Abstract
Previous studies have shown that cyclic peptides corresponding to residues 35 to 52 of the Limulus antilipopolysaccharide (anti-LPS) factor (LALF) bind and neutralize LPS-mediated in vitro and in vivo activities. Therapeutic approaches based on agents which bind and neutralize LPS activities are particularly attractive because these substances directly block the primary stimulus for the entire proinflammatory cytokine cascade. Here we describe new activities of the LALF31–52 peptide, other than its LPS binding ability. Surprisingly, supernatants from human mononuclear cells stimulated with the LALF peptide are able to induce in vitro antiviral effects on the Hep-2 cell line mediated by gamma interferon (IFN-γ) and IFN-α. Analysis of the effect of LALF31–52 on tumor necrosis factor (TNF) and nitric oxide (NO) production by LPS-stimulated peritoneal macrophages revealed that a pretreatment with the peptide decreased LPS-induced TNF production but did not affect NO generation. This indicates that the LALF peptide modifies the LPS-induced response. In a model in mice with peritoneal fulminating sepsis, LALF31–52 protected the mice when administered prophylactically, and this effect is related to reduced systemic TNF-α levels. This study demonstrates, for the first time, the anti-inflammatory properties of the LALF-derived peptide. These properties widen the spectrum of the therapeutic potential for this LALF-derived peptide and the molecules derived from it. These agents may be useful in the prophylaxis and therapy of viral and bacterial infectious diseases, as well as for septic shock.
Lipopolysaccharide (LPS) is believed to be the initiator of the systemic inflammatory cascade that culminates in the organ damage and multiorgan failure found in septic patients (17, 47). In recent years, attention has been focused on the release by macrophages of cytokines, particularly interleukin 1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α), that are important mediators of septic shock pathology (9, 39). Recent data have substantially advanced our knowledge on the complex timing and interactions among events like LPS exposure, cytokine release (32), and the development of septic shock. These data strongly involve LPS and macrophage cytokine release as primary effectors in the etiology of septic shock. They also suggest that LPS blockade, cytokine blockade, or specific cytokine modulation may be of therapeutic benefit in septic patients.
Therapeutic approaches based on molecules that bind and neutralize LPS are particularly attractive, because they directly block the primary stimulus for the proinflammatory cytokine cascade. Recent studies have mainly involved rBPI (recombinant bactericidal permeability-increasing protein) (1) and peptides derived from it (13, 36). The Limulus anti-LPS factor (LALF) is a small, basic protein found in hemocytes from both Tachypleus tridentatus and Limulus polyphemus, which inhibits the endotoxin-mediated activation of the coagulation cascade (30), apparently by binding to LPS. This protein factor reduces mortality in experimental animals when administered before or after LPS challenge or gram-negative bacterial infection (46).
A major problem with antiendotoxin therapy is that, whereas endotoxemia can be intermittent and recurrent, the administered LPS-binding proteins are, in general, rapidly cleared; consequently, LPS is only transiently neutralized by these therapies. For this reason, the development of complementary therapeutic approaches and particularly of immunomodulators is currently envisaged for the treatment of sepsis.
Recently, the use of certain biological-response modifiers that enhance the functional status of macrophages and neutrophils has increased resistance to challenge with gram-negative bacteria (48). In addition, recent alternative concepts for an intervention in the systemic immune response syndrome, other than strategies directed toward the suppression of a single target cytokine, involve inducing a shift of the immune response favoring the production of proinflammatory mediator antagonists (24). These effects were observed after granulocyte colony-stimulating factor treatment of healthy volunteers (20) and after treatment with methylxanthines that inhibit the production of TNF and other mediators (40). It has been demonstrated that there is a biphasic response in sepsis: an initial hyperinflammatory phase followed by a hypoinflammatory phase. The latter is characterized by a monocytic deactivation, which is called “immunoparalysis” by Volk (45). While anti-inflammatory therapy (e.g., anti-TNF antibodies, IL-1 receptor antagonist, and IL-10) is sensible during the initial hyperinflammatory phase, immune stimulation by removing inhibitory factors (plasmapheresis) or the administration of monocyte-activating cytokines (gamma interferon [IFN-γ], granulocyte-macrophage colony-stimulating factor, etc.) may be more useful during immunoparalysis (45).
We synthesized a peptide comprising amino acids 31 to 52 from LALF (LALF31–52), a region previously described as a potential binding site for LPS (22). In this paper, we show evidence of new activities of this LALF-derived peptide. Surprisingly, supernatants from human peripheral blood mononuclear cells (PBMC) incubated with the peptide showed antiviral properties. Furthermore, the peptide modifies the response of LPS-activated peritoneal macrophages. In mice, it is able to increase survival when prophylactically administered in induced bacteremia, an effect that is related with a reduction in systemic TNF-α levels. In this report, we present evidence demonstrating that LALF31–52 is a molecule capable not only of binding LPS, but also of modulating the inflammatory immune response, providing a wider range of protection in therapeutic application than was previously recognized.
MATERIALS AND METHODS
Reagents and strains.
Escherichia coli O111:B4 LPS, phytohemagglutinin (PHA), and NG-methyl-l-arginine (l-NMMA) were purchased from Sigma Chemical Co. (St. Louis, Mo.).
The Pseudomonas aeruginosa strain used in the animal model was obtained from the American Type Culture Collection (ATCC 1960).
Peptide synthesis.
Peptides were synthesized using a solid-phase procedure on 4-methylbenzydrilamine resin, with a loading of 1.2 mmol/g and coupling via N,N-diisopropylcarbodiimide activation. All amino acids were supplied by BACHEM. The butoxycarbonyl (Boc) group was removed with 37.5% trifluoroacetic acid in CH2Cl2. The “low-high” HF procedure was used during the deprotection step in multiple deprotection equipment. Crude peptides were extracted with a 30% acetic acid solution in water, lyophilized, and then purified by reverse-phase high-performance liquid chromatography (RP-HPLC). The molecular masses of purified peptides were verified using a JEOL JMS-HX110HF two-sector mass spectrometer equipped with a fast atom bombardment (FAB) gun. The peptides used in these studies are as follows: amino acids 86 to 99 of human LPS binding protein (follow hLBP86–99), LALF31–52 (22), and amino acids 106 to 128 of 18-kDa cationic antimicrobial protein (Cap-18106–128) (21). Peptide LALF31–52 was cyclized by oxidation of the cysteine residues.
