This cohort study assesses the association of prostate-specific antigen screening with incidence of metastatic prostate cancer in the Veterans Health Administration (VHA) from 2005 to 2019.
Key Points
Question
Are higher rates of prostate-specific antigen screening associated with lower population-level incidence of metastatic prostate cancer?
Findings
In this cohort study of male patients seen at 128 US Veterans Health Administration facilities from 2005 (n = 4 678 412) to 2019 (n = 5 371 701), facilities with higher rates of prostate-specific antigen screening had lower subsequent metastatic prostate cancer incidence rates.
Meaning
The findings suggest that variation in prostate cancer screening rates is associated with subsequent metastatic prostate cancer incidence; these data may inform shared decision-making about the potential benefits of prostate-specific antigen screening.
Abstract
Importance
There is controversy about the benefit of prostate-specific antigen (PSA) screening. Prostate-specific antigen screening rates have decreased since 2008 in the US, and the incidence of metastatic prostate cancer has increased. However, there is no direct epidemiologic evidence of a correlation between population PSA screening rates and subsequent metastatic prostate cancer rates.
Objective
To assess whether facility-level variation in PSA screening rates is associated with subsequent facility-level metastatic prostate cancer incidence.
Design, Setting, and Participants
This retrospective cohort used data for all men aged 40 years or older with an encounter at 128 facilities in the US Veterans Health Administration (VHA) from January 1, 2005, to December 31, 2019.
Exposures
Yearly facility-level PSA screening rates, defined as the proportion of men aged 40 years or older with a PSA test in each year, and long-term nonscreening rates, defined as the proportion of men aged 40 years or older without a PSA test in the prior 3 years, from January 1, 2005, to December 31, 2014.
Main Outcomes and Measures
The main outcomes were facility-level yearly counts of incident metastatic prostate cancer diagnoses and age-adjusted yearly metastatic prostate cancer incidence rates (per 100 000 men) 5 years after each PSA screening exposure year.
Results
The cohort included 4 678 412 men in 2005 and 5 371 701 men in 2019. Prostate-specific antigen screening rates decreased from 47.2% in 2005 to 37.0% in 2019, and metastatic prostate cancer incidence increased from 5.2 per 100 000 men in 2005 to 7.9 per 100 000 men in 2019. Higher facility-level PSA screening rates were associated with lower metastatic prostate cancer incidence 5 years later (incidence rate ratio [IRR], 0.91 per 10% increase in PSA screening rate; 95% CI, 0.87-0.96; P < .001). Higher long-term nonscreening rates were associated with higher metastatic prostate cancer incidence 5 years later (IRR, 1.11 per 10% increase in long-term nonscreening rate; 95% CI, 1.03-1.19; P = .01).
Conclusions and Relevance
From 2005 to 2019, PSA screening rates decreased in the national VHA system. Facilities with higher PSA screening rates had lower subsequent rates of metastatic prostate cancer. These data may be used to inform shared decision-making about the potential benefits of PSA screening among men who wish to reduce their risk of metastatic prostate cancer.
Introduction
The widespread adoption of prostate-specific antigen (PSA) screening in the 1990s was associated with a substantial increase in nonmetastatic prostate cancer incidence in the US.1 When randomized clinical trials failed to produce a consensus regarding the clinical benefit of PSA screening,2,3,4,5 in 2008, the US Preventive Services Task Force (USPSTF) recommended against PSA screening for men older than 75 years.6 This recommendation was followed by guidelines in 2012 recommending against PSA screening among men of any age.7 Following the 2012 guidelines, multiple studies demonstrated a substantial decrease in nonmetastatic prostate cancer incidence associated with a decrease in PSA screening rates.8,9,10 However, the decrease in nonmetastatic prostate cancer incidence was accompanied by a significant increase in metastatic prostate cancer incidence from approximately 2013 onward.11,12
The cause of this increase in metastatic prostate cancer incidence has been debated, but one hypothesis is that lower screening rates owing to the 2008 and 2012 USPSTF guidelines led to missed nonmetastatic prostate cancer diagnoses that subsequently progressed to metastatic prostate cancer.11,13 It is currently unknown whether variation in PSA screening rates in this period was associated with subsequent metastatic prostate cancer incidence. In this study, we used data from the US Veterans Healthcare Administration (VHA) to assess variation in facility-level PSA screening rates, prostate biopsy rates, and incident nonmetastatic prostate cancer and metastatic prostate cancer rates from 2005 to 2019. We hypothesized that lower facility-level PSA screening rates in 2005 to 2014 would be associated with higher subsequent metastatic prostate cancer incidence.
