Abstract
Immune checkpoint inhibitors (ICIs) are complicated by immune-related adverse events (irAEs), such as myositis, myocarditis, and myasthenia gravis (MG). Anti-titin antibody and anti-voltage-gated potassium channel Kv1.4 antibody are anti-striated antibodies that are frequently detected in MG patients with myositis and/or myocarditis. However, the clinical relationship between positive anti-striated antibodies and irAEs of ICIs remains unknown. We herein report a case of nivolumab-induced myositis and myocarditis with positive anti-titin antibody and anti-voltage-gated potassium channel Kv1.4 antibody in a patient with non-small-cell lung cancer. We also review reported cases of positive anti-striated antibodies related to irAEs of ICIs.
Keywords: anti-striated antibody, anti-titin antibody, anti-voltage-gated potassium channel Kv1.4 antibody, immune checkpoint inhibitors, immune-related adverse events, nivolumab
Introduction
Immune checkpoint inhibitors (ICIs) represent a novel therapeutic approach to treat unresectable tumors (1). Programmed cell death protein 1 (PD-1) inhibitors (nivolumab and pembrolizumab) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) inhibitors (ipilimumab) are effective in treating melanoma, non-small-cell lung cancer, renal cell carcinoma, and other malignancies (2). However, ICIs may be complicated by a unique spectrum of immune-related adverse events (irAEs), such as myositis, myocarditis, and various neurological irAEs that involve the central and peripheral nervous systems, including polyneuropathy and myasthenia gravis (MG), predominantly after treatment with nivolumab (3).
Anti-titin antibody and anti-voltage-gated potassium channel Kv1.4 antibody are anti-striated antibodies that are frequently detected in MG patients with myositis and/or myocarditis. In addition to anti-acetylcholine receptor (AChR) antibodies, antibodies that can recognize other components of the skeletal muscle and yield striational immunostaining have long been detected in patients with MG (4). Anti-voltage-gated potassium channel Kv1.4 antibody occurred most frequently among patients with severe MG suffering from bulbar symptoms, myasthenic crisis, thymoma, myocarditis, and prolonged QT time on electrocardiogram (ECG) registration (5). A previous study showed that MG patients positive for both antibodies had more severe manifestations and more frequent concomitant myocarditis and/or myositis than MG patients with positivity for anti-titin antibodies only (6). However, the clinical relationship between positive anti-striated antibodies and irAEs of ICIs remains unknown due to its rarity. To date, only nine cases of positive anti-striated antibodies associated with irAEs of ICIs have been reported (7-15).
We herein report a case of nivolumab-induced myositis, myocarditis, and myasthenia-like syndrome with positive anti-titin antibody and anti-voltage-gated potassium channel Kv1.4 antibody in a patient with non-small-cell lung cancer. We also review the literature on cases of positive anti-striated antibodies related to irAEs of ICIs.
Case Report
A 77-year-old Japanese woman with an Eastern Cooperative Oncology Group performance status 1 and who was treated with nivolumab as second-line therapy for advanced non-small-cell lung cancer, presented with progressive myalgia, ophthalmoplegia, and blepharoptosis 1 day after the second infusion of nivolumab (2 mg/kg, every 2 weeks). She had undergone resection of the right middle lung lobe for lung adenocarcinoma (clinical T1aN0M0, clinical stage IA) two years ago. However, pulmonary metastasis was noted during follow-up one year after the operation. She subsequently underwent five cycles of pemetrexed therapy. After a response evaluation, the patient was found to have progressive disease; thus, we switched to nivolumab as second-line therapy. She had no symptoms after the first infusion of nivolumab, but she noticed progressive myalgia, ophthalmoplegia, and blepharoptosis one day after the second infusion. Therefore, she decided to visit our hospital four days after the infusion. She denied any chest pain and dyspnea. The patient had no history of autoimmune disorders or cardiac risk factors. Her family history was unremarkable. She had no history of smoking or alcohol consumption. She was taking codeine phosphate for a cough.