Assay for antiviral activity on Hep-2 cells.
Antiviral activity was estimated by determining the inhibition of the cytopathic effect produced by viruses on Hep-2 cells, a larynx epidermoid carcinoma cell line (ATCC CCL 23). This is a common procedure for determining the antiviral activity of interferons (10). For this purpose, 96-well plastic culture plates (Nunc, Roskilde, Denmark) were used. Cells (105 per well) were grown in an Eagle minimal essential medium (MEM) supplemented with 10% fetal calf serum up to monolayer formation (24 h). Then 100 μl of serial dilutions of the samples was added to the plates and incubated for another 24 h. Cells were washed with saline and infected with 107 PFU of mengovirus (provided by The Institute of Molecular Biology, University of Zurich, Zurich, Switzerland). Eighteen hours later, cell viability was measured by fixation and staining with crystal violet, and plates were read using an Ultramicroanalytical System (SUMA) photometer. Data were calculated and corrected, and results were expressed as arbitrary units of antiviral activity (UAA) (12). These units were set according to the biological activity of the standard preparations of human IFN-γ used in this same assay. One unit of antiviral activity for the standards is defined as the quantity of IFN capable of producing a 50% inhibition of the viral cytopathic effect.
Neutralization of antiviral activity.
The antiviral effect induced by the supernatant from PBMC incubated with 5 μM LALF31–52 was neutralized using antibodies specific to human IFN-α purchased from Innogenetics (Belgium). Serial dilutions of the samples were incubated with 100 neutralizing units (NU) of anti-IFN-α in MEM for 1 h at 37°C, 3% CO2, and 95% humidity. Thereafter, 100 μl of the mixture was added to the cells and the assay for antiviral activity was carried out as described above.
Stimulation of PBMC.
Venous blood was obtained from healthy volunteers, and mononuclear cells were isolated by Ficoll-Hypaque centrifugation. Cells were washed and resuspended in an endotoxin-free RPMI-1640 culture medium supplemented with 10% fetal calf serum. Cells (2 × 106 per ml) were seeded into 24-well microtiter plates (Nunc). Cells were incubated either with LBP86–99, Cap-18108–128, on LALF31–52 at 5 μM or with 10 ng of LPS per ml. All incubations were carried out at 37°C for 18 h under a 5% CO2 atmosphere. Then cell culture supernatants were assayed for antiviral activity on Hep-2 cells by using the procedure described above.
Release of cytokines in LALF31–52-stimulated human mononuclear cells.
The presence of TNF-α and IL-6 in the supernatant was determined using a specific sandwich enzyme-linked immunosorbent assay (ELISA), kindly provided by Wim Buurman (University of Maastricht, The Netherlands) (6). LPS (10 ng/ml) was used as a cytokine inducer.
A double-monoclonal antibody (MAb) sandwich ELISA was used to determine the presence of IFN-γ as a secretion product in the supernatant of cultures. Primary and secondary biotinylated MAbs and recombinant IFN-γ were purchased from PharMingen (San Diego, Calif.) PHA at 1% (vol/vol) in the culture medium was used as an IFN-γ inducer.
Analysis of cytokine gene expression in LALF31–52-stimulated human mononuclear cells.
The expression of distinct mRNA species was analyzed on samples of total RNA from stimulated and nonstimulated human PBMC by using the PharMingen RiboQuant multiprobe RNase protection assay (RPA) system with the hck-1 probe template set (catalog no. 45031P). This set includes mRNA probes to analyze different species of cytokines (IL-5, IL-4, IL-10, IL-15, IL-9, IL-2, IL-13, and IFN-γ). The RPA is a highly sensitive and specific method for the detection, quantitation, and characterization of RNA molecules (5, 16).
Briefly, a high-specific-activity, [32P]labeled antisense cytokine RNA probe set (hck-1) is hybridized in excess to target RNA from PBMC incubated with 5 μM peptide for 18 h. Free probes and other single-stranded RNA molecules are digested with RNases, whereas annealed probe-target RNA duplexes are protected from RNase digestion. These remaining RNase-protected probes are purified, resolved on denaturing polyacrylamide gels according to size, and imaged by autoradiography. The identity and quantity of each mRNA species in the original RNA sample can then be determined based on the signal intensities given by protected probe fragment bands of the appropriate sizes. An unprotected probe set and a probe for housekeeping gene transcripts are incorporated in order to identify and quantify the RNA molecules in the original samples.
Total RNA was obtained using a single-step method of RNA isolation by acid guanidinium-phenol-chloroform (AGPC) extraction (4).
Release of NO end products by mouse macrophages.
Peritoneal macrophages were obtained from BALB/c mice (CENPALAB, Havana, Cuba). Mouse peritoneal exudate macrophages were collected from the peritoneal cavity 4 days after the intraperitoneal (i.p.) injection of 1 ml of 4% Brewer's thioglycolate broth. Cells were maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 200 U of penicillin per ml, and 200 μg of streptomycin per ml at 37°C under a 5% CO2 atmosphere. Nitrite, which is a parameter of nitric oxide (NO) release by stimulated macrophages, was detected as previously described (33). Then cells were placed in 96-well plates at 2 × 106 cells per ml and incubated at 37°C for 2 h. Nonadherent cells were removed by repeated washings with cool saline. Macrophages were incubated with 5 μM LALF31–52 for 18 h; afterwards, cells were thoroughly washed with RPMI 1640 medium and then treated with 1 μg of LPS per ml for 72 h. Cell viability was assessed by the mitochondria-dependent reduction of MTT to formazan (43). Portions (15 μl) of cultured medium were mixed with an equal volume of Griess's reagent (1% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride, 2.5% H3PO4) (19). Absorbance at 540 nm was read in a microplate reader (SUMA) with sodium nitrite as the standard. The nitrite content of a similarly incubated cell-free medium was subtracted. The concentration of TNF in supernatants of mouse macrophages was determined using a specific sandwich ELISA.
