Abstract
Brown seaweed (Ascophyllum nodosum) is an exceptional bioactive substance known for its excellent antioxidant ability. Given the potential benefits of brown seaweed, the current study was conducted to determine its efficacy on growth performance, blood biochemistry, immunoglobulins (IgG and IgM), and the antioxidant capacity of broiler chickens challenged with heat stress (HS). A total of 336 mixed-sex Ross 308 broiler chicks (one-day-old) were randomly assigned into two groups; The thermoneutral group (TN, broilers were raised at 24 ± 1°C); and the heat stress group (HS; broilers were exposed to 32°C to 34°C, 8 h/d from day 21 to 27; the temperature in the remaining time was same as TN group). All birds in each group were randomly allotted to 4 dietary treatments—Negative control (NC) (without seaweed), NC + 1 mL seaweed extract (SWE) in drinking water, NC + 2 mL SWE in drinking water, and NC + 2% seaweed meal (SWM) in feed. Each treatment was assigned to six replicates with 7 broilers/replicate. Average body weight gain (ABWG), average feed intake (AFI), average water intake (AWI), feed conversion ratio (FCR), and mortality were determined weekly. On day 28, two male birds/cage were euthanized to collect blood and immune organs for subsequent biochemical, antioxidant, and immune status analysis. Data were analyzed as a 4 × 2 factorial analysis of variance using the GLM procedure of Minitab software. Overall, 2% SWM inclusion significantly increased (P < 0.05) the AFI, ABWG, and AWI of broiler chickens irrespective of HS. HS significantly reduced (P < 0.05) AFI and increased (P < 0.05) the bird's rectal temperature, plasma concentrations of sodium, chloride, glucose, amylase, and uric acid compared to TN birds. HS increased (P < 0.05) serum IgM and IgG and decreased plasma glutathione reductase and glutathione peroxidase compared to TN birds, while the activity of superoxide dismutase was not affected by HS and dietary treatments. 1 mL SWE in water and 2% SWM in feed significantly reduced (P < 0.05) the plasma activity of alanine aminotransferase and gamma-glutamyl transferase of heat-stressed broilers, respectively compared to other treatments. Conclusively, dietary supplementation of brown seaweed improved the growth performance of birds irrespective of HS and may help to reduce the negative effects of HS by improving the plasma enzyme activities of heat-stressed birds.
Key words: brown seaweed, heat stress, growth performance, immune status, broiler chickens
INTRODUCTION
Heat stress (HS) is a significant problem in modern poultry production (Ahmed-Farid et al., 2021, Calik et al., 2022, Huang et al., 2015, Liu et al., 2020a, Quinteiro-Filho et al., 2010, Zhang et al., 2017), causing considerable economic losses, which are conjected to surge in coming years with the rise of global temperature (Abdel-Moneim et al., 2021). Current poultry genotypes experience a fast growth rate, which reduces their tolerance to heat, leading to various metabolic disorders in chickens (Varasteh et al., 2015). Due to their high metabolic rate and physiological state, broiler chickens tend to be more vulnerable to temperature-related stress (Zhang et al., 2017). Under HS conditions, the physiological blood parameters of broilers are altered, which ultimately reduces feed intake, performance, and productivity (Liu et al., 2019; Khan et al., 2021; Oke et al., 2021). High ambient temperature can also weaken the immune system of broiler chickens (Siddiqui et al., 2021). Evidence showed lowered CD3+ and CD8+ T altered cytokine expression, and reduced immune organ weights and B-lymphocyte in response to HS (Honda et al., 2015, Quinteiro-Filho et al., 2010; Xu et al., 2018; Siddiqui et al., 2021).
Moreover, inflammatory cytokines are secreted in response to stressors and can be used to determine animals’ immune status (Quinteiro-Filho et al., 2017). Proinflammatory cytokines mediate inflammatory damage while anti-inflammatory cytokines ameliorate inflammation and improve the healing process (Siddiqui et al., 2020). High expression of proinflammatory cytokines triggered by HS might be linked with reactive oxygen species (ROS) production (Jang et al., 2014). The ROS activates the production of primary regulators of inflammatory response known as cytokines via activation of the nuclear factor kappa-B pathway, which controls the expression of genes involved in inflammation and stress responses (Khan et al., 2019). High temperature can stimulate excessive ROS and cause an imbalance between oxidation and antioxidant enzymes resulting in oxidative damage in chickens (Lin et al., 2006). Previous studies have shown oxidative-induced damage in response to HS in broiler chickens (Yang et al., 2010; Huang et al., 2015). Exposure of broiler chicks to an acute temperature of 32°C for 6 h induced metabolic changes that triggered oxidative stress (Lin et al., 2006). A recent study by Liu et al. (2022a) further confirmed that chronic HS (32.5 ± 1.4°C for 8 h/d) for 4 wk induced oxidative stress in broilers evidenced by elevated MDA levels and reduced antioxidant enzymes activity in serum, muscles and small intestines. Enzymatic and non-enzymatic antioxidant defence systems can be internally synthesized or externally supplied in response to oxidative stress and increased due to defence mechanisms against heat-induced oxidative stress (Akbarian et al., 2016; Surai et al., 2019). The removal of free radicals in the body directly hinged on the depletion of the antioxidant factors (Zhang et al., 2020), which can alter the antioxidant capacity of broiler chickens (Hu et al., 2021).
Significant developments in management and nutritional strategies have been attained to mitigate HS and reduce the resulting animal production losses. However, the current increase in the incidence of heat waves due to climate change further heightens HS concerns (Chauhan et al., 2021). Recently, interests have involved the extensive use of bioactive substances to ameliorate HS in poultry (Saracila et al., 2021) due to their beneficial effects on health and production (Abdelli et al., 2021). These phytogenic feed additives can be incorporated into animal feed or water as extracts (Gadde et al., 2017). Brown seaweeds are exceptional bioactive substances, rich in phenolic compounds (phlorotannin, flavonoids), pigments (chlorophyl, fucoxanthin) and contain high content of total polysaccharides such as fucoidan, laminarin, alginic acid, and mannitol (Holdt and Kraan, 2011; Zheng et al., 2020). They have been reported to exert different biological activity, including antioxidant, anti-inflammatory, and anti-microbial roles (Lordan et al., 2013; Gullon et al., 2020; Meresse et al., 2020). For instance, fucoidan prevented liver injury in mice by deactivating the inflammatory signaling pathway (Li and Ye, 2015); laminarin enhanced growth performance, beneficial bacteria in chickens and increased the expression of claudins 1, toll-like receptor 2 and interleukins 17A in the small intestine (Venardou et al., 2021). Dietary seaweed (Enteromorpha prolifera) polysaccharide supplementation also improved broilers’ immunity and breast muscle growth (Zhao et al., 2021). The phenolic compounds in brown seaweed can protect the body against oxidative damage that occurs in response to environmental or nutrient changes (Li et al., 2017) and promote good health and productivity in animals (Michalak et al., 2022). Previous studies showed that the dietary supplementation of marine algae polysaccharides extracted from seaweed (E. prolifera) improved bursa weight, the intestinal and liver antioxidant enzymes (total superoxide dismutase, glutathione, catalase and glutathione peroxidase) and reduced malondialdehyde (MDA) contents of broilers (Liu et al., 2020b; Guo et al., 2021). In aged laying hens, marine algae polysaccharides improved the productive performance, egg quality and antioxidant capacity by increasing the activity of serum total superoxide dismutase, liver and jejunal catalase, and decreased jejunal MDA concentration (Guo et al., 2020). Studies have shown brown seaweed's capacity in alleviating the adverse effects of HS in ruminant animals (Saker et al., 2003; Kannan et al., 2007) with no available data on broiler chickens. Therefore, our current study was conducted to test the hypothesis that the dietary supplementation of 2% brown seaweed meal (in feed), and 1 and 2 mL brown seaweed extract (in water) can mitigate HS negative effects in broiler chickens. The main objective was to evaluate the potential beneficial effect of brown seaweed meal and extract on the growth performance, blood biochemistry, immune response, and antioxidant status of broiler chickens challenged with heat stress.
MATERIALS AND METHODS
Management and Housing
All experimental procedures adopted in this study were endorsed by Dalhousie University Animal Use and Care Committee (Ethical code 2021-029), and all chickens were handled in agreement with the Canadian Council on Animal Care (CCAC-Canadian Council On Animal Care, 2009) guidelines. A total of 336 one-day-old broiler chicks (Ross 308) were gotten from a commercial source. Upon arrival, birds were weighed in groups of 6, then randomly assigned to each cage (50 cm × 60 cm) at a stocking density of 0.076 m2/bird. They were raised for 28 d in a controlled environment at the Atlantic Poultry Research Center, Dalhousie University, Faculty of Agriculture (Truro, NS, Canada). Room temperature was checked daily and steadily reduced from 33 to 24°C from d 0 to 28 in the thermoneutral room. The lighting was programmed to produce 18 h of light and 6 h of darkness throughout the experimental period, and illumination was gradually reduced from 20 lux on day 0 to 5 lux (Adewole et al., 2020).
Heat Stress Protocol
Heat stress was induced on days 21 to 27 for 8 h/d. For the HS group, the temperature was gradually raised at 9 am, reached 28°C by 10 am, 32°C by noon, maintained at 34 ± 1°C from noon to 4 pm, then slowly reduced to 28°C by 5 pm and dropped to thermoneutral (TN (24°C ± 1) for the remainder of the day. TN birds were housed under the ambient temperature of 24°C ± 1 throughout the heat stress week. The TN and HS conditions were controlled by an automatic temperature control system and recorded twice daily. Relative humidity was observed and maintained at <40% for both rooms.
Seaweed products, Diets, and Experimental Design
The brown seaweed meal and extract used in this study were obtained from Sealife Seaplants (NS, Canada). Seaweed extract was derived from fresh Ascophyllum nodosum seaweed harvested from the North Atlantic coastal water of NS, Canada. The seaweed meal contained 90.4% dry matter, 3.7% crude protein, and 25.5% neutral detergent fiber on as fed basis. For both seaweed and experimental diets, dry matter was determined according to the Association of Official Analytical Chemists (AOAC, 1990), method (925.09) by oven drying a 5.0 g sample at 105°C overnight. The method of Goering and Van Soest (1970) was used to determine the content of neutral detergent fiber. The nitrogen content was determined using the combustion method (990.03; AOAC, 1990) with an N analyzer (Model Leco CN828 Carbon Nitrogen Determinator, St. Joseph, MO), and crude protein was calculated as N × 6.25. The ether extract in the diets was determined after hexane extraction (Method 920.39; AOAC, 1990) in an Ankom XT10 Fat Extractor system (Macedon, NY). The diets were further analyzed for minerals after they were ashed at 600°C for 12 h in a muffle furnace, using a Varian 725 ICP-OES inductively coupled plasma mass spectrometer (ICP-AES; Vista, Varian,Palo Alto, CA) according to the method of AOAC (2005) (method 985.01). The seaweed extract is a water-soluble viscous brownish-black liquid containing minerals and carbohydrates (alginic acid, mannitol, and laminaria). Broiler chicks were randomly allotted to 8 treatment groups containing 6 replicate cages of 7 birds each. The experiment was designed as a 4 × 2 factorial arrangement consisting of 2 environmental controlled rooms; 1) Thermoneutral (TN; 24°C ± 1 on d 21–27), Heat stress (HS; 32-34°C for 8 h/d on d 21–27) and 4 dietary treatments: 1) Corn-wheat-soybean based diet negative control (NC)); 2) NC + 1 mL seaweed extract in drinking water (1 mL SWE), 3) NC + 2 mL seaweed extract in drinking water (2 mL SWE), and 4) NC + 2% seaweed meal in feed (2% SWM). All diets, including starter (d 0–14) and grower (d 15–28), were formulated to meet Ross 308 guidelines for nutrient requirements. Broiler chickens received feed (in mash form) and water ad libitum throughout the experimental period. Dietary treatments, ingredients, and nutritional composition are shown in Table 1.
