Abstract
T-cell proliferation is an important in vitro parameter of in vivo immune function and has been used as a prognostic marker of human immunodeficiency virus type 1 (HIV-1) disease progression. The proliferative capacity of T cells in response to various stimuli is commonly determined by a radioactive assay based on incorporation of [3H]thymidine ([3H]TdR) into newly generated DNA. In order to assess techniques for application in laboratories where radioactive facilities are not present, two alternative methods were tested and compared to the [3H]TdR assay as a “gold standard.” As an alternative, T-cell proliferation was measured by flow cytometric assessment of CD38 expression on T cells and by an enzyme-linked immunosorbent assay (ELISA) based on bromo-2′-deoxyuridine (BrdU) incorporation. Peripheral blood mononuclear cells (PBMCs), either in whole blood or Ficoll-Isopaque separated, from a total of 26 HIV-1-positive and 18 HIV-1-negative Dutch individuals were stimulated with CD3 monoclonal antibody (MAb) alone, a combination of CD3 and CD28 MAbs, or phytohemagglutinin. BrdU incorporation after 3 days of stimulation with a combination of CD3 and CD28 MAbs correlated excellently with the [3H]TdR incorporation in both study groups (HIV-1 positives, r = 0.96; HIV-1 negatives, r = 0.83). A significant correlation of absolute numbers of T cells expressing CD38 with [3H]TdR incorporation, both in HIV-1-positive (r = 0.96) and HIV-1-negative (r = 0.84) individuals, was also observed under these conditions. The results of this study indicate that determination of both the number of CD38-positive T cells and BrdU incorporation can be used as alternative techniques to measure the in vitro T-cell proliferative capacity. The measurement of CD38 expression on T cells provides the additional possibility to further characterize the proliferating T-cell subsets for expression of other surface markers.
Human immunodeficiency virus (HIV) infection results in a significant quantitative loss of CD4+ T cells relatively late after seroconversion (9, 28). However, qualitative differences in the functional performance of peripheral blood mononuclear cells (PBMCs) from HIV-infected subjects can be detected much earlier. Loss or reduction of T-cell proliferative capacity to in vitro stimulation is one of these qualitative changes (4, 13, 20, 23, 31). Decreased proliferation of T cells from HIV-infected individuals has been measured in response to in vitro stimulation with CD3 monoclonal antibody (MAb), pokeweed mitogen, and recall antigens (3, 33, 34). The response to phytohemagglutinin (PHA) remains unaffected in the early phases but is significantly reduced later in infection (8, 14). It has been reported that T-cell proliferation in response to stimulation with CD3 and CD3+CD28 MAbs decreases shortly after seroconversion, before the decline in CD4+ T-cell number is observed (10, 23, 33). It has also been demonstrated that loss of T-cell reactivity to CD3 and CD3+CD28 MAbs in vitro is a strong predictive marker for progression to AIDS, independent of decline in CD4 counts (32, 33). Thus, T-cell proliferative capacity is an important independent predictor of progression to HIV disease (31, 33) and also can serve to monitor immunological improvement after therapy (1, 26).
The most commonly used method to determine T-cell proliferative capacity is based on measuring tritiated thymidine ([3H]TdR) incorporation into the DNA of proliferating cells. Since this method requires radioactive facilities, it has drawbacks in places where these facilities and the management of waste are not available. In addition, proliferation as measured by [3H]TdR incorporation does not give information on which subsets of cells are in fact proliferating to the in vitro stimuli.
CD38 is a type II transmembrane glycoprotein originally identified by the MAb OKT 10 (30). It plays a role in lymphocyte adhesion, proliferation, and cytokine production (7). Most peripheral T and B cells, as well as red blood cells, are CD38 negative (18, 30). However, CD38 is expressed on activated lymphocytes; thus, it has been used as an activation marker of T cells. In HIV-infected individuals, the in vivo expression of CD38 on T cells is elevated and reported to be predictive of progression of HIV disease to AIDS and death (2, 12, 15, 16, 27). CD38 was also expressed on immunoglobulin (Ig)-secreting plasma B cells. Finally, CD38 has also been detected on immature cells, i.e., thymocytes and germinal center B cells (17).
In this paper, two alternative methods were evaluated for assessment of T-cell proliferative capacity in response to in vitro stimulation. These methods involved flow cytometric measurement of CD38 expression on T cells and enzyme-linked immunosorbent assay (ELISA) determination of 5-bromo-2′-deoxyuridine (BrdU) incorporation into newly synthesized DNA of proliferating T cells. BrdU is a pyrimidine analogue and is incorporated in place of thymidine into DNA of proliferating cells. It is measured by ELISA using peroxidase-labeled anti-BrdU antibody.
The two methods were tested for applicability on both HIV-negative and HIV-positive samples and compared to the commonly used [3H]TdR incorporation assay as a “gold standard.”
