Abstract
Cortisol is a key element in acute stress including a severe infection. However, in coronavirus-associated disease, 20% of subjects experience hypocortisolemia due to direct or immune damage of pituitary and adrenal glands. One extreme form of adrenal insufficiency is found in 2/3 of cases with viral and post-viral adrenal infarction (AI) (with/without adrenal hemorrhage) that is mostly associated with a severe coronavirus disease 2019 (COVID-19) infection; it requires prompt glucocorticoid intervention. Some reports are incidental findings at computed tomography (CT)/magnetic resonance imaging (MRI) scans for non-adrenal complications like pulmonary spreading and others are seen on post-mortem analysis. This is a review of PubMed-accessible, English papers focusing on AI in addition to the infection, between March 1, 2020 and November 1, 2021. Exclusion criteria were acute adrenal insufficiency without the histopathological (HP) and/or imaging report of adrenal enlargement, necrosis, etc., respective adrenal failure due to pituitary causes, or non-COVID-19-related adrenal events. We identified a total of 84 patients (different levels of statistical evidence), as follows: a retrospective study on 51 individuals, two post-mortem studies comprising nine, respectively 12 patients, a case series of five subjects, seven single-case reports. HP aspects include necrosis associated with ischemia, cortical lipid degeneration (+/- focal adrenalitis), and infarcts at the level of adrenal cortex, blood clot into vessels, acute fibrinoid necrosis in arterioles and capsules, as well as subendothelial vacuolization. Collateral potential contributors to adrenal damage are thrombotic events, coagulation anomalies, antiphospholipid syndrome, endothelial dysfunction, severe COVID-19 infection with multiorgan failure, etc. Clinical picture is variable from acute primary adrenal insufficiency to asymptomatic or mild evolution, even a retrospective diagnostic; it may be a part of long COVID-19 syndrome; glucocorticoid therapy for non-adrenal considerations might mask cortisol deficient status due to AI/hemorrhage. Despite its rarity, the COVID-19-associated AI/hemorrhage represents a challenging new chapter, a condition that is essential to be recognized due to its gravity since prompt intervention with glucocorticoid replacement is lifesaving.
Keywords: stress, COVID-19, cortisol, adrenal hemorrhage, adrenal tumor
⧉ Introduction
The concept of adrenal infarction/hemorrhage
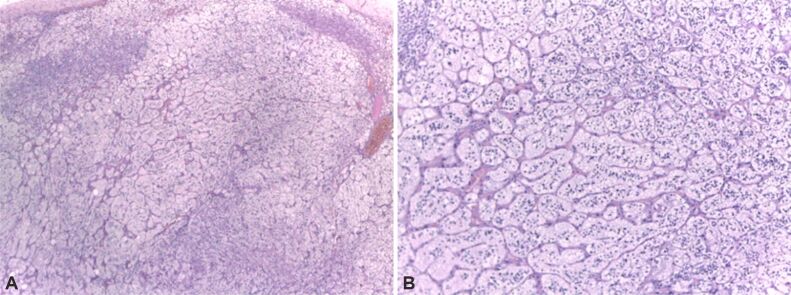
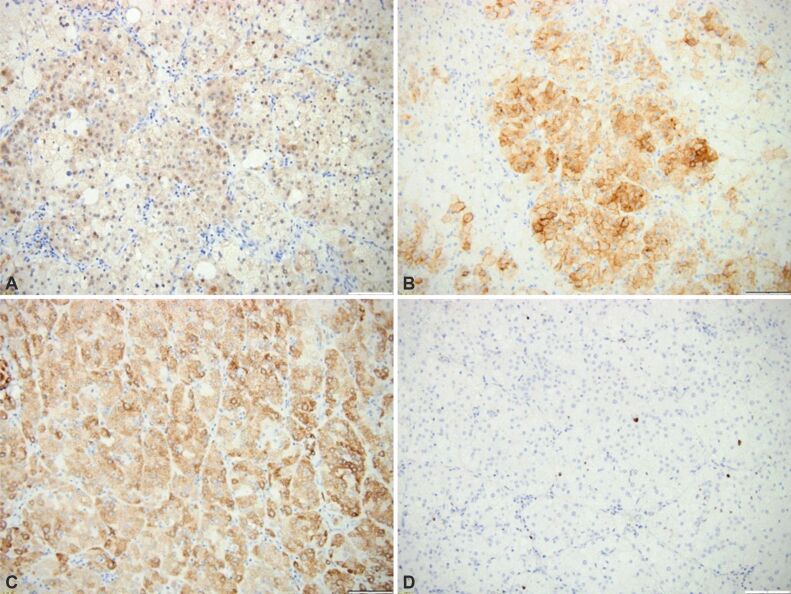
Adrenal infarction (AI) may associate or not adrenal hemorrhage; this is a very rare disease for both adults and pediatrics; it is caused or associated with sepsis, abdominal trauma, coagulation defects or use of anticoagulant medication, general conditions associating a high risk of hemorrhage (as active cancers, and neonatal stress, etc.) [1,2,3,4]. In one series, 40% of cases that were diagnosed with adrenal hemorrhage had a previous non-functioning adenoma at adrenal cortex level [1] (Figure 1, A and B). An adenoma at the level of adrenal cortex might or not be prior recognized; adrenal hemorrhage is sometimes the first presentation of the patient; histopathological (HP) report may identify both the tumor and the adrenal infection/hemorrhage; the immunohistochemistry (IHC) report on remnant adrenocortical adenoma might help its characterization [5,6,7,8,9,10] (Figure 2A,2B,2C,2D). Exceptional situations include syndromic context like TAFRO syndrome (which includes thrombocytopenia, anasarca, reticulin fibrosis of the bone marrow, renal dysfunction, and organomegaly) [11].
Figure 1.
(A and B) Pathological report of a non-functional adrenocortical adenoma: Hematoxylin–Eosin (HE) aspect. The tumor cells are uniformly disposed, with benign features. This is a 53-year-old female who suffered a right adrenalectomy for a tumor of 2.4 cm after doubling the maximum diameter after five years of follow-up. HE staining: (A) ×40; (B) ×100
Figure 2.
(A–D) Immunohistochemistry report on adrenocortical adenoma (×200): (A) Positive calretinin in tumor cells; (B) Positive synaptophysin in tumor cells; (C) Positive inhibin in tumor cells; (D) Positive Ki67 proliferation index of 2% in tumor cells
The clinical presentation of AI varies from acute adrenal insufficiency (in two thirds of cases – especially with bilateral lesions present – if left untreated it has a fatal evolution) and/or shock up to an incidental retrospective finding at different imaging evaluations, either as a necrotic adrenal mass, active hemorrhage or cystic tumor-like aspect or a post-mortem, HP report [12,13,14].
COVID-19 infection and endocrine glands
Crucial effects on endocrine glands and neuroendocrine system are related to the coronavirus disease 2019 (COVID-19) infection, with a heterogeneous pattern of presentation; among major targets are pancreas, pituitary gland, thyroid, adrenal glands, and testes [15,16]. The presence of diabetes mellitus (DM) or high blood pressure (HBP) are among the factors with a poor prognostic, as also found, for instance, for advanced age, active smoking, chronic renal failure, co-presence of prior cardio-cerebrovascular conditions [17].