Model of fulminating gram-negative peritoneal sepsis.
A model of fulminating gram-negative peritoneal sepsis in BALB/c mice was used to characterize the efficacy of the LALF peptide in protecting an immunologically intact host against serious infection. Groups of six 8-week-old mice received i.p. 5 μM LALF31–52 in 0.1 ml of saline, (0.7 μg/g of body weight) or saline alone (0.1 ml), 20 h prior to the infection inoculum. A dose of 2 × 108 P. aeruginosa CFU per mouse, which produced about 90% mortality, was used to induce experimental peritonitis. Survival was recorded every 24 h, for 120 h after bacterial injection. Blood was collected for TNF-α quantitation from the retroorbital sinus at 20, 40, 60, 90, 120, 140, 180, and 240 min after the challenge. The concentration of TNF-α was measured by ELISA.
Statistical methods.
All values in figures are expressed as means ± standard deviations (SD). A Kruskal-Wallis test was used to compare means between groups (P values below 0.05 were considered statistically significant). In survival experiments, the Kaplan-Meier survival function estimate was determined and the statistical significance was analyzed by the log rank test.
RESULTS
Antiviral effect of supernatants from LALF31–52-stimulated PBMC.
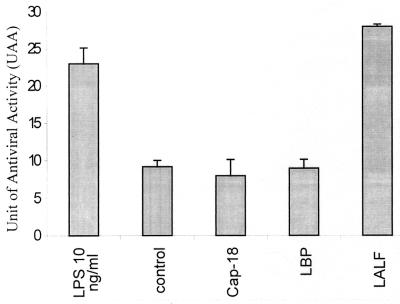
Supernatants from human mononuclear cells incubated for 18 h with LALF31–52 protected Hep-2 cells against viral infection. Figure 1 shows the antiviral effect induced by supernatants from the PBMC incubated with the different peptides or 10 ng of LPS per ml; the latter was used as an IFN inducer. The results show that only LALF31–52 or LPS was able to stimulate the human mononuclear cells and induce the release of a factor capable of giving Hep-2 cells protection against viral infection. Supernatants were diluted at least fourfold in order to estimate their ability to inhibit the viral cytopathic effect, decreasing the peptide concentration as low as 1.2 μM. It is notable that the treatment of Hep-2 cells with peptide concentrations below 5 μM did not induce cell protection against viral infection, as shown in Table 1. Thus, under these experimental conditions, the peptide was not directly inducing the antiviral effect, but another factor(s) released by stimulated cells should largely account for the antiviral activity observed. In order to determine the factors involved in the antiviral effect observed in the supernatants of LALF31–52-stimulated mononuclear cells, we analyzed cytokine gene expression using the RPA, and different experiments were also conducted to determine the presence of IFNs in the sample.
FIG. 1.
Antiviral properties observed in supernatants from PBMC incubated with different LPS-binding peptides (LBP86–99, Cap-18108–128, and LALF31–52). Human mononuclear cells were incubated with 5 μM peptide for 18 h. Supernatants were then fourfold diluted, and antiviral activity was measured by means of the cytopathic effect produced by mengovirus in Hep-2 cells. Units of antiviral activity are set in reference to the biological activity of standard preparations of human IFN-γ. Ten nanograms of LPS per milliliter was used to induce IFNs. Values are means from three experiments. Error bars, SD.
TABLE 1.
Antiviral effect of the LALF peptide on Hep-2 cells
| Treatment | UAA (mean ± SE)a |
|---|---|
| LALF peptide | |
| 1 μM | 7.0 ± 0.26 |
| 1.25 μM | 7.5 ± 0.16 |
| 2.5 μM | 7.5 ± 0.11 |
| Medium only | 7.0 ± 0.16 |
| LPS (10 ng/ml) | 30 ± 0.10 |
Values are from three experiments. The direct antiviral effect of the peptide on Hep-2 cells was determined using the antiviral activity assay described in Materials and Methods. Different amounts of peptide were incubated with the cells during 24 h and antiviral activity was measured by means of the cytopathic effect produced by mengovirus in Hep-2 cells. LPS was used to induce IFNs.
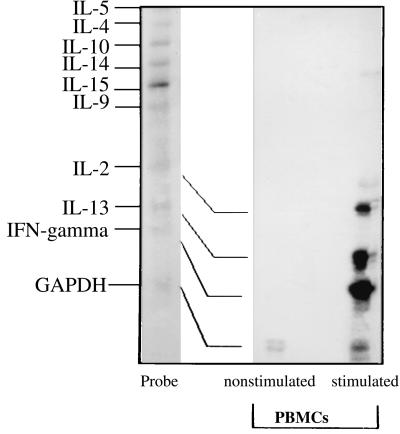
As shown in Fig. 2, 5 μM LALF31–52 was able to preferentially induce IFN-γ, IL-2, and IL-13 in human mononuclear cells after 18 h of incubation. The presence of IFN-γ in the supernatant was determined by using an ELISA system specific for this cytokine. The release of IFN-α was also observed, as shown in the neutralization experiments (Table 2). The expression and subsequent release of IFNs (IFN-α and -γ) seem to mediate the antiviral property observed in the supernatant of cells treated with LALF31–52.
FIG. 2.
Samples of total RNA from nonstimulated and stimulated human PBMC with 5 μM of LALF31–52 peptide for 18 h were analyzed for distinct mRNA species by the RPA with the hck-1 probe template set. The autoradiogram from this analysis shows hck-1 as an unprotected probe set (Probe). It also shows the corresponding RNase-protected probes following hybridization with total RNA isolated from nonstimulated PBMC and with total RNA isolated from PBMC stimulated with 5 μM LALF peptide for 18 h. Data shown are derived from a single experiment that is representative of at least three independent experiments.
