Abstract
Arterial line waveform; Blood pressure; Focal arterial dissection; Aortic dissection; Cardiothoracic surgery.
Keywords: Arterial line waveform, Blood pressure, Focal arterial dissection, Aortic dissection, Cardiothoracic surgery
1. Introduction
Dampening of the arterial line waveform during surgery is always a major concern for the operating team. During cardiovascular surgery the etiology may be very serious and require immediate diagnosis, as the surgical plan may need be altered urgently.
We present a case of a Type A aortic intramural hematoma repair where the arterial line waveform acutely dampened during weaning from cardiopulmonary bypass and the course of action that took place.
2. Case report
An 80-year-old woman with a past medical history of hypertension, type II diabetes mellitus, and hyperlipidemia developed chest pain and tightness in the morning of 2022/01/05 and was initially sent to a local hospital. The patient's chest computed tomography (CT) revealed massive aortic intramural hematoma, and she was immediately transferred to the emergency department (ED) of our hospital (tertiary medical center) for help. At the ED, the patient presented with no neurologic deficits. Her systolic blood pressure in the four limbs were taken to be 122 mmHg (right arm), 118 mmHg (left arm), 147 (right leg), 140 mmHg (left leg). Electrocardiogram (ECG) demonstrated sinus rhythm with bradycardia at 57 beats per minute; there was no ST segment elevation. Chest X-ray at the local hospital revealed widened mediastinum. The chest CT showed extension of the intramural hematoma through the ascending aorta, the aortic arch, and the descending aorta; the major divisions from the aorta seemed not affected. Cardiac echo revealed that the aortic root was dilated; the patient had no chamber dilatation or regional wall abnormalities, and her left ventricular systolic function was preserved. She had mild regurgitations in the aortic, tricuspid, and mitral valves. She had minimal pericardial effusion. No intimal flap was initially identified. Due to the acute onset of symptoms and concern for further progression, the patient was sent under family consent to the operating room to receive surgery.
Induction of anesthesia commenced. The anesthesia team inserted arterial catheters in the left and right radial arteries. Propofol was administered via target controlled infusion (Marsh model) to achieve unconsciousness at Ce = 2.5 mcg/ml (concentration in the effect-site). Fentanyl 25 mcg was injected to provide analgesia for intubation, followed by cisatracurium 8 mg for muscular relaxation. After intubation, the patient's systolic blood pressure surged to 180 mmHg then decreased to 120 mmHg. A multilumen catheter (MAC) was inserted in the right internal jugular vein followed by correct placement of the pulmonary artery catheter through the introducer port (as confirmed by transesophageal echocardiography (TEE)). TEE examination findings were compatible with intramural hematoma; there was mild aortic regurgitation, and no intimal flap was identified. Anesthesia was maintained with propofol, fentanyl, and cisatracurium infusion. The surgical team proceeded with skin incision and electrocauterization to reach the sternum, and sternotomy was performed. During this period, the systolic blood pressure remained at around 100–120 mmHg. A vessel graft was anastomosed to the right subclavian artery for cardiopulmonary bypass arterial inflow, and the right atrium was cannulated for venous drainage. The patient entered partial cardiopulmonary bypass with a mean arterial blood pressure of 50–60 mmHg. The aorta was then clamped, the root cut open, and cardioplegia performed. The sinotubular junction was reconstructed and anastomosed to a 28 mm straight graft; no dissection inlet was found in the proximal ascending aorta. Unilateral antegrade cerebral perfusion (UACP) was performed at 25 °C at 20:09 via a separate graft anastomosed to the right subclavian artery. Further exploration revealed one inlet over the lesser curvature of the aortic arch, confirming the diagnosis of dissection with false lumen thrombosis. The surgical team then completed hemiarch reconstruction with the 28mm graft and discontinued UACP at 20:41. The cross-clamp was removed.
During rewarming from hypothermia, the team observed that the waveform of the right radial arterial line was flat even though the heart had started beating. TEE examination found aortic dissection with inlet flow in the aortic arch distal to the 28 mm graft (Figure 1, B), which was not present in the pre-operation TEE examination (Figure 1, A). A left femoral arterial catheter was placed, and the waveform and the pressures were normal (systolic blood pressure was 110 mmHg). The left radial artery pressure was around 90 mmHg. After discussion, the team decided to proceed with weaning from cardiopulmonary bypass. The weaning was smooth with dopamine and dobutamine infusion support at 6 mcg/kg/min each. Sternal wound closure was done. The right hand was exposed and showed delayed capillary refill, and its temperature was colder than the left hand. The anesthesia team scanned the right radial and brachial artery with ultrasound but found no pulsatile flow under color flow Doppler.
Figure 1.
Transesophageal echocardiography (TEE) images of aortic arch before (A) and after (B) operation.
