Abstract
Objective
Aripiprazole and risperidone are 2 of the most used second-generation antipsychotics (SGAs) worldwide. Previous evidence shows a similar effect of these SGAs on weight and metabolic changes in the short term. However, a longer period is necessary for a better assessment of the SGA´s metabolic profile. We aimed to compare the long-term (1-year) metabolic profile of these 2 antipsychotics on a sample of drug-naïve first episode-psychosis (FEP) patients.
Methods
A total 188 drug-naïve patients, suffering from a first episode of non-affective psychosis (FEP), were randomly assigned to treatment with either aripiprazole or risperidone. Weight and glycemic/lipid parameters were recorded at baseline and after 1-year follow-up.
Results
We observed significant weight increments in both groups (9.2 kg for aripiprazole and 10.5 kg for risperidone) after 1 year of treatment. Despite this, weight and body mass index changes did not significantly differ between treatment groups (P > .05). Similarly, both treatment groups presented similar metabolic clinical impact with a comparable increase in the proportion of participants meeting criteria for metabolic disorders such as obesity or hypercholesterolemia, but not for metabolic syndrome (Δ9.2% vs Δ4.3%) or hypertriglyceridemia (Δ21.9% vs Δ8.0%), where aripiprazole showed worse outcomes than risperidone.
Conclusion
This study shows that aripiprazole and risperidone share a similar long-term metabolic profile. After 1 year of antipsychotic treatment, drug-naïve FEP patients in both treatment groups presented a significant increase in weight and metabolic changes, leading to a greater prevalence of metabolic disorders.
Keywords: Metabolism, weight gain, treatment-naïve, second-generation antipsychotic, schizophrenia
Significance Statement.
We studied, in a cohort of patients with a first episode of non-affective psychosis, the changes in body weight and glucose/lipid metabolisms observed during the first year after the diagnoses of psychosis and the initiation of antipsychotic treatment. At study entry, patients were randomized to either aripiprazole or risperidone, so we were able to run head-to-head comparisons between antipsychotic treatments. The results showed a significant increase in mean body weight in the whole cohort. Despite this, aripiprazole and risperidone showed a similar metabolic profile and produced comparable weight gain at long term in individuals with a first episode of psychosis. Secondary analyses showed that women had a statistically significant greater increase in body mass index than men. We did not observe sex–treatment interactions in weight gain or metabolic changes. Early preventive measures to avoid weight gain should be put in place when treating individuals for a first episode of psychosis.
Introduction
Schizophrenia is a neuropsychiatric disorder characterized by a chronic progression and lifetime prevalence of approximately 1% (Kahn et al., 2015). The primary treatment for schizophrenia is second-generation antipsychotics (SGAs) (Goff, 2021). After symptoms have been controlled in individuals with their first episode of psychosis (FEP), it is strongly advised to reduce SGA treatment to the lowest effective dose as soon as possible. This praxis not only ensures symptomatic stability but also reduces the likelihood of emergent adverse events, which would result in poor treatment acceptability and compliance (Goff et al., 2017; Correll et al., 2018). In this regard, SGAs have been widely linked to weight gain, which, along with other lifestyle risk factors, contributes to an increased risk of dyslipidemia, glucose intolerance, diabetes mellitus, metabolic syndrome (MetS), and, ultimately, cardiovascular disease (Henderson et al., 2015; Nielsen et al., 2021). These physical conditions are underlying causes of up to 70% of the excess mortality seen in schizophrenia, resulting in a life expectancy reduction of up to 20 years (Owen et al., 2016; Kurdyak et al., 2021). Recent evidence, however, indicates that there are differences in the side effects profile of antipsychotics in terms of weight gain (Leucht et al., 2013; Huhn et al., 2019). A recent meta-analysis described gradual but marked differences between a wide range of antipsychotic treatments regarding weight gain (Huhn et al., 2019).
Aripiprazole (a D2 and 5-HT1A receptor partial agonist and 5-HT2A receptor antagonist) and risperidone (a D2, 5-HT2, and NE alpha-2 receptor antagonist) are 2 of the most widely used SGAs (Toto et al., 2019; Taipale et al., 2020). As a result, head-to-head comparisons of tolerability, particularly in terms of metabolic profiles, are timely but currently scarce (Kishimoto et al., 2019). Moreover, literature on this topic showed contradictory results when comparing aripiprazole and risperidone metabolic profiles. A recent meta-analysis of patients with chronic schizophrenia found significant differences in weight gain between aripiprazole and risperidone, with the former being more effective (Huhn et al., 2019). Previous research on FEP patients found that aripiprazole (Vazquez-Bourgon et al., 2018) and risperidone (Perez-Iglesias et al., 2009) had metabolic profiles similar to other SGAs such as olanzapine or quetiapine. Similarly, a recent pooled analysis in FEP patients that included 6 different SGAs (haloperidol, olanzapine, risperidone, quetiapine, ziprasidone, and aripiprazole) and focused on treatment effectiveness found that aripiprazole and risperidone demonstrated clear advantages in effectiveness without demonstrating significant differences in weight gain after long-term follow-up (Gomez-Revuelta et al., 2020). Another recent study carrying head-to-head comparison of aripiprazole and risperidone in the acute-phase treatment of FEP patients (6 weeks after FEP diagnosis) showed no significant differences in weight gain between treatment groups (Gomez-Revuelta et al., 2021). Furthermore, Josiassen et al. (2010) demonstrated that after 8 weeks of treatment, FEP patients receiving aripiprazole gained similar weight to those receiving risperidone (7% vs 7.3% from baseline body weight).
Nonetheless, a longer timeframe may be required to properly observe clinically relevant changes in weight and metabolic parameters. Long-term studies on FEP patients show that the majority of this weight gain occurs within the first year of FEP diagnosis, indicating that this is the critical period for these side effects (Bioque et al., 2018; Vázquez-Bourgon et al., 2021).
As a result, the first aim of this study was to elucidate whether aripiprazole and risperidone may have distinct tolerability profiles in medication-naïve FEP patients in the first year of treatment.
Our main hypothesis was that based on the previous research, aripiprazole and risperidone would present with similar metabolic profiles. Second, we hypothesized that after the first year of antipsychotic treatment, FEP drug-naive patients would have a significant increase in weight and metabolic measures.
MATERIALS AND METHODS
The study was carried out in an early-psychosis intervention program in northern Spain (Programa Asistencial de las Fases Iniciales de Psicosis [PAFIP] program); this is a clinical and research program aimed at providing intensive and specialized treatment to people in Cantabria who have experienced their first episode of non-affective psychosis (Pelayo-Terán et al,. 2008). Furthermore, the study formed part of a pragmatic clinical trial to compare the effectiveness of aripiprazole and risperidone in a sample of drug-naive FEP patients (NCT02532491) (Son et al., 2021). The PAFIP program involves clinical and research activity at the outpatient and inpatient psychiatric units of the University Hospital Marqués de Valdecilla (Santander, Spain). The hospital serves as a reference center for a 580 000-person catchment area and houses the province’s only psychiatric acute inpatient unit as well as a 24-hour emergency care service. Regular meetings with all community mental health teams in Cantabria were maintained in an attempt to reach all persons suffering from FEP in Cantabria to provide them with adequate specialized treatment.
Participants
The sample for the present study was recruited between February 2011 and October 2018. These patients were referred to the PAFIP program when presenting a FEP and were admitted if they fulfilled the following criteria: (1) aged 15–60 years; (2) met the DSM-IV criteria (according to the Structured Clinical Interview for DSM-IV) 6 months after inclusion for a principal diagnosis of schizophrenia, schizophreniform disorder, schizoaffective disorder, brief reactive psychosis, or psychosis non-otherwise specified; (3) habitually living in the catchment area; (4) no prior treatment with antipsychotic medication; and (5) current psychotic symptoms of at least moderate severity, as assessed by 1 of the 5 items of the Scale for the Assessment of Positive Symptoms (SAPS). Patients were not admitted to the program if they had any of the following conditions: (1) intellectual disability, (2) a neurological disorder, or (3) a drug dependence (DSM-IV criteria). Prior to their inclusion in the study, all participants provided written informed consent, which was approved by the regional ethics committee (Clinical Research Ethics Committee of Cantabria).