IHC studies showed positive reaction for angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) at cells of the adrenal cortex [18,19]. The clinical presentation is mostly related to endocrine dysfunction, meaning low cortisol levels in critical patients [18]. Acute onset of COVID-19 infection might involve a switch from a chronic to adrenal form of primary adrenal insufficiency, previously known or unknown [20,21]. Physiologically, angiotensin system serves for maintaining normal blood pressure and adequate remodeling of blood vessels walls [21,22].
Aim
Our purpose was to correlate certain aspects of adrenal response in relationship with COVID-19 infection. This type of infection is regarded as a major stress for the human body (which was reflected by cortisol levels), thus it involves severe complications of adrenal glands as AI with or without hemorrhage.
⧉ Materials and Methods
Original data includes a narrative review of coronavirus infection with adrenal involvement (especially focusing on cortex-produced cortisol hormone). This is a review of PubMed-accessible, English scientific literature including only in extenso papers focusing on AI with or without hemorrhage in addition to the infection with coronavirus (between March 1, 2020 and November 1, 2021). The search words are in different combinations “COVID-19”, “coronavirus”, “SARS-CoV-2” and “adrenal”, “cortisol”, “stress”, “adrenal hemorrhage”, “adrenal infarction”.
Exclusion criteria were acute adrenal insufficiency without the HP and/or imaging report of adrenal enlargement, necrosis, and adrenal failure due to pituitary causes, and also non-COVID-19-related cases. Overall, 97 references are cited at different subsections. Figures 1 and 2 represent captures of pathological reports and IHC analysis that are based on authors’ clinical experience. The patients agreed to anonymously use their medical records.
⧉ Literature data
We identified articles regarding COVID-19 infection and AI with different levels of statistical significance, as follows: seven case reports, a case series of five individuals, a retrospective study on 51 subjects, two post-mortem studies comprising nine, respectively 12 patients (a total of 84 adults with different types of AI +/- hemorrhage +/- adrenal insufficiency).
COVID-19 infection: adrenal involvement
Cortisol as stress hormone
COVID-19 infection may be regarded as an acute stress of the human body, thus the outburst of stress hormones like cortisol is expected [23]. During acute stress, the general biochemistry and endocrine panel includes increased traditional biomarkers of inflammation (like fibrinogen, C-reactive protein), changes of leptin, adrenalin, procalcitonin, ferritin, lactate dehydrogenase levels, a new dynamics of interleukins (ILs) profile, etc. [24,25]. Other data particularly concerning COVID-19 infection, showed, for instance, as cortisol associates with inflammation markers, so is total testosterone and calculated free testosterone in females with pneumonia due to more intense adrenal cortex activity [26]. Particular aspects related to coronavirus infection-associated stress include anomalies of coagulation profile in addition to platelets damage as reflected by increased D-dimers, fibrinogen, and coagulation factor VIII, as well as low anticoagulant protein S and activated protein C, reduced coagulation factor XII [27].
Hypocortisolism concerning COVID-19 infection
Immune response to the virus may damage pituitary gland, regardless there is a prior hypophyseal known tumor or not, either functioning or nor (including the potential of associated morbidities like DM or HBP) [28]. Post-viral necrosis and low pituitary hormones has been described in viral hypophysitis, as traditionally seen in other circumstances like spontaneous necrosis/apoplexy, dopamine agonists and somatostatin analogues use for somatotropinoma or prolactinoma, Sheehan syndrome, etc. [28,29,30].
In severe forms of coronavirus infection, hypocortisolemia was found in approximately one fifth of the patients, depending on study [31]. This is due to the presence of antibodies against adrenocorticotropic hormone (ACTH) due to hypophysitis causing secondary adrenal insufficiency or to adrenalitis [32].
One transversal, retrospective, single-center study showed that subjects with moderate-to-severe COVID-19 infection have statistically significant more frequent hypocortisolemia versus subjects with mild coronavirus infection (which was defined in this study as an oxygen saturation of more than 94% and no other comorbidities) [33]. Central-related low levels of cortisol may also associate secondary hypothyroidism due to COVID-19 infection; during acute phase, it is difficult to assess if permanent glucocorticoid and/or Levothyroxine replacements will be required [34,35,].
After the recovery from COVID-19 infection, some authors also found a recovery of adrenal function. As an example, there is a prospective, observational study on 70 adults who were infected three months prior and had normal basal cortisol levels, as well as post-Synacthen test (performed with an intravenous dose of 250 μg) [34]. However, other authors suggested a potential adrenal involvement in long COVID-19 syndrome associating asthenia, fatigue, and even cognitive impairment within the first weeks up to several months after infection [36,37,38,39].
COVID-19 infection: glucocorticoids use
Generally, glucocorticoid therapy is highly recommended for its strong anti-inflammatory effect [40]. Early during pandemic, a particular candidates’ group was identified in subjects with severe respiratory distress symptoms and/or immunosuppression, septic shock (representing 5% of all infected cases) [41]. Systemic glucocorticoid medication is associated, depending on severity and comorbidity, with a large panel of intervention like drugs from antiviral medication, oxygen therapy, antibiotics, anticoagulants to extracorporeal blood purification therapy (EBPT) to immunomodulating drugs, renal replacement therapy, etc. [41,42,43,44,45].
Systemic corticosteroids administration reduces all-cause mortality in subjects hospitalized for coronavirus infection, and probably decreases the need for ventilation [46]. Also, their use was correlated to a reduced rate of conversion from a mild case into a severe one [47].
In cases with COVID-19 infection, as generally known, large doses and/long-term glucocorticoid therapy are expected to associate a multitude of side effects from HBP, bone damage in terms of bone turnover, reduced bone mineral density and abnormal microarchitecture as reflected by trabecular bone score, digestive anomalies, glucose variations, etc. [48,49]. Also, glucocorticoid treatment is indicated in critically ill COVID-19-positive patients, thus increasing the risk of venous thromboembolism with a poor outcome [50,51]. As an alternative, Methylprednisolone or Dexamethasone in high dose as pulse therapy may reduce the side effects [51]. Moreover, a corticosteroids regime of short-term was proposed in addition to the first dose of vaccination to reduce the reactogenicity surrounding the immunization against COVID-19; however, this type of approach still needs validation [52].
AI and COVID-19 infection
HP aspects
The virus uses ACE2 as a receptor; we already know that the receptor is displayed all over the human body, including in arterial and venous cells of endothelial type, also in adrenal glands [53]. Even before current pandemic outbreak, the severe acute respiratory syndrome coronavirus (SARS-CoV) family was known to target adrenal glands, mostly at cortex level, representing a clear pathogenic loop of the virus, thus cortisol levels are expected to be affected [54].
AI goes with or without hemorrhage, having different extends which are translated into endocrine anomalies of adrenal cortex especially in severe situations [55,56,57]. Associated adrenal insufficiency may be the main feature of the clinical picture or it may represent only a small part of an otherwise complicated presentation due to severe COVID-19 infection, especially with lung or multiorgan involvement [55,56,57].
One post-mortem study showed various adrenal lesions in 46% of the patients (a cohort of 28 subjects who died because of coronavirus complications): seven individuals with necrosis associated with ischemia, four persons with cortical lipid degeneration (two cases of them also associating lesions of focal adrenalitis), two subjects with adrenal necrosis associated with hemorrhage, and one 70-year-old male with isolated lesions of focal adrenalitis [58]. Interestingly, one of the cases with necrosis also had lesions of adrenocortical carcinoma [58]. Another post-mortem study showed a misalignment between general involvement and particular aspects concerning adrenal damage where a complex panel of changes is described: infarcts at the level of adrenal cortex, thrombi into vessels, acute fibrinoid necrosis at arterioles and glands capsule, as well as sub-endothelial vacuolization, etc. [59].
