Abstract
Background
There is an unmet need for non-medication approaches to illicit opioid discontinuation and relapse prevention. The NET (NeuroElectric Therapy) Device is a non-invasive, battery-operated, portable, re-useable device designed to deliver bilateral transcranial transcutaneous alternating current electrical stimulation, and is intended to treat opioid use disorder (OUD) without medication. The device is a CE-marked Class IIa, non-significant risk, investigational medical device.
Objective
This prospective trial (NRC021) tests the hypothesis that the NET Device provides safe and effective neurostimulation treatment for persons with OUD who express a desire to be opioid abstinent without medications for opioid use disorder (MOUD).
Methods
NRC021 is a randomized, parallel-group, sham-controlled, quintuple-blinded, single-site study. Persons with OUD entering a residential treatment facility for opioid detoxification are assigned to active or sham treatment (n = 50/group). Group assignment is stratified on presence of any current non-opioid substance use disorder and by sex. The biostatistician maintains the blinding so that the study sponsor, principal investigator, research assistants, treatment staff, and participants remain blinded. Following discharge from the inpatient facility, participants are assessed once weekly over 12 weeks for substance use (using timeline followback interview and video assessment of observed oral fluid sample provision and testing). The primary efficacy endpoint is each participant's overall percentage of weekly abstinence from illicit opioid use without use of MOUD. The secondary efficacy endpoint is each participant's percentage of non-opioid drug-free weeks. Safety outcomes are also measured.
Conclusion
NRC021 is designed to assess the efficacy of a novel non-medication treatment for OUD.
Clinical trial registration
ClinicalTrials.gov with the identifier NCT04916600.
Keywords: Opioid use disorder, Detoxification, Withdrawal, Craving, Transcranial electrical stimulation, Medications
1. Introduction
Nonmedical use of prescription opioids, illicit opioid use, and opioid use disorder (OUD) has led to unprecedented levels of health, societal and economic problems [[1], [2], [3], [4], [5]], aggravated by exposure to high-potency synthetic opioids [1,[6], [7], [8]] and psychostimulants [9,10], and complicated by the COVID-19 pandemic [[11], [12], [13], [14]]. Opioid-related overdoses, emergency department visits, and deaths have risen precipitously over the past two decades. These consequences have led to assertive federal, state, and local initiatives to combat this crisis. Opioid misuse problems were estimated to cost the United States (US) about $1 trillion in 2017 [15].
Despite these enormous problems, OUD treatment participation is unacceptably low: fewer than 15% of persons with OUD in the US currently receive treatment [[16], [17], [18]]. Food and Drug Administration (FDA)-approved medications for OUD (MOUD) are effective for promoting retention and abstinence among many but not all persons seeking pharmacotherapy; however, due to several barriers [[19], [20], [21], [22]], rates of MOUD utilization are low [[23], [24], [25]]. Furthermore, a non-trivial proportion of patients entering OUD treatment express reservations about MOUD (e.g. side effects, inconvenience, stigma) as well as positive interest in non-medication interventions for transitioning to longer-term abstinence [[26], [27], [28], [29], [30]], although these options are sparse. This gap could lead many OUD patients to avoid or drop out of otherwise lifesaving treatment.
Notably, novel treatments for OUD are not intended to replace existing approved treatments; rather, the intent is to supply patients and clinicians with safe and effective alternative/complementary interventions that promote better personal, clinical, and public health outcomes. Clinical investigations of candidate treatments should measure the independent effectiveness of the proposed intervention while respecting patient choices to use existing standards of care.
The NeuroElectric Therapy (NET) Device offers a promising non-pharmacological approach for treatment of persons with OUD but has not yet undergone rigorous clinical efficacy evaluation. Similar earlier-generation devices have been studied extensively under open-label, non-controlled conditions, and exclusively in the inpatient setting, as a possible monotherapy for medication-free detoxification from chronic substance use [[31], [32], [33], [34]]. In April 2009, the NET Device received CE-mark as a Class IIa medical device.
Results from observational pilot studies in Europe and the US indicate that treatment with the NET Device as monotherapy, i.e., without MOUD, can rapidly decrease opioid detoxification-related drug craving and withdrawal symptom elevations. In addition, approximately 85% of the followed completers tested negative for illicit opioids, methadone, and buprenorphine, and 98% tested negative for cocaine and methamphetamines. However, those pilot studies did not use intent-to-treat (ITT), sham-controlled, blinded procedures. The present study is a rigorous, randomized controlled trial that addresses these limitations.
2. Methods
2.1. NET device description
Stimulation characteristics. The NET Device delivers alternating current via surface electrodes placed transcranially (bilaterally) on the mastoid processes (Fig. 1). The device delivers multiple low-amperage waveforms at controlled frequencies and pulse widths that vary throughout each treatment day, with no net-direct current component; this approach differs from single-frequency, sinusoidal transcranial alternating current stimulation. Waveshapes were refined across several engineering iterations that adjusted for dynamic variations in skin impedance, electrode conductance, frequency and pulse-width related sensation, and orthogonal electrode pressure (e.g. from head pressure when sleeping), leading to improved rates of patient tolerability.
Fig. 1.
Upper panels: NET Device (left), mastoid-region electrode placement (middle), and cumulative distribution function of participants’ device utilization (percentage of sample with device “on”) across hours in two pilot studies conducted in Kentucky and Scotland (right). Lower panels: Time course of device intensity setting (left), and cumulative distribution functions of scores for the Subjective Opioid Withdrawal Scale (SOWS; middle) and craving (right).
Stimulation is continuously available (except when bathing) for up to 7 days via transcutaneous electrodes of size approximately 1 cm × 2 cm. Stimulation output frequency varies from 4 to 3000 Hz and pulse width from 7 to 1024 μs. Stimulation output current varies from 0 to 3.2 mA (peak) into a 15 kOhm load, and output voltage varies from 0 to 44 V (peak to peak).
Treatment is self-administered, and participants are instructed that they can control the device output intensity and duration according to perceived benefit.