MATERIALS AND METHODS
Study population.
Heparin venous blood samples were collected from HIV-1-infected participants (n = 26; medians: CD4 count, 330/μl; CD8 count, 870/μl; CD4-to-CD8 ratio, 0.45) of the Amsterdam cohort study on HIV-1 infection and AIDS and from healthy Dutch blood donors (n = 18; medians: CD4 count, 993/μl; CD8 count, 506/μl; CD4-to-CD8 ratio, 2.0) after informed consent.
Culture conditions.
Both the [3H]TdR and the CD38 experiments were performed either on whole blood or on Ficoll-Isopaque (Pharmacia, Uppsala, Sweden)-isolated PBMCs. Whole-blood lymphocyte culture was done as described previously (5). Briefly, blood samples were diluted 1:10 in Iscove's modified Dulbecco's medium supplemented with gentamycin, 2-mercaptoethanol (5 × 10−5 M), and 20% human pooled serum. A sample of 150 μl of the diluted blood was cultured in round-bottom 96-well plates. PBMCs were used at a concentration of 4 × 104 cells/well. Cells were stimulated with CD3 MAb (CLB T3/4.E, IgE; final dilution of ascites, 1:104 [35]), a combination of CD3 and CD28 MAbs (CLB-CD28/1, IgG1; final dilution of ascites, 1:103 [36]) or PHA (final concentration, 4 μg/ml; Wellcome, Dartford, United Kingdom).
[3H]TdR incorporation assay.
Both whole-blood and PBMC cultures were set up in triplicate in 96-well plates. Proliferation was measured at different time points by adding 0.2 mCi of [3H]TdR (Amersham, Little Chalfont, Buckinghamshire, United Kingdom) per well during the last 24 h of culture. Incorporated [3H]TdR was measured on washed cells, harvested using a cell harvester (Titertek, Helsinki, Finland), and analyzed in a beta counter (LKB, Bromma, Sweden). Incorporated radioactivity is expressed as counts per minute.
Measurement of CD38-positive T cells.
The whole-blood or PBMC cultures of six 96-well plate wells were pooled in order to have sufficient numbers of cells for FACScan analysis. Approximately 2.5 × 105 cells were incubated with a combination of phycoerythrin-conjugated CD3 MAb and fluorescein isothiocyanate-conjugated CD38 MAb (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) for 15 min. Red blood cells were lysed by adding 2 ml of lysing solution (FACSlyse; Becton Dickinson). Cells were washed twice with phosphate-buffered saline containing 0.1% bovine serum albumin and 0.01% sodium azide. Isotype-specific control MAbs (Becton Dickinson) were used on a daily basis to control for background fluorescence and to set the cursors. Data acquisition was done on a minimum of 3,000 CD3+ events, and analysis was performed using Cellquest software on a FACScan (Becton Dickinson).
BrdU ELISA.
For the BrdU ELISA, various concentrations of PBMCs and whole blood were tested. Whole blood yielded very high background signals, and the best signal-to-noise ratio for PBMCs was reached at 3 × 104 PBMCs per well of a 96-well plate. The ELISA method used was the Cell Proliferation ELISA System, version 2 (Amersham), which was performed according to the instructions of the manufacturer. Briefly, PBMCs were cultured in triplicate for 3 days in 96-well plates in the presence or absence of CD3 plus CD28 MAbs as a stimulant. BrdU labeling reagent (final concentration, 10 μM) was added after 48 h of culture. At 72 h, the cells were harvested by centrifugation at 300 × g for 10 min and were fixed for 30 min in ethanol solution. Blocking was done by incubating for 30 min at room temperature in 1% (wt/vol) protein in 50 mM Tris-HCl–150 mM NaCl, pH 7.4, followed by incubation with peroxidase-labeled anti-BrdU for 1 h. As a substrate, 100 μl of 3,3,5,5-tetramethylbenzidine (TMB) dissolved in 15% (vol/vol) dimethylsulfoxide was used. Optical density (OD) was determined at 450 nm using an ELISA reader (Organon Teknika, The Netherlands). Culture medium alone and cells incubated with peroxidase-labeled anti-BrdU in the absence of BrdU were used as controls for nonspecific binding.
Statistical analysis.
The association between two alternative technologies was determined by calculation of the Pearson correlation coefficient (r). A square transformation was applied to the variable describing the percentage of CD3+ cells expressing CD38 to linearize the regression model comparing it to the counts per minute as suggested by Kleinbaun et al. when the original relationship is curvelinear downwards (19).
RESULTS
Determination of optimal in vitro stimulus.