Contributor factors
Previous thrombotic events are an essential contributor factor [60]. Other collateral elements might be autoimmune conditions of endocrine and non-endocrine type, liver anomalies (including of coagulation factors), anti-phospholipid syndrome (APLS); endothelial dysfunction in severe shock accompanying coronavirus infection has been described including in pediatric population [60,61]. Endothelial injury, inflammation and hypoxia may similarly cause myocardial infarction [62,63,64]. Most probably, the fact that COVID-19 infection induced positive anti-phospholipid antibodies represents the key element which was also described in patients without coronavirus infection [65]. However, the actual level of statistical evidence is low, and we currently consider AI as a more complicated puzzle with multifactorial pathogenicity [60].
AI may be found in patients with less severe coronavirus infection, but also, it is found accompanying the general damage due to cytokine storm, as an excessively increased status of acute inflammation in critically ill infected subjects (between 1% and 5% of COVID-19 patients depending on study), in cases with multiorgan failure underling severe thromboembolic events like ischemic stroke, myocardial infarction, acute renal failure, vasculitis, etc., thus involving vessels that are connected with adrenal glands [66]. The severe clinical picture is heterogeneous since multiple systems are damaged. For instance, in one case of a 50-year-old male, bilateral adrenal hemorrhage was first accidently detected at computed tomography (CT) scan for COVID-19 pneumonia, followed by clinical manifestation of adrenal insufficiency with hypotension, which was unfortunately followed by multiple thrombosis including massive pulmonary embolism [67]. This case also associates a prior diagnosis of adrenal tumor, which is exceptionally found until now in patients with COVID-19-positive adrenal hemorrhage [67]. Another 48-year-old male with a prior diagnosed of APLS who was under therapy with vitamin K antagonists at the moment of COVID-19 infection developed ischemia at the level of left toes due to artery occlusion, and he was synchronously diagnosed with adrenal hemorrhage before the switch was done for low-molecular-weight heparin (Enoxaparin) [68]. Another 66-year-old female who was previously known with APLS was also admitted for active COVID-19 infection and adrenal hemorrhage-related adrenal insufficiency followed by a thrombosis at the level of renal vein, an evolution that confirms so called “two-hit” theory [69].
Clinical elements
Digestive symptoms and cardiovascular deterioration may be related to the infection or to adrenal infraction [64]. Digestive involvement (like abdominal pain, diarrhea, nausea, vomiting) during COVID-19 infection is reported in almost half of cases, typically after pulmonary symptoms [70,71]. These are caused by the fact that ACE2 receptors are located at the level of gastrointestinal system, and also at pancreas [72,73]. On the other hand, the clinical presentation of acute adrenal insufficiency includes hypotension, collapse, nausea, vomiting, abdominal pain, fever, lethargy, confusion, etc., which may mimic a COVID-19 infection with digestive presentation [73]. However, an infection itself (of any type) may be the trigger of on acute form of primary adrenal failure [73]. The incidence of adrenal crisis in general population is 10/100 individuals-years (a mortality of 0.5/100 patient-years) [73]. Prompt recognition and adequate glucocorticoid therapy is lifesaving [73].
Recently, a new potential differential diagnostic of acute adrenal failure with abdominal pain was established in relationship with coronavirus infection: multisystem inflammatory disease in adults (MIS-A), syndrome that was initially described in children (MIS-C syndrome), with a Kawasaki-like pattern of presentation [74,75]. MIS-A or MIS-C syndrome causes abdominal pain, cardiac failure, shock, fever, increased markers of inflammation following the actual COVID-19 infection or being synchronous to it [76,77]. This complication of acute respiratory distress syndrome increases the mortality; the use of immunoglobulin treatment, steroids, biotherapy like anti-IL antibodies may improve the prognostic to some extent [78,79].
In one retrospective study, which aimed to identify the adrenal lesions suggestive for infarction in acute coronavirus infection with severe lung complications, the adrenal lesion was incidentally detected in 23% of subjects (male predominance, a mean age of 67±11 years) [80]. Only 8% of cases had a confirmation of endocrine panel suggestive for adrenal insufficiency; the accidental CT finding was correlated with a more prolonged hospital stay [80].
Another retrospective study on nine adult UK subjects (with mean age of 73 years) with SARS (due to coronavirus as cause of death was published in 2020 [81]. Post-mortem analysis showed that 33% of studied patients had an adrenal microinfarction, while thrombotic events were confirmed in 89% of cases, mostly at pulmonary level, in association with other three major HP elements including diffuse alveolar damage (which was confirmed in all the studied individuals), immune anomalies like lymphocyte depletion [mostly cluster of differentiation (CD)-positive T-cells], as well as hemophagocytes [as otherwise seen in other severe infections like human immunodeficiency virus (HIV) infection] [81,82]. If recognized, AI-related adrenal insufficiency requires intravenous Hydrocortisone, followed by lifetime oral Hydrocortisone and Fludrocortisone [83]. As mentioned, some severe subjects are already under systemic glucocorticoid medication [84]. A 70-year-old male case was diagnosed with bilateral adrenal hemorrhage, while he was under glucocorticoid medication in addition to a complex regime for COVID-19 bilateral bronchopneumonia [85]. Because of this aspect, baseline cortisol/ACTH assessments are not feasible; the need of glucocorticoid substitution after the subject is discharged for COVID-19 infection is an argument of chronic adrenal insufficiency; however, replacement medication may be stopped under clinical surveillance to check the hormonal panel if the patient is stable to check the spontaneous hormonal balance of glucocorticoid axes [85]. A 53-year-old male with right adrenal hemorrhage diagnosed while performing a CT scan for bilateral pulmonary embolism did not associate adrenal insufficiency and the lesion was remitted according to CT exam that was performed five months after he was discharged [86] (Table 1).
Table 1.
