Abstract
The patient was a 34-year-old woman who suddenly collapsed. On arrival, she was in cardiac arrest. Cardiac ultrasound revealed cardiac tamponade; thus, urgent thoracotomy with pericardiotomy was performed. Spontaneous circulation was temporarily obtained; however, her circulation was not stabilized, and she ultimately died. An autopsy revealed a pericardial inflammatory myofibroblastic tumor (IMT) causing bloody cardiac tamponade. There were no signs of cardiac rupture, myocardial infarction or aortic dissection. We reported the first case of fatal bloody cardiac tamponade due to pericardial IMT in an adult. An autopsy is important for clarifying the etiology in cases of fatal cardiac tamponade of unknown cause.
Keywords: cardiac tamponade, pericardium, inflammatory myofibroblastic tumor
Introduction
Cardiac tamponade is caused by an abnormal increase in fluid accumulation in the pericardial sac, which, by raising intracardiac pressures, impedes normal cardiac filling and reduces cardiac output, sometimes to a dramatic extent (1). The etiologies of cardiac tamponade include malignancy, uremia, iatrogenic, myocardial infarction, aortic dissection, infection, collagen vascular, and hypothyroidism; however, idiopathic cases also occur. The etiologies of bloody cardiac tamponade have included aortic dissection, rupture of myocardial infarction, malignancy, iatrogenic, and drug usage (2-5).
Inflammatory myofibroblastic tumor (IMT) is a rare mesenchymal neoplasm of intermediate biological potential with a predilection for the lung and abdominopelvic region, although it can be found in almost all parts of the human body (6,7). IMT represents the neoplastic subset of the family of inflammatory pseudotumors, an umbrella term for spindle cell proliferations of uncertain histogenesis with a variable inflammatory component. Immunohistochemistry demonstrates myofibroblastic differentiation (6). The tumors are associated with a benign behavior, but recurrence is detected in approximately one-third of abdominal cases (6).
We herein report a case of fatal cardiac tamponade due to pericardial IMT.
Case Report
The patient was a 34-year-old woman who suddenly collapsed while walking home from a train station. A witness called an ambulance. She had no relevant history. Her mother had diabetes mellitus and a history of myocardial infarction. When emergency medical technicians checked her, she had consciousness disturbance with urinary incontinence and was complaining of dyspnea. Immediately after being transferred into the ambulance, she became restless and then experienced cardiac arrest. The initial rhythm was bradycardiac pulseless activity, but this soon changed to asystole. She received basic life support with oxygen and was transported to our hospital 10 minutes after cardiac arrest.
On arrival, she remained in cardiac arrest. She underwent tracheal intubation, then a venous route was secured, and infusion of adrenaline was initiated. Cardiac ultrasound revealed cardiac tamponade (Fig. 1). She underwent urgent thoracotomy with pericardiotomy. After the patient temporarily obtained spontaneous circulation, computed tomography (CT) was performed to investigate the cause of cardiac tamponade. CT failed to show the etiology of cardiac tamponade, but remaining hematoma was recognized (Fig. 2). After returning to the emergency room, the pericardiotomy was extended, which resulted in the drainage of massive hematoma. However, her circulation was not stabilized, even with the use of transfusion and vasopressor, and she ultimately died.
Figure 1.
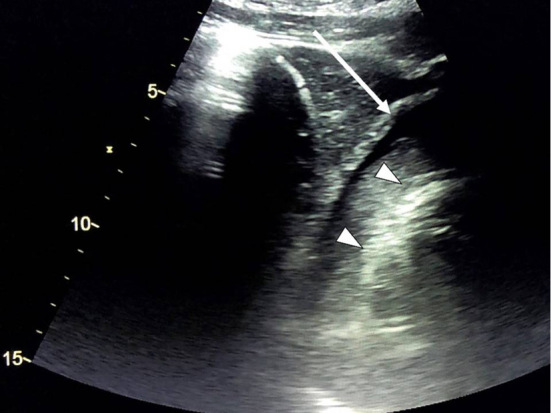
Cardiac ultrasound on arrival. Cardiac ultrasound revealed cardiac tamponade (arrow). Triangles indicate the outline of the cardiac wall. The tamponade consisted of two layers: an outer low-echoic layer and an inner high-echoic layer.