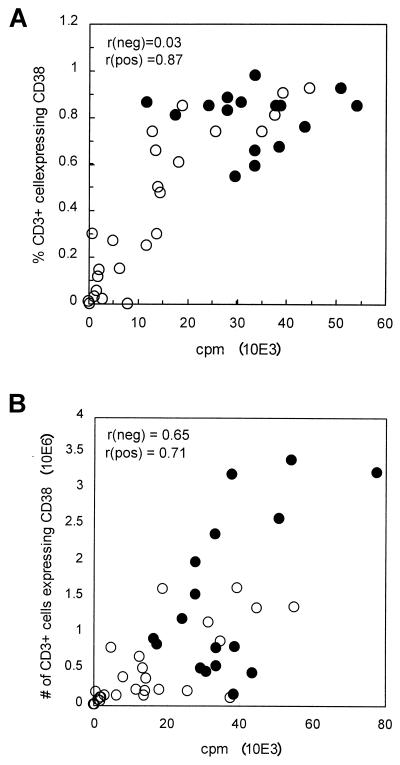
To determine the optimal conditions for using CD38 as a readout for T-cell stimulation, PBMCs were cultured for 3 days, a stimulation period which was established to be optimal for the [3H]TdR incorporation assay (5). Three different stimuli were used: (i) CD3 MAb, (ii) CD3+CD28 MAbs, and (iii) PHA. Table 1 shows the correlation of absolute numbers of CD38+ T cells with counts per minute determined by [3H]TdR incorporation assays in response to the various stimuli. Stimulation by the combination of CD3+ CD28 MAbs resulted in the strongest (r = 0.75) overall association (HIV-negative samples, r = 0.65; HIV-positive samples, r = 0.71). Stimulation with CD3 MAb yielded correlations of 0.84 (for HIV−) and 0.15 (for HIV+), with an overall correlation of 0.71, and stimulation with PHA yielded correlations of 0.55 (for HIV−) and 0.24 (for HIV+), with an overall correlation of 0.46. Thus, the combination of CD3+CD28 MAbs was used in all of the following experiments, which measured CD38 expression. Figure 1 details the correlation pattern of percentage (Fig. 1A) and absolute numbers (Fig. 1B) of CD3+ CD38+ T cells with [3H]TdR incorporation in HIV-positive (n = 26) versus HIV-negative (n = 18) individuals. As shown in Fig. 1A, the use of the percentage of CD3+ cells expressing CD38 as a readout could discriminate well between the proliferation capacity of HIV-negative and HIV-positive subjects and has an overall good correlation with the [3H]TdR incorporation assay (r = 0.81). However, as a result of a high percentage of CD3+ cells expressing CD38 in response to the in vitro stimulation in most of the HIV− subjects, the correlation with the [3H]TdR incorporation assay for this group looks poor (r = 0.03 for HIV− versus r = 0.87 for HIV+). Therefore, the absolute number of CD3+ CD38+ cells is preferred to be used as a readout for comparison with the [3H]TdR incorporation assay.
TABLE 1.
Comparison of median percentages and absolute numbers of CD3+ T cells expressing CD38 with median [3H]TdR incorporation after 3 days of stimulation of whole-blood samples with different stimulantsa
| Stimulant | Subject group (n) | % CD38+ T cells | Absolute no. of CD38+ T cells (105) | cpm (104) (range) | Correlation (r)b | P |
|---|---|---|---|---|---|---|
| Anti-CD3 | HIV− (9) | 49 (1–87) | 1.4 (0.06–8.7) | 0.5 (0.08–1.2) | 0.84 | 0.005 |
| HIV+ (8) | 12 (8–54) | 1.0 (0.5–3.5) | 0.02 (0.008–0.7) | 0.15 | NSc | |
| All (17) | 37 (1–87) | 1.2 (0.06–8.7) | 0.2 (0.008–1.2) | 0.71 | 0.001 | |
| Anti-CD3+ CD28 | HIV− (18) | 92 (74–99) | 10.5 (1.6–34.0) | 3.4 (1.2–8.3) | 0.65 | 0.004 |
| HIV+ (26) | 56 (1–96) | 2.1 (0.2–16.3) | 1.3 (0.005–5.5) | 0.71 | <0.001 | |
| All (44) | 81 (2–99) | 5.3 (0.2–33.9) | 2.5 (0.008–8.2) | 0.75 | <0.001 | |
| PHA | HIV− (9) | 87 (74–99) | 7.4 (0.5–27.5) | 3.7 (2.3–5.4) | 0.55 | NS |
| HIV+ (8) | 57 (25–85) | 2.2 (0.6–3.0) | 0.9 (0.1–4.1) | 0.24 | NS | |
| All (17) | 78 (25–99) | 2.2 (0.5–27.5) | 2.8 (0.1–5.4) | 0.46 | 0.06 |
95% ranges are shown in parentheses.
Correlations are between counts per minute and absolute numbers of CD38+ T cells.
NS, not significant.
FIG. 1.