On presentation, her Glasgow Coma Score was 15. Her body mass index was 16.4 kg/m2, with no noticeable body weight changes. She had a slight fever of 37.1°C, but her other vital signs were stable (blood pressure, 120/76 mmHg; pulse, 76/min; respiratory rate, 18/min; oxygen saturation, 96%). A physical examination showed bilateral blepharoptosis (left-side dominant) and limitation of ocular movement. She was capable of slightly moving her eyes to the left side, up, and down, but could not move them to the right side. Her visual acuity was 0.9 on the right side and 0.8 on the left side. Her manual muscle test was 4/5 at the neck, bilateral arms, hip, and proximal muscles. No murmurs or crackles were observed.
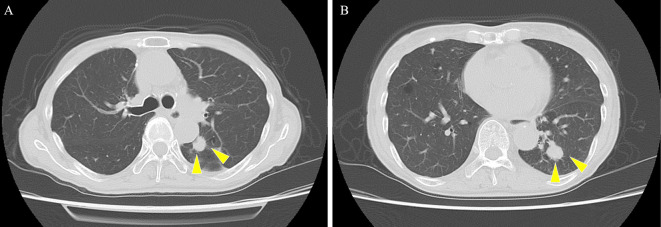
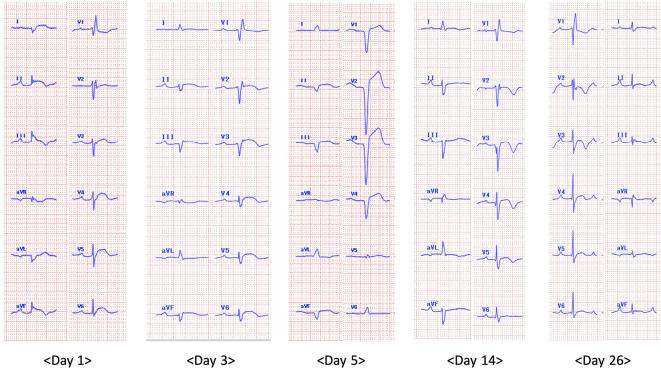
Chest radiography showed a tumor mass in the left lower lung field, and the cardiothoracic ratio was 51% without pleural effusion. Chest computed tomography demonstrated tumor masses (14 mm at the left S1+2 and 16 mm at the left S9), with no thymoma or interstitial pneumonia noted (Fig. 1). An ECG showed a bifascicular block (first-degree atrioventricular block and complete right bundle branch block) and ST-segment elevation in leads I, II, III, aVF, V3, V4, V5, and V6 without reciprocal ST-segment depression (Fig. 2). Transthoracic echocardiography showed a preserved ejection fraction without asynergy, and no pericardial effusion or obvious myocardial edema was noted.
Figure 1.
Chest computed tomography showing tumor masses of 14 mm at the left S1+2 (A) and 16 mm at the left S9 (B).
Figure 2.
Time-course changes of an electrocardiogram (ECG) after admission. The ECG on day 1 showing bifascicular block (first-degree atrioventricular block and complete right bundle branch block) and ST-segment elevation in leads I, II, III, aVF, V3, V4, V5 and V6 without reciprocal ST-segment depression. The ECG follow-up on day 3 showing trifascicular block (first-degree atrioventricular block, complete right bundle branch block, and left anterior hemiblock). The ECG on day 5 showing progression to atrioventricular dissociation. The ECG on day 14 showing the recovering trifascicular block, which transformed into a bifascicular block on day 26.