The PubMed-based, English language, full-length papers published between March 1, 2020 and November 1, 2021 concerning COVID-19 infection and AI with or without adrenal hemorrhage, with or without adrenal insufficiency related to COVID-19 infection
|
No. |
Authors / reference |
Type of study |
No. of patients / age [years] / sex |
Presentation |
Endocrine aspects |
COVID-19 aspects |
Observations |
|
1. |
Freire Santana et al. / [58] |
Post-mortem study (autopsy) |
12 out of 28 (46%) patients with adrenal lesions |
Autopsy diagnostic |
No adrenal insufficiency based on cortisol levels (sample within 1–2 days before death) |
COVID-19 as cause of death |
Prior diagnostic of APLS |
|
50, 65 / F | |||||||
|
34, 35, 48, 52, 55, 57, 65, 66, 70, 88 / M | |||||||
|
2. |
Iuga et al. / [59] |
Case series |
5 patients / between 59 and 90 / F/M ratio: 1/4 |
Autopsy diagnostic |
Not available |
COVID-19 as cause of death |
2 patients died of cardiac arrest at emergency room |
|
3 patients were hospitalized (maximum 72 hours) before death | |||||||
|
3. |
Machado et al. / [60] |
Case report |
1 patient / 46 / F |
Abdominal pain, hypotension, skin hyperpigmentation |
Na↓ |
AI after COVID-19 infection |
CT scan: bilateral adrenal enlargement (infarction) |
|
Cortisol↓ | |||||||
|
ACTH↑ |
Other contributors: prior autoimmune hepatitis, de novo positive anti-phospholipid antibodies |
||||||
|
Aldosterone↓ | |||||||
|
4. |
Kumar et al. / [64] |
Case report |
1 patient / 70 / F |
Fatigue, abdominal pain, vomiting, diarrhea |
Na↓ |
Synchronous with COVID-19 pneumonia |
CT: non-hemorrhagic AI |
|
On admission: random ACTH, cortisol, K normal → then adrenal insufficiency |
Other elements: negative anti-cardiolipin antibodies, history of HBP, hypercholesterolemia |
||||||
|
5. |
Elkhouly et al. / [67] |
Case report |
1 patient / 50 / M |
Fever, malaise, dyspnea, cough, bilateral flank discomfort |
Adrenal lesion was accidentally detected at CT |
COVID-10 pneumonia, further complicated with deep vein thrombosis, massive pulmonary embolism causing the patient’s death |
Previous diagnostic of HBP and right adrenal tumor |
|
On day 3 after hospitalization – hypotension | |||||||
|
6. |
Maria et al. / [68] |
Case report |
1 patient / 48 / M |
Fever, cough, myalgia + sudden abdominal pain |
Not available |
COVID-19 pneumonia and limb arterial ischemia |
Prior diagnostic of APLS |
|
The patient was under vitamin K antagonists at the moment of infection | |||||||
|
7. |
Frankel et al. / [69] |
Case report |
1 patient / 66 / F |
Fever, dyspnea, abdominal pain, nausea, vomiting |
Na↓ |
Synchronous with COVID-19 infection |
CT: enlargement of adrenal glands |
|
Basal cortisol↓ | |||||||
|
ACTH↑ | |||||||
|
8. |
Leyendecker et al. / [80] |
Retrospective study (March 9–April 10, 2020) |
51 out of 219 (23%) patients with CT for lung involvement / mean age 67±11 / 71% M |
Detection as incidental CT finding |
8% adrenal insufficiency |
Synchronous with COVID-19 with severe/critical lung disease |
CT: bilateral lesions in 88% of cases |
|
Correlation with a longer hospital stay versus without | |||||||
|
9. |
Hanley et al. / [81] |
Retrospective (post-mortem) study (March 1–April 30, 2020) |
9 patients / 73 (IQR 52–79) / 7 M, 3 F |
Death due to SARS |
Adrenal involvement confirmed at post-mortem analysis |
Multiple organ involvement |
|
|
10. |
Álvarez-Troncoso et al. / [85] |
Case report |
1 patient / 70 / M |
Fever, chills, asthenia, constipation, malaise, generalized weakness, anorexia, nausea, vomiting |
Na↓ |
Synchronous with COVID-19 bilateral bronchopneumonia |
CT: bilateral enlargement of adrenal glands (with blurring aspect) |
|
Hypotensive |
No hormonal assessment due to glucocorticoids therapy |
||||||
|
11. |
Sharrack et al. / [86] |
Case report |
1 patient / 53 / M |
Fever, dyspnea, pleuritic chest pain |
Normal short Synacthen test |
COVID-19-related bilateral pulmonary embolism |
CT: right adrenal hemorrhage confirmed during infection and remitted at CT scan performed after five months |
ACTH: Adrenocorticotropic hormone; AI: Adrenal infarction; APLS: Antiphospholipid syndrome; COVID-19: Coronavirus disease 2019; CT: Computed tomography; F: Female; HBP: High blood pressure; IQR: Interquartile range; K: Potassium; M: Male; Na: Sodium; SARS: Severe acute respiratory syndrome
⧉ Discussions
Our systematic review represents a new chapter in medical field, once the COVID-19 pandemic started and changed the entire worlds, not just from a strictly medical perspective [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86].
Early during pandemic, the endocrine glands were found to be affected by the coronavirus, and the adrenal glands represent a hallmark of human body survival under different circumstances on daily basis and acute, as well as chronic stress. Assessment of cortisol levels are essential tools for practitioners to assess to body capacity to adjust under these circumstances (unless the patient is already under glucocorticoid medication). The extreme clinical presentation of adrenal involvement in a patient who is infected with coronavirus or is a survivor of disease is adrenal infraction, especially associating adrenal hemorrhage. The traditional HP panel is a milestone for this condition; however, the early recognition (based on signs and symptoms of newly detected adrenal insufficiency, as well as imaging findings like enlargement of one or both glands and blurring pattern at CT scan) and further on therapy with systemic corticoids, are essential for prognostic/survival [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86].
In relationship with COVID-19 infection, the cases that are reported so far by the medical community concern adult patients who either had a severe infection with a multiorgan failure or are during post-viral period of time. Also, valuable data are provided by post-mortem analysis in subjects who died from the disease. The recognition of clinical entity may be masked by severe general involvement of multiple organs or by the fact that the patient is already under glucocorticoid medication for a different (non-adrenal) complication, thus the lifesaving therapy is already provided to the patient. Also, after surpassing the acute phase, it remains this debatable issue of long-term need for oral corticoids, including the utility of assessing the hormonal tests, which are specific for cortisol/ACTH levels, including stimulation tests, which might indicate the need of glucocorticoids even in small doses or their supplementation under different stress circumstances [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86].
Even though current level of statistical evidence is not reliable for large meta-analysis, the topic is new, it dates from 2020–2021, and it is essential to be communicated to the practitioners at the level we are currently aware of [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86].
Adrenal hemorrhage has been exceptionally described in relationship with medulla; a first case of pheochromocytoma-related hemorrhage in a 62-year-old male admitted for coronavirus-associated pneumonia was the initial sign that led to the detection of the adrenal tumor [87].
Vaccine against coronavirus might also trigger an AI. One of the mechanisms that link COVID-19 adenoviral vector-based vaccination to immune (vaccine-induced) thrombotic thrombocytopenia that may cause acute complications like acute renal failure due to thrombosis or bleeding due to low platelets count [88,89,90]. This may also represent the pathogenic factor of adrenal hemorrhage, as several cases reported it in 2021 [91,92,93,94]. Another case of a 54-year-old female presented post-vaccine disseminated intravascular coagulation due to multiple-site thrombosis including brain, lung, and bilateral adrenal hemorrhage [95]. Moreover, a first case of pheochromocytoma detection after COVID-19 vaccine was recently reported; this 63-year-old male patient had no previous history of an adrenal tumor until adrenergic crisis starting within 24 hours since vaccination [96]. Overall, despite limited level of statistical evidence, it is mandatory to address to topic of adrenal involvement amid COVID-19 pandemic on a multidisciplinary perspective.
⧉ Conclusions
Cortisol is a key element in acute stress including a severe infection. However, in coronavirus-associated infection, hypocortisolemia is found due to direct or immune damage at pituitary and adrenal glands. One extreme form of adrenal insufficiency is COVID-19-related AI with/without hemorrhage that is mostly associated with a severe form of infection; it requires prompt glucocorticoid intervention. Some of the reports are incidental findings during CT for pulmonary lesions and others are post-mortem analysis. The presence of APLS represents an aggravating circumstance. So far, the level of statistical evidence is low, but the condition is essential to be adequately recognized due to its gravity.
Conflict of interests
The authors declare that they have no conflict of interests.
