Abstract
Maternal obesity is associated with increased maternal and fetal morbidity and mortality, with an increased risk of gestational diabetes mellitus (GDM) and preeclampsia (PE). This prospective study histopathologically analyzes the placentas obtained from 34 pregnant obese women studied between October 2016 and May 2020. The 10 cases of term placentas from obese pregnancies with GDM and the 12 cases with PE were examined by the Hematoxylin–Eosin (HE), Masson’s trichrome (MT) and Periodic Acid–Schiff–Hematoxylin (PAS–H) classical stainings, and by the immunohistochemical evaluation and compared to placentae from uncomplicated term obese pregnancies (12 cases). We did not meet placental histopathological (HP) abnormalities that we could classify as characteristic only for the state of obese pregnancy, but we did find placental changes associated with PE and GDM, in the context of obese pregnancy. In the case of association with PE, there were common lesions, manifested by intra- and perivillous fibrinoid deposition, calcification, and placental infarction area, to which were added numerous syncytial knots. In the case of obese pregnancy associated with GDM, we found, in addition to common placental lesions of obesity, intravillositary vascular edema and in the terminal villi appearing chorangiosis. This study revealed a number of HP changes that occur in maternal obesity, even in uncomplicated obese pregnancies. A characteristic of obese pregnancies associated with PE was the presence of numerous syncytial knots, and in obese pregnancies associated with GDM, the most common HP lesion was placental chorangiosis. Certainly, we cannot conclude that these HP lesions are specific to a particular pathology, but they belong primarily to the status of maternal obesity.
Keywords: obesity, pregnancy, preeclampsia, gestational diabetes mellitus, pathology
⧉ Introduction
Obesity is currently a global health problem and the prevalence of obesity during pregnancy is increasing. In the United States, the incidence of obesity during pregnancy varies from 18.5% to 38.3%, while in the most European countries, the prevalence of obesity is lower, about 15% [1,2].
Pre-pregnancy obesity is associated with increased maternal and fetal morbidity and mortality, with an increased risk of gestational diabetes mellitus (GDM), preeclampsia (PE), fetal macrosomia, and late stillbirth during pregnancy [3]. It was noted that the offspring have an increased risk of developing obesity and metabolic syndrome [4], thus continuing the negative consequences of obesity in the next generation. Unfortunately, the mechanisms that determine these adverse outcomes are not yet well known.
The placenta probably mediates these complications, the consequence of obesity on placental function including the placental production of hormones and the transport of nutrients to the fetus [5]. Studies on the human placenta have shown an increased maternal inflammatory response in obese pregnancy, demonstrated by increased levels of circulating proinflammatory cytokines [interleukin-6 (IL-6)] and the presence of higher levels of placental proinflammatory cytokines [6].
Pathological analysis of the placenta is important in trying to find the underlying causes of pregnancy complications in obese women, because not much is known about the placental histopathological (HP) appearance of pregnancies with obesity, with or without adverse pregnancy outcome.
Aim
This study aimed to assess the correlation between PE and GDM and patients’ body mass index (BMI) and assessed if obesity influences the fetal growth development. Another purpose was to signal the placental structural changes associated.
⧉ Patients, Materials and Methods
This was a prospective cohort study on singleton pregnancy in obese or overweight women. Term placentas from complications with obese pregnancies, GDM (10 cases) and PE (12 cases), were examined and compared to placentae from uncomplicated term obese pregnancies (12 cases). The 34 cases were studied between October 2016 and May 2020. The study took place in the Clinic of Obstetrics and Gynecology, Filantropia Municipal Clinical Hospital, Craiova, Romania. Maternal obesity was defined as BMI >30 kg/m2, reported to the first prenatal visit, in the first trimester. BMI was calculated according to the standard formula (kg/m2), and patients were grouped according to the classification of the World Health Organization (WHO): normal BMI (≤24.9 kg/m2), overweight (25.0–29.9 kg/m2) and obese (≥30.0 kg/m2). All pregnancies were singleton and term pregnancies (defined as >37 weeks gestation).