Table 1.
Ingredients, calculated, and analyzed compositions of experimental diets (as-fed basis, % unless otherwise stated).
| Ingredients | Starter (1–14 d) |
Grower (15–28 d) |
||
|---|---|---|---|---|
| Negative control (NC) | 2% SWM | Negative control (NC) | 2% SWM | |
| Ingredients composition | ||||
| Corn (ground) | 42.42 | 39.12 | 45.75 | 42.45 |
| Soybean meal (46.5) | 40.12 | 40.58 | 36.19 | 36.65 |
| Wheat | 10.00 | 10.00 | 10.00 | 10.00 |
| Vegetable Oil Young | 2.80 | 3.92 | 3.82 | 4.94 |
| Brown seaweed meal | 0.00 | 2.00 | 0.00 | 2.00 |
| Dicalcium phosphate | 1.57 | 1.57 | 1.39 | 1.39 |
| Limestone | 1.45 | 1.34 | 1.32 | 1.21 |
| DL Methionine Premix1 | 0.61 | 0.61 | 0.53 | 0.53 |
| Vitamin-Mineral Premix2,3 | 0.50 | 0.50 | 0.50 | 0.50 |
| Salt | 0.37 | 0.21 | 0.38 | 0.22 |
| Lysine HCl | 0.16 | 0.15 | 0.12 | 0.11 |
| Total | 100 | 100 | 100 | 100 |
| Calculated composition | ||||
| Met. energy (kcal/kg) | 3000 | 3000 | 3100 | 3100 |
| Digestible Tryptophan | 0.25 | 0.25 | 0.23 | 0.23 |
| Digestible Threonine | 0.87 | 0.87 | 0.82 | 0.81 |
| Digestible Met. & Cys. | 0.95 | 0.95 | 0.87 | 0.87 |
| Digestible Lysine | 1.28 | 1.28 | 1.15 | 1.15 |
| Protein | 23.0 | 23.0 | 21.5 | 21.5 |
| Calcium | 0.96 | 0.96 | 0.87 | 0.87 |
| Available phosphorus | 0.48 | 0.48 | 0.44 | 0.44 |
| Sodium | 0.18 | 0.18 | 0.18 | 0.18 |
| Analyzed composition | ||||
| Dry Matter | 92.4 | 92.6 | 91.5 | 92.0 |
| Crude protein | 24.6 | 24.4 | 22.5 | 23.8 |
| Neutral detergent fibre | 8.70 | 9.71 | 9.15 | 10.71 |
| Crude fat | 3.08 | 4.00 | 4.10 | 6.10 |
| Calcium | 0.96 | 0.97 | 0.72 | 0.83 |
| Potassium | 0.99 | 1.02 | 0.98 | 0.99 |
| Magnesium | 0.18 | 0.18 | 0.17 | 0.18 |
| Phosphorus | 0.60 | 0.60 | 0.57 | 0.55 |
| Sodium | 0.20 | 0.15 | 0.14 | 0.18 |
Supplied/kg premix: DL-Methionine, 0.5 kg; wheat middlings, 0.5 kg.
Starter vitamin-mineral premix contained the following per kg of diet: 9750 IU vitamin A; 2000 IU vitamin D3; 25 IU vitamin E; 2.97 mg vitamin K; 7.6 mg riboflavin; 13.5 mg Dl Ca-pantothenate; 0.012 mg vitamin B12; 29.7 mg niacin; 1.0 mg folic acid, 801 mg choline; 0.3 mg biotin; 4.9 mg pyridoxine; 2.9 mg thiamine; 70.2 mg manganese; 80.0 mg zinc; 25 mg copper; 0.15 mg selenium; 50 mg ethoxyquin; 1543 mg wheat middlings; 500 mg ground limestone.
Grower and Finisher vitamin-mineral premix contained the following per kg of diet: 9750 IU vitamin A; 2000 IU vitamin D3; 25 IU vitamin E; 2.97 mg vitamin K; 7.6 mg riboflavin;13.5 mg Dl Ca-pantothenate; 0.012 mg vitamin B12; 29.7 mg niacin; 1.0 mg folic acid, 801 mg choline; 0.3 mg biotin; 4.9 mg pyridoxine;2.9 mg thiamine; 70.2 mg manganese; 80.0 mg zinc; 25 mg copper; 0.15 mg selenium; 50 mg ethoxyquin; 1543 mg wheat middling's; 500 mg ground limestone.
Growth Performance and Sample Collection
Feed intake (FI) and body weight (BW) were recorded weekly, and average feed intake (AFI), and average body weight (ABW) were calculated. The feed conversion ratio (FCR) was obtained by dividing the amount of feed consumed by body weight gain. Mortality was recorded daily and used to correct the AFI and FCR. Dead birds were sent to Animal Health Laboratory (Truro, NS) for necropsy examination. On day 28, 2 male birds per cage (12 replicates per treatment group) were randomly selected and euthanized by electrical stunning and exsanguination. Following euthanizing, blood samples were collected from the jugular vein of each bird into 10 mL serum and 10 mL heparinized tubes, and centrifuged at 2,000 × g for 15 min. The supernatant was further divided into several aliquots tubes to avoid repeated thawing. The serum and plasma samples were stored immediately at −80°C for serum enzyme-linked immunosorbent assay (ELISA) and at −20°C for plasma biochemistry and other analysis.
Plasma Biochemistry
Plasma samples for biochemical analysis were shipped on ice to Atlantic Veterinary College, University of Prince Edward Island Pathology Laboratory, for analysis using cobas6000 analyzer series (Roche Diagnostics, Indianapolis, IN).
Immune Parameters
The bursa and spleen weights collected on day 28 were recorded as a percentage of the live BW of the slaughtered chicken (g/Kg BW). Serum samples were used to analyze the concentrations of immunoglobulins G (IgG) and immunoglobulins M (IgM) using ELISA kits specified for chickens (Bethyl Laboratories, Montgomery, TX; Catalog No. E33-104-200210 and E33-102-21109, respectively) following the company protocol. Plasma samples were used to determine the concentrations of chicken interleukin 6 (IL6) and interleukin 10 (IL10) using ELISA kits (MyBioSource.com Catalog No. MBS037319 and MBS007312, respectively). The optical density (OD) values for IgG, IgM, IL6 and IL10 were determined on a microplate reader (Bio-Tek Instrument Inc., Winooski, VT) using a software program (KC4, version #3.3, Bio Tek Instruments). The 4-parameter logistic model was used to calculate immunoglobulins concentration, while curve fitting software was used to generate standard linear curves and calculate IL6 and IL10 analyte levels.
Antioxidant Activities Analysis
Antioxidant activities of Superoxide dismutase (SOD), Glutathione peroxidase (GPx), and Glutathione reductase (GR) were assessed using the Superoxide dismutase Assay Kits (Item No. 706002), Glutathione Peroxidase Assay Kit (Item No. 703102), Glutathione Reductase Assay Kits (Item No. 703202), respectively, following the manufacturers’ instructions (Cayman Chemical Company, Ann Arbor, MI). Absorbance for serum SOD was measured at 450 nm while absorbance for plasma GPx and GR were read once every minute at 340 nm using a plate reader (Bio-Tek Instrument Inc., Winooski, VT) and a software program (KC4, version #3.3, Bio Tek Instruments) to obtain at least 5-time points. The concentration of GPx and GR were expressed as nmol/min/mL while SOD was expressed as U/mL equivalent.
Stress Indicators
The rectal temperature of broilers (2 birds/replicate) on d 21 and 27 were measured using a digital thermometer (Accuflex5; A.M.G. Medical Inc.) to confirm induced HS on d 27. The entire probe was inserted into the bird's cloaca, and the data were automatically displayed on the device. Serum corticosterone was measured using a commercial ELISA kit (Corticosterone Enzyme Immunoassay Kit; Catalog Number K014-H1/H5) following the instructions and recommendations from the manufacturer (Arbor Assays, Ann Arbor, MI).
Statistical Analysis
Data were analyzed as a 4 × 2 factorial analysis of variance (ANOVA) using the General Linear Model of Minitab LLC (2019) software for data collected on day 28. Statistically significant differences among dietary treatments (NC, 1 mL SWE, 2 mL SWE and 2% SWM) were identified using one-way ANOVA before the onset of HS. Following ANOVA, significant means were separated using Tukey's honest significant difference test. Analyzed data were presented as means, standard error of the mean (SEM), and probability values. P-values less than or equal to 0.05 were considered significant.
RESULTS
Growth Performance
The effect of experimental treatments on the growth performance of broiler chickens is shown in Table 2. Two percent SWM inclusion significantly increased (P = 0.023) the body weight gain and significantly improved (P = 0.024) FCR at the early phase of growth (D 0–7) and significantly increased (P = 0.001) average feed intake at D 7–14 compared to NC. There was no significant interaction between dietary treatment and the temperature model for all the growth performance parameters (D 21–28). HS significantly reduced feed intake (P < 0.001) and FCR (P = 0.012) compared to TN birds. Among the heat-stressed group, broiler chickens fed with 2% SWM and those that received 1 mL and 2 mL SWE in drinking water had numerically improved FCR (19.4%, 26.7%, 22.1%, respectively) compared to those fed the control diet. Overall, the dietary treatment significantly affected (P < 0.05) all the growth performance parameters except for FCR. Birds fed 2% SWM had significantly higher AFI (P = 0.019), ABWG (P = 0.020), and AWI (P = 0.026) than those that were fed with the control diet alone. Birds that received 1 mL and 2 mL SWE in drinking water had marginally improved (P < 0.05) growth performance parameters, compared to the control.
Table 2.