Study Design
Patients entering the clinical trial (NCT02532491) were randomly assigned to treatment with aripiprazole (D2 and 5-HT1A receptor partial agonist, and 5-HT2A receptor antagonist) or risperidone (D2, 5-HT2, and NE alpha-2 receptor antagonist). A statistician created a computer-generated randomization list. The treatment dose ranges for aripiprazole were 5–30 mg/d and 1–6 mg/d for risperidone. Doses could be adjusted within the prescribed range as clinically indicated to target the lowest effective dose. Certain concomitant medications, such as lormetazepam and clonazepam (benzodiazepine receptor agonists, non-selective GABA-A receptor–positive allosteric modulators), were permitted for the management of agitation, general behavior disturbances, and/or insomnia. Only if clinically significant extrapyramidal symptoms occurred was anticholinergic medication (biperiden, at a dose of up to 8 mg/d) permitted. If clinically necessary, antidepressants (sertraline, a SERT reuptake inhibitor) and mood stabilizers (lithium; enzyme interactions) were also permitted. Those patients who did not respond after 6 weeks of treatment [<30% total improvement measured by SAPS (Andreasen, 1984) and the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1983) scales] or who experienced significant side effects, measured by the Udvalg für Kliniske Undersogelser (UKU) scale (Lingjaerde et al., 1987), were switched to a different antipsychotic. Patients were clinically evaluated by a senior psychiatrist at baseline and at the following time-points after initiating the treatment: 6 weeks, 12 weeks, and 1 year.
The NCT02532491 is a clinical trial aimed to evaluate and compare the effectiveness of aripiprazole and risperidone in FEP patients at long term (1 year). The clinical trial outcomes were discontinuation rates, changes in psychopathological (e.g., Brief Psychiatric Rating Scale, SAPS, and SANS) and side effects (e.g., UKU, Barnes Akathisia Scale) scales, and relapse and remission rates. Taking advantage of the clinical trial implementation and the systematic measure of anthropometric and metabolic parameters carried out in the patients included in the PAFIP program, we were able to set the following outcomes for the present exploratory analyses associated with the clinical trial. The primary outcome measures for the current study were changes in weight and body mass index (BMI), and the secondary outcomes were changes in metabolic (lipid and glycemic) parameters from baseline to 1 year after starting antipsychotic treatment.
Laboratory Analysis
All determinations were performed in our hospital, including both biochemical and endocrinology analysis. All measurements were obtained after an overnight fast. Patients and family members reported on their fasting state as well as treatment adherence. Glucose, total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides were measured using automated methods on a TechniconDax (Technicon Instruments Corp, Tarrytown, NY, USA), with reagents provided by Boehringer-Mannheim (Mannheim, Germany). Low-density lipoprotein cholesterol was determined by the Friedewald et al. calculation (Friedewald et al., 1972):
Low-density lipoprotein = total cholesterol − (HDL + [triglycerides/5])
Insulin levels were determined using an immunoradiometric assay (Immunotech, Beckman Coulter Company, Prague, Czech Republic) with a 3.3% average interassay coefficient of variation and a 2.8% intraassay coefficient of variation. The sensitivity of the method was 0.5 μU/mL. Values for normal-weight participants were 2–17 μU/mL. This assay does not show any cross-reactivity with human proinsulin and C-peptide. Homeostasis model assessment (HOMA) was used to assess insulin resistance (IR). The HOMA index was calculated using a previously described formula (Matthews et al., 1985):
HOMA = [fasting insulin (μU/mL) × fasting glucose (mmol/L)]/22.5.
Furthermore, as proposed by McLaughlin and colleagues (McLaughlin et al., 2005), we calculated the triglyceride to HDL cholesterol ratio as a predictor of IR using the optimal cutpoint of 3.5.
Statistical Analysis
We investigated the relationship between antipsychotic treatment and changes in metabolic parameters. For comparison between groups, an analysis of variance for continuous variables and a chi-square test for categorical variables were used first. A paired t test was used to determine if there were any statistically significant changes in glycemic and lipid parameters after 1 year of antipsychotic treatment. When exploring differences in longitudinal changes between antipsychotic groups, we compared mean differences resulting from 1-year data minus baseline data.
General linear model ANCOVA analyses were performed to explore longitudinal differences in weight and metabolic parameters between antipsychotic treatment groups. Measurements of each parameter at baseline, 3 months, and 1 year were entered as the dependent variable; the type of medication (treatment group) as the independent variable; and BMI at baseline, gender, and age as covariates. This analysis was carried out following an Intention-to-treat (ITT) approach (per protocol analysis was also performed). We additionally calculated the percentage of participants with pathologic values in weight and glucose and lipid parameters (according to the reference values of our laboratory) at baseline and 1 year. To evaluate significant changes in these percentages, we used the McNemar test for repeated measures.
Finally, we conducted post-hoc analysis to explore a possible differentiated effect of antipsychotic treatment regarding gender. We used ANCOVA analyses to accomplish this.
For statistical analyses, the Statistical Package for Social Science version 23.0 (IBM Corp, Armonk, NY, USA) was used. All statistical tests were 2-tailed, and significance was set at .05.
RESULTS
Enrollment and Characteristics of Study Population
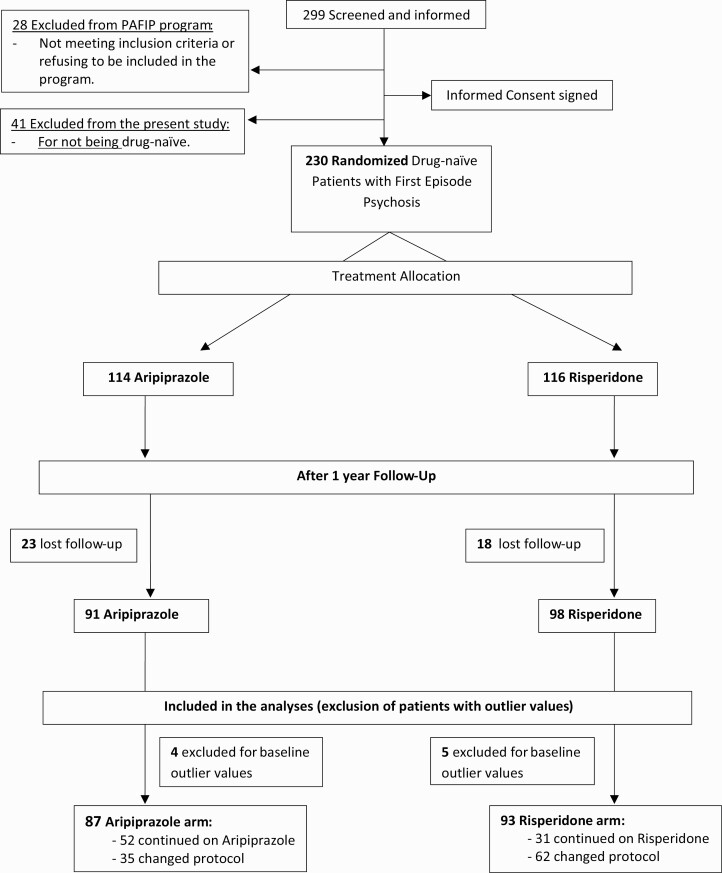
A total of 299 patients were screened and offered to enter the clinical/research program between February 2011 and October 2018. A total of 28 of these individuals were excluded because they did not meet the inclusion criteria or refused to be treated in the program. Another 41 patients were excluded from the statistical analyses for the current study solely because they were not drug free (having been on antipsychotic treatment for >2 days before entering the program). A total of 230 patients were included in the study; they had been randomized to receive (Fig. 1) aripiprazole (n = 114; 49.6%) or risperidone (n = 116; 50.4%). We had a 17.9% dropout rate at 1 year (41 patients were lost to follow-up or refused to be evaluated: 23 on the aripiprazole arm and 18 on the risperidone one). As a result, 189 patients entered the current study to assess the long-term changes in the main metabolic values. To avoid data distortion, 9 participants with outlier values for at least 1 of the glycemic/lipid parameters at baseline (value >3 SDs) were excluded from the analysis (4 of these patients were on aripiprazole and 5 on risperidone). Post-hoc statistical analyses that included the outlier participants revealed that their exclusion did not affect the reported results.
