Abstract
Introduction: Tuberous sclerosis complex (TSC) is a rare autosomal dominant condition characterized by cutaneous, cerebral, and other multiorgan involvement. Aneurysms due to TSC pathogenic mechanism are rarely present, mainly aortic, renal, or intracranial and very few associated with peripheral circulation. A TSC patient, aged 31 years, who developed brachial and subclavian arteries aneurysms is presented. The question of a random association of the aneurysms with TSC versus aneurysms within pathogenic released mammalian target of rapamycin (mTOR) pathway effect was raised. Case presentation: Patient’s file, available from the age of six months, was analyzed for demonstration of the TSC diagnosis. Patient was examined, and cerebral magnetic resonance imaging (MRI) was repeated. Surgery and angiographic reports and images were reviewed. Pathology of the aneurysmal wall available from surgery was reexamined and special stainings and immunohistochemistry markers were applied. Genetic characterization of the patient was performed. Definite TSC was diagnosed based on major criteria [ungual fibromas, shagreen patch, cortical tubers, subependymal nodules (SENs), subependymal giant cell astrocytoma (SEGA)], minor criteria (confetti skin lesions, dental enamel pits, gingival fibromas), genetic result showing heterozygous variant in exon 8 of TSC1 gene (c.733C>T-p.Arg245*). Pathology analysis revealed markedly thickened aneurysmal wall due to smooth muscle cells (SMCs) proliferation in media and neoformation vessels with similar characteristics in the aneurysmal wall. Discussions and Conclusions: This is a rare case with aneurysms related to TSC, with an exceptional peripheral localization. Pathology exam is the key investigation in demonstrating the TSC-related pathogenic mechanism. A literature review showed 73 TSC cases presenting aneurysms published until now.
Keywords: tuberous sclerosis complex, subclavian artery, aneurysms, heterozygous TSC1 variant
⧉ Introduction
Tuberous sclerosis complex (TSC) is a rare autosomal dominant disorder characterized by skin abnormalities (hypomelanotic macules, facial angiofibromas, shagreen patches, fibrous facial plaques, ungual fibromas), brain involvement [cortical tubers, subependymal nodules (SENs) and subependymal giant cell astrocytoma (SEGA), seizures, intellectual disability], also affecting other organs, such as: kidneys [angiomyolipomas (AMLs)], cysts, renal cell carcinomas), heart (rhabdomyomas), lungs [lymphangioleiomyomatosis (LAM)], etc. [1]. Vascular involvement is rare and therefore less known and studied. Central and peripheral aneurysms and large and medium size arterial stenotic-occlusive disease have been reported in patients with TSC, as well as dysplasia of small vessels [2], including fibromuscular dysplasia [3].
Aim
We present the case of a 31-year-old TSC male patient with TSC1 mutation diagnosed with two consecutive aneurysms of the right brachial and subclavian arteries, respectively, raising the question of a random association of the aneurysms with TSC, versus aneurysms within pathogenic effect of the unsuppressed mammalian target of rapamycin (mTOR) pathway.
⧉ Case presentation
Patient’s file, available from the age of six months was analyzed for demonstration of TSC diagnosis. Patient was clinically reexamined, and brain magnetic resonance imaging (MRI) was repeated. The TSC1, TSC2 genes were analyzed by polymerase chain reaction (PCR) and next-generation sequencing of both deoxyribonucleic acid (DNA) strands of the entire coding region, and the highly conserved exon–intron splice junction analysis was performed. Multiplex ligation-dependent probe amplification (MLPA) analyses were performed to test for deletions or duplications within or including the TSC1, TSC2 genes.
Surgery and angiography reports and films were reviewed together with the surgeon and the interventional radiologist. Pathology of the fragments of the aneurysmal wall available from the two surgical interventions were reexamined and special stainings for elastin, smooth muscle fibers and immunohistochemistry markers for vascular endothelium were applied: Hematoxylin–Eosin (HE), elastic van Gieson, alpha-smooth muscle actin (α-SMA), cluster of differentiation 34 (CD34) marker.
Cutaneous, mucosal, ungual and dental changes
Three ungual fibromas of the right- and left-hand fingers (Figure 1b) were documented in the patient’s file at the age of 11 months; shagreen patch of the dorso-lumbar region, confetti white spots of the skin were noted at the age of four years, one dental enamel pit and gingival fibromas at the age of seven years.
Figure 1.
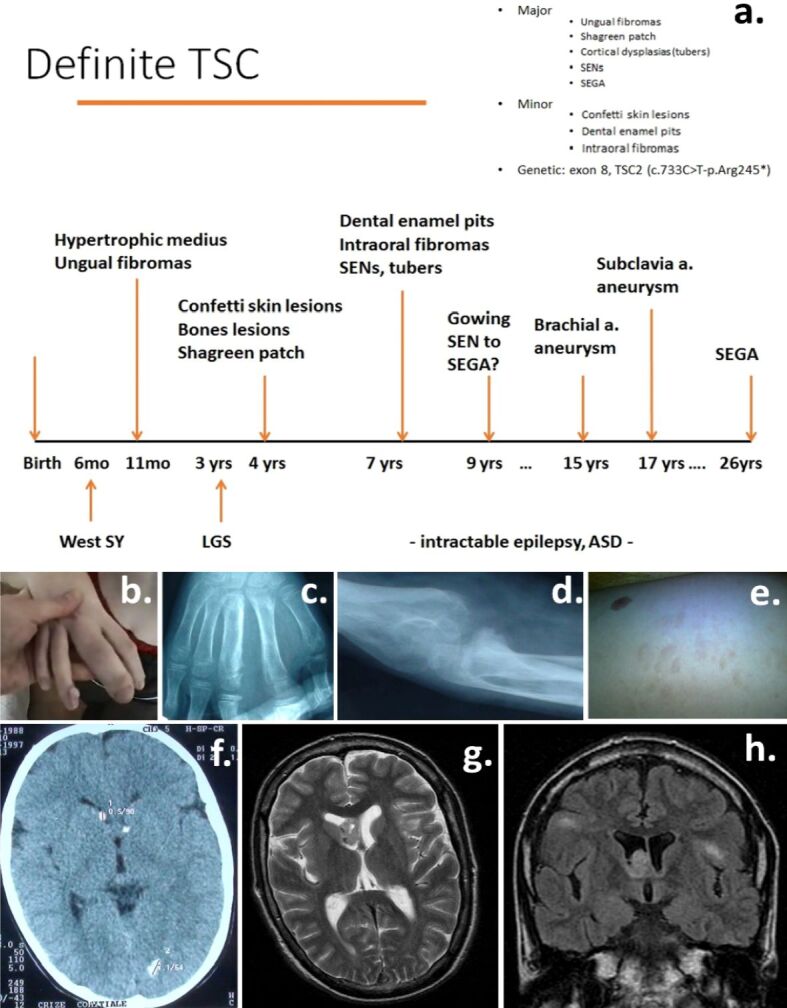
(a) Chronological development of clinical features of the case; (b) Ungual fibromas; (c and d) Bone changes – hypertrophic digitus medius of the right hand with disorganized structure of the medius phalanges, thick bones cortex, osseous cysts, and periosteal apposition of the right forearm long and small bones; (e) Shagreen patch; (f) Calcified SENs and occipital tuber; (g) Right SEGA; (h) Bilateral cortical tubers and right SEGA. ASD: Autism spectrum disorder; LGS: Lennox–Gastaut syndrome; SEGA: Subependymal giant cell astrocytoma; SEN: Subependymal nodule; SY: Syndrome; TSC: Tuberous sclerosis complex
Cerebral lesions
At the age of four years, a brain computed tomography (CT) scan revealed calcified SENs (Figure 1f); cerebral MRI performed at the age of 7, 11 and 26 years showed multiple bilateral cortical tubers and growing SENs; at the age of 26 years, SEGA was noted (Figure 1, g and h).