FIG. 5.
Regulatory effect of the LALF-derived peptide on TNF-α production by LPS-stimulated peritoneal macrophages. Macrophages were pretreated with 5 μM LALF peptide for 18 h. Cells were then activated with 1 μg of E. coli LPS. TNF-α was measured by ELISA in culture supernatants. Values are means from three different experiments. Error bars, SD. Statistical significance was determined by the Kruskal-Wallis test (∗, P < 0.05; ∗∗, P < 0.01).
TABLE 2.
Presence of IFN-α and IFN-γ in the supernatant of PBMC incubated with the LALF peptide
| Sample | Presencea of:
|
||
|---|---|---|---|
| IFN-αb (IU/ml) | Anti-IFN-αc (IU/ml) | IFN-γd (pg/ml) | |
| Supernatant from PBMC + 5 μM LALF peptide | 108 | <10.0 | 761.45 |
| Human IFN-α (internal standard) | 1 × 106 | 1.5 × 104 | |
| Supernatant from PBMC + 1% (vol/vol) PHA | 2473 | ||
| Medium only | 105.6 | ||
Values are means of three experiments.
The presence of IFN-α was determined using an antiviral activity assay described in Materials and Methods. International units per milliliter are designated in reference to the biological activity of a standard preparation of human IFN-α.
Serial dilutions of the samples were incubated with 100 NU of antibodies to human IFN-α. One hundred eight units of human IFN-α antiviral activity was neutralized by 100 NU of anti-IFN-α.
The presence of IFN-γ was estimated by means of an ELISA system specific for human IFN-γ. PHA was used as an inducer of IFN-γ.
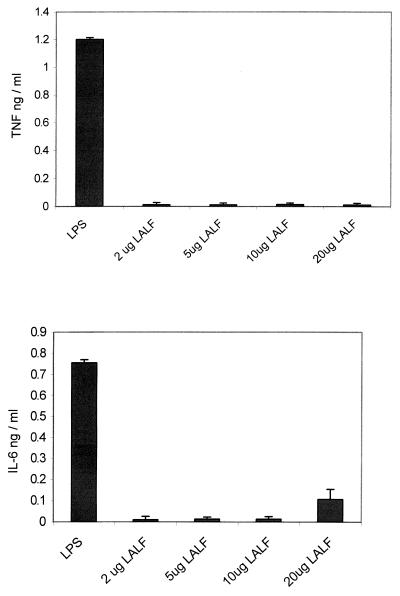
Because TNF-α and IL-6 play an important role in inflammatory events, and antiviral activity has been described for both cytokines, the levels of either TNF or IL-6 were measured in the supernatants of LALF31–52-stimulated PBMC. As shown in Fig. 3, neither TNF-α nor IL-6 was detected, indicating that the peptide does not induce the release of these cytokines.
FIG. 3.
TNF and IL-6 levels in supernatants from human PBMC incubated with different concentrations of LALF31–52. TNF and IL-6 levels were measured in the culture supernatants after 18 h of incubation with the peptide. The concentration for both cytokines was made using an ELISA system. LPS (10 ng per ml) was used as the cytokine inducer. Only supernatants from PBMC incubated with LPS showed detectable amounts of either TNF or IL-6. Values are means from three different experiments. Error bars, SD.
Regulatory effect of the LALF peptide on the activation of peritoneal macrophages by LPS.
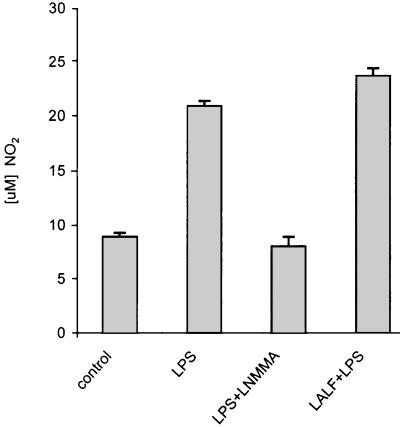
Since LALF31–52 has been primarily described as a molecule that binds and neutralizes LPS, and our principal objective was to characterize its anti-inflammatory ability, we investigated whether the peptide was able to modify LPS-induced responses. For this purpose, the release of TNF-α and NO by peritoneal macrophages was estimated after treatment with LALF31–52. Peritoneal macrophages were pretreated with LALF31–52 at 5 μM for 18 h. After this time the peptide contained in the supernatant was washed away, and macrophages were then stimulated with E. coli LPS in a fresh medium. TNF-α and NO levels were determined in cell culture supernatants. As depicted in Fig. 4, pretreatment with the peptide and subsequent stimulation with LPS slightly increased the production of NO by macrophages. As expected, l-NMMA (500 μM), a specific inhibitor of NO synthase, significantly reduced LPS-induced NO synthesis. However, the production of TNF-α was decreased in LALF peptide-treated cells, as measured at 4 and 6 h after LPS challenge (P < 0.05 and P < 0.01, respectively) (Fig. 5). These results suggest that the LALF peptide induces a differential regulation of the inflammatory mediator secretion by LPS-stimulated cells. The peptide did not affect cell viability, as measured after peptide treatment of nonstimulated and LPS-stimulated cells (data not shown). Cell viability was always above 95%.
FIG. 4.
Regulatory effect of the LALF-derived peptide on NO production by LPS-stimulated murine peritoneal macrophages. Macrophages were pretreated with 5 μM LALF peptide for 18 h. Afterwards, cells were activated with 1 μg of E. coli LPS for 72 h. Levels of NO were measured in culture supernatants using the Griess method. Values are means from three different experiments. Error bars, SD.
LALF31–52 increases survival of P. aeruginosa-infected mice.
