Abstract
We herein report a 47-year-old man with autoimmune glial fibrillary acidic protein astrocytopathy (GFAP-A) revealed by periventricular radial linear enhancement on repeated brain magnetic resonance imaging (MRI). He presented with a history of headache and a fever followed by somnolence and worsening of consciousness. On admission (16 days from the onset), although lymphocytic pleocytosis and hypoglycorrhachia in the cerebrospinal fluid (CSF) were noted, initial brain MRI demonstrated non-specific findings. At 30 days from the onset, repeated brain MRI revealed characteristic findings of GFAP-A, and we detected anti-GFAP antibodies in the CSF. Thus, repeated brain MRI provides clues for the diagnosis of GFAP-A.
Keywords: autoimmune glial fibrillary acid protein astrocytopathy (GFAP-A), MRI, periventricular radial gadolinium enhancement, meningoencephalitis
Introduction
Autoimmune glial fibrillary acidic protein (GFAP) astrocytopathy (GFAP-A) is an inflammatory disease of the central nervous system confirmed by anti-GFAP α antibodies in the cerebrospinal fluid (CSF) (1,2). GFAP-A patients usually have acute or subacute symptoms indicating infectious meningoencephalitis, such as headache and a fever, followed by meningeal signs, disturbance of consciousness, and psychosis (1,2). A CSF examination reveals lymphocytic pleocytosis with elevated total protein levels and sometimes an elevated adenosine deaminase activity (3). Periventricular radial linear enhancement on brain magnetic resource imaging (MRI) is characteristic of GFAP-A and useful for its diagnosis (3,4).
We herein report an interesting case of GFAP-A revealed by periventricular radial linear enhancement on repeated brain MRI.
Case Report
A healthy right-handed 47-year-old Japanese man was admitted to our hospital for disturbance of consciousness 16 days from the onset. He had a fever, headache, and weariness that did not improve. He visited a medical practitioner, but no influenza antigens or positive findings on polymerase chain reaction (PCR) of severe acute respiratory coronavirus 2 were noted in the nasal cavity. On the day 13 from the onset, he became somnolent, and his consciousness worsened.
On admission, his Glasgow Coma Scale score was E3V3M4. A neurological examination revealed nuchal stiffness, a positive Kernig sign, and urinary retention, but brisk tendon reflexes, pathological reflexes, cerebellar ataxia, and cranial nerve dysfunction were absent. Laboratory examinations revealed hyponatremia and elevated liver enzymes, but normal levels of ammonia, a normal thyroid function, normal glucose values, and normal quantiferon for tuberculosis, along with negative results for anti-nuclear antibodies, serine proteinase 3-anti-neutrophil cytoplasmic antibodies, myeloperoxidase-anti-neutrophil cytoplasmic antibodies, and anti-aquaporin 4 antibodies. The serum antibody titers for viruses, i.e. herpes simplex virus (HSV), varicella zoster virus (VZV), Epstein-Barr virus, and cytomegalovirus, were unremarkable. A CSF analysis revealed a normal opening pressure, pleocytosis (44/μL of monocytes), elevated total protein (140 mg/dL), and hypoglycorrhachia (46 mg/dL; simultaneous blood glucose level of 113 mg/dL). The IgG index was 0.54, and myelin basic protein and adenosine deaminase activity were normal, but the results were positive for intrathecal oligoclonal IgG bands and elevated soluble interleukin-2 receptor (417 IU/mL; that for serum being 211 IU/mL). HSV-PCR, VZV-PCR, and Gram and acid-fast staining and cultures of his CHS were all negative.
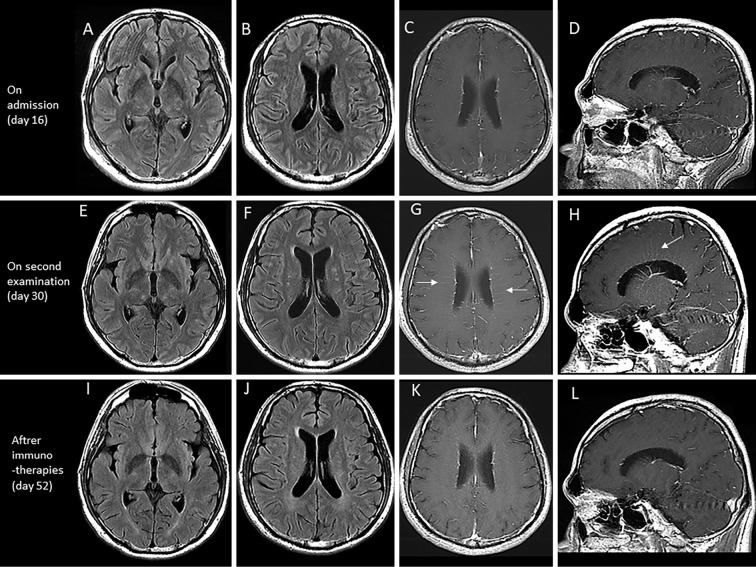
Brain MRI on admission demonstrated only small spotty pale high intensity areas in the white matter on T2 and fluid attenuated inversion recovery (FLAIR) sequences. T1-weighted gadolinium-enhanced MRI revealed only leptomeningeal enhancement (Fig. 1A-D). Spinal MRI was unremarkable. Electroencephalography demonstrated a basic rhythm of 7-8 Hz θ activity without epileptic discharges.