Figure 2.
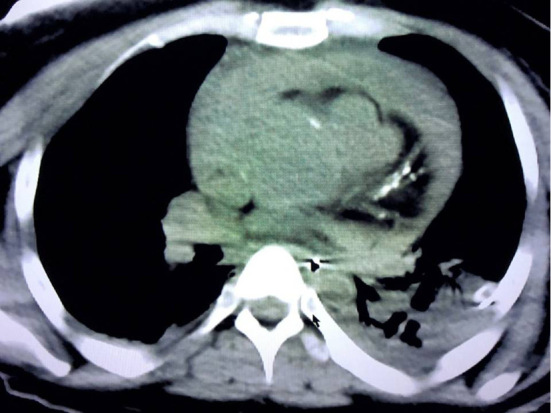
Computed tomography (CT) after obtaining spontaneous circulation. CT demonstrated residual cardiac tamponade.
The patient's blood test results are shown in Table. The estimated cardiac arrest time was relatively short (10 minutes); however, she had severe metabolic acidosis and hyperglycemia. Therefore, she might have already been in a state of diabetic ketoacidosis before collapse. An autopsy revealed pericardial IMT causing bloody cardiac tamponade (Fig. 3, 4). There were no signs of cardiac rupture, myocardial infarction, or aortic dissection.
Table.
Laboratory Data at the Time of Deterioration.
| pH | 6.84 | ||
| Base excess | -26.2 | mmol/L | |
| Lactate | 19 | mmol/L | |
| White blood cell | 10,100 | /μL | |
| Hemoglobin | 13.0 | g/dL | |
| Platelet | 35.6×104 | /μL | |
| Total protein | 6.8 | g/dL | |
| Albumin | 4.1 | g/dL | |
| Total bilirubin | 0.7 | mg/dL | |
| Aspartate aminotransferase | 45 | IU/L | |
| Alanine aminotransferase | 59 | IU/L | |
| Alkaline phosphatase | 167 | IU/L | |
| γ-glutamyltransferase | 24 | IU/L | |
| Lactate dehydrogenase | 212 | IU/L | |
| Amylase | 83 | IU/L | |
| Blood urea nitrogen | 11.8 | mg/dL | |
| Creatinine | 0.90 | mg/dL | |
| Creatine kinase | 54 | IU/L | |
| Glucose | 424 | mg/dL | |
| Sodium | 141 | mEq/L | |
| Potassium | 5.1 | mEq/L | |
| Chloride | 105 | mEq/L | |
| C-reactive protein | 0.58 | mg/dL | |
| Prothrombin time | 14.1 (11.9) | s | |
| Activated partial thromboplastin time | 26.5 (27.5) | s | |
| Fibrinogen degradation products | 15.2 | μg/mL |
Figure 3.
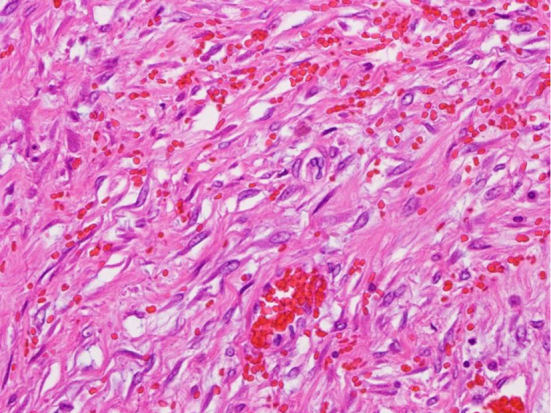
A histopathological examination of the pericardium (Hematoxylin and Eosin staining ×400). Infiltration with spindle cells and lymphocytes with hemorrhaging was observed from the pericardium to the origin of the aorta.
Figure 4.
