Abstract
Background
Although clozapine is effective for treatment-resistant schizophrenia (TRS), the rate of clozapine prescription is still low. Whereas antipsychotic monotherapy is recommended in clinical practice guidelines, the rate of antipsychotic polypharmacy is still high. There is little evidence on whether a clozapine prescription influences changes in the rate of monotherapy and polypharmacy, including antipsychotics and other psychotropics. We therefore hypothesized that the rate of antipsychotic monotherapy in patients with TRS who were prescribed clozapine would be higher than that in patients with schizophrenia who were not prescribed clozapine.
Methods
We assessed 8306 patients with schizophrenia nationwide from 178 institutions in Japan from 2016 to 2019. We analyzed the psychotropic prescription data at discharge in patients diagnosed with TRS and with no description of TRS (ND-TRS) based on the diagnosis listed in the discharge summary.
Results
The rate of antipsychotic monotherapy in the TRS with clozapine group (91.3%) was significantly higher than that in the TRS without clozapine group (45.9%; P < 2.0 × 10−16) and the ND-TRS without clozapine group (54.7%; P < 2.0 × 10−16). The rate of antipsychotic monotherapy without any other concomitant psychotropics in the TRS with clozapine group (26.5%) was significantly higher than that in the TRS without clozapine group (12.6%; P = 1.1 × 10−6) and the ND-TRS without clozapine group (17.0%; P = 5.9 × 10−6).
Conclusions
Clozapine prescription could be associated with a high rate of antipsychotic monotherapy. Patients will benefit from the correct diagnosis of TRS and thus from proper clozapine prescription.
Keywords: Treatment-resistant schizophrenia, lithium, polypharmacy, guideline, EGUIDE
Significance Statement.
Clozapine is effective for treatment-resistant schizophrenia (TRS). However, the rate of clozapine prescription is low, and the rate of polypharmacy of psychotropics, including antipsychotics, is high in many countries. This real-world nationwide study revealed that the prescription of clozapine had almost no occurrence of antipsychotic polypharmacy compared with TRS and with no description of TRS without clozapine. Furthermore, the prescription of clozapine was associated with a higher prescription rate of antipsychotic monotherapy without other concomitant psychotropics than other groups. This may be associated with a reduction in the risks of psychotropic polypharmacy and may play a key role in reducing long-term all-cause mortality. We found that the prescription of clozapine was associated with a high prescription rate of lithium in this study. There may be domestic standards for white blood cell count in Japan, and applying global standards may result in a gradual decrease in lithium prescriptions.
Introduction
Schizophrenia is a chronic and complex disease with a prevalence of approximately 0.5%–1.0% (Simeone et al., 2015). Many guidelines recommend antipsychotic monotherapy as a first-line treatment for schizophrenia (Galletly et al., 2016; Remington et al., 2017; Barnes et al., 2020; Keepers et al., 2020; Japanese Society of Neuropsychopharmacology, 2021). Antipsychotics are effective in improving symptoms of schizophrenia; however, approximately 30% of patients with schizophrenia do not respond to antipsychotics and develop treatment-resistant schizophrenia (TRS) (Kane et al., 2019). TRS is defined as an insufficient response to or insufficient tolerance to antipsychotics (Yasui-Furukori et al., 2022).
Clozapine is the only licensed antipsychotic for TRS, and clozapine monotherapy is the recommended treatment for TRS in most guidelines (Galletly et al., 2016; Remington et al., 2017; Barnes et al., 2020; Keepers et al., 2020; Japanese Society of Neuropsychopharmacology, 2021). Compared with other antipsychotics, clozapine has significant efficacy for overall symptoms, positive symptoms, and negative symptoms in the short term and significant efficacy for positive symptoms in the long term (Siskind et al., 2016); however, clozapine can cause many adverse effects, such as hypersalivation, tachycardia, constipation, glucose intolerance, weight gain, seizure, myocarditis, and pneumonia (Barnes et al., 2020; Japanese Society of Neuropsychopharmacology, 2021; Leon et al., 2022). In particular, neutropenia, including agranulocytosis, is a serious adverse effect of clozapine that can cause death (Matsui et al., 2020). A recent meta-analysis of clozapine use reported that the prevalence of neutropenia was 3.8%, the prevalence of agranulocytosis was 0.9%, and the mortality due to agranulocytosis was 2.1% (Myles et al., 2018). As a countermeasure against these adverse effects, patients treated with clozapine are registered and monitored in many countries by the Clozaril Patient Monitoring System (CPMS) (Matsui et al., 2020). These adverse effects represent one of the reasons why the rate of clozapine prescription is still low in many countries (Bachmann et al., 2017).
Antipsychotic polypharmacy is sometimes used in daily practice, such as in patients with insufficient improvement on antipsychotic monotherapy. Antipsychotic polypharmacy is associated with an increased risk of many adverse effects, such as extrapyramidal symptoms (EPSs) and hyperprolactinemia (Barnes et al., 2020; Japanese Society of Neuropsychopharmacology, 2021). Because of these adverse effects, antipsychotic polypharmacy is not recommended in guidelines (Galletly et al., 2016; Remington et al., 2017; Barnes et al., 2020; Keepers et al., 2020; Japanese Society of Neuropsychopharmacology, 2021). Nevertheless, the rate of polypharmacy is still high in several countries (Yang et al., 2018; Toto et al., 2019; Ayenew et al., 2021). We started the Effectiveness of Guidelines for Dissemination and Education (EGUIDE) Psychiatric Treatment Project in 2016. The purpose of the EGUIDE project was the dissemination of guidelines for the treatment of schizophrenia and major depressive disorder through educational programs. Furthermore, we investigated the effectiveness of our educational programs (Takaesu et al., 2019; Numata et al., 2021) as well as real-world prescribing patterns and treatment styles associated with nonadherence to the guidelines by assessing prescription rates at discharge for inpatients with schizophrenia or major depressive disorder (Ichihashi et al., 2020; Iida et al., 2020; Hashimoto et al., 2021). Regarding TRS, we reported that institutions with a low TRS examination rate had a low clozapine prescription rate (Yasui-Furukori et al., 2022).