Correlation of percentage (A) and number (B) of CD3+ cells expressing CD38 with a standard 3-day [3H]TdR incorporation assay for 18 HIV− (●) and 26 HIV+ (○) PBMCs stimulated with CD3+CD28 MAbs.
Determination of optimal stimulation time.
Time-response experiments were performed to determine the optimal period of stimulation using the above combination of CD3+CD28 MAbs. Again, day 3 [3H]TdR incorporation was used as a gold standard. As shown in Table 2, the best correlation of absolute numbers of CD3+ T cells expressing CD38 with [3H]TdR incorporation, including a proper signal-to-noise ratio, was found after 3 days of stimulation with CD3+CD28 (r = 0.96 for HIV+; r = 0.84 for HIV−).
TABLE 2.
Comparison of median percentages and absolute numbers of CD3+ T cells expressing CD38, after different periods of stimulation, with median [3H]TdR incorporation after 3 days of stimulation of whole-blood samples with CD3+ CD28 MAbsa
| Stimulation period | Subject group (n) | % CD38+ T cells | Absolute no. of CD38+ T cells (104) | cpm (104) | Correlation (r)b | P |
|---|---|---|---|---|---|---|
| 1 day | HIV− (4) | 21 (15–27) | 8.8 (5.4–14.9) | −0.66 | NSc | |
| HIV+ (8) | 11 (6–20) | 3.5 (1.2–9.2) | −0.02 | NS | ||
| All (12) | 16 (6–27) | 5.7 (1.2–14.9) | 0.18 | NS | ||
| 2 days | HIV− (4) | 37 (34–59) | 17.1 (15.1–19.3) | −0.71 | NS | |
| HIV+ (8) | 23 (17–61) | 6.7 (4.5–36.1) | 0.71 | 0.04 | ||
| All (12) | 31 (17–61) | 12.3 (4.5–36.1) | 0.69 | 0.01 | ||
| 3 days | HIV− (4) | 81 (74–92) | 80.2 (51.0–80.8) | 3.2 (1.8–3.9) | 0.84 | <0.001 |
| HIV+ (8) | 75 (24–96) | 44.2 (5.6–135.4) | 1.3 (0.06–4.5) | 0.96 | <0.001 | |
| All (12) | 81 (24–96) | 51.7 (5.6–135.4) | 1.6 (0.06–4.5) | 0.91 | <0.001 | |
| 4 days | HIV− (4) | 96 (84–97) | 174.9 (90.2–238.2) | 0.09 | NS | |
| HIV+ (8) | 93 (47–98) | 110.6 (13.6–399.8) | 0.98 | <0.001 | ||
| All (12) | 94 (47–98) | 124.7 (13.6–399.8) | 0.83 | <0.001 |
95% ranges are shown in parentheses.
Correlations are between counts per minute and absolute numbers of CD38+ T cells.
NS, not significant.
Analysis of CD38 expression on T cells after in vitro stimulation.
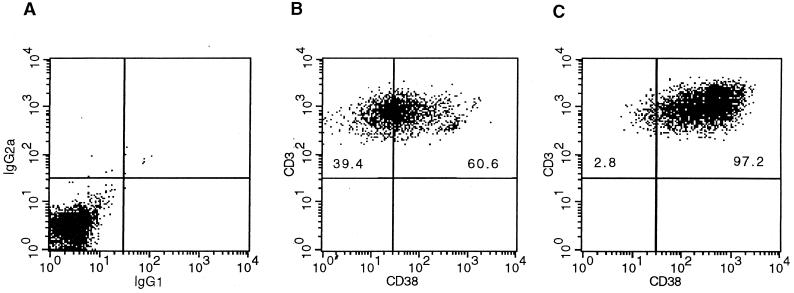
Figure 2 shows typical fluorescence-activated cell sorter (FACS) analysis dot plots of CD38 expression on CD3+ T cells for an HIV-positive individual (Fig. 2B) and an HIV-negative individual (Fig. 2C) after 3 days of culture with CD3+ CD28 MAbs. A dot plot from an isotype control tube is also shown in Fig. 2A. Invariably, the PBMCs of HIV-positive individuals show lower proportions and absolute numbers of T cells expressing CD38 after stimulation compared to those of HIV-negative individuals (in this case, 60.6% versus 97.2%).
FIG. 2.
Representative dot plots of PBMCs stained with aCD3-PE and aCD38-FITC. Data were obtained with samples from an isotype control tube (A), an HIV-positive subject (B), and an HIV-negative subject (C) after 3 days of stimulation with CD3+CD28 MAbs.
Kinetics of CD3+ and CD3+ CD38+ T cells.