All women completed a questionnaire of demographic details, obstetric history, and pregnancy symptoms before the first consultation, and informed consent was obtained from the women who agreed to participate in this study. The study was conducted in full compliance with the ethical principles contained in the “Declaration of Human Rights” adopted in Helsinki, which are in accordance with the “Rules of Good Practice in Clinical Trials” and the legal regulations in force and with the Approval of the Ethics Committee of the University of Medicine and Pharmacy of Craiova. The statistical analysis was performed in the Department of Biostatistics, University of Medicine and Pharmacy of Craiova.
The placental tissues were sent to the Research Center for Microscopic Morphology and Immunology (University of Medicine and Pharmacy of Craiova) for HP analysis. The tissues were fixed in 10% neutral buffered formalin at room temperature (RT). After fixation, the tissue pieces included in solid paraffin blocks were sectioned to a thickness of 5 μm, using the HM350 microtome, equipped with a water-based transfer system (STS microM); the tissue sections were applied on single slides and on slides treated with poly-L-lysine and left to thermostat (37°C) for one day for drying. To highlight the microscopic elements, we applied the protocol for the classical histological stainings: Hematoxylin–Eosin (HE), Masson’s trichrome (MT), Periodic Acid–Schiff–Hematoxylin (PAS–H), but also on the special immunohistochemical (IHC) ones.
For the IHC study, sections were collected on slides coated with poly-L-lysine and dried in a thermostat at 37°C for 24 hours. After this procedure, the sections were subjected to a classical protocol: deparaffinization, dehydration, rehydration, antigen unmasking by boiling the slides in a sodium citrate solution, pH 6, for 21 minutes (seven cycles of 3 minutes) in a microwave oven. The endogenous peroxidase blocking was performed by incubating the histological sections in 3% hydrogen peroxide for 30 minutes, at RT, followed by a wash in distilled water for 10 minutes and a wash in 1% phosphate-buffered saline (PBS) for five minutes. Afterwards, blocking the nonspecific sites followed by using 2% skim milk for 30 minutes. Subsequently, histological sections were coated with the primary antibody (Table 1) and introduced into the cold (4°C, for 18 hours). After 18 hours, the slides were left at RT (30 minutes), washed in PBS (3×5 minutes) and the secondary antibody was applied for one hour [mouse/rabbit immunoglobulin G (IgG) antibody, VC002-025, R&D Systems VisUCyte Horseradish peroxidase (HRP) Polymer], were washed again in PBS (3×5 minutes) and developed with 3,3’-Diaminobenzidine (DAB) (Dako). At the end, the nuclei were labeled with Hematoxylin, the slides were dehydrated in ethanol with increasing concentrations (70%, 90%, 96%, 100%) (×5 minutes each), clarified in xylene for 30–45 minutes and Canada conditioner slides were fitted.
Table 1.
Antibody used in this study
|
Primary antibody |
Producer |
Clone |
Antigenic unmasking |
Secondary antibody |
Dilution |
Marking |
|
Anti-CD34 |
Dako |
QBEnd 10 |
Citrate, pH 6 |
Monoclonal mouse anti-human CD34 Class II |
1:50 |
Small neoformation vessels |
CD34: Cluster of differentiation 34
⧉ Results
The maternal and neonatal characteristics of the 34 cases whose placentas were studied from a HP point of view (Table 2). We selected cases with similar characteristics to try to determine specific HP lesions. Regarding BMI, PE occurred mainly in obese women (>30 kg/m2), and GDM in overweight women. We noticed that fetal birth weight, interpreted as mean ± standard deviation (SD), did not exceed 50 percentiles, the lowest weight being in cases with PE, which shows that in pregnancies associated with obesity there is some restriction or slowdown of fetal growth.
Table 2.