Effect of brown seaweed meal and extract on the growth performance parameters of broiler chickens before and during HS conditions.
| Treatment1 |
Temp. Model |
P-values |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | NC | 1 mL SWE | 2 mL SWE | 2% SWM | TN2 | HS3 | SEM4 | Diet | Temp. | Diet X Temp. |
| Day 0–7 | ||||||||||
| AFI (g/bird) | 158 | 152 | 161 | 160 | - | - | 7.00 | 0.068 | - | - |
| BW(g/bird) | 105b | 109ab | 111ab | 117a | - | - | 1.44 | 0.024 | - | - |
| BWG(g/bird) | 65.5b | 68.9ab | 70.8ab | 77.5a | 1.45 | 0.023 | - | - | ||
| FCR | 2.3a | 2.2ab | 2.1ab | 1.9b | - | - | 0.11 | 0.024 | - | - |
| AWI (L) | 0.4 | 0.3 | 0.4 | 0.4 | - | - | 0.01 | 0.427 | - | - |
| Day 7–14 | ||||||||||
| AFI(g/bird) | 196b | 218ab | 209b | 235a | - | - | 7.64 | 0.001 | - | - |
| BW(g/bird) | 306b | 322ab | 323ab | 344a | - | - | 5.06 | 0.069 | - | - |
| BWG(g/bird) | 201 | 212 | 211 | 227 | - | - | 4.03 | 0.159 | - | - |
| FCR | 1.0 | 1.0 | 1.0 | 1.1 | - | - | 0.04 | 0.508 | - | - |
| AWI (L) | 0.6 | 0.6 | 0.6 | 0.6 | - | - | 0.02 | 0.793 | - | - |
| Day 14–21 | ||||||||||
| AFI(g/bird) | 537b | 572ab | 578ab | 611a | - | - | 12.4 | 0.039 | - | - |
| BW(g/bird) | 647 | 687 | 688 | 743 | - | - | 13.4 | 0.097 | - | - |
| BWG(g/bird) | 341 | 366 | 364 | 399 | - | - | 8.97 | 0.166 | - | - |
| FCR | 1.6 | 1.6 | 1.6 | 1.5 | - | - | 0.03 | 0.739 | - | - |
| AWI (L) | 0.9b | 1.0ab | 1.0ab | 1.1a | - | - | 0.02 | 0.016 | - | - |
| Day 21–28 | ||||||||||
| AFI (g/bird) | ||||||||||
| HS | 627 | 598 | 628 | 687 | 802a | 635b | 19.1 | 0.106 | <0.001 | 0.641 |
| TN | 740 | 808 | 768 | 864 | ||||||
| BW(g/bird) | ||||||||||
| HS | 1037 | 1100 | 1181 | 1272 | 1165 | 1150 | 27.6 | 0.019 | 0.773 | 0.610 |
| TN | 1007 | 1242 | 1128 | 1288 | ||||||
| BWG (g/bird) | ||||||||||
| HS | 373 | 429 | 476 | 504 | 485 | 453 | 17.8 | 0.026 | 0.237 | 0.565 |
| TN | 405 | 558 | 480 | 581 | ||||||
| FCR | ||||||||||
| HS | 1.66 | 1.39 | 1.31 | 1.36 | 1.6a | 1.4b | 0.07 | 0.161 | 0.012 | 0.582 |
| TN | 1.81 | 1.48 | 1.66 | 1.53 | ||||||
| AWI (L) | ||||||||||
| HS | 1.53 | 1.80 | 1.68 | 1.85 | 1.7 | 1.7 | 0.03 | 0.025 | 0.354 | 0.333 |
| TN | 1.65 | 1.63 | 1.60 | 1.80 | ||||||
| Total trial period | ||||||||||
| AFI(g/bird) | 1590b | 1648ab | 1662ab | 1796a | - | - | 24.7 | 0.019 | - | - |
| BW(g/bird) | 2088b | 2275ab | 2262ab | 2473a | - | - | 45.0 | 0.025 | - | - |
| BWG(g/bird) | 997b | 1130ab | 1110ab | 1241a | - | - | 27.7 | 0.020 | - | - |
| FCR | 1.6 | 1.5 | 1.5 | 1.4 | - | - | 0.03 | 0.156 | - | - |
| AWI | 3.4b | 3.7ab | 3.5ab | 3.9a | - | - | 0.05 | 0.026 | - | - |
means assigned different lowercase superscript letters are significantly different, P < 0.05 (Tukey's procedure).
Dietary treatment includes NC (negative control [corn-soybean meal-wheat–based diet]), 1 mL SWE (chicks supplied brown seaweed extract in water at 1 mL/L), 2 mL SWE (chicks supplied brown seaweed extract in water at 1 mL/L), 2% SWM (chicks fed NC + 2% brown seaweed in feed).
HS (Heat stress).
TN (Thermoneutral).
SEM, Standard error of means.
Abbreviations: AFI, average feed intake; AWI, average water intake; BW, body weight; BWG, body weight gain; FCR, feed conversion ratio; Temp, Temperature.
Plasma Biochemistry
The effect of the experimental treatments on the blood biochemical indices is presented in Table 3. There was a significant interaction between dietary treatments and HS for plasma lipase (P = 0.002) and alanine aminotransferase (ALT; P < 0.001). In the heat-stressed group, birds that received 1 mL and 2 mL SWE in drinking water had higher (P = 0.042) plasma magnesium concentrations compared to those in the NC treatment. Also, birds fed with 2% SWM had lower plasma lipase (P < 0.001), uric acid (P = 0.017) and gamma-glutamyl transferase (GGT; P = 0.033) concentrations) compared to other treatments. The plasma activity of ALT significantly reduced (P < 0.001) in the heat-stressed broilers that received 1 mL SWE in drinking water compared to those that received other treatments. In-water supplementation of 2 mL SWE significantly increased plasma albumin in heat-stressed birds, while 1 mL SWE and 2% SWM marginally increased albumin levels compared to the control treatment (P = 0.039). Concerning the temperature model, significantly higher plasma concentrations of sodium (P = 0.038), chloride (P = 0.024), glucose (P < 0.001), amylase (P = 0.046), and uric acid (P = 0.012) and reduced concentrations of urea (P = 0.002), lipase (P < 0.001), and creatine kinase (P = 0.045) concentrations were recorded among the heat-stressed chicks, compared to TN birds. The remaining tested plasma biochemistry parameters were not significantly affected by heat stress and brown seaweed products supplementation.
Table 3.
Effect of brown seaweed meal and extract on blood biochemistry parameters in broiler chickens under TN and HS conditions.
| Parameters | Thermoneutral (TN) Dietary treatments1 |
Heat stress (HS) Dietary reatments1 |
Temp. Model |
P-value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | 1 mL SWE | 2 mL SWE | 2% SWM | NC | 1 mL SWE | 2 mL | 2% SWM | TN | HS | PooledSEM2 | Diet | Temp. | Diet X Temp. | |
| Electrolytes and minerals, mmolL−1 | ||||||||||||||
| Sodium | 144 | 144 | 146 | 146 | 147 | 147 | 148 | 146 | 145.5b | 147.2a | 0.45 | 0.555 | 0.038 | 0.398 |
| Potassium | 7.56 | 6.84 | 7.10 | 6.36 | 6.5 | 7.5 | 6.6 | 6.5 | 6.72 | 6.66 | 0.16 | 0.353 | 0.805 | 0.284 |
| Chloride | 107 | 106 | 107 | 108 | 109 | 109 | 109 | 108 | 107b | 109a | 0.4 | 0.853 | 0.024 | 0.465 |
| Calcium | 1.91 | 1.70 | 1.77 | 1.94 | 1.88 | 1.89 | 2.11 | 1.93 | 1.8 | 1.9 | 0.04 | 0.606 | 0.073 | 0.477 |
| Phosphorus | 2.12 | 1.72 | 1.99 | 1.85 | 1.84 | 1.95 | 1.82 | 1.87 | 1.8 | 1.8 | 0.05 | 0.75 | 0.812 | 0.346 |
| Magnesium | 0.75 | 0.68 | 0.73 | 0.69 | 0.66b | 0.72a | 0.73a | 0.69ab | 0.7 | 0.7 | 0.01 | 0.684 | 0.106 | 0.075 |
| Metabolites, mmolL−1 | ||||||||||||||
| Urea | 0.38 | 0.40 | 0.42 | 0.42 | 0.37 | 0.33 | 0.36 | 0.34 | 0.4a | 0.3b | 0.01 | 0.861 | 0.002 | 0.532 |
| Glucose | 13.0 | 12.8 | 13.0 | 13.1 | 13.2 | 14.0 | 13.7 | 13.4 | 12.9b | 13.6a | 0.08 | 0.599 | <0.001 | 0.09 |
| Cholesterol | 2.52 | 2.68 | 2.71 | 2.59 | 2.69 | 2.69 | 2.78 | 2.57 | 2.6 | 2.7 | 0.03 | 0.326 | 0.218 | 0.817 |
| Uric acid | 320 | 336 | 338 | 349 | 340b | 386ab | 485a | 336b | 324b | 373a | 13.4 | 0.065 | 0.012 | 0.13 |
| Enzymes, UL−1 | ||||||||||||||
| Amylase | 472 | 509 | 545 | 527 | 594 | 560 | 698 | 529 | 479b | 594a | 28.6 | 0.721 | 0.046 | 0.688 |
| ALP3 | 4382 | 6266 | 4087 | 5872 | 5294 | 4043 | 5264 | 3387 | 4364 | 4420 | 282 | 0.791 | 0.91 | 0.023 |
| Creatine kinase | 14420 | 7550 | 11630 | 12502 | 4337 | 4338 | 4478 | 5002 | 6001a | 4527b | 1045 | 0.86 | 0.045 | 0.783 |
| AST4 | 232 | 192 | 220 | 241 | 174 | 176 | 194 | 180 | 197 | 183 | 7.21 | 0.434 | 0.112 | 0.802 |
| GGT5 | 10.1 | 10.7 | 10.3 | 11.3 | 11.9ab | 13.2a | 11.8ab | 9.5b | 10.3 | 11.4 | 0.34 | 0.428 | 0.122 | 0.073 |
| Lipase | 21.1 | 21.0 | 27.4 | 22.3 | 18.0a | 21.0a | 18.4a | 10.0b | 22.5a | 17.1b | 0.8 | <0.001 | <0.001 | 0.002 |
| ALT6 | 5.5c | 11.5a | 10ab | 7bc | 7.97ab | 6.27b | 9.95a | 11.03a | 7.8 | 8.9 | 0.42 | 0.014 | 0.145 | <0.001 |
| Proteins, gL−1 | ||||||||||||||
| Total protein | 25.67 | 25.42 | 25.50 | 25.83 | 25.5 | 25.1 | 27.5 | 26.0 | 25.2 | 26.2 | 0.34 | 0.441 | 0.107 | 0.495 |
| Albumin | 10.4 | 10.4 | 10.4 | 10.3 | 10.2 | 10.4 | 11.3 | 10.8 | 10.4 | 10.7 | 0.1 | 0.215 | 0.092 | 0.236 |
| Globulin | 15.3 | 15.0 | 15.1 | 15.5 | 15.1 | 14.9 | 16.3 | 15.2 | 14.8 | 15.4 | 0.28 | 0.678 | 0.215 | 0.693 |
means assigned different lowercase superscript letters are significantly different, P < 0.05 (Tukey's procedure).
