Abstract
Objective
Postoperative delirium (POD) seriously affects recovery of older persons, increasing their mortality rate after surgery. We aimed to evaluate preoperative oral saline administration on postoperative delirium in older persons undergoing spinal decompression.
Design
A randomised controlled trial in a large tertiary hospital.
Setting and Participants
A total of 76 older persons (≧65 years old) undergoing spinal surgery from May 2020 to January 2021.
Methods
Older persons (65–83 years old) who underwent elective spinal canal decompression were randomly grouped into either the control group (n = 38) or the intervention group (n = 38). The control group was forbidden from drinking 8 hours prior to the operation while the intervention group was administered 5 mL·kg−1 of normal saline 2 hours before anesthesia. Hemodynamic indicators, diagnostic biomarkers, preoperative mini-mental status scores, and intraoperative fluid dynamics were recorded at baseline and at various postoperative timepoints. Subjects were then scored for POD and postoperative pain.
Results
S100β protein was lowered in S1 (FS1 = 12.289, P <0.001) and S2 (FS2 = 12.440, P <0.001) in the intervention group while mean arterial blood pressure (FT1= 42.997, P<0.001) and heart rate (FT1= 8.974, P=0.004) were increased. The Ln c-reactive protein of the intervention group was lowered 1 day postoperatively (FS2 = 6.305, P = 0.014). The incidence of postoperative delirium in the control group was higher than in the intervention group (27.8% vs 8.3%, χ2 = 4.547, P = 0.033).
Conclusion
Preoperative oral saline can reduce the incidence of postoperative delirium in older persons by minimizing perioperative hemodynamic fluctuations and central nervous system damage.
Keywords: older persons, postoperative delirium, spinal canal decompression
Introduction
Due to the prevalence of comorbidities in the elderly, postoperative complications like postoperative delirium (POD) are predicted to rise. Meanwhile, there is evidence that POD is associated with deteriorating cognition in both the short term (months) and long term (≧ 1 year) after its occurrence which be often referred to as postoperative cognitive dysfunction (POCD).1 Many studies have shown that POD to be associated with POCD up to 12 months postsurgery and even associated with dementia up to 5 years after POD.2 Overall, POD seriously affects recovery of older persons, increasing their mortality rate after surgery.
POD is a transient psychiatric syndrome and it mainly occurs 1 to 5 days after surgery.3 The incidence of POD can be anywhere from 4% ~ 61%, with a higher incidence after cardiothoracic, orthopedic and general surgery.4,5 Specifically, delirium occurs in 3.8–40.4% of older persons after orthopedic spinal surgery and 13.6% of patients undergoing lumbar spinal surgery.6,7 Patients with POD can exhibit hypoactive (50%), hyperactive (25%), or mixed (25%) delirium subtypes. Delirium can be accompanied by apathy, confusion, emotional agitation or hallucinations, as well as sympathetic arousal.8 Increased age is the predisposing factor for the hypoactive subtype, where the prognosis is the worst.9 This is largely because it is difficult for clinicians to recognize this subtype, which leads to delays in intervention and treatment. The early prevention and treatment of POD is very important for postoperative outcome improvement. Studies have shown that about 30% to 40% of POD is preventable.10
Clinically, it is believed that restricting drinking for 6 to 8 hours before surgery is needed to complement the recommended fasting guidelines. There is no evidence that reducing the ban on drinking to just 2–3 h preoperatively will increase the risk of vomiting, aspiration and reflux. One study pointed out that the emptying half-life of neutral isotonic solutions such as 500 mL isotonic saline is 12 min, with 90% of the liquid passing through the pylorus within 1 h and almost complete elimination within 2 h.11
A large cohort study found that an independent risk factor for POD is a fasting time of >6 hours (OR = 10.6), demonstrating that prolonged prohibition of liquids before surgery is particularly unfavorable to the patient.12 A statement was advocated that an appropriate amount of water can be safely taken orally 2 hours before induction of anesthesia to maintain hydration while reducing postoperative complications.13 Despite the rising incidence of POD in the elderly, clinicians are not sufficiently aware of POD in patients undergoing spinal surgery and generally lack evidence based prevention and treatment strategies. To address this problem, this study longitudinally evaluated the effect of oral saline administration 2 h preoperatively on POD in older persons undergoing elective spinal canal decompression surgery. Inflammatory responses and neurodegeneration were explored as possible mechanisms for the protective effect of normal saline on POD.
Materials and Methods
Case Selection and Trial Grouping
Case Selection
This clinical trial (NCT01374893) complied with the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) statement. We enrolled 76 older persons who were scheduled to undergo spinal canal decompression in a large tertiary hospital from May 2020 to January 2021, with strictly controlled inclusion and exclusion criteria. Patients were randomized into either the intervention or control group, with 38 cases in each group.