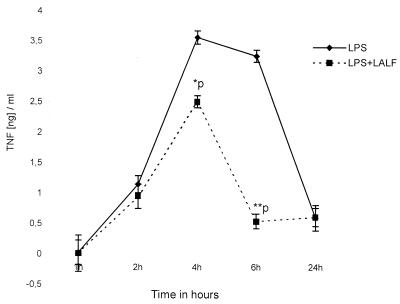
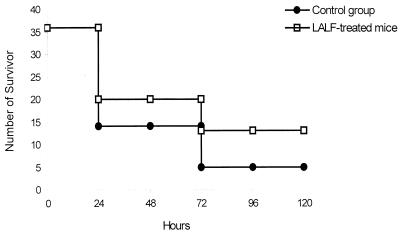
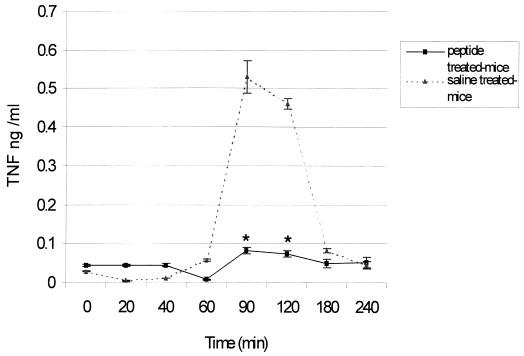
Since the peptide was able to modify in vitro LPS-induced responses, we characterized LALF peptide efficacy in protecting immunologically intact hosts against serious infection, using a model of gram-negative peritoneal sepsis. LALF31–52 at 5 μM was administered i.p. to 8-week-old mice. Twenty hours later, animals were challenged i.p. with 90% of a lethal dose of P. aeruginosa. After results from six different experiments were averaged (n = 36), it was found that survival was significantly increased (P < 0.01) in LALF31–52-treated mice (Fig. 6). Doses of 5 μM LALF31–52 increased survival up to 36%, compared to 8% in vehicle-treated mice. This protection could not be explained by the ability of the peptide to bind LPS, as reported in previous papers, because of the short half-life of peptides in plasma. In line with the results of our previous in vitro experiments, we investigated whether the peptide had modified in vivo TNF production as an inflammatory-immune response. Serum levels of TNF-α were evaluated in both LALF31–52-treated and control mice. Figure 7 shows the time course of serum TNF-α levels for both groups of animals during P. aeruginosa peritoneal infection. TNF levels in peptide-treated mice were significantly lower at 90 and 120 min after bacterial inoculation (P < 0.05). Inhibition of TNF production may play an important role in mitigating bacterial-infection-induced death, because high levels of this cytokine have been associated with lethal damage in sepsis (25).
FIG. 6.
Protection against a lethal dose of P. aeruginosa by LALF31–52. Eight-week-old mice were injected i.p. with the peptide at 5 μM (0.7 μg/g of body weight) in 0.1 ml of saline or with saline alone. Twenty hours later, mice were challenged with a lethal dose of P. aeruginosa. Survival was recorded every 24 h for 120 h.
FIG. 7.
Comparison of systemic TNF levels in peptide-treated and control mice. Eight-week-old mice were injected i.p. with 5 μM LALF31–52 in 0.1 ml of saline or with saline alone. Twenty hours later, mice were injected i.p. with a lethal dose of P. aeruginosa. Mice were bled from the retroorbital sinus at 0, 20, 40, 60, 90, 120, 180, and 240 min following the bacterial inoculation, and serum TNF concentrations were measured. Triangles, control group (n = 5); squares, LALF peptide-treated mice (n = 5). Error bars, SD. ∗, P < 0.05 by the Kruskal-Wallis test.
DISCUSSION
LALF was originally described as a basic protein from horseshoe crabs which binds and neutralizes LPS and has a strong antibacterial effect on gram-negative R-type bacteria (30). Different authors have described the apparent involvement of cationic segments from several proteins in LPS binding, and the LPS-neutralizing activities of peptides corresponding to these regions have been proven (2, 13, 21, 38, 41, 44). The high-resolution structure of recombinant LALF has been described previously, and the authors proposed that the binding site for LPS involves an extended amphipathic loop (22). Minimal requirements of recombinant LALF for endotoxin and lipid A binding with linear peptides have been investigated (27, 36). These data prompted us to synthesize a peptide comprising the amphipathic region of the LALF protein in order to study the ability of this peptide to bind to LPS and modify the deleterious response to LPS. We observed that LALF31–52 binds LPS from different sources (M. J. Araña, G. Chinea, M. Guerra, A. Rodriguez, O. Reyes, H. E. Garay, and G. Padrón, Abstr. Fifth Ann. Conf. Int. Cytokine Soc. 1997, abstr. H-51, p. 902, 1997). Furthermore, it inhibits TNF-α production induced by LPS (E. coli or P. aeruginosa) in whole-blood cultures, as well as nitrite formation by mice macrophages activated with E. coli LPS. It is well documented that proinflammatory cytokines (TNF, IL-1, and IL-8) and other mediators such as NO directly induced by LPS have deleterious effects on microvascular cells that contribute to capillary leak, tissue injury and ultimately to multiple organ failure. The attenuation of the secretion of LPS-induced mediators by LALF31–52 seems to participate in the decreased in vivo toxicity of LPS reported by us elsewhere (Araña et al., Abstr. Fifth Ann. Conf. Int. Cytokine Soc. 1997).
LALF31–52 showed other unexpected properties. In this paper, we present evidence that the peptide is able to activate human mononuclear cells, subsequently inducing the release of soluble molecules with antiviral properties. In this sense, both IFN-α and IFN-γ were identified as molecules involved in this effect (Table 2). On the other hand, IL-2 and IL-13 genes were also expressed in PBMC stimulated with the peptide. The LALF peptide might specifically modulate the host immune response, leading to releases of IFNs and other cytokines such as IL-2 and IL-13 with immunomodulatory and anti-inflammatory abilities, respectively. TNF-α is not present in the supernatants from human PBMC stimulated with the peptide (Fig. 3). This could be beneficial, considering that high systemic levels of this cytokine have been associated with in vivo deleterious effects.
