Key Points
Question
What was the prevalence of dementia and mild cognitive impairment (MCI) in the US in 2016?
Findings
This nationally representative cross-sectional study found that approximately one-third of 3496 individuals 65 years and older had dementia or MCI. Prevalence rates were similar by sex but varied by age, education, and race and ethnicity.
Meaning
The results suggest there may be disparities in dementia and MCI among Black and Hispanic older adults and people with lower educational attainment.
This cross-sectional study estimates the prevalence of dementia and mild cognitive impairment in the US in 2016 by age, race, ethnicity, and sex.
Abstract
Importance
Nationally representative data are critical for understanding the causes, costs, and outcomes associated with dementia and mild cognitive impairment (MCI) in the US and can inform policies aimed at reducing the impact of these conditions on patients, families, and public programs. The nationally representative Health and Retirement Study (HRS) is an essential resource for such data, but the HRS substudy providing dementia diagnostic information was fielded more than 20 years ago and more recent data are needed.
Objective
The Harmonized Cognitive Assessment Protocol (HCAP) was developed to update national estimates of the prevalence of MCI and dementia in the US and examine differences by age, race, ethnicity, and sex.
Design, Setting, and Participants
HRS is an ongoing longitudinal nationally representative study of people 51 years and older with staggered entry dates from 1992 to 2022 and follow-up ranging from 4 to 30 years. HCAP is a cross-sectional random sample of individuals in HRS who were 65 years or older in 2016. Of 9972 age-eligible HRS participants, 4425 were randomly selected for HCAP, and 3496 completed a comprehensive neuropsychological test battery and informant interview, none of whom were excluded. Dementia and MCI were classified using an algorithm based on standard diagnostic criteria and comparing test performance to a robust normative sample.
Exposures
Groups were stratified by age, sex, education, race, and ethnicity.
Main Outcomes and Measures
National prevalence estimates using population weights.
Results
The mean (SD) age of the study population sample (N = 3496) was 76.4 (7.6) years, and 2095 participants (60%) were female. There were 551 participants who self-identified as Black and not Hispanic (16%), 382 who self-identified as Hispanic regardless of race (16%), 2483 who self-identified as White and not Hispanic (71%), and 80 who self-identified as another race (2%), including American Indian or Alaska Native, Asian, Native Hawaiian or Pacific Islander, or another self-described race. A total of 393 individuals (10%; 95% CI, 9-11) were classified as having dementia and 804 (22%; 95% CI, 20-24) as having MCI. Every 5-year increase in age was associated with higher risk of dementia (weighted odds ratio [OR], 1.95 per 5-year age difference; 95%, CI, 1.77-2.14) and MCI (OR, 1.17 per 5-year age difference, 95% CI, 1.09-1.26). Each additional year of education was associated with a decrease in risk of dementia (OR, 0.93 per year of school, 95% CI, 0.89-0.97) and MCI (OR, 0.94, 95% CI, 0.91-0.97). Dementia was more common among non-Hispanic Black individuals (OR, 1.81; 95% CI, 1.20-2.75) and MCI in Hispanic individuals (OR, 1.42; 95% CI, 1.03-1.96) compared with non-Hispanic White individuals. Other group comparisons by race and ethnicity were not possible owing to small numbers. No differences in prevalence were found between female individuals and male individuals.
Conclusions and Relevance
Using a comprehensive neuropsychological test battery and large sample, the national prevalence of dementia and MCI in 2016 found in this cross-sectional study was similar to that of other US-based studies, indicating a disproportionate burden of dementia and MCI among older Black and Hispanic adults and those with lower education.
Introduction
Dementia is a highly prevalent condition characterized by cognitive difficulties that typically begin in adulthood and affect a person’s ability to independently perform everyday activities. Alzheimer disease is the most common cause of dementia, accounting for approximately 60% to 80% of all dementia cases.1,2 Mild cognitive impairment (MCI) is a clinical classification assigned to people who are thought to be transitioning between normal aging and dementia.3,4,5 Because age is the most potent risk factor for dementia and MCI, the number of adults with these conditions is projected to rise dramatically in the US and around the world due to demographic trends that have transformed populations from mostly young adults to mostly older adults.2 As dementia progresses, individuals need increased supervision and help making decisions and may ultimately require full-time long-term care. The impact of dementia and MCI on older adults and their families, health care capacity and costs, and care and services for people with dementia and their caregivers poses a considerable challenge to future social and financial well-being across the globe. The economic impact of dementia, including the large burden of unpaid family caregiving, has been estimated at $257 billion per year in the US2 and $800 billion worldwide.6
Based on a community cohort of older adults in Chicago, Illinois, the prevalence of dementia due to Alzheimer disease in the US in 2021 was estimated at 11.3% of those 65 years and older,1,2 which translates to about 6.2 million adults. Other recent estimates have been somewhat lower,7,8,9,10 likely related to differences in study sampling methods, setting-specific social and structural inequalities in risk and prevalence, and diagnostic criteria used.11 Similarly, different implementation of MCI criteria and cohort characteristics drive variability in MCI prevalence and rates of progression to dementia across cohorts.12,13,14,15 It is well established that annual progression rates of MCI to dementia in population- or community-based studies are lower (4% to 15%) than in clinic-based studies (12% to 17%).
