Purpose of review
Coronavirus disease (COVID-19)-associated pulmonary aspergillosis (CAPA) may concern up to one third of intensive care unit (ICU) patients. The purpose of this review is to discuss the diagnostic criteria, the pathogenesis, the risk factors, the incidence, the impact on outcome, and the diagnostic and therapeutic management of CAPA in critically ill patients.
Recent findings
The incidence of CAPA ranges 3--28% of critically ill patients, depending on the definition used, study design, and systematic or triggered screening. COVID-19 is associated with direct damage of the respiratory epithelium, immune dysregulation, and common use of immunosuppressive drugs which might promote Aspergillus respiratory tract colonization and invasion. Positive Aspergillus tests among COVID-19 critically patients might reflect colonization rather than invasive disease. CAPA usually appears during the second week after starting invasive mechanical ventilation and is independently associated with ICU mortality.
Summary
Further studies are needed to validate CAPA case definitions, to determine the accurate incidence of CAPA in comparison to adequate controls, and its evolution during the pandemic. A pro-active diagnostic strategy, based on risk stratification, clinical assessment, and bronchoalveolar lavage could be recommended to provide early antifungal treatment in patients with high probability of CAPA and clinical deterioration.
Keywords: Aspergillus, COVID-19, intensive care, invasive aspergillosis, SARS-CoV-2
INTRODUCTION
Over the past 20 years, incidence of invasive pulmonary aspergillosis (IPA) has markedly decreased in critically ill patients with classical host factors, i.e., severely immunosuppressed, mainly thanks to antifungal prophylaxis [1]. IPA in patients with hematological malignancies now mostly affects patients with prolonged neutropenia and allogeneic stem cell transplant recipient, particularly those with active graft versus host disease [2]. Further, a shift towards less immunocompromised intensive care unit (ICU) populations has been widely reported. New well established risk factors include chronic obstructive pulmonary disease (COPD) [3], severe alcoholic hepatitis, cirrhosis [4], acquired postsepsis immunoparalysis, prolonged corticosteroid therapy, acute respiratory distress syndrome (ARDS) [5] and severe influenza [6]. Since the start of the coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), more and more studies have reported cases of COVID-19-associated pulmonary aspergillosis (CAPA), raising the question of the burden of this secondary infection among critically ill patients. This review aims to discuss the gradual adaptation of IPA case definitions for critically ill patients, recent data on mycological diagnostic, pathogenesis, risk factors, incidence, and outcome of CAPA, that support current diagnostic and therapeutic management strategies.
Box 1.
no caption available
INVASIVE PULMONARY ASPERGILLOSIS CASE DEFINITIONS FOR INTENSIVE CARE UNIT PATIENTS
The histopathological, clinical, and radiological features of IPA, as well as diagnostic accuracy of mycological tests, highly depend on the severity of the underlying immune deficiency. Neutropenic patients will present typical computed tomography patterns, such as the halo sign, the air crescent or a cavity, due to significant Aspergillus hyphae invasion and necrosis. The diagnosis of IPA is generally more challenging in nonneutropenic patients, in whom tissue invasion is less extensive, clinical picture and computed tomography lesions are not specific, and respiratory mycological tests hardly distinguish between Aspergillus colonization and invasive infection.
Current IPA case definitions for critically ill patients are shown in Table 1 . European Organization for Research and Treatment of Cancer / Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) criteria, updated in 2020, fail to classify most cases of IPA among critically ill patients [7▪]. Lung biopsy, which defines proven cases, is rarely performed, and ICU patients do not meet the classical host factors.
Table 1.
Invasive pulmonary aspergillosis case definitions for critically ill patients
The AspICU algorithm has been first proposed as an alternative to address this issue [8]. Putative IPA combines a positive culture of any lower respiratory tract specimen, compatible clinical signs, any infiltrate on chest X-ray or computed tomography scan, and in the absence of a host risk factor, a positive culture of bronchoalveolar lavage (BAL). If one criterion is not met, the case is classified as Aspergillus colonization. Importantly, AspICU is the only classification for IPA cases that has been validated using a histopathological gold standard in a large international study. BAL is therefore considered, in all subsequent case definitions, as a cornerstone for IPA diagnosis among ICU nonneutropenic patients.