Dietary treatment includes NC (negative control [corn-soybean meal-wheat–based diet]), 1 mL SWE (chicks supplied brown seaweed extract in water at 1 mL/L), 2 mL SWE (chicks supplied brown seaweed extract in water at 1 mL/L), 2% SWM (chicks fed NC + 2% brown seaweed in feed).
SEM, Standard error of means.
ALP, Alkaline phosphatase.
AST, Aspartate Aminotransferase.
GGT, Gamma-Glutamyl Transferase.
ALT, Alanine Transferase.
Stress Indicators, Immune Response, and Antioxidants Status
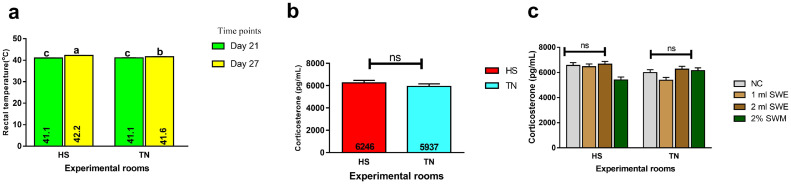
The effect of HS on broilers' rectal temperature and corticosterone concentration is shown in Figure 1. On d 21, before the exposure to HS, the rectal temperatures of birds in the 2 rooms were not different (average of 41.1°C in each room). However, on d 27, high ambient temperature significantly elevated (P < 0.001) the rectal temperature of birds in the HS room (average of 42.2°C) compared to those in the TN rooms (average of 41.6°C). The dietary treatments had no significant effect on the bird's rectal temperature (P = 0.752). Corticosterone level was not affected by HS (P = 0.459) and dietary treatments (P = 0.621), though the corticosterone level of heat-stressed birds was numerically higher than the TN birds (5.2%).
Figure 1.
(a) Effect of heat stress on the rectal temperature (˚C) of broiler chickens at different time points. Means with the different letters are significantly different; (b) Corticosterone concentration (pg/mL) in the serum of broiler chickens subjected to heat stress or thermoneutral temperature; (c) Effect of brown seaweed meal and extract on serum corticosterone level of broiler chickens subjected to heat stress or thermoneutral temperature. ns=not significant.
The effect of SWM and SWE on the relative weight of immune organs, serum IgG and IgM, interleukins, and antioxidant status of broiler chickens challenged or unchallenged with HS is shown in Table 4. There was no significant interaction between dietary treatments and the temperature model. The immune organs’ weights (bursa and spleen), serum IgG, IgM, plasma IL6, IL10, serum SOD, plasma GPx, and GR were not significantly affected by SWM and SWE supplementation in-feed and in-water. HS increased plasma IL6 (P < 0.001), serum IgM (P < 0.001) and IgG (P = 0.002) concentrations, compared to TN birds. Broiler chickens challenged with HS exhibited lower (P < 0.001) plasma GR and GPx compared to TN birds, while the activity of serum SOD, plasma IL10, and immune organ weights were not affected by HS.
Table 4.
Effect of brown seaweed meal and extract on relative immune organ weight, serum immunoglobulins, interleukins, and antioxidant status in broiler chickens challenged with or without HS.
| Dietary treatment1 |
Temp. model |
P-values |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | NC | 1 mL SWE |
2 mL SWE |
2% SWM |
TN2 | HS3 | SEM4 | Diet | Temp. | Diet X Temp. |
| Bursa (g / Kg BW) | 0.20 | 0.21 | 0.20 | 0.21 | 0.20 | 0.20 | 0.006 | 0.801 | 0.906 | 0.349 |
| Spleen (g / Kg BW) | 0.09 | 0.08 | 0.08 | 0.08 | 0.10 | 0.10 | 0.002 | 0.381 | 0.196 | 0.364 |
| Serum IgG (mg/mL) | 3.32 | 4.13 | 4.08 | 4.13 | 2.9b | 5.20a | 0.44 | 0.789 | 0.002 | 0.773 |
| Serum IgM (mg/mL) | 0.20 | 0.15 | 0.16 | 0.19 | 0.10b | 0.20a | 0.02 | 0.088 | <0.001 | 0.805 |
| Plasma IL 6 (pg/mL)5 | 18.7 | 23.1 | 17.5 | 26.4 | 17.0b | 26.0a | 2.04 | 0.185 | 0.005 | 0.527 |
| Plasma IL 10 (pg/mL)6 | 52.3 | 43.8 | 41.2 | 54.9 | 51.2 | 44.5 | 2.56 | 0.073 | 0.119 | 0.143 |
| Serum SOD (U/mL)7 | 0.50 | 0.48 | 0.66 | 0.50 | 0.50 | 0.50 | 0.03 | 0.234 | 0.669 | 0.798 |
| Plasma GR (nmol/min/mL)8 | 23.7 | 20.0 | 21.7 | 20.0 | 28.5a | 14.6b | 1.05 | 0.261 | <0.001 | 0.619 |
| Plasma GPx (nmol/min/mL)9 | 461 | 471 | 492 | 455 | 500a | 436b | 9.11 | 0.233 | <0.001 | 0.404 |
means assigned different lowercase superscript letters are significantly different, P < 0.05 (Tukey's procedure).
Dietary treatment includes NC (negative control (corn-soybean meal-wheat–based diet;)), 1 mL SWE (chicks supplied brown seaweed extract in water at 1 mL/L),
2 mL SWE (chicks supplied brown seaweed extract in water at 1 mL/L), 2% SWM (chicks fed NC + 2% brown seaweed in feed).
HS (Heat stress).
TN (Thermoneutral).
SEM, Standard error of means.
IL 6, Interleukin 6.
IL 10, Interleukin 10
SOD, superoxide dismutase.
GR, Glutathione reductase.
GPx, Glutathione peroxidase.
DISCUSSION
Seaweed contains unique bioactive compounds and has been applied in poultry feed because of its richness in carbohydrates, minerals, protein, vitamins, and dietary fibers, with relatively stable amino acid profiles and growth-stimulating properties (Kulshreshtha et al., 2020). Several studies have reported the potential benefits of different species of seaweed in layers (Choi et al., 2014; Kulshreshtha et al., 2014; Borzouie et al., 2020), ducks (El-Deek and Brikaa, 2009), and broiler chickens (Abudabos et al., 2013; Mohammadigheisar et al., 2020) in terms of performance, blood parameters and gut health. However, there are limited studies on the potential effect of brown seaweed (Ascophyllum nodosum) meal and extract on broiler chickens challenged with HS. Here, we investigated for the first time the effect of brown seaweed meal and extract on the growth performance, plasma biochemistry indices, immune response, and antioxidant capacity of broiler chickens challenged or unchallenged with HS.
Our study showed that 2% SWM inclusion improved the BWG, FCR, AFI, and AWI overall regardless of HS. This was in accordance with Choi et al. (2014) and Kulshreshtha et al. (2014), who found a significant improvement in the growth performance of layers fed with 0.5% brown and red seaweed, respectively. In a study by Kumar (2018), dietary supplementation of Sargassum wightii (genus of brown seaweed) at 1%, 2%, 3%, and 4% enhanced BW, FI, FCR, and meat quality of broilers. Conversely, 0.5%, 1%, and 2% brown seaweed in broiler chickens’ diet did not influence the ABG, FI, or FCR of broiler chickens in the study by Bonos et al. (2016). The authors attributed this to several factors like housing conditions, basal diet, and production system (Bonos et al., 2016). The growth-promoting effects of brown seaweed leading to the increment of AFI and BWG in our study could be ascribed to its richness in fiber content (Table 1) and laminarin. Evidence revealed that dietary fiber could improve digestibility, feed intake, and overall growth performance in poultry production (Rezaei et al., 2014; Varastegani and Dahlan, 2014; Nassar et al., 2019). Laminarin interacts with a surface layer of the small intestine to improve nutrient digestibility, positively affecting broiler chickens' growth performance parameters (Sweeney et al., 2017). In addition, HS reduced the AFI of broiler chickens by 20.9% (about 167 g/bird) compared to TN birds which was consistent with past findings (Awad et al., 2020; Kikusato et al., 2021; Iyasere et al., 2021) while the body weight was unaffected by HS in our study. The decrease in feed intake among HS birds is a recovery mechanism to reduce body-heat increment (Song et al., 2013). There was no significant interaction between the dietary treatments and HS for growth performance parameters; however, among heat-stressed birds, we observed that the inclusion of brown seaweed meal and 2 mL brown seaweed extract numerically improved FCR by 18 and 21%, respectively, compared to NC. Studies on ruminants indicated that dietary supplementation with 2 and 4% brown seaweed meal improved productive performance through improving growth rate and blood constituents in heat-stressed Barki ram lamb (Ibrahim et al., 2020). Similarly, 4% Sargassum latifolium significantly improved body weight gain in heat-stressed exposed sheep (Ellamie et al., 2020). Meanwhile, dietary supplementation of seaweed (Ulva lactuca) did not enhance production performance in growing lambs reared under HS conditions (Abdoun et al., 2014). Taken together, our results suggest that dietary supplementation of 2% SWM improved palatability while increasing AFI, leading to improved BWG of broiler chickens compared to those fed the control diets.
The cloaca temperature and corticosterone level can reflect the degree of HS in broiler chickens. The cloaca temperature is a sign of balance between heat loss and gain, reflecting the central body temperature (Farag and Alagawany, 2018). The balance will be disrupted when the thermoregulatory mechanisms of birds are compromised (Selvam et al., 2018). The present results revealed that the rectal temperature of broiler chickens under HS treatment significantly increased, following HS exposure at 8hr/day for 7 d, indicating that the birds were stressed. Similarly, Altan et al. (2000) reported that broiler chickens challenged with HS (38 ± 1°C) for 2 h had significantly increased rectal temperature compared to the TN group. He et al. (2019) revealed that heat-stressed birds had significantly increased rectal temperature from the third time point to the end of the cyclic HS while a polyphenolic compound suppressed the increase. In our study, dietary treatments did not influence the rectal temperature. Aside from rectal temperature, corticosterone is secreted in response to physiological stress (Zeng et al., 2022). Studies have shown that cyclic HS markedly increased serum corticosterone concentration in broilers compared to the control group (Cheng et al., 2018; He et al., 2018). On the contrary, the corticosterone level was not affected by HS in our study; this agrees with Sun et al. (2015), who reported that exposing broiler chickens to 10 h HS (32°C) for 7 d did not influence their plasma corticosterone. The authors further explained that the effect of HS on plasma corticosterone is associated with the length of heat exposure (Sun et al., 2015). Acute heat exposure (32°C) for 3 and 6 h did not change the corticosterone level of birds as well (Lin et al., 2006). HS triggers the activity of the neuroendocrine system, resulting in activation of the hypothalamic-pituitary-adrenal axis, which subsequently elevates corticosterone levels (He et al., 2018).