The Health and Retirement Study (HRS)16 provides a core resource for researchers who require US population-level data on dementia prevalence and incidence, as well as risk factors, care, costs, and other outcomes associated with dementia and MCI. The critical role of this resource is reflected in the hundreds of published studies that used data and dementia classifications from the Aging, Demographics, and Memory Study (ADAMS),17 which administered a comprehensive neuropsychological battery and informant measures to a subset of 856 HRS participants 71 years and older. ADAMS data were used to estimate prevalence of dementia in the US18 and calibrated to brief measures administered in the HRS core to develop cutoff points for individuals with cognitive impairment but not dementia and those with dementia who had the same population distribution of cognitive states estimated by ADAMS.19 ADAMS was fielded between 2001 and 2003 and, because of trends in dementia prevalence20 and incidence,21 should be updated. The ADAMS substudy was small, and the limited inclusion of Black, Hispanic, and American Indian or Alaska Native participants contributed to lack of precision of estimates among minoritized racial and ethnic groups that have been shown to experience a higher burden of cognitive impairment and dementia.22
To update nationally representative estimates of the prevalence of MCI and dementia in the US, improve on the design of ADAMS, and establish the methods and data infrastructure for calibration to the larger HRS cohort, we developed the Harmonized Cognitive Assessment Protocol (HCAP). HCAP has been implemented in a number of HRS international partner studies to facilitate international comparisons of the burden of cognitive decline and dementia in countries around the world.23 The goal of the current study was to use the HCAP assessment to provide national estimates of the prevalence of MCI and dementia in the US in 2016 and examine differences in prevalence by age, race, ethnicity, and sex.
Methods
Overview
Details of HCAP have been previously published23,24 and are available at the HRS website.25 Briefly, neuropsychological measures and informant reports were piloted, then selected to comprehensively characterize cognitive function across multiple cognitive domains and detect cognitive decline in older adults with the potential for harmonization to other ongoing longitudinal studies of cognition and aging as well as adaptation to other countries and languages.26 Participants were randomly selected from the HRS cohort that completed the core survey in 2016. For this cross-sectional study, the HCAP battery was administered to participants in English or Spanish, depending on their preference (proficiency in either English or Spanish is required for participation in HRS). Participants and their informants provided written informed consent to participate. The HRS and HCAP study protocols were approved by the University of Michigan Institutional Review Board. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Participants
Of 9972 age-eligible HRS participants, 4425 were randomly selected for HCAP and 3496 completed the HCAP assessment between June 2016 and October 2017 for a final response rate of 79% (eTable 1 in the Supplement). The sample for the HCAP study was randomly selected from HRS panel respondents who were 65 years or older within 2 months of the date they completed their 2016 HRS core interview. If 2 eligible people lived in 1 household, 1 was randomly selected to participate in HCAP. For every participant, an informant was selected from 1 of up to 3 individuals nominated by the respondent. Informant relationships with the respondent included spouse or partner (1438 [41%]), child (859 [25%]), friend (377 [11%]), sibling (141 [4%]), grandchild (77 [2%]), parent (45 [1%]), neighbor (27 [1%]), guardian (5 [0.1%]), or other relationship (214 [6%]). The mean (SD; range) number of years an informant reported knowing the respondent was 30 (21; 1-87). The HCAP was administered in English among 3312 participants (95%), Spanish among 178 (5%), or both among 3 (0.1%). Race and ethnicity in the HCAP study were assessed as markers of exposure to evolving systems of racism, not as a proxy for genetic variation or any other biological variable. Race and ethnicity were self-reported by participants according to options defined by the 2000 US Census. There were 149 HCAP participants where only an informant interview was collected because the HCAP respondent was not able to conduct an interview but an informant had been nominated and enrolled. A total of 313 HCAP participants were respondent-only participants where an informant had not been nominated by the respondent or where a nominated informant was not enrolled. Overall, 3033 of 3496 HCAP participants (87%) included both a respondent and informant interview.
HCAP Assessment
The HCAP assessment included a 1-hour in-person battery of neuropsychological tests and an informant interview lasting about 20 minutes (eTables 6 and 7 in the Supplement show a comprehensive list of HCAP tests and instruments). In brief, the cognitive battery was administered in the participant’s home and included measures of word list learning and memory, story recall, object naming, comprehension, semantic fluency, attention, speed, set shifting, reasoning, constructional praxis, and orientation. Subjective cognitive worsening was assessed using a single item included in the HRS core interview, where respondents were asked whether their memory was better, the same, or worse than 2 years ago. Informant questionnaires included items assessing the participant’s ability to carry out independent activities of daily living, loss of cognitive function, change in abilities over a 10-year period, and attribution of changes to mental, physical, or both mental and physical causes.