Galactomannan has been previously described as a valuable tool for IPA diagnosis among immunocompromised critically ill patients [9]. As a significant rate of BAL culture are negative for Aspergillus spp. in IPA complicating viral pneumonia in particular, galactomannan has been included in mycological diagnostic criteria for further IPA case definitions, since modified AspICU [6]. Galactomannan can be measured in serum and BAL, and shows high specificity for the diagnosis of IPA. BAL galactomannan is more sensitive than serum galactomannan among nonneutropenic critically ill patients [10]. False negative results of serum galactomannan are frequent, maybe except for patients with influenza-associated pulmonary aspergillosis (IAPA) [6].
More recently, an expert panel proposed a specific case definition for IAPA or CAPA among ICU patients [11▪▪]. Probable IPA are defined as any pulmonary infiltrate with a positive culture of BAL, or a positive galactomannan in BAL or serum. A positive culture of tracheal aspirate or sputum is enough, in case of cavitating infiltrate. However, some strongly suggestive computed tomography features for IPA, such as multiple nodules or lung cavitation, can be seen in COVID-19 patients with extensive lung destruction, without CAPA. The expert panel also defined Aspergillus tracheobronchitis, notably reported in ICU patients with severe influenza [12▪], which requires a bronchoscopic evidence for airway plaque, pseudomembrane or ulcer, with any positive mycological test.
Last, 2020 European Confederation of Medical Mycology and International Society for Human and Animal Mycology (ECMM/ISHAM) case definitions for CAPA were specifically designed for critically ill covid-19 patients [13▪▪]. Probable CAPA are defined, in addition to previous mycological criteria, by two positive Aspergillus polymerase chain reaction (PCR) test in serum or one in BAL with a cycle threshold cut-off of 36, or a combination of positive PCR in serum and BAL. However, although Aspergillus PCR, particularly in BAL, shows high diagnostic accuracy in severe immunocompromised patients such as those with hematological malignancy or recipients of hematological stem cell or solid organ transplants with suspected IPA, data are insufficient to recommend their use among critically ill patients without those conditions [10,14]. In addition, nonbronchoscopic lavage (NBL), a blind application of 10–20 mL saline recovered by aspiration via a closed suction system in a patient who is intubated, is suggested to define possible CAPA cases. NBL has been used as a substitute to BAL, at a time when intensivists were reluctant to perform bronchoscopy in patients infected with SARS-CoV-2, due to the risk of aerosol generation and virus exposure [15]. However, positive mycological tests in NBL may reflect Aspergillus upper airway colonization, and their diagnostic accuracy for IPA has never been evaluated.
PATHOPHYSIOLOGY OF CORONAVIRUS DISEASE (COVID-19)-ASSOCIATED PULMONARY ASPERGILLOSIS
Multiple factors contribute to Aspergillus respiratory tract colonization and further invasion in critically ill patients infected with SARS-CoV-2 [16▪]. Underlying immunocompromised conditions or structural lung disease, direct lytic effects of the virus on respiratory epithelium resulting in defective muco-ciliary clearance and further lung and tracheobronchial injury, additional virus-related immune dysregulation increasing susceptibility to fungal infections [17], and effects of immunomodulatory drugs suppressing antifungal host defense pathways, are all aggregated factors that promote the occurrence of CAPA (Fig. 1).
FIGURE 1.
Pathogenesis of COVID-19-associated pulmonary aspergillosis. Factors promoting Aspergillus respiratory tract colonization and invasion in critically ill patients infected with SARS-CoV-2. ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; EORTC/MSGERC, European Organization for Research and Treatment of Cancer / Mycoses Study Group Education and Research Consortium; ICU, intensive care unit; IL-6, interleukin-6.