HS can further cause metabolic disorders in broiler chickens. Our blood biochemistry analysis showed altered plasma electrolytes and metabolites in response to HS. The increase in plasma sodium, chloride, glucose, and uric acid among heat-stressed birds has been confirmed by past studies (Harsini et al., 2012; Hajati et al., 2015; Xie et al., 2015; Huang et al., 2018). Huang et al. (2018) observed increased sodium and decreased chloride concentration among birds exposed to 2 h HS (38 ± 1°C). Alterations in sodium and chloride concentrations can indicate kidney damage. As sodium and chloride excretion levels increase, their loss could result in acid-base disorder, which is detrimental to the kidney (Farag and Alagawany, 2018). Glucose and uric acid levels are good indicators of stress. Uric acid is excreted as an end product of nitrogen metabolism and can scavenge free radicals in birds (Hajati et al., 2015; Kikusato et al., 2021). Under HS conditions, catecholamines depletes liver glycogen and increased glucose level in the blood (Wasti et al., 2020). In the current study, dietary treatments did not influence sodium, chloride, and glucose level but 2% SWM supplementation suppressed plasma uric acid concentration in heat-stressed treated chickens. Hence, 2% SWM might mitigate oxidative damage by eliminating the need to produce uric acid (Kikusato et al., 2021). Phytogenic feed additives can lower blood uric acid through their antioxidant ability to depress the adeno-corticotropic hormone responsible for uric acid production (Kanani et al., 2016).
The increase in blood enzymes levels denote consequent enzyme leakage and could result from hepatocyte deterioration (Tessari et al., 2010). Specifically, high levels of AST, ALT, and GGT are sensitive indicators of liver damage (Will Castro et al., 2016). HS can cause liver damage that can enhance the serum levels of AST and ALT in chickens because of enzyme leakages from hepatocytes (Chang et al., 2020). In the current study, 2% SWM and 1 mL SWE decreased the plasma ALT and GGT of birds under HS conditions; respectively, which implies an amelioration of hepatic damage (Erinle et al., 2022) among treated birds. Dietary inclusion of galactofucan sulfate in brown seaweed reduced the activity of ALT, GGT, and AST in rats (Will Castro et al., 2016). We speculated that the hepatoprotective activity of seaweed on heat-stressed birds is associated with its richness in fucoidans that have been found to prevent induced liver damage via regulating inflammatory mediators (Lim et al., 2015). Fucoidan, a sulfated polysaccharide extract from brown seaweeds, consistently reduced plasma activity of ALT and AST and ameliorated hepatic accumulation in mice (Zheng et al., 2018). Brown seaweed meal and extract also increased plasma albumin concentration among heat-stressed birds in our study, indicating better liver functioning (Nhlane et al., 2020). Choi et al. (2018) reported the greatest blood albumin level among birds fed with brown seaweed products compared to other treatments. A linear increase in albumin concentration due to seaweed supplementation was previously observed by Balasubramanian et al. (2021).
Moreover, HS can impair intestinal absorption by reducing plasma digestive enzymes (Al-Zghoul et al., 2019). In the current study, HS decreased lipase activity compared to TN birds, and this finding agrees with Song et al. (2018), who reported reduced jejunal lipase activity after HS treatments. The lipase activity was enhanced by 1 mL SWE supplementation compared to 2% SWM in heat-stressed birds in our study, indicating that 1 mL SWE exerts a positive effect on the digestive capacity of broilers under HS and could be related to the anti-inflammatory and antibacterial activity of the seaweed extract (Wu, 2018). Several studies have revealed that polysaccharides from bioactive substances can improve the activities of lipase in broiler chickens (Hashemipour et al., 2013; Long et al., 2020). The significant difference in the effects of brown seaweed extract and meal on digestive enzymes might be due to the ability of the polyphenol contents of seaweed to bind with feed and gut contents forming insoluble complexes (Abdel-Moneim et al., 2020). In-water treatments are generally absorbed better than in-feed treatments. We also observed an increase in amylase activity in response to HS, contrary to previous studies (Huang et al., 2018; Jaiswal et al., 2018). The reason amylase activity increased with HS was not entirely understood; a possible explanation may be due to modulatory actions of brown seaweed in HS groups. An alternative hypothesis could be that, during heat stress, the body wants to limit the overall heat produced by reducing feed intake. It is possible that a higher amylase activity may help this process by improving starch digestion and that ultimately may help to reduce the feed intake. Among the heat-stressed birds, birds that received SWM and SWE had higher amylase levels than the control diet; however, this was not statistically significant. Overall, our results indicated that brown seaweed products might serve as a mitigative nutritional strategy to sustain liver functioning and improve chickens' digestive enzymes under HS.
Regarding immunoglobulins, there was no interaction between the dietary treatment and HS in the current study. Meanwhile, HS increased serum concentrations of IgG and IgM compared to TN birds in our present study, similar to Honda et al. (2015) findings. Our findings also agree with Nanto-HARA et al. (2021), who reported that HS (33°C for 14 d) activated immune responses by increasing plasma levels of immunoglobulins (IgY and IgM). Contradictorily, several articles have shown lower levels of IgA, IgM, and IgG in broiler chickens exposed to HS (Awad et al., 2020; Hu et al., 2022; Abdel-Moneim et al., 2022). Variation in results might be attributed to the duration of HS exposure, level of HS, time of sample collection, and presence of other stressors. For example, Honda et al. (2015) reported increased levels of IgG and IgM in heat-stressed chickens on d 7, and other results revealed lower concentrations of IgM on d 19 in heat-stressed vaccinated birds. Stressors can increase the levels of immunoglobulins at least 24 h after the end of stress stimuli application (Honda et al., 2015). Acute HS has also been discussed to improve immune function, while chronic heat stress has been shown to suppress immunity (Han et al., 2010). Furthermore, the present study had no treatment effect on the relative weight of the bursa and spleen. Matshogo et al. (2020) reported no changes on the internal organs except the decrease in the spleen in response to SWM intake in broilers. On the other hand, the dietary inclusion of Laminaria japonica, a type of seaweed, increased the weights of spleen, bursa, and thymus in 21 and 41-day-old broilers (Bai et al., 2019). Additionally, 0.4% red seaweed (Chondrus crispus) increased the relative weights of thymus and bursa (Martínez et al., 2019). Immune organ weights can reflect the immune status of chickens. The present findings signify that our HS protocol and dietary brown seaweed used in this study had no detrimental effects on the immune organs of chickens.
Cytokines are also important markers of immunity that can enhance host defence for controlling infection (Kaiser et al., 2009). Interleukins are major cytokines that play vital roles in stimulating inflammation and immune responses under HS conditions (Goel et al., 2021), in which excessive expression may lead to tissue damage. The present study showed an increase in the secretion of IL6 in response to HS while the IL10 remain unaltered. IL6 could act as a proinflammatory and anti-inflammatory cytokine. IL10 is produced simultaneously with proinflammatory cytokines and plays a central role in the anti-inflammatory response by preventing inflammatory pathology (Hietbrink et al., 2006). Brown seaweed extract can inhibit inflammatory responses by reducing the secretion of IL6 (Saeed et al., 2021). Similarly, dietary supplementation of seaweed-derived polysaccharides reduced the levels of pro-inflammatory cytokines and splenic inflammatory response of heat-stressed birds by suppressing the nuclear factor-kappa B signaling (Liu et al., 2022b). Nonetheless, dietary supplementation of brown seaweed meal and extract had no significant effects on the expression of IL6, and IL10 in chickens challenged or unchallenged with HS in the current study.
Brown seaweed can also improve the animal antioxidant defence systems and regulate their inflammatory responses (Ramadan et al., 2020; Michalak et al., 2022). Our results showed that dietary supplementation of brown seaweed meal and extract did not influence antioxidant enzymes (GPx, GR, and SOD). This agrees with Paul et al. (2020) that red seaweed (Kappaphycus alvarezii) did not affect most of the antioxidant enzymes in broiler chickens. In contrast, Kannan et al. (2007) observed that the dietary supplementation of brown seaweed powder increased the antioxidant status of goats exposed to preslaughter stress. Liu et al. (2021) found that dietary algae-derived polysaccharides supplementation improved the antioxidant capacity in the duodenum of broilers through the regulation of the nuclear factor erythroid 2-related factor 2 signaling pathway observed in the study (Liu et al., 2021). Ellamie et al. (2020) reported that 4% Sargassum latifolium improved the total antioxidant capacity and the activities of catalase and SOD in the sheep exposed to HS (Ellamie et al., 2020). Similarly, Saker et al. (2004) observed increased GPx and SOD and decreased MDA in heat-stressed lambs fed with brown seaweed (Tasco). SOD, GPx, and GR are essential antioxidants enzymes in the body, and their activities indirectly show the body's capability to combat oxidative stress (Hu et al., 2020). SOD is the first line of defence against ROS, while GPx catalyzes the reduction of hydrogen peroxides by reduced glutathione to preserve cells from oxidative damage (Nagami et al., 2005). HS further reduced GPx and GR while SOD was unaffected. The decreased antioxidant enzymes observed in this study confirmed the claim from multiple studies (Ahmed-Farid et al., 2021, Song et al., 2018; Hu et al., 2021; Wang et al., 2021) that HS can induce oxidative stress by disrupting the redox status (Wang et al., 2021). The antioxidant capacity and radical scavenging activity of seaweed are positively correlated with flavonoid concentration and the presence of flavonoids can be influenced by seaweed geographical area, harvest time, period of collection and extraction methods (Cotas et al., 2020). The effect of brown seaweed on the antioxidant status of chicken in our present study might be affected by several factors such as concentration and composition of polyphenols, the bioavailability of molecules and the type of chickens (Gessner et al., 2017). Liu et al. (2022b) speculated that the effect of seaweed treatments on the antioxidants-related gene expression could be dose-dependent. Lowered polyphenol contents in seaweed species are associated with oxidation and polymerization of phlorotannins, reflecting the antiproliferative effects and the quantity of secondary metabolites in seaweed (Yuan and Walsh, 2006).
In conclusion, our results indicated that HS reduced feed intake and affected blood biochemical parameters and immunoglobulins, while dietary supplementation of brown seaweed products improved the growth performance of birds irrespective of HS and may help to mitigate the negative effects of HS by improving the plasma and digestive enzyme activities of heat-stressed birds.
