Abstract
Alteration of the intercellular adhesion system plays an essential role in the initiation and progression of bladder carcinomas. We followed the immunoexpression of adhesion molecules, E-cadherin, β-catenin and Claudin-1, in relation to the histopathological grade and the pT category in a number of 50 urothelial carcinomas of the bladder, based on a final staining score (FSS), calculated on the basis of reaction intensity and labeled cells number. E-cadherin immunoexpression was identified in the membrane of tumor cells, low FSS being associated with invasive high-grade carcinomas. β-catenin reactions were membranous in the case of low-grade noninvasive carcinomas and predominantly cytoplasmic and nuclear in the case of high-grade invasive ones, for which high FSS were associated. Claudin-1 was identified at the membrane level, the high FSS values being more frequent in the case of high-grade invasive carcinomas, although there were no significant statistical associations. Loss of E-cadherin expression and the associated positive linear relation of β-catenin and Claudin-1 indicate the usefulness of the analyzed markers in identifying the invasive aggressive phenotype of urothelial bladder carcinomas.
Keywords: urothelial carcinomas, E-cadherin, β-catenin, Claudin-1
⧉ Introduction
There are a growing number of studies that suggest that disruption of the intercellular adhesion system may be involved in the initiation and progression of bladder cancer.
Currently, certain studies indicated that decreasing of E-cadherin immunoexpression is related with poor outcome in distinct carcinomas [1,2,3,4]. Nevertheless, the E-cadherin contribution in the bladder carcinomas evolution remains questionable. In this context, some studies support a poor evolution for patients with underexpression of E-cadherin [5,6], while other indicated no relation among E-cadherin and final outcome [7,8,9,10].
During tumor progression, when E-cadherin expression decreases or when cytosolic β-catenin degradation is inhibited, cytoplasmic β-catenin accumulates and migrates to the nucleus, the interaction between nuclear β-catenin downstream transcriptional factors causing abnormal gene activations [11]. Mutations and β-catenin overexpression are associated with adverse prognosis in many cancers, including bladder cancer [12].
Proteins that form tight junctions (claudins) could play a central role in neoplastic progression by coupling the extracellular environment to intracellular signaling and cytoskeleton pathways [13]. For urothelial carcinomas (UCs), the expression of Claudin-1, -3, -4 and -7 was reported in over 80% of cases, which were associated with advanced stage and higher tumor grade [14].
Aim
The present study concerned the evaluation of the immunoexpression of E-cadherin, β-catenin and Claudin-1 in relation to the tumor grade and the pT category of UCs of the bladder.
⧉ Materials and Methods
We investigated 50 UCs of the bladder from the Clinic of Urology, Emergency County Hospital, Craiova, Romania. Surgical excision specimens (cystectomy or transurethral tumor resection) were introduced in 10% neutral buffered formalin, followed by paraffin inclusion and then Hematoxylin–Eosin (HE) stained. The classification of lesions was performed according to the recommendations of the World Health Organization (WHO) [15], revealed in 11 cases low-grade noninvasive urothelial carcinoma (LGNUC), in 14 cases high-grade noninvasive urothelial carcinoma (HGNUC), in nine cases low-grade invasive urothelial carcinoma (LGIUC) and in 16 cases high-grade invasive urothelial carcinoma (HGIUC).
From the paraffin blocks, we made serial sections that were processed by immunohistochemistry using the Labeled Streptavidin–Biotin (LSAB) 2 detection system (Dako, code K0675). The detection of reactions was done by using 3,3’-Diaminobenzidine (DAB) tetrahydrochloride chromogen (Dako, code 3467), and to validate the reactions we included external positive/negative controls (Table 1).
Table 1.
Used antibodies: clone, dilution, antigen retrieval and external control
|
Antibodies |
Clone / Producer |
Dilution |
Antigen retrieval |
Positive external control |
|
E-cadherin |
Monoclonal mouse anti-human NCH-38 / Dako |
1:50 |
Microwaving in citrate buffer, pH 6 |
Mammary gland |
|
β-catenin |
Monoclonal mouse anti-human β-catenin-1 / Dako |
1:100 |
Microwaving in citrate buffer, pH 6 |
Liver |
|
Claudin-1 |
Polyclonal rabbit anti-human / Thermo Fisher Scientific |
1:200 |
Microwaving in citrate buffer, pH 6 |
Skin |
The semi-quantitative quantification of the immunoexpression of adhesion molecules investigated was performed by an adapted system, by two specialists, who appreciated the intensity of staining and the positive cells percentage [16]. The score for intensity was considered mild (score 1), moderate (score 2) or strong (score 3), while the positive cells percentage range was considered 6–25% (score 1), 26–50% (score 2), 51–75% (score 3) and >75% (score 4). Multiplying the two scores (intensity/percentage), let us the calculation of the final staining score (FSS). The FSS values of 1–4 were considered low, while the values of 6–12 were high. The cutoff value for positivity was 5% positive cells regardless of the intensity of the reaction, below this value the FSS being considered negative. For the analyzed proteins, the immunostaining was considered for quantification regardless of cell location.