Possible mechanisms of action. Earlier studies demonstrated that transcranial electrostimulation of the type delivered by the NET Device can attenuate the severity of opioid withdrawal [[35], [36], [37], [38], [39]]. Such neurostimulation is thought to modulate endogenous opioid, dopaminergic and serotonergic systems and the autonomic nervous system [[40], [41], [42]], which are dysregulated in the opioid-dependent state [[43], [44], [45], [46], [47], [48], [49]]. We hypothesize that self-titrated NET Device stimulation of these multiple interacting neurochemical systems may promote neuroplastic changes [[50], [51], [52], [53]] that normalize functioning of these systems and support longer-lasting changes in drug-abstinence behavior. Although the mechanism of action is not well-understood and is a subject for future investigation, treatment duration (≤7 days, self-administered) is consistent with FDA-approved percutaneous nerve stimulators, and the outcome measurement period (up to 16 weeks from start of treatment) is consistent with results of open-label NET pilot studies in Kentucky and Scotland.
Safety. As a Non-Significant Risk device, FDA does not require submitting an Investigational Device Exemption for this study. Safety of the NET Device has not been established for persons who: (1) are pregnant, breastfeeding, or <18 years old; (2) have serious heart conditions or a cardiac pacemaker; (3) have suffered a stroke, brain tumor, or brain injury; (4) have current epilepsy; (5) are suffering serious psychotic illness; or (6) are taking medications such as neurotransmitter blockers.
2.2. Study design
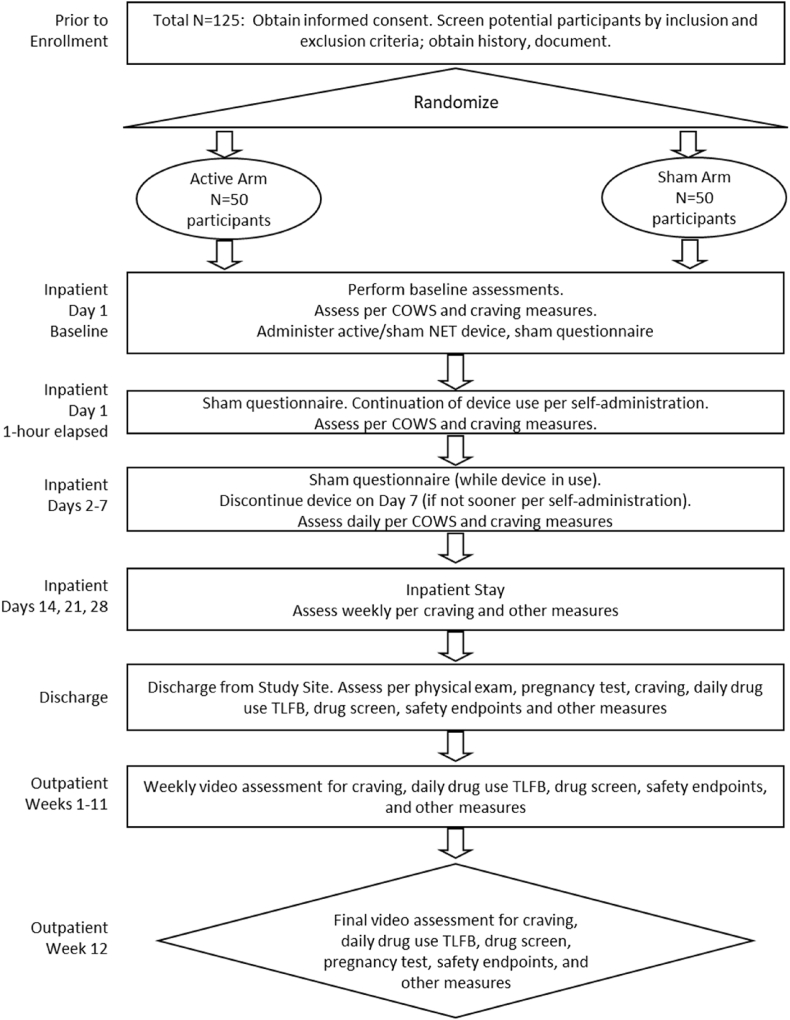
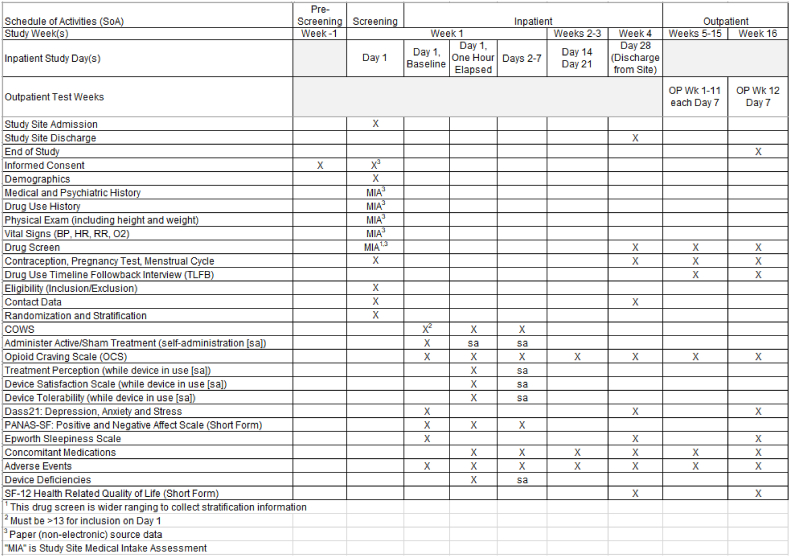
This ITT, randomized, single-site trial uses a superiority design that compares active treatment to sham control. This trial will evaluate efficacy and safety endpoints during opioid discontinuation within an inpatient setting, and subsequent outpatient assessment of opioid and non-opioid substance use following discharge (primary and secondary endpoints, respectively; section 2.4). Fifty participants will be randomized to each of the two treatment arms (active and sham) and prospectively followed. Fig. 2 illustrates the study schema. Fig. 3 illustrates the schedule of activities.
Fig. 2.
Study schema Notes: “NET”, NeuroElectric Therapy. “COWS”, Clinical Opiate Withdrawal Scale. “TLFB”, Timeline Followback.
Fig. 3.
Schedule of Activities Notes: “OP”, outpatient. “Wk”, week. “sa”, self-administered. “BP”, blood pressure. “COWS”, Clinical Opiate Withdrawal Scale, “HR”, heart rate. “RR”, respiration rate. “O2”, oxygen saturation.
