Abstract
BACKGROUND
Parkinson’s disease (PD) is a common neurogenerative disease marked by the characteristic triad of bradykinesia, rigidity, and tremor. A significant percentage of patients with PD also demonstrate postural abnormalities (camptocormia) that limit ambulation and accelerate degenerative pathologies of the spine. Although deep brain stimulation (DBS) is a well-established treatment for the motor fluctuations and tremor seen in PD, the efficacy of DBS on postural abnormalities in these patients is less clear.
OBSERVATIONS
The authors present a patient with a history of PD and prior lumbosacral fusion who underwent bilateral subthalamic nucleus DBS and experienced immediate improvement in sagittal alignment and subjective relief of mechanical low-back pain.
LESSONS
DBS may improve postural abnormalities seen in PD and potentially delay or reduce the need for spinal deformity surgery.
Keywords: sagittal alignment, camptocormia, deep brain stimulation, Parkinson’s disease
ABBREVIATIONS : CT = computed tomography, DBS = deep brain stimulation, PD = Parkinson’s disease, STN = subthalamic nucleus, SVA = sagittal vertical axis
Parkinson’s disease (PD) is a common neurodegenerative disease afflicting 1% of the population over the age of 60.1 Patients with PD typically demonstrate characteristic bradykinesia, rigidity, and tremor. Approximately 6.9% of these patients also demonstrate postural abnormalities such as camptocormia.2 Camptocormia is a nonfixed forward flexion of the thoracolumbar spine that worsens with walking and resolves with recumbency.3 Numerous medical and surgical treatment options exist for camptocormia in PD, however, there is no consensus in the literature on which option is most effective.
A recent systemic review in 2015 addressed this question and concluded that deep brain stimulation (DBS) offers sustained improvement in camptocormia with low postoperative morbidity compared to spinal deformity surgery.4 A similar review in 2019 found that PD patients receiving subthalamic nucleus (STN) DBS within 2 years of camptocormia onset had increased odds of achieving at least a 15-degree improvement in their sagittal plane bending angle as compared to patients with longer disease duration.5 As the prevalence of PD continues to increase, so does the demand for a unified treatment algorithm to address camptocormia in these patients. Patients who are first treated for degenerative spine pathology without addressing the underlying PD often experience poor outcomes with high rates of complications and revision surgeries.6,7 Therefore, early identification and treatment of camptocormia and other postural abnormalities in PD with DBS may reduce the need for spinal deformity surgeries, although further investigation is required.
We present a patient with a history of PD and prior lumbosacral fusion who underwent bilateral STN DBS and experienced immediate improvement in sagittal alignment and subjective relief of mechanical low-back pain.
Illustrative Case
A 69-year-old male with a history of PD first diagnosed more than 5 years ago was seen in the functional neurosurgery clinic. His primary symptoms at diagnosis were bradykinesia, tremor, and low-back pain with bilateral lower extremity radiculopathy. He was treated initially with standard medications (carbidopa-levodopa, entacapone, pramipexole) however his PD symptoms progressed. He also describes a history of prior lumbosacral fusion from L2 to S1 approximately 2 years earlier at an outside facility.
Following that surgery, he experienced progressively worsening lower-back pain and bilateral lower extremity radiculopathy. A computed tomography (CT) scan showed considerable lucency around the S1 pedicle screw and subtle lucency at the left L5 pedicle screw worrisome for pseudoarthrosis. He underwent revision surgery to remove the loose S1 instrumentation and decompress the left L5–S1 foramen by an outside surgeon. Despite this surgery, the patient continued to report mechanical low-back pain and bilateral radiculopathy with decreased sensation and strength. Repeat imaging showed L5 on S1 spondylolisthesis with severe narrowing of the bilateral neural foramina. CT myelogram demonstrated stenosis and vacuum disc at L5–S1, characteristic of pseudoarthrosis (Fig. 1).
FIG. 1.