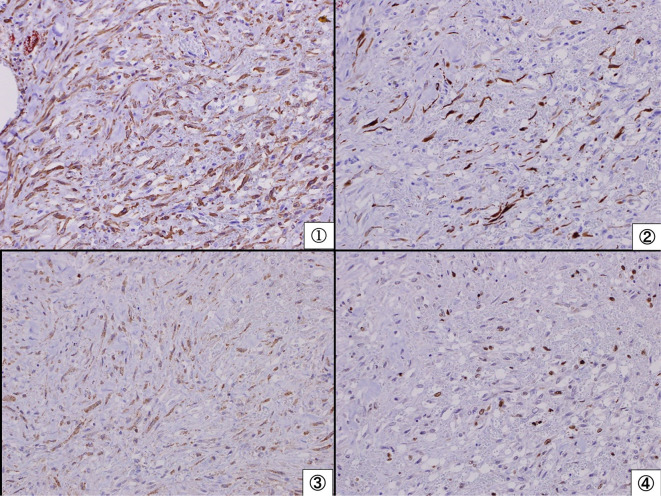
Results of an immunohistochemical examination of the pericardium. Spindle cells were positive for smooth muscle actin (①), desmin (②), anaplastic lymphoma kinase (③), and Ki67 (④) and negative for CD31/CD34. These results did not contradict a diagnosis of an inflammatory myofibroblastic tumor.
Discussion
We reported a case of fatal cardiac tamponade due to pericardial IMT in an adult patient. We performed a PubMed search to identify any related articles using the key words “inflammatory myofibroblastic tumor” and “cardiac tamponade.” We only found one case report by Agarwala et al.; however, in that case, the cause of tamponade was pus due to purulent pericarditis, not IMT (8). An additional article reported by Blanco et al. was identified by a manual search (9). Their 18-year-old patient showed cardiac tamponade with pain, a fever, leukocytosis, elevated cardiac enzymes, and second-degree atrioventricular block, requiring subxifoid pericardiocentesis, which resulted in drainage of a cloudy liquid. A survival outcome was achieved with anti-inflammatory treatment. Accordingly, ours is the first case of fatal bloody cardiac tamponade due to pericardial IMT in an adult patient.
Among reports of IMT of the heart, Eilers et al. recently reviewed the largest number of patients (n=57) (10-12). In their report, the average age was 19 years old (range, 1 day to 72 years old) (10). Furthermore, cardiac IMT, occurred more frequently in pediatric patients (12 months to 18 years old, 38.2%) than in adults (>18 years old, 34.2%) or infants (<12 months old, 27.3%) (10). A female predominance was also demonstrated (54.6% vs. 45.4%) (10). When the location of cardiac IMT was evaluated, 56.4% were located on the right side of the heart, 32.7% were located on the left side, and 10.9% involved both right- and left-sided structures (10). Achouh et al. reported an asymptomatic case of pericardial IMT compressing the coronary artery; thus, pericardial-origin IMT is also extremely rare (13). The most common single clinical presentation was cardiac (19.3%), which included chest pain, heart failure, myocardial ischemia, murmur, and/or arrhythmia (10). A constitutional presentation (a fever, lethargy, weight loss, malaise, myalgia, and/or decreased appetite) alone or in combination with another presentation occurred most frequently (32.7%) (9). We were unable to investigate any complaints before collapse in the present case. Previous reports showed six cases of sudden death associated with cardiac IMT (14-19). Among them, five cases were caused by myocardial ischemia because of occlusion of the coronary arteries by the IMT. In the remaining case, the IMT obstructed the outflow tract of the left ventricle, resulting in circulatory failure.
Bloody cardiac tamponade by an IMT, as was observed in the present case, has not been previously reported. Neither autopsy imaging nor blood biochemistry was useful for clarifying the cause of sudden death in the present case. Accordingly, a conventional autopsy is still important for clarifying the etiology in cases of fatal cardiac tamponade of unknown cause.