As the platform for this trial, all participants receive treatment as usual (TAU), except MOUD, which they choose (as part of informed consent) not to receive as a condition of inclusion in this study. Participants could experience clinical deterioration during inpatient discontinuation of illicit opioids, prescribed opioid agonist medications (buprenorphine, methadone), and other illicit substances. Participants are instructed that device use (active or sham treatment) is self-administered, they may discontinue device use at any time and for any reason and can receive TAU for their clinical condition including MOUD and comfort medications.
The rationale for the minimum 1-h device treatment period is to ensure a controlled degree of exposure to the device (active or sham) for all participants; based on prior open-label studies, the active device produces benefits in about 15–20 min. Completing this 1-hr period triggers study follow-up assessments. The rationale for the 7-day maximum device treatment period is to limit variability in the duration of exposure (to be treated as a covariate in analyses); as Fig. 1 indicates, the majority of participants stop active device use after about 7 days.
2.3. Randomization and blinding
The biostatistician (author SG) block-randomizes assignment of the ITT population to treatment (sham vs. active) using a 1:1 allocation ratio and, within each treatment group, stratification by sex (male/female) and non-opioid substance use disorder (presence/absence). The master codebook for randomization is kept in a secure location at Wayne State University and only the biostatistician and a research assistant (backup person) can access it.
Sham treatment, which controls for placebo effects, is designed to minimize sham recognition by the sponsor (author JRW), principal investigator (author MKG), participants, research assistants and treatment staff (i.e. quintuple blinding). Participants in the active arm receive standard NET treatment. Participants in the sham arm receive the same standard NET treatment as the active arm but will receive no electrical stimulation (cable is disabled beforehand). Sham credibility is evaluated for both arms based on surveys following the 1-hr treatment phase, and daily during device use. A single ‘treatment perception’ question asks: “How confident are you that you are receiving the real device treatment and not the placebo (sham) treatment?” Three ‘device satisfaction’ questions ask: (1) “How satisfied are you with the NET Device?” (2) “How willing are you to use the NET Model 901 Device?” and (3) “How willing are you to recommend the NET Model 901 Device to other people undergoing opioid discontinuation?”
Active and sham arms will receive identical device care and use instructions, identical equipment, attachment methods and locations, and identical daily reviews of device operation, electrode attachment, and withdrawal severity. The treatment apparatus presents both active- and sham-assigned participants with visual cues from the device's “heartbeat” indicator (a blinking green light-emitting diode which indicates the device is active) during treatment. Research staff instruct each participant that the equipment is designed to be self-administered, that s/he can set the level of stimulation wherever it is comfortable, that stimulation is not always (and does not need to be) sensate, and that device use may be discontinued when s/he feels it is providing no benefit.
The sham and active interventions both use the NET Device without alteration. The sham intervention uses lead wires that have been rendered non-conductive beforehand, preventing any electrical stimulation from being delivered to the participant. Before study initiation, the biostatistician allocated a random and unique Study Device Number (SDN) to each identical-looking active and sham lead wire, applying pre-printed SDN heat shrink. At the study site, the research assistant is notified electronically of participant inclusion, and selects a device and lead wires by SDN from a pre-printed randomization table for delivery of active or sham treatment. At the end of the study, all lead wires will be delivered to the biostatistician for verification against the group assignment by measuring conductivity using the sponsor-supplied validation circuit.
2.4. Outcome measures
During the 12-week outpatient period, participants will be interviewed for safety and efficacy endpoints and undergo a remote drug screen. Remote interviews reduce risk of COVID-19 exposure for the participant and research assistant, and decrease transportation barriers (increased feasibility). Each week, the research assistant hosts a live video call with the participant, and conducts a timeline follow-back (TLFB) review of opioid, MOUD, stimulant, sedative, cannabis and alcohol use for each of the prior 7 days. At discharge from the inpatient facility, each participant will be given 12 kits (plus one spare) of the Premier Biotech OralTox OT-80605, an FDA 510(K) cleared 6-panel rapid oral fluid drug screen. Each test covers amphetamines and methamphetamine (positive cutoffs = 50 ng/ml), opioids and THC (positive cutoffs = 40 ng/ml), oxycodone and cocaine (positive cutoffs = 20 ng/ml). Each individually packaged test is sealed in foil and is marked with a lot number and expiration date. Each individual test contains a QR code that encodes a unique identification number that will be used to validate authenticity and uniqueness of the screen. The participant receives printed and verbal instructions for use from the research assistant, is observed during each test, and test results with authenticating QR code are captured as an image file by the research assistant and saved for validation. In order to conduct this FDA-qualifying study during the COVID-19 pandemic, remote test methods were evaluated for use during the outpatient phase. When this study began, FDA had only approved a limited selection of oral fluid rapid tests for use in research. The authors selected TLFB self-report as the primary source for data, supplemented by such relevant oral fluid tests as were FDA approved.
The primary efficacy endpoint is each participant's overall percentage of weekly abstinence from illicit opioid use without use of MOUD. This is defined as the percentage of each participant's negative oral fluid samples and self-reports of illicit opioid, methadone, buprenorphine, and naltrexone use per week throughout the 12-week outpatient study period. The rationale for choosing this endpoint is to perform a rigorous test of the isolated efficacy of NET, with the safety net of allowing (and measuring) participants' use of MOUDs.
During a given week, oral fluid samples and self/pharmacy reports that both indicate no illicit opioid, methadone, buprenorphine, or naltrexone use will be considered “opioid-abstinence without MOUD”. Cumulative distribution function, time (days) to first use of illicit opioids, time (days) to first use of MOUD, and duration (weeks) of continuous abstinence without MOUD will be evaluated as supportive outcomes. Response profiles for each treatment arm are based on each participant's individual rates of weekly illicit opioid or MOUD use, including negative oral fluid results and daily self (TLFB) of illicit opioid or MOUD use/non-use. Table 1 presents explanatory cases of the primary efficacy endpoint measurement. The null hypothesis is that “opioid abstinence without MOUD” will not significantly differ between active and sham groups, whereas the alternative hypothesis is that “opioid abstinence without MOUD” will be superior in the active (relative to sham) treatment arm.
Table 1.