Maternal and neonatal characteristics
|
|
Uncomplicated term deliveries in obese pregnancy (n=12) |
GDM in obese pregnancy (n=10) |
PE in obese pregnancy (n=12) |
|
Maternal characteristics | |||
|
Age [years] |
28±3.96 |
27±4.05 |
28.5±4.38 |
|
BMI [kg/m2] |
28.45±7.42 |
29.20±5.84 |
33.15±7.42 |
|
Nulliparity [%] |
83.33 |
60 |
83.33 |
|
Normal vaginal delivery [%] |
25 |
10 |
10 |
|
C-section [%] |
75 |
90 |
90 |
|
Fetal characteristics | |||
|
GA at birth [years] |
38.4±1.39 |
38.25±0.85 |
38.1±1.10 |
|
Birthweight (percentile) [kg] |
46±30.80 |
44±23.27 |
34±24.14 |
|
Apgar score at five minutes |
9±0.51 |
9±0.63 |
8.5±0.96 |
|
SGA (<10 percentile) [%] |
8.33 |
10 |
25 |
|
LGA (>90 percentile) [%] |
0 |
0 |
8.33 |
|
AGA (10–90 percentile) [%] |
91.66 |
90 |
66.67 |
AGA: Appropriate for gestational age; BMI: Body mass index; C-section; Caesarean section; GA: Gestational age; GDM: Gestational diabetes mellitus; LGA: Large for gestational age; n: No. of cases; PE: Pre-eclampsia; SGA: Small for gestational age
The present study revealed at the HP examination of the placental sections obtained from obese mothers, several changes of the placenta, which we tried to distribute according to the pathology presented: PE, GDM or pregnancies with normal evolution but associated with obesity.
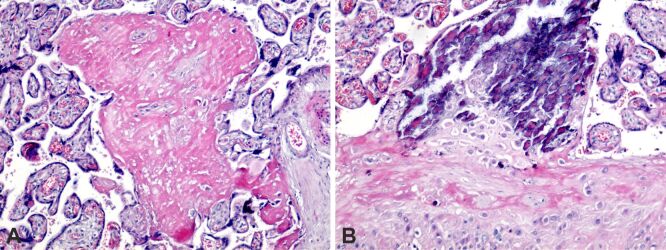
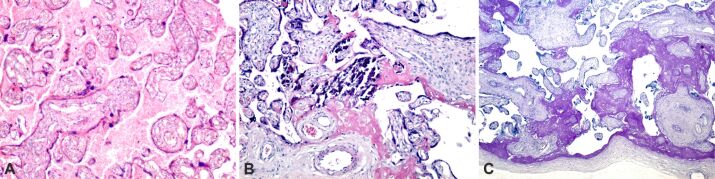
In uncomplicated term deliveries from obese pregnancy, abnormal placental histopathology has been observed, but that may be an aspect of obese uncomplicated term pregnancies because it has been found in almost all obese pregnancies. Intra- and perivillous fibrinoid deposition, placental infarction, perilesional fibrinoid deposition, extravillous calcification were present, and at the level of the chorionic plaque we noticed fibrinoid deposition (Figure 1, A and B).
Figure 1.
Placental structural analysis in obese pregnancy: (A) The presence of intra- and perivillous fibrinoid deposition (intense pink), small areas of extravillous calcification and the area of massive placental infarction that included several terminal placental villi, with perilesional fibrinoid deposition; (B) The presence of intra- and perivillous fibrinoid deposition (intense pink color), massive area of extravillous calcification (pink-purple color), perichorial and fibrinoid deposition in the structure of the chorionic plaque. HE staining: (A and B) ×100. HE: Hematoxylin–Eosin
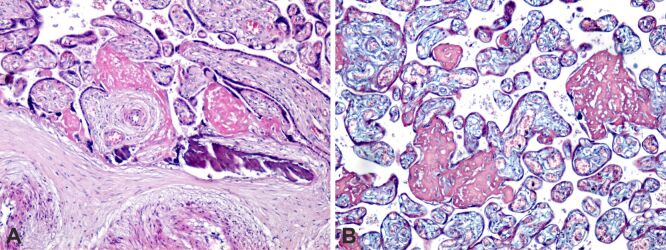
We found no placental HP abnormalities that we could classify as characteristic only for the state of obese pregnancy, but placental changes associated with PE and GDM were found. In case of association with PE, common lesions were present, manifested by intra- and perivillous fibrinoid deposition, calcification, and placental infarction area, with perilesional, intravillous and pericotyledonary fibrinoid deposition (Figure 2, A and B).
