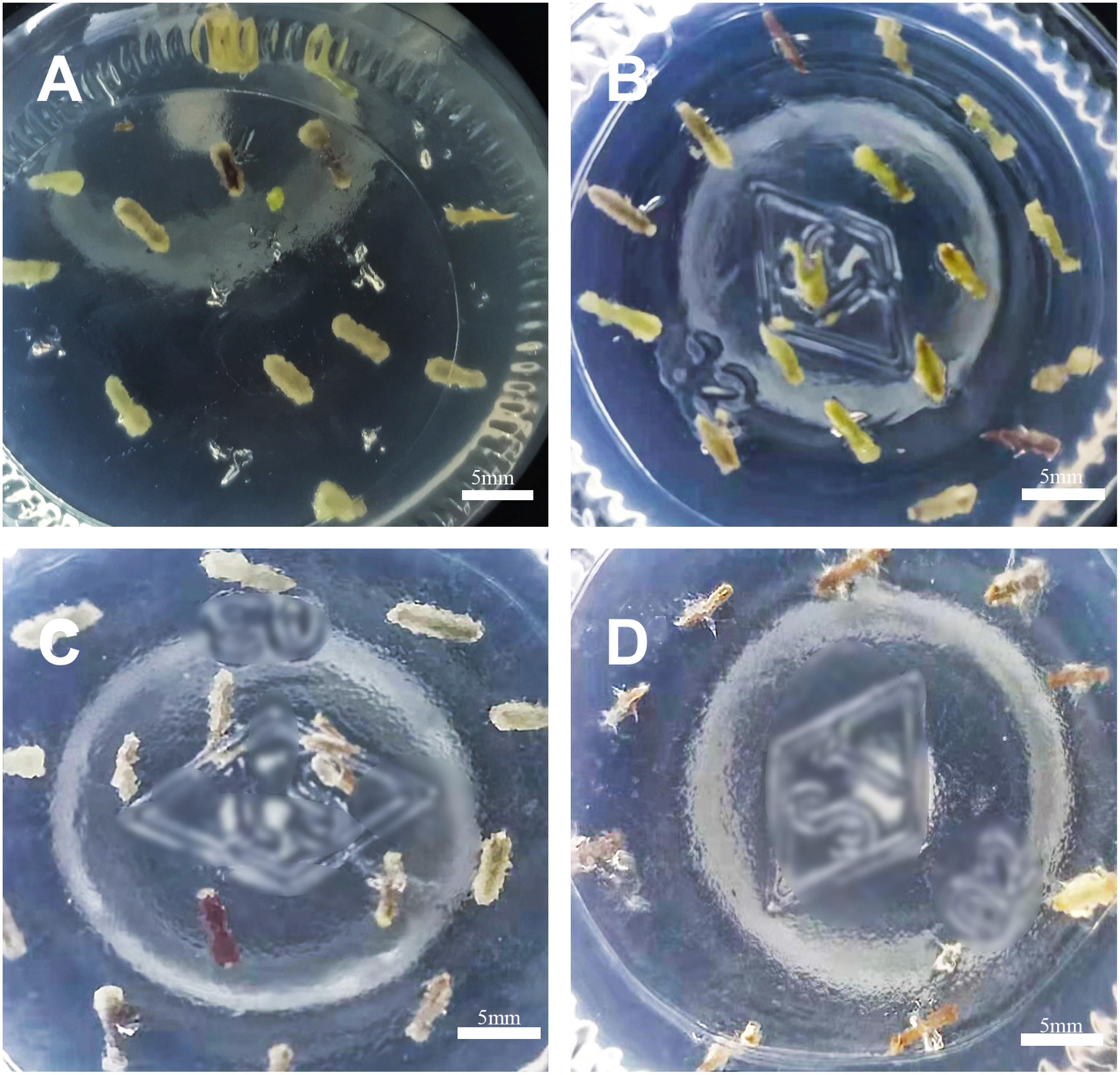
Table 2. Effect of hygromycin concentration on the induction of callus in C. pilosula.
| Hygromycin concentration (mg l−1) | Number of samples | Number of calli | Callus induction rate (%)* |
|---|---|---|---|
| 0 | 33 | 30 | 91.39±7.47a |
| 0.5 | 33 | 31 | 92.22±6.94a |
| 1 | 45 | 34 | 74.97±6.71b |
| 1.5 | 44 | 36 | 81.75±4.32ab |
| 2 | 36 | 10 | 27.98±1.78c |
*Different letters indicated the significant differences (p<0.05).