Epilepsy, intellectual disability, and autism spectrum disorder
Patient had West syndrome starting at the age of six months (epileptic spasms, hypsarrhythmia, developmental arrest), further evolving to Lennox–Gastaut syndrome, with multiple types of seizures: tonic seizures while awake and in sleep, generalized tonic-clonic seizures, atypical absences, focal seizures resistant to most antiseizure medications tried in monotherapy or different combinations. He had delayed milestones achievement and evolved to a profound intellectual disability [intelligence quotient (IQ)<20 at the age of 31], associating autism spectrum disorder with moderate aggressiveness from early childhood.
Other clinical changes
Bone changes (hypertrophic digitus medius of the right hand, with disorganized structure of the phalanges, thick bones cortex, osseous cysts, and periosteal apposition of the right forearm long and small bones) were noted starting around the age of four years (Figure 1a,1b,1c,1d,1e,1f,1g,1h). No cardiac, renal, pulmonary or eye changes were present.
Aneurysms
Patient developed a growing tumor in the right arm starting at the age of 13 (Figure 2, a and b). Right arm MRI with Gadolinium and further angiography showed a giant aneurysmal dilatation of the brachial artery and important surrounding soft tissue changes secondary to local ischemia (Figure 2c,2d,2e,2f). Aneurysm and damaged tissue were surgically removed. Angiographic checkup after one year showed a second murine aneurysm at the right subclavian level (Figure 3a). Ligature and partial resection of the aneurysm were performed (Figure 3b). Angiography of the arms, neck and head arteries did not detect other aneurysms. Aortic, renal, or lower limb vessels were not investigated with angiography, but chest CT scan and abdominopelvic MRI with contrast agent were negative.
Figure 2.
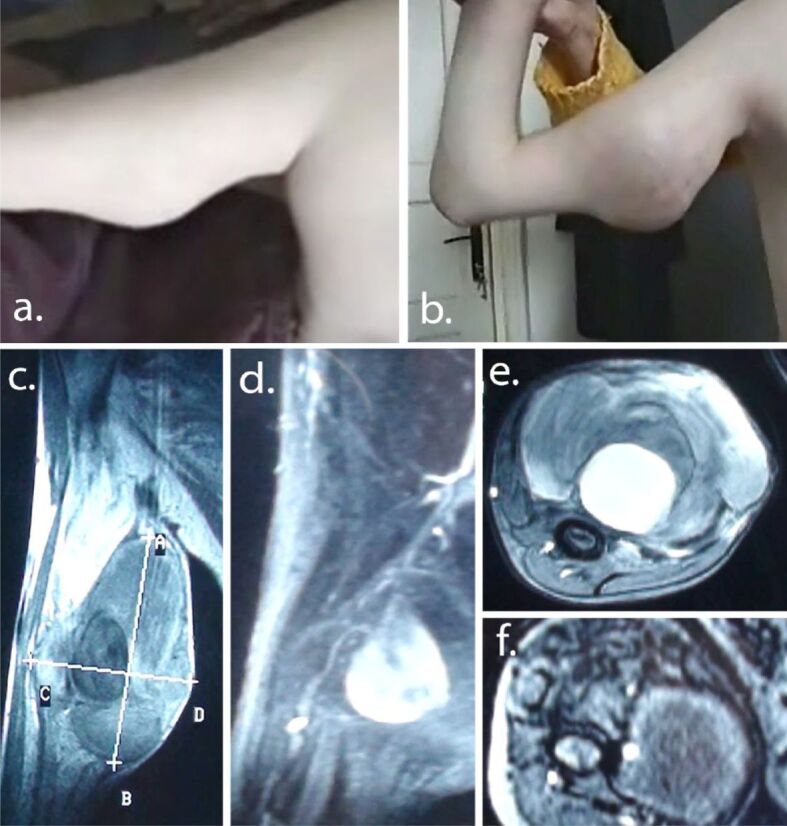
(a and b) Brachial artery aneurysm: clinical progression (one year apart); (c–f) Preoperatory MRI with contrast agent showing massive saccular (5.5/6 cm) dilatation of middle part of brachial artery and destruction of surrounding tissue. MRI: Magnetic resonance imaging
Figure 3.
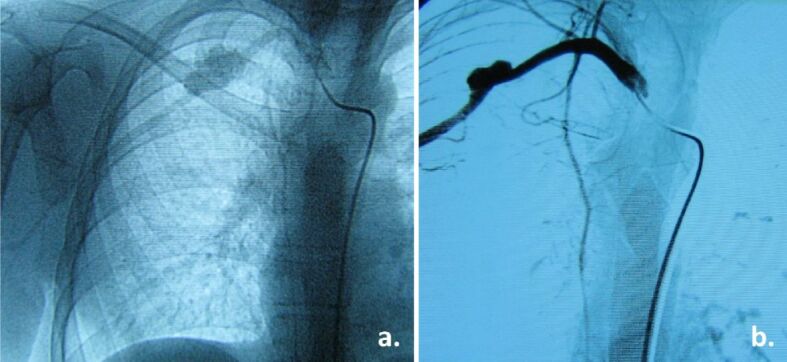
(a) Angiography at one year after surgery for brachial aneurysm shows a second aneurysm – subclavian artery; (b) Same aneurysm after ligature (partial resection)
Genetics and clinical family examination
Genetic analysis showed a heterozygous mutation in exon 8 of the TSC1 gene (c.733C>T-p.Arg245*). Father, who had periungual fibromas, declined further assessment (clinical, imaging, or genetic). Mother stated that her husband had only one generalized tonic-clonic seizure at the age of 42 for which he didn’t receive treatment. Other family members (mother, sister) showed no TSC clinical signs.
Pathology examination
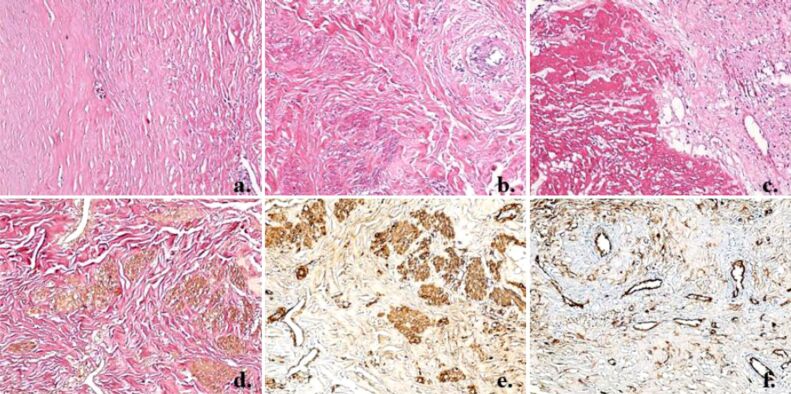
Pathology examination of the surgically removed fragments revealed a markedly thickened fibrous aneurysmal wall, with disorganized structure. Thickening was due to excessive proliferation of smooth muscle cells (SMCs) within media layer; the smooth muscle fibers were fragmented by overdeveloped collagen fibers, arranged in islands, and showing markedly disorganized arrangement. Middle tunic elastic layer was disorganized and fragmented by the collagen fibers. The intimate tunic showed large deposits of fibrin. Proliferation of small (neoformation) vessels in the outer tunic of the aneurysmal wall was observed (Figure 4a,4b,4c,4d,4e,4f).