Laboratory data showed elevated levels of aspartate aminotransferase (757 U/L, normal range; 0-31 U/L), alanine aminotransferase (225 U/L, normal range; 0-41 U/L), lactate dehydrogenase (1,634 U/L, normal range; 120-240 U/L), C-reactive protein (1.21 mg/dL, normal range; 0-30 mg/dL), brain natriuretic peptide (88.1 pg/mL, normal range; 0-18.4 pg/mL), creatine kinase (CK) (11,297 U/L, normal range; 32-187 U/L), CK-MB (204 U/L, normal range; 0-12 U/L), and troponin T (1,478 ng/L, normal range; 0-14 ng/L). Thyroid functions [thyroid-stimulating hormone of 1.572 μIU/mL (normal range; 0.35-4.94 μIU/mL) and free thyroxine of 1.03 ng/dL (normal range; 0.70-1.48 ng/dL) and thyroid stimulating hormone receptor antibody (0.423 U/L, normal range; <2.0 U/L)] were within normal. Arterial blood gas on room air revealed a pH of 7.475, pCO2 of 39.6 mmHg, pO2 of 87.5 mmHg, and HCO3- of 28.5 mEq/L. She underwent coronary angiography, but no clinically significant stenosis of the coronary artery was observed. Therefore, we clinically diagnosed the patient with autoimmune myocarditis induced by nivolumab.
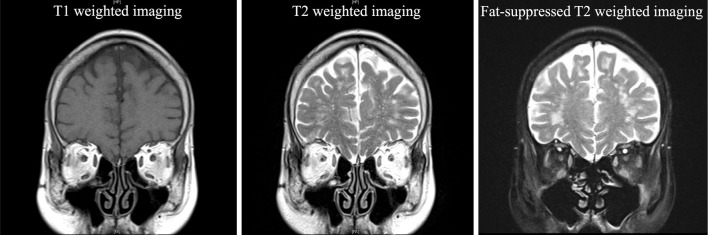
After admission, she underwent head magnetic resonance imaging, which revealed a high signal in the extraocular muscles on fat-suppressed T2-weighted imaging (Fig. 3). We first suspected MG induced by nivolumab since she felt muscular fatigability; however, the degree of blepharoptosis and diplopia did not show diurnal variation, and fluctuating muscle weakness was unclear. In addition, the ice pack test, anti-AChR antibody test, and anti-muscle-specific kinase (MuSK) antibody tests were negative. All myositis antibodies were undetected. Furthermore, a repetitive nerve stimulation test of the left facial nerve did not reveal a waning pattern. However, anti-titin antibody and anti-voltage-gated potassium channel Kv1.4 antibody were positive. Thus, we were able to diagnose the patient with nivolumab-induced ocular myositis.
Figure 3.
Magnetic resonance imaging revealing a high signal in the extraocular muscles on fat-suppressed T2-weighted imaging.
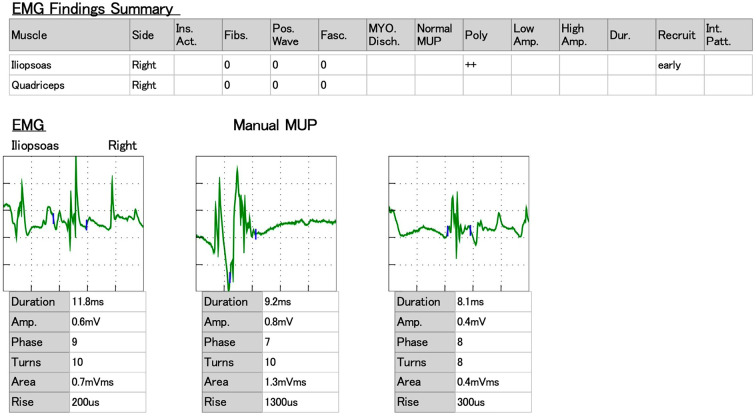
In addition, magnetic resonance imaging of the lower extremity showed a high signal on the femoral muscle in short tau inversion recovery imaging (Fig. 4). Furthermore, needle electromyography (EMG) of the iliopsoas and quadriceps muscle revealed no fibrillation potential but pathological recruitment from the iliopsoas muscle (Fig. 5). These findings also suggested myositis. Pulmonary function tests revealed restrictive ventilatory impairment with %VC of 73.6% and FEV1.0 of 92.9% on admission.