Callus induction in C. pilosula
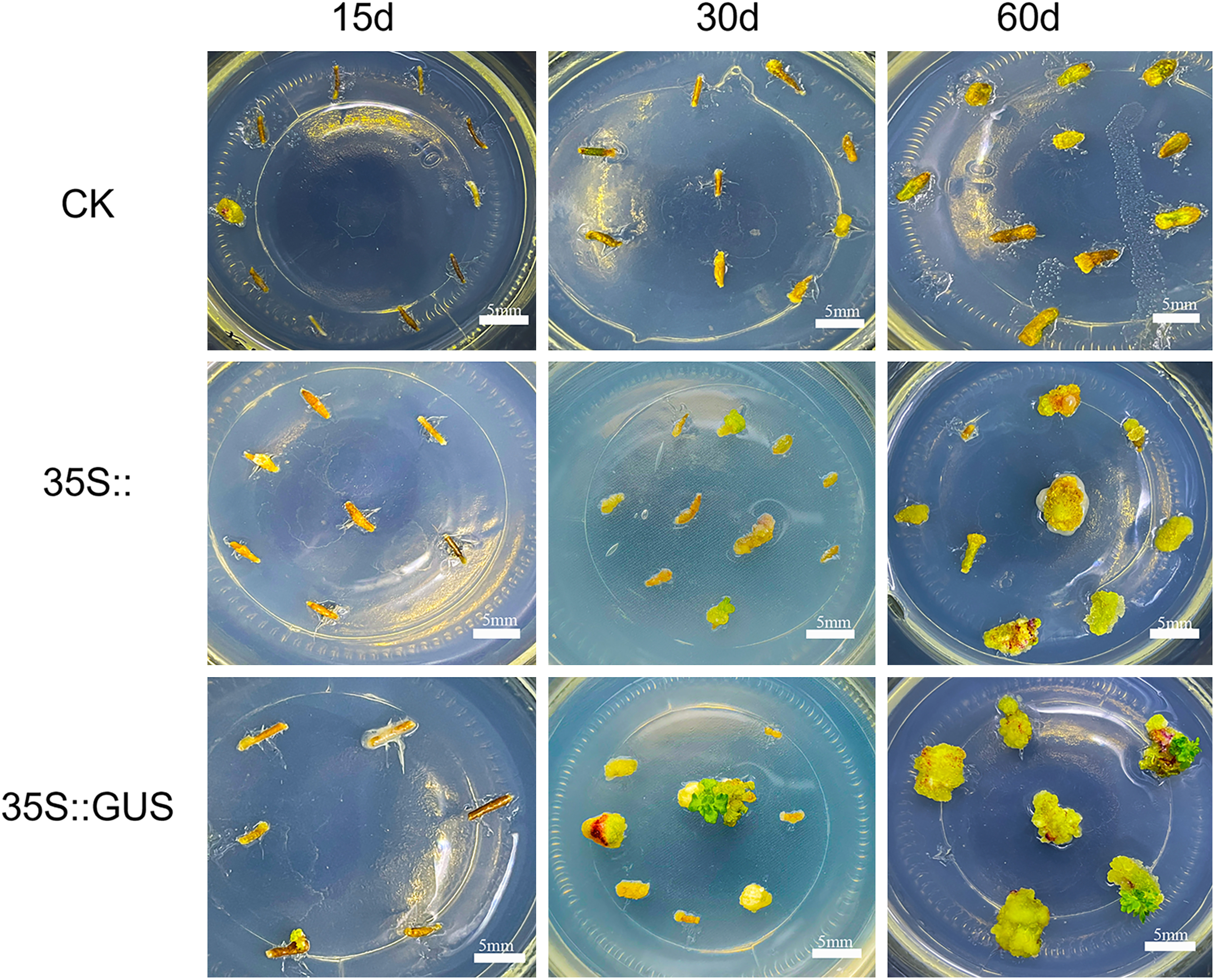
The orthogonal test design indicated that the induction rate for transgenic callus was optimal with a 3-day pre-cultivation period, a bacterial concentration of OD600 value of 0.3, an infection time of 15 min, a 1-day co-cultivation period, and a 12-day delayed cultivation period. Based on the data shown in Table 1, induction rates with the optimal protocol were highest, i.e., 90.55±3.27%, followed by only 70.00±10.00% for the protocol with a 1-day pre-cultivation period, an OD600 value of 0.9 for bacterial concentration, an infection time of 10 min and a 12-day delayed cultivation period. No calli were induced in groups 4, 7, 11, and 12, which might be due to the high bacterial concentration, short pre-cultivation duration and delayed cultivation, or long duration of infection and co-cultivation.