Selection Criteria
① Age ≥ 65 years;
② The patient agrees to participate in the study;
③ Complete collection of medical records;
④ Open elective spinal canal decompression.
Exclusion Criteria
① Incomplete collection of medical records;
② Patients have a history of mental illness and take relevant drugs or preoperative mini-mental state examination (MMSE) <23 points (MMSE evaluates preoperative cognitive function 1 day before surgery);
③ Hearing and language barriers that affect communication;
④ Preoperative delirium;
⑤ Patients with oral eating contraindications, gastroesophageal reflux and gastric emptying disorders, patients with gastrointestinal obstruction, patients with history of upper gastrointestinal tumors;
⑥ Patients with airway difficulties.
⑦ Patients receiving any corticosteroid therapy.
Grouping
The control group observed a normal diet 1 day before surgery, avoiding spicy, irritating, and greasy food. Patients ate a semi-liquid diet 1 night before surgery, avoiding drinking 8 hours before surgery. The intervention group similarly observed a normal diet 1 day before surgery and a semi-liquid diet 1 night before surgery. Patients in the intervention group received 5 mL·kg−1 oral administration of normal saline 2 hours before surgery, with the total volume < 400 mL.
Main Reagents
S100β ELISA kit (Wuhan Elite Biotechnology Co., Ltd. Lot number: E-EL-H1297c), CRP test box (Beckman, USA, batch number: GS621M)
Research Plan
Researcher Arrangement
A random number table was used to determine patient allocation. Patients were randomised (1: 1) to be selected for the control or intervention group according to inclusion and exclusion criteria. A researcher conducted preoperative visits and interviewed candidates who met the inclusion criteria and signed the informed consent. The other investigator assigned normal saline to patients in the intervention group based on the time of the previous surgery. The perioperative management was performed by the same group of physicians. Anesthesiologist, outcome assessors and the surgical team were blind to the distribution of participants in the study. Nursing delirium screening scale (nursing delirium screening scale, Nu-DESC) was used in the post-anesthesia care unit (PACU). Postoperative confusion assessment method (CAM), and visual analogue scale (VAS) scoring were performed for postoperative continuous 3 days.
Preoperative Evaluation
Patients were evaluated the day before surgery in a quiet environment by the Preoperative Simple Mental State Scale. Patients with an MMSE score of <23 were excluded from the study.
Diagnosis of POD
The CAM scale was used for the detection of POD, and the specific diagnostic criteria were: ① acute change of mental state with volatility; ② attention disorder; ③ confusion of thinking; ④ change of consciousness level. ① and ② existing at the same time, plus any one of ③ or ④. Specific scales included acute onset, attention disorder, confusion, changes in the level of consciousness, disorientation, memory loss, perceptual disturbance, psychomotor agitation, psychomotor retardation, volatility, and sleep-wake cycle changes. A score of 19 or less indicated that the patient did not have delirium while a score of 20 to 22 indicated the suspicion of delirium and a score of 22 or more indicated delirium.
Patients were sent to the PACU where they were scored by Nu-DESC including 5 clinical features. Each item was recorded as 0 ~ 2 points according to the presence or absence of clinical symptoms and severity. The highest score possible was 10 points, and a total score of ≥ 2 points was regarded as delirium.
Perioperative Management
Patients were given mask oxygen and peripheral venous access was opened. Electrocardiogram (ECG), blood pressure (BP), pulse oximetry (SpO2) and EEG bispectral index (business information system, BIS) were monitored. Radial artery puncture was used for intraoperative monitoring of BP and intraoperative blood gas. All patients were anesthetized by tracheal intubation and general anesthesia, no dexmedetomidine or penehyclidine hydrochloride was used. All patients received postoperative patient controlled intravenous analgesia (PCIA) with 2.5 μg·kg−1 sufentanil, 4 mg·kg−1 flurbiprofen axetil and 0.2 mg·kg−1 tropisetron (total volume of 100 mL, including 0.9% normal saline, bolus 2 mL, basal rate 2 mL/h, and lockout time 15 mins) for 48 hours after surgery.
Observation Indicators
A detailed medical history of each research subject was taken one day preoperatively. The content included Basic information, presence of medical diseases, history of sleep disorders, history of alcohol intake, operative blood loss and surgical time.
Venous blood was taken 5 minutes before operation (S0), 30 minutes after awakening from anesthesia (S1), and 24 hours after operation (S2) for evaluation of S100β. CRP analysis was taken from S0 and S2 blood samples. After sitting at room temperature for 2 h and centrifugation for 20 min at 4°C (The centrifugal force is 1000 g, by model TDL-5L table low speed centrifuge), the supernatant was frozen at −80 °C for evaluation of S100β.