Host factors of immunosuppression (including long-term corticosteroids), older age, COPD, longer duration of mechanical ventilation, extracorporeal membrane oxygenation, and treatment with interleukin (IL)-6 inhibitors or a combination of corticosteroids and IL-6 inhibitors were reported as independent risk factor for CAPA in several cohorts (Table 2) [18▪▪,19,20,21▪]. Further, implementation of negative air pressure in ICU rooms, recommended at the beginning of the COVID-19 pandemic to protect caregivers and other patients from SARS-CoV-2 transmission, could be the source of contamination of room air by Aspergillus spp. and increase the risk of IPA among patients at high risk [22].
Table 1 (Continued).
Invasive pulmonary aspergillosis case definitions for critically ill patients
Table 2.
Multicentre cohorts specifically addressing Coronavirus disease (COVID-19)-associated pulmonary aspergillosis and including more than 100 critically ill patients
Interestingly, Aspergillus may exhibit a higher ability to reach the angioinvasion threshold in patients with influenza, rather than in patients with COVID-19, due to more severe influenza-related epithelial damage and immune dysregulation, via NADPH oxidase complex suppression [16▪]. Conversely, COVID-19 is characterized by early endothelial injury, with delayed and less extensive airway epithelium destruction [23]. CAPA occurrence may be mostly promoted by an additional effect of corticosteroids and IL-6 inhibitors used in critically ill patients. These pathophysiological hypotheses are supported by a higher incidence of IPA, a more frequent positivity of serum GM, and earlier occurrence after ICU admission in critically ill patients with influenza compared to patients with COVID-19 [6,24▪].
INCIDENCE OF CORONAVIRUS DISEASE (COVID-19)-ASSOCIATED PULMONARY ASPERGILLOSIS AMONG CRITICALLY ILL PATIENTS
Table 2 summarizes the main results of the multicenter cohorts, mostly retrospective, specifically addressing CAPA and including more than 100 critically ill patients. The reported incidence of proven or probable/putative CAPA is highly variable, with rates ranging from 3 to 28%. The French multicenter Mycovid study, which is the largest published cohort of mechanically ventilated patients with systematic respiratory screening for IPA, reports a 15% rate of proven and probable CAPA, based on ECMM/ISHAM case definitions [13▪▪]. The median time from ICU admission to CAPA diagnosis varies between 4 and 13 days, the shortest time being reported in a study with a systematic screening including BAL immediately upon admission [25▪].
The real incidence of CAPA is difficult to determine in the face of such wide ranges in the literature and may be significantly impacted by publication bias. First of all, an overlap of included patients among the main cohorts in the field has to be acknowledged, which interferes with our interpretation of the disease burden. The prospective or retrospective design also impacts the reported incidences. Besides, several studies included nonintubated patients, which might underestimate the incidence of CAPA. On the opposite, six studies excluded patients who had no or incomplete mycological testing, leading to an overestimation of reported rates of CAPA. Further, different case definitions and screening methods were used, with heterogeneous ability to differentiate invasive aspergillosis from Aspergillus colonization. In a same study, the choice of ECMM/ISHAM or IAPA expert case definitions, as compared to AspICU algorithm, can increase the incidence of CAPA from single to double [25▪,26]. Studies with systematic respiratory screening of COVID-19 critically ill patients, including BAL, are likely to minimize the risk of missed cases [18▪▪,25▪,26], but real-life scenario designs, without any standardized protocol for mycological samples, are more relevant for detecting patients with compatible clinical presentation, i.e., respiratory deterioration.
Misclassification of CAPA cases is supported by further surprising findings. First, detection of Aspergillus by any mycological test in BAL does not prove tissue invasion, and the likelihood of invasive infection is increased if circulating galactomannan is detected. However, serum galactomannan is rarely positive in reported cases of CAPA (in less than 20% of cases), which reflects, at least, the absence of angioinvasion [19,25▪,27]. Moreover, probability of survival significantly decreases with number of positive mycological tests [27], which indicates different levels of severity, or diagnostic certainty, associated with CAPA. In addition, there is a discrepancy between high rates of CAPA reported in several studies and the lack of histopathological evidence found in the literature. CAPA is, surprisingly, an uncommon autopsy-finding in COVID-19. In a systematic review of autopsy series, autopsy-proven CAPA occurred in 8 (1.2%) of 677 decedents with COVID-19 [28]. In another small case series of six patients with ARDS diagnosed with probable CAPA, none of them were confirmed by histologic examination of ultrasound and computed tomography-guided postmortem needle core biopsy of both lungs [29▪].