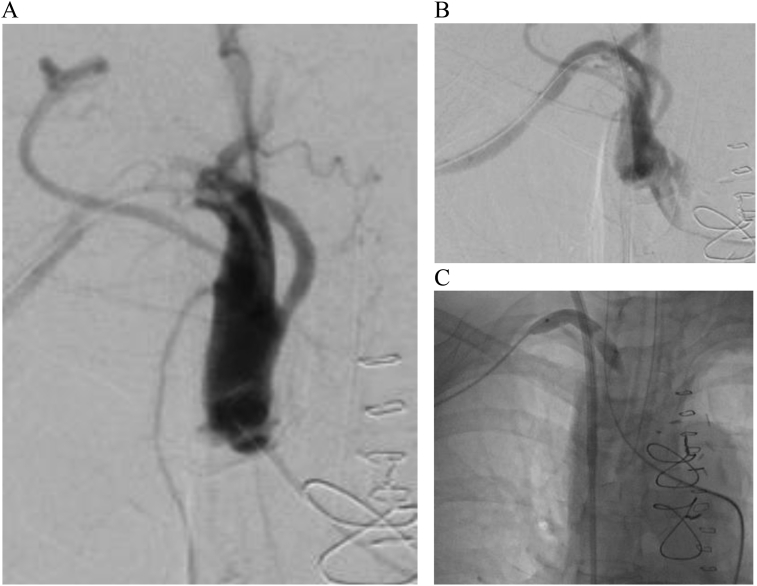
The patient was quickly transferred to the hybrid operating room for angiography. A 5 Fr. sheath was inserted into the left femoral artery to provide access; angiography revealed occlusion with focal dissection of the right subclavian artery distal to the right vertebral artery (Figure 2, A). The right brachial artery was cut-down for insertion of an 8 Fr. sheath; wiring through the lesion improved the right radial systolic blood pressure to 90 mmHg (Figure 2, B). An 8 mm balloon was passed through the lesion and inflated for percutaneous transluminal angioplasty (PTA) (Figure 2, C). At this time, the left radial artery waveform was dampened, and the systolic pressure measured around 60 mmHg. The patient was transferred to the intensive care unit (ICU).
Figure 2.
Angiography revealing near total obstruction of flow (A), contrast enhancement after wiring (B), and the process of balloon transluminal angioplasty (C).
The patient's condition was stable at the ICU, and she was soon weaned from dopamine and dobutamine support. New onset atrial fibrillation was treated with intravenous amiodarone, and the patient's heart rate reverted to sinus rhythm. She was transferred to the cardiovascular ward on 2022/01/08. Her cuffed blood pressure measured 113 mmHg (right arm) and 91 mmHg (left arm). The patient was eventually discharged on 2022/01/14 under stable condition.
3. Discussion
The flattening of the arterial line waveform during major vessel and cardiac surgery poses a diagnostic challenge. In our case, we tried to differentiate between the different possible causes of the flat right radial arterial waveform during and after weaning from cardiopulmonary bypass (Table 1).
Table 1.
Etiologies of a flat arterial line waveform. BP, blood pressure; CAD, coronary artery disease; SVR, systemic vascular resistance; TEE, transesophageal echocardiography. US, ultrasound.
| Etiology | Conditions | Causes | Confirmation |
|---|---|---|---|
| Occlusion | Dissection | Fragile intimal tissue Aorta cross-clamp Poor BP control |
TEE Angiography |
| Thrombosis | Thrombi migration | Angiography | |
| Surgical compression/manipulation | Surgical needs or inadvertent compression | Observe the surgical field | |
| Hypotension | Poor cardiac output | Delayed recovery from cardioplegia Hypovolemia Unaddressed arrhythmia Myocardial ischemia Protamine induced hypotension |
TEE Compare with aorta main trunk or femoral artery pressure |
| Low SVR | Deep anesthesia Vasodilatory agents in use Allergy Protamine induced hypotension Sepsis |
As above | |
| Device malfunction | Catheter kink | Folded wrist or skin folds | Exposure Direct examination |
| Occluded pressure transducer line Pressure set dysfunction |
Inadvertent clamping of transducer line Physical impact on sensor |
Check entire set | |
| Vasospasm | Constriction of small diameter arteries | Multiple insertion attempts Prolonged catheter placement time |
Peripheral US |
| Local swelling | Tissue edema | Over flushing of arterial line set | Exposure Peripheral US |
| Hematoma | Multiple insertion attempts | Exposure Peripheral US |
3.1. Occlusion of vasculature or true hypotension
Hypotension may be a result of low cardiac output, low systemic vascular resistance, or both. The heart may still be recovering from cardiopulmonary bypass, or there may be fibrillations which deprive the heart of the atrial kick, which accounts for 25% of the cardiac output of a normal beating heart. New onset myocardial ischemia as a result of coronary artery compression or air embolism can lead to ventricular hypokinesis. Protamine can suppress sympathetic outflow by increasing NO in the central nervous system and induce release of relaxing factors in the vessels, thus lowering systemic vascular resistance and cardiac output [1, 2].