Figure 1.
Participant flow in the randomized clinical trial.
The clinical and socio-demographic characteristics of the sample are shown in Table 1. The 2 treatment groups were comparable on a wide range of baseline data, including age, ethnicity, substance use, and clinical severity.
Table 1.
Socio-Demographic and Clinical Characteristics of Study Population
| Patients’ characteristics | Aripiprazole (n = 87) |
Risperidone (n = 93) |
Total (n = 180) |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age at inclusion, y | 32.01 | 10.69 | 32.50 | 10.21 | 32.26 | 10.42 |
| DUP, mo | 10.60 | 25.70 | 15.01 | 42.93 | 12.91 | 35.74 |
| Weight at inclusion, kg | 65.24 | 14.13 | 63.72 | 15.66 | 64.46 | 14.91 |
| BMI at inclusion, kg/m2 | 22.43 | 3.76 | 22.47 | 4.44 | 22.45 | 4.12 |
| SAPS at inclusion | 15.03 | 5.00 | 15.85 | 5.23 | 15.46 | 5.12 |
| SANS at inclusion | 5.81 | 6.23 | 4.78 | 5.46 | 5.28 | 5.85 |
| n | % | n | % | n | % | |
| Sex, male | 49 | 56.32 | 49 | 52.69 | 98 | 54.44 |
| Student | 23 | 27.06 | 19 | 20.65 | 42 | 23.73 |
| Education level, secondary or lower | 43 | 51.19 | 39 | 42.39 | 82 | 46.59 |
| Family socioeconomic status, low | 42 | 50.60 | 55 | 61.11 | 97 | 56.07 |
| Living with family | 67 | 79.76 | 66 | 71.74 | 133 | 75.57 |
| Single | 63 | 74.12 | 59 | 64.13 | 122 | 68.93 |
| Unemployed | 33 | 39.29 | 35 | 38.46 | 68 | 38.86 |
| Obesity at inclusion | 3 | 3.45 | 7 | 7.61 | 10 | 5.59 |
| Diagnosis, schizophrenia | 41 | 47.13 | 36 | 38.71 | 77 | 42.78 |
| Hospitalization at inclusion | 68 | 78.16 | 74 | 79.57 | 142 | 78.89 |
| Drug consumption | ||||||
| Tobacco | 38 | 45.78 | 46 | 51.69 | 84 | 48.84 |
| Cannabis | 34 | 39.08 | 36 | 38.71 | 70 | 38.89 |
| Alcohol | 27 | 31.76 | 32 | 35.96 | 59 | 33.91 |
| Concomitant treatments | ||||||
| Anticholinergics 3 mo | 26 | 32.10 | 26 | 28.57 | 52 | 30.23 |
| Anticholinergics 6 mo | 13 | 16.46 | 14 | 16.09 | 27 | 16.27 |
| Anticholinergics 1 y | 9 | 11.11 | 12 | 13.48 | 21 | 12.35 |
| Antidepressants 3 mo | 10 | 12.35 | 19 | 20.88 | 29 | 16.86 |
| Antidepressants 6 mo | 19 | 24.05 | 28 | 31.82 | 47 | 28.14 |
| Antidepressants 1 y | 21 | 25.30 | 26 | 29.21 | 47 | 27.33 |
| Benzodiazepines 3 mo | 19 | 23.46 | 29 | 31.87 | 48 | 27.91 |
| Benzodiazepines 6 mo | 14 | 17.72 | 23 | 26.14 | 37 | 22.16 |
| Benzodiazepines 1 y | 15 | 18.07 | 19 | 21.35 | 34 | 19.77 |
| Mood stabilizers 3 mo | 3 | 3.70 | 4 | 4.40 | 7 | 4.07 |
| Mood stabilizers 6 mo | 2 | 2.53 | 5 | 5.68 | 7 | 4.19 |
| Mood stabilizers 1 y | 2 | 2.41 | 4 | 4.49 | 6 | 3.49 |
| Hypnotics 3 mo | 20 | 24.69 | 20 | 21.98 | 40 | 23.26 |
| Hypnotics 6 mo | 14 | 17.72 | 8 | 9.09 | 22 | 13.17 |
| Hypnotics 1 y | 12 | 14.46 | 14 | 15.73 | 26 | 15.12 |
Abbreviations: BMI, body mass index (obesity defined as BMI ≥ 30 kg/m2); DUP, duration of untreated psychosis; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms.
Statistical analyses: Un-adjusted ANOVA for continuous variables and chi-square test for categorical variables. Non-significant group differences, all P > .05.
At the end of follow-up, 46.1% (n = 83) of the 180 patients evaluated continued with the same antipsychotic treatment assigned at baseline (59.8% of aripiprazole group vs 33.3% of risperidone group). After 1 year of treatment, 97 patients did not complete the treatment protocol (supplementary Material, Tables 1, 2, 3A, 3B). The reasons for the treatment switch included side effects (41.2%), inefficacy (38.1%), non-adherence (16.5%), and others (4.1%). Despite the high rate of treatment switches due to side effects, neither weight gain nor metabolic disorders were among the reasons for antipsychotic change. Detailed data on the reasons for the antipsychotic switch in both treatment groups, as well as data on which treatments were prescribed to those patients who discontinued the initial treatment protocol, are available (supplementary Table 1).
Mean daily baseline doses (after initial titration) were 9.1 mg (SD = 2.5) for aripiprazole and 2.0 mg (SD = .4) for risperidone. At the 1-year follow-up (per protocol), mean daily doses were 8.3 mg (SD = 3.9) for aripiprazole and 2.3 mg (SD = 1.3) for risperidone. Mean antipsychotic doses using chlorpromazine equivalents (Gardner et al., 2010) (where 600 mg chlorpromazine is equivalent to 30 mg aripiprazole or 6 mg risperidone) at baseline were 181.6 mg (SD = 49.5) aripiprazole and 203.2 mg (SD = 40.2) risperidone (P < .001); at 1-year follow-up (intention-to-treat [ITT]), doses were 243.4 mg (SD=133.0) in the aripiprazole group and 287.3 mg (SD = 200.9) in the risperidone group (P > .05).
Weight and Metabolic Changes After the First Year of Antipsychotic Therapy
Results from paired t tests in the whole FEP sample showed significant changes in weight as well as glycemic and lipid parameters after 1 year of antipsychotic treatment (Table 2). Thus, FEP patients had a significant increase in weight and BMI (t = 16.49, P < .001 and t = 16.62, P < .001, respectively), with a mean increase of 9.9 kg in the first year after starting antipsychotic treatment, reflected in a 3.5-point increase in BMI.
Table 2.