Figure 4.
(a) Markedly thickened fibrous aneurysmal wall; (b) Middle tunic of the vascular wall with markedly disorganized arrangement of the collagen fibers; (c) Large deposits of fibrin in the intimate tunic of the vascular wall; (d) Medium tunic elastic fibers, disorganized and fragmented by overdeveloped collagen fibers, with no visible continuous elastic layer; (e) Smooth muscle fibers proliferation in the middle tunic of the vascular wall, fragmented by collagen fibers, arranged in islands; (f) Microscopic image from the outer tunic of the vascular wall, which shows the presence of many blood vessels, including angiogenesis vessels. HE staining: (a–c) ×100. van Gieson staining: (d) ×100. Immunostaining with anti-α-SMA antibody: (e) ×100. Immunostaining with anti-CD34 antibody: (f) ×100. α-SMA: Alpha-smooth muscle actin; CD34: Cluster of differentiation 34; HE: Hematoxylin–Eosin
⧉ Discussions
This patient was diagnosed with definite TSC based on five major criteria (ungual fibromas, shagreen patch, cortical tubers, SENs, SEGA), three minor criteria (confetti skin lesions, dental enamel pits, gingival fibromas), the genetic result showing a heterozygous pathogenic variant in exon 8 of the TSC1 gene (c.733C>T-p.Arg245*). The disease was most probably transmitted by the father, who had periungual nodules and a single tonic-clonic epileptic seizure at the age of 42, but no other clinical and genetic data are available; father declined treatment and investigations.
The case presented here showed two aneurysms in the arteries of the right arm, brachial and subclavian arteries. According to Boronat et al. (2014), aneurysms are rarely described, but twice more frequent in TSC patients (0.74%) compared to the general population (0.35%) [4]. Literature review underlines the rarity of TSC cases associating aneurysms – only 73 cases published since 1971 (Table 1). The most frequent arterial location is intracranial (53 aneurysms), usually involving the internal carotid artery (33 aneurysms); the second frequent aneurysmal location is aortic (36 aneurysms, 30 developed in the abdominal aorta). Peripheral aneurysms are very rare – only six cases have been previously described, presenting 10 aneurysms, in the iliac, iliofemoral, axillary, subclavian, or brachial arteries (Table 1). Patients usually have single aneurysms, only 12 cases presented multiple affected arteries and among these, only one in the arm. The patient discussed here presented two aneurysms in the arm, an exceptional aneurysmal location. Due to this rare disposition, an obvious question arose: are the aneurysms of the described case randomly associated with TSC or a direct result of the disturbed mTOR pathway and therefore a vascular TSC manifestation?
Table 1.
Location of TSC-associated aneurysms (literature review)
|
Author(s), year |
Ref. No. |
Aneurysm location |
No. of patients |
Age |
Genetics |
|
Freycon et al., 1971 |
5 |
Abdominal aorta |
1 |
|
|
|
Larbre et al., 1971 |
6 |
Abdominal aorta |
1 |
|
|
|
Davidson, 1974 |
7 |
Multiple (2): internal carotid arteries (bilateral, fusiform) |
1 |
Child |
|
|
Snowdon, 1974 |
8 |
Intracranial |
1 |
Child |
|
|
Dutton & Singleton, 1975 |
9 |
Abdominal aorta |
1 |
Child |
|
|
Hagood et al., 1976 |
10 |
Abdominal aorta |
1 |
Infant |
|
|
Ho, 1980 |
11 |
Intraventricular |
1 |
26 years |
|
|
Beall & Delaney, 1983 |
12 |
Multiple (2): internal carotid; anterior communicating artery |
1 |
17 years |
|
|
Guttman et al., 1984 |
13 |
Giant intracranial |
1 |
53 years |
|
|
Brill et al., 1985 |
14 |
Giant intracranial |
1 |
Child |
|
|
Copley, 1985 |
15 |
Intracranial |
1 |
|
|
|
Blumenkopf & Huggins, 1985 |
16 |
Multiple fusiform intracranial aneurysms |
1 |
6 years |
|
|
Martin et al., 1987 |
17 |
Giant intracranial |
1 |
|
|
|
Towbin et al., 1987 |
18 |
Abdominal aorta |
1 |
9 months |
|
|
Ng et al., 1988 |
19 |
Abdominal aorta |
1 |
24 years |
|
|
Libby et al., 1989 |
20 |
Axillary |
1 |
|
|
|
Shepherd et al., 1991 |
21 |
Thoracic aorta |
1/355 (causes of death) |
Child |
|
|
Occhionorelli et al., 1991 |
22 |
Abdominal aorta |
1 |
Adult |
|
|
van Reedt Dortland et al., 1991 |
23 |
Abdominal aorta |
1 |
5 years |
|
|
Lavocat et al., 1992 |
24 |
Abdominal aorta (giant) |
1 |
4.5 months |
|
|
Engel, 1992 |
25 |
Arterial circle of Willis |
1 |
1 year |
|
|
Tsukui et al., 1995 |
26 |
Abdominal aorta |
1 |
4 years |
|
|
Paraf & Bruneval, 1996 |
3 |
Abdominal aorta (pathology – fibromuscular dysplasia) |
1 |
|
|
|
Spangler et al., 1997 |
27 |
Multiple (3): internal carotid (fusiform), anterior cerebral (ectasia), middle cerebral (ectasia), all same side (left) |
1 |
5 months |
|
|
Longa et al., 1997 |
28 |
Middle cerebral (asymptomatic) |
1 |
30 years |
Large TSC2/PKD1 deletion |
|
Tamisier et al., 1997 |
29 |
Abdominal aorta |
1 |
Child |
|
|
Swarnkar et al., 1998 |
30 |
Intracranial |
1 |
Child |
|
|
Hite et al., 1998 |
31 |
Multiple (2): axillary, brachial |
1 |
10 months |
|
|
Jarrett et al., 1998 |
32 |
Carotid |
1 |
|
|
|
Beltramello et al., 1999 |
2 |
Internal carotid (giant) |
1 |
11 years |
|
|
Ko et al., 2000 |
33 |
Abdominal aorta (hamartomatous) |
1 |
22 years |
|
|
Bavdekar et al., 2000 |
34 |
Aorta |
1 |
Child |
|
|
Baker & Furnival, 2000 |
35 |
Abdominal aorta |
1 |
12 months |
|
|
Chen et al., 2001 |
36 |
Internal carotid (paraclinoid) |
1 |
19 years |
|
|
Jost et al., 2001 |
37 |
Abdominal aorta |
1 |
9 years |
|
|
Thoracic aorta |
1 |
41 years |
|
||
|
Patzer et al., 2002 |
38 |
Internal carotid |
1 |
|
|
|
Jones et al., 2002 |
39 |
Intracranial: midbasilar (giant) |
1 |
19 months |
|
|
Burrows & Johnson, 2004 |
40 |
Pulmonary |
1 |
52 years |
|
|
Lee et al., 2004 |
41 |
Abdominal aorta |