Methods
Data Source and Study Design
This cohort study used electronic medical record data from the Veterans Affairs Corporate Data Warehouse, which includes electronic medical record data for all veterans receiving care through VHA facilities. This study was approved by the University of Utah institutional review board, which is the institutional review board of record for Salt Lake City VA Medical Center. Informed consent and Health Insurance Portability and Accountability Act authorization were waived because the study analyzed retrospective data and involved minimal risk to human participants. We evaluated time trends in PSA screening rates, prostate biopsy rates, and incidence rates of newly diagnosed nonmetastatic prostate cancer or metastatic prostate cancer within the VHA from January 1, 2005, through December 31, 2019; 2020 and 2021 were excluded owing to changes in PSA screening behavior during the COVID-19 pandemic.14 Counts of patients undergoing PSA screening, prostate biopsies, and incident prostate cancer cases were calculated yearly and aggregated by age group (40-54, 55-69, and ≥70 years, reflecting current USPSTF guidelines),15 self-reported race and ethnicity (Black, non-Hispanic White, other [Asian and Hispanic], and unknown), and VHA facility. Race and ethnicity were included in the study owing to the higher prostate cancer incidence among Black men.16 Of 130 total VHA facilities represented in the Corporate Data Warehouse, 2 were excluded owing to incomplete data, leaving 128 facilities in our analyses. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
PSA Screening and Prostate Biopsy Rates
Our primary exposures included yearly PSA screening rates and long-term nonscreening rates from January 1, 2005, to December 31, 2014. Total PSA tests were obtained through Corporate Data Warehouse laboratory data and were validated using Current Procedural Terminology codes for PSA testing (eMethods in the Supplement). The PSA screening rate was defined as the number of unique male patients aged 40 years or older who received a PSA test at a VHA facility in each year, divided by the number of unique male patients aged 40 years or older who received any care at a VHA facility in that year. The long-term nonscreening rate was defined as the number of unique male patients aged 40 years or older without any PSA tests within the prior 3 years, divided by the number of unique male patients aged 40 years or older who received any care at a VHA facility each year. For example, a patient seen in 2010 who did not have a PSA test from 2007 to 2010 would be counted in the numerator of the 2010 long-term nonscreening rate. The numerators and denominators excluded patients with a previous diagnosis of prostate cancer. The prostate biopsy rate was defined as the number of prostate biopsy procedures (defined by Current Procedural Terminology, International Classification of Diseases, Ninth Revision [ICD-9], and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] codes) divided by the total male population with a VHA encounter in each year. The prostate biopsy rate was age adjusted to the 2000 US standard population by the direct method.17
Prostate Cancer Incidence Rates
Incidence rates for nonmetastatic prostate cancer and metastatic prostate cancer were defined as the number of patients with incident prostate cancer each year divided by the number of unique male patients who received any care at a VHA facility in that year. Incident nonmetastatic prostate cancer was defined as the first presence of Gleason score documentation in clinical notes or pathology reports within 90 days of a diagnosis code for prostate cancer (ICD-9 code 185.x or ICD-10 code C61.x) and without documentation of metastatic disease within 90 days of the code. Incident metastatic prostate cancer was defined as documentation of metastatic prostate cancer within 90 days of the first prostate cancer diagnosis code. Gleason score was obtained through natural language processing of clinical notes and pathology reports (eMethods in the Supplement). Documentation of metastatic disease was obtained through natural language processing of clinical notes and pathology reports as previously described.18 To help account for differing age distributions across facilities, incidence rates were age adjusted to the 2000 US standard population by the direct method.17
Statistical Analysis
To evaluate the association between PSA screening rates and subsequent rates of incident metastatic prostate cancer, we developed a regression model in which the primary independent variable was the annual facility-level PSA screening rate and the dependent variable was the facility-level metastatic prostate cancer incidence 5 years later. We used screening frequencies from 2005 to 2014 and incident metastatic prostate cancer rates from 2010 to 2019. The lag time of 5 years was chosen owing to the observed interval between randomization and decreases in population-level metastatic prostate cancer incidence in the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial.2 In sensitivity analyses, we varied this lag time to 3 and 7 years.
The primary analysis used a multivariable negative binomial mixed-effects regression to estimate the association between facility-level metastatic prostate cancer case counts and PSA screening rates. The model included an offset term for the facility-level total male population in each metastatic prostate cancer incidence year to account for different facility population sizes and random intercepts by facility to account for within-facility correlation. To account for potential confounders, we controlled for several additional facility-level variables that were assessed in each metastatic prostate cancer incidence year. These included the population percentage of Black men, percentage of men in the group aged 70 years or older, availability of novel positron emission tomography (PET) tracers, facility proportion of patients with incident prostate cancer who underwent magnetic resonance imaging (MRI) of the pelvis within 3 months of diagnosis, calendar year, and facility region according to the VHA classification of facility geographic regions.19 Availability of novel PET tracers was defined as a binary indicator of whether each facility used any novel PET tracers in that year or any previous years, including novel PET performed by non-VHA providers but reimbursed by the VHA; tracer use was assessed by Current Procedural Terminology codes and included 18F sodium fluoride, 68Ga or 18F prostate specific membrane antigen, 18F fluciclovine, C11 choline, and 18F piflufolastat. Calendar year was modeled with a natural cubic spline with 4 degrees of freedom, chosen using the Akaike information criterion among models containing a linear term or splines with 2 to 5 degrees of freedom.
To permit a more flexible correlation structure within facilities, in sensitivity analyses we used a Poisson generalized estimating equation approach with an unstructured covariance matrix and robust SEs with the same covariate set. To investigate the potential effects of non-VHA care on our results, in additional sensitivity analyses, we restricted the yearly at-risk population to frequent VHA users, defined as men with a VHA encounter in each of the 3 years before each at-risk year. For example, PSA screening rates and metastatic prostate cancer rates in 2012 would be calculated only among men with an encounter each year from 2009 to 2012. All statistical tests were 2 sided with P < .05 indicating statistical significance. Analyses were performed with SAS, version 9.4 (SAS Institute Inc) and R, version 4.0.5 (R Project for Statistical Computing).