Changes in Weight and in Glycemic and Lipid Parameters After 1 Year of Antipsychotic Treatment in a Population of Drug-Naïve Patients
| Weight and metabolic parameters | 1-Year follow-up | Baseline | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Change | n | t | P | |
| Anthropometric parameters | ||||||||
| Weight, kg | 74.22 | 16.49 | 64.35 | 14.95 | 9.873 | 177 | 16.487 | <.001 |
| Aripiprazole | 74.38 | 14.83 | 65.17 | 14.20 | 9.208 | 86 | 11.193 | <.001 |
| Risperidone | 74.07 | 18.00 | 63.57 | 15.68 | 10.502 | 91 | 12.118 | <.001 |
| BMI, kg/m2 | 25.90 | 4.67 | 22.44 | 4.14 | 3.463 | 177 | 16.618 | <.001 |
| Aripiprazole | 25.60 | 4.02 | 22.42 | 3.78 | 3.189 | 86 | 11.378 | <.001 |
| Risperidone | 26.18 | 5.22 | 22.46 | 4.47 | 3.721 | 91 | 12.170 | <.001 |
| Glycemic parameters (fasting) | ||||||||
| Glucose, mg/dL | 89.17 | 11.66 | 84.11 | 9.27 | 5.057 | 175 | 5.347 | <.001 |
| Aripiprazole | 88.21 | 11.97 | 85.27 | 8.84 | 2.941 | 85 | 2.182 | .032 |
| Risperidone | 90.08 | 11.36 | 83.02 | 9.59 | 7.056 | 90 | 5.430 | <.001 |
| Insulin, µU/mL | 11.40 | 9.15 | 9.35 | 9.28 | 2.049 | 174 | 2.362 | .019 |
| Aripiprazole | 10.30 | 4.94 | 9.06 | 7.06 | 1.236 | 85 | 1.551 | .125 |
| Risperidone | 12.45 | 11.80 | 9.63 | 11.02 | 2.825 | 89 | 1.863 | .066 |
| HOMA index | 2.61 | 2.71 | 2.02 | 2.16 | 0.590 | 173 | 2.509 | .013 |
| Aripiprazole | 2.24 | 1.13 | 1.96 | 1.62 | 0.277 | 85 | 1.480 | .143 |
| Risperidone | 2.96 | 3.61 | 2.06 | 2.58 | 0.892 | 88 | 2.102 | .038 |
| Triglyceride/HDL index | 2.46 | 1.98 | 1.68 | 0.94 | 0.780 | 143 | 5.269 | <.001 |
| Aripiprazole | 2.53 | 1.84 | 1.79 | 0.99 | 0.740 | 71 | 4.067 | <.001 |
| Risperidone | 2.39 | 2.11 | 1.57 | 0.88 | 0.821 | 72 | 3.501 | .001 |
| Lipid parameters (fasting) | ||||||||
| Cholesterol, mg/dL | 190.51 | 41.57 | 170.10 | 33.53 | 20.410 | 173 | 7.341 | <.001 |
| Aripiprazole | 190.81 | 46.16 | 167.44 | 30.86 | 23.376 | 85 | 5.253 | <.001 |
| Risperidone | 190.23 | 36.86 | 172.68 | 35.92 | 17.545 | 88 | 5.200 | <.001 |
| LDL-cholesterol, mg/dL | 116.51 | 35.78 | 101.06 | 28.70 | 15.443 | 140 | 6.254 | <.001 |
| Aripiprazole | 117.71 | 40.26 | 100.41 | 28.80 | 17.300 | 70 | 4.409 | <.001 |
| Risperidone | 115.30 | 30.89 | 101.71 | 28.79 | 13.586 | 70 | 4.512 | <.001 |
| HDL-cholesterol, mg/dL | 51.89 | 13.32 | 52.33 | 13.27 | −0.441 | 143 | -0.394 | .694 |
| Aripiprazole | 51.38 | 13.86 | 50.92 | 12.83 | 0.465 | 71 | 0.338 | .736 |
| Risperidone | 52.39 | 12.85 | 53.72 | 13.64 | −1.333 | 72 | -0.758 | .451 |
| Triglycerides, mg/dL | 111.91 | 72.20 | 79.51 | 32.03 | 32.399 | 148 | 5.976 | <.001 |
| Aripiprazole | 114.34 | 66.74 | 82.34 | 31.35 | 32.000 | 73 | 4.456 | <.001 |
| Risperidone | 109.53 | 77.51 | 76.75 | 32.64 | 32.787 | 75 | 4.024 | <.001 |
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; HOMA, homeostasis model assessment; LDL, low-density lipoprotein.
Statistical analyses: paired t test comparison.
These changes were also observed when we separately examined both treatment groups and also maintained statistical significance in most of the parameters (Table 2). For example, in the first year of treatment, patients in the aripiprazole and risperidone groups gained 9.2 kg and 10.5 kg, respectively (t = 11.19, P < .001 and t = 12.12, P < .001, respectively), increasing their mean BMI by 3.2 and 3.7 points.
Comparison Between Treatment Groups on Weight and Metabolic Longitudinal Changes During the First Year of Antipsychotic Therapy
Through general linear model ANCOVA tests, we explored whether there were significant differences between treatment groups in longitudinal changes in weight and metabolic parameters.
These tests revealed no significant differences (all P > .05) in any of the weight and metabolic measures compared between treatment groups (aripiprazole vs risperidone) (Table 3). Following per protocol analysis approach, no significant differences were observed between treatment groups (supplementary Material 2; supplementary Table 2).
Table 3.
Comparison of Longitudinal Changes in Weight and Metabolic Parameters Between Treatment Groups
| Weight and metabolic parameters | Aripiprazole | Risperidone | Total | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3M | 1Y | Baseline | 3M | 1Y | Baseline | 3M | 1Y | |||||||||||||||
| n | Mean | SD | Mean | SD | Mean | SD | n | Mean | SD | Mean | SD | Mean | SD | n | Mean | SD | Mean | SD | Mean | SD | F | P | |
| Anthropometric changes | |||||||||||||||||||||||
| Weight, kg | 81 | 64.9 | 14.1 | 69.6 | 14.9 | 74.2 | 15.1 | 87 | 63.5 | 15.9 | 69.1 | 15.4 | 74.1 | 17.9 | 168 | 64.2 | 15.0 | 69.3 | 15.1 | 74.2 | 16.5 | 0.050 | .824 |
| BMI, kg/m2 | 81 | 22.5 | 3.8 | 24.0 | 4.0 | 25.7 | 4.1 | 87 | 22.4 | 4.5 | 24.4 | 4.3 | 26.2 | 5.2 | 168 | 22.4 | 4.2 | 24.2 | 4.1 | 25.9 | 4.7 | 0.315 | .576 |
| Lipid parameters | |||||||||||||||||||||||
| Cholesterol, mg/dL | 80 | 168.5 | 30.7 | 187.3 | 35.0 | 191.6 | 47.1 | 81 | 171.7 | 34.4 | 191.0 | 35.5 | 191.7 | 37.5 | 161 | 170.1 | 32.5 | 189.2 | 35.2 | 191.6 | 42.4 | 0.196 | .658 |
| Triglycerides, mg/dL | 69 | 83.4 | 31.8 | 99.4 | 41.6 | 116.3 | 67.7 | 70 | 77.2 | 33.0 | 94.5 | 43.3 | 111.7 | 79.7 | 139 | 80.3 | 32.4 | 97.0 | 42.4 | 114.0 | 73.7 | 0.294 | .588 |
| HDL cholesterol, mg/dL | 67 | 51.0 | 13.1 | 52.4 | 13.1 | 51.7 | 14.1 | 67 | 53.1 | 13.1 | 56.1 | 15.4 | 52.6 | 13.2 | 134 | 52.0 | 13.1 | 54.3 | 14.4 | 52.1 | 13.6 | 0.406 | .525 |
| LDL cholesterol, mg/dL | 66 | 100.9 | 29.0 | 114.3 | 31.8 | 118.4 | 41.3 | 65 | 102.1 | 28.9 | 114.6 | 32.5 | 116.5 | 31.3 | 131 | 101.5 | 28.8 | 114.5 | 32.0 | 117.5 | 36.6 | 0.014 | .905 |
| Glycemic parameters | |||||||||||||||||||||||
| Glucose, mg/dL | 80 | 85.3 | 8.9 | 86.0 | 8.7 | 88.4 | 12.3 | 83 | 82.9 | 9.9 | 85.3 | 8.2 | 89.8 | 11.5 | 163 | 84.1 | 9.4 | 85.6 | 8.4 | 89.1 | 11.8 | 0.326 | .569 |
| HOMA index | 79 | 2.0 | 1.7 | 2.2 | 1.2 | 2.3 | 1.2 | 79 | 2.1 | 2.7 | 2.1 | 1.3 | 2.9 | 3.8 | 158 | 2.0 | 2.2 | 2.1 | 1.2 | 2.6 | 2.8 | 1.142 | .287 |
| HOMA index men | 42 | 1.9 | 1.7 | 2.4 | 1.4 | 2.5 | 1.4 | 40 | 2.4 | 3.4 | 2.3 | 1.3 | 3.1 | 3.7 | 82 | 2.2 | 2.7 | 2.4 | 1.4 | 2.7 | 2.8 | 1.121 | .293 |