Figure 1.
Brain magnetic resource imaging (MRI) at 16 days (on admission) (A-D), 30 days (two weeks from admission) (E-H), and 52 days (after immunotherapies) (I-L) after the onset. Brain MRI on admission showed high intensity areas on fluid attenuated inversion recovery (FLAIR) imaging (A, B), but no evidence of periventricular gadolinium enhancement on T1 imaging (C, D). After two weeks, brain MRI showed increased high intensity areas on FLAIR imaging (E, F), and periventricular gadolinium enhancement (arrows) on T1 imaging (G, H). Brain MRI after immunotherapies showed that the spotty lesions had become paler (I, J), and there was a reduction in the enhancement (K, L).
Since we were unable to rule out the possibility of HSV encephalitis, we treated him with intravenous acyclovir (10 mg/kg, every 8 h) and intravenous methylprednisolone (1,000 mg/day, for 3 days). His consciousness improved slightly, but he was still disoriented and apathetic and needed some support or care for his activities of daily life, such as eating or going to the toilet. The results of higher-brain function tests, including the Revised Hasegawa Dementia Scale (HDS-R), Mini-Mental State Examination (MMSE), Frontal Assessment Battery (FAB), and Japanese version of Trail Making Test Parts A and B (J-TMT Parts A and B), are shown in the Table.
Table.
Results of Higher-brain Function Tests.
| Pre-immunotherapy | Post-immunotherapy | |||
|---|---|---|---|---|
| HDS-R (/30) | 20 | 28 | ||
| MMSE (/30) | 25 | 29 | ||
| FAB (/18) | 12 | 17 | ||
| TMT-J Part A (s) | 69 | 26 | ||
| TMT-J Part B (s) | 201 | 76 |
HDS-R: revised Hasegawa dementia scale, MMSE: mini-mental state examination, FAB: frontal assessment battery, J-TMT: Japanese version of trail making test
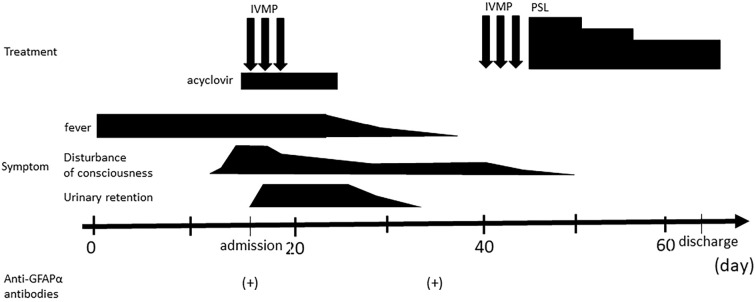
Second brain MRI performed 30 days from the onset revealed periventricular radial linear enhancement on T1-gadolinium sequence in addition to spotty, pale lesions on T2 and FLAIR sequences (Fig. 1E-H). We suspected a diagnosis of GFAP-A and examined anti-GFAP α antibodies in CSF samples obtained on admission and on day 14 of hospitalization. As a result, anti-GFAP α antibodies were detected in both samples, so we diagnosed him with GFAP-A (Fig. 2).
Figure 2.
Clinical course of the present case. IVMP: intravenous methylprednisolone, PSL: prednisolone
We again administered intravenous methylprednisolone (1,000 mg/day, for 3 days) and oral prednisolone (50 mg/day, followed by tapering and maintenance at 30 mg/day). His symptoms improved remarkably, and he became independent in activities of daily life. His disorientation gradually improved (Fig. 2), as did his higher-brain function test results (Table). Follow-up MRI showed a reduction in the characteristic enhancement for GFAP-A (Fig. 1I-L). A CSF examination showed decreased cell counts (16/μL) and normal protein levels (42 mg/dL).
Discussion
We encountered a Japanese patient with periventricular linear gadolinium enhancement revealed by repeated brain MRI. Regarding brain MRI, almost half of patients with GFAP-A reportedly showed abnormal periventricular hyperintensity lesions on T2-weighted and FLAIR images, and a T1-gadolinium sequence revealed periventricular linear radial enhancement (3-5). This sign is a hallmark of GFAP-A, being seen in almost half of patients with GFAP-A (3,4), but the ideal timing of MRI has not been explored in detail. Although how this sign arises remains unclear, neuropathological examination of some autopsy cases of GFAP-A have revealed extensive inflammation around the vessels and microglial activation in their brains, and the marked inflammatory responses in perivascular regions were consistent with periventricular linear radial gadolinium enhancement (6). The brain MRI findings on admission were unremarkable, but a re-examination performed two weeks later, after the first intravenous methylprednisolone pulse therapy, showed the characteristic sign of GFAP-A. This phenomenon suggests that the advanced perivascular inflammation in his brain may have been superior to immunotherapy at that time. This finding led us to examine for GFAP α antibodies in his CSF. Thus, repeated brain MRI is required for the diagnosis of GFAP-A.