Acknowledgments
Acknowledgments
This paper was supported by 26/8C/23.06.2021 grant: “Assessment of the neuroendocrine effects of stress on a school population during the COVID-19 pandemic”.
References
- 1.Karwacka IM, Obołończyk Ł, Sworczak K. Adrenal hemorrhage: a single center experience and literature review. Adv Clin Exp Med. 2018;27(5):681–687. doi: 10.17219/acem/68897. [DOI] [PubMed] [Google Scholar]
- 2.An P, Chen K, Yang GQ, Dou JT, Chen YL, Jin XY, Wang XL, Mu YM, Wang QS. Diffuse large B cell lymphoma with bilateral adrenal and hypothalamic involvement: a case report and literature review. World J Clin Cases. 2019;7(23):4075–4083. doi: 10.12998/wjcc.v7.i23.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toti MS, Ghirri P, Bartoli A, Caputo C, Laudani E, Masoni F, Mele L, Bernardini R. Adrenal hemorrhage in newborn: how, when and why - from case report to literature review. Ital J Pediatr. 2019;45(1):58–58. doi: 10.1186/s13052-019-0651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jimidar N, Ysebaert D, Twickler M, Spinhoven M, Dams K, Jorens PG. Bilateral adrenal haemorrhage after a high energetic trauma: a case report and review of current literature. Acta Chir Belg. 2020;120(2):131–135. doi: 10.1080/00015458.2018.1515339. [DOI] [PubMed] [Google Scholar]
- 5.Dumitrascu MC, Sandru F, Carsote M. From oral anticoagulants to Prednisone. Medical Image Database. 2021;4(1):31–32. https://www.medicalimage.ro/index.php/MID/article/view/104 [Google Scholar]
- 6.Valea A, Carsote M, Petrova E, Dumitrascu MC, Sandru F. Pale skin: an adrenal cancer. Rom J Med Pract. 2019;14(4):460–463. https://rjmp.com.ro/rpm-vol-xiv-nr-4-an-2019/ [Google Scholar]
- 7.Dumitrascu MC, Trandafir AI, Petrova E, Ghemigian A, Carsote M, Sandru F, Mehedintu C. Bilateral adrenal hemorrhage. Rom J Med Pract. 2021;16(4):413–421. https://rjmp.com.ro/rpm-vol-xvi-nr-4-an-2021/ [Google Scholar]
- 8.Valea A, Botezan O, Turturea R, Carsote M, Rusu C. Surgery for primary hyperparathyroidism diagnosed as acute renal failure. J Surg Sci. 2018;5(2):108–112. https://journalofsurgicalsciences.com/index.php/jss/article/view/161 [Google Scholar]
- 9.Dumitrascu MC, Rentea DE, Zugravu S, Mehedintu C, Carsote M, Sandru F. Follow-up of second adrenal tumor after remission of Cushing syndrome. Rom J Med Pract. 2021;16(4):509–515. https://rjmp.com.ro/rpm-vol-xvi-nr-4-an-2021/ [Google Scholar]
- 10.Sandru F, Dumitrascu MC, Petca RC, Mehedintu C, Carsote M. Adrenal surgery amid COVID-19 pandemic. Rom Med J. 2021;68(4):429–432. https://rmj.com.ro/rmj-vol-lxviii-nr-4-an-2021/ [Google Scholar]
- 11.Yonezaki S, Nagasaki K, Yamaguchi H, Kobayashi H. Bilateral adrenal infarctions as an initial manifestation of TAFRO syndrome: a case report and review of the literature. Intern Med. 2022;61(5):743–747. doi: 10.2169/internalmedicine.7976-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soltanmohammadi S, Vakhshoori M, Sajadi G, Heidarpour M. Bilateral adrenal hemorrhage and adrenal insufficiency in the context of polycythemia vera: a case report and review of the literature. Case Rep Med. 2022;2022:5335543–5335543. doi: 10.1155/2022/5335543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carsote M, Ghemigian A, Terzea D, Gheorghisan-Galateanu AA, Valea A. Cystic adrenal lesions: focus on pediatric population (a review) Clujul Med. 2017;90(1):5–12. doi: 10.15386/cjmed-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terzea D, Carsote M. Inside of an adrenal cyst. Medical Image Database. 2019;2(2) http://www.medicalimage.ro/index.php/MID/article/view/35 [Google Scholar]
- 15.Moroti R, Badiu C. Endocrine effects of COVID 19: difficulties in the management of endocrine disorders from individual to societies. Acta Endocrinol (Bucharest) 2020;16(1):74–77. doi: 10.4183/aeb.2020.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kazakou P, Paschou SA, Psaltopoulou T, Gavriatopoulou M, Korompoki E, Stefanaki K, Kanouta F, Kassi GN, Dimopoulos MA, Mitrakou A. Early and late endocrine complications of COVID-19. Endocr Connect. 2021;10(9):R229–R239. doi: 10.1530/EC-21-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi C, Wang L, Ye J, Gu Z, Wang S, Xia J, Xie Y, Li Q, Xu R, Lin N. Predictors of mortality in patients with coronavirus disease 2019: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):663–663. doi: 10.1186/s12879-021-06369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao Y, Xu B, Guan W, Xu D, Li F, Ren R, Zhu X, Gao Y, Jiang L. The adrenal cortex, an underestimated site of SARS-CoV-2 infection. Front Endocrinol (Lausanne) 2021;11:593179–593179. doi: 10.3389/fendo.2020.593179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45–45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashim M, Athar S, Gaba WH. New onset adrenal insufficiency in a patient with COVID-19. BMJ Case Rep. 2021;14(1):e237690–e237690. doi: 10.1136/bcr-2020-237690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akbas EM, Akbas N. COVID-19, adrenal gland, glucocorticoids, and adrenal insufficiency. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2021;165(1):1–7. doi: 10.5507/bp.2021.011. [DOI] [PubMed] [Google Scholar]
- 22.Lite C, Ahmed SSSJ, Juliet M, Freddy AJ. SARS-CoV-2/human interactome reveals ACE2 locus crosstalk with the immune regulatory network in the host. Pathog Dis. 2021;79(2):ftab005–ftab005. doi: 10.1093/femspd/ftab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasile CM, Udriştoiu AL, Ghenea AE, Popescu M, Gheonea C, Niculescu CE, Ungureanu AM, Udriştoiu Ş, Drocaş AI, Gruionu LG, Gruionu G, Iacob AV, Alexandru DO. Intelligent diagnosis of thyroid ultrasound imaging using an ensemble of deep learning methods. Medicina (Kaunas) 2021;57(4):395–395. doi: 10.3390/medicina57040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel E, Zill-E-Huma R, Demertzidou E. Spontaneous adrenal haemorrhage in pregnancy and review of the literature. BMJ Case Rep. 2022;15(5):e246240–e246240. doi: 10.1136/bcr-2021-246240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Wang Z. Identification of risk factors for in-hospital death of COVID-19 pneumonia – lessons from the early outbreak. BMC Infect Dis. 2021;21(1):113–113. doi: 10.1186/s12879-021-05814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Stasi V, Rastrelli G, Inglese F, Beccaria M, Garuti M, Di Costanzo D, Spreafico F, Cervi G, Greco GF, Pecoriello A, Todisco T, Cipriani S, Maseroli E, Scavello I, Glingani C, Franchini M, Maggi M, De Donno G, Vignozzi L. Higher testosterone is associated with increased inflammatory markers in women with SARS-CoV-2 pneumonia: preliminary results from an observational study. J Endocrinol Invest. 2021;45(3):639–648. doi: 10.1007/s40618-021-01682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calderon-Lopez MT, Garcia-Leon N, Gomez-Arevalillo S, Martin-Serrano P, Matilla-Garcia A. Coronavirus disease 2019 and coagulopathy: other prothrombotic coagulation factors. Blood Coagul Fibrinolysis. 2021;32(1):44–49. doi: 10.1097/MBC.0000000000000996. [DOI] [PubMed] [Google Scholar]