| HOMA index women | 37 | 2.0 | 1.7 | 2.0 | 0.9 | 2.0 | 0.9 | 39 | 1.8 | 1.6 | 1.8 | 1.2 | 2.8 | 3.9 | 76 | 1.9 | 1.6 | 1.9 | 1.1 | 2.4 | 2.8 | 0.170 | .681 |
| Triglyceride/HDL index | 67 | 1.8 | 1.0 | 2.1 | 1.2 | 2.6 | 1.9 | 67 | 1.6 | 0.9 | 1.9 | 1.2 | 2.4 | 2.2 | 134 | 1.7 | 1.0 | 2.0 | 1.2 | 2.5 | 2.0 | 0.192 | .662 |
| Insulin total,μU/mL | 79 | 9.0 | 7.3 | 10.4 | 5.1 | 10.3 | 5.1 | 80 | 9.9 | 11.6 | 9.8 | 5.9 | 12.4 | 12.2 | 159 | 9.4 | 9.6 | 10.1 | 5.5 | 11.4 | 9.4 | 0.967 | .327 |
| Insulin men | 42 | 9.0 | 8.1 | 11.1 | 6.0 | 11.0 | 5.9 | 41 | 11.4 | 14.8 | 10.9 | 6.4 | 12.9 | 11.0 | 83 | 10.2 | 11.9 | 11.0 | 6.2 | 12.0 | 8.8 | 1.247 | .268 |
| Insulin women | 37 | 9.1 | 6.3 | 9.7 | 3.9 | 9.5 | 3.8 | 39 | 8.3 | 6.6 | 8.7 | 5.1 | 11.9 | 13.5 | 76 | 8.7 | 6.4 | 9.1 | 4.5 | 10.7 | 10.0 | 0.029 | .865 |
| Hormonal levels | |||||||||||||||||||||||
| Leptin total, ng/mL | 77 | 10.4 | 12.5 | 12.8 | 14.4 | 14.5 | 11.6 | 80 | 8.9 | 11.6 | 13.7 | 13.6 | 16.3 | 14.3 | 157 | 9.6 | 12.0 | 13.3 | 14.0 | 15.4 | 13.0 | 0.023 | .880 |
| Leptin men | 41 | 7.6 | 10.3 | 8.0 | 8.8 | 9.5 | 8.2 | 41 | 5.7 | 6.2 | 9.1 | 7.9 | 12.8 | 11.4 | 82 | 6.6 | 8.5 | 8.5 | 8.3 | 11.1 | 10.0 | 0.447 | .506 |
| Leptin women | 36 | 13.6 | 14.1 | 18.3 | 17.5 | 20.3 | 12.3 | 39 | 12.3 | 14.7 | 18.6 | 16.5 | 20.0 | 16.1 | 75 | 12.9 | 14.3 | 18.5 | 16.9 | 20.1 | 14.3 | 0.073 | .787 |
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; HOMA, homeostasis model assessment; LDL, low-density lipoprotein.
Statistical analyses: GLM ANCOVA model, where measures of each parameters at baseline, 3 months, and 1 year were the dependent variable; treatment group the independent variable; and baseline BMI, sex, and age were the covariates.
Both treatment groups followed comparable trajectories of weight and BMI gain throughout the first year of antipsychotic treatment (Fig. 2).
Figure 2.
Differences in weight and body mass index (BMI) changes between treatment groups.
Clinical Impact of Weight and Metabolic Changes
Similarly, after the first year of antipsychotic treatment, McNemar tests revealed a significant increase in the percentage of FEP patients meeting clinical criteria for obesity and metabolic disorders (Table 4). For example, after comparing the rate of patients with pathological levels before and 1 year after initiating antipsychotic treatment, we found a significant increase in the percentage of patients with MetS (2.8% vs 9.4%, P = .010), obesity (BMI ≥ 30 kg/m2) (5.6% vs 15.3%; P < .001), hypercholesterolemia (cholesterol > 200 mg/dL) (14.5% vs 35.3%; P < .001), and hypertriglyceridemia (triglycerides > 150 mg/dL) (3.4% vs 18.2%; P < .001). Except the triglyceride to HDL IR index (6.3% vs 21%, P < .001), we did not find a significant increase in the percentage of patients with pathological criteria for glycemic metabolism measures.
Table 4.
Comparison of Proportion of Participants With Pathologic Parameters in Weight, Fasting Glucose, and Lipid Levels at Baseline and at 1-Year Follow-up in Each Treatment Group
| Metabolic conditions | 1-Year follow-up | Baseline | |||
|---|---|---|---|---|---|
| % (n) | % (n) | % Difference | n | P a | |
| BMI ≥30 kg/m2 | |||||
| Aripiprazole group | 10.5 (9) | 3.5 (3) | 10.0 | 86 | .041 |
| Risperidone group | 19.8 (18) | 7.7 (7) | 12.1 | 91 | .003 |
| Total | 15.3 (27) | 5.6 (10) | 9.6 | 177 | <.001 |
| Metabolic syndrome | |||||
| Aripiprazole group | 10.3(9) | 1.1(1) | 9.2 | 87 | .027 |
| Risperidone group | 8.6(8) | 4.3(4) | 4.3 | 93 | .289 |
| Total | 9.4(17) | 2.8(5) | 6.7 | 180 | .010 |
| Glucose>110 mg/dL | |||||
| Aripiprazole group | 2.4 (2) | 0 | 2.3 | 85 | .480 |
| Risperidone group | 3.3 (3) | 0 | 3.3 | 90 | .248 |
| Total | 2.9 (5) | 0 | 2.9 | 175 | .074 |
| Insulin, µU/mL; men>15.7, women>17.3 | |||||
| Aripiprazole group | 9.4(8) | 7.1(6) | 2.4 | 85 | .773 |
| Risperidone group | 14.6(13) | 7.9(7) | 6.7 | 89 | .211 |
| Total | 12.1(21) | 7.5(13) | 4.6 | 174 | .186 |
| HOMA; men>3.5, women>3.9 | |||||
| Aripiprazole group | 7.1(6) | 5.9(5) | 1.2 | 85 | 1.000 |
| Risperidone group | 12.5(11) | 8.0(7) | 4.5 | 88 | .423 |
| Total | 9.8(17) | 6.9(12) | 2.9 | 173 | .424 |
| Triglyceride/HDL index>3.5 | |||||
| Aripiprazole group | 22.5 (16) | 7.0 (5) | 15.5 | 7 | .010 |
| Risperidone group | 19.4 (14) | 5.6 (4) | 13.9 | 72 | .009 |
| Total | 21.0 (30) | 6.3 (9) | 14.7 | 143 | <.001 |
| Cholesterol >200mg/dL | |||||
| Aripiprazole group | 36.5 (31) | 11.8 (10) | 24.7 | 85 | <.001 |
| Risperidone group | 34.1 (30) | 17.0 (15) | 17.1 | 88 | .002 |
| Total | 35.3 (61) | 14.5 (25) | 20.8 | 173 | <.001 |
| LDL cholesterol >130mg/dL | |||||
| Aripiprazole group | 34.3 (24) | 14.3 (10) | 20.0 | 70 | .002 |
| Risperidone group | 28.6 (20) | 12.9 (9) | 15.7 | 70 | .010 |
| Total | 31.4 (44) | 13.6 (19) | 17.9 | 140 | <.001 |
| HDL cholesterol <40mg/dL | |||||
| Aripiprazole group | 19.7 (14) | 18.3 (13) | 1.4 | 71 | 1.000 |
| Risperidone group | 12.5 (9) | 12.5 (9) | 0 | 72 | 1.000 |
| Total | 16.1 (23) | 15.4 (22) | 0.7 | 143 | 1.000 |
| Triglycerides >150mg/dL | |||||
| Aripiprazole group | 23.3 (17) | 1.4 (1) | 21.9 | 73 | <.001 |
| Risperidone group | 13.3 (10) | 5.3 (4) | 8.0 | 75 | .149 |
| Total | 18.2 (27) | 3.4 (5) | 14.9 | 148 | <.001 |
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; HOMA, homeostasis model assessment; LDL, low-density lipoprotein.
a McNemar test for repeated measures.
When looking at each treatment group separately, we observed similar results in both groups, with increments after 1 year in the proportion of the participant reaching the criteria for obesity and other metabolic disorders. However, although the increase in the percentage of patients meeting MetS criteria (from 1.1% to 10.3%, P = .027) and hypertriglyceridemia (from 1.4% to 23.3%, P < .001) was statistically significant in the aripiprazole group, it was not statistically significant in the risperidone group (MetS: from 4.3% to 8.6%, P = .289; hypertriglyceridemia: from 5.3% to 13.3%, P = .149).