Figure 4.
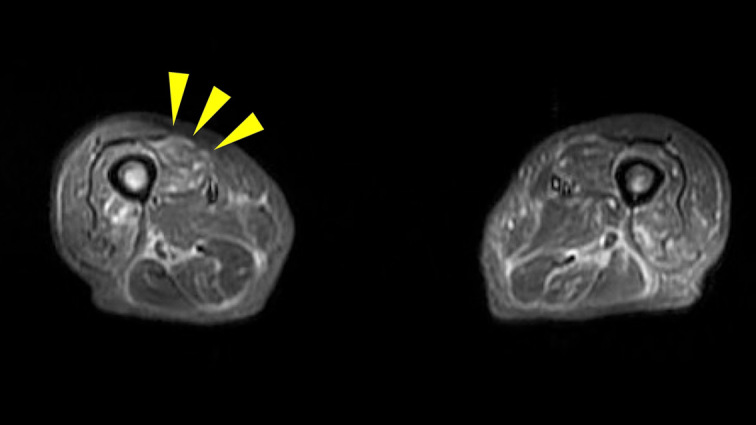
Magnetic resonance imaging of the lower extremity showing a high signal in the femoral muscle (arrowheads) on short tau inversion recovery imaging.
Figure 5.
Needle electromyography (EMG) findings of the iliopsoas muscle revealing pathological recruitment.
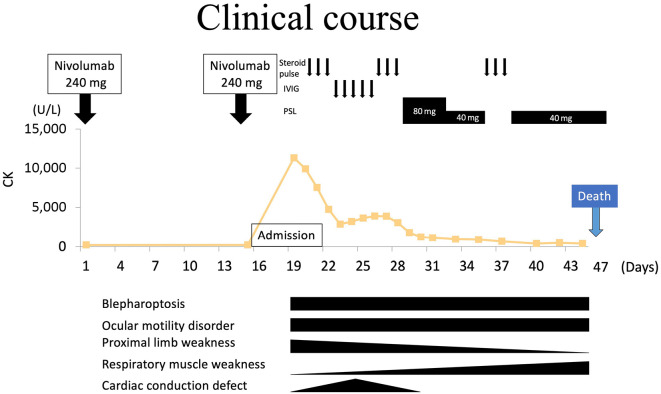
The clinical course is shown in Fig. 6. She was given a steroid pulse of 1 g methylprednisolone for 3 consecutive days, followed by intravenous injection of immunoglobulin (0.4 g/kg/day) for five consecutive days. The serum CK level had improved to 2,896 U/L on day 5 after admission but proceeded to decrease again. ECG follow-up showed trifascicular block (first-degree atrioventricular block, complete right bundle branch block, and left anterior hemiblock) on day 3, which progressed to atrioventricular dissociation on day 5; thus, a temporary pacemaker was inserted (Fig. 2). She received a second cycle of steroid pulse from days 9 to 11, and her proximal limb weakness and cardiac conduction defect gradually improved; however, blepharoptosis and ocular motility disorder persisted. The temporary pacemaker was removed on day 13 owing to the improvement of the atrioventricular dissociation. She continued taking 80 mg/day of prednisolone, which was eventually tapered to 40 mg/day; however, her respiratory muscle weakness gradually worsened. A pulmonary function test on day 19 revealed progression of restrictive ventilatory impairment with a %VC of 21.9% and FEV1.0 of 87.0%. She was administered steroid pulse again and underwent respiratory support. The patient passed away on day 29 after admission due to respiratory failure.
Figure 6.
Clinical course of the patient. CK: creatine kinase, IVIG: intravenous immunoglobulin, PSL: prednisolone
Discussion
ICIs are associated with a unique spectrum of side effects linked to irAEs, and ICI-induced myopathy has been reported to overlap with MG-like symptoms (16). Although anti-striated antibodies, which react with epitopes on the muscle proteins titin, ryanodine receptor, and Kv1.4, are frequently detected in MG patients, positive anti-striated antibodies associated with irAEs of ICIs have rarely been reported (17). Recently, anti-striated antibodies have been detected in the serum of these patients and are expected to be serological markers of serious irAEs (18). Therefore, the major clinical features of the nine previously reported cases of positive anti-striated antibodies related to irAEs of ICIs in our case are summarized in Table (7-15).