Finally, almost all the published cohorts are coming from Europe and USA, and report first waves data, when corticosteroid therapy was not yet the standard of care [30]. Geographical location and current practice of immunomodulation may significantly impact rates of CAPA.
CORONAVIRUS DISEASE (COVID-19)-ASSOCIATED PULMONARY ASPERGILLOSIS VERSUS INFLUENZA-ASSOCIATED PULMONARY ASPERGILLOSIS
Very few studies have compared the incidence of IPA among critically ill COVID-19 patients to an adequate control group, which is essential to further assess the risk of IPA in this population. For example, IPA has been reported to be independently associated with influenza in a large retrospective multicenter cohort of ICU nonimmunocompromised patients with influenza or noninfluenza related community-acquired pneumonia (control group) (14 versus 5%, adjusted odds ratio (OR) 5.2, 95% confidence interval (CI) 2.6–10.3) [6]. However, the association between severe influenza and IPA remains controversial. A recent French retrospective multicenter cohort reports much lower rate of IPA among 524 critically ill patients admitted for severe influenza (1.9%), based on the validated AspICU algorithm [31], which again underlines the important issue of the case definitions choice.
In a single-center retrospective study including 172 patients, fewer cases of putative IPA, according to AspICU algorithm, were observed in patients with COVID-19-related ARDS as compared to patients with non-SARS-CoV-2 viral ARDS (2% versus 15%, P = 0.003) [32]. In a planned ancillary analysis of the coVAPid European retrospective cohort, including 1047 patients who needed invasive mechanical ventilation for at least 48 h, the 28-day cumulative incidence of putative IPA was significantly lower in patients with SARS-CoV-2 (2.5%) compared with patients with influenza pneumonia (6%), even after adjusting for unbalanced risk factors for IPA, such as COPD and immunosuppression (adjusted cause-specific hazard ratio (HR) 3.29, 95% CI 1.53–7.02). As previously mentioned, median time from intubation to IPA diagnosis was longer (11 versus 6 days), and serum galactomannanwas less frequently positive (50% versus 77%) in COVID-19 patients as compared to influenza patients, which supports the hypothesis of a late angioinvasion in cases of CAPA, as compared to IAPA. However, the evaluation of the two diseases was not done simultaneously because of the absence of influenza during COVID-19 pandemic.
IMPACT OF CORONAVIRUS DISEASE (COVID-19)-ASSOCIATED PULMONARY ASPERGILLOSIS ON MORTALITY
Mortality is high among critically ill patients with CAPA, ranging from 36 to 74% (Table 2). In multivariate analyses, three out of seven multicenter cohorts have identified CAPA as an independent risk factor for death, with different effect size [18▪▪,19,25▪]. After adjustment for confounders with a logistic regression model, highest OR for 30-day mortality (11.6, 95% CI 3.2–41.3) was seen in patients with putative IPA, based on AspICU algorithm, as compared to patients without CAPA [25▪].
Surprisingly, appropriate antifungal treatment does not improve survival in critically ill COVID-19 patients with CAPA, even early diagnosed with a systematic screening protocol [18▪▪,25▪,27]. Although no study was designed to evaluate the efficacy of early antifungal treatment among patients with CAPA, these data raise the double question of the relevance of diagnostic criteria that were used, and of the real impact of IPA on the course of the COVID-19, as a nonmodifiable marker of severity for mechanically ventilated patients.