For group 15, explants expanded and white calli were observed 30 days after exposure to A. tumefaciens GV3101 carrying pCAMBIA1381-35S and pCAMBIA1381-35S::GUS, both containing a hygromycin resistance gene. In contrast, the explants exposed to native GV3101 did not differentiate into calli until 60 days after infection due to the lack of the hygromycin resistance gene (Figure 2). After transformed with the strain GV3101 harboring the expression vectors for 60 days, the white calli differentiated into green calli. And the green ones further differentiated into green buds. These results indicated that the optimal genetic transformation medium system with 2.0 mg l−1 hygromycin in the screening medium could screen out the positive calli. The GUS gene in the vector did not affect the induction rate and growth of calli of C. pilosula.
Figure 2. Callus of C. pilosula induced from stem explants at 15 days, 30 days, 60 days after infection by Agrobacterium tumefaciens GV3101 harboring pCAMBIA1381-35S and pCAMBIA1381-35S::GUS. The native GV3101 strain was used as control (CK).
Molecular detection of transformed transgenic callus using PCR
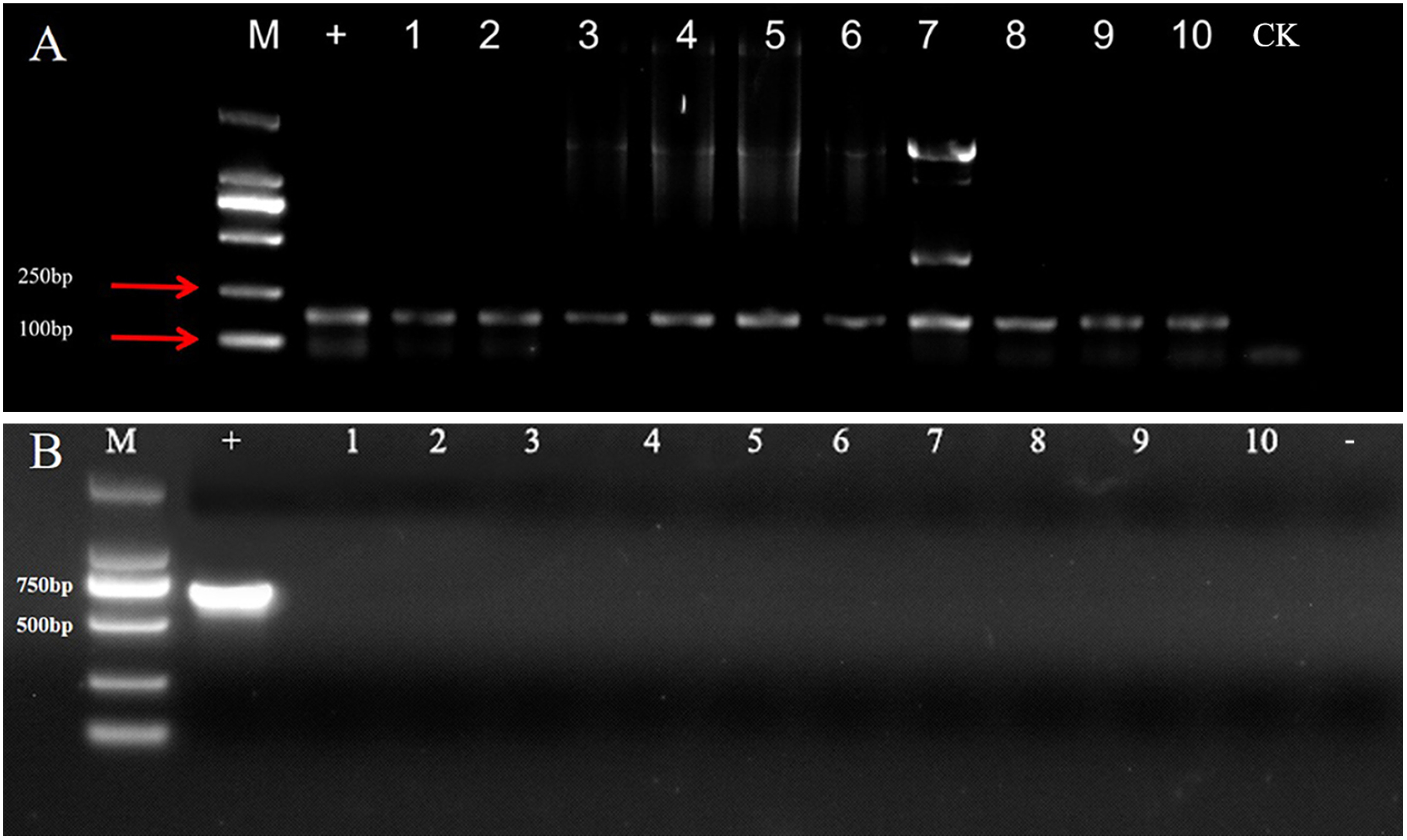
The GUS reporter gene was detected by PCR amplification using GUS-F and GUS-R gene-specific primers to confirm the successful transformation of the callus lines with the heterologous gene. The expected 150 bp band was amplified in the 10 callus lines transformed with pCAMBIA1381-35S::GUS, whereas no band was observed in the callus lines transformed with pCAMBIA1381-35S (Figure 3A). Meanwhile, no fragments from recombinant vector pCAMBIA1381-35S::GUS were amplified in the callus cells (Lane 1–10), while a 656-bp fragment was amplified in the sample with bacterial harboring plasmid pCAMBIA1381-35S::GUS as template (+) (Figure 3B), excluding the possibility of Agrobacterium cell contamination. All these indicated that the transgenic callus cells no longer contain bacteria and would be able to claim that the signal comes from stably transformed GUS genes.