To our knowledge, there is little real-world evidence regarding whether the prescription rates of monotherapy and polypharmacy, including not only antipsychotics but also other psychotropics, differed between patients with TRS who were and were not prescribed clozapine. We hypothesized that the prescription rate of monotherapy for patients with TRS who were prescribed clozapine would be higher than that for patients with schizophrenia who were not prescribed clozapine, and we investigated the characteristics of prescribed pharmacotherapy at discharge between these groups.
METHODS
Patients
This study was a continuous, nationwide, cross-sectional study. We performed this study as part of the previously reported EGUIDE project (Takaesu et al., 2019; Iida et al., 2020; Hashimoto et al., 2021; Numata et al., 2021; Furihata et al., 2022; Yasui-Furukori et al., 2022). We recruited psychiatrists beginning in October 2016. All participants provided written informed consent after the chief researcher had fully explained the procedures at the institution. We gathered the medical record information of patients at each institution with opt-out consent. Ethical approval was obtained from the ethics committees of the National Center of Neurology and Psychiatry (B2020-106) and each participating university, hospital, and clinic. The study procedures were conducted according to the Declaration of Helsinki. The protocol of this study was registered in the University Hospital Medical Information Network registry (UMIN000022645).
We gathered data from each participating institution from April to September of each year from 2016 to 2019 using a standardized data collection method in which participating institutions’ medical records were checked and the data were manually entered into an Excel sheet. The data manager then double-checked the data. We gathered the data of 8306 patients diagnosed with schizophrenia at discharge from 178 institutions. We recorded all types and doses of prescribed psychotropics, such as antipsychotics, antidepressants, anti-Parkinson drugs, hypnotics and anxiolytics, mood stabilizers/antiepileptics, and other types of drugs, at both admission and discharge, as we reported previously (Hashimoto et al., 2021; Furihata et al., 2022). We calculated the dose of psychotropics using psychotropic dose equivalence, such as the chlorpromazine equivalent, imipramine equivalent, biperiden equivalent, and diazepam equivalent (Inada and Inagaki, 2015). We also recorded information on the diagnosis at discharge, modified electroconvulsive therapy during each hospitalization, and the number of rehospitalizations for the patients. We defined lithium, sodium valproate, carbamazepine, and lamotrigine as mood stabilizers in this study because they were approved as mood stabilizers in Japan. In this study, we included patients who were prescribed antipsychotics at discharge from their first hospitalization for schizophrenia during the data collection period.
Finally, we analyzed a total of 7186 patients from 177 institutions. We show the characteristics of the patients in Table 1.
Table 1.
Characteristics of Patients
| Variables | TRS with clozapine | TRS without clozapine | ND-TRS without clozapine | P 1 | P 2 | P 3 |
|---|---|---|---|---|---|---|
| n | 426 | 414 | 6346 | |||
| Female (%) | n = 236 (55.4) | n = 235 (56.8) | n = 3465 (54.6) | 1.0 | 1.0 | 1.0 |
| Age (y) | 41.6 (11.8) | 45.8 (14.3) | 46.2 (15.8) | 1.4 × 10−4 | 1.1 × 10−8 | 1.0 |
| Prescription clozapine at admission (%) | n = 189 (44.4) | n = 8 (1.9) | n = 7 (0.1) | < 2.0 × 10−16 | < 2.0 × 10−16 | 2.4 × 10−6 |
| Mean dose of clozapine at admission (mg/d)4 | 364.6 (175.6) | 370.3 (168.2) | 332.1 (192.1) | 1.0 | 1.0 | 1.0 |
| Electroconvulsive therapy during hospitalization (%) | n = 36 (8.5) | n = 100 (24.2) | n = 264 (4.2) | 1.8 × 10−9 | 4.1 × 10−4 | < 2.0 × 10−16 |
Abbreviations: ND-TRS, no description of TRS; TRS, treatment-resistant schizophrenia. Values are expressed as the mean (SD) except for (%). Fisher’s exact test or the Mann–Whitney U test was adjusted by Bonferroni’s correction for post hoc analyses, and P < 6.2 × 10−4 (0.05/81) was defined as significant.
1Comparison between the TRS with clozapine and TRS without clozapine groups using the Mann–Whitney U test or Fisher’s exact test with Bonferroni correction.
2Comparison between the TRS with clozapine and ND-TRS without clozapine groups using the Mann–Whitney U test or Fisher’s exact test with Bonferroni correction.
3Comparison between the TRS without clozapine and ND-TRS without clozapine groups using the Mann–Whitney U test or Fisher’s exact test with Bonferroni correction.
4The mean dose of clozapine in patients for whom clozapine was prescribed at admission.
Procedure
The definition of TRS of the Clozapine Appropriate Use Committee in Japan was applied in this study. An insufficient response was defined when symptoms did not respond to more than 2 adequate doses of antipsychotic therapy (i.e., >600 mg of chlorpromazine equivalent per day) (Inada and Inagaki, 2015), including more than one of the atypical antipsychotics for at least 4 weeks (Yasui-Furukori et al., 2022), for adequate periods of time with adequate medication adherence. Insufficient tolerance was defined when more than 2 types of atypical antipsychotics were taken as monotherapy, but the dose of any of the antipsychotics could not be increased to an adequate dose because of the occurrence of adverse effects, such as EPSs (Matsui et al., 2020).
Patients with TRS were those for whom the diagnosis at discharge was listed as TRS in the discharge summary. Patients without TRS listed in the discharge summary were classified as having no description of TRS (ND-TRS) because we could not determine whether the possibility of TRS was considered for each patient. We analyzed whether patients were prescribed clozapine at discharge. All patients prescribed clozapine at discharge had been diagnosed with TRS. We compared the data of patients with TRS who were prescribed clozapine, patients with TRS who were not prescribed clozapine, and patients with ND-TRS who were not prescribed clozapine.
In this study, we defined antipsychotic monotherapy as the prescription of a single antipsychotic regardless of concomitant use of other psychotropics, and we defined psychotropic monotherapy as the prescription of a single antipsychotic without concomitant use of other psychotropics.