Results
Trends in PSA Screening, Long-term Nonscreening, and Biopsy Rates
The overall VHA cohort included 4 678 412 men in 2005. Facility-level characteristics are shown in eFigure 1 in the Supplement. In 2012, the median proportion of Black patients was 9.2% (IQR, 3.4%-18.9%); White patients, 76.7% (IQR, 59.1%-85.5%), other race and ethnicity, 2.5% (IQR, 1.5%-6.3%), and unknown race and ethnicity, 7.1% (IQR, 4.9%-10.4%). The median proportion of patients aged 70 years or older was 33.9% (IQR, 29.5%-38.3%). Overall PSA screening rates increased from 47.2% in 2005 to a maximum of 50.8% in 2008, then decreased to 37.0% in 2019 (Table 1 and eFigure 2 in the Supplement). The long-term nonscreening rate, representing patients not receiving a PSA test in the prior 3 years, increased from a low of 20.9% in 2009 to a high of 33.2% in 2019 (eFigure 2 in the Supplement). When examined separately by age, all age groups experienced a decrease in the PSA screening rate, although the rate in the group aged 70 years or older decreased earlier than rates in the other groups starting in 2008 (Figure 1A and B and eTable 1 in the Supplement). All race and ethnicity groups had decreases in PSA screening rates and increases in long-term nonscreening rates, although Black patients had higher PSA screening rates (Figure 2A), higher biopsy rates, and lower long-term nonscreening rates (Figure 2B) throughout the study period compared with other racial and ethnic groups (eTable 2 in the Supplement). Prostate biopsy rates were stable or decreased over the study period for most race and ethnicity and age groups, although there was a slight increase in the group aged 70 years or older from 2014 to 2019 (eTable 1 in the Supplement).
Table 1. Overall Screening and Diagnosis Rates per Year.
| Year | Men with VHA encounter, No. | Incident metastatic prostate cancer cases, No. | Screening PSA, %a | Prostate biopsy rateb | Incident nonmetastatic prostate cancer cases with Gleason score of 8-10, % | Incidence rate, per 100 000 menb | ||
|---|---|---|---|---|---|---|---|---|
| Yes | None in prior 3 y | Nonmetastatic prostate cancer | Metastatic prostate cancer | |||||