Figure 3. Molecular analysis of GUS gene (A) and the sequence outside the T-DNA region of the recombinant vector (B) in different transformed calli of C. pilosula. M, 2,000 bp DNA Marker; −, negative control; +, bacteria harboring plasmid pCAMBIA1381-35S::GUS; CK, callus lines transformed with pCAMBIA1381-35S; 1–10, callus lines transformed with pCAMBIA1381-35S::GUS.
GUS staining method to detect callus induction rate
Because the pCAMBIA1381-35S::GUS vector carried the GUS reporter gene, we further examined the protein expression level of the GUS reporter gene in transgenic calli. Among the 21 randomly tested samples, 19 were positive. The results show that the conversion rate was as high as 90.47% (Figure 4E). Blue staining was not observed in the negative control group (Figure 4D).
Figure 4. Histochemical GUS assay of C. pilosula transgenic callus infected by pCAMBIA1381-35S and pCAMBIA1381-35S::GUS. (A) Transformed transgenic callus infected by A. tumefaciens GV3101 strain harboring pCAMBIA1381-35S. (B, C) Transformed transgenic callus after 15 days (B) and 60 days (C) of infection by GV3101 strain harboring pCAMBIA1381-35S::GUS. (D) GUS activity in transformed transgenic callus infected by GV3101 strain harboring pCAMBIA1381-35S. (E, F) GUS activity in transformed transgenic calli after 15 days (E) and 60 days (F) of infection by GV3101 strain harboring pCAMBIA1381-35S::GUS.
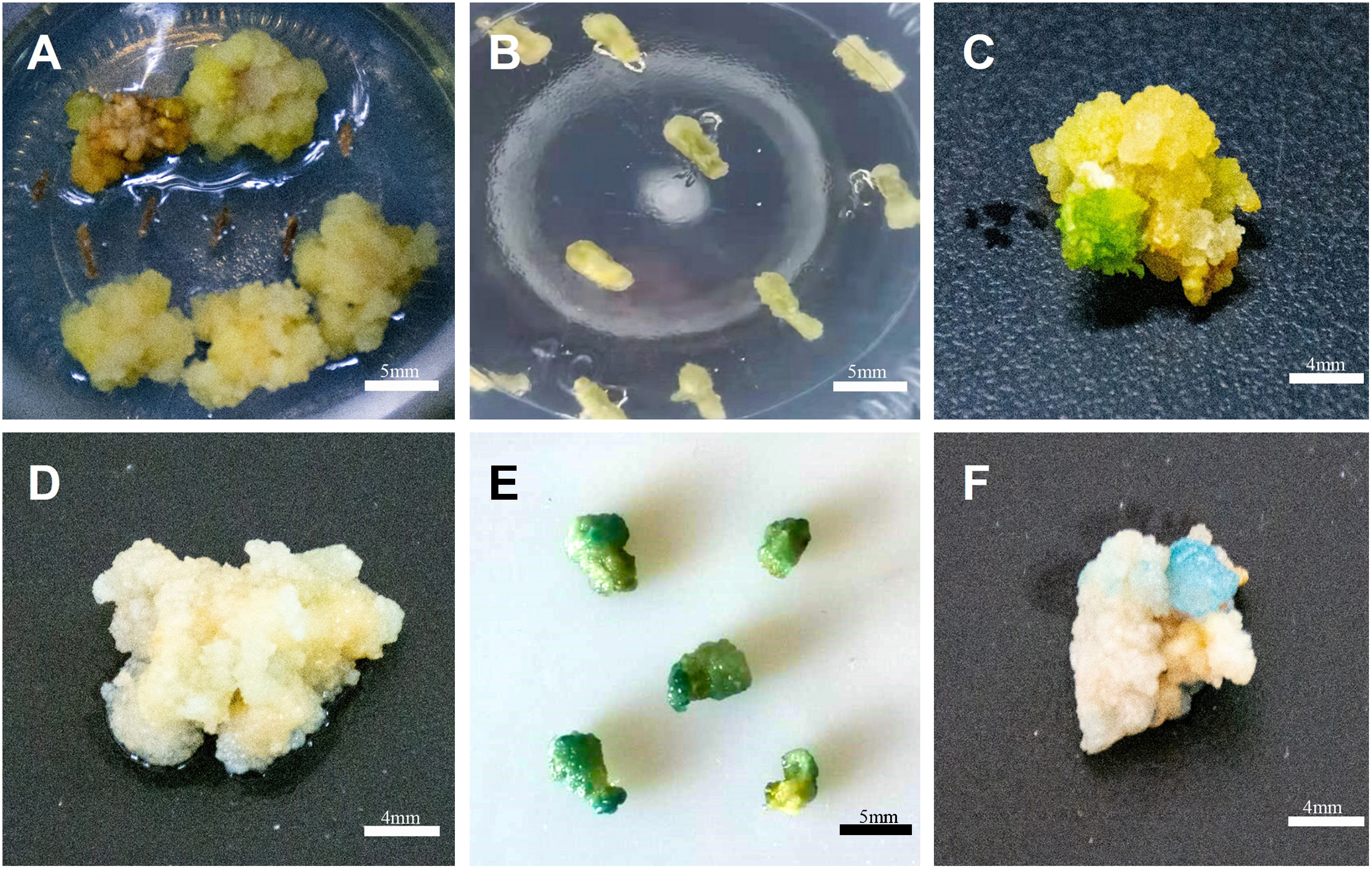
We analyzed GUS activity in proliferated transgenic calli successfully infected by strain GV3101 harboring pCAMBIA1381-35S::GUS confirmed by PCR and cultured for 60 days. GUS activity was observed in all transgenic callus lines tested, whereas no such activity was detected in the negative control lines.
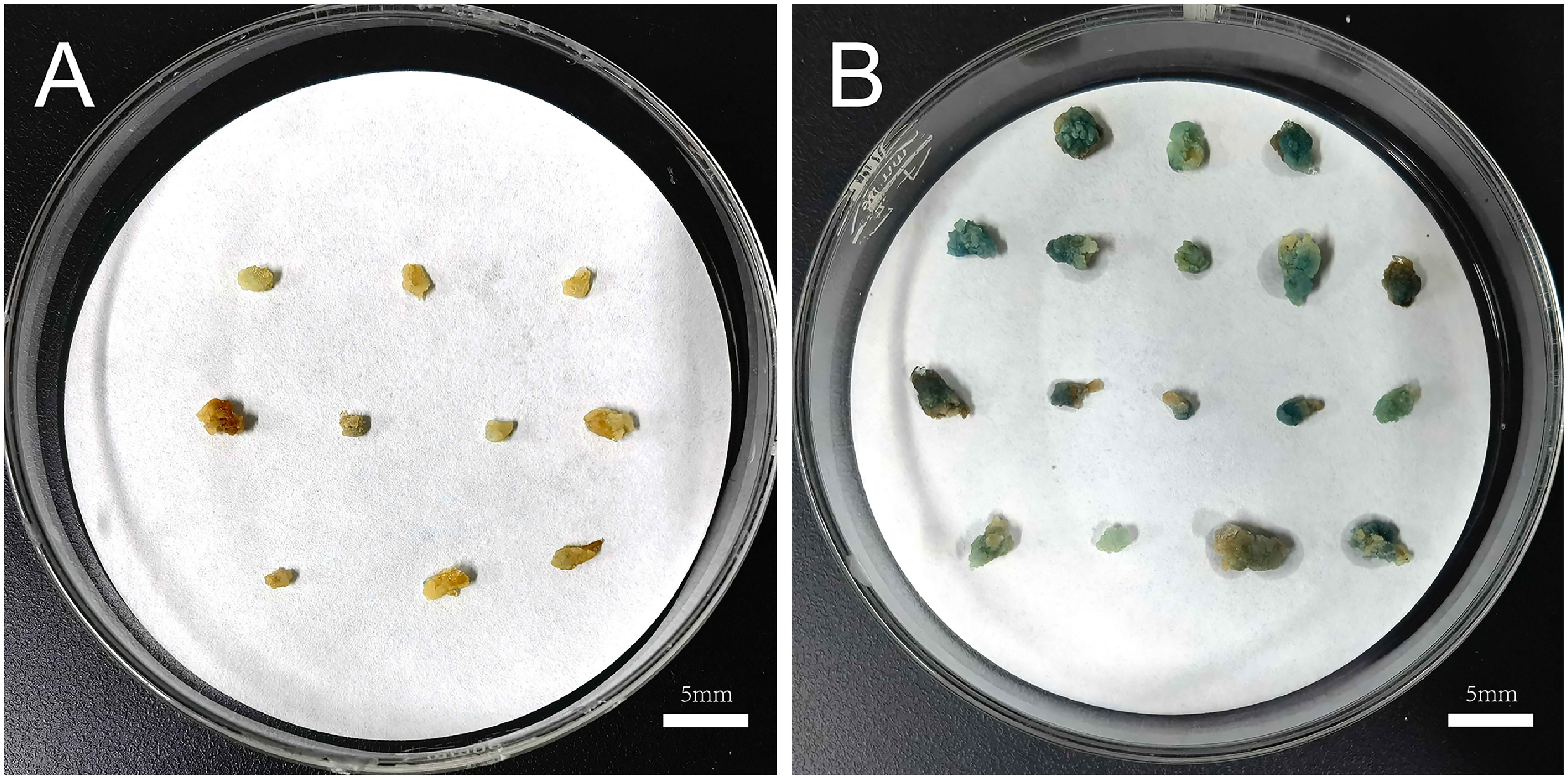
We verified the transformation rate of the calli using GUS staining. According to the optimal protocol of the callus genetic transformation system, calli were successfully induced at an induction rate that reached 91.07%. Additionally, 92.13% of calli showed the characteristic intense blue color. No blue coloration was observed in the control group. (Figure 5).