1 |
8 months |
|
|
Patiño Bahena et al., 2005 |
42 |
Abdominal aorta (giant) and aortic dissection |
1 |
8 months |
|
|
Kimura et al., 2005 |
43 |
Descending aorta |
1 |
2 years |
|
|
Wong et al., 2006 |
44 |
Abdominal aorta (infradiaphragmatic) |
1 |
4 years |
|
|
Araújo et al., 1996 |
45 |
Internal carotid (giant) |
1 |
9 years |
TSC2 deletion |
|
Carette et al., 2006 |
46 |
Pulmonary |
1 |
|
|
|
Koch et al., 2008 |
47 |
Subclavian |
1 |
4 years |
|
|
Calcagni et al., 2008 |
48 |
Iliofemoral |
1 |
20 months |
|
|
Hung et al., 2008 |
49 |
Multiple intracranial (2): internal carotid and middle cerebral |
1 |
8 months |
|
|
Moon et al., 2009 |
50 |
Abdominal aorta |
1 |
9 months |
|
|
Salerno et al., 2010 |
51 |
Abdominal aorta |
1 |
14 months |
|
|
Aissi et al., 2010 |
52 |
Internal carotid (intracavernous) |
1 |
34 years |
|
|
Sabat et al., 2010 |
53 |
Multiple intracranial (2): posterior communicating (bilateral) |
1 |
Adult |
|
|
Cao et al., 2010 |
54 |
Multiple aortic (2): thoracoabdominal aorta and thoracic aorta |
1 |
3 years |
TSC2 del.5340-5371 |
|
Shelton et al., 2011 |
55 |
Internal carotid (intracavernous) |
1 |
9 months |
|
|
Denne et al., 2011 |
56 |
Abdominal aorta |
1 |
10 months |
TSC2 |
|
Ye et al., 2012 |
57 |
Abdominal aorta |
1/6 |
17 months |
|
|
Yi et al., 2012 |
58 |
Internal carotid |
1 |
13 months |
|
|
Koroknay-Pál et al., 2012 |
59 |
Multiple intracranial (4) fusiform aneurysms – three giant: one basilar, two internal carotid artery at bifurcation (bilateral), one posterior communicating artery |
1/114 |
6 years |
|
|
Kirkwood et al., 2013 |
60 |
Extracranial carotid |
2/3 monozygotic twins |
32 years |
|
|
Intracranial |
Maternal grandmother |
|
|
||
|
Bailey et al., 2013 |
61 |
Thoracoabdominal aorta |
1 |
3 years |
|
|
Dunet et al., 2013 |
62 |
Multiple pulmonary artery |
1 |
Adolescence |
TSC1 (exon 10, c.990insT) |
|
Sawan et al., 2015 |
63 |
Thoracoabdominal aorta |
1 |
5 years |
|
|
Wang et al., 2017 |
64 |
Superior mesenteric artery, right subclavian artery aneurysm |
1 |
22 years |
|
|
Dueppers et al., 2017 |
65 |
Abdominal aortic aneurysm |
1 |
9 years |
|
|
Wang et al., 2017 |
66 |
Carotid artery aneurysm |
1 |
21 months |
Large deletion 16p13.3. (TSC2/PKD1 contiguous gene syndrome) |
|
Geiger et al., 2019 |
67 |
Thoracic aortic aneurysm |
1 |
26 years |
|
|
Wiemer-Kruel et al., 2020 |
68 |
Two aortic aneurysms and congenital segmental lymphedema of the left leg |
1 |
7 years |
TSC2 (exon 38, c.5024C>T; p.(Pro1675Leu) |
|
Hedin et al., 2021 |
69 |
Abdominal aorta (infrarenal) |
1 |
2 years |
|
|
Byrne et al., 2022 |
70 |
Thoracic aorta |
1 |
Pediatric patient |
|
|
Olvera et al., 2022 |
71 |
Thoraco-abdominal aorta (three aneurysms) |
1 |
4 years followed-up to 18 years |
|
PKD1: Polycystin 1, transient receptor potential channel interacting; TSC: Tuberous sclerosis complex
The pathogeny of aneurysms formation in people without TSC is a complex remodeling process of synthesis and degradation of matrix proteins, the most striking feature being a thin media due to elastin fragmentation (leading to a compromised elastin network) [72,73], decreased SMCs density by 74% by apoptosis, adventitial collagen synthesis, inflammation playing a role in the interaction between mesenchymal cells (SMCs and fibroblasts) and inflammatory cells (lymphocytes and macrophages) [74]. By contrast, TSC aneurysmal pathogeny is characterized by increased proliferation of the SMCs within the media, disorganized structure also involving the elastic layer [54]. The pathology examination of the aneurysmal wall of the patient showed characteristic changes for activation of the mTOR pathway, with the typical important thickening of the aneurysmal wall based on media SMCs proliferation.
We report a pathogenic variant in exon 8 of the TSC1 gene (c.733C>T-p.Arg245*). c.733C>T variant has been previously described; in the Leiden Open Variation Database (LOVD) (http://chromium.lovd.nl/LOVD2/TSC/home.php?select_db=TSC1&used_old_url), c.733C>T variant was reported 45 times. This was the only mutation in 40 reports, another variant being associated in the rest of the five reports, including TSC2 gene mutations. Usually, TSC2 variants are associated with severe phenotypes [75]. Only 10 of the LOVD reports were published in five articles [76,77,78,79,80]. None of the published cases with c.733C>T variant associated aneurysms.
In the literature review of the published TSC cases associating aneurysms, very few discussed the causal variant (Table 1). Both TSC1 and TSC2 variants have been described in individuals with TSC and aneurysms. TSC pathogeny has a role in the genesis of aneurysms, considering that aneurysms are twice more frequent in the TSC population compared to the general population. Why are not all TSC patients presenting aneurysms? Why is this clinical manifestation so rare? Most probably other modifying genes and/or epigenetic factors are involved.
The patients with TSC and palpable vascular masses, hypertension, abdominal pain, or other symptoms, which may suggest aneurysms, should be screened using duplex ultrasound as the initial diagnostic approach of choice [51]. Multislice CT or MRI is recommended as a complementary screening and diagnostic tool [48]. Because TSC families with positive history of aneurysms do exist (although rare), it was suggested that systematic screening for aneurysms should be added as standard care of the asymptomatic TSC patients with vascular positive family history [60]. Routine screening for aneurysms is not recommended in patients with TSC and no positive history for aneurysms, due to the very low incidence of the aneurysms in the TSC population (0.74%) [4, 51].