Patients with GFAP-A often have flu-like symptoms and antecedent meningoencephalitis-like symptoms (7). A few patients with GFAP-A that developed after viral encephalitis (4,8) have been reported. On admission, our patient was positive for anti-GFAP α antibodies in the CSF without characteristic brain MRI findings or positive results for viral antibodies or PCR findings for viruses. These findings suggest that viral meningoencephalitis might not have preceded GFAP-A in this case.
Periventricular linear gadolinium enhancement is not always seen in GFAP-A patients, as in the initial MRI findings in our patient. If MRI does not show any abnormal findings, the detection of GFAP α antibodies in the CSF with a cell-based assay involving rat frozen brain sections is another valuable tool for making a diagnosis of GFAP-A (1,3). Since we did not strongly suspect that he had GFAP-A at first, we did not check for antibodies on admission. Previously, a 31-year-old woman who had been receiving oral corticosteroids for 7 years, and initially been diagnosed with chronic lymphocytic inflammation with pontine perivascular enhancement-responsive lesions was reevaluated at the time of the seventh relapse, when haracteristic gadolinium enhancement of GFAP-A was first observed (9). Furthermore, a rapidly progressive demented 54-year-old man whose symptoms mimicked Creuzfeldt-Jakob disease was confirmed to have GFAP-A at six months from the first brain MRI (10). These patients were similar to our own in that characteristic enhancement was revealed after initially unremarkable MRI findings, but their clinical courses involved relapse and remission or were insidious. Some patients with GFAP-A experience relapses (9) or chronic progression (10,11), but this is not common for GFAP-A. Our patient showed an acute meningoencephalitis-like disease course, which is representative of GFAP-A, and characteristic enhancement was observed even after intravenous methylprednisolone therapy in the acute phase. Subsequent brain MRI with gadolinium led to the correct diagnosis of GFAP-A. Thus, repeated brain MRI provides clues for the diagnosis of GFAP-A.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Fang B, McKeon A, Hinson SR, et al. Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol 73: 1297-1307, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Flaganan EP, Hinson SR, Lennon VA, et al. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: analysis of 102 patients. Ann Neurol 82: 298-309, 2017. [DOI] [PubMed] [Google Scholar]
- 3. Kimura A, Takekoshi A, Yoshikura N, Hayashi Y, Shimohata T. Clinical characteristics of autoimmune GFAP astrocytopathy. J Neuroimmunol 332: 91-98, 2019. [DOI] [PubMed] [Google Scholar]
- 4. Dubey D, Hinson SR, Jolliffe EA, et al. Autoimmune GFAP astrocytopathy: prospective evaluation of 90 patients in 1 year. J Neuroimmunol 321: 157-163, 2018. [DOI] [PubMed] [Google Scholar]
- 5. Xiao J, Chen X, Shang K, et al. Clinical, neuroradiological, diagnostic and prognostic profile of autoimmune glial fibrillary acidic protein astrocytopathy: a pooled analysis of 324 cases from published data and a single-center retrospective study. J Neuroimmunol 360: 577718, 2021. [DOI] [PubMed] [Google Scholar]
- 6. Long Y, Liang J, Xu H, et al. Autoimmune glial fibrillary acidic protein astrocytopathy in Chinese patients: a retrospective study. Eur J Neurol 25: 477-483, 2018. [DOI] [PubMed] [Google Scholar]
- 7. Kunchok A, Zekeridou A, McKeon A. Autoimmune glial fibrillary acidic protein astrocytopathy. Curr Opin Neurol 32: 452-458, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li J, Xu Y, Ren H, Zhu Y, Peng B, Cui L. Autoimmune GFAP astrocytopathy after viral encephalitis: a case report. Mult Scler Relat Disord 21: 84-87, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Yin HX, Zhou Y, Xu Y, et al. A case report of autoimmune glial fibrillary acidic protein astrocytopathy diagnosed after long term diagnosis of chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids. Front Neurol 11: e598650, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toledano-Illán C, Esparragosa Vázquez I, Zelaya Huerta MV, et al. Autoimmune glial fibrillary acidic protein astrocytopathy: case report of a treatable cause of rapidly progressive dementia. J Neurol 268: 2256-2258, 2021. [DOI] [PubMed] [Google Scholar]
- 11. Natori T, Shindo K, Okumura A, Kimura A, Takiyama Y. A treatable case of autoimmune GFAP astrocytopathy presenting chronic progressive cognitive impairment. Neurol Sci 41: 2999-3002, 2020. [DOI] [PubMed] [Google Scholar]