Post-Hoc Analyses Exploring Sex–Treatment Interaction on Metabolic and Weight Changes
Secondary analysis carried out dividing the sample according to sex did not find sex–treatment interaction in weight/BMI or metabolic changes after 1 year of treatment. Complete data are available as supplementary material (supplementary material 3; supplementary Tables 3A and 3B).
Discussion
We discovered that aripiprazole and risperidone have a similar long-term metabolic profile in FEP patients, resulting in a comparable increase in weight gain and an analogous incidence of metabolic alterations after a long-term follow-up.
Head-to-Head Comparison Regarding Weight and Metabolic Changes
When we explored the possible differential effect of treatments on weight gain and related metabolic side effects, both through intention-to-treat and per-protocol analysis, we observed no significant differences between aripiprazole and risperidone treatment groups (Tables 2 and 3; supplementary Material 2). The analogous trajectories of weight and BMI gain throughout the first year of treatment demonstrate this similarity in the metabolic impact of both antipsychotic treatments (Fig. 2).
The lack of significant differences between aripiprazole and risperidone metabolic profiles is contrary to previous evidence in multi-episode schizophrenia patients. A recent meta-analysis found significant differences in weight gain between aripiprazole and risperidone, where the latter was associated with a greater weight gain (Huhn et al., 2019). In this regard, risperidone has been widely described as one of the antipsychotics linked to increased weight gain and metabolic changes, whereas aripiprazole has traditionally been associated with a lower weight gain. Actually, it was even proposed that aripiprazole may be a weight gain–neutral drug (Kane et al., 2002; McEvoy et al., 2007). Furthermore, several studies have shown that switching from certain antipsychotic treatments to aripiprazole improves weight and metabolic parameters (Siskind et al., 2021). In contrast, studies on FEP patients that excluded confounding factors such as chronicity and antipsychotic polypharmacy found similar results to those found in our study. The current study’s significant increase in weight and BMI matches previous long-term studies from other FEP samples (Perez-Iglesias et al., 2009; Vazquez-Bourgon et al., 2018). In those studies, FEP patients presented significant increments in body weight and significant changes in metabolic variables, reaching pathological values. Former long-term follow-up studies conducted by our group on different FEP samples discovered that aripiprazole (Vazquez-Bourgon et al., 2018) and risperidone (Perez-Iglesias et al., 2009) had metabolic profiles similar to other SGAs such as olanzapine or quetiapine. Consistent with these findings, a recent pooled analysis comparing the efficacy of 6 different antipsychotics found that aripiprazole and risperidone had clear advantages in terms of efficacy, with no significant differences in weight gain after a 3-year follow-up (Gomez-Revuelta et al., 2020). Furthermore, Mustafa and colleagues (2019) studied predictors of antipsychotic discontinuation in FEP patients and did not find significant differences in weight change among 3 SGAs (olanzapine, risperidone, and aripiprazole) after 1 year of initiating antipsychotic treatment (Mustafa et al., 2019).
In any case, given the high rate of treatment switch in our sample and the differences in protocol discontinuation characteristics between both treatment groups, our findings should be interpreted with caution. For instance, patients initially randomized to risperidone discontinued the protocol more frequently than those randomized to aripiprazole. Those discontinuing aripiprazole, on the other hand, did so sooner and due to poor response, whereas those discontinuing risperidone did so primarily due to side effects and more evenly throughout the 1-year follow-up. Interestingly, in none of the groups was weight gain or other metabolic changes cited as the primary reason for protocol discontinuation. The same number of patients in each group (n = 20) switched to olanzapine or clozapine, 2 antipsychotics known to produce greater weight gain.
Head-to-Head Comparison Regarding Clinical Impact
After 1 year of treatment, we observed an increase in the proportion of participants meeting the criteria for obesity in both treatment groups (Δ12.1% in the risperidone group and Δ10.0% in the aripiprazole group). Contrary to our results, Cheng and colleagues (2019) reported significant differences in the short term (8 weeks) in FEP patients between aripiprazole and risperidone, with a lower prevalence of patients with clinically significant (i.e., ≥7%) weight gain (17% vs 32.5%) in the aripiprazole group compared with the risperidone group (Cheng et al., 2019). According to a recent systematic review and meta-analysis (Barton et al., 2020), although all antipsychotic groups (including aripiprazole and risperidone) significantly increased weight gain, aripiprazole did not cause clinically significant weight gain. Furthermore, the risk of presenting clinically relevant weight gain was higher in risperidone patients than in aripiprazole patients with established schizophrenia (relative risk 3.64 vs 1.86) (Huhn et al., 2019).
Our results revealed some differences concerning the metabolic clinical impact between treatment groups. The aripiprazole group showed a greater increase in the proportion of participants with MetS (Δ9.2%, P = .027), whereas this increment in the risperidone group (Δ4.3%, P > .05) did not reach statistical significance. Equally, the increments in the proportion of patients with hypertriglyceridemia were statistically significant in the aripiprazole group (Δ21.9%, P < .001), but not in the risperidone group (Δ8.0%, P < .05). A previous study of 151 FEP patients discovered that the prevalence of MetS was comparable between the aripiprazole and risperidone groups (18.2% vs 23.5%) (Ventriglio et al., 2018). However, recent research on established schizophrenia found that risperidone was associated with a higher risk of MetS than aripiprazole (odds ratio = 2.21 and 1.74, respectively) (Zhang et al., 2020).
Head-to-Head Comparison in Other Populations
Our results are consistent with studies comparing risperidone and aripiprazole in other patient populations. The use of antipsychotics in the treatment of other disorders and populations, such as children and adolescents with behavioral disorder or autism, has allowed researchers to compare their metabolic impact on these populations. A recent large-scale systematic meta-review showed that both treatments presented an increased risk for weight gain in children and adolescents (Solmi et al., 2020). In this regard, there has been an increase in evidence comparing the effects of these 2 antipsychotics on these populations in recent years (Ishitobi et al., 2013; Wink et al., 2014; Nicol et al., 2018; Schoemakers et al., 2019; Cicala et al., 2020). Head-to-head comparison studies between aripiprazole and risperidone in these populations showed similar results on weight gain and lipid/glycemic parameters, in both the short term (6–12 weeks) (Ishitobi et al., 2013; Nicol et al., 2018) and long-term (1 year) (Wink et al., 2014; Schoemakers et al., 2019; Cicala et al., 2020).
Strengths and Limitations
The study has several limitations. Firstly, its design, as a pragmatic clinical trial, makes it difficult to control for confounding factors with a possible effect on weight changes. For example, diet and physical activity have a clear impact on weight changes (Erickson et al., 2016; Nyboe et al., 2019) but were not recorded in this study. Secondly, because of the significant percentage of patients who discontinued the protocol treatment during the study period, the results for head-to-head comparisons (ITT approach) should be interpreted with caution. In addition, although the results from the per protocol analyses followed the same direction, the all-cause discontinuation rate was higher for risperidone than for aripiprazole. Thirdly, we conducted multiple (uncorrected) comparisons in this exploratory study, so the results should be interpreted with caution. On the other hand, this study presents several strengths. First, its longitudinal prospective design enables reliable analysis of the changes and progression of weight and metabolic parameters. Second, such a long-term follow-up period (1 year) is uncommon and aids in the detection of metabolic disturbances, which typically take a long time to develop. Third, by limiting the study to drug-naive patients with FEP, we were able to avoid the confounding effect of chronicity and long-term exposure to previous medications (van der Esch et al., 2020). Finally, the results of this study may be highly generalizable due to its pragmatic randomized clinical trial design and high retention rate for a long-term follow-up study, which may reflect real-world clinical practice.
Conclusions
In this study, we demonstrated that aripiprazole and risperidone caused similar weight changes and had a similar metabolic profile. The study highlights the significant and rapid weight gain associated with both treatments in the first year following the psychosis diagnoses as well as the early metabolic repercussions produced in these young patients. As a result, greater efforts must be made to prevent these weight and metabolic changes from the earliest phases of the psychosis.