In the case of transurethral tumor resection fragments, the inclusion criterion in the study was the presence of smooth muscle fiber bundles, and the exclusion criterion was the presence of electrocoagulation or processing artifacts that could interfere with the immunoexpression of the analyzed proteins.
The analysis of the results by statistical processing used mean values, standard deviations, and tests of comparison [χ2 (chi-squared) test, Pearson’s test], within Statistical Package for the Social Sciences (SPSS) 10 software, the values for p less than 0.05 being considered significant.
We use for the image capture the Motic Panthera DL microscope provided with Motic Images Plus 3.0 ML software.
The study was approved by the local Ethics Committee, for the investigated group being obtained the informed consent.
⧉ Results
Histopathological analysis for the 50 UCs of the bladder, invasive or noninvasive, with varying degrees of differentiation, classified into different pT categories (Table 2), indicated: 25 cases corresponding to the pTa category (noninvasive – 11 LGNUC cases, 14 HGNUC cases), 18 cases to the pT1 category (invasion of the lamina propria – nine LGIUC cases, nine HGIUC cases), five cases of the pT2 category (invasion of muscularis propria) and respectively two cases in the pT3 category (perivesical invasion) (seven HGIUC cases).
Table 2.
Distribution of urothelial carcinomas in relation to tumor grade and pT category according to FSS values
|
Immunomarker |
Stage / n |
LGNUC |
HGNUC |
LGIUC |
HGIUC |
||||
|
n |
FSS |
n |
FSS |
n |
FSS |
n |
FSS |
||
|
E-cadherin |
pTa / 25 |
11 |
11.1 |
14 |
7.3 |
– |
– |
– |
– |
|
pT1 / 14 |
– |
– |
– |
– |
9 |
4.6 |
5 |
4.4 |
|
|
pT2 / 2 |
– |
– |
– |
– |
– |
– |
2 |
4 |
|
|
pT3 / 0 |
– |
– |
– |
– |
– |
– |
– |
– |
|
|
β-catenin |
pTa / 19 |
9 |
5.1 |
10 |
5.3 |
– |
– |
– |
– |
|
pT1 / 14 |
– |
– |
– |
– |
7 |
6.8 |
7 |
9.2 |
|
|
pT2 / 4 |
– |
– |
– |
– |
– |
– |
4 |
9.5 |
|
|
pT3 / 2 |
– |
– |
– |
– |
– |
– |
2 |
10 |
|
|
Claudin-1 |
pTa / 21 |
10 |
5.8 |
11 |
6.2 |
– |
– |
– |
– |
|
pT1 / 14 |
– |
– |
– |
– |
7 |
6 |
7 |
10.7 |
|
|
pT2 / 5 |
– |
– |
– |
– |
– |
– |
5 |
10.8 |
|
|
pT3 / 2 |
– |
– |
– |
– |
– |
– |
2 |
12 |
|
FSS: Final staining score; HGIUC: High-grade invasive urothelial carcinoma; HGNUC: High-grade noninvasive urothelial carcinoma; LGNUC: Low-grade noninvasive urothelial carcinoma; LGIUC: Low-grade invasive urothelial carcinoma; n: No. of cases
E-cadherin expression
E-cadherin expression analysis was identified in 41 (82%) cases, with membranous pattern, variable intensity of reaction and an average number of 58.7±21.5 labeled cells, for the whole positive group the mean FSS value being 7.2.
For noninvasive UCs (pTa), E-cadherin reactions indicated strong intensity in the case of LGNUC, a number of 76±10.4 labeled cells and a mean FSS value of 11.1. By comparison, in HGNUC, the intensity of the reactions was moderate or strong, the number of labeled cells was 66.4±18.7 and the mean FSS value was 7.3 (Figure 1, A and B).