Classification of MCI and Dementia
Determination of MCI and dementia prevalence in HCAP involved 3 phases: selecting a robust normative sample (n = 1787), standardizing within the normative sample with respect to sociodemographic characteristics, and developing an algorithm for the classification of dementia status using additional informant and self-report information from all participants in the HCAP study (N = 3496). Each of these phases is described in detail in the eMethods in the Supplement.
Briefly, in the first phase, a robust normative sample was selected within HCAP to identify expected levels of cognitive performance on the battery of HCAP neuropsychological tests for each person, given their age, sex, education, race, and ethnicity.12,27 The exclusion criteria used to select the robust normative sample from the full HCAP sample are shown in eTable 5 in the Supplement. These criteria were blind to the HCAP neuropsychological test scores, but were designed to exclude from the normative sample individuals with evidence at HCAP baseline or follow-up of conditions that cause or are associated with cognitive impairment and cognitive decline, such as stroke, neurodegenerative disease, severe cognitive or functional impairment, and death.
In the second phase, performance on the HCAP neuropsychological test battery was calibrated using latent variable models described previously24 and was standardized according to demographic variables. Briefly, we subjected test scores (N = 3347) to a series of factor analysis models and derived factors and factor score estimates based on HCAP performance in 5 domains: memory, executive functioning, language, visuospatial, and orientation (eTable 4 in the Supplement). Factor scores were normalized and then standardized with respect to demographic variables, and t scores with a mean (SD) of 50 (10) were calculated.
In the third phase, a classification algorithm was derived for MCI and dementia using informant report of cognitive or functional impairment or self-report of subjective memory worsening (eTable 8 in the Supplement). Classification of MCI and dementia and MCI were based on diagnostic criteria from the National Institute on Aging and Alzheimer’s Association workgroups.3,28 Classification of dementia required that at least 2 cognitive domains were below thresholds for impairment (>1.5 SDs below the mean or a t score of 35) and an informant report of functional impairment. People who did not meet criteria for cognitive impairment in any domain were classified as undergoing normal aging. If 1 cognitive domain was in the impaired range, people were classified as undergoing normal aging only if their informant did not report concerns about their function and they had no self-reported cognitive concerns. All other participants with at least 1 impaired cognitive domain who were not classified as having dementia were classified as having MCI.
Statistical Analyses
The national prevalence of dementia and MCI were estimated using sampling weights for the HCAP sample derived from HRS population weights (eMethods and eTables 2 and 3 in the Supplement) for adults 65 years and older within categories stratified by age, sex, race, ethnicity, and educational attainment. Logistic regression analyses estimated the likelihood of dementia and MCI as a function of age (where people aged 65 to 74 years were the reference group), sex (with male individuals as the reference group), race and ethnicity (with White participants as the reference group), and education (with participants with college degrees or higher as the reference group). All statistical analyses were conducted with Stata version 16.1 (Stata Corp) and R version 4.1.1 (R Foundation). Sampling weights were used in statistical analyses, and summary statistics that reflect the use of sampling weights are highlighted in the results. Missing values for cognitive performance data or informant data were singly imputed as bayesian plausible values using Mplus software version 8.2 (Muthén & Muthén).29 Language of administration was not considered formally in the current analyses but is being considered in ongoing harmonization activities for HCAP.
Results
The mean (SD) age of the study population sample (N = 3496) was 76.4 (7.6) years, and 2095 participants (60%) were female. There were 551 participants who self-identified as Black and not Hispanic (16%), 382 who self-identified as Hispanic regardless of race (16%), 2483 who self-identified as White and not Hispanic (71%), and 80 who self-identified as another race (2%), including American Indian or Alaska Native, Asian, Native Hawaiian or Pacific Islander, or another self-described race. Due to small sample sizes and risk of identification, HRS uses a pooled category for people in these groups. Participants who were selected for the robust norms sample were younger, more likely to be non-Hispanic White, had more years of school, and had higher MMSE scores than participants who were not selected; however, both subgroups were inclusive of people across the range of demographic characteristics (Table 1).
Table 1. Participant Characteristics.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Total sample | Robust norms sample | Not in robust norms sample | |
| Total | 3496 (100) | 1787 (100) | 1709 (100) |
| Age, mean (SD), y | 76.4 (7.6) | 73.9 (6.3) | 79 (7.9) |
| Female | 2095 (60) | 1074 (60) | 1021 (60) |
| Male | 1401 (40) | 713 (40) | 688 (40) |
| Race and ethnicitya | |||
| Black | 551 (16) | 265 (15) | 286 (17) |
| Hispanic | 382 (11) | 178 (10) | 204 (12) |
| White | 2484 (71) | 1300 (73) | 1184 (69) |
| Otherb | 79 (2) | 44 (2) | 35 (2) |
| Years of education, mean (SD) | 12.7 (3.2) | 13.3 (2.8) | 12 (3.4) |
| MMSE score, 0-30, mean (SD)c | 26.6 (3.9) | 27.9 (2.1) | 25.1 (4.8) |
Abbreviation: MMSE, Mini-Mental State Examination.