Everolimus, an mTOR inhibitor, is now indicated for treatment of SEGA and AMLs, leading to their size reduction, but also has a favorable effect on other clinical elements of the disease, such as angiofibromas, or skin lesions [81]. mTOR is a serine/threonine kinase regulated by phosphoinositide-3-kinase (PI3K) and regulates cellular metabolism, cell growth, motility, angiogenesis. The PI3K-mTOR pathway members are implicated in the pathogeny of vascular anomalies by dysregulation of angiogenesis and lymphangiogenesis, protein overgrowth, cellular hypermetabolism [82,83]. It was shown in an experimental rat model that Rapamycin (mTOR inhibitor) suppressed the aortic aneurysm growth [84], but the pathogeny of this model may differ from that of the activated mTOR pathway. Therefore, mTOR inhibitors are predicted to be effective in other disorders in which the growth control factor is affected. There is no data in the literature presenting the effects of Everolimus treatment on aneurysms in TSC patients, but it is very tempting to think that Everolimus will have a favorable effect on TSC aneurysms, as Rapamycin (Sirolimus) proved to be effective in vascular anomalies such as vascular malformations or vascular tumors (Kaposiform hemangioendothelioma, capillary lymphatic venous malformation, diffuse microcystic lymphatic malformation), with significant response/improvement to Sirolimus [85]. In a recent published case of a 7-year-old TSC male patient with TSC2 mutation, multiple aneurysms have been described: one growing aneurysm of the abdominal aorta near the emergence of both renal arteries, one of the common iliac, one of the left external iliac, two of the left internal iliac arteries. Because of an additional lymphatic malformation of the left leg, he was treated with Everolimus for six weeks, then stopped, four weeks before an urgent operation of the growing aortic aneurysm at the age of 17 months. One month after surgery, the treatment with Everolimus was resumed for the lymphatic malformation, without long-term side effects (more than 3.3 years with 3.5 mg/day). In a routine control of the aorta by CT angiography at five months, a new aneurysm was seen at the junction of the aorta and renal arteries, above the graft with Everolimus therapy. It remained stable over time, not growing with Everolimus treatment of 5 mg/day [68]. It is possible that the growth of the first aneurysm was due to an insufficient treatment duration. Arrest aneurysm growth in this single case report points at possible favorable effect of Everolimus for this pathology. It is known that in TSC aneurysms, massive SMCs proliferation within aneurysmal wall leads to elastic fibers fragmentation and impressive loss of organization of the trilaminar vascular wall structure [54]. Hypothetically, if Everolimus treatment is used and SMCs proliferation is stopped or even reversed, one may speculate that a thin and fragile aneurysmal wall (with damaged elastic layer) may result, and this might lead to an increased probability of rupture. Currently no proof exists on this effect of Everolimus treatment on SMCs. Further research is needed to clarify this issue.
⧉ Conclusions
A case with a severe phenotype having a TSC1 gene mutation is presented; usually, the severe phenotype is associated with the TSC2 variants. This is a rare case presenting aneurysms related to TSC, with an exceptional localization. Pathology examination is the key investigation for demonstrating TSC-related pathogenic mechanism. A literature review of TSC cases presenting with aneurysms was performed (73 cases). Very few TSC patients with aneurysms were genetically analyzed, and in most cases strict analysis of the TSC1 and TSC2 genes was performed. To determine if other modifying genes or epigenetic factors are involved in the pathogenesis of the TSC-associated aneurysms, further research is needed. The role of Everolimus in the treatment of TSC aneurysms must be unraveled.
Conflict of interests
The authors declare that they have no conflict of interests.
Acknowledgments
Acknowledgments
Many thanks to Dragoş-Paul Hagiu, who kindly offered his time and talent to organize the figures for this article and to Horea Constantin Chirilă for technical support.
Role of the funding source
The analysis of genetic background of this patient was performed using an educational grant from Novartis Pharma. Novartis had no role in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication. I, first author, had full access to all the data in this case and I take complete responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Northrup H, Krueger DA, International Tuberous Sclerosis Complex Consensus Group Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49(4):243–254. doi: 10.1016/j.pediatrneurol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beltramello A, Puppini G, Bricolo A, Andreis IA, el-Dalati G, Longa L, Polidoro S, Zavarise G, Marradi P. Does the tuberous sclerosis complex include intracranial aneurysms? A case report with a review of the literature. Pediatr Radiol. 1999;29(3):206–211. doi: 10.1007/s002470050573. [DOI] [PubMed] [Google Scholar]
- 3.Paraf F, Bruneval P. Dysplasie fibromusculaire artérielle et sclérose tubéreuse de Bourneville [Arterial fibromuscular dysplasia and Bourneville’s tuberous sclerosis] Ann Pathol. 1996;16(3):203–206. [PubMed] [Google Scholar]
- 4.Boronat S, Shaaya EA, Auladell M, Thiele EA, Caruso P. Intracranial arteriopathy in tuberous sclerosis complex. J Child Neurol. 2014;29(7):912–919. doi: 10.1177/0883073813492386. [DOI] [PubMed] [Google Scholar]
- 5.Freycon F, Mollard P, Hermier M, Guibaud P, Chazalette JP, Weill B, Flattot M, Jeune M. Anévrysme de l’aorte abdominale au cours d’une sclérose tubéreuse de Bourneville [Abdominal aorta aneurysm during Bourneville’s tuberous sclerosis] Pediatrie. 1971;26(4):421–417. [PubMed] [Google Scholar]
- 6.Larbre F, Loire R, Guibaud P, Lauras B, Weill B. Observation clinique et anatomique d’un anévrisme de l’aorte au cours d’une sclérose tubéreuse de Bourneville [Clinical and anatomical case of an aortic aneurysm in the course of Bourneville’s tuberous sclerosis] Arch Fr Pediatr. 1971;28(9):975–984. [PubMed] [Google Scholar]
- 7.Davidson S. Tuberous sclerosis with fusiform aneurysms of both internal carotid arteries manifested by unilateral visual loss and papilledema. Bull Los Angeles Neurol Soc. 1974;39(3):128–132. [PubMed] [Google Scholar]