We compared the prescription rate of antipsychotic monotherapy and the dose of antipsychotics among the 3 groups, and we compared the mean number of types of psychotropics prescribed and the prescription rate of psychotropic monotherapy among the 3 groups. In addition, we compared the prescription rate and the prescribed dose of other psychotropics, such as anti-Parkinson drugs, antidepressants, anxiolytics, and hypnotics, and each mood stabilizer among the 3 groups.
Statistical Analysis
We performed statistical analysis with SPSS 22.0 software (IBM Co., Armonk, NY, USA) and EZR 1.54 software (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) and a modified version of R commander designed to add statistical functions for biostatistics (Kanda, 2013). We used the Shapiro–Wilk test as a test of normality. To compare the TRS with clozapine group, TRS without clozapine group, and ND-TRS without clozapine group, Fisher’s exact test was used for categorical variables (such as sex, prescription rate of monotherapy, prescription rate of electroconvulsive therapy, and prescription rate of each psychotropic), the Kruskal–Wallis test was used for continuous and ordered variables (such as age, number of types of psychotropics prescribed and dose of each psychotropic prescribed), and P < 1.9 × 10−3 (0.05/27) was considered statistically significant. Then, Fisher’s exact test and the Mann–Whitney U test were adjusted by Bonferroni’s correction for post hoc analyses, and P < 6.2 × 10−4 (0.05/81) was defined as significant. The lower limit of detection of EZR was 2.0 × 10−16. Descriptive statistics are expressed as the mean ± SD.
RESULTS
Patient Characteristics
The number of patients was 426 in the TRS with clozapine group, 414 in the TRS without clozapine group, and 6346 in the ND-TRS without clozapine group, and there was no significant sex difference among the groups (Table 1).
Prescription Rate of Antipsychotic Monotherapy and Psychotropic Monotherapy
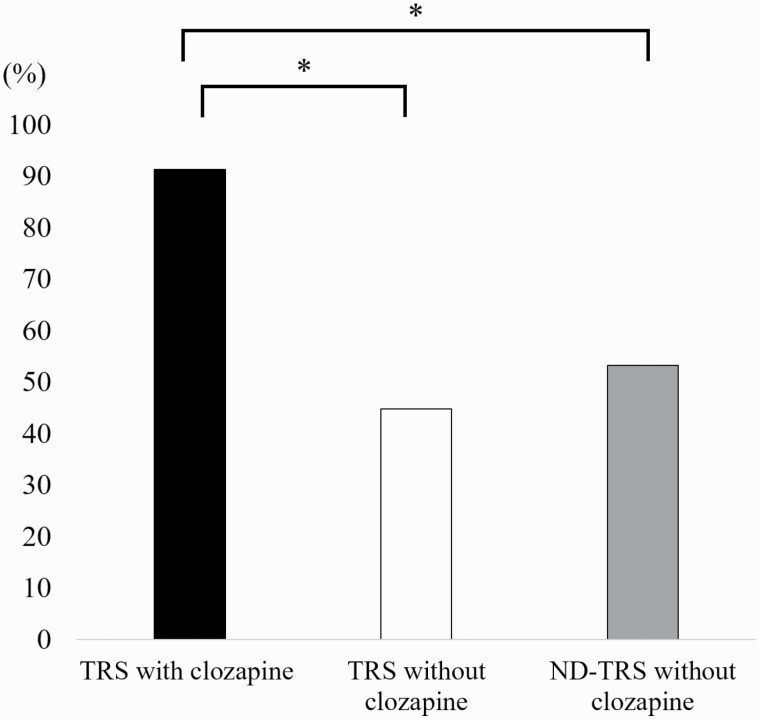
We show the prescription rates of psychotropics at discharge in Table 2. The prescription rate of antipsychotic monotherapy in the TRS with clozapine group (91.3%) was significantly higher than that in the TRS without clozapine group (45.9%; P < 2.0 × 10−16) and the ND-TRS without clozapine group (54.7%; P < 2.0 × 10−16) (Fig. 1).
Table 2.
Prescription of Psychotropics at Discharge
| Variables | TRS with clozapine | TRS without clozapine | ND-TRS without clozapine | P 2 | P 3 | P 4 |
|---|---|---|---|---|---|---|
| Mean dose of clozapine at discharge (mg/d) | 344.8 (163.9) | |||||
| Antipsychotic monotherapy (%) | n = 389 (91.3) | n = 190 (45.9) | n = 3469 (54.7) | < 2.0 × 10−16 | < 2.0 × 10−16 | 1.9 × 10−3 |
| Mean dose of total antipsychotics (mg/d)1 | 716.4 (357.6) | 886.6 (591.2) | 687.8 (464.8) | 1.4 × 10−3 | 1.8 × 10−3 | 2.8 × 10−12 |
| Mean no. of all types of psychotropics (n/d) | 2.5 (1.3) | 3.8 (1.9) | 3.4 (1.9) | < 2.0 × 10−16 | < 2.0 × 10−16 | 1.4 × 10−5 |
| Antipsychotic monotherapy without concomitant other psychotropics (%) | n = 113 (26.5) | n = 52 (12.6) | n = 1079 (17.0) | 1.1 × 10−6 | 5.9 × 10−6 | 5.2 × 10−2 |
| Prescription rate of anti-Parkinson drugs (%) | n = 47 (11.0) | n = 146 (35.3) | n = 1870 (29.5) | < 2.0 × 10−16 | < 2.0 × 10−16 | 4.4 × 10−2 |
| Prescription rate of anxiolytic and hypnotics (%) | n = 213 (50.0) | n = 295 (71.3) | n = 4218 (66.5) | 8.8 × 10−10 | 4.8 × 10−11 | 1.4 × 10−1 |
| Prescription rate of antidepressants (%) | n = 23 (5.4) | n = 39 (9.4) | n = 566 (8.9) | 1.0 × 10−1 | 3.8 × 10−2 | 1.0 |
| Prescription rate of mood stabilizers (%) | n = 163 (38.3) | n = 106 (25.6) | n = 1506 (23.7) | 2.6 × 10−4 | 3.5 × 10−10 | 1.0 |
| Prescription rate of lithium (%) | n = 118 (27.7) | n = 21 (5.1) | n = 340 (5.4) | < 2.0 × 10−16 | < 2.0 × 10−16 | 1.0 |
| Prescription rate of mood stabilizers except lithium (%) | n = 60 (14.1) | n = 92 (22.2) | n = 1265 (19.9) | 6.9 × 10−3 | 8.9 × 10−3 | 7.8 × 10−1 |
Abbreviations: ND-TRS, no description of TRS; TRS, treatment-resistant schizophrenia. Values are expressed as the mean (SD) except for (%). Fisher’s exact test or the Mann–Whitney U test was adjusted by Bonferroni’s correction for post hoc analyses, and P < 6.2 × 10−4 (0.05/81) was defined as significant.