- 28.Raishan S, Alsabri M, Hanna AM, Brett M. Resolution of pituitary microadenoma after coronavirus disease 2019: a case report. J Med Case Rep. 2021;15(1):544–544. doi: 10.1186/s13256-021-03127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valea A, Ghervan C, Carsote M, Morar A, Iacob I, Tomesc F, Pop DD, Georgescu C. Effects of combination therapy: somatostatin analogues and dopamine agonists on GH and IGF1 levels in acromegaly. Clujul Med. 2015;88(3):310–313. doi: 10.15386/cjmed-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valea A, Carsote M, Ghervan C, Georgescu C. Glycemic profile in patients with acromegaly treated with somatostatin analogue. J Med Life. 2015;8(Spec Issue):82–86. [PMC free article] [PubMed] [Google Scholar]
- 31.Leow MKS, Kwek DSK, Ng AWK, Ong KC, Kaw GJL, Lee LSU. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS) Clin Endocrinol (Oxf) 2005;63(2):197–202. doi: 10.1111/j.1365-2265.2005.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piticchio T, Le Moli R, Tumino D, Frasca F. Relationship between betacoronaviruses and the endocrine system: a new key to understand the COVID-19 pandemic – a comprehensive review. J Endocrinol Invest. 2021;44(8):1553–1570. doi: 10.1007/s40618-020-01486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das L, Dutta P, Walia R, Mukherjee S, Suri V, Puri GD, Mahajan V, Malhotra P, Chaudhary S, Gupta R, Jayant SS, Agrawal K, Kumar V, Sachdeva N, Rastogi A, Bhadada SK, Ram S, Bhansali A. Spectrum of endocrine dysfunction and association with disease severity in patients with COVID-19: insights from a cross-sectional, observational study. Front Endocrinol (Lausanne) 2021;12:645787–645787. doi: 10.3389/fendo.2021.645787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chua MWJ, Chua MPW. Delayed onset of central hypocortisolism in a patient recovering from COVID-19. AACE Clin Case Rep. 2021;7(1):2–5. doi: 10.1016/j.aace.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De La Flor Merino JC, Mola Reyes L, Linares Gravalos T, Roel Conde A, Rodeles Del Pozo M. An unusual case of severe acute hyponatremia in patient with COVID-19 infection. Nefrologia (Engl Ed) 2020;40(3):356–358. doi: 10.1016/j.nefro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarke SA, Phylactou M, Patel B, Mills EG, Muzi B, Izzi-Engbeaya C, Choudhury S, Khoo B, Meeran K, Comninos AN, Abbara A, Tan T, Dhillo WS. Normal adrenal and thyroid function in patients who survive COVID-19 Infection. J Clin Endocrinol Metab. 2021;106(8):2208–2220. doi: 10.1210/clinem/dgab349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salzano C, Saracino G, Cardillo G. Possible adrenal involvement in long COVID syndrome. Medicina (Kaunas) 2021;57(10):1087–1087. doi: 10.3390/medicina57101087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabrera Martimbianco AL, Pacheco RL, Bagattini ÂM, Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: a systematic review. Int J Clin Pract. 2021;75(10):e14357–e14357. doi: 10.1111/ijcp.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Churilov LP, Kanduc D, Ryabkova VA. COVID-19: adrenal response and molecular mimicry. Isr Med Assoc J. 2021;23(10):618–619. [PubMed] [Google Scholar]
- 40.Hu Z, Lv Y, Xu C, Sun W, Chen W, Peng Z, Chen C, Cui X, Jiao D, Cheng C, Chi Y, Wei H, Hu C, Zeng Y, Zhang X, Yi Y. Clinical use of short-course and low-dose corticosteroids in patients with non-severe COVID-19 during pneumonia progression. Front Public Health. 2020;8:355–355. doi: 10.3389/fpubh.2020.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim MX, Fong KK, Yeap TB. Use of extracorporeal blood purification therapy (ECBPT) as an adjuvant to high-dose corticosteroids in a severely ill COVID-19 patient with concomitant bacterial infection. BMJ Case Rep. 2021;14(10):e245639–e245639. doi: 10.1136/bcr-2021-245639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smail SW, Saeed M, Twana A, Khudhur ZO, Younus DA, Rajab MF, Abdulahad WH, Hussain HI, Niaz K, Safdar M. Inflammation, immunity and potential target therapy of SARS-CoV-2: a total scale analysis review. Food Chem Toxicol. 2021;150:112087–112087. doi: 10.1016/j.fct.2021.112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan J, Yuan Y, Xu C, Song C, Liu D, Ma D, Gao Q. A retrospective comparison of drugs against COVID-19. Virus Res. 2021;294:198262–198262. doi: 10.1016/j.virusres.2020.198262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ansems K, Grundeis F, Dahms K, Mikolajewska A, Thieme V, Piechotta V, Metzendorf MI, Stegemann M, Benstoem C, Fichtner F. Remdesivir for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;8(8):CD014962–CD014962. doi: 10.1002/14651858.CD014962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burke E, Haber E, Pike CW, Sonti R. Outcomes of renal replacement therapy in the critically ill with COVID-19. Med Intensiva. 2021;45(6):325–331. doi: 10.1016/j.medin.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagner C, Griesel M, Mikolajewska A, Mueller A, Nothacker M, Kley K, Metzendorf MI, Fischer AL, Kopp M, Stegemann M, Skoetz N, Fichtner F. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;8(8):CD014963–CD014963. doi: 10.1002/14651858.CD014963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao C, He L, Ma J, Chen M, Li Y, Jiang Q, Wu S, Yu L, Huang W, Qian G, Zhu C, Chu J, Chen X. Clinical features and predictors for patients with severe SARS-CoV-2 pneumonia at the start of the pandemic: a retrospective multicenter cohort study. BMC Infect Dis. 2021;21(1):666–666. doi: 10.1186/s12879-021-06335-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esslami GG, Moienafshar A. Neonatal bilateral adrenal hemorrhage and adrenal insufficiency accompanied by subgaleal hematoma: a case report with brief review of literature. BMC Pediatr. 2022;22(1):248–248. doi: 10.1186/s12887-022-03314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cardoza-Jiménez KJ, Carranza-Zavala B, Manrique-Franco K, Espinoza-Morales F, Mejia CR. Daily glucose variation influenced by the use of corticosteroids in COVID-19 patients treated in Lima – Peru. Diabetes Metab Syndr. 2021;15(4):102188–102188. doi: 10.1016/j.dsx.2021.102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarfraz A, Sarfraz Z, Razzack AA, Patel G, Sarfraz M. Venous thromboembolism, corticosteroids and COVID-19: a systematic review and meta-analysis. Clin Appl Thromb Hemost. 