Figure 2.
Placental structural analysis in obese pregnancy associated with pre-eclampsia: (A) Intra- and perivillous fibrinoid deposition (intense pink color), extravillous calcification and placental infarction areas, with perilesional, intravillous and pericotyledonary fibrinoid deposition; (B) Intra- and perivillous fibrinoid deposition (red), small areas of extra- and intravillous calcification and old placental infarction, with perilesional fibrinoid development. HE staining: (A) ×100. MT staining: (B) ×100. HE: Hematoxylin–Eosin; MT: Masson’s trichrome
To all these lesions were added the presence of syncytial knots, which are constantly present in the placenta at term, but increased syncytial knots are frequently associated with maternal vascular malperfusion, as occurs in PE (Figure 3A,3B,3C).
Figure 3.
Placental structural analysis in obese pregnancy associated with preeclampsia: (A) Intra- and perivillous fibrinoid deposition (intense pink), small areas of extravillous calcification, old placental infarction, with perilesional fibrinoid development and syncytial knots; (B) Intra- and perivillous fibrinoid deposition (intense pink), small areas of extravillous calcification; the presence of syncytial knots and intervillous extravasation was identified; (C) Intra- and perivillous fibrinoid deposition (intense pink) and the presence of small syncytial knots. HE staining: (A and B) ×100. MT staining: (C) ×100. HE: Hematoxylin–Eosin; MT: Masson’s trichrome
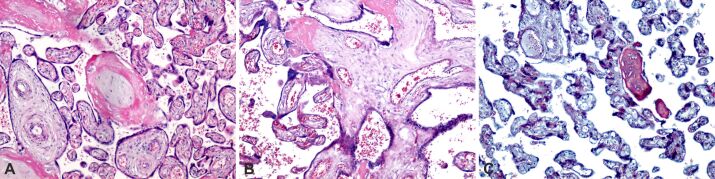
At the level of the chorionic plaque, in the case of obese pregnancy associated with PE, we noticed a massive deposition of subchorial and intrachorial fibrinoid, as well as areas of chorial infarction (Figure 4A,4B,4C).
Figure 4.
Chorionic plate structural analysis in obese pregnancy associated with preeclampsia: (A) Chorionic plaque with fibrinoid deposit (red areas) and small areas of chorionic infarction; (B) Area of massive deposition of subchorial and intrachorial fibrinoid (intense pink), small areas of perichorial calcification; (C) Intra- and perivillous fibrinoid deposition (intense pink), small extravillous calcification areas, and the subchorial fibrinoid deposition area that includes chorionic plaque cells. MT staining: (A) ×100. PAS–H staining: (B) ×100. HE staining: (C) ×100. HE: Hematoxylin–Eosin; MT: Masson’s trichrome; PAS–H: Periodic Acid–Schiff–Hematoxylin
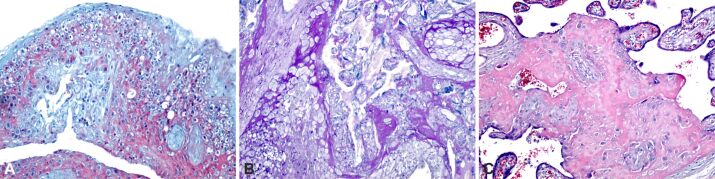
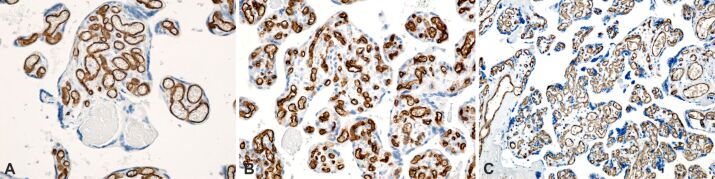
In the case of obese pregnancy associated with GDM, we found, in addition to common placental lesions of obesity, intravillositary vascular edema, massive deposition of subchorial and subamniotic fibrinoids, perichorial and intercotyledonary calcification (Figure 5A,5B,5C). In this context, in the terminal villi develop several branches from a single capillary, appearing a phenomenon of hypervascularity, known as chorangiosis. Chorangiosis appears to be a placental adaptation for better placental efficacy in GDM. CD34 cells with a positive immunoreaction were found in the endothelium of vascular tree villi in cases with GDM, for the appearance of chorangiosis (Figure 6A,6B,6C).