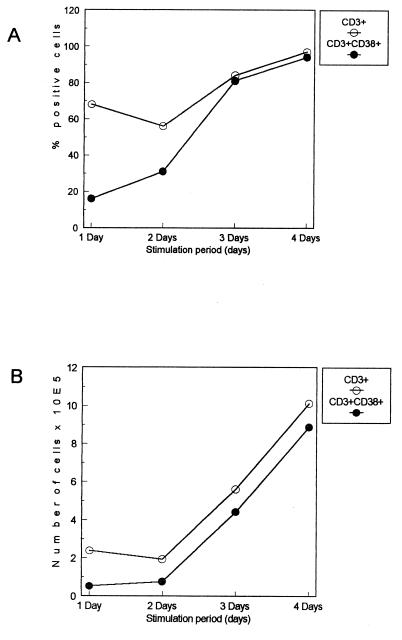
We followed the kinetics of the percentage and number of CD3+ T cells and their CD38-expressing subsets over 4 days in anti-CD3+CD28-stimulated cells from 12 HIV-positive and -negative subjects. As shown in Fig. 3, the percentage and number of CD3+ T cells increase continuously. The percentage of CD3+ CD38+ T cells also increases in parallel, and the increase seems to be sharp after 2 days of culture.
FIG. 3.
Kinetics of percentage and absolute number of CD3+ and CD3+ CD38+ T cells over 4 days in cells stimulated with anti-CD3+CD38. Each point represents median percentage and absolute numbers from 12 HIV-negative and HIV-positive subjects.
BrdU ELISA.
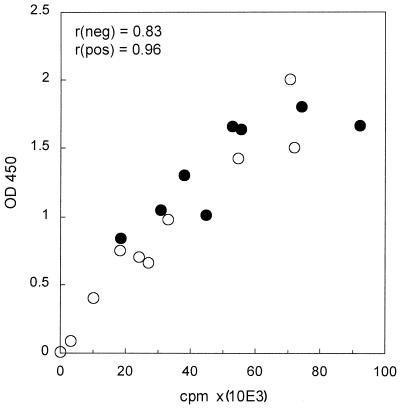
The BrdU ELISA procedure, which utilizes the incorporation of BrdU into the DNA of proliferating cells, was performed on Ficoll-Isopaque-isolated PBMCs only. The method was not found to be suitable for whole-blood culture, because of the high background due to red blood cells interfering with the ELISA reader OD450 measurements. As shown in Fig. 4, the OD450 results of the BrdU ELISA correlated strongly (overall, r = 0.82; HIV−, r = 0.83; HIV+, r = 0.96) with the counts per minute of the [3H]TdR incorporation assay. This correlation was further improved (overall, r = 0.92) when the final substrate reaction supernatant was transferred into a new 96-well plate before measuring OD450.
FIG. 4.
Correlation of OD450 of BrdU ELISA with standard 3-day [3H]TdR incorporation assay for 8 HIV− (●) and 10 HIV+ (○) PBMCs stimulated with CD3+CD28 MAbs. Samples from a group of subjects different from those presented in Fig. 1 were used for this assay.
DISCUSSION
The [3H]TdR incorporation assay is widely applied as a measure of T-cell proliferation in vitro and thus as an indirect quantification of T-cell activation. However, this technology gives only information on the overall proliferative responses, not detailing the specific cell subsets involved in these responses. In addition, this technology is only applicable in laboratories where radioactive facilitates are present and proper waste management is implemented.
In this paper, two alternative methods to measure T-cell stimulation are described, one which measures the expression of the T-cell activation marker CD38 and the other which measures BrdU incorporation into newly generated DNA.
The optimal reaction conditions for using CD38 expression as an alternative readout to [3H]TdR incorporation proved to be 3 days of stimulation with the combination of CD3+CD28 MAbs. When CD3 MAb alone was used, the overall stimulation and also its correlation with [3H]TdR incorporation was lower. This is in accordance with previous reports that, for optimal mitogenic stimulation, signaling not only through the T-cell receptor (via CD3) but also through costimulatory molecules (like CD28) is essential (21).
As presented in this report, CD38 expression paralleled DNA synthesis as measured by the [3H]TdR incorporation assay. This observation may indicate that the cells expressing CD38 after the in vitro stimulation are proliferating. Paradoxically, T cells obtained from HIV+ patients with high expression of CD38 in vivo were found to be confined to the nonproliferative phase of the cell cycle (22). This may suggest that T cells expressing CD38 have different characteristics in terms of proliferation depending on whether the activation is in vivo or in vitro.
Studies have reported that CD38 binding elicits activation and proliferation programs in T cells, and the involvement of CD38 in signal transduction and cell adhesion is indicated (11). Furthermore, an important association between a reduced T-cell proliferation and reduced percentage of CD38+ T cells has been shown for people with depression (6). Our results may further support the importance of CD38 in T-cell proliferation.