Table.
Literature Review of Cases with Positive Anti-striated Antibody Related to Immune-related Adverse Events of ICIs.
| Case | (Reference) | Age | Sex | Initial presentation | Type of cancer | Type of ICI | Cycles of ICIs | Anti-AChR antibody | Type of anti-striated antibody | Thymoma | Peak CK (U/L) | Myositis | Myasthenia-like syndrome | Myocarditis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | (7) | 73 | M | Lower back pain, profound weakness, ptosis, and extraocular muscle weakness | Transitional cell cancer of the renal pelvis | Ipilimumab/ Nivolumab |
2 | No | ND | ND | 13,710 | Yes | Yes | No | Steroid pulse, IVIG, PSL, plasmapheresis, infliximab | Died at 4 months |
| 2 | (8) | 49 | F | Nausea | Melanoma | Ipilimumab/ Nivolumab |
1 | ND | ND | ND | 335 | No | No | Yes | PSL, IVIG | Alive at 275 days |
| 3 | (9) | 87 | M | Double vision, ptosis, muscle weakness, and nasal voice | Urothelial cancer | Atezolizumab | 2 | Yes | ND | No | 1,542 | Yes | Yes | Yes | PSL, IVIG, pyridostigmine | Died at 3 days |
| 4 | (10) | 78 | M | Dyspnea and mild weakness of the neck and upper limbs | Bladder cancer | Pembrolizumab | 1 | Yes | Aanti-titin and anti-Kv1.4 | No | 2,015 | Yes | Yes | No | Steroid pulse, PSL, IVIG | Alive at 5 months |
| 5 | (11) | 78 | F | Diplopia, muscle weakness, and myalgias | Melanoma | Ipilimumab/ Nivolumab |
1 | ND | ND | ND | 9,198 | Yes | Yes | Yes | Steroid pulse, IVIG, plasmapheresis | ND in detail (palliative care) |
| 6 | (12) | 76 | F | General fatigue | Urothelial cancer | Pembrolizumab | 3 | No | Anti-titin | No | 3,527 | Yes | Yes | Yes | PSL, IVIG | Alive at 3 months |
| 7 | (13) | 73 | M | Diplopia | Lung adenocarcinoma | Pembrolizumab | 1 | No | Anti-titin | ND | 7,311 | Yes | Yes | No | Steroid pulse, PSL | Alive at 4 months |
| 8 | (14) | 86 | M | Decreased vision, fatigue, and lower back and bilateral hip pain | Cutaneous squamous cell carcinoma | Cemiplimab | 1 | Yes | ND | ND | 6,407 | Yes | Yes | Yes | Steroid pulse, IVIG, plasmapheresis | Dead |
| 9 | (15) | 75 | M | Generalized muscle weakness, shortness of breath, double vision and ptosis | Malignant mesothelioma | Pembrolizumab | 2 | No | Anti-titin | ND | 3,480 | Yes | Yes | Yes | PSL, plasmapheresis | Alive at 1 year |
| 10 | (Our case) | 77 | F | Myalgia, ophthalmoplegia, and ptosis | Non-small cell lung cancer | Nivolumab | 2 | No | Aanti-titin and anti-Kv1.4 | No | 11,297 | Yes | Yes | Yes | Steroid pulse, PSL, IVIG | Dead at 29 days |
AChR: acetylcholine receptor, CK: creatine kinase, F: female, ICI: immune checkpoint inhibitor, IVIG: intravenous injection of immunoglobulin, M: male, ND: not described, PSL: prednisolone
The characteristics of these cases (7 men and 3 women) showed that the average age at presentation was 75.2 (range 49-87) years old. The initial presenting symptoms were similar to myasthenia-like syndrome, including ophthalmoplegia, blepharoptosis, and head drop (n=7), followed by muscle weakness (n=5), myalgia (n=4), fatigue (n=2), and respiratory symptoms (n=1). The types of cancer were carcinoma of the renal urinary system (n=4), melanoma (n=2), lung cancer (n=2), cutaneous squamous cell carcinoma (n=1), and malignant mesothelioma (n=1). Nivolumab and pembrolizumab are the major