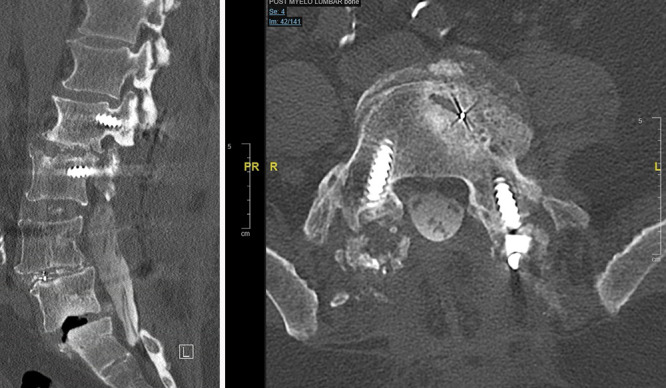
Preoperative postmyelography CT demonstrated pseudoarthrosis and spondylolisthesis at L5–S1.
The patient sought an additional spine recommendation, and an L5–S1 anterior lumbar interbody fusion and T10-pelvis posterior instrumentation and fusion was proposed. However, due to the concern for an increased risk of proximal junctional kyphosis in patients with PD, the patient was referred first for treatment of his underlying PD with DBS prior to any further spinal or deformity correction surgery. Therefore, the patient underwent bilateral MRI-guided STN DBS (Fig. 2). At the 1- and 3-month follow-up visits, the patient reported improvement in his PD symptoms, postural complaints, back pain, and radiculopathy. Two-month postoperative scoliosis radiographs demonstrated a significant improvement in his sagittal balance (Fig. 3) after DBS alone. Although our patient’s sagittal vertical axis (SVA) improved, his pelvic tilt increased, likely as a compensatory mechanism.
FIG. 2.
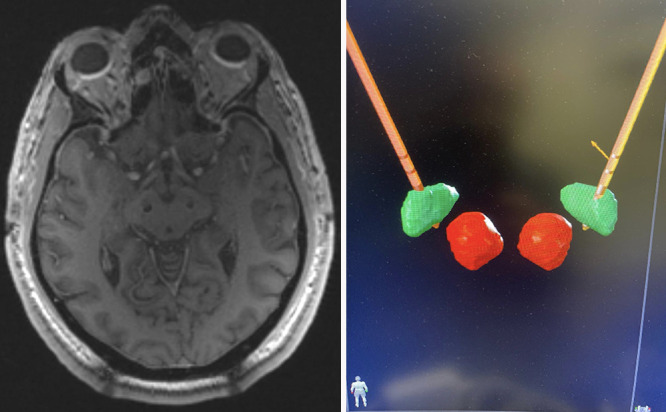
Left: Placement of bilateral STN depth electrodes using Clearpoint 2. Right: BrainLab atlas postoperative reconstruction confirming DBS placement within bilateral STNs (green). The red nucleus is depicted in red.
FIG. 3.
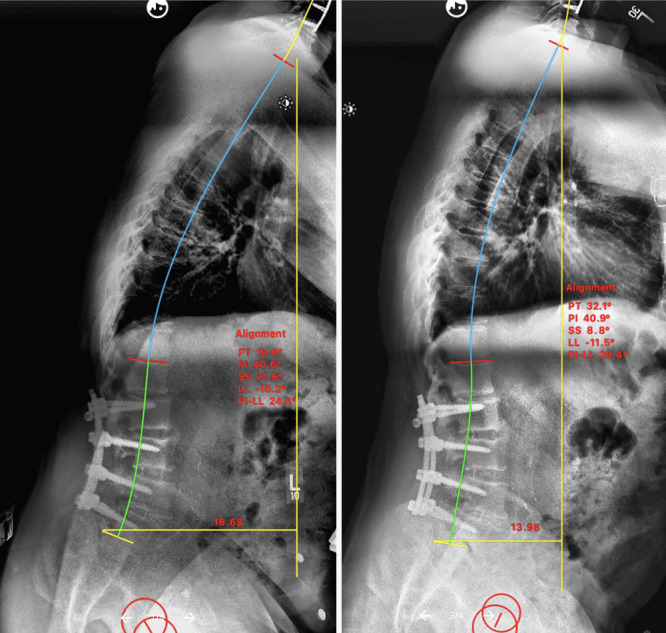
Sacropelvic parameters before (left) and after (right) DBS surgery for PD. Note the improvement in SVA after DBS.