We have shown that measurement of CD38 expression can be exploited as an alternative readout for the capacity of T cells to proliferate in response to different stimuli. The correlation of the absolute number of CD38+ T cells with the [3HTdR] assay varied for the three stimuli used, being higher for the CD3+CD28 MAb. This may be because of the variation in the capacity of the three stimuli used to activate the two pathways. Despite this difference, the flow cytometric method as the [3H]TdR incorporation assay was also capable of discriminating between the T-cell response in the HIV-1-infected and noninfected subjects. Furthermore, the percentage and number of CD3+ T cells increased in parallel with the percentage and absolute number of CD38-expressing T cells. However, in our opinion, the direct measurement of CD3+ T cells cannot be used as an alternative since it can be affected by the apparent individual variation in baseline values.
Several studies by us and others (2, 12, 24) have shown that CD38 expression on circulating CD4+ and CD8+ T cells is increased during HIV-1 infection. The data presented here on the expression of CD38 on in vitro-stimulated T cells show that the percentage of CD38-expressing cells in HIV-positive individuals are already low after 1 day in culture. As shown in Table 1, the median CD38 expression for HIV+ subjects is 11% (range, 6 to 20%), which is lower than previously reported in vivo values for HIV subjects at different clinical stages: 31 to 69% for CD8+ T cells (2), 37 to 55% for CD4+ T cells and 44 to 81% for CD8+ T cells (24); and 55 to 68% for total lymphocytes, 66 to 78% for CD4+ T cells, and 75 to 87% for CD8+ T cells (27). After 1 day in culture, the difference in the percentage of CD38-positive cells between the HIV-negative and HIV-positive subjects was not large compared to what has been observed for the in vivo situation. As also suggested by other studies (29), this may indicate that the in vivo-activated cells fail to survive in vitro and undergo apoptosis after overnight incubation.
It has been reported that CD38 expression, especially on CD8 cells, is high in children and it decreases with age (23). Therefore, the use of the CD38 expression method to measure T-cell proliferation capacity in children needs to be further studied.
We observed that the BrdU ELISA also showed a good association with the [3H]TdR incorporation (overall correlation, r = 0.82). The correlation was further improved (r = 0.92) when the final reaction supernatant was transferred to a new 96-well plate before measuring OD450. This may be due to background signals caused by the fixed cells.
The CD38 method needs equal investments in terms of laboratory equipment as compared to the [3H]TdR incorporation assay. However, no radioactivity is used, and in addition, the nature of the proliferating T-cell subsets can be studied in much more detail by combining CD38 MAbs with other reagents for FACS analysis. Furthermore, the signal-to-noise ratio is higher (up to 20:1) than that of the BrdU ELISA (up to 10:1). The broad expression of CD38 on stimulated T cells may indicate its potential use for detection of low-frequency responses.
The BrdU method is clearly much more economical than the above two methods; however, its sensitivity is limited by the OD450 readout range (between 0.000 and 3.000) and it does not allow for subspecifying the contribution of certain T-cell subsets to the overall proliferation signal. Also, the assay proved to be only feasible on isolated PBMCs and it is not suitable for whole-blood cultures, because of the high background due to the presence of red blood cells interfering with the OD450 reading. However, it is worth mentioning that the BrdU method is currently being used successfully in our laboratory to measure proliferation responses in PHA- and purified protein derivative-stimulated PBMCs from both HIV-negative as well as HIV-positive Ethiopians (M. Legesse, personal communication).
In conclusion, the data presented in this paper show that the BrdU method, followed by the CD38 expression method, showed a significant agreement with the widely used [3H]TdR incorporation assay in measuring T-cell proliferation in response to stimulation by PHA, anti-CD3, and anti-CD3+CD28, indicating that both methods can be used as alternative techniques to measure in vitro T-cell proliferative capacity in response to the above stimulants. However, the use of these methods for measuring low-frequency responses (responses to recombinant antigens) needs to be assessed.
ACKNOWLEDGMENTS
This study is part of the Ethio-Netherlands AIDS Research Programme (ENARP), a collaborative effort of the Ethiopian Health and Nutrition Research Institute (EHNRI), the Amsterdam Municipal Health Service (GG/GD), the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service (CLB), and the Academic Medical Center of the University of Amsterdam (AMC). ENARP is financially supported by the Netherlands Ministry of Foreign Affairs and the Ethiopian Ministry of Health (MOH) as a bilateral project.