Example calculations of the primary efficacy endpoint measurement.
| Cases measuring “abstinence without MOUD”: | ||
|---|---|---|
| Heroin use, no MOUD | {1,1,1,h,h,1,1,1,1,1,1,h} | = 9/12 |
| Heroin use, no MOUD, missing weeks | {1,1,1,h,h,1,-,-,1,1,-,-} | = 6/12 |
| MOUD use, no Heroin | {1,1,m,m,m,m,1,1,1,1,1,1} | = 8/12 |
| MOUD use, no Heroin, missing weeks | {1,1,m,m,m,m,1,1,-,-} | = 4/12 |
| Heroin use, MOUD use | {1,1,h,h,h + m,m,m,m,m,h,1,1} | = 4/12 |
| Heroin use, MOUD use, missing weeks | {1,1,h,h,h + m,m,-,-,m,h,-,-} | = 2/12 |
Legend.
1 = all drug tests and self-reports are negative.
h = illicit opioid (e.g., heroin) drug screen or self-report is positive.
m = medication for opioid use disorder (MOUD) or self-report is positive.
‘-‘ = missing value.
The secondary efficacy endpoint is each participant's percentage of non-opioid drug-free weeks during the 12-week outpatient period. Non-opioids assessed include cocaine, sedatives, and stimulants. Use of alcohol and cannabis will be measured, but are not included in the secondary efficacy analysis, due to their legal status. Response profiles for each arm are based on each participant's individual rates of weekly abstinence data, including negative oral fluid results and daily self-reports of non-opioid drug use/non-use (TLFB).
The secondary safety endpoint is the prevalence of all adverse events (AEs), serious adverse events (SAEs), adverse device effects (ADEs), serious adverse device effects (SADEs), unanticipated adverse device effects (UADEs), and device deficiencies.
Supportive and exploratory measures. Treatment retention will be measured throughout the inpatient and outpatient phases. Self-stimulation intensity (in 5-min bins), duration, and device on/off data from each NET Device are automatically transmitted to a computer server and processed offline. Opioid withdrawal symptoms, craving severity, and positive/negative affect are measured daily during the first inpatient week. Measures of depression, anxiety and stress, sleepiness, and health-related quality of life are obtained at the conclusion of inpatient and outpatient phases.
2.5. Participant selection criteria
Screening includes informed consent, HIPAA authorization, assessment of demographics, contact data, pregnancy testing, contraception methods, medical history, and urine drug testing (including fentanyl, buprenorphine, and methadone). Assessments are aligned with the treatment facility's standard of care such that electronic case report forms (eCRFs) can be rapidly reviewed for study eligibility by the remote investigator, given the time-sensitive nature of enrollment.
During the recruitment and informed consent process, participants are repeatedly told they can receive FDA-approved MOUD at the treatment facility instead of participating in the study. They are also told that upon discontinuation of device stimulation, and/or if they drop out of the study, they can receive TAU (including MOUD) at any time. All participants are given a “loss-of-opioid tolerance” warning (i.e. stopping opioids leads to reduced tolerance, and subsequent opioid use increases their risk of overdose and death), which they must sign. Participants are also given a naloxone kit upon discharge from the treatment facility and educated on its use. All of these procedures are documented in the medical record and case report forms.
Table 2 presents study inclusion and exclusion criteria. For inclusion, participants ages 18–65 years old must have current OUD, present in otherwise good health, be seeking opioid discontinuation at the treatment facility, self-report that they wish to become/remain abstinent without using MOUD, be willing to follow study procedures, provide informed consent and use medically-accepted highly effective contraception. An additional criterion (following informed consent) is that device use will not begin until the Clinical Opiate Withdrawal Scale (COWS [54]) total score is 13 or greater (at least moderate withdrawal); thus, a consented participant could be dis-enrolled if the latter criterion is not met. Exclusion criteria include pregnancy or lactation, serious current psychiatric disorder (schizophrenia, bipolar) or use of neuropsychiatric medications that may overlap with NET's proposed mechanisms of action (e.g. anxiolytics, antidepressants, anticonvulsants, sedating H1-receptor antihistamines, prescription or over-the-counter stimulants), need for detoxification from alcohol or benzodiazepines, past 300-day exposure to extended-release buprenorphine, certain chronic illnesses (especially seizures), unstable medical conditions, or presence of cardiac pacemaker.
Table 2.
Participant selection criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.6. Data & safety monitoring
Oversight of data management and quality assurance is the responsibility of the investigator with input from the Study Monitor designated by the sponsor. Trial data are managed with a sponsor-designed electronic source data system. This system provides source data capture, modification, correction and access based on pre-defined roles, e.g. only the investigator can determine inclusion/exclusion. Specific eCRF data are exported to a FDA-compliant electronic data capture (Advarra) system at Wayne State University, where the biostatistical team manages and analyzes outcome measures. There is no data safety monitoring board for this trial.
2.7. Statistical analysis
Sample size determination. Sample size is based on statistical power analyses for the primary hypothesis based on single primary outcome (opioid abstinence without MOUD). A conservative approach was used to preserve power with a smaller effect size than observed from prior uncontrolled, non-blinded intervention studies with the NET Device. Power analyses examined sample sizes required to detect clinically meaningful group differences between sham and active groups. This represents standardized difference between the two groups after adjusting for the effect of the covariate “duration of inpatient residence” (which is capped at 28 days). Sample size determination was carried out via simulation in R-software; 5000 iterations were run in which group means were generated using a mixed-effect additive model. Sample size is a function of hypothesized effect size (standardized group difference) and the sign and magnitude of the regression coefficient (duration of inpatient residence). With projected N = 100 (enrolled) there will be sufficient power to detect a standardized effect size of 0.45 or higher.
Missing data. In this ITT design, a participant is “included” (becomes part of the evaluable ITT data set) when he/she completes 1-hr of device use. We expect only 5% attrition, i.e. for 100 participants who complete 1-hr of device use, we expect to consent 105 individuals. A comparable trial of a percutaneous electrical stimulation device for treating opioid withdrawal symptoms found that 71 of 73 enrolled participants completed 1-hr of device use, only 2.8% attrition [55]. Table 1 explains how missing data are handled, i.e. for the primary efficacy endpoint, missing data are imputed as “not opioid abstinent without MOUD”. The definition of the primary endpoint (“opioid abstinence without MOUD”) and secondary endpoint (“non-opioid abstinence”) thus covers any intermittent missing values and loss to follow-up, and is not expected to impact power of the primary analysis. Premature study termination (e.g., death, relocation, or adverse events) may result in missing data, although this attrition is expected to be low. The projected sample size (N = 125) was increased to cover unforeseen events.