ACKNOWLEDGMENTS
The authors thank Sealife Seaplants, NS, Canada, for supplying the seaweed products used in this study and appreciate the staff of the Atlantic Poultry Research Centre – Michael McConkey, Sarah Macpherson, and Krista Budgell for help with animal care and other logistics. Thanks to Janice MacIsaac for diet formulation and other logistics, and Jamie Fraser for diet preparation. Appreciation goes to Taiwo Erinle, Taiwo Makinde, and Samson Oladokun for helping with feeding and sample collections. Funding for this study was provided by Nova Scotia Canadian Agricultural Partnership (CAP), Chicken Farmers of Nova Scotia, Chicken Farmers of Canada, and Mitacs.
DISCLOSURES
The authors declare no conflict of interest.
Footnotes
Part of this work was presented at the 2022 PSA Annual Meeting, San Antonio, TX, United States.
REFERENCES
- Abdelli N., Solà-Oriol D., Pérez J.F. Phytogenic feed additives in poultry: achievements, prospective and challenges. Animals. 2021;11:3471. doi: 10.3390/ani11123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Moneim A.E., Shehata A.M., Alzahrani S.O., Shafi M.E., Mesalam N.M., Taha A.E., Abd El-Hack M.E. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2020;104:1851–1866. doi: 10.1111/jpn.13455. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.-M.E., Shehata A.M., Khidr R.E., Paswan V.K., Ibrahim N.S., El-Ghoul A.A., Ebeid T.A. Nutritional manipulation to combat heat stress in poultry – a comprehensive review. J. Therm. Biol. 2021;98 doi: 10.1016/j.jtherbio.2021.102915. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.M.E., Shehata A.M., Mohamed N.G. Synergistic effect of Spirulina platensis and selenium nanoparticles on growth performance, serum metabolites, immune responses, and antioxidant capacity of heat-stressed broiler chickens. Biol. Trace. Elem. Res. 2022;200:768–779. doi: 10.1007/s12011-021-02662-w. [DOI] [PubMed] [Google Scholar]
- Abdoun K.A., Okab A.B., El-Waziry A.M., Samara E.M., Al-Haidary A.A. Dietary supplementation of seaweed (Ulva lactuca) to alleviate the impact of heat stress in growing lambs. Pak. Vet. J. 2014;34:108–111. [Google Scholar]
- Abudabos A.M., Okab A.B., Aljumaah R.S., Samara E.M., Abdoun K.A., Al-Haidary A.A. Nutritional value of green seaweed (Ulva lactuca) for broiler chickens. Ital. J. Anim. Sci. 2013;12:e28. doi: 10.2527/jas.2013-6719. [DOI] [PubMed] [Google Scholar]
- Adewole D., MacIsaac J., Fraser G., Rathgeber B. Effect of oat hulls incorporated in the diet or fed as free choice on growth performance, carcass yield, gut morphology and digesta short chain fatty acids of broiler chickens. Sustainability. 2020;12:3744. [Google Scholar]
- Ahmed-Farid A., Salah O., Nassan A.S., El-Tarabany M.A. Effects of chronic thermal stress on performance, energy metabolism, antioxidant activity, brain serotonin, and blood biochemical indices of broiler chickens. Animals. 2021;11:2554. doi: 10.3390/ani11092554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016;7:37. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altan O., Altan A., Oguz I., Pabuçcuoglu A., Konyalioglu S. Effects of heat stress on growth, some blood variables and lipid oxidation in broilers exposed to high temperature at an early age. Br. Poult. Sci. 2000;41:489–493. doi: 10.1080/713654965. [DOI] [PubMed] [Google Scholar]
- Al-Zghoul M.B., Alliftawi A.R.S., Saleh K.M.M., Jaradat Z.W. Expression of digestive enzyme and intestinal transporter genes during chronic heat stress in the thermally manipulated broiler chicken. Poult. Sci. 2019;98:4113–4122. doi: 10.3382/ps/pez249. [DOI] [PubMed] [Google Scholar]
- AOAC . 15th ed. Association of Official Analytical Chemists; Washington, DC: 1990. Official Methods of Analysis. [Google Scholar]
- Awad E., Najaa M., Zulaikha Z., Zulkifli I., Soleimani A. Effects of heat stress on growth performance, selected physiological and immunological parameters, caecal microflora, and meat quality in two broiler strains. Anim. Biosci. 2020;33:778–787. doi: 10.5713/ajas.19.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J., Wang R., Yan L., Feng J. Co-Supplementation of Dietary Seaweed Powder and Antibacterial Peptides Improves Broiler Growth Performance and Immune Function. Braz. J Poult. Sci. 2019;21 doi: 10.1590/1806-9061-2018-0826. [DOI] [Google Scholar]
- Balasubramanian B., Shanmugam S., Park S., Recharla N., Koo J.S., Andretta I., Kim I.H. Supplemental impact of marine red seaweed (Halymenia palmata) on the growth performance, total tract nutrient digestibility, blood profiles, intestine histomorphology, meat quality, fecal gas emission, and microbial counts in broilers. Animals. 2021;11:1244. doi: 10.3390/ani11051244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonos E., Kargopoulos A., Nikolakakis I., Florou-Paneri P., Christaki E. The seaweed Ascophyllum nodosum as a potential functional ingredient in chicken nutrition. J. Oceanogr. Mar. Res. 2016;4:140. [Google Scholar]
- Borzouie S., Rathgeber B.M., Stupart C.M., MacIsaac J., MacLaren L.A. Effects of dietary inclusion of seaweed, heat stress and genetic strain on performance, plasma biochemical and hematological parameters in laying hens. Animals. 2020;10:1570. doi: 10.3390/ani10091570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calik A., Emami N.K., Schyns G., White M.B., Walsh M.C., Romero L.F., Dalloul R.A. Influence of dietary vitamin E and selenium supplementation on broilers subjected to heat stress, Part II: oxidative stress, immune response, gut integrity, and intestinal microbiota. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CCAC-Canadian Council On Animal Care . CCAC; Ottawa: 2009. The Care and Use of Farm Animals in Research, Teaching and Testing; pp. 12–15. [Google Scholar]
- Chang Q., Lu Y., Lan R. Chitosan oligosaccharides as an effective feed additive to maintain growth performance, meat quality, muscle glycolytic metabolism and oxidative status in yellow-feather broilers under heat stress. Poult. Sci. 2020;99:4824–4831. doi: 10.1016/j.psj.2020.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan S.S., Rashamol V.P., Bagath M., Sejian V., Dunshea F.R. Impacts of heat stress on immune responses and oxidative stress in farm animals and nutritional strategies for amelioration. Int. J. Biometeorol. 2021;65:1231–1244. doi: 10.1007/s00484-021-02083-3. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Du M., Xu Q., Chen Y., Wen C., Zhou Y. Dietary mannan oligosaccharide improves growth performance, muscle oxidative status, and meat quality in broilers under cyclic heat stress. J Therm. Biol. 2018;75:106–111. doi: 10.1016/j.jtherbio.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Choi Y., Lee E., Na Y., Lee S. Effects of dietary supplementation with fermented and non-fermented brown algae by-products on laying performance, egg quality, and blood profile in laying hens. Anim. Biosci. 2018;31:1654–1659. doi: 10.5713/ajas.17.0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.J., Lee S.R., Oh J.-.W. Effects of dietary fermented seaweed and seaweed fusiforme on growth performance, carcass parameters and immunoglobulin concentration in broiler chicks. Asian-Australas. J. Anim. Sci. 2014;27:862–870. doi: 10.5713/ajas.2014.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotas J., Leandro A., Monteiro P., Pacheco D., Figueirinha A., Gonçalves A.M.M., Pereira L. Seaweed phenolics: from extraction to applications. Marine Drugs. 2020;18:384. doi: 10.3390/md18080384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Deek A.A., Brikaa A.M. Effect of different levels of seaweed in starter and finisher diets in pellet and mash form on performance and carcass quality of ducks. Int. J. Poult. Sci. 2009;8:1014–1021. [Google Scholar]
- Ellamie A.M., Fouda W.A., Ibrahim W.M., Ramadan G. Dietary supplementation of brown seaweed (Sargassum latifolium) alleviates the environmental heat stress-induced toxicity in male Barki sheep (Ovis aries) J. Therm. Biol. 2020;89 doi: 10.1016/j.jtherbio.2020.102561. [DOI] [PubMed] [Google Scholar]
- Erinle T.J., Oladokun S., MacIsaac J., Rathgeber B., Adewole D. Dietary grape pomace–effects on growth performance, intestinal health, blood parameters, and breast muscle myopathies of broiler chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag M.R., Alagawany M. Physiological alterations of poultry to the high environmental temperature. J Therm. Biol. 2018;76:101–106. doi: 10.1016/j.jtherbio.2018.07.012. [DOI] [PubMed] [Google Scholar]
- Gadde U., Kim W., Oh S., Lillehoj H. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Gessner D.K., Ringseis R., Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017;101:605–628. doi: 10.1111/jpn.12579. [DOI] [PubMed] [Google Scholar]
- Goel A., Ncho C.M., Choi Y.H. Regulation of gene expression in chickens by heat stress. J. Animal Sci. Biotechnol. 2021;12:11. doi: 10.1186/s40104-020-00523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering H.K., Van Soest P.J. Vol. 379. Agricultural Research Service, United States Department of Agriculture; Washington, DC: 1970. (Forage Fiber Analyses: Apparatus, Reagents, Procedures, and Some Applications). [Google Scholar]
- Gullón B., Gagaoua M., Barba F.J., Gullón P., Zhang W., Lorenzo J.M. Seaweeds as promising resource of bioactive compounds: overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Tech. 2020;100:1–18. [Google Scholar]
- Guo Y., Balasubramanian B., Zhao Z.H., Liu W.C. Marine algal polysaccharides alleviate aflatoxin B1-induced bursa of Fabricius injury by regulating redox and apoptotic signaling pathway in broilers. Poult. Sci. 2021;100:844–857. doi: 10.1016/j.psj.2020.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Zhao Z.H., Pan Z.Y., An L.L., Balasubramanian B., Liu W.C. New insights into the role of dietary marine-derived polysaccharides on productive performance, egg quality, antioxidant capacity, and jejunal morphology in late-phase laying hens. Poult. Sci. 2020;99:2100–2107. doi: 10.1016/j.psj.2019.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajati H., Hassanabadi A., Golian A., Nassiri-Moghaddam H., Nassiri M.R. The effect of grape seed extract and vitamin C feed supplementation on some blood parameters and HSP70 gene expression of broiler chickens suffering from chronic heat stress. Ital. J Anim. Sci. 2015;14:3273. [Google Scholar]