Race and ethnicity data were gathered via self-report at the time of first interview and are considered to be markers of exposure to evolving systems of racism, not as a proxy for genetic variation or any other biological variable. Race was self-selected by participants at the time of the first interview from a list of options defined by the 2000 US Census criteria.
Other includes a pooled group of participants who identified as American Indian or Alaska Native, Asian, Native Hawaiian or Pacific Islander, or another self-described race, consolidated due to small sample sizes and risk of identification.
Higher scores indicate better cognitive function.
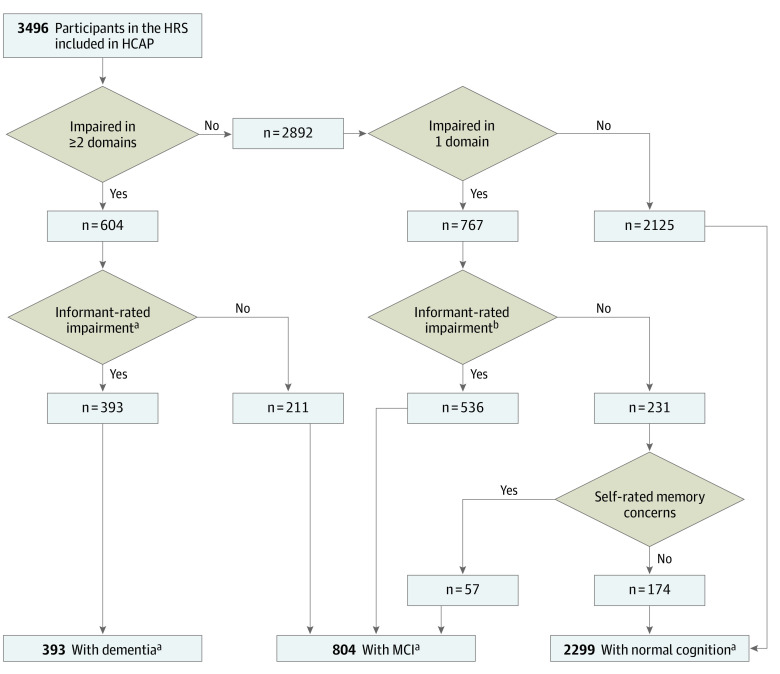
The results of the classification algorithm are displayed in the Figure and detailed in eTable 9 in the Supplement. A total of 393 individuals (weighted percentage, 10%; 95% CI, 9-11) (mean [SD] age, 82.3 [7.4] years; 243 female [62%] and 150 male [38%]) were classified as having dementia, and 804 (weighted percentage, 22%; 95% CI, 20-24) individuals were classified as having MCI (mean [SD] age, 76.8 [7.8] years; 474 female [59%] and 330 male [41%]).
Figure. Flow Diagram Showing the Harmonized Cognitive Assessment Protocol (HCAP) Classification Algorithm for Dementia and Mild Cognitive Impairment (MCI) .
HCAP sampling weights were used to determine percentages within each diagnostic classification. HRS indicates the Health and Retirement Study.
aJorm Informant Questionnaire on Cognitive Decline in the Elderly score 3.4 or greater or Blessed Dementia Rating Scale Part I score 2 or greater.
bJorm Informant Questionnaire on Cognitive Decline in the Elderly score greater than 3.0 or Blessed Dementia Rating Scale Part I score greater than 0.
Numbers and weighted estimates are presented for the classification of dementia and MCI (Table 2) among all persons recruited to HCAP. People with dementia were older (weighted odds ratio [OR], 1.95 per 5-year age difference; 95% CI, 1.77-2.14), attended fewer years of school (weighted and age-adjusted OR, 0.93 per year of school; 95% CI, 0.89-0.97), and were more likely to be Black than White (weighted and age-adjusted OR, 1.81 for Black; 95% CI, 1.20-2.75). MCI was more frequent in people who were older (weighted OR, 1.17 per 5-year age difference; 95% CI, 1.09-1.26), had fewer years of school (weighted and age-adjusted OR, 0.94 per year of school; 95% CI, 0.91-0.97), and Hispanic (weighted and age-adjusted OR, 1.42 for Hispanic vs White; 95% CI, 1.03-1.96).