2021;27:1076029621993573–1076029621993573. doi: 10.1177/1076029621993573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.López Zúñiga MÁ, Moreno-Moral A, Ocaña-Granados A, Padilla-Moreno FA, Castillo-Fernández AM, Guillamón-Fernández D, Ramírez-Sánchez C, Sanchez-Palop M, Martínez-Colmenero J, Pimentel-Villar MA, Blázquez-Roselló S, Moreno-Sánchez JJ, López-Vílchez M, Prior-Sánchez I, Jódar-Moreno R, López Ruz MÁ. High-dose corticosteroid pulse therapy increases the survival rate in COVID-19 patients at risk of hyper-inflammatory response. PLoS One. 2021;16(1):e0243964–e0243964. doi: 10.1371/journal.pone.0243964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J, Ko JH, Baek JY, Hong J, Ha S, Lee B, Huh K, Cho SY, Kang CI, Chung DR, Kim YJ, Kang ES, Peck KR. Effects of short-term corticosteroid use on reactogenicity and immuno-genicity of the first dose of ChAdOx1 nCoV-19 vaccine. Front Immunol. 2021;12:744206–744206. doi: 10.3389/fimmu.2021.744206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pal R. COVID-19, hypothalamo–pituitary–adrenal axis and clinical implications. Endocrine. 2020;68(2):251–252. doi: 10.1007/s12020-020-02325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wheatland R. Molecular mimicry of ACTH in SARS – implications for corticosteroid treatment and prophylaxis. Med Hypotheses. 2004;63(5):855–862. doi: 10.1016/j.mehy.2004.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cancio M, Ciccocioppo R, Rocco PRM, Levine BL, Bronte V, Bollard CM, Weiss D, Boelens JJ, Hanley PJ. Emerging trends in COVID-19 treatment: learning from inflammatory conditions associated with cellular therapies. Cytotherapy. 2020;22(9):474–481. doi: 10.1016/j.jcyt.2020.04.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bellastella G, Maiorino MI, Esposito K. Endocrine complications of COVID-19: what happens to the thyroid and adrenal glands. J Endocrinol Invest. 2020;43(8):1169–1170. doi: 10.1007/s40618-020-01311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clotman K, Twickler MB. Diabetes or endocrinopathy admitted in the COVID-19 ward. Eur J Clin Invest. 2020;50(7):e13262–e13262. doi: 10.1111/eci.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freire Santana M, Borba MGS, Baía-da-Silva DC, Val F, Alexandre MAA, Brito-Sousa JD, Melo GC, Queiroga MVO, Leão Farias ME, Camilo CC, Naveca FG, Xavier MS, Monteiro WM, Augusto Pivoto João G, Hajjar LA, Ordi J, Lacerda MVG, Ferreira LCL. Case Report: Adrenal pathology findings in severe COVID-19: an autopsy study. Am J Trop Med Hyg. 2020;103(4):1604–1607. doi: 10.4269/ajtmh.20-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iuga AC, Marboe CC, Yilmaz MM, Lefkowitch JH, Gauran C, Lagana SM. Adrenal vascular changes in COVID-19 autopsies. Arch Pathol Lab Med. 2020;144(10):1159–1160. doi: 10.5858/arpa.2020-0248-LE. [DOI] [PubMed] [Google Scholar]
- 60.Machado IFR, Menezes IQ, Figueiredo SR, Coelho FMA, Terrabuio DRB, Ramos DV, Fagundes GFC, Maciel AAW, Claudia Latronico A, Fragoso MCBV, Cancado ELR, Mendonca BB, Almeida MQ. Primary adrenal insufficiency due to bilateral adrenal infarction in COVID-19. J Clin Endocrinol Metab. 2022;107(1):e394–e400. doi: 10.1210/clinem/dgab557. [DOI] [PubMed] [Google Scholar]
- 61.Borgel D, Chocron R, Grimaud M, Philippe A, Chareyre J, Brakta C, Lasne D, Bonnet D, Toubiana J, Angoulvant F, Desvages M, Renolleau S, Smadja DM, Oualha M. Endothelial dysfunction as a component of severe acute respiratory syndrome coronavirus 2-related multisystem inflammatory syndrome in children with shock. Crit Care Med. 2021;49(11):e1151–e1156. doi: 10.1097/CCM.0000000000005093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Darvishi M, Shahali H. Acute cardiac tamponade: a case of life-threatening coronavirus disease 2019 complication during air medical transportation. Air Med J. 2021;40(3):179–181. doi: 10.1016/j.amj.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mirza SA, Sheikh AAE, Barbera M, Ijaz Z, Javaid MA, Shekhar R, Pal S, Sheikh AB. COVID-19 and the endocrine system: a review of the current information and misinformation. Infect Dis Rep. 2022;14(2):184–197. doi: 10.3390/idr14020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar R, Guruparan T, Siddiqi S, Sheth R, Jacyna M, Naghibi M, Vrentzou E. A case of adrenal infarction in a patient with COVID 19 infection. BJR Case Rep. 2020;6(3):20200075–20200075. doi: 10.1259/bjrcr.20200075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warriach SA, Mustafa M, O’Keeffe D, Watts M. Unusual case of antiphospholipid syndrome presenting as adrenal insufficiency. BMJ Case Rep. 2020;13(3):e233631–e233631. doi: 10.1136/bcr-2019-233631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, Rajagopal S, Pai AR, Kutty S. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM Consortium Position Paper. Front Immunol. 2020;11:1648–1648. doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elkhouly MMN, Elazzab AA, Moghul SS. Bilateral adrenal hemorrhage in a man with severe COVID-19 pneumonia. Radiol Case Rep. 2021;16(6):1438–1442. doi: 10.1016/j.radcr.2021.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maria ATJ, Diaz-Cau I, Benejean JM, Nutz A, Schiffmann A, Biron-Andreani C, Guilpain P. Flare of antiphospholipid syndrome in the course of COVID-19. TH Open. 2020;4(3):e207–e210. doi: 10.1055/s-0040-1716735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frankel M, Feldman I, Levine M, Frank Y, Bogot NR, Benjaminov O, Kurd R, Breuer GS, Munter G. Bilateral adrenal hemorrhage in coronavirus disease 2019 patient: a case report. J Clin Endocrinol Metab. 2020;105(12):dgaa487–dgaa487. doi: 10.1210/clinem/dgaa487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35(5):744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 71.Patel KP, Patel PA, Vunnam RR, Hewlett AT, Jain R, Jing R, Vunnam SR. Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19. J Clin Virol. 2020;128:104386–104386. doi: 10.1016/j.jcv.2020.104386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saeed U, Sellevoll HB, Young VS, Sandbaek G, Glomsaker T, Mala T. Covid-19 may present with acute abdominal pain. Br J Surg. 2020;107(7):e186–e187. doi: 10.1002/bjs.11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murali N, El Hayek SM. Abdominal pain mimics. Emerg Med Clin North Am. 2021;39(4):839–850. doi: 10.1016/j.emc.