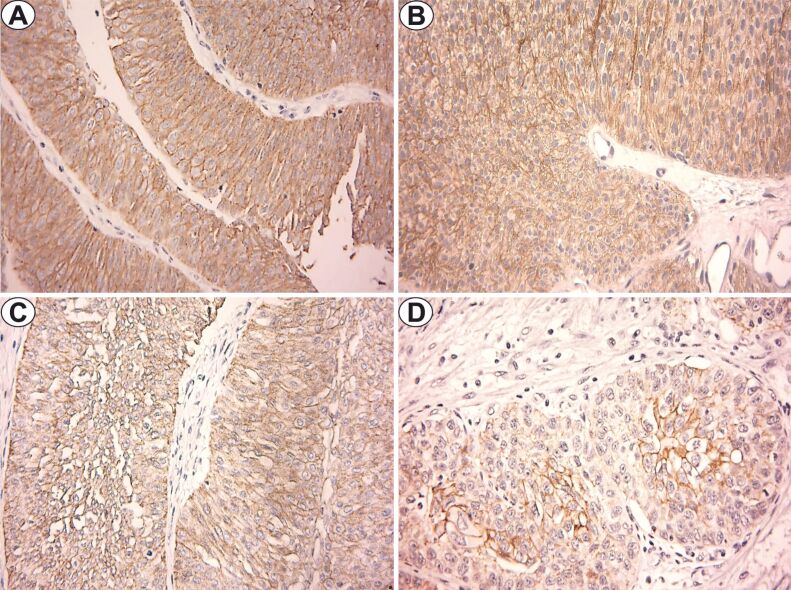
Figure 1.
E-cadherin immunostaining (×400): (A) LGNUC – pTa; (B) HGNUC – pTa; (C) LGIUC – pT1; (D) HGIUC – pT2. HGIUC: High-grade invasive urothelial carcinoma; HGNUC: High-grade noninvasive urothelial carcinoma; LGIUC: Low-grade invasive urothelial carcinoma; LGNUC: Low-grade noninvasive urothelial carcinoma
In LGIUC and HGIUC of the pT1 category, the positive reactions had predominantly moderate intensity, an average number of labeled cells of 38.8±13.6 and 40±16.9, respectively, and mean FSS values of 4.6 and 4.4, respectively (Figure 1C).
In the case of HGIUC from the pT2 category, we observed membrane positivity with moderate or mild intensity, an average number of 45% labeled cells and a mean FSS value of 4. For tumors of pT3 category, the cases were negative for this immunomarker (Figure 1D).
β-catenin expression
Analysis of β-catenin expression was observed in 39 (78%) cases, with variable intensity and pattern, with an average number of 68.2±12.8 labeled cells and a mean FSS value of 6.9. In LGNUC and HGNUC, the immunostaining had a membranous pattern, with mild or moderate intensity, with an average number of labeled cells of 62.7±15 and 60±24, respectively, and a mean FSS value of 5.1 for LGNUC and 5.3 for HGNUC (Figure 2, A and B).
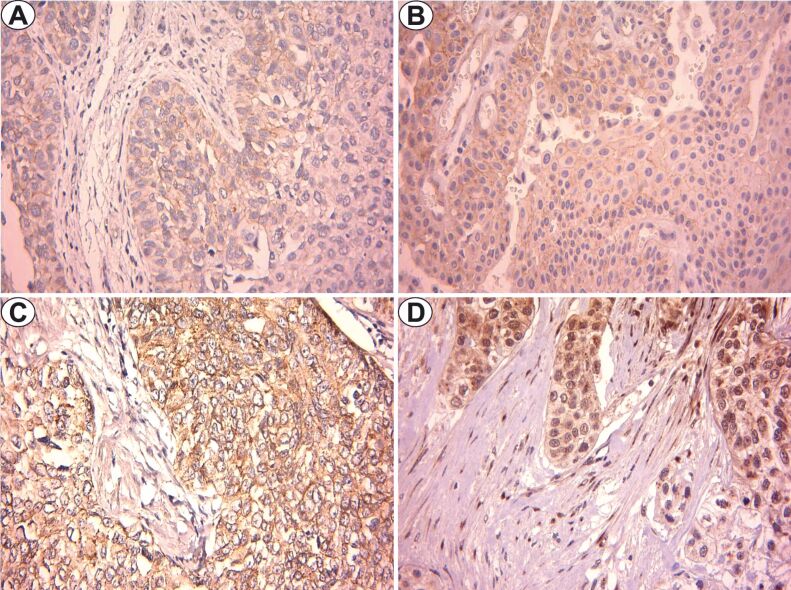
Figure 2.
β-catenin immunostaining (×400): (A) LGNUC – pTa; (B) HGNUC – pTa; (C) LGIUC – pT1; (D) HGIUC – pT3. HGIUC: High-grade invasive urothelial carcinoma; HGNUC: High-grade noninvasive urothelial carcinoma; LGIUC: Low-grade invasive urothelial carcinoma; LGNUC: Low-grade noninvasive urothelial carcinoma
In the case of LGIUC, the immunostaining was also membranous and cytoplasmic, with moderate or strong intensity, a number of 70.7±10.9 labeled cells and a mean FSS value of 6.8 (Figure 2C).