Participant withdrawal. Participants can withdraw from the study at any time on request. Discontinuation of device use prior to completing the minimum 1-hr treatment period constitutes withdrawal from the study. The investigator may discontinue or withdraw a participant from the study for the following reasons: (1) significant study intervention non-compliance; (2) if any clinical adverse event (AE), laboratory abnormality, or other medical condition or situation occurs such that continued participation in the study is not in the best interest of the participant; (3) disease progression which requires discontinuation of the study intervention; (4) if the participant meets an exclusion criterion (either newly developed or not previously recognized) that precludes further study participation; or (5) participant is unable to receive treatment following enrollment.
Participants who sign the informed consent form and are randomized but do not receive the study intervention will be replaced. Participants who sign the informed consent form, are randomized, receive the study intervention, and subsequently withdraw or are withdrawn, will be replaced.
Participants who withdraw from the study will receive TAU as defined by treatment staff (who are independent of the research staff), where such care may include MOUD.
Lost to follow-up. A participant is considered lost to follow-up if s/he becomes unavailable for all video assessments following discharge from the inpatient environment.
Populations for analysis. The ITT analysis dataset and the safety analysis dataset will contain all randomized participants. The per-protocol analysis dataset will contain the subset of the ITT dataset who: (1) present with a COWS score of moderate or above; (2) receive at least 1 h treatment from the device; and (3) complete at least one post-discharge video interview.
Efficacy endpoints. The primary analysis will assume that data came from a mixed-effect linear model. Each model will include a random intercept, fixed effect for treatment, participant-specific random effect and covariate corresponding to duration of inpatient residence. The primary hypothesis involves testing the main effect for treatment difference between the groups (sham vs. active). A likelihood ratio test will examine the group effect focusing on the primary hypothesis.
Secondary endpoint analyses are not powered, but may be used to demonstrate additional benefits of the treatment, provided it has been demonstrated that the primary endpoint shows clear statistical significance, as well as clinical meaningful benefits of the treatment [56]. Analysis of the secondary endpoint resembles primary endpoint analysis, using mixed-effect model testing for the between-group main effect.
Given that participants can control the level of device stimulation, exploratory regression analyses will seek to identify potential relationships between device utilization (e.g. number of minutes device ‘on’ [non-zero intensity], average intensity while device ‘on’) and efficacy endpoints.
Expected outcomes. We selected a single primary outcome (abstinence without MOUD). From our past experience at this same treatment facility, the expected group means for sham and NET arms are 0.48 and 3.97 weeks of average opioid abstinence with standard deviations 1.19 and 3.46, respectively. This results in effect size of 1.12. This effect size based on pilot data is rather large, which is often the case due to limited scope of the pilot study. Thus, we took a conservative approach as suggested by Kraemer et al. [57] with moderate effect size of 0.45.
Safety endpoints. Secondary safety endpoints (AEs, SAEs, ADEs, SADEs, UADEs and device deficiencies) will be summarized using descriptive statistics.
Subgroup analysis. The analysis will stratify by sex (male/female) and non-opioid SUD (presence/absence). The expected outcome is that allocation on those variables will be balanced between the two groups. It is not anticipated that the primary outcome (opioid abstinence without MOUD) will differ across these subgroups. If significant baseline group imbalance is detected on any variable and its correlation with outcome (≥0.30), that variable will be included as a covariate in the inferential analyses. If systematic variation is noted by any subgroups for primary or secondary outcomes, further exploration will be conducted via cluster analysis.
3. Results
The Wayne State University Institutional Review Board approved this trial August 23, 2021. The electronic data capture system for this study was established in early November 2021. This trial enrolled the first participant November 24, 2021, and is expected to conclude follow-up in Spring 2023.
It was decided a priori not to perform interim analysis of the data.
4. Discussion
The opioid epidemic has worsened, current MOUD treatments are under-utilized and not universally effective, and some patients seek non-medication approaches to aid their recovery from OUD. Novel approaches are needed, but must be evaluated for safety and efficacy. The NET Device is a non-pharmacological, self-administered intervention under investigation for treating persons with OUD. This sham-controlled, randomized, blinded trial is assessing the efficacy of NET primarily on outpatient opioid abstinence (independent of MOUDs), and secondarily on outpatient non-opioid drug abstinence, as well as safety. Findings from this innovative, rigorous trial will inform the development of this approach.
The trial design was predicated on observations that, despite the established efficacy of MOUDs, some patients choose to discontinue medication [[26], [27], [28], [29], [30],58] and many treatment facilities under-utilize MOUD treatment [59]. Also, some patients seek non-pharmacological treatment options [[23], [24], [25]], and there is growing scientific interest in the potential value of developing medical devices for treating OUD [60]. To provide alternative options to patients, ethically-responsible controlled trials are needed to evaluate the efficacy of medical devices while allowing use of medications (e.g. if the patient changes his/her mind). We considered a study design where NET treatment is delivered (active and sham) as adjunctive therapy for participants receiving MOUD. Such a design would avoid exposing the vulnerable population in the sham condition to ineffective treatment. Such a design, however, would expose the active population to stimulation concurrent with MOUD. This is contrary to recommended use of FDA-approved percutaneous nerve stimulators for substance use disorders. To minimize the impact of ineffective treatment on the sham population, participants may stop device treatment and may request TAU at any time after 1 h has elapsed.
Furthermore, we recognize that NET Device stimulation is limited to the first week of a 4-week inpatient stay, whereas the primary efficacy endpoint is opioid abstinence without MOUD during the post-discharge 12-week outpatient period. Maximum stimulation duration of 7 days was selected based on self-administration and withdrawal severity data from open-label studies in Kentucky and Scotland (Fig. 1). Anecdotal clinician reports suggest that stimulation beyond the point of perceived benefit results in patient irritation and dissatisfaction. We did not provide devices to study participants on an outpatient basis for two reasons: (1) manufacturing and supply limitations, and (2) prior open-label studies only provided device access during the acute opioid discontinuation period.
If it can be demonstrated that patients with OUD who seek non-MOUD treatment can benefit from this intervention by improved rates of opioid abstinence, then NET could be supplied to these individuals with appropriate monitoring. Whereas MOUD treatment has become standard-of-care, underutilization of these therapies and findings that some patients do not achieve abstinence, drop out from MOUD treatment, or seek alternatives, implies that additional options could be useful.