Conclusion
We reported the first case of fatal bloody cardiac tamponade due to pericardial IMT in an adult patient. An autopsy is important for clarifying the etiology of fatal cardiac tamponade of unknown cause.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported in part by a Grant-in-Aid for Special Research in Subsidies for ordinary expenses of private schools from The Promotion and Mutual Aid Corporation for Private Schools of Japan. (No grant number)
References
- 1. Appleton C, Gillam L, Koulogiannis K. Cardiac tamponade. Cardiol Clin 35: 525-537, 2017. [DOI] [PubMed] [Google Scholar]
- 2. Hoit BD. Pericardial effusion and cardiac tamponade in the new millennium. Curr Cardiol Rep 19: 57, 2017. [DOI] [PubMed] [Google Scholar]
- 3. Asad ZUA, Ijaz SH, Chaudhary AMD, Khan SU, Pakala A. Hemorrhagic cardiac tamponade associated with apixaban: a case report and systematic review of literature. Cardiovasc Revasc Med 20: 15-20, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Čanádyová J, Zmeko D, Mokráček A. Re-exploration for bleeding or tamponade after cardiac operation. Interact Cardiovasc Thorac Surg 14: 704-707, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yanagawa Y, Jitsuiki K, Ota S, et al. Significance of medical intervention for non-traumatic hemorrhagic cardiac tamponade. Am J Emerg Med 50: 636-639, 2021. [DOI] [PubMed] [Google Scholar]
- 6. Surabhi VR, Chua S, Patel RP, Takahashi N, Lalwani N, Prasad SR. Inflammatory myofibroblastic tumors: current update. Radiol Clin North Am 54: 553-563, 2016. [DOI] [PubMed] [Google Scholar]
- 7. Leuschner I. Inflammatory myofibroblastic tumor. Pathologe 31: 106-108, 2010(in German). [DOI] [PubMed] [Google Scholar]
- 8. Agarwala BN, Ruschhaupt DG, Sand ME. Pseudotumor of the pericardium. Pediatr Cardiol 18: 429-431, 1997. [DOI] [PubMed] [Google Scholar]
- 9. Blanco M, Fulquet E, Laguna G, et al. Cardiac inflammatory myofibroblastic tumor in a young male patient with myopericarditis. Circulation 132: e386-e387, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Eilers AL, Nazarullah AN, Shipper ES, Jagirdar JS, Calhoon JH, Husain SA. Cardiac inflammatory myofibroblastic tumor: a comprehensive review of the literature. World J Pediatr Congenit Heart Surg 5: 556-564, 2014. [DOI] [PubMed] [Google Scholar]
- 11. Kato T, Tomita S, Tamaki M, Yutani C, Okawa Y. Inflammatory myofibroblastic tumor of the heart. Heart Vessels 29: 123-128, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Mizia-Malarz A, Sobol-Milejska G, Buchwald J, Woś H. Inflammatory myofibroblastic tumor of the heart in the infant: review of the literature. J Pediatr Hematol Oncol 38: e298-e230, 2016. [DOI] [PubMed] [Google Scholar]
- 13. Achouh PE, Zegdi R, Azarine A, Fabiani JN. Cardiac epicardial tumor with coronary artery compression. Eur J Cardiothorac Surg 35: 362, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Suzuki S, Ohtani M, Matsuo Y, et al. A forensic autopsy case: sudden unexpected death due to cardiac inflammatory myofibroblastic tumor. Leg Med (Tokyo) 53: 101931, 2021. [DOI] [PubMed] [Google Scholar]
- 15. Li L, Burke A, He J, Chang L, Zielke HR, Fowler DR. Sudden unexpected death due to inflammatory myofibroblastic tumor of the heart: a case report and review of the literature. Int J Legal Med 125: 81-85, 2011. [DOI] [PubMed] [Google Scholar]
- 16. Burke A, Li L, Kling E, Kutys R, Virmani R, Miettinen M. Cardiac inflammatory myofibroblastic tumor: a “benign” neoplasmthat may result in syncope, myocardial infarction, and sudden death. Am J Surg Pathol 31: 1115-1122, 2017. [DOI] [PubMed] [Google Scholar]
- 17. Dickens P, Lam AK. Sudden death associated with cardiac inflammatory pseudotumour. Forensic Sci Int 49: 89-93, 1991. [DOI] [PubMed] [Google Scholar]
- 18. Rose AG, McCormick S, Cooper K, Titus JL. Inflammatory pseudotumor (plasma cell granuloma) of the heart. Report of two cases and literature review. Arch Pathol Lab Med 120: 549-554, 1996. [PubMed] [Google Scholar]
- 19. Xu B, Fraser RS, Renaud C, Youssef S, Gottesman RD, Bernard C. Inflammatory myofibroblastic tumor of the aortic valves causing sudden cardiac death: a case report and review of the literature. Pediatr Dev Pathol 17: 231-239, 2014. [DOI] [PubMed] [Google Scholar]