- Han A.Y., Zhang M.H., Zuo X.L., Zheng S.S., Zhao C.F., Feng J.H., Cheng C. Effect of acute heat stress on calcium concentration, proliferation, cell cycle, and interleukin-2 production in splenic lymphocytes from broiler chickens. Poult. Sci. 2010;89:2063–2070. doi: 10.3382/ps.2010-00715. [DOI] [PubMed] [Google Scholar]
- Harsini G.S., Habibiyan M., Moeini M.M. Effects of dietary selenium, vitamin e, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol. Trace Elem. Res. 2012;148:322–330. doi: 10.1007/s12011-012-9374-0. [DOI] [PubMed] [Google Scholar]
- Hashemipour H., Kermanshahi H., Golian A., Veldkamp T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013;92:2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- He S., Li S., Arowolo M.A., Yu Q., Chen F., Hu R., He J. Effect of resveratrol on growth performance, rectal temperature and serum parameters of yellow-feather broilers under heat stress. Anim. Sci. J. 2019;90:401–411. doi: 10.1111/asj.13161. [DOI] [PubMed] [Google Scholar]
- He S.P., Arowolo M.A., Medrano R.F., Li S., Yu Q.F., Chen J.Y., He J.H. Impact of heat stress and nutritional interventions on poultry production. World's Poult. Sci. J. 2018;74:647–664. [Google Scholar]
- Hietbrink F., Koenderman L., Rijkers G., Leenen L. Trauma: the role of the innate immune system. World J. Emerg. Surg. 2006;1:15. doi: 10.1186/1749-7922-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdt S.L., Kraan S. Bioactive compounds in seaweed: functional food applications and legislation. J. Appl. Phycol. 2011;23:543–597. [Google Scholar]
- Honda B.T.B., Calefi A.S., Costola-de-Souza C., Quinteiro-Filho W.M., da Silva Fonseca J.G., de Paula V.F., Palermo-Neto J. Effects of heat stress on peripheral T and B lymphocyte profiles and IgG and IgM serum levels in broiler chickens vaccinated for Newcastle disease virus. Poult. Sci. 2015;94:2375–2381. doi: 10.3382/ps/pev192. [DOI] [PubMed] [Google Scholar]
- Hu H., Bai X., Xu K., Zhang C., Chen L. Effect of phloretin on growth performance, serum biochemical parameters and antioxidant profile in heat-stressed broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Chen L., Dai S., Li J., Bai X. Effect of glutamine on antioxidant capacity and lipid peroxidation in the breast muscle of heat-stressed broilers via antioxidant genes and HSP70 pathway. Animals. 2020;10:404. doi: 10.3390/ani10030404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.Y., Mohammed A.A., Murugesan G.R., Cheng H.W. Effect of a synbiotic supplement as an antibiotic alternative on broiler skeletal, physiological, and oxidative parameters under heat stress. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Jiao H., Song Z., Zhao J., Wang X., Lin H. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 2015;93:2144–2153. doi: 10.2527/jas.2014-8739. [DOI] [PubMed] [Google Scholar]
- Huang S., Yang H., Rehman M.U., Tong Z. Acute heat stress in broiler chickens and its impact on serum biochemical and electrolyte parameters Indian. J. Anim. Res. 2018;52:683–686. [Google Scholar]
- Ibrahim N.H., Ellamie Ashgan.M., Fouda Wafaa.A., Younis F.E. Physiological and behavioral responses of growing barki ram lambsexposed to heat stress and fed brown seaweed as additives under semi-arid conditions. J. Anim. Poult. Prod. 2020;11:55–56. [Google Scholar]
- Iyasere O.S., Bateson M., Beard A.P., Guy J.H. Provision of additional cup drinkers mildly alleviated moderate heat stress conditions in broiler chickens. J. Appl. Anim. Welf. Sci. 2021;24:188–199. doi: 10.1080/10888705.2020.1846534. [DOI] [PubMed] [Google Scholar]
- Jaiswal S.K., Tyagi J.S., Raza M., Uniyal S., Mishra A., Chandrakar C. Effect of protein synthesis modulator and acute heat stress on jejunal digestive enzymes in broiler chicken. Pharma Innovat. 2018;7:884–887. [Google Scholar]
- Jang I.S., Ko Y.H., Moon Y.S., Sohn S.H. Effects of Vitamin C or E on the pro-inflammatory cytokines, heat shock protein 70 and antioxidant status in broiler chicks under summer conditions. Asian-Australas. J. Anim. Sci. 2014;27:749–756. doi: 10.5713/ajas.2013.13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser P., Wu Z., Rothwell L., Fife M., Gibson M., Poh T.-Y., Shini S. Prospects for understanding immune-endocrine interactions in the chicken. Gen. Comp. Endocrinol. 2009;163:83–91. doi: 10.1016/j.ygcen.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Kanani B., Daneshyar P., Najafi M. Effects of cinnamon(Cinnamomum zeylanicum) and turmeric (Curcuma longa) powders on performance, enzyme activity, and blood parameters of broiler chickens under heat stress. Poult. Sci. J. 2016;1:47–53. [Google Scholar]
- Kannan G., Saker K.E., Terrill T.H., Kouakou B., Galipalli S., Gelaye S. Effect of seaweed extract supplementation in goats exposed to simulated preslaughter stress. Small Rum. Res. 2007;73:221–227. [Google Scholar]
- Khan A.Z., Khan I.U., Khan S., Afzal S., Hamid M., Tariq Selenium-enriched probiotics improve hepatic protection by regulating pro-inflammatory cytokines and antioxidant capacity in broilers under heat stress conditions. J. Adv. Vet. Anim. Res. 2019;6:355–361. doi: 10.5455/javar.2019.f354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R.U., Naz S., Ullah H., Ullah Q., Laudadio V., Qudratullah. Bozzo G., Tufarelli V. Physiological dynamics in broiler chickens under heat stress and possible mitigation strategies. Anim. Biotechnol. 2021;2:1–10. doi: 10.1080/10495398.2021.1972005. [DOI] [PubMed] [Google Scholar]
- Kikusato M., Xue G., Pastor A., Niewold T.A., Toyomizu M. Effects of plant-derived isoquinoline alkaloids on growth performance and intestinal function of broiler chickens under heat stress. Poult. Sci. 2021;100:957–968. doi: 10.1016/j.psj.2020.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulshreshtha G., Hincke M.T., Prithiviraj B., Critchley A. A review of the varied uses of macroalgae as dietary supplements in selected poultry with special reference to laying hen and broiler chickens. J. Mar. Sci. Eng. 2020;8:536. [Google Scholar]
- Kulshreshtha G., Rathgeber B., Stratton G., Thomas N., Evans F., Critchley A., Prithiviraj B. Feed supplementation with red seaweeds, Chondrus crispus and Sarcodiotheca gaudichaudii, affects performance, egg quality, and gut microbiota of layer hens. Poult. Sci. 2014;93:2991–3001. doi: 10.3382/ps.2014-04200. [DOI] [PubMed] [Google Scholar]
- Kumar A.K. Effect of Sargassum wightii on growth, carcass and serum qualities of broiler chickens. Vet. Sci. Res. 2018;3:156. [Google Scholar]
- Li X.J., Ye Q.F. Fucoidan reduces inflammatory response in a rat model of hepatic ischemia-reperfusion injury. Can. J. Physiol. Pharmacol. 2015;93:999–1005. doi: 10.1139/cjpp-2015-0120. [DOI] [PubMed] [Google Scholar]
- Li Y., Fu X., Duan D., Liu X., Xu J., Gao X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs. 2017;15:49. doi: 10.3390/md15020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J., Lee S., Kim T., Jang S.-A., Kang S.E., Koo H., Sohn E., Bak J., Namkoong S., Kim H., Song I.N., Kim N., Sohn E.-H., Han J. Fucoidan from Fucus vesiculosus protects against alcohol- induced liver damage by modulating inflammatory mediators in mice and HepG2 cells. Mar. Drugs. 2015;13:1051–1067. doi: 10.3390/md13021051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Decuypere E., Buyse J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. Part A. 2006;144:11–17. doi: 10.1016/j.cbpa.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Liu L., Ren M., Ren K., Jin Y., Yan M. Heat stress impacts on broiler performance: a systematic review and meta-analysis. Poult. Sci. 2020;99:6205–6211. doi: 10.1016/j.psj.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Yuan Y., Sun C., Balasubramanian B., Zhao Z., An L. Effects of dietary betaine on growth performance, digestive function, carcass traits, and meat quality in indigenous yellow-feathered broilers under long-term heat stress. Animals. 2019;9:506. doi: 10.3390/ani9080506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.C., Guo Y., Zhao Z.H., Jha R., Balasubramanian B. Algae-derived polysaccharides promote growth performance by improving antioxidant capacity and intestinal barrier function in broiler chickens. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.601336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.C., Pan Z.Y., Zhao Y., Guo Y., Qiu S.J., Balasubramanian B., Jha R. Effects of heat stress on production performance, redox status, intestinal morphology and barrier-related gene expression, cecal microbiome, and metabolome in indigenous broiler chickens. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.890520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.-C., Zhu Y.-R., Zhao Z.-H., Jiang P., Yin F.-Q. Effects of dietary supplementation of algae-derived polysaccharides on morphology, tight junctions, antioxidant capacity and immune response of duodenum in broilers under heat stress. Animals. 2021;11:2279. doi: 10.3390/ani11082279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.-C., Zhuang D.-P., Zhao Y., Balasubramanian B., Zhao Z.-H. Seaweed-derived polysaccharides attenuate heat stress-induced splenic oxidative stress and inflammatory response via regulating Nrf2 and NF-κB signaling pathways. Mar. Drugs. 2022;20:358. doi: 10.3390/md20060358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L.N., Kang B.J., Jiang Q., Chen J.S. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult. Sci. 2020;99:744–751. doi: 10.1016/j.psj.2019.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lordan S., Smyth T.J., Soler-Vila A., Stanton C., Ross R.P. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013;141:2170–2176. doi: 10.1016/j.foodchem.2013.04.123. [DOI] [PubMed] [Google Scholar]
- Martínez Y., Ayala L., Hurtado C., Más D., Rodríguez R. Effects of dietary supplementation with red algae powder (Chondrus crispus) on growth performance, carcass traits, lymphoid organ weights and intestinal pH in broilers. Braz. J. Poult. Sci. 2019;21 doi: 10.1590/1806-9061-2019-1015. [DOI] [Google Scholar]
- Matshogo T.B., Mnisi C.M., Mlambo V. Dietary green seaweed compromises overall feed conversion efficiency but not blood parameters and meat quality and stability in broiler chickens. Agriculture. 2020;10:547. [Google Scholar]