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chung H, Seo H, Park S, Kim H, Jung J, Chong YP, Kim SH, Lee SO, Choi SH, Kim YS, Kim MJ. The first case of multi-system inflammatory syndrome in adult after COVID-19 in Korea. J Korean Med Sci. 2021;36(25):e181–e181. doi: 10.3346/jkms.2021.36.e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pereira MFB, Litvinov N, Farhat SCL, Eisencraft AP, Gibelli MABC, Carvalho WB, Fernandes VR, Fink TT, Framil JVS, Galleti KV, Fante AL, Fonseca MFM, Watanabe A, Paula CSY, Palandri GG, Leal GN, Diniz MFR, Pinho JRR, Silva CA, Marques HHS, Pediatric COVID HC-FMUSP Study Group. Rossi Junior A, Delgado AF, Andrade APM, Schvartsman C, Sabino EC, Rocha MC, Kanunfre KA, Okay TS, Carneiro-Sampaio MMS, Jorge PPD. Severe clinical spectrum with high mortality in pediatric patients with COVID-19 and multisystem inflammatory syndrome. Clinics (Sao Paulo) 2020;75:e2209–e2209. doi: 10.6061/clinics/2020/e2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cattalini M, Della Paolera S, Zunica F, Bracaglia C, Giangreco M, Verdoni L, Meini A, Sottile R, Caorsi R, Zuccotti G, Fabi M, Montin D, Meneghel A, Consolaro A, Dellepiane RM, Maggio MC, La Torre F, Marchesi A, Simonini G, Villani A, Cimaz R, Ravelli A, Taddio A, Rheumatology Study Group of the Italian Pediatric Society Defining Kawasaki disease and pediatric inflammatory multisystem syndrome-temporally associated to SARS-CoV-2 infection during SARS-CoV-2 epidemic in Italy: results from a national, multicenter survey. Pediatr Rheumatol Online J. 2021;19(1):29–29. doi: 10.1186/s12969-021-00511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alsaied T, Tremoulet AH, Burns JC, Saidi A, Dionne A, Lang SM, Newburger JW, de Ferranti S, Friedman KG. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation. 2021;143(1):78–88. doi: 10.1161/CIRCULATIONAHA.120.049836. [DOI] [PubMed] [Google Scholar]
- 78.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, Ramnarayan P, Fraisse A, Miller O, Davies P, Kucera F, Brierley J, McDougall M, Carter M, Tremoulet A, Shimizu C, Herberg J, Burns JC, Lyall H, Levin M, PIMS-TS Study Group and EUCLIDS and PERFORM Consortia Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sperotto F, Friedman KG, Son MBF, VanderPluym CJ, Newburger JW, Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. 2021;180(2):307–322. doi: 10.1007/s00431-020-03766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leyendecker P, Ritter S, Riou M, Wackenthaler A, Meziani F, Roy C, Ohana M. Acute adrenal infarction as an incidental CT finding and a potential prognosis factor in severe SARS-CoV-2 infection: a retrospective cohort analysis on 219 patients. Eur Radiol. 2021;31(2):895–900. doi: 10.1007/s00330-020-07226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R, Al-Sarraj S, Abdolrasouli A, Swann OC, Baillon L, Penn R, Barclay WS, Viola P, Osborn M. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1(6):e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chiperi LE, Ionescu AD, Marcu CT, Itu-Mureşan C, Pantelemon C. Hemophagocytic lymphohistiocytosis in a child with human immunodeficiency virus infection – a case report. Rom J Morphol Embryol. 2021;62(1):279–282. doi: 10.47162/RJME.62.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paladugu S, Donato AA. Recommendations for caring for patients with severe and nonsevere COVID-19. Ann Intern Med. 2020;173(2):JC3–JC3. doi: 10.7326/ACPJ202007210-004. [DOI] [PubMed] [Google Scholar]
- 84.Mattos-Silva P, Felix NS, Silva PL, Robba C, Battaglini D, Pelosi P, Rocco PRM, Cruz FF. Pros and cons of corticosteroid therapy for COVID-19 patients. Respir Physiol Neurobiol. 2020;280:103492–103492. doi: 10.1016/j.resp.2020.103492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.lvarez-Troncoso J, Zapatero Larrauri M, Montero Vega MD, Gil Vallano R, Palmier Peláez E, Martín Rojas-Marcos P, Martín-Luengo F, Lázaro Del Campo P, Herrero Gil CR, Trigo Esteban E. Case Report: COVID-19 with bilateral adrenal hemorrhage. Am J Trop Med Hyg. 2020;103(3):1156–1157. doi: 10.4269/ajtmh.20-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharrack N, Baxter CT, Paddock M, Uchegbu E. Adrenal haemorrhage as a complication of COVID-19 infection. BMJ Case Rep. 2020;13(11):e239643–e239643. doi: 10.1136/bcr-2020-239643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rebollo-Román A, Alhambra-Expósito MR, Herrera-Martínez Y, Leiva-Cepas F, Alzas C, Muñoz-Jiménez C, Ortega-Salas R, Molina-Puertas MJ, Gálvez-Moreno MA, Herrera-Martínez AD. Catecholaminergic crisis after a bleeding complication of COVID-19 infection: a case report. Front Endocrinol (Lausanne) 2021;12:693004–693004. doi: 10.3389/fendo.2021.693004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin CY, Huang LY, Wu KA, Chan JS, Wu KL, Shyu HY, Hsiao PJ. Response to bilateral adrenal haemorrhage in the differential diagnosis of abdominal pain in vaccine-induced thrombosis with thrombocytopenia. QJM. 2022;114(12):910–911. doi: 10.1093/qjmed/hcab239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Al Rawahi B, BaTaher H, Jaffer Z, Al-Balushi A, Al-Mazrouqi A, Al-Balushi N. Vaccine-induced immune thrombotic thrombocytopenia following AstraZeneca (ChAdOx1 nCOV19) vaccine – a case report. Res Pract Thromb Haemost. 2021;5(6):e12578–e12578. doi: 10.1002/rth2.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuter DJ. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. Br J Haematol. 2021;195(3):365–370. doi: 10.1111/bjh.17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang YH, Huang LY, Chen YL, Chan JS, Chiang WF, Lin CY, Chen MH, Shyu HY, Hsiao PJ. ChAdOx1 COVID-19 vaccine-induced thrombocytopenia syndrome. QJM. 2021;114(10):733–734. doi: 10.1093/qjmed/hcab221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Varona JF, García-Isidro M, Moeinvaziri M, Ramos-López M, Fernández-Domínguez M. Primary adrenal insufficiency associated with Oxford–AstraZeneca ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia (VITT) Eur J Intern Med. 2021;91:90–92. doi: 10.1016/j.ejim.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taylor P, Allen L, Shrikrishnapalasuriyar N, Stechman M, Rees A. Vaccine-induced thrombosis and thrombocytopenia with bilateral adrenal haemorrhage. Clin Endocrinol (Oxf) 2022;97(1):26–27. doi: 10.1111/cen.14548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb Haemost. 2021;19(7):1771–1775. doi: 10.1111/jth.15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.D’Agostino V, Caranci F, Negro A, Piscitelli V, Tuccillo B, Fasano F, Sirabella G, Marano I, Granata V, Grassi R, Pupo D, Grassi R. A rare case of cerebral venous thrombosis and disseminated intravascular coagulation temporally associated to the COVID-19 vaccine administration. J Pers Med. 2021;11(4):285–285. doi: 10.3390/jpm11040285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haji N, Ali S, Wahashi EA, Khalid M, Ramamurthi K. Johnson and Johnson COVID-19 vaccination triggering pheochromocytoma multisystem crisis. Cureus. 2021;13(9):e18196–e18196. doi: 10.7759/cureus.18196. [DOI] [PMC free article] [PubMed] [Google Scholar]