| 2005 | 4 678 412 | 844 | 47.2 | 23.8 | 200.6 | 15.5 | 99.3 | 5.2 |
| 2006 | 4 713 876 | 845 | 48.7 | 22.8 | 196.2 | 16.6 | 98.3 | 5.2 |
| 2007 | 4 735 010 | 798 | 49.6 | 21.7 | 209.4 | 16.6 | 105.3 | 4.9 |
| 2008 | 4 759 413 | 786 | 50.8 | 21.0 | 196.0 | 16.7 | 99.6 | 4.6 |
| 2009 | 4 910 792 | 914 | 50.1 | 20.9 | 185.6 | 17.5 | 97.3 | 5.3 |
| 2010 | 4 989 335 | 890 | 48.2 | 21.3 | 175.7 | 18.3 | 92.5 | 5.0 |
| 2011 | 5 101 613 | 984 | 47.5 | 21.5 | 176.5 | 18.5 | 91.9 | 5.3 |
| 2012 | 5 175 058 | 1039 | 43.2 | 22.9 | 152.9 | 18.9 | 79.8 | 5.4 |
| 2013 | 5 207 538 | 1087 | 40.6 | 24.7 | 141.8 | 18.9 | 75.1 | 5.6 |
| 2014 | 5 269 477 | 1130 | 39.4 | 26.6 | 129.6 | 20.4 | 70.6 | 5.8 |
| 2015 | 5 280 469 | 1251 | 38.6 | 29.0 | 136.9 | 21.0 | 73.8 | 6.5 |
| 2016 | 5 290 551 | 1403 | 37.9 | 30.7 | 137.3 | 21.4 | 73.8 | 7.0 |
| 2017 | 5 312 173 | 1651 | 37.8 | 31.7 | 137.5 | 22.8 | 74.2 | 8.2 |
| 2018 | 5 340 725 | 1722 | 37.5 | 32.4 | 142.0 | 23.2 | 79.5 | 8.0 |
| 2019 | 5 371 701 | 1700 | 37.0 | 33.2 | 138.5 | 23.8 | 77.2 | 7.9 |
Abbreviations: PSA, prostate-specific antigen; VHA, Veterans Health Administration.
Among men aged 40 years or older.
Age adjusted to the 2000 US standard population.
Figure 1. Prostate-Specific Antigen (PSA) Screening, Prostate Biopsy, and Prostate Cancer Incidence Rates by Age Group.
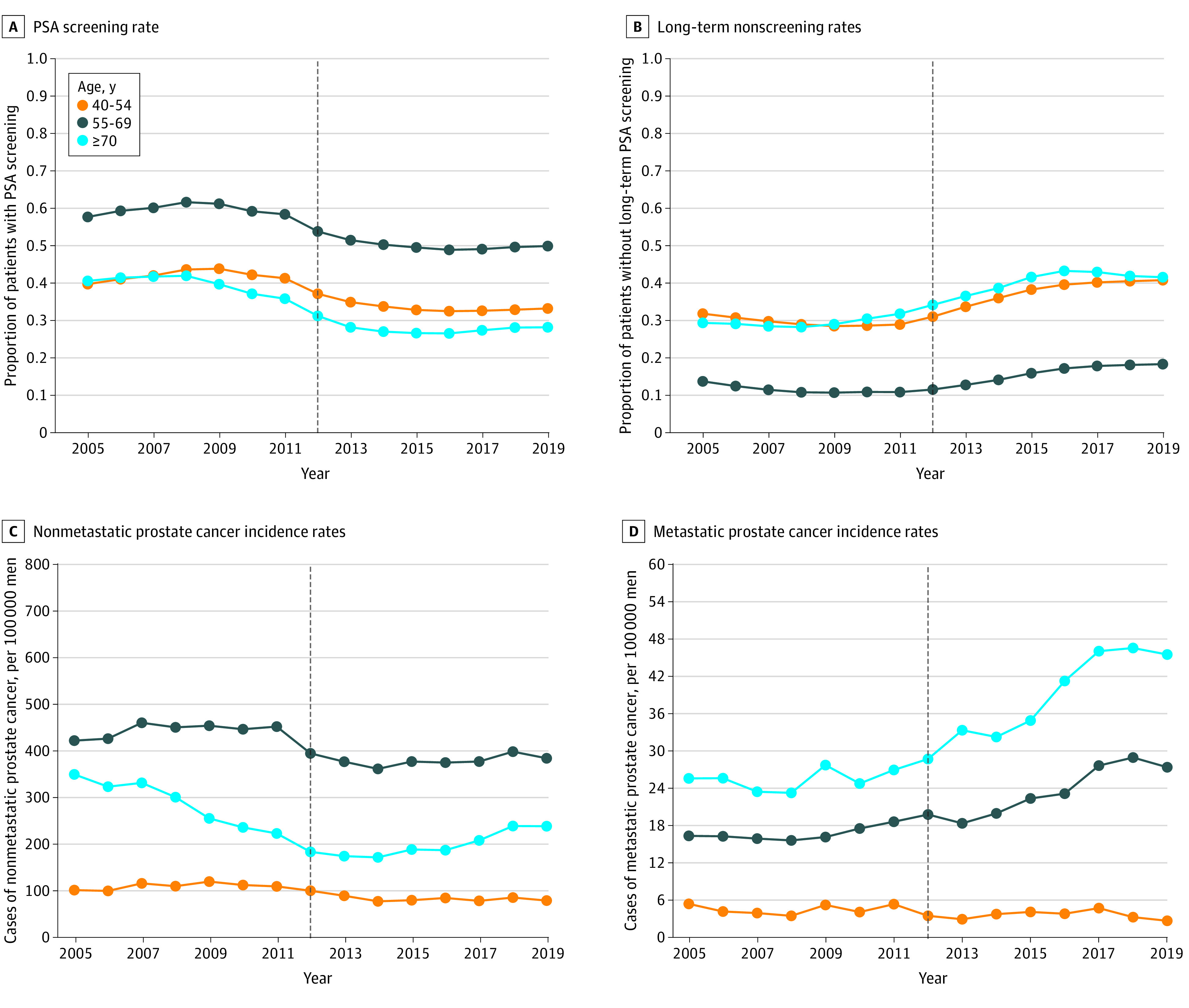
Long-term nonscreening rates represent the proportion of patients without a PSA screening test in the prior 3 years. All rates were pooled across 128 Veterans Health Administration facilities. Vertical dashed line represents the year of US Preventive Services Task Force screening guideline publication in 2012.
Figure 2. Prostate-Specific Antigen (PSA) Screening, Prostate Biopsy, and Prostate Cancer Incidence Rates by Racial and Ethnic Group.
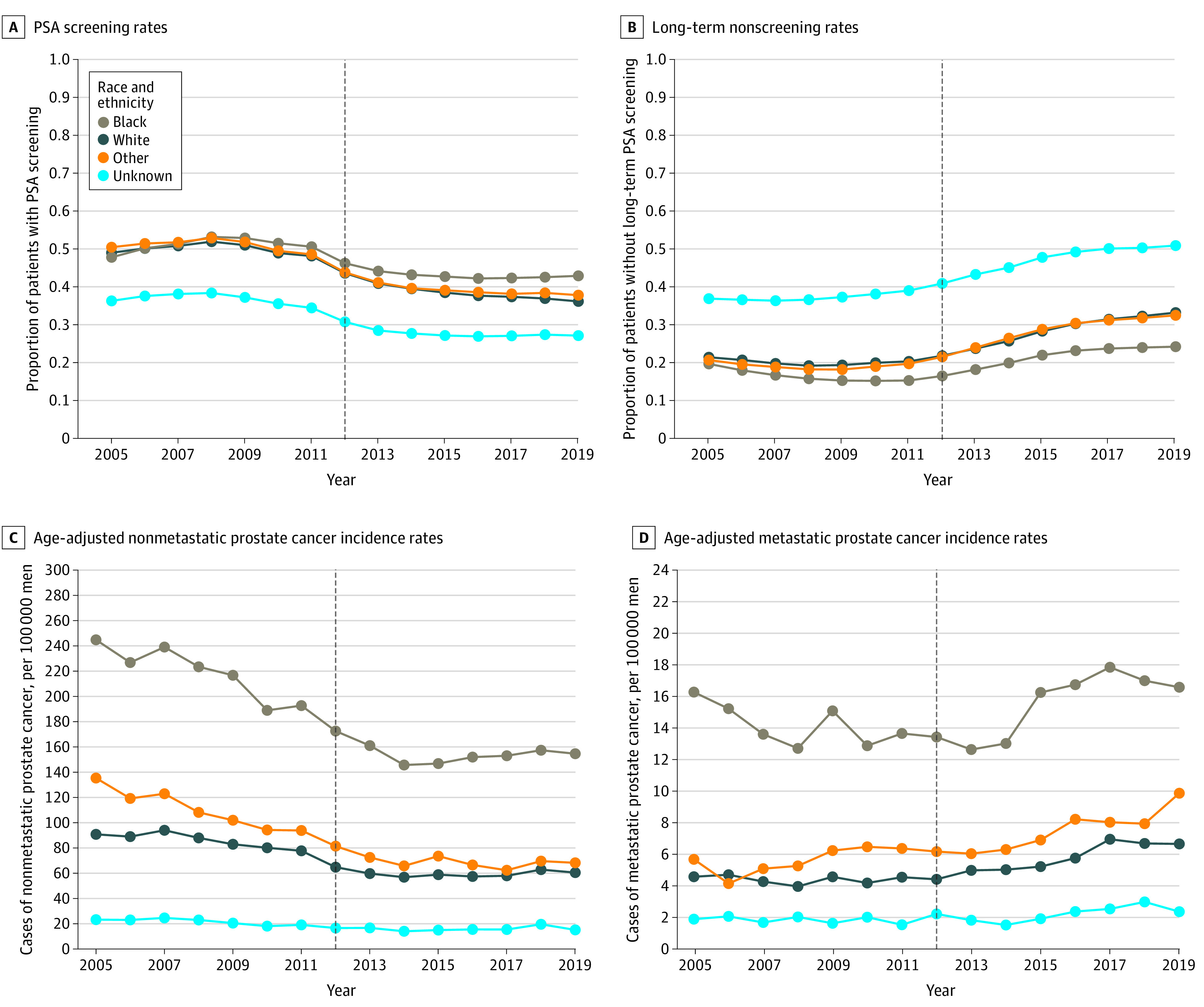
Long-term nonscreening rates represent the proportion of patients without a PSA screening test in the prior 3 years. All rates were pooled across 128 Veterans Health Administration facilities. “Other” included Asian and Hispanic men. Vertical dashed line represents the year of US Preventive Services Task Force screening guideline publication in 2012.