The mean arterial blood pressure (MAP) and heart rate (HR) were measured at 4 time points: 5 min before anesthesia (T0), 1 min after induction of anesthesia (T1), 5 min after surgery (T2), and 5 min after extubation (T3).
Perioperative pain was scored using the VAS score sheet (0 ~ 10 points) preoperatively (S0), after awakening from anesthesia (S1), the first postoperative day (S2), the second postoperative day (S3), and the third postoperative day (S4). And the patients were monitored for nausea and vomiting at all five research time points.
Statistical Analysis
According to the study, the highest incidence of POD in spinal surgery elderly patients was 40.5%. Power And Sample Size were used to calculate the Sample Size. In the end, the sample size of each group was finally determined to be 38, with a total of 76 patients in the two groups. SPSS 25.0 statistical software was used for analysis. Continuous data was expressed as mean ± standard deviation (± s) or median (¼, ¾). All continuous data with normal distribution were compared by independent sample t-test while the Mann–Whitney U nonparametric test was used for abnormally distributed data. Multiple time points within the group were analyzed by repeated measures analysis of variance. The comparison of continuous data was performed by chi-square test or Fisher’s exact probability method, P <0.05.
Results
Baseline Data
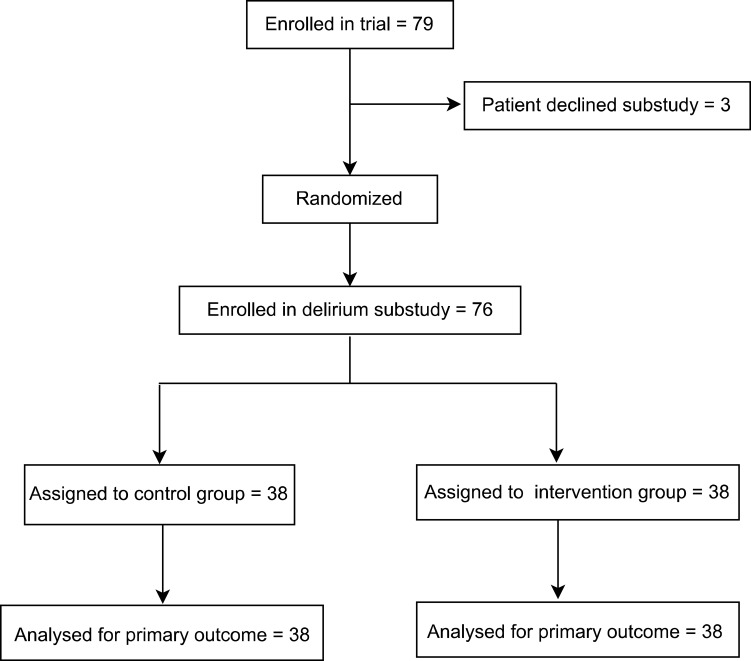
In this study intention-to-treat analysis was used and a total of 76 patients were analyzed (see Figure 1). There were no statistically significant differences in the age, gender, BMI, ASA classification, length of hospitalization, comorbidities, history of alcohol and sleep disorders, education level, surgical type, bleeding volume, operation time, preoperative MMSE score, preoperative VAS score, preoperative blood glucose, preoperative Hb, or preoperative albumin (see Table 1).
Figure 1.
Study flow chart.
Table 1.