For HGIUC, the staining pattern was varied according to the pT stage. The pT1 tumors indicated membranous and cytoplasmic staining, strong intensity and sometimes moderate, a number of 74.2±3.1 labeled cells and a mean FSS value of 9.2. In the case of pT2 and pT3 tumors, the staining had a cytoplasmic and nuclear pattern, with strong or moderate intensity, an average number of labeled cells of 75±5.7 and 75%, respectively, and the highest mean FSS values of 9.5 and 10, respectively (Figure 2D).
Claudin-1 expression
Claudin-1 expression was identified in 42 (84%) cases, with cytoplasmic and membranous pattern and variable intensity, for the whole group the average number of positive cells was 70±12.3, with a mean FSS value of 7.7. In the LGNUC classified in the pTa category, the intensity of the reactions was moderate, the mean number of positive cells was 64.5±12.1, and the mean FSS value was 5.8 (Figure 3A).
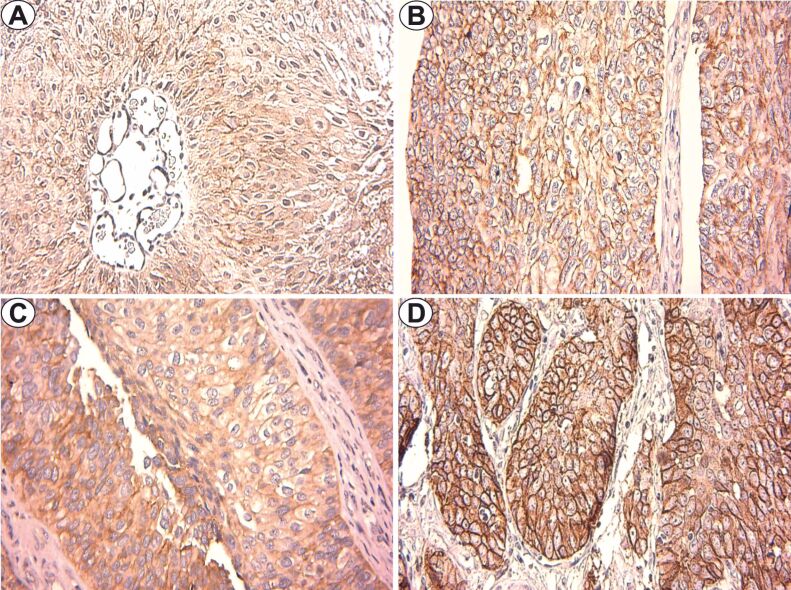
Figure 3.
Claudin-1 immunostaining (×400): (A) LGNUC – pTa; (B) HGNUC – pTa; (C) LGIUC – pT1; (D) HGIUC – pT2. HGIUC: High-grade invasive urothelial carcinoma; HGNUC: High-grade noninvasive urothelial carcinoma; LGIUC: Low-grade invasive urothelial carcinoma; LGNUC: Low-grade noninvasive urothelial carcinoma
In contrast, for HGNUC, the membrane pattern revealed moderate or strong intensity, a mean value of 63.6±15.3 labeled cells and a mean FSS value of 6.2 (Figure 3B).
In LGIUC, we observed reactions with moderate intensity membrane pattern, the average number of positive cells being 70.7±9.4 and the mean FSS value of 6 (Figure 3C). In HGIUC from the categories pT1, pT2 and pT3, the membrane immunostaining indicated increased intensity, with a number of labeled cells of 77.1±5.6, 79±4.1 and 82.5%, respectively, and a mean FSS values of 10.7, 10.8 and 12, respectively (Figure 3D).