- 8.Snowdon JA. Cerebral aneurysm, renal cysts and hamartomas in a case of tuberous sclerosis. Br J Urol. 1974;46(5):583–583. doi: 10.1111/j.1464-410x.1974.tb03860.x. [DOI] [PubMed] [Google Scholar]
- 9.Dutton RV, Singleton EB. Tuberous sclerosis: a case report with aortic aneurysm and unusual rib changes. Pediatr Radiol. 1975;3(3):184–186. doi: 10.1007/BF01006909. [DOI] [PubMed] [Google Scholar]
- 10.Hagood CO, Garvin DD, Lachina FM, Polsky WS, Ball TP, Bobroff LM. Abdominal aortic aneurysm and renal hamartoma in an infant with tuberous sclerosis. Surgery. 1976;79(6):713–715. [PubMed] [Google Scholar]
- 11.Ho KL. Intraventricular aneurysm associated with tuberous sclerosis. Arch Neurol. 1980;37(6):385–386. doi: 10.1001/archneur.1980.00500550087017. [DOI] [PubMed] [Google Scholar]
- 12.Beall S, Delaney P. Tuberous sclerosis with intracranial aneurysm. Arch Neurol. 1983;40(13):826–827. doi: 10.1001/archneur.1983.04050120076015. [DOI] [PubMed] [Google Scholar]
- 13.Guttman M, Tanen SM, Lambert CD. Visual loss secondary to a giant aneurysm in a patient with tuberous sclerosis. Can J Neurol Sci. 1984;11(4):472–474. doi: 10.1017/s0317167100046035. [DOI] [PubMed] [Google Scholar]
- 14.Brill CB, Peyster RG, Hoover ED, Keller MS. Giant intracranial aneurysm in a child with tuberous sclerosis: CT demonstration. J Comput Assist Tomogr. 1985;9(2):377–380. doi: 10.1097/00004728-198503000-00031. [DOI] [PubMed] [Google Scholar]
- 15.Copley DJ. Case of the season. Diagnosis: intracranial aneurysms in a patient with tuberous sclerosis. Semin Roentgenol. 1985;20(2):107–109. doi: 10.1016/0037-198x(85)90060-4. [DOI] [PubMed] [Google Scholar]
- 16.Blumenkopf B, Huggins MJ. Tuberous sclerosis and multiple intracranial aneurysms: case report. Neurosurgery. 1985;17(5):797–800. doi: 10.1227/00006123-198511000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Martin N, de Broucker T, Cambier J, Marsault C, Nahum H. MRI evaluation of tuberous sclerosis. Neuroradiology. 1987;29(5):437–443. doi: 10.1007/BF00341739. [DOI] [PubMed] [Google Scholar]
- 18.Towbin RB, Ball WS, Kaufman RA. Pediatric case of the day. Abdominal aortic aneurysm in a patient with tuberous sclerosis. Radiographics. 1987;7(4):818–821. doi: 10.1148/radiographics.7.4.3329364. [DOI] [PubMed] [Google Scholar]
- 19.Ng SH, Ng KK, Pai SC, Tsai CC. Tuberous sclerosis with aortic aneurysm and rib changes: CT demonstration. J Comput Assist Tomogr. 1988;12(4):666–668. doi: 10.1097/00004728-198807000-00031. [DOI] [PubMed] [Google Scholar]
- 20.Libby PA, Maitem AN, Strauss EB. Axillary artery aneurysm in a patient with tuberous sclerosis. Pediatr Radiol. 1989;20(1-2):94–94. doi: 10.1007/BF02010644. [DOI] [PubMed] [Google Scholar]
- 21.Shepherd CW, Gomez MR, Lie JT, Crowson CS. Causes of death in patients with tuberous sclerosis. Mayo Clin Proc. 1991;66(8):792–796. doi: 10.1016/s0025-6196(12)61196-3. [DOI] [PubMed] [Google Scholar]
- 22.Occhionorelli S, Mascoli F, Romano D, Taddia MC, Donini A, Vasquez G, Santini M, Galeotti R, Cavagna E. Aneurisma dell’aorta addominale e stenosi da compressione del tripode celiaco in giovane donna con sclerosi tuberosa già trovata affetta da nefroma mesoblastico [Aneurysm of the abdominal aorta and stenosis caused by compression of the celiac tripod in a young woman with tuberous sclerosis and previous mesoblastic nephroma] Minerva Chir. 1991;46(23–24):1271–1274. [PubMed] [Google Scholar]
- 23.van Reedt Dortland RW, Bax NM, Huber J. Aortic aneurysm in a 5-year-old boy with tuberous sclerosis. J Pediatr Surg. 1991;26(12):1420–1422. doi: 10.1016/0022-3468(91)91054-3. [DOI] [PubMed] [Google Scholar]
- 24.Lavocat MP, Teyssier G, Allard D, Tronchet M, Freycon F. Anévrysme de l’aorte abdominale et sclérose tubéreuse de Bourneville [Abdominal aortic aneurysm and Bourneville’s tuberous sclerosis] Pediatrie. 1992;47(7–8):517–519. [PubMed] [Google Scholar]
- 25.Engel U. Hirnbasisaneurysma und tuberöse Hirnsklerose [Aneurysm at the base of the brain and tuberous sclerosis] Zentralbl Pathol. 1992;138(1):67–70. [PubMed] [Google Scholar]
- 26.Tsukui A, Noguchi R, Honda T, Tobita T, Fukuda S, Shimoji K. Aortic aneurysm in a four-year-old child with tuberous sclerosis. Paediatr Anaesth. 1995;5(1):67–70. doi: 10.1111/j.1460-9592.1995.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 27.Spangler WJ, Cosgrove GR, Moumdjian RA, Montes JL. Cerebral arterial ectasia and tuberous sclerosis: case report. Neurosurgery. 1997;40(1):191–193; discussion 193-194. doi: 10.1097/00006123-199701000-00042. [DOI] [PubMed] [Google Scholar]
- 28.Longa L, Scolari F, Brusco A, Carbonara C, Polidoro S, Valzorio B, Riegler P, Migone N, Maiorca R. A large TSC2 and PKD1 gene deletion is associated with renal and extrarenal signs of autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 1997;12(9):1900–1907. doi: 10.1093/ndt/12.9.1900. [DOI] [PubMed] [Google Scholar]
- 29.Tamisier D, Goutière F, Sidi D, Vaksmann G, Bruneval P, Vouhé P, Leca F. Abdominal aortic aneurysm in a child with tuberous sclerosis. Ann Vasc Surg. 1997;11(6):637–639. doi: 10.1007/s100169900104. [DOI] [PubMed] [Google Scholar]
- 30.Swarnkar A, Jungreis CA, Peel RL. Central odontogenic fibroma and intracranial aneurysm associated with tuberous sclerosis. Am J Otolaryngol. 1998;19(1):66–69. doi: 10.1016/s0196-0709(98)90069-2. [DOI] [PubMed] [Google Scholar]
- 31.Hite SH, Kuo JS, Cheng EY. Axillary artery aneurysm in tuberous sclerosis: cross-sectional imaging findings. Pediatr Radiol. 1998;28(7):554–556. doi: 10.1007/s002470050412. [DOI] [PubMed] [Google Scholar]
- 32.Jarrett ME, Libertiny G, Gould SJ, Morris P. Carotid artery aneurysm in a child with tuberous sclerosis. Eur J Vasc Endovasc Surg. 1998;16(1):80–81. doi: 10.1016/s1078-5884(98)80097-x. [DOI] [PubMed] [Google Scholar]
- 33.Ko Y, Horikoshi S, Mizuno A, Aoki I, Taguchi S. A surgical case of abdominal aortic aneurysm accompanied by hamartomatous changes in the vascular wall] Nihon Geka Gakkai Zasshi. 2000;101(11):809–813. [PubMed] [Google Scholar]
- 34.Bavdekar SB, Vaideeswar P, Bakune RH, Sahu DK, Kamat JR. Aortic aneurysm in a child with tuberous sclerosis. Indian Pediatr. 2000;37(3):319–322. [PubMed] [Google Scholar]