TNF production decreased but nitrite generation increased, although only slightly, in peritoneal macrophages pretreated with the peptide and activated with LPS. Although TNF has been reported as an INOS inducer (42), other cytokines such as IFN-γ and IL-2 are also involved in the induction of INOS activity (14, 35, 50). The source of some of these cytokines could be remnant lymphocytes present in the primary culture of peritoneal macrophages (34). Nevertheless, our data suggest that the LALF-derived peptide specifically induces a modulation of the response in LPS-activated cells.
We demonstrated that the prophylactic administration of the LALF peptide partially protected mice against lethal infection with P. aeruginosa. The LPS-neutralizing activity of the peptide does not mediate this effect, since the short serum half-life of peptides is well documented and under our experimental conditions LALF31–52 is administered 20 h before the infective inoculum. Results of in vitro experiments suggest that the LALF peptide could modulate host responses to insulting agents, leading, in this case, to protection against bacterial infection in challenged animals. Interestingly, Robert et al., reported that LALF prevents mortality in mice late in the course of endotoxemia, and they cannot rule out the possibility that LALF is acting through mechanisms other than the neutralization of LPS (37).
Since systemic TNF levels were diminished in peptide-treated mice during bacterial infection, the effect of the LALF peptide mitigating bacterial-infection-induced death may be attributed, at least partially, to the inhibition of this cytokine. TNF is a pleiotropic proinflammatory cytokine, and it is well established that plasma TNF levels are elevated during sepsis, especially during the early stage (18). As a consequence of high levels of circulating TNF, multiorgan failure, including septic liver failure, might occur in humans as well as in experimental animals (28, 29). However, other mediators could be involved in this effect, assuming the complexity of the pathogenic events.
The action of the peptide may be particularly effective in mobilizing host immune defenses leading to protection from bacterial infections in treated hosts. The peptide may enhance the microbicidal response and decrease the lethal consequences of the systemic inflammatory response. Other laboratories have shown that the specific activation of macrophages decreases septic sequelae and improves survival in humans and experimental animals (3, 7, 11). Besides human macrophages, other immune-related cells respond to different molecules increasing resistance to infection (23, 24, 51). In this setting, for example, the involvement of T cells in the enhanced resistance to Klebsiella pneumoniae septicemia in mice treated with liposome-encapsulated muramyl tripeptide has been demonstrated (19).
Recently, agents with immunomodulating ability have been described which attenuate the systemic release of endogenous TNF during endotoxin shock and potently up-regulate the early production of circulating IL-10 (9). Similarly, LALF31–52 may exert a protective effect modulating cytokine expression. The release of anti-inflammatory mediators such as transforming growth factor β and the soluble TNF receptor could regulate proinflammatory responses during bacterial infection, diminishing organ damage and increasing survival. In addition, the effects of LALF31–52 in modulating other molecules, which may be of importance in the mediation of septic sequelae, such as IL-1, chemokines, and adhesion molecules, are currently under investigation.
The findings described herein strengthen the therapeutic potential of LALF31–52. This peptide was able to prevent deleterious effects observed during bacterial infection in vivo and to induce the release of IFNs (IFN-γ and -α) in PBMC stimulated with the peptide. LALF31–52 could be a promising molecule in acute and chronic inflammatory processes as well as in immunosuppression states. In this regard, different studies have demonstrated host-protective effects for endogenously regulated cytokines and NO mediators in different autoimmune diseases (15, 26, 31, 49). Furthermore, diminishing TNF levels has been effective in rheumatoid arthritis (8). The diverse functional properties of LALF31–52 highlight its advantages as a therapeutic agent for endotoxin shock and infectious diseases. Our results suggest that the LALF31–52 peptide may have a greater therapeutic potential than that originally contemplated for an LPS-binding molecule.
ACKNOWLEDGMENTS
This work was partially supported by Third World Academy of Sciences grant 97-143 RG/BIO/LA.
We thank Wim Buurman from Maastricht University for providing TNF ELISA reagents. We are grateful to Laydis Duany for conducting assays testing the inhibition of the viral cytopathic effect.