Table 2. Group Differences in Prevalence of Dementia and Mild Cognitive Impairment (MCI) Among Harmonized Cognitive Assessment Protocol (HCAP) Participants.
| Variable | Total | Dementia | MCI | ||||
|---|---|---|---|---|---|---|---|
| Observed No. | %a (95% CI) | ORb (95% CI) | Observed No. | %a (95% CI) | ORb (95% CI) | ||
| Overall | 3496 | 393 | 10 (9-11) | NA | 804 | 22 (20-24) | NA |
| Age group, y | |||||||
| 65-69 | 821 | 25 | 3 (1-4) | 1 [Reference] | 186 | 22 (18-25) | 1 [Reference] |
| 70-74 | 667 | 31 | 4 (2-6) | 1.4 (0.8-2.7) | 138 | 20 (17-24) | 0.9 (0.7-1.3) |
| 75-79 | 844 | 76 | 9 (6-11) | 3.3 (1.8-5.8) | 194 | 21 (18-24) | 1.0 (0.7-1.3) |
| 80-84 | 611 | 105 | 18 (14-22) | 7.6 (4.3-13.3) | 156 | 25 (21-29) | 1.2 (0.9-1.7) |
| 85-89 | 345 | 84 | 26 (20-31) | 11.9 (6.7-21.2) | 78 | 22 (17-27) | 1.0 (0.7-1.5) |
| ≥90 | 208 | 72 | 35 (28-43) | 18.8 (10.3-34.4) | 52 | 27 (20-35) | 1.4 (0.9-2.1) |
| Sexc | |||||||
| Female | 2095 | 243 | 10 (9-11) | 1.1 (0.8-1.4) | 474 | 22 (19-24) | 0.9 (0.8-1.2) |
| Male | 1401 | 150 | 10 (8-11) | 1 [Reference] | 330 | 22 (20-25) | 1 [Reference] |
| Race and ethnicityc,d | |||||||
| Black | 551 | 63 | 15 (10-19) | 1.8 (1.2-2.7) | 126 | 22 (17-27) | 1.0 (0.8-1.4) |
| Hispanic | 382 | 43 | 10 (7-13) | 1.1 (0.7-1.7) | 112 | 28 (22-34) | 1.4 (1.0-2.0) |
| White | 2484 | 264 | 11 (10-13) | 1 [Reference] | 566 | 23 (21-25) | 1 [Reference] |
| Othere | 79 | 12 | 26 (13-39) | 3.3 (1.4-7.6) | 26 | 45 (30-59) | 2.7 (1.5-5.1) |
| Educational attainmentc | |||||||
| <High school | 715 | 111 | 13 (10-16) | 1.6 (1.1-2.3) | 214 | 30 (25-34) | 1.6 (1.2-2.2) |
| High school | 1166 | 128 | 9 (7-11) | 1.0 (0.7-1.4) | 234 | 19 (16-21) | 0.9 (0.7-1.2) |
| Some college | 764 | 65 | 9 (6-11) | 0.9 (0.6-1.4) | 170 | 23 (19-26) | 1.1 (0.8-1.5) |
| ≥College degree | 851 | 89 | 9 (7-11) | 1 [Reference] | 186 | 21 (17-24) | 1 [Reference] |
Abbreviations: NA, not applicable; OR, odds ratio.
Indicates prevalence of dementia or MCI in variable category, estimated with sampling weights.
ORs estimated with sampling weights.
Marginal prevalence estimates for sex, race and ethnicity, and educational attainment reflect adjustment for age.
Race and ethnicity data were gathered via self-report at the time of first interview and are considered to be markers of exposure to evolving systems of racism, not as a proxy for genetic variation or any other biological variable. Race was self-selected by participants at the time of the first interview from a list of options defined by the 2000 US Census criteria.
Other includes a pooled group of participants who identified as American Indian or Alaska Native, Asian, Native Hawaiian or Pacific Islander, or another self-described race, consolidated due to small sample sizes and risk of identification.
Discussion
Using a comprehensive cognitive testing battery and informant reports in a cross-sectional nationally representative subsample of the HRS and a diagnostic classification algorithm based on National Institute on Aging and Alzheimer’s Association criteria, we estimated the prevalence of dementia and MCI among individuals 65 years and older in the US in 2016 to be 10% and 22%, respectively. Our results confirm that the burden of cognitive impairment and dementia in the US is associated with increasing age. As longevity increases and as so-called baby boomer generation ages, the burden of cognitive impairment is projected to increase in the decades ahead for individuals, families, and programs that provide care and services for people with dementia. We found similar rates of MCI and dementia in male and female individuals, which is consistent with other large studies30,31,32,33 that account for greater survival among female individuals and also apply appropriate normative expectations for neuropsychological test performance among male and female individuals.
Our findings are similar to other recent estimates of dementia prevalence in the US. For instance, data from 10 342 participants in the Chicago Health and Aging Project (CHAP)1 were used to estimate the 2020 to 2060 Census-standardized prevalence of Alzheimer disease dementia in adults 65 years and older. The overall estimated prevalence in 2020 was 11.3% (95% CI, 10.7-11.93), which translates to about 6.07 million cases of dementia in the US, somewhat higher than the 2016 HCAP estimate of about 4.92 million cases of dementia in the US. The higher CHAP estimate is likely related to diagnostic criteria in that study, which relied on cognitive test criteria only and did not require an informant report of disability.11 In HCAP, we found that older Black adults were at higher risk for dementia, but the disparity was not as striking as in the CHAP-based estimates, where dementia prevalence among Black individuals was nearly twice that among non-Hispanic White individuals.