Figure 5.
Placental structural analysis in obese pregnancy associated with gestational diabetes mellitus: (A) Intra- and perivillous fibrinoid deposition (intense pink), small areas of extravillous calcification and intense extravillous hemorrhagic process with areas of intravillous vascular edema; (B) Intra- and extravillous fibrinoid deposition, with massive area of central villous calcification and small areas of perivillous calcification; (C) Area of massive deposition of subchorial and subamniotic fibrinoid (intense pink), small areas of perichorial and intercotyledonary calcification. HE staining: (A and B) ×100. PAS–H staining, ×100. HE: Hematoxylin–Eosin; PAS–H: Periodic Acid–Schiff–Hematoxylin
Figure 6.
Placental structural analysis in obese pregnancy associated with gestational diabetes mellitus. The basement membrane of the capillary endothelium was immunohistochemically labeled in brown: (A) Placental villi with chorangiosis (>10 intravillous capillaries) and the presence of minor intravillous infarcts; (B) Placental villi with chorangiosis; (C) Placental villi with the presence of normal looking intravillous capillaries and the area of placental villi with chorangiosis. Anti-CD34 antibody immunomarking: (A–C) ×200. CD34: Cluster of differentiation 34
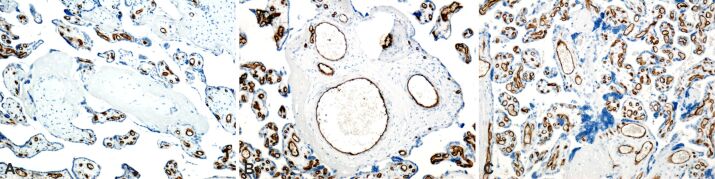
In the IHC study performed, we also found a number of aspects that were common for placentas from obese women with associated PE and GDM: placental villi infarcted completely with absent vascular capillaries, perivillous calcification, placental cotyledons with extremely dilated blood vessels and edema (Figure 7A,7B,7C).
Figure 7.
Placental structural analysis in obese pregnancy associated with gestational diabetes mellitus and preeclampsia. The basement membrane of the capillary endothelium was immunohistochemically labeled in brown: (A) Placental villi with the presence of normal-looking intravillous capillaries and placental villi with total infarction, with absent vascular capillaries; (B) Placental villi with the presence of normal-looking intravillous capillaries and placental cotyledons with extremely dilated, edematous blood vessels; in the dilated vessels, there is a decrease in the thickness of the vascular endothelium; (C) Placental villi with the presence of intravillous capillaries, with normal appearance and placental villi with total infarction, which do not have intravillous vascular capillaries; the presence of perivillous calcification areas was identified. Anti-CD34 antibody immunomarking: (A–C) ×200. CD34: Cluster of differentiation 34
This study found that HP examination of placentas obtained from obese mothers did not reveal placental HP abnormalities specific only to the state of obese pregnancy, but only placental changes associated with PE and GDM, associated pathologies that were followed.
⧉ Discussions
The placenta is a unique extraembryonic organ that is present only during pregnancy and is responsible for fetal intrauterine development [7]. The HP study of the placenta may reveal aspects of fetal intrauterine life, which promises a clarification of the “mysteries” that cause adverse pregnancy outcome in several maternal pathologies, including obesity.