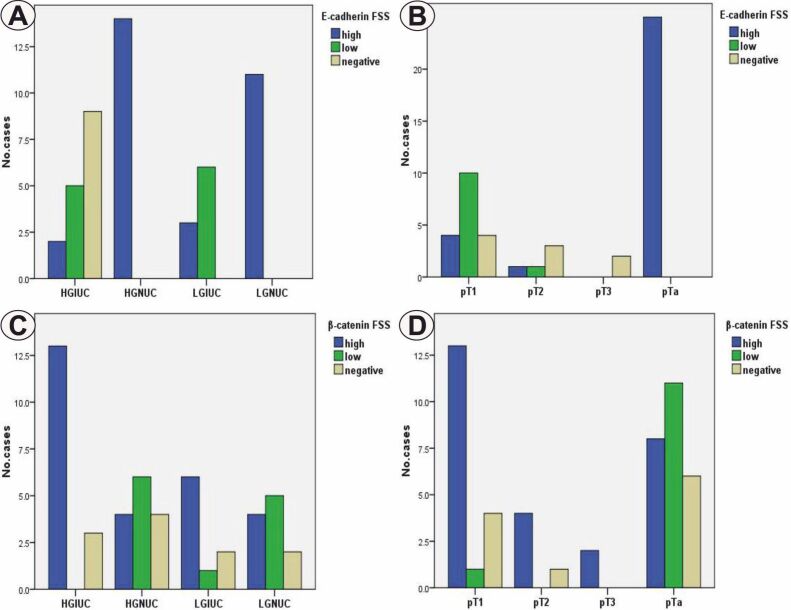
Statistical analysis indicated significantly lower differences in E-cadherin immunoexpression in high-grade (p<0.001, χ2 test) and deep invasive (p<0.001, χ2 test) tumors (Figure 4, A and B). In the case of β-catenin, the reaction scores were significantly higher in the high-grade lesions (p=0.031, χ2 test) and in the invasive pT2–pT3 ones (p=0.034, χ2 test) (Figure 4, C and D).
Figure 4.
Distribution of cases depending on tumor grade (A and C), the depth of the invasion (B and D) and FSS of E-cadherin (A and B) and β-catenin (C and D). FSS: Final staining score; HGIUC: High-grade invasive urothelial carcinoma; HGNUC: High-grade noninvasive urothelial carcinoma; LGIUC: Low-grade invasive urothelial carcinoma; LGNUC: Low-grade noninvasive urothelial carcinoma
In the case of Claudin-1, the differences were statistically non-significant, although the high scores were more frequently associated with high-grade UCs (p=0.394, χ2 test) and invasive pT1–pT3 tumors (p=0.369, χ2 test).
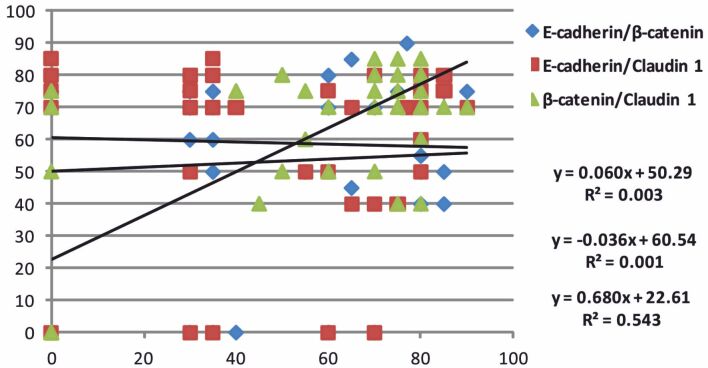
The analysis of the percentage values distribution for the investigated markers indicated a non-significant borderline linear positive correlation between E-cadherin/β-catenin (p=0.685, Pearson’s test), a negative linear non-significant correlation of E-cadherin/Claudin-1 (p=0.792, Pearson’s test) and a significant positive linear correlation in the case of β-catenin/Claudin-1 (p<0.001, Pearson’s test) (Figure 5).
Figure 5.
Distribution of E-cadherin, β-catenin and Claudin-1 percentage values
⧉ Discussions
Dysfunction of cell adhesion molecules was correlated with the early stages of tumor invasion, but also with the development of metastases in UC of the bladder [17].
There are many types of carcinomas, including of the bladder, for which the decrease of cadherin expression is associated with the reserved prognosis [1,2,3,4]. The E-cadherin impact of in the final outcome of bladder carcinomas remains under question, given that the results of different studies indicate low expression as being associated [5,6] or not associated [7,8,9,10] with an unfavorable prognosis.
The immunoreaction for E-cadherin was identified in 82% of cases, with membranous pattern and variable intensity. FSS values were significantly lower in the case of high-grade and invasive carcinomas, with pT3 HGIUC being completely negative for this immunomarker.
Tumor invasion in UC is often associated with impaired E-cadherin expression, concomitant with suppression of cellular junctions, while decreased E-cadherin expression has been reported in high-grade UC [18]. The reduced expression of E-cadherin or its absence requires clinical monitoring of patients even if the initial diagnosis is non-invasive UC, due to the high rate of transformation into invasive carcinoma, compared with recurrent cases [19].
Disruption of cadherin control over intercellular adhesion and activation of β-catenin/Wnt induced signals are essential stages in initiation and development of several tumors [20]. The β-catenin/Wnt signaling system is involved in the urogenital system growth and, therefore, its abnormal activation is significantly involved in the pathogenesis of bladder cancer [21].