The ADAMS study18 estimated a 2002 US dementia prevalence of 13.9% and a cognitive impairment without dementia prevalence of 22.2% among those 71 years and older .13 A number of diagnostic classification algorithms based on ADAMS have used cognitive and other data from the HRS to classify all HRS respondents across multiple waves of HRS data.34 Dementia prevalence estimates for those 65 years and older in 2012 have ranged from 8.8%20 to 10.5%7 across different HRS algorithms, a range which includes our new HCAP dementia prevalence estimate of 10% for 2016.
The overall rates of MCI in this study (22%) are roughly comparable to those in other population-based cohorts in the US.1,12,35 Few population-based samples in the US have had the statistical power to compare rates of MCI across ethnic and racial groups. In CHAP, older Black adults had a higher prevalence of MCI than older White adults.1 However, in a community-based cohort in Washington Heights in New York City, New York (WHICAP),12 no racial or ethnic differences in MCI prevalence were found. Like the current study, WHICAP used a robust norms approach for diagnosis of MCI, whereas CHAP did not. It is possible that the robust norms approach is more effective at identifying dementia in minoritized racial and ethnic groups than MCI. In addition, lower rates of survival to older ages among older Black and Hispanic adults may mask group differences in MCI.
Our estimated overall dementia prevalence rate is also comparable to recent studies that used completely different measurement strategies than the direct cognitive and functional assessment used in HCAP. Using data from the American Community Survey, a recent report36 showed a prevalence of 10% of reported serious cognitive problems among people 65 years and older in 2017, a decline from 2008, when the estimated prevalence was 12.2%. The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 201937 used meta-analytic techniques and bayesian compartmental modeling to estimate that 5.27 (95% uncertainty interval, 4.90-5.71) million people had dementia in the US in 2019, which is approximately 9.7% of the 54.1 million people older than 65 years in the US at that time.
A declining trend in dementia incidence and prevalence rates, perhaps due to rising educational attainment and better control of cardiovascular risk factors, has been reported by many studies in high-income countries around the world,21,38 including studies using HRS data.7,20 Given a leveling of educational attainment among more recent birth cohorts, as well as rising rates of cardiovascular risk factors such as obesity and diabetes, it will be important to track dementia incidence and prevalence in the years ahead to monitor a potential reversal of the favorable trends in recent decades, which would have serious implications for the burden of dementia on families and public programs. In addition, tracking whether disparities in dementia risk are closing or widening over time may help shape potential public health and public policy responses. A recent analysis39 of HRS data showed that, while overall dementia prevalence declined between 2000 and 2016, the greater risk of dementia among Black individuals compared to White individuals did not change. The HRS-HCAP assessment and data infrastructure can be used to cost-effectively track dementia incidence and prevalence over time in the US and in other countries around the world.
Estimation of dementia and MCI rates in HRS also provides a critical resource for addressing methodological issues in sampling and generalizability that are fundamental to understanding risks for cognitive impairment and costs and outcomes associated with dementia, yet these potential sources of bias are often overlooked. Selection bias can transform and in some cases reverse associations between exposures and outcomes, threatening the utility of conclusions drawn from convenience samples.40,41 The HCAP-based dementia classifications made available by this study may be valuable in transporting estimation of risk-outcome associations from small highly selected studies with neuroimaging biomarkers or neuropathology to nationally representative samples using statistical techniques like inverse odds selection weights.42
The strengths of this study include its large representative sample that facilitates estimates within groups that experience health disparities and enables tracking of national trends in cognitive impairment and dementia. This study advances neuropsychological assessment of older adults by introducing psychometric methods for determination of cognitive factors and normative adjustment. MCI and dementia status from this study can be used to predict clinically relevant outcomes via linkage to Medicare, as well as nursing home placement and mortality.
Limitations
This study has limitations. The in-person HCAP assessment was comprehensive, but not as thorough as what is typically obtained for gold-standard diagnoses in clinical settings; as a result, dementia subtype information is not available for HCAP. This is a cross-sectional study of prevalence of MCI and dementia, and thus cannot assess incidence of cognitive impairment or rates of progression among people with MCI. Prior reports from several longitudinal studies found MCI classification to be variable, with high rates of people who had reverted to normal cognition at follow-up.43,44,45,46 Without follow-up data, we were unable to examine the stability of diagnoses over time in HCAP. The cross-sectional design does not allow for examination of survival bias, which could inflate prevalence if some groups are living longer with dementia or decrease estimates in groups with higher mortality. While the HCAP sample is nationally representative, the sampling of some groups is too small to examine heterogeneity within certain groups (eg, Spanish-speaking male and female).