Discussion
Spinal deformities such as scoliosis, camptocormia, Pisa syndrome, and antecollis are common among patients with PD.7,8 The underlying cause has not been fully elucidated, but there are several theories including medication effects, soft tissue changes, myopathy, and dystonia. Dystonia and myopathies can lead to spinal deformity by affecting paraspinal musculature causing subsequent deformities.7–9 This theory is supported by electromyographical, histological, and imaging studies in patients with PD showing increased myopathic and dystonic pathologies in the paraspinal muscles.7,8
Similarly, the involvement of paraspinal musculature in patients with PD leads to postural instability, which is a biomechanical problem that significantly affects quality of life. Postural instability in patients with PD tends to appear later in the disease course. The narrow stance and inclined posture classic in PD are due to a combination of impaired proprioception and increased muscle tone.10 This instability and balance impairment portends to increased frequency of falls and contributes to spinal deformities. Various interventions including DBS, physical therapy, and spinal cord stimulation have been shown to improve gait difficulties.11 However, surgery is still commonly performed to address spinal deformity in patients with PD.
Observations
Our patient had multiple spine surgeries for low-back pain and bilateral radiculopathy. It has been well reported in the literature that patients with PD have increased complications from spine surgery and high reoperation rates.12–14 Sheu et al.13 examined 66 patients with PD who underwent spine surgeries at their institution and found that 29% of patients required revision surgery. They also reported that instrumentation of more than three surgical levels and patients undergoing corrective osteotomies had earlier reoperations. Similar studies found patients with PD had revision rates between 21% and 86%.13,14 The reasons for revision surgeries included hardware failure, worsening kyphosis, vertebral fractures, and other surgery-related complications.
There are several suspected reasons for the increased complication and reoperation rate in patients with PD. Because patients with PD are often older, they naturally have age-related bone loss and poor bone quality.14,15 However, the decreased bone quality in patients with PD is multimodal in nature. Studies looking at the bone mineral density in patients with PD matched with patients without PD found that patients with PD had a lower bone mineral density.16 Decreased bone density has been shown to increase the risks of fracture, instrumentation failure, and pseudoarthrosis. Patients with PD also have reduced activity levels due to the inherent nature of the disease. It has been shown that decreased physical activity leads to decreased mechanical stress on bones, further exacerbating bone loss.15 Furthermore, the flexed posture classically seen in PD and many other sagittal plane deformities increases the biomechanical stress on instrumentation leading to failure.14,15
Lessons
Spinal deformities are common among patients with PD, but there are several factors which make patients with PD poor candidates for spinal deformity surgery. Therefore, it may be important to first treat PD to reduce the complications from spine surgery or the need for multiple operations. We present a patient with a history of PD and multiple spine surgeries who underwent bilateral STN DBS and experienced immediate improvement in sagittal alignment and subjective relief of mechanical low-back pain. The role of DBS in the management of spinal deformities in patients with PD should be further investigated with larger, multicenter prospective studies. In addition to improving the classic symptoms of PD such as bradykinesia and tremor, DBS has the potential to improve postural abnormalities and reduce or delay the need for spinal deformity surgeries in patients with PD.
Disclosures
Dr. Saifi reported personal fees from Nuvasive outside the submitted work; consulting and shares (stock) for Restor3d; consulting from Alphatec Spine; and shares (stock) for Vertera. No other disclosures were reported.
Author Contributions
Conception and design: Bhenderu, Saifi, Faraji. Acquisition of data: Guerrero, Bhenderu, Faraji. Analysis and interpretation of data: Guerrero, Bhenderu, Cruz-Garza, Faraji. Drafting the article: Guerrero, Bhenderu, Cruz-Garza, Faraji. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Guerrero. Administrative/technical/material support: Faraji. Study supervision: Saifi, Faraji.