Comparison of the General Conditions of the Two Groups of Patients (n = 38)
| General Information | Control | Intervention Group | t, c2 or Z | P |
|---|---|---|---|---|
| Age | 71.63 ± 4.55 | 71.87 ± 4.79 | −0.221 | 0.826 |
| Male [n(%)] | 22 (57.80) | 19(50.00) | 0.477 | 0.490 |
| BMI (kg/m2) | 23.73 ± 3.31 | 23.98 ± 3.74 | −0.315 | 0.754 |
| ASA grade | 0.000 | 1.000 | ||
| II [n(%)] | 19 (50.00) | 19 (50.00) | – | – |
| III [n(%)] | 19 (50.00) | 19 (50.00) | – | – |
| Days of hospitalization(d) | 14.00(9.75, 20.25) | 13.50(9.00, 18.25) | −0.599 | 0.549 |
| Comorbidity [n(%)] | 20(52.60) | 26(68.40) | 1.983 | 0.159 |
| History of alcohol consumption [n(%)] | 3(7.80) | 1(2.60) | 0.264 | 0.603 |
| Education level [n(%)] | 36(94.70) | 35(92.10) | 0.000 | 1.000 |
| History of sleep disorders [n(%)] | 10(26.30) | 5(13.10) | 2.077 | 0.150 |
| Type of surgery | – | 1.000 | ||
| Cervical spine surgery [n(%)] | 13(34.20) | 13(34.20) | – | – |
| Thoracic spine surgery [n(%)] | 2(5.20) | 2(5.20) | – | – |
| Lumbar spine surgery [n(%)] | 23(60.50) | 23(60.50) | – | – |
| Bleeding volume(mL) | 250(188, 400) | 200(188, 300) | −0.704 | 0.481 |
| Operation time(h) | 3.32 ± 1.38 | 3.02 ± 1.10 | 1.047 | 0.299 |
| Preoperative VAS score(score) | 3.00(2.00, 3.00) | 2.00(2.00, 3.00) | −1.367 | 0.172 |
| Preoperative MMSE score(score) | 27(26, 28) | 28(27, 28) | −1.483 | 0.352 |
| Preoperative blood glucose(mmol/L) | 5.50(5.00, 7.00) | 5.60(4.99, 6.85) | −0.156 | 0.876 |
| Preoperative Hb(g/L) | 135.70 ± 15.43 | 132.84 ± 14.94 | 0.822 | 0.768 |
| Preoperative albumin(g/L) | 41.47 ± 3.74 | 42.25 ± 4.70 | −0.805 | 0.720 |
| Preoperative CRP(mg/L) | 3.35 (2.01, 6.27) | 2.74 (1.97, 4.56) | −0.691 | 0.490 |
Notes: (Normally distributed continuous data are represented by mean±SD and non-normally distributed data are represented by M (¼, ¾); BMI, body mass index; MMSE score, simple intelligence score; VAS score, pain visual analog score; Hb, Hemoglobin; CRP, C-reactive protein).
Comparison of the Incidence of POD
The incidence of POD in the control group was higher than that in the intervention group (27.8% vs 8.3%, χ2=4.547, P=0.033) (see Table 2).
Table 2.
Comparison of the Incidence of POD Between the Two Groups (n = 38 [n(%)])
| Group | Number of Cases of Delirium [n(%)] |
|---|---|
| Control | 10(27.8) |
| Intervention | 3(8.3) |
Note: (χ2= 4.547, P= 0.033).
Comparison of Serum S100β and CRP at Different Time Points
Compared with S0, the S100β of both groups gradually increased over time (F control group = 94.655, P <0.001; F intervention group = 57.825, P <0.001). By the S1 timepoint, S100β was significantly lower in the intervention group (FS1 = 12.289, P <0.001), echoed in S2 (FS2 = 12.440, P <0.001) (see Table 3).
Table 3.
Comparison of Serum Log CRP and S100β Levels Between the Two Groups of Patients (n = 38)
| Indicators | Group | S0 | S1 | S2 | F | P |
|---|---|---|---|---|---|---|
| Log CRP | Control | 5.25 ± 0.79 | – | 18.02 ± 2.06a | 35.780 | < 0.001 |
| Intervention | 4.47 ± 0.79 | – | 10.73 ± 2.06a | 8.588 | 0.004 | |
| FLog CRP | 0.491 | – | 6.305 | – | – | |
| PLog CRP | 0.486 | – | 0.014 | – | – | |
| S100β (pg/mL) | Control | 45.52 ± 6.66 | 76.08 ± 23.03a | 80.06 ± 20.79a | 94.655 | <0.001 |
| Intervention | 41.51 ± 6.45 | 59.72 ± 17.26ab | 73.65 ± 14.20ab | 57.825 | < 0.001 | |
| FS100β | 7.079 | 12.289 | 12.440 | – | – | |
| PS100β | 0.009 | < 0.001 | < 0.001 | – | – |
Notes: (Continuous data is expressed by mean±SD; S0: preoperatively, S1: 30 minutes after awakening from anesthesia, S2: 1 day after operation; intra-group comparison: a: Compared with T0 in the same group, aP <0.05, b: Compare with the control group, bP<0.05).
Log CRP in both groups increased significantly 1 day after surgery (F control group = 35.780, P <0.001; F intervention group = 8.588, P = 0.004). While no significant difference was observed between the two groups preoperatively (FS1 = 0.491, P = 0.486), Log CRP of the intervention group was significantly lower than the control group (FS2 = 6.305, P = 0.014) 1 day postoperatively (See Table 3).