STRATEGY FOR DIAGNOSIS AND TREATMENT AT THE BEDSIDE
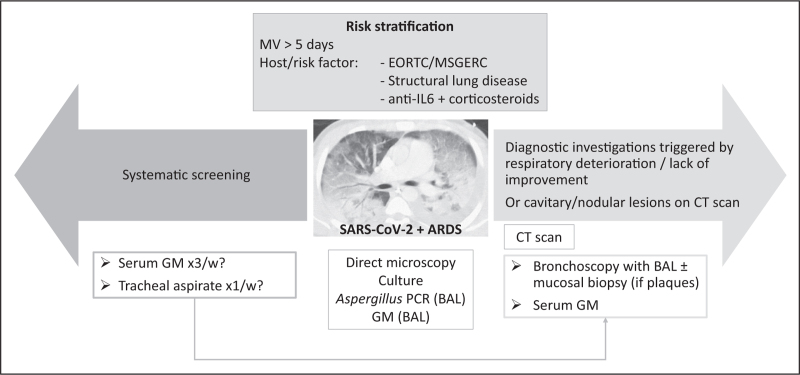
The 2020 ECMM/ISHAM guidelines suggest systematic screening for CAPA, using serum GM thrice a week, accompanied by respiratory samples, such as tracheal aspirate or NBL weekly [13▪▪]. They recommend starting antifungal treatment in possible CAPA while performing further investigations, using BAL to confirm the diagnosis. However, as discussed above, routine screening for CAPA is probably not justified and might result in overdiagnoses and inappropriate treatment. Risk factors, other than SARS-CoV-2 infection, should be taken into account for CAPA suspicion (Fig. 2). In a recent taskforce, experts in the field suggest searching for CAPA in patients with respiratory deterioration, lack of improvement, or cavitary or nodular lesions on computed tomography scan [33▪▪]. Empirical antifungal treatment should be started in patients with high probability of CAPA and a compatible clinical presentation, based on recent guidelines [34,35]. Voriconazole or isavuconazole are first-line treatments if no azole resistance is suspected. Further, decision to stop or taper concomitant corticosteroids has to be individualized, considering previous dose and duration, hyperinflammatory status, evidence for angioinvasive CAPA and response to antifungal treatment. Recent studies suggested beneficial effects of prophylactic antifungal treatment in COVID-19 patients [36,37]. However, these studies were observational, performed in single-centers, and a small number of patients were included.
FIGURE 2.
Diagnostic strategy for COVID-19-associated pulmonary aspergillosis in the ICU, based on recent recommendations. Systematic screening is suggested by the 2020 ECMM/ISHAM guidelines [13▪▪]. Diagnostic procedure triggered by clinical assessment or specific computed tomography lesions is recommended by an international taskforce of experts in the field [33▪▪]. ARDS, acute respiratory distress syndrome; BAL, bronchoalveolar lavage; CT, computed tomography; EORTC/MSGERC, European Organization for Research and Treatment of Cancer / Mycoses Study Group Education and Research Consortium; GM, galactomannan; IL-6, interleukin-6; MV, mechanical ventilation; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
CONCLUSION
CAPA is reported in 3–28% of critically ill COVID-19 patients and has been identified as an independent risk factor for death in several cohorts. Positive Aspergillus tests might reflect colonization rather than invasive disease in this population. Classification of CAPA cases is complex, and identification of patients in whom antifungal therapy would be beneficial is challenging. Further studies should validate the new case definitions, determine the impact of routine corticosteroid use on CAPA incidence, and evaluate the interest of prophylactic antifungal treatment. Finally, a pro-active diagnostic strategy based on risk stratification, clinical assessment, and BAL, triggering early empirical treatment in patients with high probability of IPA might be helpful to improve outcomes and should be further evaluated in critically ill COVID-19 patients.
Acknowledgements
None.
Financial support and sponsorship
Prof. Ignacio Martin-Loeches has been supported by SFI (Science Foundation Ireland), grant number 20/COV/0038.
Conflicts of interest
A.R.: MSD (lecture), I.M.L.: Gilead, Pfizer and Mundipharma (board and lectures), S.N.: Pfizer, Gilead, MSD, Biomérieux, Bio-Rad, Fisher and Payekel (lectures).
Contributor Information
Anahita Rouzé, Email: anahita.rouze@chru-lille.fr.