References
- 1. Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 2017;124(8):901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 2. Doherty KM, van de Warrenburg BP, Peralta MC, et al. Postural deformities in Parkinson’s disease. Lancet Neurol. 2011;10(6):538–549. doi: 10.1016/S1474-4422(11)70067-9. [DOI] [PubMed] [Google Scholar]
- 3. Spindler P, Alzoobi Y, Kühn AA, Faust K, Schneider GH, Vajkoczy P. Deep brain stimulation for Parkinson’s disease-related postural abnormalities: a systematic review and meta-analysis. Neurosurg Rev. 2022;45(5):3083–3092. doi: 10.1007/s10143-022-01830-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chieng LO, Madhavan K, Wang MY. Deep brain stimulation as a treatment for Parkinson’s disease related camptocormia. J Clin Neurosci. 2015;22(10):1555–1561. doi: 10.1016/j.jocn.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 5. Chan AK, Chan AY, Lau D, et al. Surgical management of camptocormia in Parkinson’s disease: systematic review and meta-analysis. J Neurosurg. 2018;131(2):368–375. doi: 10.3171/2018.4.JNS173032. [DOI] [PubMed] [Google Scholar]
- 6. Kimura H, Fujibayashi S, Otsuki B, et al. Lumbar spinal surgery in patients with Parkinson disease: a multicenter retrospective study. Clin Spine Surg. 2017;30(6):E809–E818. doi: 10.1097/BSD.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 7. Ruttiman R, Eltorai AEM, Daniels AH. Etiology and management of spinal deformity in patients with Parkinson’s disease. Int J Spine Surg. 2018;12(1):15–21. doi: 10.14444/5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abe K, Uchida Y, Notani M. Camptocormia in Parkinson’s disease. Parkinsons Dis. 2010;2010:267640. doi: 10.4061/2010/267640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farah K, Prost S, Meyer M, et al. Surgery for spinal deformity in Parkinson’s disease patients: what are we missing? Neurochirurgie. 2022;68(2):183–187. doi: 10.1016/j.neuchi.2021.08.004. [DOI] [PubMed] [Google Scholar]
- 10. Viseux FJF, Delval A, Defebvre L, Simoneau M. Postural instability in Parkinson’s disease: review and bottom-up rehabilitative approaches. Neurophysiol Clin. 2020;50(6):479–487. doi: 10.1016/j.neucli.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 11. Debû B, De Oliveira Godeiro C, Lino JC, Moro E. Managing Gait, Balance, and Posture in Parkinson’s Disease. Curr Neurol Neurosci Rep. 2018;18(5):23. doi: 10.1007/s11910-018-0828-4. [DOI] [PubMed] [Google Scholar]
- 12. Spindler P, Tkatschenko D, Alzoobi Y, et al. Thoracolumbar instrumentation surgery in patients with parkinson’s disease: a case-control study. J Neurol Surg A Cent Eur Neurosurg. doi: 10.1055/s-0041-1741535. Published online January 31, 2022. doi: 10.1055/s-0041-1741535. [DOI] [PubMed] [Google Scholar]
- 13. Sheu H, Liao JC, Lin YC. The fate of thoracolumbar surgeries in patients with Parkinson’s disease, and analysis of risk factors for revision surgeries. BMC Musculoskelet Disord. 2019;20(1):106. doi: 10.1186/s12891-019-2481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Babat LB, McLain RF, Bingaman W, Kalfas I, Young P, Rufo-Smith C. Spinal surgery in patients with Parkinson’s disease: construct failure and progressive deformity. Spine (Phila Pa 1976) 2004;29(18):2006–2012. doi: 10.1097/01.brs.0000138306.02425.21. [DOI] [PubMed] [Google Scholar]
- 15. Sapkas G, Lykomitros V, Soultanis K, Papadopoulos EC, Papadakis M. Spinal surgery in patients with Parkinson’s disease: unsatisfactory results, failure and disappointment. Open Orthop J. 2014;8:264–267. doi: 10.2174/1874325001408010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu B, Chen G, Yu Z, et al. Bone mineral density and related scores in Parkinson’s disease: a systematic review and meta-analysis. World Neurosurg. 2021;146:e1202–e1218. doi: 10.1016/j.wneu.2020.11.132. [DOI] [PubMed] [Google Scholar]