We observed β-catenin expression in 78% of cases, with varied membrane, cytoplasmic and nuclear pattern. If in LGNUC and HGNUC the staining had a membranous pattern, for LGIUC the staining pattern was membranous and cytoplasmic, and in the case of HGIUC the pattern was variable depending on the pT category, cytoplasmic and nuclear reactions predominating in the case of pT2–pT3 tumors. In this study, high β-catenin scores were associated with invasive and invasive carcinomas.
During tumorigenesis, with decreased E-cadherin expression, proteasomal degradation of cytoplasmic β-catenin is inhibited, leading to accumulation and migration of catenin into the nucleus. Some studies have indicated that β-catenin nuclear expression was statistically higher in urinary bladder carcinoma, and associated with poor outcome [22,23]. Mutations and overexpression of β-catenin have been associated with the prognosis reserved for many types of carcinomas, including bladder cancer [12].
Claudin-1 has been shown to exert its oncogenic function via the β-catenin signaling pathway [24]. Claudin-1 is essential for maintaining epithelial integrity, its aberrant expression and correlation with β-catenin has been identified in several malignancies [25,26,27]. While a relation between the reduced immunoexpression of Claudin-1 and poorer outcome was indicated, the involvement of Claudin-1 in different malignancies remain unclear [28,29].
Immunoreaction for Claudin-1 was identified in 84% of the investigated cases, with cytoplasmic and membranous pattern. In this study, the high scores of Claudin-1 were more frequent in the case of HGIUCs, the aspects being statistically non-significant. We also found a significant positive linear relation between β-catenin and Claudin-1, aspects that support the cooperation of the two proteins in stimulating the progression of UCs.
Kokenek-Unal et al. reported that most UCs with muscle invasion (80%) presented intense staining, and generally Claudin-1 had significantly lower expression in low-grade UCs compared with high-grade ones [30]. Nakanishi et al. observed increases in Claudin-1, -3 and -4 expression in advanced stages of UCs in the upper urinary tract, associated with reduced survival, and in addition, the expression of Claudin-1 and -4 was significantly associated with tumor stage [14].
⧉ Conclusions
The study indicated decreased E-cadherin expression and increased β-catenin and Claudin-1 expression in HGIUCs. The translocation of β-catenin from the membrane level into the cytoplasm and nucleus support the involvement in the acquisition of the invasive behavior of these lesions. The positive linear relation of β-catenin and Claudin-1 supports the presence of synergistic dependent mechanisms with role in the progression of bladder carcinomas. The results obtained can be used to identify the aggressive invasive phenotype of UCs of the bladder.
Conflict of interests
The authors declare that they have no conflict of interests.
References
- 1.Li Z, Yin S, Zhang L, Liu W, Chen B. Prognostic value of reduced E-cadherin expression in breast cancer: a meta-analysis. Oncotarget. 2017;8(10):16445–16455. doi: 10.18632/oncotarget.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren X, Wang J, Lin X, Wang X. E-cadherin expression and prognosis of head and neck squamous cell carcinoma: evidence from 19 published investigations. Onco Targets Ther. 2016;9:2447–2453. doi: 10.2147/OTT.S98577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xing XB, Tang YB, Yuan G, Wang Y, Wang JH, Yang Y, Chen MH. The prognostic value of E-cadherin in gastric cancer: a meta-analysis. Int J Cancer. 2013;132(11):2589–2596. doi: 10.1002/ijc.27947. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Zhao J, Ma R, Lin H, Liang X, Cai X. Prognostic significance of E-cadherin expression in hepatocellular carcinoma: a meta-analysis. PLoS One. 2014;9(8):e103952–e103952. doi: 10.1371/journal.pone.0103952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang N, He YL, Pang LJ, Zou H, Liu CX, Zhao J, Hu JM, Zhang WJ, Qi Y, Li F. Down-regulated E-cadherin expression is associated with poor five-year overall survival in bone and soft tissue sarcoma: results of a meta-analysis. PLoS One. 2015;10(3):e0121448–e0121448. doi: 10.1371/journal.pone.0121448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breyer J, Gierth M, Shalekenov S, Aziz A, Schäfer J, Burger M, Denzinger S, Hofstädter F, Giedl C, Otto W. Epithelial-mesenchymal transformation markers E-cadherin and survivin predict progression of stage pTa urothelial bladder carcinoma. World J Urol. 2016;34(5):709–716. doi: 10.1007/s00345-015-1690-5. [DOI] [PubMed] [Google Scholar]
- 7.Otto W, Breyer J, Herdegen S, Eder F, Bertz S, May M, Mayr R, Lausenmeyer EM, Denzinger S, van Rhijn BWG, Burger M, Hartmann A. WHO 1973 grade 3 and infiltrative growth pattern proved, aberrant E-cadherin expression tends to be of predictive value for progression in a series of stage T1 high-grade bladder cancer after organ-sparing approach. Int Urol Nephrol. 2017;49(3):431–437. doi: 10.1007/s11255-016-1491-9. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J, Dong D, Sun L, Zhang G, Sun L. Prognostic significance of the epithelial-to-mesenchymal transition markers E-cadherin, Vimentin and Twist in bladder cancer. Int Braz J Urol. 2014;40(2):179–189. doi: 10.1590/S1677-5538.IBJU.2014.02.07. [DOI] [PubMed] [Google Scholar]
- 9.Gudjónsson S, Bendahl PO, Chebil G, Höglund M, Lindgren D, Lundberg LM, Lövgren K, Fernö M, Månsson W, Liedberg F. Can tissue microarray-based analysis of protein expression predict recurrence of stage Ta bladder cancer. Scand J Urol Nephrol. 2011;45(4):270–277. doi: 10.3109/00365599.2011.568956. [DOI] [PubMed] [Google Scholar]
- 10.Mhawech-Fauceglia P, Fischer G, Alvarez V, Ahmed A, Herrmann FR. Predicting outcome in minimally invasive (T1a and T1b) urothelial bladder carcinoma using a panel of biomarkers: a high throughput tissue microarray analysis. BJU Int. 2007;100(5):1182–1187. doi: 10.1111/j.1464-410X.2007.07090.x. [DOI] [PubMed] [Google Scholar]
- 11.Kraus C, Liehr T, Hülsken J, Behrens J, Birchmeier W, Grzeschik KH, Ballhausen WG. Localization of the human beta-catenin gene (CTNNB1) to 3p21: a region implicated in tumor development. Genomics. 1994;23(1):272–274. doi: 10.1006/geno.1994.1493. [DOI] [PubMed] [Google Scholar]
- 12.Chen YT, Wu CC, Liu XP, Chai CY. Aberrant β-catenin expression in urothelial carcinomas in blackfoot disease-endemic areas. Kaohsiung J Med Sci. 2017;33(1):11–16. doi: 10.1016/j.kjms.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Singh AB, Sharma A, Dhawan P. Claudin family of proteins and cancer: an overview. J Oncol. 2010;2010:541957–541957. doi: 10.1155/2010/541957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakanishi K, Ogata S, Hiroi S, Tominaga S, Aida S, Kawai T. Expression of occludin and claudins 1, 3, 4, and 7 in urothelial carcinoma of the upper urinary tract. Am J Clin Pathol. 2008;130(1):43–49. doi: 10.1309/U77A6BTEXVCA5D0E. [DOI] [PubMed] [Google Scholar]
- 15.Moch H, Humphrey PA, Ulbright TM, Reuter VE, editors. World Health Organization (WHO) Classification of tumours of the urinary system and male genital organs. Lyon, France: International Agency for Research on Cancer (IARC) Press; 2016. https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-The-Urinary-System-And-Male-Genital-Organs-2016 [Google Scholar]
- 16.Wang WS, Yu SL, Yang XS, Chang SD, Hou JQ. Expression and significance of Twist and E-cadherin in ovarian cancer tissues. Asian Pac J Cancer Prev. 2013;14(2):669–672. doi: 10.7314/apjcp.2013.14.2.669. [DOI] [PubMed] [Google Scholar]
- 17.Kashibuchi K, Tomita K, Schalken JA, Kume H, Yamaguchi T, Muto S, Horie S, Kitamura T. The prognostic value of E-cadherin, alpha-, beta-, and gamma-catenin in urothelial cancer of the upper urinary tract. Eur Urol. 2006;49(5):839–845; discussion 845. doi: 10.1016/j.eururo.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 18.Ismail AF, Oskay Halacli S, Babteen N, De Piano M, Martin TA, Jiang WG, Khan MS, Dasgupta P, Wells CM. PAK5 mediates cell: cell adhesion integrity via interaction with E-cadherin in bladder cancer cells. Biochem J. 2017;474(8):1333–1346. doi: 10.1042/BCJ20160875. [DOI] [PubMed] [Google Scholar]