This prospective study evaluated the HP findings of the placentas of obese mothers both with the association of PE and GDM, and a pregnancy with obesity without any associated pathology. Our observations showed a significant increase in the frequency of intra- and perivillous fibrinoid deposition, fibrinoid deposition that was also present in the chorionic plaque, and placental infarction that included several terminal placental villi. As reported by some studies and authors, this lesion is due to increased muscle layer of the vessels, which causes a stasis of maternal blood in the intervillous space, which leads to a coagulation reflex [6, 8]. In their study, Jarmuzek et al. [9] showed that placental HP lesions in GDM pregnancies show some typical features, such as villous immaturity, villous fibrinoid necrosis, chorangiosis, and increased angiogenesis, but the authors point out that the type of lesion depends on how early the pathology set in [9].
The classical definition of chorangiosis refers to the marked increase in the number of vessels (>10 capillaries in more than 10 villi in several areas of the placenta) from non-infarcted and non-ischemic placental areas [10,11,12,13].
Suzuki et al. [14] appreciate that chorangiosis is a compensatory response to the status of chronic hypoxia associated with common conditions, including GDM and PE, facilitating vascular remodeling to adapt to low oxygen intake [15].
Redline [16] also reported that chorangiosis could be the hallmark of delayed villous maturation, which occurs in placentas from obese pregnancies, due to increased insulin resistance and hypoxia [16]. An independent factor for hypoxia is fetal hyperinsulinemia, which is associated with maternal obesity [17]. Probably that is why in our analysis we identified GDM-associated obese pregnancy as being associated with placental chorangiosis much more frequently than PE-associated obese pregnancy.
Another element in our study of the placental lesion found in placentas from obese pregnancies, was syncytial knots, which was more common in obese pregnancies associated with PE. Syncytial knots increase with increasing gestational age (GA) and assess villous maturity. But a high increase in syncytial knots may call into question maternal vascular malperfusion, which may be associated with adverse pregnancy outcomes, including PE and small for gestational age (SGA) in newborns [18,19].
Syncytial knots are a feature of pregnancy associated with PE, as previous studies have shown, with numerous syncytial knots being combinations of reduced perfusion [20,21,22]. According to Burton et al. [23], the presence of oxidative stress and the generation of reactive oxygen species could underlie abnormal vascular remodeling and the appearance of increased syncytial knots [21]. As we mentioned, in our study we found an increase in syncytial knots especially in cases with obese pregnancies and associated PE, but we also found this HP lesion in cases with GDM or uncomplicated obese pregnancies, but less frequently. These findings are in accordance with other studies [24,25,26].
All the placental changes determined chorionic villi changes and in histoarchitecture and chorionic villi function with serious consequences on vascular, procoagulant status and areas with reduced blood flow [27,28], having significant influence in the fetal outcome [28], with consequences on trophoblastic vascularization [29].
⧉ Conclusions
This study revealed a number of HP placental changes that occur in maternal obesity, reflecting the adaptability of this unique organ for fetal protection. Even in uncomplicated pregnancies, maternal obesity can cause characteristic changes in placental histology. Thus, in uncomplicated obese pregnancies we can find increase in fibrinoid deposition, and placental infarction that included several terminal placental villi, intravillous vascular edema. A characteristic of obese pregnancies associated with PE was the presence of numerous syncytial knots, and in obese pregnancies associated with GDM, the most common HP lesion was placental chorangiosis. We cannot conclude that these HP lesions are specific to a particular pathology, but they may be primarily related to maternal obesity status.
Conflict of interests
The authors declare that they have no conflict of interests.
Authors’ contribution
Maria Carmen Tabacu and Anca-Maria Istrate-Ofiţeru equally contributed to this article.
Acknowledgments
Microscopic images have been acquired in the Research Center for Microscopic Morphology and Immunology, University of Medicine and Pharmacy of Craiova, Romania (Manager: Laurenţiu Mogoantă, Professor, MD, PhD).