- Méresse S., Fodil M., Fleury F., Chénais B. Fucoxanthin, a marine-derived carotenoid from brown seaweeds and microalgae: a promising bioactive compound for cancer therapy. Int. J. Mol. Sci. 2020;21:9273. doi: 10.3390/ijms21239273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak I., Tiwari R., Dhawan M., Alagawany M., Farag Mayada R., Sharun K., Emran T.B., Dhama K. Antioxidant effectsof seaweeds and their active compounds on animal health and production – a review. Vet. Q. 2022;42:48–67. doi: 10.1080/01652176.2022.2061744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadigheisar M., Shouldice V.L., Sands J.S., Lepp D., Diarra M.S., Kiarie E.G. Growth performance, breast yield, gastrointestinal ecology and plasma biochemical profile in broiler chickens fed multiple doses of a blend of red, brown and green seaweeds. Br. Poult. Sci. 2020;61:590–598. doi: 10.1080/00071668.2020.1774512. [DOI] [PubMed] [Google Scholar]
- Nagami H., Yoshimoto N., Umakoshi H., Shimanouchi T., Kuboi R. Liposome-assisted activity of superoxide dismutase under oxidative stress. J. Biosci. Bioeng. 2005;99:423–428. doi: 10.1263/jbb.99.423. [DOI] [PubMed] [Google Scholar]
- Nanto-HARA F., Ohtsu H., Yamazaki1 M., Hirakawa T., Sato K., Murakami H. Effects of dietary brown rice on the growth performance, systemic oxidative status, and splenic inflammatory responses of broiler chickens under chronic heat stress. J. Poult. Sci. 2021;58:154–162. doi: 10.2141/jpsa.0200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar M.K., Lyu S., Zentek J., Brockmann G.A. Dietary fiber content affects growth, body composition, and feed intake and their associations with a major growth locus in growing male chickens of an advanced intercross population. Livest. Sci. 2019;227:135–142. [Google Scholar]
- Nhlane L.T., Mnisi C.M., Mlambo V., Madibana M.J. Nutrient digestibility, growth performance, and blood indices of boschveld chickens fed seaweed-containing diets. Animals. 2020;10:1296. doi: 10.3390/ani10081296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke O.E., Uyanga V.A., Iyasere O.S., Oke F.O., Majekodunmi B.C., Logunleko M.O., Onagbesan O.M. Environmental stress and livestock productivity in hot-humid tropics: alleviation and future perspectives. J. Therm. Biol. 2021;100 doi: 10.1016/j.jtherbio.2021.103077. [DOI] [PubMed] [Google Scholar]
- Paul S.S., Venkata H.G.R.V., Raju M.V.L.N., Rao S.V.R., Nori S.S., Suryanarayan S., Prasad C.S. Dietary supplementation of extracts of red sea weed (Kappaphycus alvarezii) improves growth, intestinal morphology, expression of intestinal genes and immune responses in broiler chickens. J. Sci. Food. Agric. 2020;101:997–1008. doi: 10.1002/jsfa.10708. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Calefi A.S., Cruz D., Aloia T., Zager A., Astolfi-Ferreira C.S., Piantino Ferreira J.A., Sharif S., Palermo-Neto J. Heat stress decreases expression of the cytokines, avian β-defensins 4 and 6 and Toll-like receptor 2 in broiler chickens infected with Salmonella Enteritidis. Vet. Immunol. Immunopathol. 2017;186:19–28. doi: 10.1016/j.vetimm.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sakai M., Sa L.R.M., Ferreira A.J.P., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Ramadan G., Fouda W.A., Ellamie A.M., Ibrahim W.M. Dietary supplementation of Sargassum latifolium modulates thermo-respiratory response, inflammation, and oxidative stress in bacterial endotoxin-challenged male Barki sheep. Environ. Sci. Pollut. Res. 2020;27:33863–33871. doi: 10.1007/s11356-020-09568-5. [DOI] [PubMed] [Google Scholar]
- Rezaei M., Torshizi M.A.K., Shariatmadari F. Inclusion of processed rice hulls as insoluble fiber in the diet on performance and digestive traits of Japanese quails. J. Anim. Sci. Adv. 2014;4:962–972. [Google Scholar]
- Saeed M., Arain M.A., Fazlani A.S. A comprehensive review on the health benefits and nutritional significance of fucoidan polysaccharide derived from brown seaweeds in human, animals and aquatic organisms. Aquacult. Nutr. 2021;27:633–654. [Google Scholar]
- Saker K.E., Fike J.H., Veit H., Ward D.L. Brown seaweed- (TascoTM) treated conserved forage enhances antioxidant status and immune function in heat-stressed wether lambs. J. Anim. Physiol. Anim. Nutr. 2004;88:122–130. doi: 10.1111/j.1439-0396.2003.00468.x. [DOI] [PubMed] [Google Scholar]
- Saracila M., Panaite T.D., Mironeasa S., Untea A.E. Dietary supplementation of some antioxidants as attenuators of heat stress on chicken meat characteristics. Agriculture. 2021;11:638. [Google Scholar]
- Selvam R., Suresh S., Saravanakumar M., Chandrasekaran C.V., Prashanth D. Alleviation of heat stress by a polyherbal formulation, phytocee™: impact on zootechnical parameters, cloacal temperature, and stress markers. Phcog. Res. 2018;10:1–8. doi: 10.4103/pr.pr_138_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui. Kang D., Park J., Khan M., Belal S.A., Shin D., Shim K. Altered relationship between gluconeogenesis and immunity in broilers exposed to heat stress for different durations. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui S.H., Kang D., Park J., Khan M., Shim K. Chronic heat stress regulates the relation between heat shock protein and immunity in broiler small intestine. Sci. Rep. 2020;10:1–11. doi: 10.1038/s41598-020-75885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Jiao L.F., Xiao K., Luan Z.S., Hu C.H., Shi B., Zhan X.A. Cello-oligosaccharide ameliorates heat stress-induced impairment of intestinal microflora, morphology and barrier integrity in broilers. Anim. Feed. Sci. Technol. 2013;185:175–181. [Google Scholar]
- Song Z.H., Cheng K., Zheng X.C., Ahmad H., Zhang L.L., Wang T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018;97:430–437. doi: 10.3382/ps/pex312. [DOI] [PubMed] [Google Scholar]
- Sun X., Zhang H., Sheikhahmadi A. Effects of heat stress on the gene expression of nutrient transporters in the jejunum of broiler chickens (Gallus gallus domesticus) Int. J. Biometeorol. 2015;59:127–135. doi: 10.1007/s00484-014-0829-1. [DOI] [PubMed] [Google Scholar]
- Surai Kochish., Fisinin, and Kidd Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants. 2019;8:235. doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney T., Meredith H., Vigors S., McDonnell M.J., Ryan M., Thornton K., O'Doherty J.V. Extracts of laminarin and laminarin/fucoidan from the marine macroalgal species Laminaria digitata improved growth rate and intestinal structure in young chicks, but does not influence Campylobacter jejuni colonisation. Anim. Feed. Sci. Technol. 2017;232:71–79. [Google Scholar]
- Tessari E.N., Kobashigawa E., Cardoso A.L., Ledoux D.R., Rottinghaus G.E., Oliveira C.A. Effects of Aflatoxin B1 and Fumonisin B1 on blood biochemical parameters in broilers. Toxins. 2010;2:453–460. doi: 10.3390/toxins2040453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varastegani A., Dahlan I. Influence of dietary fiber levels on feed utilization and growth performance in poultry. J. Anim. Prod. Adv. 2014;4:422–429. [Google Scholar]
- Varasteh S., Braber S., Akbari P., Garssen J., Fink-Gremmels J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venardou B., O'Doherty J.V., Vigors S., O'Shea C.J., Burton E.J., Ryan M.T., Sweeney T. Effects of dietary supplementation with a laminarin-rich extract on the growth performance and gastrointestinal health in broilers. Poultr. Sci. 2021;100 doi: 10.1016/j.psj.2021.101179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zhao F., Li Z., Jin X., Chen X., Geng Z., Zhang C. Effects of resveratrol on growth performance, intestinal development, and antioxidant status of broilers under heat stress. Animals. 2021;11:1427. doi: 10.3390/ani11051427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasti S., Sah N., Mishra B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals. 2020;10:1266. doi: 10.3390/ani10081266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will Castro L.S.E.P., Gomes Castro A.J., da S Nascimento Santos M., de Sousa Pinheiro T., de Quevedo Florentin K., Alves L.G., Leite E.L. Effect of galactofucan sulfate of a brown seaweed on induced hepatotoxicity in rats, sodium pentobarbital-induced sleep, and anti-inflammatory activity. J. Appl. Phycol. 2016;28:2005–2017. [Google Scholar]
- Wu S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018;97:3489–3493. doi: 10.3382/ps/pey220. [DOI] [PubMed] [Google Scholar]
- Xie J., Tang L., Lu L., Zhang L., Lin X., Liu H.C., Luo X. Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders. Poult. Sci. 2015;94:1635–1644. doi: 10.3382/ps/pev105. [DOI] [PubMed] [Google Scholar]
- Xu Y., Lai X., Li Z., Zhang X., Luo Q. Effect of chronic heat stress on some physiological and immunological parameters in different breed of broilers. Poult. Sci. 2018;11:4073–4082. doi: 10.3382/ps/pey256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Tan G.Y., Fu Y.Q., Feng J.H., Zhang M.H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Physiol. Part C. 2010;151:204–208. doi: 10.1016/j.cbpc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Yuan Y.V., Walsh N.A. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem. Toxicol. 2006;44:1144–1150. doi: 10.1016/j.fct.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Zeng L., Liu Q., Wang T., Yang Y., Jado A., Elhadidi Y., Pan J. Effects of monochromatic blue light on reducing the adverse impact of induced cyclic chronic heat stress during the thermal manipulation of broiler embryos. Oxid. Med. Cell Longev. [e-pub ahead of print] 2022 doi: 10.1155/2022/9898311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wang C., Zhao X., Chen K., Geng Z. Effect of L-theanine on meat quality, muscle amino acid profiles, and antioxidant status of broilers. Anim. Sci. J. 2020;91:e13351. doi: 10.1111/asj.13351. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhao X.H., Yang L., Chen X.Y., Jiang R.S., Jin S.H., Geng Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017;96:4325–4332. doi: 10.3382/ps/pex266. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Balasubramanian B., Guo Y., Qiu S.J., Jha R., Liu W.C. Dietary Enteromorpha polysaccharides supplementation improves breast muscle yield and is associated with modification of mRNA transcriptome in broiler chickens. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.663988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L.-X., Chen X.-Q., Cheong K.-L. Current trends in marine algae polysaccharides: the digestive tract, microbial catabolism, and prebiotic potential. Int. J Biol. Macromol. 2020;151:344–354. doi: 10.1016/j.ijbiomac.2020.02.168. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Liu T., Wang Z., Xu Y., Zhang Q., Luo D. Low molecular weight fucoidan attenuates liver injury via SIRT1/AMPK/PGC1α axis in db/db mice. Int. J. Biol. Macromol. 2018;112:929–936. doi: 10.1016/j.ijbiomac.2018.02.072. [DOI] [PubMed] [Google Scholar]