The secondary endpoint of non-opioid drug abstinence explores the impact of treatment on non-opioid substance use for individuals with primary OUD. Different electronic waveforms corresponding to subtypes of polysubstance use have been iterated based on human and animal studies, and from clinical observation. These waveforms are programmed into the device at treatment entry and vary based on drug screen results at the time of treatment initiation. If it can be demonstrated that active NET is superior to sham for promoting non-opioid drug abstinence, this would also be a major benefit because we presently have very limited FDA-approved therapies for treating these other conditions. Polysubstance use is the norm rather than the exception in patients with primary OUD; thus, any intervention that could be used alone, or combined with other approved therapies, could play an important role in promoting recovery from substance use-related problems.
The duration of the outpatient trial period (12 weeks) is relatively brief for the chronic, relapsing condition of OUD, but captures the period of greatest vulnerability to relapse following discharge from inpatient detoxification. If it can be demonstrated that NET produces superior outcomes to sham over this interval, then future studies would examine longer-term outcomes.
Plans are also underway to evaluate neurochemical mechanisms of action that may underlie the efficacy of NET. Understanding the mode of action of this intervention could provide useful data for determining whether it may complement other types of interventions including MOUDs.
In conclusion, this pivotal randomized controlled trial of the NET Device will provide timely and important evidence to fill a gap in the treatment of OUD.
Authors’ contributions (CRediT roles)
MKG: Conceptualization; Investigation; Methodology; Project administration; Supervision; Writing – original draft and editing. SG: Conceptualization; Data curation; Formal analysis; Writing – review and editing. JRW: Conceptualization; Data curation; Funding; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization (figure creation); Writing – review and editing.
Funding
This trial is funded solely by NET Recovery Corp. MKG effort is supported by the Gertrude Levin Endowed Chair in Addiction and Pain Biology, and Michigan Department of Health and Human Services (Lycaki/Young Funds).
Availability of data and materials
Requests for data required to support the protocol will be evaluated by the sponsor and provided if deemed appropriate. All study-related information will be regarded as confidential.
Ethics approval
This study is conducted in full accordance with all US federal and state laws and regulations including 45 Code of Federal Regulations (CFR) 46, HIPAA Privacy Rule, and approval of the Wayne State University Institutional Review Board. Any episode of noncompliance will be documented. The Investigator will perform the study in accordance with this protocol and will report unexpected problems in accordance with all federal and IRB requirements. Collection, recording, and reporting of data will be accurate and will ensure the privacy, health, and welfare of research participants during and after the study.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: MKG consults for Indivior Inc, which makes buprenorphine products. Indivior played no role in this study. SG declares no competing interests. JRW is chief executive officer and co-founder of NET Recovery Corp., which sponsors this study.
Acknowledgements
NET Recovery Corp. employees Myrrh Winston, Eric Moyer, Owen Fielding, and Lorne Patterson contributed to the implementation and execution of the study procedures. Cheryl Lockett (BlueVector Limited) is the independent study monitor. The authors thank staff members at the treatment facility for their many contributions to the study. Finally, we thank study participants for their cooperation and assistance.
References
- 1.Centers for Disease Control and Prevention . 2021. Increase in Fatal Drug Overdoses across the United States Driven by Synthetic Opioids before and during the COVID-19 Pandemic.https://emergency.cdc.gov/han/2020/pdf/CDC-HAN-00438.pdf (Aug 1) [Google Scholar]
- 2.Degenhardt L., Charlson F., Mathers B., et al. The global epidemiology and burden of opioid dependence: results from the global burden of disease 2010 study. Addiction. 2014;109:1320–1333. doi: 10.1111/add.12551. [DOI] [PubMed] [Google Scholar]
- 3.Gomes T., Mamdani M.M., Dhalla I.A., Cornish S., Paterson J.M., Juurlink D.N. The burden of premature opioid-related mortality. Addiction. 2014;109:1482–1488. doi: 10.1111/add.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphreys K., Shover C.L., Andrews C.M., et al. Responding to the opioid crisis in North America and beyond: recommendations of the Stanford-Lancet commission. Lancet. 2022 Feb 5;399(10324):555–604. doi: 10.1016/S0140-6736(21)02252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCance-Katz E.F. 2018. SAMHSA/HHS: an Update on the Opioid Crisis.https://www.samhsa.gov/sites/default/files/aatod_2018_final.pdf [Google Scholar]
- 6.Frank R.G., Pollack H.A. Addressing the fentanyl threat to public health. N. Engl. J. Med. 2017;376:605–607. doi: 10.1056/NEJMp1615145. [DOI] [PubMed] [Google Scholar]
- 7.Jones C.M., Bekheet F., Park J.N., Alexander G.C. The evolving overdose epidemic: synthetic opioids and rising stimulant-related harms. Epidemiol. Rev. 2020;42:154–166. doi: 10.1093/epirev/mxaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ochalek T.A., Parker M.A., Higgins S.T., Sigmon S.C. Fentanyl exposure among patients seeking opioid treatment. J. Subst. Abuse Treat. 2019;96:23–25. doi: 10.1016/j.jsat.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciccarone D. The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Curr. Opin. Psychiatr. 2021;34:344–350. doi: 10.1097/YCO.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frost M.C., Lampert H., Tsui J.I., Iles-Shih M.D., Williams E.C. The impact of methamphetamine/amphetamine use on receipt and outcomes of medications for opioid use disorder: a systematic review. Addiction Sci. Clin. Pract. 2021;16:62. doi: 10.1186/s13722-021-00266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Society of Addiction Medicine . 2020. Caring for Patients during the COVID-19 Task Force.https://www.asam.org/quality-care/clinical-guidelines/covid [Google Scholar]