Figure 5. Validation of the transformation efficiency of the optimal callus genetic transformation system. (A) GUS activity in transformed calli infected by strain GV3101 harboring pCAMBIA1381-35S. (B) GUS activity in transformed calli infected by strain GV3101 harboring pCAMBIA1381-35S::GUS.
Discussion
In traditional Chinese medicine, C. pilosula strengthens the spleen and lungs, nourishes the blood, and promotes fluid (Gao et al. 2018). Polysaccharides are some of the main ingredients. The fructans were separated into CP-A and CP-B (Li JK et al. 2018; Li J 2020), and CP-A was found to have an anti-gastric ulcer effect (Li JK et al. 2017). However, most studies have focused on the chemical composition and pharmacological action of C. pilosula. Reports on the function of genes related to polysaccharide synthesis are severely limited because of the lack of a genetic transformation system (Cao et al. 2020; Wang et al. 2018).
Callus can be utilized for plant regeneration and as a biological reactor for producing bioactive compounds from medicinal plants (Guillon et al. 2006; Wang et al. 2012). Establishing a genetic transformation system lays the foundation for studying plant functional genes. Targeted genes can be introduced into plants by Agrobacterium transformation for functional analysis. The establishment of the C. pilosula callus genetic transformation system will lay the foundation for functional analysis of genes related to bioactive ingredient biosynthesis.
Several factors, including the pre-incubation time, bacterial concentration, infection time, period of co-cultivation, and delayed cultivation, influence the genetic transformation rate. The highest transformation frequency was found with stems of C. pilosula pre-cultured for three days, infected for 15 min with 0.3 OD600, and followed by co-cultivation for 1 day and delayed cultivation for 12 days. Pre-cultivation of the explants before infection can reduce the stress damage of Agrobacterium during the infection period and promote cell division. This makes the explant cells more sensitive to Agrobacterium to facilitate its adsorption, thereby improving the transformation efficiency of explants (Zhu et al. 2013). In this study, when the infection time was 15 min, the same as the that in optimal protocol, the transformation rate was decreased along with the decreasing of the pre-cultivation time, less than 10%. The callus transformation rate increased with prolonged pre-cultivation time, consistent with the findings of Zhang et al. (2020). The concentration of Agrobacterium and the infection time can affect the transformation rate (Wang et al. 2002). A too high bacterial concentration or too long infection time can easily lead to Agrobacterium contamination and browning or necrosis of explants (Sun and Wang 2007). We found that the transformation rate was less than 15% in the group with a long infection period (20 min) and short pre-cultivation period (less than 2 days). All groups with high bacterial concentrations showed a low transformation rate of less than 20%. If the concentration of the bacterial solution is too low or the infection time is too short, cell adsorption is insufficient, which will result in a low transformation rate. However, we found that a lower bacterial concentration led to a higher transformation rate in the optimal group, which might be due to the long delayed-cultivation period. The delayed cultivation medium was supplemented with cephalosporin to inhibit the growth of Agrobacterium. However, the lack of antibiotics for screening positive transgenic plants in a short time could enhance the adaptability to screening pressure, which could reduce the browning of the explants (Li et al. 2003). Wu et al. (2020) found that within a certain range, the induction and differentiation rates of resistant calli showed an increasing trend with increasing delays in cultivation time (Wu et al. 2020). In this study, we found that when the delayed cultivation period was less than 4 days, the transformation rate was lower.