Trends in Nonmetastatic Prostate Cancer and Metastatic Prostate Cancer Incidence
Age-adjusted nonmetastatic prostate cancer incidence rates decreased from 2007 to 2014 but were mostly stable from 2014 to 2019 (eFigure 2 in the Supplement). A larger decrease in nonmetastatic prostate cancer incidence was observed in the group aged 70 years or older from 351 cases per 100 000 men in 2005 to 173 cases per 100 000 men in 2014 followed by a slight increase from 2014 to 2019 (Figure 1C). The proportion of incident nonmetastatic prostate cancer cases with a Gleason score of 8 to 10 increased throughout the study period in the groups aged 55 to 69 years and 70 years or older but was stable in the group aged 40 to 54 years (eTable 1 in the Supplement).
Overall age-adjusted metastatic prostate cancer incidence increased from 4.6 cases per 100 000 men in 2008 to a high of 8.2 cases per 100 000 men in 2017, a 78% relative increase (eFigure 2 in the Supplement). The increase in metastatic prostate cancer incidence was prominent in groups aged 55 to 69 years and 70 years or older, but rates in the group aged 40 to 54 years were stable or slightly decreased after 2012 (Figure 1D and eTable 1 in the Supplement). Age-adjusted metastatic prostate cancer rates were highest among Black patients, although increases were seen across racial and ethnic groups (Figure 2D and eTable 2 in the Supplement).
Association of PSA Screening Rates and Subsequent Metastatic Prostate Cancer Incidence
In the mixed-effects negative binomial regression model, higher facility-level PSA screening rates were associated with lower subsequent metastatic prostate cancer rates (incidence rate ratio [IRR], 0.91 per 10% increase in PSA screening rate; 95% CI, 0.87-0.96; P < .001) (Figure 3A). An association was also revealed in the generalized estimating equations model (IRR, 0.91 per 10% increase in PSA screening rate; 95% CI, 0.87-0.95; P < .001) and in sensitivity analyses using a 7-year lag (IRR, 0.89 per 10% increase in PSA screening rate; 95% CI, 0.84-0.95; P < .001), although there was no association when using a 3-year lag (IRR, 0.96 per 10% increase in PSA screening rate; 95% CI, 0.91-1.01; P = .10). Similar results were found in the analyses of long-term nonscreening rates, with higher nonscreening rates associated with higher subsequent metastatic prostate cancer rates in the primary analysis (IRR 1.11 per 10% increase in long-term nonscreening rate; 95% CI, 1.03-1.19; P = .01) (Figure 3B), in the generalized estimating equations model (IRR, 1.13 per 10% increase in PSA screening rate; 95% CI, 1.05-1.22; P = .002), and in sensitivity analyses using a 7-year lag (IRR, 1.13 per 10% increase in PSA screening rate; 95% CI, 1.03-1.23; P = .008) and 3-year lag (IRR, 1.08 per 10% increase in PSA screening rate; 95% CI, 1.02-1.15; P = .01). Other variables associated with higher metastatic prostate cancer incidence rates included higher population percentage of Black patients, patients aged 70 years or older, and patients living in the Pacific region (Table 2). These results were consistent in sensitivity analyses restricted to frequent VHA users (eTable 3 in the Supplement).
Figure 3. Association of Prostate-Specific Antigen Screening Rates and Long-term Nonscreening Rates With Subsequent Metastatic Prostate Cancer Incidence.