MAP and HR at Different Time Points
MAP values ranked over time were T1 <T2 <T3 <T0 while HR values were T2 <T1 <T3 <T0 in the intervention group. At T1, the MAP (FT1 = 42.997, P <0.001) and the HR (FT1 = 8.974, P = 0.004) of the intervention group was significantly higher than that of the control group (see Table 4).
Table 4.
Comparison of MAP, HR Between the Two Groups at Different Time Points (n=38)
| Indicators | Group | T0 | T1 | T2 | T3 | F | P |
|---|---|---|---|---|---|---|---|
| MAP (mmHg) | Control | 112.78 ± 15.60 | 79.81 ± 8.05a | 85.44 ± 9.21a | 97.35 ± 4.71a | 81.697 | < 0.001 |
| Intervention | 112.20 ± 18.96 | 91.15 ± 6.31ab | 89.25 ± 12.25a | 98.54 ± 7.65a | 31.303 | < 0.001 | |
| FMAP | 0.004 | 42.997 | 2.154 | 0.609 | – | – | |
| PMAP | 0.949 | < 0.001 | 0.147 | 0.438 | – | – | |
| HR (bpm) | Control | 77.14 ± 10.33 | 60.80 ± 7.51a | 57.63 ± 6.04a | 68.3 ± 11.96a | 38.041 | < 0.001 |
| Intervention | 77.40 ± 13.98 | 70.20 ± 16.98ab | 60.63 ± 8.49a | 74.8 ± 11.50ab | 31.445 | < 0.001 | |
| FHR | 0.008 | 8.974 | 2.901 | 5.300 | – | – | |
| PHR | 0.931 | 0.004 | 0.093 | 0.024 | – | – |
Notes: (The measurement data is expressed by mean±SD; T0, before anesthesia, T1, 1 min after induction of anesthesia, T2, 5 min after the start of the operation, T3, 5 min after extubation; intra-group comparison: aCompared with T0 in the same group, aP <0.05; comparison between groups: bCompared with the control group, bP <0.05).
Postoperative Complications
The incidence of thirst in the intervention group was significantly lower than that in the control group (36.80% vs 10.50%, χ2 = 7.280, P = 0.007). There was no significant difference in nausea or vomiting nor reflux aspiration or VAS scores between groups at either of the 5 time points (P > 0.05) (see Table 5).
Table 5.
Comparison of Postoperative Complications Among the Two Groups of Patients (n = 38)
| Parameter | Control | Intervention | χ2 or Z | P |
|---|---|---|---|---|
| Thirst [n(%)] | 14(36.80) | 4(10.50) | 7.280 | 0.007 |
| Nausea [n(%)] | 1(2.60) | 2(5.20) | 0.000 | 1.000 |
| Vomiting [n(%)] | 1(2.60) | 1(2.60) | 0.000 | 1.000 |
| Reflux aspiration [n(%)] | 0(0) | 0(0) | – | – |
| VAS score | ||||
| S0 | 3.00(2.00, 3.00) | 2.00(2.00, 3.00) | −1.251 | 0.211 |
| S1 | 2.00(2.00, 3.00) | 2.50(2.00, 3.00) | −0.996 | 0.319 |
| S2 | 2.00(2.00, 2.00) | 2.00(1.00, 2.00) | −1.142 | 0.254 |
| S3 | 1.00(1.00, 2.00) | 1.00(1.00, 2.00) | −0.653 | 0.513 |
| S4 | 2.00(1.00, 2.00) | 2.00(1.00, 2.00) | −0.750 | 0.453 |
Notes: (Continuous data is represented by M(¼, ¾); S0, preoperative; S1, Awakening from anesthesia; S2, 1d after operation; S3, 2d after operation; S4, 3d after operation; VAS score, pain visual analog score).
Discussion
This study found that oral administration of normal saline 2 hours before surgery can reduce the incidence of POD in older persons undergoing spinal decompression. Compared to other surgeries, spinal surgery is more complicated, involving heavier bleeding, longer operation time, and increased occurrence in older persons. The perioperative hemodynamic fluctuations are also more obvious in this type of surgery, thus, the incidence of POD is higher. Also, our study found that the incidence of POD in older persons receiving spinal decompression was higher, while the incidence of POD in the intervention group was significantly lower than that in the control group. In addition to lowered S100β levels, hemodynamics (MAP, HR) fluctuations in the intervention group were smaller than in the control group.