Conclusions
In conclusion, the HCAP study found a similar prevalence of dementia and MCI among older adults in the US to that found in other recent studies in the US. These updated dementia prevalence estimates from 2016 show a disproportionate burden of dementia among older Black adults, of MCI among older Hispanic adults, and of both among people with lower educational attainment.
eTable 1. Characteristics of the HRS-HCAP sample
eMethods
eTable 2. 2016 HCAP sampling weight descriptive statistics
eTable 3. Weighted comparison of HCAP respondents and 2016 HRS reference sample
eTable 4. Description of factor scores and performance summaries based on domains of neuropsychological functioning and cognitive test performance
eTable 5. Selection of robust normative sample from HCAP participants
eTable 6. HRS Harmonized Cognitive Assessment Protocol (HCAP) Respondent Tests
eTable 7. HRS Harmonized Cognitive Assessment Protocol (HCAP) Informant Report
eTable 8. Criteria for Dementia and MCI
References
- 1.Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 2021;17(12):1966-1975. doi: 10.1002/alz.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17(3):327-406. doi: 10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- 3.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bondi MW, Edmonds EC, Jak AJ, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis. 2014;42(1):275-289. doi: 10.3233/JAD-140276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303-308. doi: 10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- 6.Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. 2016;8(1):23. doi: 10.1186/s13195-016-0188-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudomiet P, Hurd MD, Rohwedder S. Dementia prevalence in the United States in 2000 and 2012: estimates based on a nationally representative study. J Gerontol B Psychol Sci Soc Sci. 2018;73(suppl 1):S10-S19. doi: 10.1093/geronb/gbx169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement. 2018;14(2):121-129. doi: 10.1016/j.jalz.2017.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Global status report on the public health response to dementia. Published online 2021. Accessed September 28, 2022. https://www.who.int/publications/i/item/9789240033245
- 10.GBD 2016 Dementia Collaborators . Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88-106. doi: 10.1016/S1474-4422(18)30403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson RS, Weir DR, Leurgans SE, et al. Sources of variability in estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimers Dement. 2011;7(1):74-79. doi: 10.1016/j.jalz.2010.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manly JJ, Bell-McGinty S, Tang MX, Schupf N, Stern Y, Mayeux R. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol. 2005;62(11):1739-1746. doi: 10.1001/archneur.62.11.1739 [DOI] [PubMed] [Google Scholar]
- 13.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427-434. doi: 10.7326/0003-4819-148-6-200803180-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephan BCM, Brayne C, McKeith IG, Bond J, Matthews FE; Medical Research Council Cognitive Function and Ageing Study . Mild cognitive impairment in the older population: Who is missed and does it matter? Int J Geriatr Psychiatry. 2008;23(8):863-871. doi: 10.1002/gps.2013 [DOI] [PubMed] [Google Scholar]
- 15.Matthews FE, Stephan BCM, McKeith IG, Bond J, Brayne C; Medical Research Council Cognitive Function and Ageing Study . Two-year progression from mild cognitive impairment to dementia: to what extent do different definitions agree? J Am Geriatr Soc. 2008;56(8):1424-1433. doi: 10.1111/j.1532-5415.2008.01820.x [DOI] [PubMed] [Google Scholar]
- 16.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JWR, Weir DR. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43(2):576-585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langa KM, Plassman BL, Wallace RB, et al. The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiology. 2005;25(4):181-191. doi: 10.1159/000087448 [DOI] [PubMed] [Google Scholar]
- 18.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1-2):125-132. doi: 10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66(suppl 1):i162-i171. doi: 10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langa KM, Larson EB, Crimmins EM, et al. A Comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51-58. doi: 10.1001/jamainternmed.2016.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolters FJ, Chibnik LB, Waziry R, et al. Twenty-seven–year time trends in dementia incidence in Europe and the United States: the Alzheimer Cohorts Consortium. Neurology. 2020;95(5):e519-e531. doi: 10.1212/WNL.0000000000010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Power MC, Gianattasio KZ, Ciarleglio A. Implications of the use of algorithmic diagnoses or Medicare claims to ascertain dementia. Neuroepidemiology. 2020;54(6):462-471. doi: 10.1159/000510753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langa KM, Ryan LH, McCammon RJ, et al. The Health and Retirement Study Harmonized Cognitive Assessment Protocol Project: study design and methods. Neuroepidemiology. 2020;54(1):64-74. doi: 10.1159/000503004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones R, Manly J, Langa K, et al. Factor structure of the Harmonized Cognitive Assessment Protocol neuropsychological battery in the Health and Retirement Study. PsyArXiv. Preprint posted online October 17, 2020. doi: 10.31234/osf.io/rvmhj [DOI] [PMC free article] [PubMed]