Supplementary Material
Acknowledgments
This study was conducted as part of the randomized clinical trial “Effectiveness of Second Generation Antipsychotics in First Episode Psychosis Patients: 1-Year Follow-up (PAFIP3_1Y).” ClinicalTrials.gov Identifier: NCT02532491. The authors thank all Programa Asistencial de las Fases Iniciales de Psicosis (PAFIP) research team and all patients and family members who participated in the study.
This work was supported by the Instituto de Investigación Sanitaria Valdecilla (INT/A20/04, INT/A21/10) and the Instituto de Salud Carlos III (PI020499, PI050427, PI060507). Unrestricted educational and research grants from AstraZeneca, Pfizer, Bristol-Myers Squibb, Janssen Johnson & Johnson, and Lundbeck provided support for PAFIP activities. No pharmaceutical industry or institutional sponsors participated in the study concept and design, data collection, analysis and interpretation of the results, or drafting the manuscript.
Contributor Information
Javier Vázquez-Bourgon, Department of Psychiatry, University Hospital Marqués de Valdecilla - Instituto de Investigación Marqués de Valdecilla (IDIVAL), Santander, Spain; Centro de Investigación Biomédica en Red en Salud Mental (CIBERSAM), Sevilla, Spain; Department of Medicine and Psychiatry, School of Medicine, Universidad de Cantabria, Santander, Spain.
Víctor Ortiz-García de la Foz, Department of Psychiatry, University Hospital Marqués de Valdecilla - Instituto de Investigación Marqués de Valdecilla (IDIVAL), Santander, Spain.
Marcos Gómez-Revuelta, Department of Psychiatry, University Hospital Marqués de Valdecilla - Instituto de Investigación Marqués de Valdecilla (IDIVAL), Santander, Spain; Department of Medicine and Psychiatry, School of Medicine, Universidad de Cantabria, Santander, Spain.
Jacqueline Mayoral-van Son, Centro de Investigación Biomédica en Red en Salud Mental (CIBERSAM), Sevilla, Spain; Department of Psychiatry, School of Medicine, University Hospital Virgen del Rocío-IBiS, Sevilla, Spain.
María Juncal-Ruiz, Department of Medicine and Psychiatry, School of Medicine, Universidad de Cantabria, Santander, Spain; Department of Psychiatry, Sierrallana Hospital - Instituto de Investigación Marqués de Valdecilla (IDIVAL), Torrelavega, Spain.
Nathalia Garrido-Torres, Centro de Investigación Biomédica en Red en Salud Mental (CIBERSAM), Sevilla, Spain; Department of Psychiatry, School of Medicine, University Hospital Virgen del Rocío-IBiS, Sevilla, Spain.
Benedicto Crespo-Facorro, Centro de Investigación Biomédica en Red en Salud Mental (CIBERSAM), Sevilla, Spain; Department of Psychiatry, School of Medicine, University Hospital Virgen del Rocío-IBiS, Sevilla, Spain.
Contributors
B.C.-F. designed the study, wrote the protocol, obtained the financial support, and evaluated the patients. J.M.-v.S. and M.G.-R. evaluated the patients and collected the study variables. V.O.-G. built and maintained the dataset. J.V.-B. managed the literature searches and wrote the first draft of the manuscript. J.V.-B. and V.O.-G. undertook the statistical analyses. B.C.-F., M.G.-R., M.J.-R., and N.G.-T. contributed to the interpretation of the data and critically revised the manuscript. All authors revised and approved the final manuscript.
Interest Statement
J.V.-B has received funding support from IDIVAL and honoraria for his participation as a consultant and/or speaker at educational events from Janssen, Johnson & Johnson, and Lundbeck. V.O.-G.F., J.M.-v.S., M.G.-R., M.J.-R., and N.G.-T. report no conflicts of interest. B.C.-F. has received unrestricted research funding from ISCIII, MINECO, Gobierno de Cantabria, CIBERSAM, the 7th European Union Framework Program, and Lundbeck. He has also received honoraria for his participation as a consultant and/or speaker at educational events from Janssen, Johnson & Johnson, Lundbeck, and Otsuka Pharmaceuticals.
References
- Andreasen N (1983) Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa. [Google Scholar]
- Andreasen NC (1984) Scale for the Assessment of Positive Symptoms (SAPS). Iowa City: University of Iowa. [Google Scholar]
- Barton BB, Segger F, Fischer K, Obermeier M, Musil R. (2020) Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Saf 19:295–314. [DOI] [PubMed] [Google Scholar]
- Bioque M, Garcia-Portilla MAP, Garcia-Rizo C, Cabrera B, Lobo A, Gonzalez-Pinto A, Diaz-Caneja CM, Corripio I, Vieta E, Castro-Fornieles J, Bobes J, Gutierrez-Fraile M, Rodriguez-Jimenez R, Mezquida G, Llerena A, Saiz-Ruiz J, Bernardo M, GROUP PE. (2018) Evolution of metabolic risk factors over a two-year period in a cohort of first episodes of psychosis. Schizophr Res 193:188–196. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Yuan Y, Han X, Yang L, Cai S, Yang F, Lu Z, Wang C, Deng H, Zhao J, Xiang Y, Correll CU, Yu X. (2019) An open-label randomised comparison of aripiprazole, olanzapine and risperidone for the acute treatment of first-episode schizophrenia: eight-week outcomes. J Psychopharmacol 33:1227–1236. [DOI] [PubMed] [Google Scholar]
- Cicala G, Barbieri MA, Santoro V, Tata C, Colucci PV, Vanadia F, Drago F, Russo C, Cutroneo PM, Gagliano A, Spina E, Germano E. (2020) Safety and tolerability of antipsychotic drugs in pediatric patients: data from a 1-year naturalistic study. Front Psychiatry 11:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Rubio JM, Kane JM (2018) What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry 17:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson ZD, Mena SJ, Pierre JM, Blum LH, Martin E, Hellemann GS, Aragaki DR, Firestone L, Lee C, Lee P, Kunkel CF, Ames D. (2016) Behavioral interventions for antipsychotic medication-associated obesity: a randomized, controlled clinical trial. J Clin Psychiatry 77:e183–e189. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502. [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. (2010) International consensus study of antipsychotic dosing. Am J Psychiatry 167:686–693. [DOI] [PubMed] [Google Scholar]
- Goff DC (2021) The pharmacologic treatment of schizophrenia-2021. JAMA 325:175–176. [DOI] [PubMed] [Google Scholar]
- Goff DC, Falkai P, Fleischhacker WW, Girgis RR, Kahn RM, Uchida H, Zhao J, Lieberman JA. (2017) The long-term effects of antipsychotic medication on clinical course in schizophrenia. Am J Psychiatry 174:840–849. [DOI] [PubMed] [Google Scholar]
- Gomez-Revuelta M, Pelayo-Teran JM, Juncal-Ruiz M, Vazquez-Bourgon J, Suarez-Pinilla P, Romero-Jimenez R, Setien Suero E, Ayesa-Arriola R, Crespo-Facorro B. (2020) Antipsychotic treatment effectiveness in first episode of psychosis: PAFIP 3-year follow-up randomized clinical trials comparing haloperidol, olanzapine, risperidone, aripiprazole, quetiapine, and ziprasidone. Int J Neuropsychopharmacol 23:217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Revuelta M, Pelayo-Teran JM, Vazquez-Bourgon J, Ortiz-Garcia de la Foz V, van Son JM, Ayesa-Arriola R, Crespo-Facorro B. (2021) Aripiprazole vs risperidone for the acute-phase treatment of first-episode psychosis: a 6-week randomized, flexible-dose, open-label clinical trial. Eur Neuropsychopharmacol 47:74–85. [DOI] [PubMed] [Google Scholar]
- Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. (2015) Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry 2:452–464. [DOI] [PubMed] [Google Scholar]
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, Arndt T, Backers L, Rothe P, Cipriani A, Davis J, Salanti G, Leucht S. (2019) Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 394:939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitobi M, Kosaka H, Takahashi T, Yatuga C, Asano M, Tanaka Y, Ueno K, Okazaki R, Omori M, Hiratani M, Tomoda A, Wada Y. (2013) Effectiveness and tolerability of switching to aripiprazole from risperidone in subjects with autism spectrum disorders: a prospective open-label study. Clin Neuropharmacol 36:151–156. [DOI] [PubMed] [Google Scholar]