In this study, the incidence of POD in older persons undergoing spinal decompression surgery was 17.1%, which is slightly lower than previous studies.14 With the advancement of surgery and the strengthening of anesthesia management, the shortening of surgical operation time and the reduction of bleeding may have contributed to reductions in the incidence of POD. Compared with previous studies, patients in this study were not administered benzodiazepine or anticholinergic drugs, which may have also contributed to reductions in the incidence of POD as benzodiazepines may increase the risk of POD.15,16 In addition, the observation time of this study was relatively short (only 3 consecutive days after surgery), and the number of daily evaluations was minimal (only once a day), which could have led to missed diagnosis of delirium cases, particularly hypoactive delirium, resulting in an underestimation of POD cases. One study pointed out that the incidence of POD in patients who underwent cervical spine surgery was higher than those undergoing lumbar surgery.17 In this study, anesthesia was delivered under the guidance of the depth monitoring, minimizing the risk of brain damage and possibly contributing to the reduced incidence of POD.18,19
Prolonged abstinence before surgery is known to increase perioperative insulin resistance, contributing to a patient’s physical and mental discomfort. Simultaneously, surgery (especially heart and orthopedics surgery) can cause endocrine and inflammatory stress responses leading to perioperative insulin resistance simulating a transient and reversible type 2 diabetes.20,21 Single-photon emission computed tomography (SPECT) evaluation has demonstrated that hyperglycemia can aggravate cerebral blood flow decline in older persons, which can trigger POD.22 Thus, administering normal saline 2 hours before surgery may improve insulin sensitivity, facilitating anabolism. The current study found no correlation between perioperative hyperglycemia and POD. This may be related to differences in the research subjects and/or research design. Further exploration of perioperative insulin resistance and POD is needed.
Subclinical POD may be related to cerebrovascular events, such as intraoperative hemodynamic fluctuations related to transient cerebral hypoperfusion. Obvious intraoperative hypotension often requires the use of vasoactive drugs. One observational study found that POD is related to the frequent use of vasoactive drugs during surgery.23 Interestingly, Taipale et al also reported that changes in intraoperative MAP is one of the physiological variables closely related to the occurrence of POD.24 The results of this study are consistent with the above research reports, with the MAP and HR of the control group decreasing significantly after induction of anesthesia, followed by gradually increase. Meanwhile, the hemodynamic changes of the intervention group during induction of anesthesia were smaller.
The results of this study confirmed that perioperative inflammation in patients with delirium was significantly increased, and that CRP levels were significantly increased on the first day after surgery. CRP is a positive protein in the acute phase of the inflammatory response, and an objective and reliable indicator of inflammatory response and tissue damage.25 High levels of serum CRP can increase the permeability of the blood-brain barrier, indirectly reflect increased inflammation in the central nervous system, a risk factor for delirium.26 Glumac et al showed that preoperative dexamethasone administration improve cognitive outcome following cardiac surgery because dexamethasone could reduce inflammation response, particularly postoperative CRP protein levels.27 A number of clinical studies have suggested that CRP can predict the occurrence and recovery of delirium in elderly and ICU patients.28 The results of this study are consistent, as increases in CRP 1 day after surgery were correlated with the occurrence of POD. Under inflammatory conditions, activated astrocytes release of S100β, a calmodulin protein regulating calcium homeostasis, axon growth and neuronal differentiation. Thus, postoperative S100β increases may indicate brain dysfunction, such as cognitive impairment. The release of S100β into the peripheral blood is related to increased BBB permeability, with the subsequent inflammatory response working to reduce the BBB permeability. Glumac et al showed that there is no significant relationship between cognitive dysfunction and S100β.27 However, Hughes et al corroborate that increased S100β is related to the occurrence of POD.29 In this study, the control group experienced a significant increase in S100β protein after awakening from anesthesia and 1 day postoperatively, particularly in patients experiencing delirium, suggesting that S100β protein may help clinicians identify POD.
While at present there is no experimental evidence to explain the complex mechanisms which may reduce POD by preoperative normal saline, some mechanisms can be speculated. First, patients with prolonged drinking prohibition are prone to insulin resistance during the perioperative period, leading to blood sugar increases which may alter cerebral blood flow and increase the occurrence of POD. Secondly, the blood volume of patients who have been prohibited from drinking for long periods of time may be relatively insufficient, predisposing patients to perioperative hemodynamic fluctuations which can increase the incidence of POD. Third, patients with extended periods of prohibitive drinking have disordered blood electrolyte and acid-base balances, leading to an increased stress response, sympathetic-adrenal medulla system excitement, gastrointestinal vasoconstriction, reduced blood flow, gastrointestinal mucosal ischemia and hypoxia, gastrointestinal mucosal damage, and even severe acidosis which weakens the mucosal barrier and promotes the translocation of intestinal bacterial, toxin absorption, and enhanced systemic inflammatory response- all of which can lead to the occurrence of POD.