1Presented as chlorpromazine equivalent.
2Comparison between the TRS with clozapine and TRS without clozapine groups using the Mann–Whitney U test or Fisher’s exact test with Bonferroni correction.
3Comparison between the TRS with clozapine and ND-TRS without clozapine groups using the Mann–Whitney U test or Fisher’s exact test with Bonferroni correction.
4Comparison between the TRS without clozapine and ND-TRS without clozapine groups using the Mann–Whitney U test or Fisher’s exact test with Bonferroni correction.
Figure 1.
The prescription rate of antipsychotic monotherapy at discharge. The prescription rate of antipsychotic monotherapy at discharge in the treatment-resistant schizophrenia (TRS) with clozapine group was significantly higher than that in the other groups. Fisher’s exact test adjusted by Bonferroni correction. *P < 6.2 × 10−4 was defined as significant. ND-TRS, no description of TRS.
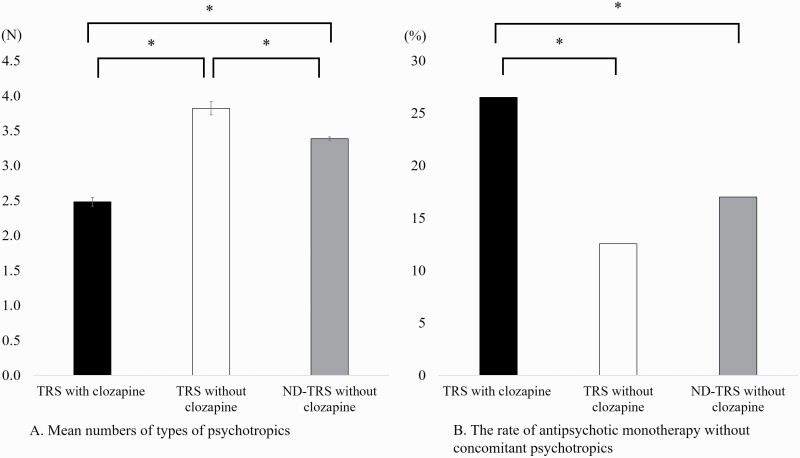
The mean number of all types of psychotropics prescribed in the TRS with clozapine group (2.5 ± 1.3/d) was significantly lower than that in the TRS without clozapine group (3.8 ± 1.9/d; P < 2.0 × 10−16) and the ND-TRS without clozapine group (3.4 ± 1.9/d; P < 2.0 × 10−16) (Fig. 2A). The prescription rate of psychotropic monotherapy in the TRS with clozapine group (26.5%) was significantly higher than that in the TRS without clozapine group (12.6%; P = 1.1 × 10−6) and the ND-TRS without clozapine group (17.0%; P = 5.9 × 10−6) (Fig. 2B).
Figure 2.
The mean number of psychotropics prescribed and the prescription rate of antipsychotic monotherapy without other concomitant psychotropics at discharge. (A) The mean number of all types of psychotropics prescribed at discharge in the treatment-resistant schizophrenia (TRS) with clozapine group was significantly lower than that in the other groups. On the other hand, the mean number of all types of psychotropics prescribed at discharge in the TRS without clozapine group was significantly higher than that in the other groups. (B) The prescription rate of antipsychotic monotherapy without other concomitant psychotropics in the TRS with clozapine group was significantly higher than that in the other groups. Error bars show the mean (SE). The Mann–Whitney U test or Fisher’s exact test adjusted by Bonferroni correction. *P < 6.2 × 10−4 was defined as significant. ND-TRS, no description of TRS.
Prescription Rates of Anti-Parkinson Drugs, Antidepressants, Anxiolytics and Hypnotics, and Mood Stabilizers, Especially Lithium
The prescription rate of anti-Parkinson drugs in the TRS with clozapine group (11.0%) was significantly lower than that in the TRS without clozapine group (35.3%; P < 2.0 × 10−16) and the ND-TRS without clozapine group (29.5%; P < 2.0 × 10−16) (Table 2). The prescription rate of anxiolytics and hypnotics in the TRS with clozapine group (50.0%) was significantly lower than that in the TRS without clozapine group (71.3%; P = 8.8 × 10−10) and the ND-TRS without clozapine group (66.5%; P = 4.8 × 10−11) (Table 2). There was no significant difference in the prescription rate of antidepressants among the groups (Table 2). There was no significant difference in the prescribed mean doses of anti-Parkinson drugs, antidepressants, or anxiolytics and hypnotics among the groups (supplementary Table 1). The prescription rate of mood stabilizers in the TRS with clozapine group (38.3%) was significantly higher than that in the TRS without clozapine group (25.6%; P = 2.6 × 10−4) and the ND-TRS without clozapine group (23.7%; P = 3.5 × 10−10) (Table 2). The prescription rate of lithium in the TRS with clozapine group (27.7%) was significantly higher than that in the TRS without clozapine group (5.1%; P < 2.0 × 10−16) and the ND-TRS without clozapine group (5.4%; P < 2.0 × 10−16) (Table 2). There was no significant difference in the prescription rate of mood stabilizers except lithium among the groups (Table 2). There was no significant difference in the mean dose of lithium prescribed among the groups (supplementary Table 1).
Discussion
The main results of this study were that the prescription rates of antipsychotic monotherapy and psychotropic monotherapy in the TRS with clozapine group were significantly higher than those in the other groups. The prescription rates of other psychotropics in the TRS with clozapine group were significantly lower and the prescription rate of lithium was significantly higher than those in the other groups.