causes of irAEs in ICIs. A combination of nivolumab and ipilimumab was administrated in three cases. Cemiplimab and atezolizumab have also been reported. It is important to note that all cases of irAEs occurred in three cycles of ICIs. Only three patients were positive for anti-AChR antibodies. In the present case, the patient was negative for anti-AChR and anti-MuSK antibodies, even though she had myasthenia-like syndrome, including blepharoptosis. Touat et al. reported that clinical manifestations in patients with ICI-related myositis and myocarditis were dominated by acute or subacute myalgia and limb-girdle, axial, and oculomotor weakness, and patients were negative for anti-AChR antibodies or myositis-associated antibodies (19). Oculomotor weakness sometimes resembles MG, but the key feature to determine the difference between oculomotor myositis and MG is the diurnal variation of ocular symptoms. Therefore, anti-AChR and anti-MuSK antibodies are not necessarily positive in patients with oculomotor myositis. In general, anti-AChR antibodies can be identified in both ICI-induced and idiopathic MG. In idiopathic MG, anti-striated antibodies are associated with myositis and/or myocarditis, suggesting that a similar pathogenic process may occur in irAEs. More severe outcomes, including fatality, were seen in ICI-induced MG with anti-striated antibodies compared to seronegative cases (11). In patients with anti-striated antibodies, five cases were positive for anti-titin antibody, and two cases were positive for anti-Kv1.4 antibody. Although anti-titin antibodies have been closely associated with the presence of thymoma, our review revealed no cases of thymoma related to irAEs of ICIs (4). In a previous study, the ratio of thymoma in the ICI-related group was significantly lower than that in the non-ICI-related group (18).
The peak CK levels varied from 335 to 13,710 U/L. Notably, in cases of positive anti-striated antibody related to irAE of ICIs, the triad of myositis, myasthenia-like syndrome, and myocarditis was seen in seven cases, suggesting that the presence of anti-striated antibodies can lead to this triad. Mechanisms underlying this triad may involve common antigens in these muscles. For example, the orbital layer of the extraocular muscles, paraspinal muscle, and diaphragmatic muscle commonly contains slow-twitch aerobic fibers with primitive myosin, and the impairment of these muscles can cause myasthenia-like syndrome (20). In addition, previous studies have shown that myocarditis often occurs in patients with MG with positive anti-striated muscle antibodies, and its mechanism may be related to immune injury mediated by the binding of antibodies to specific antigens under the myocardial cell membrane. Human leukocyte antigen immune-related genes and cellular immunity may also be involved in the pathogenesis of myocarditis (18). Treatment consists of intravenous immunoglobulin, corticosteroids, pyridostigmine, plasma exchange, and withdrawal of ICI. Our review revealed that half of the cases ultimately led to fatal outcomes. Previous studies reported that mortality was high (30%), and myocarditis and respiratory muscle paralysis are two major causes of death in cases of ICI-induced myositis (10,20).