Ignacio Martin-Loeches, Email: drmartinloeches@gmail.com.
Saad Nseir, Email: saadalla.nseir@chu-lille.fr.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Meersseman W, Vandecasteele SJ, Wilmer A, et al. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med 2004; 170:621–625. [DOI] [PubMed] [Google Scholar]
- 2.Pardo E, Lemiale V, Mokart D, et al. Invasive pulmonary aspergillosis in critically ill patients with hematological malignancies. Intensive Care Med 2019; 45:1732–1741. [DOI] [PubMed] [Google Scholar]
- 3.Delsuc C, Cottereau A, Frealle E, et al. Putative invasive pulmonary aspergillosis in critically ill patients with chronic obstructive pulmonary disease: a matched cohort study. Crit Care Lond Engl 2015; 19:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levesque E, Ait-Ammar N, Dudau D, et al. Invasive pulmonary aspergillosis in cirrhotic patients: analysis of a 10-year clinical experience. Ann Intensive Care 2019; 9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contou D, Dorison M, Rosman J, et al. Aspergillus-positive lower respiratory tract samples in patients with the acute respiratory distress syndrome: a 10-year retrospective study. Ann Intensive Care 2016; 6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schauwvlieghe AFAD, Rijnders BJA, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med 2018; 6:782–792. [DOI] [PubMed] [Google Scholar]
- 7▪.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis Off Publ Infect Dis Soc Am 2020; 71:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]; Revised definitions of invasive fungal disease from EORTC/MSGERC
- 8.Blot SI, Taccone FS, Van den Abeele A-M, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med 2012; 186:56–64. [DOI] [PubMed] [Google Scholar]
- 9.Meersseman W, Lagrou K, Maertens J, et al. Galactomannan in bronchoalveolar lavage fluid: a tool for diagnosing aspergillosis in intensive care unit patients. Am J Respir Crit Care Med 2008; 177:27–34. [DOI] [PubMed] [Google Scholar]
- 10.Haydour Q, Hage CA, Carmona EM, et al. Diagnosis of fungal infections. a systematic review and meta-analysis supporting American Thoracic Society Practice Guideline. Ann Am Thorac Soc 2019; 16:1179–1188. [DOI] [PubMed] [Google Scholar]
- 11▪▪.Verweij PE, Rijnders BJA, Brüggemann RJM, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med 2020; 46:1524–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent review on influenza-associated invasive pulmonary aspergillosis
- 12▪.Nyga R, Maizel J, Nseir S, et al. Invasive tracheobronchial aspergillosis in critically ill patients with severe influenza. A clinical trial. Am J Respir Crit Care Med 2020; 202:708–716. [DOI] [PubMed] [Google Scholar]; Largest multicenter study on invasive tracheobronchial aspergillosis in critically ill patients with severe influenza.
- 13▪▪.Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis 2021; 21:e149–e162. [DOI] [PMC free article] [PubMed] [Google Scholar]; ECMM/ISHAM consensus on CAPA case definitions.
- 14.Hage CA, Carmona EM, Epelbaum O, et al. Microbiological laboratory testing in the diagnosis of fungal infections in pulmonary and critical care practice. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2019; 200:535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doggett N, Chow C-W, Mubareka S. Characterization of experimental and clinical bioaerosol generation during potential aerosol-generating procedures. Chest 2020; 158:2467–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16▪.van de Veerdonk FL, Brüggemann RJM, Vos S, et al. COVID-19-associated Aspergillus tracheobronchitis: the interplay between viral tropism, host defence, and fungal invasion. Lancet Respir Med 2021; 9:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]; Narrative review on COVID-19-associated Aspergillus tracheobronchitis, including comparative pathophysiological insights with influenza
- 17.Arastehfar A, Carvalho A, van de Veerdonk FL, et al. COVID-19 associated pulmonary aspergillosis (CAPA)-from immunology to treatment. J Fungi Basel Switz 2020; 6:E91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪▪.Gangneux J-P, Dannaoui E, Fekkar A, et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French multicentre MYCOVID study. Lancet Respir Med 2022; 10:180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]; Largest multicenter study on incidence and outcome of fungal disease in critically ill COVID-19 patients.