To the best of our knowledge, we are the first to reveal that the TRS with clozapine group had almost no prescription of antipsychotic polypharmacy compared with both the TRS without clozapine group and the ND-TRS without clozapine group in a large nationwide sample. These findings are supported by a recent small study (n = 62) reporting that the prescription rate of antipsychotic monotherapy was significantly increased after the prescription of clozapine (Akamine et al., 2021). A recent meta-analysis reported that compared with antipsychotic monotherapy, antipsychotic polypharmacy in schizophrenia did not show efficacy in high-quality, double-blinded, randomized controlled trials (Galling et al., 2017). This meta-analysis also reported that aripiprazole for augmentation was associated with prolactin reduction and weight loss. In addition, a nationwide cohort study reported that as antipsychotic polypharmacy, clozapine with aripiprazole was associated with the lowest risk of hospitalization in patients with schizophrenia, followed by clozapine monotherapy (Tiihonen et al., 2019), although there was a lack of evidence on whether polypharmacy with more than 3 types of antipsychotics was effective. Future studies on aripiprazole augmentation with clozapine are needed to clarify the improvements to adverse effects of clozapine, such as weight gain. Although it is unclear what treatment options are prioritized for TRS, a 12-month follow-up study in the United Kingdom reported that the prescription rate of clozapine in patients with TRS was only 2.4%, but the prescription rate of antipsychotic polypharmacy, instead of clozapine, in patients with TRS was approximately 46.9%; the prescription rate of >3 antipsychotics was approximately 9.1% (Stokes et al., 2020). In Japan, as in the United Kingdom, there is a strong tendency to choose multiple antipsychotic medications for the treatment of TRS (Yang et al., 2018; Yasui-Furukori et al., 2022). This could be for several reasons. One reason is that there are still few clozapine-licensed facilities and clozapine-licensed doctors in Japan because clozapine became available in 2009, later than in other countries. The second reason is that polypharmacy in Japan may be related to the traditional oriental herbal therapeutic concept in which a mixture of many ingredients is the best prescription (Inada and Inagaki, 2015). The other reason is that there may still be some psychiatrists who do not sufficiently utilize evidence-based clinical practice guidelines for treatment. We need to disseminate the guidelines for the treatment of schizophrenia through educational programs such as the EGUIDE project.
Antipsychotic polypharmacy may be considered in some clinically limited situations; however, clozapine monotherapy for TRS should at least be attempted before antipsychotic polypharmacy is prescribed.
We also revealed for the first time, to our knowledge, that there was a significantly higher prescription rate of psychotropic monotherapy in the TRS with clozapine group than in the TRS without clozapine group and in the ND-TRS without clozapine group. In addition, significantly lower prescription rates of anti-Parkinson drugs and anxiolytics and hypnotics in the TRS with clozapine group than in the TRS without clozapine and ND-TRS without clozapine groups were found, which is in line with a previous study reporting that the prescription rates of benzodiazepines and anti-Parkinson drugs were significantly decreased after the prescription of clozapine (Akamine et al., 2021). A recent meta-analysis reported that only clozapine treatment was significantly associated with a decrease in the prescription rate of anti-Parkinson drugs (Huhn et al., 2019). Continuous prescription of clozapine was shown to significantly reduce long-term all-cause mortality compared with continuous prescription of other antipsychotics (Vermeulen et al., 2018). Furthermore, many factors, such as an increase in the number of antipsychotics, anti-Parkinson drugs, and benzodiazepines prescribed, were associated with an increased risk of neuroleptic malignant syndrome (Guinart et al., 2021) and with potential risks, including drug-drug interactions (Yasui-Furukori and Shimoda, 2020). Although a previous study in Europe reported that the prescription rate of psychotropic polypharmacy increased from 2000 to 2015 and that 44.7% of patients received more than 3 psychotropics, the prescription rate of clozapine did not change over time (Toto et al., 2019). These findings support the results of this study. Based on our findings and the findings of the literature mentioned above, an increase in the prescription of psychotropic monotherapy could be associated with a reduction in the risks of psychotropic polypharmacy and might play a key role in reducing long-term all-cause mortality associated with the prescription of clozapine.
We also found a higher prescription rate of lithium in the TRS with clozapine group than in the other groups. Although clozapine with concomitant lithium for augmentation is not recommended in guidelines (Barnes et al., 2020; Japanese Society of Neuropsychopharmacology, 2021), lithium is reported to lead to the proliferation of granulocytes, increase production of white blood cells (WBCs) and reduce the risk of neutropenia (Hager et al., 2002). In addition, lithium has widely been reported to prevent or treat neutropenia during clozapine treatment (Adityanjee, 1995; Boshes et al., 2001; Kanaan and Kerwin, 2006; Brunoni et al., 2008; Suraweera et al., 2014; Aydin et al., 2016), although concomitant lithium does not always prevent agranulocytosis (Valevski et al., 1993). Furthermore, there may be a domestic factor that would increase the concomitant use of lithium in this study. All Japanese clozapine users are also registered in the CPMS, but there are some differences between Japan and other countries (Nielsen et al., 2016; Myles et al., 2019; Matsui et al., 2020; Yasui-Furukori et al., 2022). The criteria for the initiation of clozapine treatment are stricter in Japan. The WBC count in Japan must be >4000/mm3 compared with >3500/mm3 in many other countries (Yasui-Furukori et al., 2022). Furthermore, leukopenia is defined as a WBC count <3000/mm3, and this definition is higher than that in many other countries (e.g., <3500/mm3) (Inada et al., 2018). However, applying global standards to the CPMS in Japan may result in a gradual decrease in lithium prescription.