The expression of hptII, widely used as a selectable marker in plant transformation systems, allows transformed plants to grow on media supplemented with antibiotics (Chattopadhyay et al. 2011; Rizvi et al. 2015). C. pilosula was highly sensitive to hygromycin, and the induction rate of transformed hairy roots was only 28.5% on the selection medium with 2 mg l−1 hygromycin (Yang et al. 2020), consistent with the results of the experiments described here.
In conclusion, we successfully established a genetic transformation system for C. pilosula calli with a transformation rate higher than 90%. In addition, calli can be subcultured or further induced into regenerated plants (not shown in the text). It provides a new method for studying the function of genes in C. pilosula, and lays the foundation for the establishment of a genetic transformation system for C. pilosula plant regeneration.
Acknowledgments
This research was funded by the National Key Research and Development Program of China (2018YFC1706301, 2018YFC1706304, 2019YFC1710800), the Key Projects of Key Research and Development Program of Shanxi Province (201603D3111005). We would like to thank Editage (www.editage.cn) for English language editing.
Abbreviations
- Cef
cefotaxime sodium
- Hyg
hygromycin
- Kan
kanamycin
- MS
callus-inducing medium
- Rif
rifampicin
- 6-BA
6-Benzylaminopurine
- 2,4-D
2,4-Dichlorophenoxyacetic acid
Author contributions
Z-YL and J-JJ designed the study and wrote the manuscript. Z-YL performed experiments and analyzed the data. X-RT and FJ prepared the cefotaxime sodium and hygromycin. J-KL and J-PG supervised the analysis and critically revised the manuscript.
References
- Bai RB, Zhang YJ, Fan JM, Jia XS, Li D, Wang YP, Zhou J, Yan Q, Hu FD (2020) Immune-enhancement effects of oligosaccharides from Codonopsis pilosula on cyclophosphamide induced immunosuppression in mice. Food Funct 11: 3306–3315 [DOI] [PubMed] [Google Scholar]
- Cao SB, Wang XL, Ji JJ, Wang YR, Gao JP (2020) Cloning and expression analysis of CpPMM gene in Codonopsis pilosula. Zhongguo Zhong Yao Za Zhi 45: 4382–4391 (in Chinese) [DOI] [PubMed] [Google Scholar]
- Chattopadhyay T, Roy S, Mitra A, Maiti KM (2011) Development of a transgenic hairy root system in jute (Corchorus capsularis L.) with gusA reporter gene through Agrobacterium rhizogenes mediated co-transformation. Plant Cell Rep 30: 485–493 [DOI] [PubMed] [Google Scholar]
- Chilton MD, Tepfer DA, Petit A, David C, Casse-Delbart F, Tempé J (1982) Agrobacterium rhizogenes inserts T-DNA into the genomes of the host plant root cells. Nature 295: 432–434 [Google Scholar]
- Gao JP, Wang D, Cao LY, Sun HF (2015) Transcriptome sequencing of Codonopsis pilosula and identification of candidate genes involved in polysaccharide biosynthesis. PLoS One 10: e0117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SM, Liu JS, Wang M, Cao TT, Qi YD, Zhang BG, Sun XB, Liu HT, Xiao PG (2018) Traditional uses, phytochemistry, pharmacology and toxicology of Codonopsis: A review. J Ethnopharmacol 219: 50–70 [DOI] [PubMed] [Google Scholar]
- Gao SM, Liu JS, Wang M, Liu YB, Meng XB, Zhang T, Qi YD, Zhang BG, Liu HT, Sun XB, et al. (2019) Exploring on the bioactive markers of Codonopsis Radix by correlation analysis between chemical constituents and pharmacological effects. J Ethnopharmacol 236: 31–41 [DOI] [PubMed] [Google Scholar]
- Ghosh R, Paul S, Ghosh SK, Roy A (2009) An improved method of DNA isolation suitable for PCR-based detection of begomoviruses from jute and other mucilaginous plants. J Virol Methods 159: 34–39 [DOI] [PubMed] [Google Scholar]
- Guillon S, Tremouillaux-Guiller J, Pati PK, Rideau M, Gantet P (2006) Hairy root research: Recent scenario and exciting prospects. Curr Opin Plant Biol 9: 341–346 [DOI] [PubMed] [Google Scholar]
- He JY, Ma N, Zhu S, Komatsu K, Li ZY, Fu WM (2015) The genus Codonopsis (Campanulaceae): A review of phytochemistry, bioactivity and quality control. J Nat Med 69: 1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idnurm A, Bailey AM, Cairns TC, Elliott CE, Foster GD, Ianiri G, Jeon J (2017) A silver bullet in a golden age of functional genomics: The impact of Agrobacterium-mediated transformation of fungi. Fungal Biol Biotechnol 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji JJ, Feng Q, Sun HF, Zhang XJ, Li XX, Li JK, Gao JP (2019) Response of bioactive metabolite and biosynthesis related genes to methyl jasmonate elicitation in Codonopsis pilosula. Molecules 24: 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Zhao K, Xie B, Zhang B, Luo K (2003) Establishment of a highly efficient transformation system for pepper (Capsicum annuum L.). Plant Cell Rep 21: 785–788 [DOI] [PubMed] [Google Scholar]