References
- 1.Bhattacharya S, Campbell DM, Liston WA, Bhattacharya S. Effect of body mass index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health. 2007;7:168–168. doi: 10.1186/1471-2458-7-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poston L, Caleyachetty R, Cnattingius S, Corvalán C, Uauy R, Herring S, Gillman MW. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016;4(12):1025–1036. doi: 10.1016/S2213-8587(16)30217-0. [DOI] [PubMed] [Google Scholar]
- 3.Denison FC, Price J, Graham C, Wild S, Liston WA. Maternal obesity, length of gestation, risk of postdates pregnancy and spontaneous onset of labour at term. BJOG. 2008;115(6):720–725. doi: 10.1111/j.1471-0528.2008.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010;140(3):387–398. doi: 10.1530/REP-10-0077. [DOI] [PubMed] [Google Scholar]
- 5.Block T, El-Osta A. Epigenetic programming, early life nutrition and the risk of metabolic disease. Atherosclerosis. 2017;266:31–40. doi: 10.1016/j.atherosclerosis.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Roberts KA, Riley SC, Reynolds RM, Barr S, Evans M, Statham A, Hor K, Jabbour HN, Norman JE, Denison FC. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32(3):247–254. doi: 10.1016/j.placenta.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Woods L, Perez-Garcia V, Hemberger M. Regulation of placental development and its impact on fetal growth - new insights from mouse models. Front Endocrinol (Lausanne) 2018;9:570–570. doi: 10.3389/fendo.2018.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Ali H, Al-Allaf L. The effect of maternal obesity on the placental histology. Ann Coll Med Mosul. 2020;42(2):148–156. https://mmed.mosuljournals.com/article_167530.html [Google Scholar]
- 9.Jarmuzek P, Wielgos M, Bomba-Opon D. Placental pathologic changes in gestational diabetes mellitus. Neuro Endocrinol Lett. 2015;36(2):101–105. [PubMed] [Google Scholar]
- 10.Altshuler G. Chorangiosis. An important placental sign of neonatal morbidity and mortality. Arch Pathol Lab Med. 1984;108(1):71–74. [PubMed] [Google Scholar]
- 11.Berceanu C, Tetileanu AV, Ofiţeru AM, Brătilă E, Mehedinţu C, Voicu NL, Szasz FA, Berceanu S, Vlădăreanu S, Navolan DB. Morphological and ultrasound findings in the placenta of diabetic pregnancy. Rom J Morphol Embryol. 2018;59(1):175–186. [PubMed] [Google Scholar]
- 12.Tetileanu AV, Berceanu C, Brătilă E, Navolan D, Ciortea R, Berceanu S, Cîrstoiu MM, Ofiţeru AM, Bohîlţea RE, Stepan AE, Mehedinţu C. Morphologic and ultrasound survey in type 2 diabetic placenta. . Gineco.eu. 2018;14(1):5–11. http://gineco.eu/index.php/arhiv/74363 [Google Scholar]
- 13.Istrate-Ofiţeru AM, Berceanu C, Berceanu S, Busuioc CJ, Roşu GC, Diţescu D, Grosu F, Voicu NL. The influence of gestational diabetes mellitus (GDM) and gestational hypertension (GH) on placental morphological changes. Rom J Morphol Embryol. 2020;61(2):371–384. doi: 10.47162/RJME.61.2.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki K, Itoh H, Kimura S, Sugihara K, Yaguchi C, Kobayashi Y, Hirai K, Takeuchi K, Sugimura M, Kanayama N. Chorangiosis and placental oxygenation. Congenit Anom (Kyoto) 2009;49(2):71–76. doi: 10.1111/j.1741-4520.2009.00226.x. [DOI] [PubMed] [Google Scholar]
- 15.Petersen SS, Khangura R, Davydov D, Zhang Z, Sangha R. Placental chorangiosis: increased risk for cesarean section. Case Rep Obstet Gynecol. 2017;2017:5610945–5610945. doi: 10.1155/2017/5610945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redline RW. Classification of placental lesions. Am J Obstet Gynecol. 2015;213(4 Suppl):S21–S28. doi: 10.1016/j.ajog.2015.05.056. [DOI] [PubMed] [Google Scholar]