The surgeon quickly punctured the aorta main trunk and femoral artery to obtain the pressures at each site; pressures from both sites were in the normal range (SBP = 100–110 mmHg) and had normal pulsatile waveform. Pulmonary artery catheter derived cardiac output measured between 2.8 to 3.6L/min. This suggested that the patient was hemodynamically stable, but the systemic blood pressure did not translate to a pulsatile waveform via the right radial arterial setup.
TEE examination showed ventricular contractions that were compatible with the measured cardiac output. Notably, under TEE examination, the aortic arch and the descending aorta lumen had become distorted; the shape of the intramural hematoma became irregular, and a site was discovered where trans-intimal flow was visible through colour Doppler. This proved that there was progression of aortic dissection distal to the graft. Focal dissection in the right upper limb circulation could not be confirmed by TEE examination. As mentioned above, we directly examined the patient's right upper limb and found poorer capillary refill and lower temperature compared with the left upper limb. Ultrasound color Doppler found no pulsatile signals in the right arm, thus supporting the diagnosis of arterial occlusion.
Cardiopulmonary bypass, deep hypothermic circulatory arrest, and vascular injury all contribute to thrombi formation [3]. In addition, heparin does not completely inhibit thrombin formation [4, 5], and even though the activated clotting time (ACT) was used to assess a patient's coagulation status, great variability is observed. As such, there was also the possibility that a thrombus, which may have formed during the circulatory arrest period despite adequate activated clotting time (ACT, then 408 s), occluded the subclavian artery upon resumption of circulation.
3.2. Device malfunction
We had an experience with another case where the pressure tubing was clamped by a draping fixation clamp, and the tube sustained half way through the surgery before collapsing under the continuous pressure. For the current case, we checked the pressure kit plugs, did multiple zeroing, and traced the pressure transferring lines for kinks. No abnormalities were detected, and the arterial pressure waveform remained non-pulsatile at around 40 mmHg. Later after the sterile draping was removed, we inspected the arterial catheter itself at the insertion site and identified no kink.
3.3. Vasospasm and local swelling
Capillary refill took greater than 3 seconds to gradually take place. There was grade I edematous change in the right wrist, likely a result of long period immobilization and frequent flushing of arterial line. We utilized the ultrasound to visualize the arteries in the right arm and demonstrated that the radial artery and the brachial artery were both non-pulsatile. This hinted that obstruction might have occurred in the right arm vasculature.
Finally, after discussion with the surgical team, a swift decision to move the patient to the hybrid OR for angiography was made, and weaning from the cardiopulmonary bypass was not delayed. Angiography and subsequent wiring and balloon dilatation restored blood flow to the right arm, and the arterial line waveform from the right radial artery returned to the normal contour. The angiography procedure confirmed the diagnosis of focal dissection in the subclavian artery distal to the right vertebral artery. The left arm blood pressure at the conclusion of the surgery suggested that the left arm vasculature was also affected, but the team decided to treat the condition conservatively due to prolonged surgical time.
In this case, we maintained good communication between the anesthesia team, the surgical team, and the cardiopulmonary bypass team. We did not neglect the dampening of the right arm arterial line waveform, which was actually the result of progressing arterial dissection. Meticulous confirmation was performed to rule out relevant causes.
Declarations
Author contribution statement
All authors listed have significantly contributed to the investigation, development and writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Hamada Y., Kameyama Y., Narita H., Benson K.T., Goto H. Protamine after heparin produces hypotension resulting from decreased sympathetic outflow secondary to increased nitric oxide in the Central Nervous System. Anesth. Analg. 2005;100(1):33–37. doi: 10.1213/01.ANE.0000139357.87358.94. [DOI] [PubMed] [Google Scholar]
- 2.Protamine releases endothelium-derived relaxing factor from systemic arteries. A possible mechanism of hypotension during heparin neutralization. J. Cardiothorac. Vasc. Anesth. 1993;7(1):118. doi: 10.1161/01.cir.86.1.289. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa S., Tanaka K.A. High mortality associated with intracardiac and intrapulmonary thromboses after cardiopulmonary bypass. J. Anesth. 2011;26(1):9–19. doi: 10.1007/s00540-011-1253-x. [DOI] [PubMed] [Google Scholar]
- 4.Edmunds L.H., Colman R.W. Thrombin during cardiopulmonary bypass. Ann. Thorac. Surg. 2006;82(6):2315–2322. doi: 10.1016/j.athoracsur.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 5.Weitz J.I. Low-molecular-weight heparins. N. Engl. J. Med. 1997;337(10):688–698. doi: 10.1056/NEJM199709043371007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.