- Li J, Wang Y, Zhang X, Cao L, Ji J, Zheng Q, Gao J (2020) Isolation and structural identification of a novel fructan from Radix Codonopsis. J Carbohydr Chem 39: 163–174 [Google Scholar]
- Li JK, Wang T, Zhu ZC, Yang FR, Cao LY, Gao JP (2017) Structure features and anti-gastric ulcer effects of inulin-type fructan CP-A from the roots of Codonopsis pilosula (Franch.) Nannf. Molecules 22: 2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JK, Zhang X, Cao LY, Ji JJ, Gao JP (2018) Three inulin-type fructans from Codonopsis pilosula (Franch.) Nannf. Roots and their prebiotic activity on Bifidobacterium longum: Molecules 23: 3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Huang HY, Zeng B (2020) Induction of cluster buds on the basal stem of Lycium ruthenicum and establishment of an efficient plant regeneration system. Chin Herb Med 51: 3545–3553 [Google Scholar]
- Liu SW, Ma JJ, Liu HM, Guo YT, Li W, Niu SH (2020) An efficient system for Agrobacterium-mediated transient transformation in Pinus tabuliformis. Plant Methods 16: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao YY, Hu YY, Yi SY, Zhang XJ, Tan NH (2021) Establishment of hairy root culture of Rubia yunnanensis Diels: Production of Rubiaceae-type cyclopeptides and quinones. J Biotechnol 341: 21–29 [DOI] [PubMed] [Google Scholar]
- Olhoft PM, Flagel LE, Donovan CM, Somers DA (2003) Efficient soybean transformation using hygromycin B selection in the cotyledonary-node method. Planta 216: 723–735 [DOI] [PubMed] [Google Scholar]
- Rizvi NF, Cornejo M, Stein K, Weaver J, Cram EJ, Lee-Parsons CW (2015) An efcient transformation method for estrogen-inducible transgene expression in Catharanthus roseus hairy roots. Plant Cell Tissue Organ Cult 120: 475–487 [Google Scholar]
- Stepanova AY, Solov’eva AI, Malunova MV, Salamaikina SA, Panov YM, Lelishentsev AA (2021) Hairy roots of scutellaria spp. (Lamiaceae) as promising producers of antiviral flavones. Molecules 26: 3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WS, Wang YJ (2007) Study on the inhibition of Agrbacterium in plant genetic transformation. Anhui Nongye Kexue 31: 9913–9914 [Google Scholar]
- Wang D, Zhu CX, Zheng CC, Wen FJ (2002) Optimization of factors affecting the genetic transformation of Potato by Afrobacterium tumefacins. J Shandong Agric Univ (Nat Sci) 1: 23–27 [Google Scholar]
- Wang MM, Ma SW, Long L, Huo SD (2012) Effects of hormones on callus induction of Codonopsis pilosula and its polysaccharide content. Chin J Clin Ration Drug Use 5: 18–19 [Google Scholar]
- Wang XL, Ji JJ, Gao JP (2018) Clone and expression of CpGAPDH gene in Codonopsis pilosula. China J Chin Mater Med 43: 712–720 [DOI] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J (2006) Transformation of agrobacterium using the freeze-thaw method. CSH protoc 006: pdf.prot4666. [DOI] [PubMed] [Google Scholar]
- Wu ZP, Gao YK, Fan M, Gao YH (2020) Construction of regeneration and transformation system of Chrysanthemum ‘Jinbudiao’. Mol Plant Breed 18: 150–158 [Google Scholar]
- Yang J, Guo ZL, Wang WT, Cao X, Yang XY (2021) Genome-wide characterization of SPL gene family in Codonopsis pilosula reveals the functions of CpSPL2 and CpSPL10 in promoting the accumulation of secondary metabolites and growth of C. pilosula hairy root. Genes (Basel) 12: 1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yang XZ, Li B, Lu XY, Kang JF, Cao XY (2020) Establishment of in vitro culture system for Codonopsis pilosula transgenic hairy roots. 3 Biotech 10: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen F, Lu Y, Yuan Y, Chen QX (2020) Preliminary study of genetic transformation system of jasmine stem segment. Subtrop Plant Sci (Beijing, China) 49: 358–362 [Google Scholar]
- Zhu Y, Liu YX, Huang YH, Qiu R, Liu ZY (2013) Research of genetic transformation mediated by Agrobacterium tumefaciens on potato. Seed 32: 42–44 [Google Scholar]