- 17.HAPO Study Cooperative Research Group Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG. 2010;117(5):575–584. doi: 10.1111/j.1471-0528.2009.02486.x. [DOI] [PubMed] [Google Scholar]
- 18.Loukeris K, Sela R, Baergen RN. Syncytial knots as a reflection of placental maturity: reference values for 20 to 40 weeks’ gestational age. Pediatr Dev Pathol. 2010;13(4):305–309. doi: 10.2350/09-08-0692-OA.1. [DOI] [PubMed] [Google Scholar]
- 19.Novac MV, Niculescu M, Manolea MM, Dijmărescu LA, Iliescu DG, Novac MB, Rotaru LT, Stoenescu M, Tabacu C, Tudorache Ş, Busuioc CJ, Gheonea IA. Placental findings in pregnancies complicated with IUGR – histopathological and immunohistochemical analysis. Rom J Morphol Embryol. 2018;59(3):715–720. [PubMed] [Google Scholar]
- 20.Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol. 2008;61(12):1254–1260. doi: 10.1136/jcp.2008.055236. [DOI] [PubMed] [Google Scholar]
- 21.Berceanu C, Mehedinţu C, Berceanu S, Voicu NL, Brătilă E, Istrate-Ofiţeru AM, Navolan DB, Niculescu M, Szasz FA, Căpitănescu RG, Văduva CC. Morphological and ultrasound findings in multiple pregnancy placentation. Rom J Morphol Embryol. 2018;59(2):435–453. [PubMed] [Google Scholar]
- 22.Voicu NL, Berceanu S, Paitici Ş, Roşu CG, Iovan L, Berceanu C, Bohîlţea RE, Istrate-Ofiţeru AM. Clinical and morphological study of single and twin pregnancies placenta. Curr Health Sci J. 2020;46(1):44–55. doi: 10.12865/CHSJ.46.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton GJ, Woods AW, Jauniaux E, Kingdom JCP. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30(6):473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sankar KD, Bhanu PS, Kiran S, Ramakrishna BA, Shanthi V. Vasculosyncytial membrane in relation to syncytial knots complicates the placenta in preeclampsia: a histomorphometrical study. Anat Cell Biol. 2012;45(2):86–91. doi: 10.5115/acb.2012.45.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Askar E, Selim S, Sibai H. Histological changes of human placenta in early intrauterine growth restriction with and without preeclampsia. J Med Histol. 2019;3(1):65–76. https://jmh.journals.ekb.eg/article_82776.html [Google Scholar]
- 26.Malathi BG, Ashok M. The study on morphology of placenta in gestational diabetes mellitus. IP Arch Cytol Histopathol Res. 2019;4(3):253–258. https://www.achr.co.in/article-details/9806 [Google Scholar]
- 27.Berceanu C, Ciurea EL, Cirstoiu MM, Berceanu S, Ofiteru AM, Mehedintu C, Berbece SI, Ciortea R, Stepan AE, Balseanu TA. Maternal–fetal management in thrombophilia related and placenta-mediated pregnancy complications. Rev Chim (Bucharest) 2018;69(9):2396–2401. https://revistadechimie.ro/Articles.asp?ID=6541 [Google Scholar]
- 28.Voicu NL, Bohîlţea RE, Berceanu S, Busuioc CJ, Roşu GC, Paitici Ş, Istrate-Ofiţeru AM, Berceanu C, Diţescu D. Evaluation of placental vascularization in thrombophilia and intrauterine growth restriction (IUGR) Rom J Morphol Embryol. 2020;61(2):465–476. doi: 10.47162/RJME.61.2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pătru CL, Marinaş MC, Tudorache Ş, Căpitănescu RG, Sîrbu OC, Zorilă GL, Cernea N, Istrate-Ofiţeru AM, Roşu GC, Iovan L, Iliescu DG. The performance of hyperadherence markers in anterior placenta praevia overlying the Caesarean scar. Rom J Morphol Embryol. 2019;60(3):861–867. [PubMed] [Google Scholar]