In conclusion, irAE of ICIs may cause myositis, myocarditis, and myasthenia-like syndrome in those with positive anti-striated antibodies. It is essential that patients with ICI-induced myasthenia-like syndrome be screened for anti-striated antibodies and myocarditis, which is a potentially fatal complication.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Hottinger AF. Neurologic complications of immune checkpoint inhibitors. Curr Opin Neurol 29: 806-812, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12: 252-264, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rota E, Varese P, Agosti S, et al. Concomitant myasthenia gravis, myositis, myocarditis and polyneuropathy, induced by immune-checkpoint inhibitors: a life-threatening continuum of neuromuscular and cardiac toxicity. eNeurologicalSci 14: 4-5, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamamoto AM, Gajdos P, Eymard B, et al. Anti-titin antibodies in myasthenia gravis: tight association with thymoma and heterogeneity of nonthymoma patients. Arch Neurol 58: 885-890, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Romi F, Suzuki S, Suzuki N, Petzold A, Plant GT, Gilhus NE. Anti-voltage-gated potassium channel Kv1.4 antibodies in myasthenia gravis. J Neurol 259: 1312-1316, 2012. [DOI] [PubMed] [Google Scholar]
- 6. Suzuki S, Utsugisawa K, Nagane Y, et al. Classification of myasthenia gravis based on autoantibody status. Arch Neurol 64: 1121-1124, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Bilen MA, Subudhi SK, Gao J, Tannir NM, Tu SM, Sharma P. Acute rhabdomyolysis with severe polymyositis following ipilimumab-nivolumab treatment in a cancer patient with elevated anti-striated muscle antibody. J Immunother Cancer 4: 36, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Norwood TG, Westbrook BC, Johnson DB, et al. Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer 5: 91, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thakolwiboon S, Karukote A, Wilms H. De novo myasthenia gravis induced by atezolizumab in a patient with urothelial carcinoma. Cureus 11: e5002, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sekiguchi K, Hashimoto R, Noda Y, et al. Diaphragm involvement in immune checkpoint inhibitor-related myositis. Muscle Nerve 60: E23-E25, 2019. [DOI] [PubMed] [Google Scholar]
- 11. Fazel M, Jedlowski PM. Severe myositis, myocarditis, and myasthenia gravis with elevated anti-striated muscle antibody following single dose of ipilimumab-nivolumab therapy in a patient with metastatic melanoma. Case Rep Immunol 2019: 2539493, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takahashi S, Mukohara S, Hatachi S, Yamashita M, Kumagai S. A case of myositis with dropped head syndrome and anti-titin antibody positivity induced by pembrolizumab. Scand J Rheumatol 49: 509-511, 2020. [DOI] [PubMed] [Google Scholar]
- 13. Onda A, Miyagawa S, Takahashi N, et al. Pembrolizumab-induced ocular myasthenia gravis with anti-titin antibody and necrotizing myopathy. Intern Med 58: 1635-1638, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeyakumar N, Etchegaray M, Henry J, et al. The terrible triad of checkpoint inhibition: a case report of myasthenia gravis, myocarditis, and myositis induced by cemiplimab in a patient with metastatic cutaneous squamous cell carcinoma. Case Rep Immunol 2020: 5126717, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schiopu SRI, Kasmann L, Schonermarck U, et al. Pembrolizumab-induced myocarditis in a patient with malignant mesothelioma: plasma exchange as a successful emerging therapy - case report. Transl Lung Cancer Res 10: 1039-1046, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonzalez NL, Puwanant A, Lu A, Marks SM, Zivkovic SA. Myasthenia triggered by immune checkpoint inhibitors: new case and literature review. Neuromuscul Disord 27: 266-268, 2017. [DOI] [PubMed] [Google Scholar]
- 17. Suzuki S, Utsugisawa K, Nagane Y, Suzuki N. Three types of striational antibodies in myasthenia gravis. Autoimmune Dis 2011: 740583, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng W, Sun T, Liu C, et al. A systematic review of myasthenia gravis complicated with myocarditis. Brain Behav 11: e2242, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Touat M, Maisonobe T, Knauss S, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 91: e985-e994, 2018. [DOI] [PubMed] [Google Scholar]
- 20. Fazal M, Prentice DA, Kho LK, Fysh E. Nivolumab-associated myositis myocarditis and myasthenia and anti-striated muscle antibodies. Intern Med J 50: 1003-1006, 2020. [DOI] [PubMed] [Google Scholar]