In this study, the number of patients in the ND-TRS without clozapine group was much higher than the number of patients in the other groups. We previously reported that there was a significant correlation between the prescription rate of clozapine and the diagnosis rate of TRS (Yasui-Furukori et al., 2022). Beck reported that 56% of patients had been diagnosed with TRS, and 52% of patients who had been diagnosed with TRS had never been prescribed clozapine (Beck et al., 2019). Although the evidence for the efficacy of clozapine is strong (Siskind et al., 2016; Vermeulen et al., 2018; Huhn et al., 2019; Tiihonen et al., 2019), many TRS patients have not been prescribed clozapine (Bachmann et al., 2017). The correct diagnosis of TRS is a worldwide problem. Furthermore, previous studies reported that there were many barriers to using clozapine for TRS, such as adverse effects and the lack of knowledge and confidence of clinicians (Kelly et al., 2018; Farooq et al., 2019). In Japan, in addition to these barriers, there are Japan-specific barriers. One factor is the criteria of WBC count for the initiation of clozapine, which we described above. A second factor is that excessive caution is urged for diabetic patients taking clozapine in Japan. This can be because olanzapine and quetiapine, which have similar pharmacological profiles to clozapine, are contraindicated in diabetes patients in Japan. Furthermore, in Japan, patients must be hospitalized at the time of clozapine initiation. In addition, there are still insufficient numbers of clozapine-licensed facilities and clozapine-licensed doctors (Yasui-Furukori et al., 2022). These factors are Japan-specific barriers to clozapine use in Japan. Worldwide, we should consider education and emphasis on the importance of correctly diagnosing TRS and correctly prescribing clozapine.
This study has several limitations. First, this was a cross-sectional study, and we could not clarify the causal relationships between the prescription rate of clozapine and an increase in antipsychotic monotherapy. However, in this study, the sample size was relatively large, and we analyzed control data (i.e., data from the TRS without clozapine group). Second, there may be selection bias and sampling bias because, in this study, all participating institutions voluntarily cooperated, were not selected randomly, and did not include all patients within the study period. However, we standardized the method of data collection and gathered nationwide data from Japan. Because all inpatients during the sampling period were included, the sampling bias was low, although there was institutional bias. Third, we did not assess the clinical symptoms and severity of schizophrenia using rating scales such as the Positive and Negative Syndrome Scale or the Brief Psychiatric Rating Scale. Fourth, we could not assess why the TRS without clozapine group was not prescribed clozapine or why clozapine was not continued, such as due to inefficacy or severe adverse effects such as neutropenia. Further prospective clinical studies, including randomized controlled trials, are needed to clarify these causal relationships in detail. Fifth, the number of patients in the TRS groups was relatively small, and some TRS patients may have been included in the ND-TRS without clozapine group because we analyzed the data based on the medical records from each institution. Thus, we could not strictly distinguish whether schizophrenia that was not described as TRS was not TRS. However, the prescription rate of clozapine was much lower in Japan than in other countries; furthermore, we previously reported that the low prescription rate of clozapine was correlated with the low diagnosis rate of TRS (Yasui-Furukori et al., 2022). Hence, these data could reflect real-world evidence. Therefore, in the future, we should emphasize the importance of correctly diagnosing TRS to adequately prescribe clozapine, such as through educational programs like the EGUIDE project.
In conclusion, the findings from our analysis suggested that the prescription of clozapine for TRS could be the ideal antipsychotic therapy as indicated by the guidelines. Therefore, clinicians should appropriately diagnose TRS and constructively consider the prescription of clozapine for the treatment of TRS.
Supplementary Material
Acknowledgments
The authors thank all patients and the individuals who participated in this study.
This work was supported by the Japan Agency for Medical Research and Development (AMED) (grant nos. JP18dk0307060, JP19dk0307083, and JP20dk0307081), Health and Labor Sciences Research Grants (grant nos. H29-Seishin-Ippan-001 and 19GC1201), the Japanese Society of Neuropsychopharmacology, and the Japanese Society of Mood Disorders. No funders had any role in the study design, data collection, data analysis, preparation of this manuscript or decision to publish this manuscript.
Contributor Information
Shinichiro Ochi, Department of Neuropsychiatry, Molecules and Function, Ehime University Graduate School of Medicine, Ehime, Japan.
Hiromi Tagata, Department of Neuropsychiatry, Toho University School of Medicine, Tokyo, Japan.
Naomi Hasegawa, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan.
Norio Yasui-Furukori, Department of Psychiatry, Dokkyo Medical University School of Medicine, Tochigi, Japan.
Jun-ichi Iga, Department of Neuropsychiatry, Molecules and Function, Ehime University Graduate School of Medicine, Ehime, Japan.
Hiroko Kashiwagi, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan; Department of Forensic Psychiatry, National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan.
Fumitoshi Kodaka, Department of Psychiatry, The Jikei University School of Medicine, Tokyo, Japsan.
Hiroshi Komatsu, Department of Psychiatry, Tohoku University Hospital, Miyagi, Japan.
Takashi Tsuboi, Department of Neuropsychiatry, Kyorin University School of Medicine, Tokyo, Japan.
Akira Tokutani, Department of Pharmacy, The Hospital of Hyogo College of Medicine, Hyogo, Japan.
Shusuke Numata, Department of Psychiatry, Graduate School of Biomedical Science, Tokushima University, Tokushima, Japan.
Kayo Ichihashi, Department of Neuropsychiatry, University of Tokyo Hospital, Tokyo, Japan.
Toshiaki Onitsuka, Department of Neuroimaging Psychiatry, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
Hiroyuki Muraoka, Department of Psychiatry, Tokyo Women’s Medical University, Tokyo, Japan.
Hitoshi Iida, Department of Psychiatry, Faculty of Medicine, Fukuoka University, Fukuoka, Japan.
Kazutaka Ohi, Department of Psychiatry, Gifu University Graduate School of Medicine, Gifu, Japan.
Kiyokazu Atake, Nippon Telegraph and Telephone West Corporation Kyushu Health Administration Center, Fukuoka, Japan.
Taishiro Kishimoto, Hills Joint Research Laboratory for Future Preventive Medicine and Wellness, Keio University School of Medicine, Tokyo, Japan.
Hikaru Hori, Department of Psychiatry, Faculty of Medicine, Fukuoka University, Fukuoka, Japan.
Yoshikazu Takaesu, Department of Neuropsychiatry, Graduate School of Medicine, University of the Ryukyus, Okinawa, Japan.
Masahiro Takeshima, Department of Neuropsychiatry, Akita University Graduate School of Medicine, Akita, Japan.