There are some limitations in this study: 1) Uncertainty of the clinical procedure time makes it difficult to control the preoperative abstinence period; 2) The study limited detection of S100β protein and CRP to only the first postoperative day for practical reasons despite POD occurring up to 5 days after surgery; 3) The study is a small sample and single-center test.
Conclusion
Oral administration of normal saline 2 hours before surgery can reduce the incidence of POD in older persons undergoing spinal decompression, though the specific mechanisms remain unclear and require experimental evidence. The protective effect of preoperative normal saline may include reduced inflammation, inhibition of S100β, and reduction in perioperative blood flow fluctuations. In clinical practice, anesthesiologists and surgeons should formulate a reasonable and individualized plan for older persons, considering factors which may help prevent POD and improve the prognosis.
Acknowledgments
We would like to thank the participants and peers for their participation in this study. Jinzhuan Chen and Siyu Xie are co-first authors for this study.
Funding Statement
The authors received no funding for this study.
Abbreviations
ASA, American Society of Anesthesiologists; BBB, Blood Brain Barrier; BIS, Business Information System; BMI, Body Mass Index; CAM, Confusion Assessment Method; CNS, Central Nervous System; CRP, C-reactive Protein; Hb, Hemoglobin; HR, Heart Rate; IL-6, Interleukin-6; MAP, Mean Arterial Blood Pressure; MMSE, Mini-mental State Examination; Nu-DESC, Nursing Delirium Screening Scale; PACU, Post-anesthesia Care Unit; PCIA, Patient Controlled Intravenous Analgesia; POD, Postoperative Delirium; POCD, Postoperative Cognitive Dysfunction; S100β, S100β Protein; VAS, Visual Analogue Scale.
Registration Information
Registration number: ChiCTR2100048640 Registration website: http://www.chictr.org.cn/ This study has been registered at 12/7/2021 The first patient registered in this study at 25/5/2020.
Data Sharing Statement
The data is available from the corresponding author upon the reasonable request.
Ethical Approval and Consent to Participate
This study was a single center, randomized controlled study. It was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University (Ethics Medical Research [2020] No. 155). All procedures performed in studies were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All participants in this study have provided informed written consent prior to enrollment.
Consent for Publication
Informed consent was obtained from all the participants and/or their legal guardian to publish their information.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
- 1.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39. doi: 10.1056/NEJMoa1112923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wacker P, Nunes PV, Cabrita H, et al. Post-operative delirium is associated with poor cognitive outcome and dementia. Dement Geriatr Cogn Disord. 2006;21(4):221–227. doi: 10.1159/000091022 [DOI] [PubMed] [Google Scholar]
- 3.Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34(4):192–214. doi: 10.1097/EJA.0000000000000594 [DOI] [PubMed] [Google Scholar]
- 4.Austin CA, O’gorman T, Stern E, et al. Association between postoperative delirium and long-term cognitive function after major nonemergent surgery. JAMA Surg. 2019;154(4):328–334. doi: 10.1001/jamasurg.2018.5093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berian JR, Zhou L, Russell MM, et al. Postoperative delirium as a target for surgical quality improvement. Ann Surg. 2018;268(1):93–99. doi: 10.1097/SLA.0000000000002436 [DOI] [PubMed] [Google Scholar]
- 6.Zhang HJ, Ma XH, Ye JB, et al. Systematic review and meta-analysis of risk factor for postoperative delirium following spinal surgery. J Orthop Surg Res. 2020;15(1):509. doi: 10.1186/s13018-020-02035-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JK, Park YS. Delirium after spinal surgery in Korean population. Spine. 2010;35(18):1729–1732. doi: 10.1097/BRS.0b013e3181c423fc [DOI] [PubMed] [Google Scholar]
- 8.Su X, Feng X, Terrando N, et al. Dysfunction of inflammation-resolving pathways is associated with exaggerated postoperative cognitive decline in a rat model of the metabolic syndrome. Mol Med. 2013;18(1):1481–1490. doi: 10.2119/molmed.2012.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson TN, Raeburn CD, Tran ZV, et al. Motor subtypes of postoperative delirium in older adults. Arch Surg. 2011;146(3):295–300. doi: 10.1001/archsurg.2011.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarin A, Chen LL, Wick EC. Enhanced recovery after surgery-preoperative fasting and glucose loading-A review. J Surg Oncol. 2017;116(5):578–582. doi: 10.1002/jso.24810 [DOI] [PubMed] [Google Scholar]