REFERENCES
- 1.Ammons W S, Kohn F R, Kung A H. Protective effects of an N-terminal fragment of bactericidal/permeability-increasing protein in rodent models of Gram-negative sepsis: role of bactericidal properties. J Infect Dis. 1994;170:1473–1482. doi: 10.1093/infdis/170.6.1473. [DOI] [PubMed] [Google Scholar]
- 2.Battafarano R J, Dahlberg P S, Ratz C A. Peptide derivatives of three distinct lipopolysaccharide binding proteins inhibit lipopolysaccharide-induced TNF secretion in vitro. Surgery. 1995;118:318–324. doi: 10.1016/s0039-6060(05)80340-x. [DOI] [PubMed] [Google Scholar]
- 3.Browder W, Williams D, Pretus H, Olivero G, Enrichens F, Mao P, Franchello A. Beneficial effect of enhanced macrophage function in the trauma patient. Ann Surg. 1990;211:605–613. [PMC free article] [PubMed] [Google Scholar]
- 4.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 5.Dent A L, Shaffer Y X, Allman D, Staudt L M. Cytokine expression and germinal center formation by Bcl-2. Science. 1997;276:589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 6.Dentener M A, Bazil V, Von Asmuth E J, Ceska M. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993;150:2885–2891. [PubMed] [Google Scholar]
- 7.Döcke W D, Syrbe U, Meinicke A, Platzer C, Makki A, Asadullah K, Klug C, Zuckermann H, Reinke P, Brunner H, Von Baehr R, Volk H D. Reinhart (ed.), Update in intensive care and emergency medicine—1994. Berlin, Germany: Springer; 1994. Improvement of monocytic function: a new therapeutic approach in sepsis? pp. 473–488. [Google Scholar]
- 8.Elliott M J, Maini R N, Feldmann M, Kalden J R, Antoni C, Smolen J S. Randomised double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 9.Ertel W, Morrison M H, Wang P, Ba Z F, Ayala A, Chaudry I H. The complex pattern of cytokines in sepsis. Association between prostaglandins, cachectin, and interleukins. Ann Surg. 1991;214:141–148. doi: 10.1097/00000658-199108000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Famillietti P C, Rubinstein S, Pestka S. Assay of interferon activity. Methods Enzymol. 1981;78:387–396. doi: 10.1016/0076-6879(81)78146-1. [DOI] [PubMed] [Google Scholar]
- 11.Felippe J, Silva M, Maciel F M. Infection prevention in patients with severe multiple trauma with the immunomodulator beta 1–3 polyglucose (glucan) Surg Gynecol Obstet. 1993;177:383–388. [PubMed] [Google Scholar]
- 12.Ferrero J, Ochagavia M E, Aguilera A. Titulación de la actividad antiviral de interferones utilizando el sistema de equipos SUMA. Biotechnol Apl. 1994;11:25–30. [Google Scholar]
- 13.Fletcher M A, Kloczewiak M A, Loiselle P M, Ogata M, Vermeulen M W, Zanzot E M, Warren H S. A novel peptide-IgG conjugate, Cap-18-IgG, that binds and neutralizes endotoxin and kills gram-negative bacteria. J Infect Dis. 1997;175:621–632. doi: 10.1093/infdis/175.3.621. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara H, Zou J P, Herrmann S, Hamaoka T. A sequence of cellular and molecular events involved in IL-12-induced tumor regression. Res Immunol. 1995;6:638–644. doi: 10.1016/0923-2494(96)83042-2. [DOI] [PubMed] [Google Scholar]
- 15.Gabbai F B, Boggiano C, Peter T, Khang S, Archer C, Gold D P, Kelly C J. Inhibition of inducible nitric oxide synthase intensifies injury and functional deterioration in autoimmune interstitial nephritis. J Immunol. 1998;159:6266–6275. [PubMed] [Google Scholar]
- 16.Gilman M. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1998. pp. 4.7.1–4.7.8. [Google Scholar]
- 17.Goris R J A. Sepsis and multiple organ failure: the result of whole body inflammation. In: Faist E, Meakins J, Schildberg F W, editors. Host defense dysfunction in trauma, shock and sepsis. Berlin, Germany: Springer-Verlag; 1993. pp. 161–170. [Google Scholar]
- 18.Groote M A, Martin M A, Densen P, Pfaller M, Wenzel R P. Plasma tumor necrosis factor levels in patients with presumed sepsis. Results in those treated with antilipid A antibody versus placebo. JAMA. 1989;262:249–251. doi: 10.1001/jama.262.2.249. [DOI] [PubMed] [Google Scholar]
- 19.Hagen T L, van Vianen W, Savelkoul H F, Heremans H, Buurman W A, Bakker-Woudenberg I A. Involvement of T cells in enhanced resistance to Klebsiella pneumoniae septicemia in mice treated with liposome-encapsulated muramyl tripeptide phosphatidylethanolamine or gamma interferon. Infect Immun. 1998;66:1962–1967. doi: 10.1128/iai.66.5.1962-1967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartung T, Doecke W D, Gantner F. Effect of granulocyte colony-stimulating factor treatment on ex vivo blood cytokine response in human volunteers. Blood. 1995;85:2482–2489. [PubMed] [Google Scholar]
- 21.Hirata M, Zhong J, Wright S C, Larrick J W. Structure and functions of endotoxin-binding peptides derived from Cap-18. Prog Clin Biol Res. 1997;392:317–326. [PubMed] [Google Scholar]
- 22.Hoess A, Watson S, Siber G R, Liddington R. Crystal structure of an endotoxin-neutralizing protein from the horseshoe crab, Limulus anti-LPS factor, at 1.5 Å resolution. EMBO J. 1993;112:3351–3356. doi: 10.1002/j.1460-2075.1993.tb06008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakobsen P H, Koch C, Bendtzen K. Inhibition of LPS and Plasmodium falciparum induced cytokine secretion by pentoxifylline and two analogues. Scand J Immunol. 1997;45:546–550. doi: 10.1046/j.1365-3083.1997.d01-425.x. [DOI] [PubMed] [Google Scholar]
- 24.Jilg S, Barsig J, Leist M. Enhanced release of interleukin-10 and soluble tumor necrosis factor receptors as novel principles of methylxanthine action in murine models of endotoxin shock. J Pharmacol Exp Ther. 1996;278:421–431. [PubMed] [Google Scholar]
- 25.Jones A L, Selby P. Tumor necrosis factor: clinical relevance. Cancer Surv. 1989;8:817. [PubMed] [Google Scholar]