- 19.Prattes J, Wauters J, Giacobbe DR, et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients-a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 2022; 28:580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fekkar A, Lampros A, Mayaux J, et al. Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. Am J Respir Crit Care Med 2021; 203:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪.Janssen NAF, Nyga R, Vanderbeke L, et al. Multinational observational cohort study of COVID-19-associated pulmonary aspergillosis1. Emerg Infect Dis 2021; 27:2892–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]; Large multicenter international cohort on the incidence of CAPA.
- 22.Ichai P, Saliba F, Baune P, et al. Impact of negative air pressure in ICU rooms on the risk of pulmonary aspergillosis in COVID-19 patients. Crit Care Lond Engl 2020; 24:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020; 383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24▪.Rouzé A, Lemaitre E, Martin-Loeches I, et al. Invasive pulmonary aspergillosis among intubated patients with SARS-CoV-2 or influenza pneumonia: a European multicenter comparative cohort study. Crit Care Lond Engl 2022; 26:11. [DOI] [PMC free article] [PubMed] [Google Scholar]; Large European cohort comparing invasive pulmonary aspergillosis incidence between severe influenza and COVID-19 critically ill patients.
- 25▪.Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: a prospective study. Clin Infect Dis Off Publ Infect Dis Soc Am 2021; 73:e3606–e3614. [DOI] [PMC free article] [PubMed] [Google Scholar]; First prospective multicenter study on CAPA incidence and its impact on outcome.
- 26.White PL, Dhillon R, Cordey A, et al. A National Strategy to Diagnose Coronavirus Disease 2019-associated invasive fungal disease in the intensive care unit. Clin Infect Dis Off Publ Infect Dis Soc Am 2021; 73:e1634–e1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bretagne S, Sitbon K, Botterel F, et al. COVID-19-associated pulmonary aspergillosis, fungemia, and pneumocystosis in the intensive care unit: a retrospective multicenter observational cohort during the first French pandemic wave. Microbiol Spectr 2021; 9:e0113821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kula BE, Clancy CJ, Hong Nguyen M, Schwartz IS. Invasive mould disease in fatal COVID-19: a systematic review of autopsies. Lancet Microbe 2021; 2:e405–e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪.Flikweert AW, Grootenboers MJJH, Yick DCY, et al. Late histopathologic characteristics of critically ill COVID-19 patients: Different phenotypes without evidence of invasive aspergillosis, a case series. J Crit Care 2020; 59:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]; Interesting case-series of histological cases of COVID-19 patients.
- 30.Collaborative Group RECOVERY, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coste A, Frérou A, Raute A, et al. The extent of aspergillosis in critically ill patients with severe influenza pneumonia: a multicenter cohort study. Crit Care Med 2021; 49:934–942. [DOI] [PubMed] [Google Scholar]
- 32.Razazi K, Arrestier R, Haudebourg AF, et al. Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to Coronavirus 19 disease. Crit Care Lond Engl 2020; 24:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪▪.Verweij PE, Brüggemann RJM, Azoulay E, et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med 2021; 47:819–834. [DOI] [PMC free article] [PubMed] [Google Scholar]; Taskforce on CAPA diagnosis and treatment in critically ill patients.
- 34.Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 2018; 24:e1–e38. [DOI] [PubMed] [Google Scholar]
- 35.Patterson TF, Thompson GR, Denning DW, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis Off Publ Infect Dis Soc Am 2016; 63:e1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatzl S, Reisinger AC, Posch F, et al. Antifungal prophylaxis for prevention of COVID-19-associated pulmonary aspergillosis in critically ill patients: an observational study. Crit Care 2021; 25:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Ackerbroeck S, Rutsaert L, Roelant E, et al. Inhaled liposomal amphotericin-B as a prophylactic treatment for COVID-19-associated pulmonary aspergillosis/aspergillus tracheobronchitis. Crit Care Lond Engl 2021; 25:298. [DOI] [PMC free article] [PubMed] [Google Scholar]