- 25.Weir DR, Langa KM, Ryan LH. Cognition data. Accessed September 28, 2022. https://hrs.isr.umich.edu/data-products/cognition-data
- 26.Weir DR, Langa KM, Ryan LH. 2016 Harmonized Cognitive Assessment Protocol (HCAP) study protocol summary. Accessed September 28, 2022. http://hrsonline.isr.umich.edu/index.php?p=shoavail&iyear=ZU
- 27.Sliwinski M, Lipton RB, Buschke H, Stewart W. The effects of preclinical dementia on estimates of normal cognitive functioning in aging. J Gerontol B Psychol Sci Soc Sci. 1996;51(4):217-225. doi: 10.1093/geronb/51B.4.P217 [DOI] [PubMed] [Google Scholar]
- 28.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muthén B, Muthén BO. Statistical Analysis With Latent Variables. Vol 123. Wiley; 2009. [Google Scholar]
- 30.Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer’s disease greater for women than for men? Am J Epidemiol. 2001;153(2):132-136. doi: 10.1093/aje/153.2.132 [DOI] [PubMed] [Google Scholar]
- 31.Edland SD, Rocca WA, Petersen RC, Cha RH, Kokmen E. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester, Minn. Arch Neurol. 2002;59(10):1589-1593. doi: 10.1001/archneur.59.10.1589 [DOI] [PubMed] [Google Scholar]
- 32.Chêne G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement. 2015;11(3):310-320. doi: 10.1016/j.jalz.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer’s disease. the impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49(6):1498-1504. doi: 10.1212/WNL.49.6.1498 [DOI] [PubMed] [Google Scholar]
- 34.Gianattasio KZ, Wu Q, Glymour MM, Power MC. Comparison of methods for algorithmic classification of dementia status in the Health and Retirement Study. Epidemiology. 2019;30(2):291-302. doi: 10.1097/EDE.0000000000000945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts R, Knopman DS. Classification and Epidemiology of MCI. Clin Geriatr Med. 2013;29(4):753-772. doi: 10.1016/j.cger.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuller-Thomson E, Ahlin KM. A decade of decline in serious cognitive problems among older Americans: a population-based study of 5.4 million respondents. J Alzheimers Dis. 2022;85(1):141-151. doi: 10.3233/JAD-210561 [DOI] [PubMed] [Google Scholar]
- 37.Nichols E, Szoeke CEI, Vollset SE; GBD 2016 Dementia Collaborators . Global, regional, and national burden of Alzheimer's disease and other dementias, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(1):88-106. doi: 10.1016/S1474-4422(18)30403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time—current evidence. Nat Rev Neurol. 2017;13(6):327-339. doi: 10.1038/nrneurol.2017.63 [DOI] [PubMed] [Google Scholar]
- 39.Power MC, Bennett EE, Turner RW, et al. Trends in relative incidence and prevalence of dementia across non-Hispanic Black and White individuals in the United States, 2000-2016. JAMA Neurol. 2021;78(3):275-284. doi: 10.1001/jamaneurol.2020.4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gleason CE, Norton D, Zuelsdorff M, et al. Association between enrollment factors and incident cognitive impairment in Blacks and Whites: data from the Alzheimer’s Disease Center. Alzheimers Dement. 2019;15(12):1533-1545. doi: 10.1016/j.jalz.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayeda ER, Filshtein TJ, Tripodis Y, Glymour MM, Gross AL. Does selective survival before study enrolment attenuate estimated effects of education on rate of cognitive decline in older adults? a simulation approach for quantifying survival bias in life course epidemiology. Int J Epidemiol. 2018;47(5):1507-1517. doi: 10.1093/ije/dyy124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayes-Larson E, Mobley TM, Mungas D, et al. Accounting for lack of representation in dementia research: generalizing KHANDLE study findings on the prevalence of cognitive impairment to the California older population. Alzheimers Dement. Published online February 1, 2022. doi: 10.1002/alz.12522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JPG, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63(4):494-506. doi: 10.1002/ana.21326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angevaare MJ, Vonk JMJ, Bertola L, et al. Predictors of incident mild cognitive impairment and its course in a diverse community-based population. Neurology. 2022;98(1):e15-e26. doi: 10.1212/WNL.0000000000013017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koepsell TD, Monsell SE. Reversion from mild cognitive impairment to normal or near-normal cognition: risk factors and prognosis. Neurology. 2012;79(15):1591-1598. doi: 10.1212/WNL.0b013e31826e26b7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malek-Ahmadi M. Reversion from mild cognitive impairment to normal cognition: a meta-analysis. Alzheimer Dis Assoc Disord. 2016;30(4):324-330. doi: 10.1097/WAD.0000000000000145 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of the HRS-HCAP sample
eMethods
eTable 2. 2016 HCAP sampling weight descriptive statistics
eTable 3. Weighted comparison of HCAP respondents and 2016 HRS reference sample
eTable 4. Description of factor scores and performance summaries based on domains of neuropsychological functioning and cognitive test performance
eTable 5. Selection of robust normative sample from HCAP participants
eTable 6. HRS Harmonized Cognitive Assessment Protocol (HCAP) Respondent Tests
eTable 7. HRS Harmonized Cognitive Assessment Protocol (HCAP) Informant Report
eTable 8. Criteria for Dementia and MCI