- Josiassen RC, Shaughnessy RA, Filymer DM, Donohue AM, Kacso M, Finkel N, Curtis J, Audino B, Skuban N. (2010). Early intervention with second-generation antipsychotics in first-episode psychosis: results of an 8-week naturalistic study. Early Interv Psychiatry. 4:57–63. doi:10.1111/j.1751-7893.2010.00163.x [DOI] [PubMed] [Google Scholar]
- Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, O’Donovan M, Correll CU, Kane JM, van Os J, Insel TR. (2015) Schizophrenia. Nat Rev Dis Primers 1:15067. [DOI] [PubMed] [Google Scholar]
- Kane JM, Carson WH, Saha AR, McQuade RD, Ingenito GG, Zimbroff DL, Ali MW. (2002) Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 63:763–771. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Hagi K, Nitta M, Kane JM, Correll CU. (2019) Long-term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry 18:208–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdyak P, Mallia E, de Oliveira C, Carvalho AF, Kozloff N, Zaheer J, Tempelaar WM, Anderson KK, Correll CU, Voineskos AN. (2021) Mortality after the first diagnosis of schizophrenia-spectrum disorders: a population-based retrospective cohort study. Schizophr Bull 47:864–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lassig B, Salanti G, Davis JM. (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382:951–962. [DOI] [PubMed] [Google Scholar]
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. (1987) The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 334:1–100. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Daniel DG, CarsonWH, Jr., McQuade RD, Marcus RN. (2007) A randomized, double-blind, placebo-controlled, study of the efficacy and safety of aripiprazole 10, 15 or 20 mg/day for the treatment of patients with acute exacerbations of schizophrenia. J Psychiatr Res 41:895–905. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, Simon J, Krauss RM. (2005) Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol 96:399–404. [DOI] [PubMed] [Google Scholar]
- Mustafa S, Joober R, Iyer S, Shah J, Lepage M, Malla A. (2019) Early stabilization of weight changes following treatment with olanzapine, risperidone, and aripiprazole: a 12-month naturalistic study of first episode psychosis. J Clin Psychiatry 80:18m12717. [DOI] [PubMed] [Google Scholar]
- Nicol GE, Yingling MD, Flavin KS, Schweiger JA, Patterson BW, Schechtman KB, Newcomer JW. (2018) Metabolic effects of antipsychotics on adiposity and insulin sensitivity in youths: a randomized clinical trial. JAMA Psychiatry 75:788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen RE, Banner J, Jensen SE (2021) Cardiovascular disease in patients with severe mental illness. Nat Rev Cardiol 18:136–145. [DOI] [PubMed] [Google Scholar]
- Nyboe L, Lemcke S, Moller AV, Stubbs B. (2019) Non-pharmacological interventions for preventing weight gain in patients with first episode schizophrenia or bipolar disorder: a systematic review. Psychiatry Res 281:112556. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Sawa A, Mortensen PB (2016) Schizophrenia. Lancet 388:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelayo-Terán JM, Pérez-Iglesias R, Ramírez-Bonilla M, et al. (2008) Epidemiological factors associated with treated incidence of firstepisode non-affective psychosis in Cantabria: insights from the Clinical Programme on Early Phases of Psychosis. Early Interv Psychiatry 2:178–187. [DOI] [PubMed] [Google Scholar]
- Perez-Iglesias R, Mata I, Pelayo-Teran JM, Amado JA, Garcia-Unzueta MT, Berja A, Martinez-Garcia O, Vazquez-Barquero JL, Crespo-Facorro B. (2009) Glucose and lipid disturbances after 1 year of antipsychotic treatment in a drug-naive population. Schizophr Res 107:115–121. [DOI] [PubMed] [Google Scholar]
- Schoemakers RJ, van Kesteren C, van Rosmalen J, Eussen M, Dieleman HG, Beex-Oosterhuis MM. (2019) No differences in weight gain between risperidone and aripiprazole in children and adolescents after 12 months. J Child Adolesc Psychopharmacol 29:192–196. [DOI] [PubMed] [Google Scholar]
- Siskind D, Gallagher E, Winckel K, Hollingworth S, Kisely S, Firth J, Correll CU, Marteene W. (2021) Does switching antipsychotics ameliorate weight gain in patients with severe mental illness? A systematic review and meta-analysis. Schizophr Bull 47:948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solmi M, Fornaro M, Ostinelli EG, Zangani C, Croatto G, Monaco F, Krinitski D, Fusar-Poli P, Correll CU. (2020) Safety of 80 antidepressants, antipsychotics, anti-attention-deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: a large scale systematic meta-review of 78 adverse effects. World Psychiatry 19:214–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JM, Gomez-Revuelta M, Ayesa-Arriola R, Vazquez-Bourgon J, Foz VO, Ruiz-Veguilla M, Garrido N, Tordesillas-Gutierrez D, Setien-Suero E, Crespo-Facorro B. (2021) Comparison of aripiprazole and risperidone effectiveness in first episode non-affective psychosis: rationale and design of a prospective, randomized, 3-phase, investigator-initiated study (PAFIP-3). Rev Psiquiatr Salud Ment 14:157–163. [DOI] [PubMed] [Google Scholar]
- Taipale H, Puranen A, Mittendorfer-Rutz E, Tiihonen J, Tanskanen A, Cervenka S, Lahteenvuo M. (2020) Antipsychotic use among persons with schizophrenia in Sweden and Finland, trends and differences. Nord J Psychiatry 75:315–322. [DOI] [PubMed] [Google Scholar]
- Toto S, Grohmann R, Bleich S, Frieling H, Maier HB, Greil W, Cordes J, Schmidt-Kraepelin C, Kasper S, Stubner S, Degner D, Druschky K, Zindler T, Neyazi A. (2019) Psychopharmacological treatment of schizophrenia over time in 30 908 inpatients: data from the AMSP study. Int J Neuropsychopharmacol 22:560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Esch CCL, Kloosterboer SM, van der Ende J, Reichart CG, Kouijzer MEJ, de Kroon MMJ, van Daalen E, Ester WA, Rieken R, Dieleman GC, Hillegers MHJ, van Gelder T, Koch BCP, Dierckx B. (2020) Risk factors and pattern of weight gain in youths using antipsychotic drugs. Eur Child Adolesc Psychiatry 30:1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Bourgon J, Perez-Iglesias R, Ortiz-Garcia de la Foz V, Suarez Pinilla P, Diaz Martinez A, Crespo-Facorro B. (2018) Long-term metabolic effects of aripiprazole, ziprasidone and quetiapine: a pragmatic clinical trial in drug-naive patients with a first-episode of non-affective psychosis. Psychopharmacology (Berl) 235: 245–255. [DOI] [PubMed] [Google Scholar]
- Vázquez-Bourgon J, Gómez-Revuelta M, Mayoral-van Son J, Labad J, Ortiz-García de la Foz V, Setién-Suero E, Tordesillas-Gutiérrez D, Ayesa-Arriola R, Juncal-Ruiz M, Crespo-Facorro B. (2021) Pattern of long-term weight and metabolic changes after a first-episode of non-affective psychosis: 10 years follow-up study of the PAFIP cohort. Psychological Medicine Submitted and under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventriglio A, Baldessarini RJ, Vitrani G, Bonfitto I, Cecere AC, Rinaldi A, Petito A, Bellomo A. (2018) Metabolic syndrome in psychotic disorder patients treated with oral and long-acting injected antipsychotics. Front Psychiatry 9:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink LK, Early M, Schaefer T, Pottenger A, Horn P, McDougle CJ, Erickson CA. (2014) Body mass index change in autism spectrum disorders: comparison of treatment with risperidone and aripiprazole. J Child Adolesc Psychopharmacol 24:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. (2020) Metabolic effects of 7 antipsychotics on patients with schizophrenia: a short-term, randomized, open-label, multicenter, pharmacologic trial. J Clin Psychiatry 81:19m12785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.