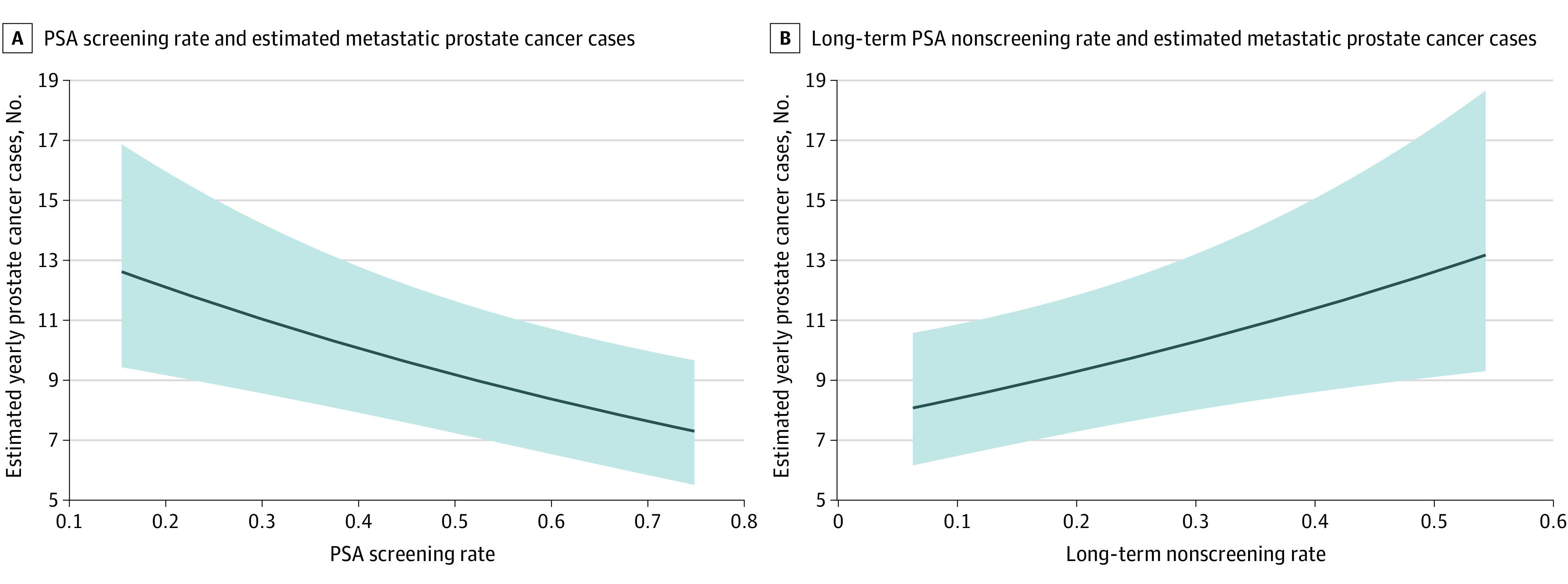
Predicted case count estimates were generated from multivariable mixed-effects negative binomial models using a random effect of 0, continuous covariates at their mean values, Pacific region, and facility size of 40 889 men. Shaded areas indicate 95% CIs.
Table 2. Results of Mixed-Effects Negative Binomial Regressions for Metastatic Prostate Cancer Rates.
| Variable | Model for PSA screening rate | Model for long-term nonscreening rate | ||
|---|---|---|---|---|
| IRR (95% CI)a | P value | IRR (95% CI)a | P value | |
| PSA screening rate or long-term nonscreening rateb | 0.91 (0.87-0.96) | <.001 | 1.11 (1.03-1.19) | .01 |
| Percentage of Black patients, per 10% increase | 1.19 (1.09-1.29) | <.001 | 1.20 (1.11-1.30) | <.001 |
| Calendar year (spline)c | NA | <.001 | NA | <.001 |
| Percentage of patients aged ≥70 y, per 10% increase | 1.12 (0.99-1.27) | .08 | 1.11 (0.98-1.26) | .10 |
| Availability of novel PET tracers | 1.03 (0.95-1.11) | .50 | 1.02 (0.95-1.11) | .60 |
| Use of MRI of the pelvis for prostate cancer workup, per 10% increased | 1.01 (0.97-1.04) | .80 | 1.00 (0.97-1.04) | .80 |
| Region | ||||
| Pacific | 1.00 [Reference] | NA | 1.00 [Reference] | NA |
| Continental | 0.73 (0.53-0.99) | .04 | 0.71 (0.52-0.98) | .04 |
| Midwest | 0.91 (0.68-1.23) | .60 | 0.89 (0.66-1.20) | .40 |
| North Atlantic | 0.73 (0.54-0.98) | .04 | 0.71 (0.53-0.96) | .03 |
| Southeast | 0.73 (0.52-1.03) | .07 | 0.70 (0.50-0.99) | .046 |
Abbreviations: IRR, incidence rate ratio; MRI, magnetic resonance imaging; NA, not applicable; PET, positron emission tomography; PSA, prostate-specific antigen.
Coefficients reflect the metastatic prostate cancer incidence rate ratio associated with a unit change in each variable.
Long-term nonscreening rates represent the percentage of men aged 40 years or older without a history of prostate cancer who had not received a PSA test in the prior 3 years.
P value for spline term generated by the likelihood ratio test.
Defined as the proportion of newly diagnosed prostate cancer cases with MRI of the pelvis performed within 3 months of the date of diagnosis.
Discussion
In this population-based study of PSA screening patterns and prostate cancer incidence among veterans aged 40 years or older from 2005 (n = 4 678 412) to 2019 (n = 5 371 701), we found a decrease in PSA screening rates between 2008 and 2019 across all age groups. This coincided with a subsequent increase in long-term nonscreening rates, decreases in prostate biopsy rates, and an increase in incident metastatic prostate cancer beginning in 2012. We found that higher yearly metastatic prostate cancer incidence rates from 2010 to 2019 were associated with lower facility-level PSA screening rates 5 years earlier; similar results were observed for long-term nonscreening rates. These results were robust in sensitivity analyses varying analytical technique and lag times. Taken together, these data suggest that facility-level PSA screening behaviors are associated with variation in metastatic prostate cancer incidence.
The observed 10% to 15% absolute decrease in PSA screening rates among veterans around the time of the introduction of the 2008 and 2012 USPSTF guidelines is consistent with other reports showing a 5% to 15% absolute decrease in PSA screening in this period.9,10 The PSA screening rate began decreasing in the group aged 70 years or older in 2008, coinciding with the publication of USPSTF guidelines recommending against screening in patients older than 75 years.6 The negative mortality results from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial were published in 2009 and may also have been associated with decreasing screening rates.4 Screening rates in the groups aged 40 to 54 years and 55 to 69 years began decreasing more steeply in 2011 and 2012, coinciding with the publication of USPSTF draft guidance in 2011 and final guidelines in 2012 recommending against PSA screening for all men.7 Of note, the VHA system maintains a recommendation of shared decision-making for screening decisions among men aged 55 to 69 years.20 We observed decreases in PSA screening in all age groups, contrary to a recent study from the VHA21; these differences may be attributable to differing choices of population denominators and methods of capturing PSA tests. The observed screening decreases were followed by an increase in the long-term nonscreening rate from 2012 to approximately 2017. Prostate biopsy rates and nonmetastatic prostate cancer incidence rates showed modest decreases that coincided with these changes in PSA screening and long-term nonscreening rates.