- 12.Radtke FM, Franck M, Macguill M, et al. Duration of fluid fasting and choice of analgesic are modifiable factors for early postoperative delirium. Eur J Anaesthesiol. 2010;27(5):411–416. doi: 10.1097/EJA.0b013e3283335cee [DOI] [PubMed] [Google Scholar]
- 13.Gan TJ, Scott M, Thacker J, et al. American society for enhanced recovery and perioperative medicine. Anesth Analg. 2018;126(6):1870–1873. doi: 10.1213/ANE.0000000000002925 [DOI] [PubMed] [Google Scholar]
- 14.Shi C, Yang C, Gao R, et al. Risk factors for delirium after spinal surgery: a meta-analysis. World Neurosurg. 2015;84(5):1466–1472. doi: 10.1016/j.wneu.2015.05.057 [DOI] [PubMed] [Google Scholar]
- 15.Memtsoudis S, Cozowicz C, Zubizarreta N, et al. Risk factors for postoperative delirium in patients undergoing lower extremity joint arthroplasty: a retrospective population-based cohort study. Reg Anesth Pain Med. 2019;44:934–943. doi: 10.1136/rapm-2019-100700 [DOI] [PubMed] [Google Scholar]
- 16.Weinstein SM, Poultsides L, Baaklini LR, et al. Postoperative delirium in total knee and Hip arthroplasty patients: a study of perioperative modifiable risk factors. Br J Anaesth. 2018;120(5):999–1008. doi: 10.1016/j.bja.2017.12.046 [DOI] [PubMed] [Google Scholar]
- 17.Pan Z, Huang K, Huang W, et al. The risk factors associated with delirium after lumbar spine surgery in elderly patients. Quant Imaging Med Surg. 2019;9(4):700–710. doi: 10.21037/qims.2019.04.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bocskai T, Kovács M, Szakács Z, et al. Is the bispectral index monitoring protective against postoperative cognitive decline? A systematic review with meta-analysis. PLoS One. 2020;15(2):e0229018. doi: 10.1371/journal.pone.0229018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackenzie KK, Britt-Spells AM, Sands LP, et al. Processed electroencephalogram monitoring and postoperative delirium: a systematic review and meta-analysis. Anesthesiology. 2018;129(3):417–427. doi: 10.1097/ALN.0000000000002323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott MJ, Baldini G, Fearon KC, et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 1: pathophysiological considerations. Acta Anaesthesiol Scand. 2015;59(10):1212–1231. doi: 10.1111/aas.12601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palermo NE, Gianchandani RY, Mcdonnell ME, et al. Stress Hyperglycemia hyperglycemia during surgery and anesthesia: pathogenesis and clinical implications. Curr Diab Rep. 2016;16(3):33. doi: 10.1007/s11892-016-0721-y [DOI] [PubMed] [Google Scholar]
- 22.Hirao K, Hanyu H, Sato T, et al. A longitudinal SPECT study of different patterns of regional cerebral blood flow in Alzheimer’s disease with or without diabetes. Dement Geriatr Cogn Dis Extra. 2011;1(1):62–74. doi: 10.1159/000323865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neerland BE, Krogseth M, Juliebø V, et al. Perioperative hemodynamics and risk for delirium and new onset dementia in Hip fracture patients; A prospective follow-up study. PLoS One. 2017;12(7):e0180641. doi: 10.1371/journal.pone.0180641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taipale PG, Ratner PA, Galdas PM, et al. The association between nurse-administered midazolam following cardiac surgery and incident delirium: an observational study. Int J Nurs Stud. 2012;49(9):1064–1073. doi: 10.1016/j.ijnurstu.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 25.Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125(4):492–504. doi: 10.1016/j.bja.2020.06.063 [DOI] [PubMed] [Google Scholar]
- 26.Pinato DJ, Bains J, Irkulla S, et al. Advanced age influences the dynamic changes in circulating C-reactive protein following injury. J Clin Pathol. 2013;66(8):695–699. doi: 10.1136/jclinpath-2012-201374 [DOI] [PubMed] [Google Scholar]
- 27.Glumac S, Kardum G, Sodic L, et al. Effects of dexamethasone on early cognitive decline after cardiac surgery: a randomised controlled trial. Eur J Anaesthesiol. 2017;34(11):776–784. doi: 10.1097/EJA.0000000000000647 [DOI] [PubMed] [Google Scholar]
- 28.Hsuchou H, Kastin AJ, Mishra PK, et al. C-reactive protein increases BBB permeability: implications for obesity and neuroinflammation. Cell Physiol Biochem. 2012;30(5):1109–1119. doi: 10.1159/000343302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes CG, Pandharipande PP, Thompson JL, et al. Endothelial activation and blood-brain barrier injury as risk factors for delirium incritically ill patients. Crit Care Med. 2016;44(9):e809–17. doi: 10.1097/CCM.0000000000001739 [DOI] [PMC free article] [PubMed] [Google Scholar]