- 19.Balci MG, Tayfur M. Loss of E-cadherin expression in recurrent non-invasive urothelial carcinoma of the bladder. Int J Clin Exp Pathol. 2018;11(8):4163–4168. [PMC free article] [PubMed] [Google Scholar]
- 20.Osterheld MC, Bian YS, Bosman FT, Benhattar J, Fontolliet C. Beta-catenin expression and its association with prognostic factors in adenocarcinoma developed in Barrett esophagus. Am J Clin Pathol. 2002;117(3):451–456. doi: 10.1309/1db6-gfvh-ra6w-q07y. [DOI] [PubMed] [Google Scholar]
- 21.Hrbácek J, Brisuda A, Babjuk M. Involvement of epithelial-mesenchymal transition in urinary bladder cancer progression. A review. Debates on Bladder Cancer. 2011;3:1–6. https://scholar.google.com/scholar_lookup?journal=Debates+on+Bladder+Cancer&title=Involvement+of+epithelial-mesenchymal+transition+in+urinary+bladder+cancer+progression.+A+review&author=J+Hrb%C3%A1cek&author=A+Brisuda&author=M+Babjuk&volume=3&publication_year=2011&pages=1-6& [Google Scholar]
- 22.Urakami S, Shiina H, Enokida H, Kawakami T, Tokizane T, Ogishima T, Tanaka Y, Li LC, Ribeiro-Filho LA, Terashima M, Kikuno N, Adachi H, Yoneda T, Kishi H, Shigeno K, Konety BR, Igawa M, Dahiya R. Epigenetic inactivation of Wnt inhibitory factor-1 plays an important role in bladder cancer through aberrant canonical Wnt/beta-catenin signaling pathway. Clin Cancer Res. 2006;12(2):383–391. doi: 10.1158/1078-0432.CCR-05-1344. [DOI] [PubMed] [Google Scholar]
- 23.Kastritis E, Murray S, Kyriakou F, Horti M, Tamvakis N, Kavantzas N, Patsouris ES, Noni A, Legaki S, Dimopoulos MA, Bamias A. Somatic mutations of adenomatous polyposis coli gene and nuclear b-catenin accumulation have prognostic significance in invasive urothelial carcinomas: evidence for Wnt pathway implication. Int J Cancer. 2009;124(1):103–108. doi: 10.1002/ijc.23917. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Xiao J, Zhao C, Zhao C, Han Z, Wang F, Yang Y, Jiang Y, Fang F. Claudin1 promotes the proliferation, invasion and migration of nasopharyngeal carcinoma cells by upregulating the expression and nuclear entry of β-catenin. Exp Ther Med. 2018;16(4):3445–3451. doi: 10.3892/etm.2018.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J, Li J, Qu Y, Zhang J, Zhang L, Chen X, Liu B, Zhu Z. The expression of claudin 1 correlates with β-catenin and is a prognostic factor of poor outcome in gastric cancer. Int J Oncol. 2014;44(4):1293–1301. doi: 10.3892/ijo.2014.2298. [DOI] [PubMed] [Google Scholar]
- 26.Xiong L, Wen Y, Miao X, Yang Z. Expressions of cell junction regulatory proteins and their association with clinicopathologic parameters in benign and malignant gallbladder lesions. Am J Med Sci. 2011;342(5):388–394. doi: 10.1097/MAJ.0b013e31821e12af. [DOI] [PubMed] [Google Scholar]
- 27.Hoellen F, Waldmann A, Banz-Jansen C, Holtrich U, Karn T, Oberländer M, Habermann JK, Hörmann M, Köster F, Ribbat-Idel J, Thill M, Rody A, El-Balat A, Hanker L. Claudin-1 expression in cervical cancer. Mol Clin Oncol. 2017;7(5):880–884. doi: 10.3892/mco.2017.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyo JS, Kim NY, Cho WJ. Prognostic role of claudin-1 immunohistochemistry in malignant solid tumors: a meta-analysis. J Pathol Transl Med. 2019;53(3):173–179. doi: 10.4132/jptm.2019.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouchagier KA, Assimakopoulos SF, Karavias DD, Maroulis I, Tzelepi V, Kalofonos H, Karavias DD, Kardamakis D, Scopa CD, Tsamandas AC. Expression of claudins-1, -4, -5, -7 and occludin in hepatocellular carcinoma and their relation with classic clinicopathological features and patients’ survival. In Vivo. 2014;28(3):315–326. [PubMed] [Google Scholar]
- 30.Kokenek-Unal TD, Coban I, Oguz-Erdogan AS, Seneldir H, Gurcay N, Alper M. Differential expression of claudin-1, claudin-3, and claudin-4 in bladder lesions. J Cancer Tumor Int. 2015;2(3):117–127. https://journaljcti.com/index.php/JCTI/article/view/19097 [Google Scholar]