REFERENCES
- 1.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects on combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 2.Autran B, Giorgi J V. Activated CD8+ cells in HIV-related diseases. In: Janossy G, Autran B, Miedema F, editors. Immunodeficiency in HIV infection and AIDS. Basel, Switzerland: Karger Basel; 1992. pp. 171–184. [Google Scholar]
- 3.Ballet J J, Coudrec L J, Rabian-Herzog C, Duval-Roy C, Janier F, Danon M, Clauvel J P, Seligmann M. Impaired T-lymphocyte dependent immune responses to microbial antigens in patients with HIV-1 associated persistent generalized lymphadenopathy. AIDS. 1988;2:291–297. doi: 10.1097/00002030-198808000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Bentin J, Tsoukas C, McCutchan J A, Spector S E, Richman D D, Vaugfan J H. Impairment in T-lymphocyte responses during early infection with the human immunodeficiency virus. J Clin Immunol. 1989;9:159–168. doi: 10.1007/BF00916944. [DOI] [PubMed] [Google Scholar]
- 5.Bloemena E, Roos M T L, van Heijst J L A M, Vossen J M J J, Schellekens P T A. Whole-blood lymphocyte cultures. J Immunol Methods. 1989;122:161–167. doi: 10.1016/0022-1759(89)90260-3. [DOI] [PubMed] [Google Scholar]
- 6.Castle S, Wilkins S, Heck E, Tanzy K, Fahey J. Depression in caregivers of demented patients is associated with altered immunity: impaired proliferative capacity, increased CD8+, and a decline in lymphocytes with surface signal transduction molecules (CD38+) and a cytotoxicity marker (CD56+CD8+) Clin Exp Immunol. 1995;101:487–493. doi: 10.1111/j.1365-2249.1995.tb03139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cesano A, Visonneau S, Deaglio S, Malavasi F, Santoli D. Role of CD38 and its ligand in the regulation of MHC-nonrestricted cytotoxic T-cells. J Immunol. 1998;160:1106–1115. [PubMed] [Google Scholar]
- 8.Clerici M, Stocks N I, Zajac R A, Boswell R N, Lucey D R, Via C S, Shearer G M. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic human immunodeficiency virus-seropositive patients. J Clin Investig. 1989;84:1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Wolf F, Lange J M A, Houweling J T M, Coutinho R A, Schellekens P T, van der Noorda J, Goudsmit J. Numbers of CD4+ cells and the levels of core antigens of and antibodies to the human immunodeficiency virus as predictors of AIDS among seropositive homosexual men. J Infect Dis. 1988;158:615–622. doi: 10.1093/infdis/158.3.615. [DOI] [PubMed] [Google Scholar]
- 10.Dolan J M, Clerici M, Blatt S P, Hendrix C W, Melcher G P, Boswell R N, Freeman T M, Ward W, Hensley R, Shearer G M. In vitro T-cell function, delayed-type hypersensitivity skin testing and CD4 T-cell subset phenotyping independently predict survival time in patients infected with human immunodeficiency virus. J Infect Dis. 1995;172:79–87. doi: 10.1093/infdis/172.1.79. [DOI] [PubMed] [Google Scholar]
- 11.Funaro A, Spagnoli G C, Ausielo C M, Alessio M, Roggero S, Delia D, Zaccolo M, Malavasi F. Involvement of the multilineage CD38 molecule in a unique pathway of cell activation and proliferation. J Immunol. 1990;145:2390–2396. [PubMed] [Google Scholar]
- 12.Giorgi J V, Ho H N, Hirji K, Chou C C, Hultin L E, O'Rourke S, Park L, Margolick J B, Ferbas J, Phair J P the Multicenter AIDS Cohort Study Group. CD8+ lymphocyte activation at human immunodeficiency virus type 1 seroconversion: development of HLA− DR+ CD38−CD8+ cells is associated with subsequent stable CD4+ cell levels. J Infect Dis. 1994;170:775–781. doi: 10.1093/infdis/170.4.775. [DOI] [PubMed] [Google Scholar]
- 13.Gruters R A, Terpstra F G, De Jong R, Van Noesel C J M, Van Lier R A W, Miedema F. Selective loss of T-cell functions in different stages of HIV infections. Eur J Immunol. 1990;20:1039–1044. doi: 10.1002/eji.1830200514. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann B, Jakobsen K D, Odum N, Dickmeiss E, Platz P, Ryder L P, Pedersen C, Mathiesen L, Bygbjerg I, Faber V, Sveffaard A. HIV induced immunodeficiency. Relatively preserved phytohemagglutinin as opposed to decreased pokeweed mitogen responses may be due to possibly preserved responses via CD2/phytohemaglutinin pathway. J Immunol. 1989;142:1874–1880. [PubMed] [Google Scholar]
- 15.Holter W, Majdic O, Liszka K, Stockinger H, Knapp W. Kinetics of activation antigen expression by in vitro stimulated human T lymphocytes. Cell Immunol. 1985;90:322–330. doi: 10.1016/0008-8749(85)90197-2. [DOI] [PubMed] [Google Scholar]