- 12.Henderson R., McInnes A., Mackey L., et al. Opioid use disorder treatment disruptions during the early COVID-19 pandemic and other emergent disasters: a scoping review addressing dual public health emergencies. BMC Publ. Health. 2021;21:1471. doi: 10.1186/s12889-021-11495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melamed O.C., deRuiter W.K., Buckley L., Selby P. Coronavirus disease 2019 and the impact on substance use disorder treatments. Psychiatr. Clin. 2022;45:95–107. doi: 10.1016/j.psc.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niles J.K., Gudin J., Radcliff J., Kaufman H.W. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020. Popul. Health Manag. 2021;24(S1):S43–S51. doi: 10.1089/pop.2020.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florence C., Luo F., Rice K. The economic burden of opioid use disorder and fatal opioid overdose in the United States, 2017. Drug Alcohol Depend. 2021;218 doi: 10.1016/j.drugalcdep.2020.108350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant B.F., Saha T.D., Ruan W.J., et al. Epidemiology of DSM-5 drug use disorder: results from the national epidemiologic survey on alcohol and related conditions-III. JAMA Psychiatr. 2016;73:39–47. doi: 10.1001/jamapsychiatry.2015.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saloner B., Karthikeyan S. Changes in substance abuse treatment use among individuals with opioid use disorders in the United States, 2004-2013. JAMA. 2015;314:1515–1517. doi: 10.1001/jama.2015.10345. [DOI] [PubMed] [Google Scholar]
- 18.Substance Abuse and Mental Health Services Administration . Center for Behavioral Health Statistics and Quality; Rockville, MD: 2021. Key Substance Use and Mental Health Indicators in the United States: Results from the 2020 National Survey on Drug Use and Health (HHS Publication No. PEP21-07-01-003, NSDUH Series H-56)https://www.samhsa.gov/data/ Substance Abuse and Mental Health Services Administration. [Google Scholar]
- 19.Hall N.Y., Le L., Majmudar I., Mihalopoulos C. Barriers to accessing opioid substitution treatment for opioid use disorder: a systematic review from the client perspective. Drug Alcohol Depend. 2021;221 doi: 10.1016/j.drugalcdep.2021.108651. [DOI] [PubMed] [Google Scholar]
- 20.Mackey K., Veazie S., Anderson J., Bourne D., Peterson K. Barriers and facilitators to the use of medications for opioid use disorder: a rapid review. J. Gen. Intern. Med. 2020;35(Suppl 3):954–963. doi: 10.1007/s11606-020-06257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madras B.K., Ahmad N.J., Wen J., Sharfstein J. 2020. Improving Access to Evidence-Based Medical Treatment for Opioid Use Disorder: Strategies to Address Key Barriers within the Treatment System. the Prevention, Treatment and Recovery Working Group of the Action Collaborative on Countering the U.S. Opioid Epidemic. NAM Perspectives. Discussion paper, Washington, DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Academy of Sciences . The National Academies Press; Washington, DC: 2019. Medications for Opioid Use Disorder Save Lives. Engineering, and Medicine. [DOI] [PubMed] [Google Scholar]
- 23.Jones C.M., Campopiano M., Baldwin G., McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am. J. Publ. Health. 2015;105:e55–e63. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krawczyk N., Jent V., Hadland S.E., Cérda M. Utilization of medications for opioid use disorder across US states: relationship to treatment availability and overdose mortality. J. Addiction Med. 2022;16:114–117. doi: 10.1097/ADM.0000000000000820. [DOI] [PubMed] [Google Scholar]
- 25.Wakeman S.E., Larochelle M.R., Ameli O., et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2019.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosten T.R., Baxter L.E. Effective management of opioid withdrawal symptoms: a gateway to opioid dependence treatment. Am. J. Addict. 2019;28:55–62. doi: 10.1111/ajad.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larney S.L., Zador D., Sindicich N., Dolan K. A qualitative study of reasons for seeking ceasing opioid substitution treatment in prisons in New South Wales, Australia. Drug Alcohol Rev. 2017;36:305–310. doi: 10.1111/dar.12442. [DOI] [PubMed] [Google Scholar]
- 28.Muthulingam D., Bia J., Madden L.M., Farnum S.O., Barry D.T., Altice F.L. Using nominal group technique to identify barriers, facilitators, and preferences among patients seeking treatment for opioid use disorder: a needs assessment for decision making support. J. Subst. Abuse Treat. 2019;100:18–28. doi: 10.1016/j.jsat.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein M.D., Anderson B.J., Bailey G.L. Preferences for aftercare among persons seeking short-term opioid detoxification. J. Subst. Abuse Treat. 2015;59:99–103. doi: 10.1016/j.jsat.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uebelacker L.A., Bailey G., Herman D., Anderson B., Stein M. Patients' beliefs about medications are associated with stated preference for methadone, buprenorphine, naltrexone, or no medication-assisted therapy following inpatient opioid detoxification. J. Subst. Abuse Treat. 2016;66:48–53. doi: 10.1016/j.jsat.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gariti P., Auriacombe M., Incmikoski R., et al. A randomized double-blind study of NeuroElectric Therapy in opiate and cocaine detoxification. J. Subst. Abuse. 1992;4:299–308. doi: 10.1016/0899-3289(92)90037-x. [DOI] [PubMed] [Google Scholar]
- 32.Patterson M.A. Acupuncture and neuro-electric therapy in the treatment of alcohol and drug addictions. Austral. J. Alcohol Drug Depend. 1975;2:90–95. [Google Scholar]
- 33.Patterson M.A. Electrostimulation and opiate withdrawal. Br. J. Psychiatry. 1985;146:213. doi: 10.1192/bjp.146.2.213. [DOI] [PubMed] [Google Scholar]
- 34.Patterson M.A., Patterson L., Patterson S.I. Electrostimulation: addiction treatment for the coming millennium. J. Alternative Compl. Med. 1996;2:485–491. doi: 10.1089/acm.1996.2.485. [DOI] [PubMed] [Google Scholar]
- 35.Alling F.A., Johnson B.D., Elmoghazy E. Cranial electrostimulation (CES) use in the detoxification of opiate-dependent patients. J. Subst. Abuse Treat. 1990;7:173–180. doi: 10.1016/0740-5472(90)90019-m. [DOI] [PubMed] [Google Scholar]