Our results suggest an association between lower facility-level PSA screening rates and higher metastatic prostate cancer incidence rates, which may implicate PSA screening behaviors in subsequent metastatic prostate cancer incidence. This would be consistent with findings from the ERSPC trial,2 which demonstrated decreased metastatic prostate cancer incidence in the PSA screening arm compared with the usual care arm. In contrast, the PLCO trial found no benefit of PSA screening in decreasing metastasis22 or mortality rates4 compared with usual care. Interpretation of the PLCO trial is limited by the high rates of PSA screening in the control arm, and post hoc analyses suggested that the results were largely consistent with the ERSPC trial after accounting for screening intensity in each arm.23 Our finding of an association between lower facility-level PSA screening rates with higher subsequent metastatic prostate cancer incidence provides epidemiological support of the ERSPC trial results, although we emphasize that no causal claims can be drawn from the present study. Our results also do not directly implicate changes in USPSTF recommendations or the publication of PSA screening trial results to the absolute increase in metastatic prostate cancer incidence since 2012, and the magnitude of our observed incidence rate ratios are not consistent with changes in PSA screening patterns being the sole factor associated with the increase in metastatic prostate cancer incidence. However, these data suggest that variation in absolute PSA screening rates is associated with subsequent metastatic prostate cancer incidence and that policies to increase PSA screening among eligible veterans might be associated with decreases in future metastatic prostate cancer incidence. The findings also highlight a potential need for policies to reduce interfacility variation in PSA screening behaviors, particularly in older age groups.
We found higher annual PSA screening rates and lower long-term nonscreening rates among Black veterans compared with veterans from other racial and ethnic groups, particularly after 2012. This may reflect physician awareness of the higher risk of incident prostate cancer among Black men and is reflected in observed higher rates of prostate biopsies, nonmetastatic prostate cancer, and metastatic prostate cancer among Black men in our study. These findings are the opposite of screening patterns seen in many other data sets showing lower screening rates among Black men compared with individuals from other racial and ethnic groups.24,25,26 This difference may be attributable to the equal-access nature of the VHA and is consonant with other results showing similar prostate cancer treatment outcomes among Black and White veterans.27,28,29
Limitations
This study has limitations. First, our results are focused on the US veteran population, which differs from civilian populations in age distribution, comorbidity, and socioeconomic factors,30 and may have unique environmental exposures associated with increased baseline risk of prostate cancer31; as such, our results may not generalize to other populations. Second, while our denominators represent patients with an inpatient or outpatient encounter in each VHA facility each year, some veterans may have received care at several facilities each year and others may have moved between primary facilities during the study period. This could have biased our results if patients with higher biologic risk of metastatic prostate cancer predominantly moved from facilities with high screening rates to facilities with low screening rates during the study period—a possibility we consider unlikely. Third, 7.1% of individuals in the cohort reported unknown race and ethnicity; these patients tended to have lower screening rates and higher long-term nonscreening rates. Patients with unknown race and ethnicity may represent patients who do not regularly receive care in the VHA, and inclusion of these patients may have produced slight downward bias in our calculated screening and incidence rates. In addition, although we attempted to control for care outside the VHA by performing a sensitivity analysis restricted to frequent VHA users and including novel PET procedures performed outside the VHA system but reimbursed by the VHA, it remains possible that our results were confounded by care outside the VHA system and should be interpreted with caution.
Conclusions
This cohort study found that from 2005 to 2019, PSA screening rates decreased among men aged 40 years or older in the VHA system. Facilities with higher PSA screening rates had lower subsequent rates of metastatic prostate cancer. These data may be used to inform shared decision-making about the potential benefits of PSA screening among men who wish to reduce their risk of metastatic prostate cancer
eMethods
eFigure 1. Characteristics of 128 Veterans Healthcare Administration Facilities Included in the Primary Analysis
eFigure 2. Pooled PSA Screening, Prostate Biopsy, and Prostate Cancer Incidence Rates
eTable 1. Screening and Diagnosis Rates by Age Group
eTable 2. Screening and Diagnosis Rates by Race
eTable 3. Results of Mixed Effects Negative Binomial Regressions for Metastatic Pca Rates Among Frequent Veterans Healthcare Administration Users
References
- 1.Potosky AL, Miller BA, Albertsen PC, Kramer BS. The role of increasing detection in the rising incidence of prostate cancer. JAMA. 1995;273(7):548-552. doi: 10.1001/jama.1995.03520310046028 [DOI] [PubMed] [Google Scholar]
- 2.Schröder FH, Hugosson J, Carlsson S, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur Urol. 2012;62(5):745-752. doi: 10.1016/j.eururo.2012.05.068 [DOI] [PubMed] [Google Scholar]
- 3.Schröder FH, Hugosson J, Roobol MJ, et al. ; ERSPC Investigators . Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384(9959):2027-2035. doi: 10.1016/S0140-6736(14)60525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andriole GL, Crawford ED, Grubb RL III, et al. ; PLCO Project Team . Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310-1319. doi: 10.1056/NEJMoa0810696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andriole GL, Crawford ED, Grubb RL III, et al. ; PLCO Project Team . Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104(2):125-132. doi: 10.1093/jnci/djr500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Preventive Services Task Force . Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(3):185-191. doi: 10.7326/0003-4819-149-3-200808050-00008 [DOI] [PubMed] [Google Scholar]