- 35.Baker PC, Furnival RA. Tuberous sclerosis presenting with bowel obstruction and an aortic aneurysm. Pediatr Emerg Care. 2000;16(4):255–257. doi: 10.1097/00006565-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Chen YL, Luo CB, Hsu SW, Rodesch G, Lasjaunias P. Tuberous sclerosis complex with an unruptured intracranial aneurysm: manifestations of contiguous gene syndrome. Interv Neuroradiol. 2001;7(4):337–341. doi: 10.1177/159101990100700410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jost CJ, Gloviczki P, Edwards WD, Stanson AW, Joyce JW, Pairolero PC. Aortic aneurysms in children and young adults with tuberous sclerosis: report of two cases and review of the literature. J Vasc Surg. 2001;33(3):639–642. doi: 10.1067/mva.2001.111976. [DOI] [PubMed] [Google Scholar]
- 38.Patzer L, Basche S, Misselwitz J. Renal artery stenosis and aneurysmatic dilatation of arteria carotis interna in tuberous sclerosis complex. Pediatr Nephrol. 2002;17(3):193–196. doi: 10.1007/s00467-001-0799-5. [DOI] [PubMed] [Google Scholar]
- 39.Jones BV, Tomsick TA, Franz DN. Guglielmi detachable coil embolization of a giant midbasilar aneurysm in a 19-month-old patient. AJNR Am J Neuroradiol. 2002;23(7):1145–1148. [PMC free article] [PubMed] [Google Scholar]
- 40.Burrows NJ, Johnson SR. Pulmonary artery aneurysm and tuberous sclerosis. Thorax. 2004;59(1):86–86. doi: 10.1136/thx.2003.016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee EJ, Yim SJ, Kim SM, Jeong DC, Kang JH, Chung SY. A case of tuberous sclerosis associated with abdominal aneurysm. Korean J Pediatr. 2004;47(5):583–587. https://www.koreamed.org/SearchBasic.php?RID=1654797 [Google Scholar]
- 42.Patiño Bahena E, Calderón-Colmenero J, Buendía A, Juanico A. Giant aortic aneurysm and rhabdomyomas in infant with tuberous sclerosis (case report) Arch Cardiol Mex. 2005;75(4):448–450. [PubMed] [Google Scholar]
- 43.Kimura Y, Sugimura H, Toda M, Nakamura Y, Shibuya K, Murakami A, Wakita S, Yanagawa Y, Kato H. A case of 2-year-old boy with tuberous sclerosis complicated with descending aortic aneurysm. Pediatr Int. 2005;47(2):224–226. doi: 10.1111/j.1442-200x.2005.02031.x. [DOI] [PubMed] [Google Scholar]
- 44.Wong H, Hadi M, Khoury T, Geary D, Rubin B, Filler G. Management of severe hypertension in a child with tuberous sclerosis-related major vascular abnormalities. J Hypertens. 2006;24(3):597–599. doi: 10.1097/01.hjh.0000209994.33680.11. [DOI] [PubMed] [Google Scholar]
- 45.Araújo JF, Santori RK, Sperlescu A, Marins JL, Balbo RJ. Aneurisma intracraniano gigante em criança de nove anos. Relato de caso [Giant intracranial aneurysm in a 9 year-old child. Case report] Arq Neuropsiquiatr. 1996;54(4):673–676. doi: 10.1590/s0004-282x1996000400020. [DOI] [PubMed] [Google Scholar]
- 46.Carette MF, Antoine M, Bazelly B, Cadranel J, Khalil A. Primary pulmonary artery aneurysm in tuberous sclerosis: CT, angiography and pathological study. Eur Radiol. 2006;16(10):2369–2370. doi: 10.1007/s00330-006-0173-x. [DOI] [PubMed] [Google Scholar]
- 47.Koch AME, Janka R, Lang W, Dittrich S. Arterial aneurysm in tuberous sclerosis. Eur Heart J. 2008;29(10):1282–1282. doi: 10.1093/eurheartj/ehm568. [DOI] [PubMed] [Google Scholar]
- 48.Calcagni G, Gesualdo F, Tamisier D, Brunelle F, Sidi D, Ou P. Arterial aneurysms and tuberous sclerosis: a classic but little known association. Pediatr Radiol. 2008;38(7):795–797. doi: 10.1007/s00247-008-0813-1. [DOI] [PubMed] [Google Scholar]
- 49.Hung PC, Wang HS, Chou ML, Wong AMC. Tuberous sclerosis complex with multiple intracranial aneurysms in an infant. Pediatr Neurol. 2008;39(5):365–367. doi: 10.1016/j.pediatrneurol.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 50.Moon SB, Shin WY, Park YJ, Kim SJ. An abdominal aortic aneurysm in an 8-month-old girl with tuberous sclerosis. Eur J Vasc Endovasc Surg. 2009;37(5):569–571. doi: 10.1016/j.ejvs.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Salerno AE, Marsenic O, Meyers KE, Kaplan BS, Hellinger JC. Vascular involvement in tuberous sclerosis. Pediatr Nephrol. 2010;25(8):1555–1561. doi: 10.1007/s00467-010-1466-5. [DOI] [PubMed] [Google Scholar]
- 52.Aissi M, Younes-Mhenni S, Jerbi-Ommezzine S, Boughammoura-Bouatay A, Frih-Ayed M, Sfar MH. Anévrismes intracrâniens et sclérose tubéreuse de Bourneville: une association rare [Tuberous sclerosis and intracranial aneurysms: a rare association] Rev Neurol (Paris) 2010;166(11):935–939. doi: 10.1016/j.neurol.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Sabat SB, Cure J, Sullivan J, Gujrathi R. Tuberous sclerosis with multiple intracranial aneurysms: atypical tuberous sclerosis diagnosed in adult due to third nerve palsy. Acta Neurol Belg. 2010;110(1):89–92. [PubMed] [Google Scholar]
- 54.Cao J, Gong L, Guo DC, Mietzsch U, Kuang SQ, Kwartler CS, Safi H, Estrera A, Gambello MJ, Milewicz DM. Thoracic aortic disease in tuberous sclerosis complex: molecular pathogenesis and potential therapies in Tsc2+/- mice. Hum Mol Genet. 2010;19(10):1908–1920. doi: 10.1093/hmg/ddq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shelton JB, Ramakrishnaiah R, Glasier CM, Phillips PH. Cavernous sinus syndrome from an internal carotid artery aneurysm in an infant with tuberous sclerosis. J AAPOS. 2011;15(4):389–391. doi: 10.1016/j.jaapos.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 56.Denne C, Gerstl EM, Mayer K, Steinborn M, Hahn H, Burdach S. Présentation inhabituelle de la sclérose tubéreuse de Bourneville de chez un nourrisson: à propos d’un cas [Uncommon presentation of tuberous sclerosis in an infant] Arch Pediatr. 2011;18(6):660–664. doi: 10.1016/j.arcped.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 57.Ye C, Yin H, Lin Y, Zhou L, Ye R, Li X, Han A, Wang S. Abdominal aorta aneurysms in children: single-center experience of six patients. Ann Thorac Surg. 2012;93(1):201–205. doi: 10.1016/j.athoracsur.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 58.Yi JL, Galgano MA, Tovar-Spinoza Z, Deshaies EM. Coil embolization of an intracranial aneurysm in an infant with tuberous sclerosis complex: a case report and literature review. Surg Neurol Int. 2012;3:129–129. doi: 10.4103/2152-7806.102944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koroknay-Pál P, Lehto H, Niemelä M, Kivisaari R, Hernesniemi J. Long-term outcome of 114 children with cerebral aneurysms. J Neurosurg Pediatr. 2012;9(6):636–645. doi: 10.3171/2012.2.PEDS11491. [DOI] [PubMed] [Google Scholar]