Masahide Usami, Department of Child and Adolescent Psychiatry, Kohnodai Hospital, National Center for Global Health and Medicine, Chiba, Japan.
Manabu Makinodan, Department of Psychiatry, Nara Medical University School of Medicine, Nara, Japan.
Naoki Hashimoto, Department of Psychiatry, Hokkaido University Graduate School of Medicine, Hokkaido, Japan.
Michiko Fujimoto, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan; Department of Psychiatry, Osaka University Graduate School of Medicine, Osaka, Japan.
Ryuji Furihata, Kyoto University Health Service, Kyoto, Japan.
Tatsuya Nagasawa, Department of NeuroPsychiatry Kanazawa Medical University, Ishikawa, Japan.
Hisashi Yamada, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan; Department of Neuropsychiatry, Hyogo College of Medicine, Hyogo, Japan.
Junya Matsumoto, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan.
Kenichiro Miura, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan.
Mikio Kido, Toyama City Hospital, Toyama, Japan; Department of Neuropsychiatry, University of Toyama Graduate School of Medicine and Pharmaceutical Sciences, Toyama, Japan.
Akitoyo Hishimoto, Department of Psychiatry, Yokohama City University Graduate School of Medicine, Kanagawa, Japan.
Shu-ichi Ueno, Department of Neuropsychiatry, Molecules and Function, Ehime University Graduate School of Medicine, Ehime, Japan.
Koichiro Watanabe, Department of Neuropsychiatry, Kyorin University School of Medicine, Tokyo, Japan.
Ken Inada, Department of Psychiatry, Kitasato University, School of Medicine, Kanagawa, Japan.
Ryota Hashimoto, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan.
Interest Statement
The authors declare no conflicts of interest.
References
- Adityanjee (1995) Modification of clozapine-induced leukopenia and neutropenia with lithium carbonate. Am J Psychiatry 152:648–649. [DOI] [PubMed] [Google Scholar]
- Akamine Y, Kikuchi Y, Miura M (2021) Effects on monotherapy and reduction of antipsychotic drugs by clozapine therapy in Japanese patients with treatment-resistant schizophrenia. J Clin Pharm Ther 46:1312–1318. [DOI] [PubMed] [Google Scholar]
- Aydin M, Ilhan BC, Calisir S, Yildirim S, Eren I (2016) Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol 6:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayenew W, Asmamaw G, Bitew T (2021) Antipsychotic polypharmacy among patients with schizophrenia in Africa: a systematic review and meta-analysis. Int J Neuropsychopharmacol 24:956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann CJ, et al. (2017) International trends in clozapine use: a study in 17 countries. Acta Psychiatr Scand 136:37–51. [DOI] [PubMed] [Google Scholar]
- Barnes TR, et al. (2020) Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol 34:3–78. [DOI] [PubMed] [Google Scholar]
- Beck K, McCutcheon R, Stephenson L, Schilderman M, Patel N, Ramsay R, Howes OD (2019) Prevalence of treatment-resistant psychoses in the community: a naturalistic study. J Psychopharmacol 33:1248–1253. [DOI] [PubMed] [Google Scholar]
- Boshes RA, Manschreck TC, Desrosiers J, Candela S, Hanrahan-Boshes M (2001) Initiation of clozapine therapy in a patient with preexisting leukopenia: a discussion of the rationale of current treatment options. Ann Clin Psychiatry 13:233–237. [DOI] [PubMed] [Google Scholar]
- Brunoni A, Ferreira L, Gallucci-Neto J, Elkis H, Velloso E, Zanetti M (2008) Lithium as a treatment of clozapine-induced neutropenia: a case report. Prog Neuropsychopharmacol Biol Psychiatry 32:2006–2007. [DOI] [PubMed] [Google Scholar]
- Farooq S, Choudry A, Cohen D, Naeem F, Ayub M (2019) Barriers to using clozapine in treatment-resistant schizophrenia: systematic review. Bjpsych Bull 43:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furihata R, et al. (2022) Hypnotic medication use among inpatients with schizophrenia and major depressive disorder: results of a nationwide study. Sleep Med 89:23–30. [DOI] [PubMed] [Google Scholar]
- Galletly C, Castle D, Dark F, Humberstone V, Jablensky A, Killackey E, Kulkarni J, McGorry P, Nielssen O, Tran N (2016) Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry 50:410–472. [DOI] [PubMed] [Google Scholar]
- Galling B, Roldán A, Hagi K, Rietschel L, Walyzada F, Zheng W, Cao XL, Xiang YT, Zink M, Kane JM, Nielsen J, Leucht S, Correll CU (2017) Antipsychotic augmentation vs. monotherapy in schizophrenia: systematic review, meta-analysis and meta-regression analysis. World Psychiatry 16:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinart D, Taipale H, Rubio JM, Tanskanen A, Correll CU, Tiihonen J, Kane JM (2021) Risk factors, incidence, and outcomes of neuroleptic malignant syndrome on long-acting injectable vs oral antipsychotics in a nationwide schizophrenia cohort. Schizophr Bull 47:1621–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager ED, Dziambor H, Winkler P, Höhmann D, Macholdt K (2002) Effects of lithium carbonate on hematopoietic cells in patients with persistent neutropenia following chemotherapy or radiotherapy. J Trace Elem Med Biol 16:91–97. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, et al. (2021) Characteristics of discharge prescriptions for patients with schizophrenia or major depressive disorder: real-world evidence from the Effectiveness of Guidelines for Dissemination and Education (EGUIDE) psychiatric treatment project. Asian J Psychiatry 63:102744. [DOI] [PubMed] [Google Scholar]
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, Arndt T, Bäckers L, Rothe P, Cipriani A, Davis J, Salanti G, Leucht S (2019) Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 394:939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi K, et al. (2020) Prescription patterns in patients with schizophrenia in Japan: first-quality indicator data from the survey of “Effectiveness of Guidelines for Dissemination and Education in psychiatric treatment (EGUIDE)” project. Neuropsychopharmacol Rep 40:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H, et al. (2020) Unmet needs of patients with major depressive disorder – Findings from the “Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)” project: a nationwide dissemination, education, and evaluation study. Psychiatry Clin Neurosci 74:667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T, Inagaki A (2015) Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci 69:440–447. [DOI] [PubMed] [Google Scholar]