- 7.Moyer VA; US Preventive Services Task Force . Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120-134. doi: 10.7326/0003-4819-157-2-201207170-00459 [DOI] [PubMed] [Google Scholar]
- 8.Aslani A, Minnillo BJ, Johnson B, Cherullo EE, Ponsky LE, Abouassaly R. The impact of recent screening recommendations on prostate cancer screening in a large health care system. J Urol. 2014;191(6):1737-1742. doi: 10.1016/j.juro.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 9.Drazer MW, Huo D, Eggener SE. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;33(22):2416-2423. doi: 10.1200/JCO.2015.61.6532 [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314(19):2054-2061. doi: 10.1001/jama.2015.14905 [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Culp MB, Ma J, Islami F, Fedewa SA. Prostate cancer incidence 5 years after US Preventive Services Task Force recommendations against screening. J Natl Cancer Inst. 2021;113(1):64-71. doi: 10.1093/jnci/djaa068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004-2013). Prostate Cancer Prostatic Dis. 2016;19(4):395-397. doi: 10.1038/pcan.2016.30 [DOI] [PubMed] [Google Scholar]
- 13.Fleshner K, Carlsson SV, Roobol MJ. The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat Rev Urol. 2017;14(1):26-37. doi: 10.1038/nrurol.2016.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman HW, Chen Z, Niles JK, Radcliff J, Fesko Y. Patterns of prostate-specific antigen testing and prostate biopsies during the COVID-19 pandemic. JCO Clin Cancer Inform. 2021;5:1028-1033. doi: 10.1200/CCI.21.00074 [DOI] [PubMed] [Google Scholar]
- 15.Grossman DC, Curry SJ, Owens DK, et al. ; US Preventive Services Task Force . Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(18):1901-1913. doi: 10.1001/jama.2018.3710 [DOI] [PubMed] [Google Scholar]
- 16.Tsodikov A, Gulati R, de Carvalho TM, et al. Is prostate cancer different in black men? answers from 3 natural history models. Cancer. 2017;123(12):2312-2319. doi: 10.1002/cncr.30687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naing NN. Easy way to learn standardization: direct and indirect methods. Malays J Med Sci. 2000;7(1):10-15. [PMC free article] [PubMed] [Google Scholar]
- 18.Alba PR, Gao A, Lee KM, et al. Ascertainment of veterans with metastatic prostate cancer in electronic health records: demonstrating the case for natural language processing. JCO Clin Cancer Inform. 2021;5:1005-1014. doi: 10.1200/CCI.21.00030 [DOI] [PubMed] [Google Scholar]
- 19.Jones AL, Pettey WBP, Carter ME, et al. Regional variations in documentation of sexual trauma concepts in electronic medical records in the United States Veterans Health Administration. AMIA Annu Symp Proc. 2020;2019:514-522. [PMC free article] [PubMed] [Google Scholar]
- 20.Screening for Prostate Cancer . Accessed August 29, 2022. http://vaww.prevention.va.gov/CPS/Screening_for_Prostate_Cancer.asp
- 21.Becker DJ, Rude T, Walter D, et al. The Association of veterans’ PSA screening rates with changes in USPSTF recommendations. J Natl Cancer Inst. 2021;113(5):626-631. doi: 10.1093/jnci/djaa120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinsky PF, Black A, Daugherty SE, et al. Metastatic prostate cancer at diagnosis and through progression in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer. 2019;125(17):2965-2974. doi: 10.1002/cncr.32176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med. 2017;167(7):449-455. doi: 10.7326/M16-2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jindal T, Kachroo N, Sammon J, et al. Racial differences in prostate-specific antigen-based prostate cancer screening: state-by-state and region-by-region analyses. Urol Oncol. 2017;35(7):460.e9-460.e20. doi: 10.1016/j.urolonc.2017.01.023 [DOI] [PubMed] [Google Scholar]
- 25.Moses KA, Zhao Z, Bi Y, et al. The impact of sociodemographic factors and PSA screening among low-income Black and White men: data from the Southern Community Cohort Study. Prostate Cancer Prostatic Dis. 2017;20(4):424-429. doi: 10.1038/pcan.2017.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kensler KH, Pernar CH, Mahal BA, et al. Racial and ethnic variation in PSA testing and prostate cancer incidence following the 2012 USPSTF recommendation. J Natl Cancer Inst. 2021;113(6):719-726. doi: 10.1093/jnci/djaa171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi: 10.1002/cncr.33224 [DOI] [PubMed] [Google Scholar]
- 28.Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non-Hispanic white men with prostate cancer in an equal-access health care system. Cancer. 2020;126(8):1683-1690. doi: 10.1002/cncr.32666 [DOI] [PubMed] [Google Scholar]
- 29.Dess RT, Hartman HE, Mahal BA, et al. Association of Black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(7):975-983. doi: 10.1001/jamaoncol.2019.0826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eibner C, Krull H, Brown KM, et al. Current and Projected Characteristics and Unique Health Care Needs of the Patient Population Served by the Department of Veterans Affairs. Rand Health Q 2016;5(4):13. [PMC free article] [PubMed] [Google Scholar]
- 31.Chamie K, DeVere White RW, Lee D, Ok JH, Ellison LM. Agent Orange exposure, Vietnam War veterans, and the risk of prostate cancer. Cancer. 2008;113(9):2464-2470. doi: 10.1002/cncr.23695 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1. Characteristics of 128 Veterans Healthcare Administration Facilities Included in the Primary Analysis
eFigure 2. Pooled PSA Screening, Prostate Biopsy, and Prostate Cancer Incidence Rates
eTable 1. Screening and Diagnosis Rates by Age Group
eTable 2. Screening and Diagnosis Rates by Race
eTable 3. Results of Mixed Effects Negative Binomial Regressions for Metastatic Pca Rates Among Frequent Veterans Healthcare Administration Users