- 60.Kirkwood ML, Chung J, Timaran CH, Valentine RJ. Extracranial carotid artery aneurysms in two of three monozygotic triplets with tuberous sclerosis complex. J Vasc Surg. 2013;57(4):1120–1122. doi: 10.1016/j.jvs.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 61.Bailey MA, Rashid ST, Bridge KI, Griffin KJ, Brown E, Guerrero RR, Patel JV, Scott DJ. Images in vascular medicine. Large thoraco-abdominal aneurysm in a 3-year-old boy with tuberous sclerosis. Vasc Med. 2013;18(3):147–148. doi: 10.1177/1358863X13475690. [DOI] [PubMed] [Google Scholar]
- 62.Dunet V, Qanadli SD, Lazor R, Beigelman-Aubry C. Multiple pulmonary artery aneurysms in tuberous sclerosis complex. BMJ Case Rep. 2013;2013:bcr2012007911–bcr2012007911. doi: 10.1136/bcr-2012-007911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sawan EB, Henaine R, Daou L, Jebara V. Successful operation for thoracoabdominal aortic aneurysm in a 5-year-old boy with tuberous sclerosis. Ann Thorac Surg. 2015;100(5):e119–e120. doi: 10.1016/j.athoracsur.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Zhang X, Liu P, Jiang G, Liu W. Duodenal angiomyolipoma with multiple systemic vascular malformations and aneurysms: a case report and literature review. Oncol Lett. 2017;14(6):6659–6663. doi: 10.3892/ol.2017.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dueppers P, Duran M, Grabitz K, Schelzig H. Open repair for abdominal aortic aneurysm in a young boy with tuberous sclerosis and review of the literature. Ann Vasc Surg. 2017;39:286e1–286e5. doi: 10.1016/j.avsg.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 66.Wang B, Tu YF, Tsai YS. Teaching NeuroImages: huge carotid artery aneurysm in TSC2/PKD1 contiguous gene syndrome. Neurology. 2017;89(8):e93–e94. doi: 10.1212/WNL.0000000000004269. [DOI] [PubMed] [Google Scholar]
- 67.Geiger MA, Cantador AA, Guillaumon AT. Thoracic aortic aneurysm in a patient with tuberous sclerosis. J Vasc Bras. 2019;18:e20160017–e20160017. doi: 10.1590/1677-5449.160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiemer-Kruel A, Mayer H, Ewert P, Martinoff S, Eckstein HH, Kriebel T, Bissler J, Franz D, Bast T. Congenital lymphatic malformation and aortic aneurysm in a patient with TSC2 mutation. Neuropediatrics. 2020;51(1):57–61. doi: 10.1055/s-0039-1694985. [DOI] [PubMed] [Google Scholar]
- 69.Hedin U, Brunnström H, Dahlin M, Backman T, Perez de Sa V, Tran PK. Resolving the biological paradox of aneurysm formation in children with tuberous sclerosis complex. JVS Vasc Sci. 2021;2:72–78. doi: 10.1016/j.jvssci.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Byrne RD, Lahiri S, Bansal M, Jacob B, Stapleton G. Endovascular repair of a descending thoracic aortic aneurysm in a pediatric patient with tuberous sclerosis: a case report and review of the literature. Pediatr Cardiol. 2022;43(1):238–243. doi: 10.1007/s00246-021-02717-8. [DOI] [PubMed] [Google Scholar]
- 71.Olvera A, Besho JM, Tanaka A, Safi HJ, Estrera AL. Multiple interventions to thoracoabdominal aortic aneurysm in a child with tuberous sclerosis. Ann Thorac Surg. 2022;0003-4975(22):00343–5. doi: 10.1016/j.athoracsur.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 72.Halloran BG, Baxter BT. Pathogenesis of aneurysms. Semin Vasc Surg. 1995;8(2):85–92. [PubMed] [Google Scholar]
- 73.Samouillan V, Dandurand J, Lacabanne C, Stella A, Gargiulo M, Degani A, Gandaglia A, Spina M. Characterization of aneurysmal aortas by biochemical, thermal, and dielectric techniques. J Biomed Mater Res A. 2010;95(2):611–619. doi: 10.1002/jbm.a.32835. [DOI] [PubMed] [Google Scholar]
- 74.López-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150(3):993–1007. [PMC free article] [PubMed] [Google Scholar]
- 75.Jones AC, Shyamsundar MM, Thomas MW, Maynard J, Idziaszczyk S, Tomkins S, Sampson JR, Cheadle JP. Comprehensive mutation analysis of TSC1 and TSC2 – and phenotypic correlations in 150 families with tuberous sclerosis. Am J Hum Genet. 1999;64(5):1305–1315. doi: 10.1086/302381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dabora SL, Sigalas I, Hall F, Eng C, Vijg J, Kwiatkowski DJ. Comprehensive mutation analysis of TSC1 using two-dimensional DNA electrophoresis with DGGE. Ann Hum Genet. 1998;62(Pt 6):491–504. doi: 10.1046/j.1469-1809.1998.6260491.x. [DOI] [PubMed] [Google Scholar]
- 77.Au KS, Williams AT, Roach ES, Batchelor L, Sparagana SP, Delgado MR, Wheless JW, Baumgartner JE, Roa BB, Wilson CM, Smith-Knuppel TK, Cheung MY, Whittemore VH, King TM, Northrup H. Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med. 2007;9(2):88–100. doi: 10.1097/gim.0b013e31803068c7. [DOI] [PubMed] [Google Scholar]
- 78.Dabora SL, Jozwiak S, Franz DN, Roberts PS, Nieto A, Chung J, Choy YS, Reeve MP, Thiele E, Egelhoff JC, Kasprzyk-Obara J, Domanska-Pakiela D, Kwiatkowski DJ. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet. 2001;68(1):64–80. doi: 10.1086/316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roberts PS, Dabora S, Thiele EA, Franz DN, Jozwiak S, Kwiatkowski DJ. Somatic mosaicism is rare in unaffected parents of patients with sporadic tuberous sclerosis. J Med Genet. 2004;41(5):e69–e69. doi: 10.1136/jmg.2003.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mayer K, Ballhausen W, Rott HD. Mutation screening of the entire coding regions of the TSC1 and the TSC2 gene with the protein truncation test (PTT) identifies frequent splicing defects. Hum Mutat. 1999;14(5):401–411. doi: 10.1002/(SICI)1098-1004(199911)14:5<401::AID-HUMU6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 81.Krueger DA, Care MM, Agricola K, Tudor C, Mays M, Franz DN. Everolimus long-term safety and efficacy in subependymal giant cell astrocytoma. Neurology. 2013;80(6):574–580. doi: 10.1212/WNL.0b013e3182815428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC–mTOR pathway in human disease. Nat Genet. 2005;37(1):19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 83.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 84.Lawrence DM, Singh RS, Franklin DP, Carey DJ, Elmore JR. Rapamycin suppresses experimental aortic aneurysm growth. J Vasc Surg. 2004;40(2):334–338. doi: 10.1016/j.jvs.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 85.Hammill AM, Wentzel MS, Gupta A, Nelson S, Lucky A, Elluru R, Dasgupta R, Azizkhan RG, Adams DM. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer. 2011;57(6):1018–1024. doi: 10.1002/pbc.23124. [DOI] [PubMed] [Google Scholar]