- Inada K, Oshibuchi H, Ishigooka J, Nishimura K (2018) Analysis of clozapine use and safety by using comprehensive national data from the Japanese Clozapine Patient Monitoring Service. J Clin Psychopharmacol 38:302–306. [DOI] [PubMed] [Google Scholar]
- Japanese Society of Neuropsychopharmacology (2021) Japanese Society of Neuropsychopharmacology: “Guideline for pharmacological therapy of schizophrenia.” Neuropsychopharmacol Rep 41:266–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan RA, Kerwin RW (2006) Lithium and clozapine rechallenge: a retrospective case analysis. J Clin Psychiatry 67:756–760. [DOI] [PubMed] [Google Scholar]
- Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl 48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JM, Agid O, Baldwin ML, Howes O, Lindenmayer J-P, Marder S, Olfson M, Potkin SG, Correll CU (2019) Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry 80:18com12123. [DOI] [PubMed] [Google Scholar]
- Keepers GA, Fochtmann LJ, Anzia JM, Benjamin S, Lyness JM, Mojtabai R, Servis M, Walaszek A, Buckley P, Lenzenweger MF, Young AS, Degenhardt A, Hong SH; (Systematic Review) (2020) The American Psychiatric Association Practice Guideline for the treatment of patients with schizophrenia. Am J Psychiatry 177:868–872. [DOI] [PubMed] [Google Scholar]
- Kelly DL, Freudenreich O, Sayer MA, Love RC (2018) Addressing barriers to clozapine underutilization: a national effort. Psychiatry Serv 69:224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon J de, et al. (2022) An international adult guideline for making clozapine titration safer by using six ancestry-based personalized dosing titrations, CRP, and clozapine levels. Pharmacopsychiatry 55:73–86. [DOI] [PubMed] [Google Scholar]
- Matsui K, Ishibashi M, Kawano M, Oshibuchi H, Ishigooka J, Nishimura K, Inada K (2020) Clozapine-induced agranulocytosis in Japan: Changes in leukocyte/neutrophil counts before and after discontinuation of clozapine. Hum Psychopharmacol 35:e2739. [DOI] [PubMed] [Google Scholar]
- Myles N, Myles H, Xia S, Large M, Kisely S, Galletly C, Bird R, Siskind D (2018) Meta-analysis examining the epidemiology of clozapine-associated neutropenia. Acta Psychiatry Scand 138:101–109. [DOI] [PubMed] [Google Scholar]
- Myles N, Myles H, Xia S, Large M, Bird R, Galletly C, Kisely S, Siskind D (2019) A meta-analysis of controlled studies comparing the association between clozapine and other antipsychotic medications and the development of neutropenia. Aust N Z J Psychiatry 53:403–412. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Young C, Ifteni P, Kishimoto T, Xiang YT, Schulte PF, Correll CU, Taylor D (2016) Worldwide differences in regulations of clozapine use. CNS Drugs 30:149–161. [DOI] [PubMed] [Google Scholar]
- Numata S, et al. (2021) Improvements in the degree of understanding the treatment guidelines for schizophrenia and major depressive disorder in a nationwide dissemination and implementation study. Neuropsychopharmacol Rep 41:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M (2017) Guidelines for the pharmacotherapy of schizophrenia in adults. Can J Psychiatry 62:604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone JC, Ward AJ, Rotella P, Collins J, Windisch R (2015) An evaluation of variation in published estimates of schizophrenia prevalence from 1990─2013: a systematic literature review. BMC Psychiatry 15:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind D, McCartney L, Goldschlager R, Kisely S (2016) Clozapine vs. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry 209:385–392. [DOI] [PubMed] [Google Scholar]
- Stokes I, Griffiths SL, Jones R, Everard L, Jones PB, Fowler D, Hodgekins J, Amos T, Freemantle N, Sharma V, Marshall M, Singh SP, Birchwood M, Upthegrove R (2020) Prevalence of treatment resistance and clozapine use in early intervention services. Bjpsych Open 6:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suraweera C, Hanwella R, de Silva V (2014) Use of lithium in clozapine-induced neutropenia: a case report. BMC Res Notes 7:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaesu Y, et al. (2019) Improvement of psychiatrists’ clinical knowledge of the treatment guidelines for schizophrenia and major depressive disorders using the “Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)” project: a nationwide dissemination, education, and evaluation study. Psychiat Clin Neuros 73:642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Taipale H, Mehtälä J, Vattulainen P, Correll CU, Tanskanen A (2019) Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry 76:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toto S, Grohmann R, Bleich S, Frieling H, Maier HB, Greil W, Cordes J, Schmidt-Kraepelin C, Kasper S, Stübner S, Degner D, Druschky K, Zindler T, Neyazi A (2019) Psychopharmacological treatment of schizophrenia over time in 30 908 inpatients: data from the AMSP study. Int J Neuropsychopharmacol 22:560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valevski A, Modai I, Lahav M, Weizman A (1993) Clozapine—lithium combined treatment and agranulocytosis. Int Clin Psychopharm 8:63–66. [DOI] [PubMed] [Google Scholar]
- Vermeulen JM, Rooijen G van, Kerkhof MPJ van de, Sutterland AL, Correll CU, Haan L de (2018) Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1–12.5 years. Schizophrenia Bull 45:315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, et al. (2018) Polypharmacy and psychotropic drug loading in patients with schizophrenia in Asian countries: fourth survey of research on Asian prescription patterns on antipsychotics. Psychiatry Clin Neurosci 72:572–579. [DOI] [PubMed] [Google Scholar]
- Yasui-Furukori N, et al. (2022) Association between the examination rate of treatment-resistant schizophrenia and the clozapine prescription rate in a nationwide dissemination and implementation study. Neuropsychopharmacol Rep 42:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui-Furukori N, Shimoda K (2020) Recent trends in antipsychotic polypharmacy in the treatment of schizophrenia. Neuropsychopharmacol Rep 40:208–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.