- 26.Klareskog J, Rönnelid J, Holm G. Immunopathogenesis and immunotherapy in rheumatoid arthritis: an area in transition. J Intern Med. 1995;238:191–206. doi: 10.1111/j.1365-2796.1995.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 27.Kloczewiak M, Black K M, Loiselle P, Cavaillon J M, Wainwright N, Warren H S. Synthetic peptides that mimic the binding site of horseshoe crab antilipopolysaccharide factor. J Infect Dis. 1994;170:1490–1497. doi: 10.1093/infdis/170.6.1490. [DOI] [PubMed] [Google Scholar]
- 28.Leist M, Gantner F, Jilg S, Wendel A. Activation of the 55 kDa TNF receptor is necessary and sufficient for TNF-induced liver failure, hepatocyte apoptosis, and nitrite release. J Immunol. 1995;154:1307–1316. [PubMed] [Google Scholar]
- 29.Mathison J C, Wolfson E, Ulevitch R. Participation of tumor necrosis factor in the mediation of gram-negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Investig. 1988;81:1925. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morita T, Ohtsubo S, Nakamura T. Isolation and biological activities of Limulus anticoagulant (anti-LPS factor) which interact with lipopolysaccharide (LPS) J Biochem. 1985;97:1611–1620. doi: 10.1093/oxfordjournals.jbchem.a135218. [DOI] [PubMed] [Google Scholar]
- 31.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–148. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 32.Natanson C, Hoffman W D, Suffredini A F, Eichacker P Q, Danner R L. Selected treatment strategies for septic shock based on proposed mechanisms of pathogenesis. Ann Intern Med. 1994;120:771–783. doi: 10.7326/0003-4819-120-9-199405010-00009. [DOI] [PubMed] [Google Scholar]
- 33.Nathan J F, Radzioch C, Ding A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88:417–426. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 34.Nibbering P H, Langermans J A M, van de Gevel J S, van der Hulst M B, van Furth R. Nitrite production by activated murine macrophages correlates with their toxoplasmastatic activity, Ia antigen expression, and production of H2O2. Immunobiology. 1991;184:93–105. doi: 10.1016/S0171-2985(11)80575-9. [DOI] [PubMed] [Google Scholar]
- 35.Nussler A, Drapier J C, Renia L, Pied S, Miltgen F, Gentilini M, Mazier D. l-Arginine-dependent destruction of intrahepatic malaria parasites in response to TNF and/or IL-6 stimulation. Eur J Immunol. 1991;21:227–230. doi: 10.1002/eji.1830210134. [DOI] [PubMed] [Google Scholar]
- 36.Ried C, Wahl C, Miethke T, Wellnhofer G, Landgraf C, Schneider-Mergener J, Hoess A. High affinity endotoxin-binding and neutralizing peptides based on the crystal structure of recombinant Limulus anti-LPS factor. J Biol Chem. 1996;271:28120–28127. doi: 10.1074/jbc.271.45.28120. [DOI] [PubMed] [Google Scholar]
- 37.Robert I R, Donghui S, Child A H, Wainwright N R, Levin J. Limulus antilipopolysaccharide factor prevents mortality late in the course of endotoxemia. J Infect Dis. 1998;177:388–394. doi: 10.1086/514204. [DOI] [PubMed] [Google Scholar]
- 38.Schumann R R, Lamping N, Hoess A. Interchangeable endotoxin-binding domains in proteins with opposite lipopolysaccharide-dependent activities. J Immunol. 1997;159:5599–5605. [PubMed] [Google Scholar]
- 39.Seatter S C, Bennet T, Li M H. Macrophage endotoxin tolerance: tumor necrosis factor and interleukin-1 regulation by lipopolysaccharide pretreatment. Arch Surg. 1994;129:1263–1270. doi: 10.1001/archsurg.1994.01420360053006. [DOI] [PubMed] [Google Scholar]
- 40.Sinha B, Semmler J, Eisenhut T. Enhanced tumor necrosis factor suppression and cycle adenosine monophosphate accumulation by combination of phosphodiesterase inhibitors and prostanoids. Eur J Immunol. 1995;25:147–153. doi: 10.1002/eji.1830250125. [DOI] [PubMed] [Google Scholar]
- 41.Srimal S, Surolia N, Balasubramanian S, Surolia A. Titration calorimetric studies to elucidate the specificity of the interactions of polymyxin B with LPS and lipid A. Biochem J. 1996;315:679–686. doi: 10.1042/bj3150679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steege J C, van de Ven M W, Forget P P, Brouckaert P, Buurman W A. The role of endogenous IFN-gamma, TNF-alpha and IL-10 in LPS-induced nitric oxide release in a mouse model. Cytokine. 1998;10:115–123. doi: 10.1006/cyto.1997.0263. [DOI] [PubMed] [Google Scholar]
- 43.Szabo C, Mitchell J A, Gross S S, Thiemermann C, Vane J R. Nifedipine inhibits the induction of nitric oxide synthase by bacterial lipopolysaccharide. J Pharmacol Exp Ther. 1992;265:674–680. [PubMed] [Google Scholar]
- 44.Taylor A H, Heavner G, Nedelman M, Sherris D, Brunt E, Knight D, Ghrayed J. Lipopolysaccharide (LPS) neutralizing peptides reveal a lipid A binding site of LPS binding protein. J Biol Chem. 1995;270:17934–17938. doi: 10.1074/jbc.270.30.17934. [DOI] [PubMed] [Google Scholar]
- 45.Volk H D, Reinke P, Krausch D, Zuckermann H, Asadullah K, Muller J M, Docke W D, Kox W J. Monocyte deactivation—rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 1996;22:S474–S481. doi: 10.1007/BF01743727. [DOI] [PubMed] [Google Scholar]
- 46.Warren H S, Glennon M L, Wainwright N. Binding and neutralization of endotoxin by Limulus antilipopolysaccharide factor. Infect Immun. 1992;60:2506–2513. doi: 10.1128/iai.60.6.2506-2513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welbourn C R, Young Y. Endotoxin, septic shock and acute lung injury: neutrophils, macrophages and inflammatory mediators. Br J Surg. 1992;79:998–1003. doi: 10.1002/bjs.1800791006. [DOI] [PubMed] [Google Scholar]
- 48.Williams D L, Mueller A, Browder W. Glucan-based macrophage stimulators. Clin Immunother. 1996;5:392–399. [Google Scholar]
- 49.Woo P. The cytokine network in juvenile chronic arthritis. Pediatr Rheumatol. 1997;23:491–498. doi: 10.1016/s0889-857x(05)70344-6. [DOI] [PubMed] [Google Scholar]
- 50.Xie Q W, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by IFN-γ and bacterial lipopolysaccharide. J Exp Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zoltan H, György H, Szabo C, Vizi E S. Amrinone and theophylline differentially regulate cytokine and nitric oxide production in endotoxemic mice. Shock. 1997;7:371–375. doi: 10.1097/00024382-199705000-00010. [DOI] [PubMed] [Google Scholar]