- 36.Auriacombe M., Tignol J., Le Moal M., Stinus L. Transcutaneous electrical stimulation with Limoge current potentiates morphine analgesia and attenuates opiate abstinence syndrome. Biol. Psychiatr. 1990;28:650–656. doi: 10.1016/0006-3223(90)90451-7. [DOI] [PubMed] [Google Scholar]
- 37.Dougherty P.M., Dafny N. Trans-cranial electrical stimulation attenuates the severity of naloxone-precipitated morphine withdrawal in rats. Life Sci. 1989;44:2051–2056. doi: 10.1016/0024-3205(89)90351-2. [DOI] [PubMed] [Google Scholar]
- 38.Ellison F., Ellison W., Daulouede J.P., et al. Opiate withdrawal and electro-stimulation. Double blind experiments. Encephale. 1987;13:225–229. [PubMed] [Google Scholar]
- 39.Meade C.S., Lukas S.E., McDonald L.J., et al. A randomized trial of transcutaneous acupoint electric stimulation as adjunctive treatment for opioid detoxification. J. Subst. Abuse Treat. 2010;38:12–21. doi: 10.1016/j.jsat.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng R.S., Pomeranz B. Electroacupuncture analgesia could be mediated by at least two pain-relieving mechanisms: endorphin and non-endorphin systems. Life Sci. 1979;25:1957–1962. doi: 10.1016/0024-3205(79)90598-8. [DOI] [PubMed] [Google Scholar]
- 41.Han J.S., Chen X.H., Sun S.L., et al. Effect of low- and high-frequency TENS on Met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain. 1991;47:295–298. doi: 10.1016/0304-3959(91)90218-M. [DOI] [PubMed] [Google Scholar]
- 42.Cheng L.-L., Ding M.-X., Xiong C., Zhou M.-Y., Qiu Z.-Y., Wang Q. Effects of electroacupuncture of different frequencies on the release profile of endogenous opioid peptides in the central nerve system of goats. Evid. Based Complement. Alternative Med. 2012 doi: 10.1155/2012/476457. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diana M., Muntoni A.L., Pistis M., Melis M., Gessa G.L. Lasting reduction in mesolimbic dopamine neuronal activity after morphine withdrawal. Eur. J. Neurosci. 1999;11:1037–1041. doi: 10.1046/j.1460-9568.1999.00488.x. [DOI] [PubMed] [Google Scholar]
- 44.García-Pérez D., López-Bellido R., Rodríguez R.E., Laorden M.L., Núnez C., Milanés M.V. Dysregulation of dopaminergic regulatory mechanisms in the mesolimbic pathway induced by morphine and morphine withdrawal. Brain Struct. Funct. 2015;220:1901–1919. doi: 10.1007/s00429-014-0761-5. [DOI] [PubMed] [Google Scholar]
- 45.Gardner E.L. Addiction and brain reward and antireward. Adv. Psychosom. Med. 2011;30:22–60. doi: 10.1159/000324065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Georges F., Stinus L., Bloch B., Le Moine C. Chronic morphine exposure and spontaneous withdrawal are associated with modifications of dopamine receptor and neuropeptide gene expression in the rat striatum. Eur. J. Neurosci. 1999;11:481–490. doi: 10.1046/j.1460-9568.1999.00462.x. [DOI] [PubMed] [Google Scholar]
- 47.Koob G.F. Hedonic homeostatic dysregulation as a driver of drug-seeking behavior. Drug Discov. Today Dis. Models. 2008;5:207–215. doi: 10.1016/j.ddmod.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi J., Li S.-X., Zhang X.-L., et al. Time-dependent neuroendocrine alterations and drug craving during the first month of abstinence in heroin addicts. Am. J. Drug Alcohol Abuse. 2009;35:267–272. doi: 10.1080/00952990902933878. [DOI] [PubMed] [Google Scholar]
- 49.Shi W., Zhang Y., Zhao G., et al. Dysregulation of dopaminergic regulatory factors TH, Nurr1, and Pitx3 in the ventral tegmental area associated with neuronal injury induced by chronic morphine dependence. Int. J. Mol. Sci. 2019;20:250. doi: 10.3390/ijms20020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cirillo G., Di Pino G., Capone F., et al. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017;10:1–18. doi: 10.1016/j.brs.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Paulus W. Transcranial electrical stimulation (tES – tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil. 2011;21:602–617. doi: 10.1080/09602011.2011.557292. [DOI] [PubMed] [Google Scholar]
- 52.Huang Y.-Z., Lu M.-K., Antal A., et al. Plasticity induced by non-invasive transcranial brain stimulation: a position paper. Clin. Neurophysiol. 2017;128:2318–2329. doi: 10.1016/j.clinph.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 53.He W., Fong P.-Y., Leung T.W.H., et al. Protocols of non-invasive brain stimulation for neuroplasticity induction. Neurosci. Lett. 2020;719 doi: 10.1016/j.neulet.2018.02.045. [DOI] [PubMed] [Google Scholar]
- 54.Wesson D.R., Ling W. Clinical opiate withdrawal Scale (COWS) J. Psychoact. Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- 55.Miranda A., Taca A. Neuromodulation with percutaneous electrical nerve field stimulation is associated with reduction in signs and symptoms of opioid withdrawal: a multisite, retrospective assessment. Am. J. Drug Alcohol Abuse. 2018;44:56–63. doi: 10.1080/00952990.2017.1295459. [DOI] [PubMed] [Google Scholar]
- 56.O'Neill R.T. Secondary endpoints cannot be validly analyzed if the primary endpoint does not demonstrate clear statistical significance. Control. Clin. Trials. 1997;18:550–556. doi: 10.1016/s0197-2456(97)00075-5. [DOI] [PubMed] [Google Scholar]
- 57.Kraemer H.C., Mintz J., Noda A., et al. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch. Gen. Psychiatr. 2006;63:484–489. doi: 10.1001/archpsyc.63.5.484. [DOI] [PubMed] [Google Scholar]
- 58.Kleber H.D. Pharmacological treatments for opioid dependence: detoxification and maintenance options. Dialogues Clin. Neurosci. 2007;9:455–470. doi: 10.31887/DCNS.2007.9.2/hkleber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma A., Kelly S.M., Mitchell S.G., et al. Update on barriers to pharmacotherapy for opioid use disorders. Curr. Psychiatr. Rep. 2017;19:35. doi: 10.1007/s11920-017-0783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Comer S.D., Dworkin R.H., Strain E.C. Medical devices to prevent opioid use disorder. Innovative approaches to addressing the opioid crisis. JAMA Psychiatr. 2019;76:351–352. doi: 10.1001/jamapsychiatry.2018.4379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests for data required to support the protocol will be evaluated by the sponsor and provided if deemed appropriate. All study-related information will be regarded as confidential.