- 16.Hong H N, Hultin L E, Mitsuyasu R T, Matud J L, Hausner M A, Bockstoce D, Chou C C, O'Rourke S, Taylor J M G, Giorgi J V. Circulating HIV specific CD8+ cytotoxic T cells express CD38 and HLA-DR antigens. J Immunol. 1993;150:3070–3079. [PubMed] [Google Scholar]
- 17.Jackson G D, Bell I J. Isolation of a cDNA encoding the human CD38(T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J Immunol. 1990;144:2811–2815. [PubMed] [Google Scholar]
- 18.Janosy G, Tidman N, Papageorgiou S E, Kung P C, Goldstein G. Distribution of T-lymphocyte subsets in the human bone marrow and thymus: an analysis with monoclonal antibodies. J Immunol. 1981;126:1608–1613. [PubMed] [Google Scholar]
- 19.Kleinbaun D G, Kupper L L, Muller K E. Applied regression analysis and other multivariate methods. Boston, Mass: PWS-Kent Publishing Company; 1988. [Google Scholar]
- 20.Lane H C, Depper J L, Greene W C, Whalen G, Waldmann T A, Fauci A S. Qualitative analysis of immune function patients with the acquired immunodeficiency syndrome. N Engl J Med. 1985;313:79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- 21.Linsely P S, Clark E A, Ledbetter J A. T-cell antigen CD28 mediates adhesion with B-cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci USA. 1990;87:5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahalingam M, Pozniak A, Mcmanus T J, Vergani D, Peakman M. Cell cycling in HIV infection: analysis of in vivo activated lymphocytes. Clin Exp Immunol. 1995;102:481–486. doi: 10.1111/j.1365-2249.1995.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCloskey T W, Cavaliere T, Bakshi S, Harper R, Fagin J, Kohn N, Pahwa S. Immunophenotyping of T lymphocytes by three-color flow cytometry in healthy newborns, children and adults. Clin Immunol Immunopathol. 1997;84:46–55. doi: 10.1006/clin.1997.4370. [DOI] [PubMed] [Google Scholar]
- 24.Messele T, Abdulkadir M, Fontanet A L, Petros B, Hamann D, Koot M, Roos M T L, Schellekens P T A, Miedema F, Rinke de Wit T F. Reduced naïve and increased activated CD4 and CD8 cells in healthy adult Ethiopians compared with their Dutch counterparts. Clin Exp Immunol. 1999;115:443–450. doi: 10.1046/j.1365-2249.1999.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miedema F, Petit A J C, Tepstra F G, Schattenkerk J K M E, De Wolf F, Al B J M, Roos M, Lange J M A, Danner S A, Goudsmit J, Schellekens P T A. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J Clin Investig. 1988;82:1908–1914. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pakker N G, Roos M T L, van Leeuwen R, de Jong M D, Koot M, Reiss P, Lange J M A, Miedema F, Danner S A, Schellekens P T A. Patterns of T-cell repopulation, virus load reduction, and restoration of T-cell function in HIV-infected persons during therapy with different antiretroviral agents. J Acquir Immune Defic Syndr. 1997;16:318–326. doi: 10.1097/00042560-199712150-00002. [DOI] [PubMed] [Google Scholar]
- 27.Plaeger S, Bass H Z, Nishanian P, Thomas J, Aziz N, Detels R, King J, Cumberland W, Kemeny M, Fahey J. The prognostic significance in HIV infection of immune activation represented by cell surface antigen and plasma activation marker changes. Clin Immunol. 1999;90:238–246. doi: 10.1006/clim.1998.4646. [DOI] [PubMed] [Google Scholar]
- 28.Polk B F, Fox R, Brookmeyer R, Kanchanaraksa S, Kaslow R, Visscher R, Rinaldo C, Phair J. Predictors of the acquired immunodeficiency syndrome developing in a cohort of seropositive homosexual men. N Engl J Med. 1987;316:61–66. doi: 10.1056/NEJM198701083160201. [DOI] [PubMed] [Google Scholar]
- 29.Prince H E, Jensen E R. HIV-related alterations in CD8 cell subsets defined by in vitro survival characteristics. Cell Immunol. 1991;134:276–286. doi: 10.1016/0008-8749(91)90302-r. [DOI] [PubMed] [Google Scholar]
- 30.Reinherz E L, Kung P C, Goldstein G, Levey R H, Schlossman S F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci USA. 1980;77:1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roos M T L, Miedema F, Koot M, Tersmette M, Schaasberg W P, Coutinho R A, Schellekens P T A. T-cell function in vitro is an independent progression marker for AIDS in human immunodeficiency virus (HIV)-infected asymptomatic individuals. J Infect Dis. 1995;171:531–536. doi: 10.1093/infdis/171.3.531. [DOI] [PubMed] [Google Scholar]
- 32.Roos M T L, Miedema F, Meinesz A A P, De Leeuw N A S M, Pakker N G, Lange J M A, Coutinho R A, Schellekens P T A. Low T-cell reactivity to combined CD3 plus CD28 stimulation is predictive for progression to AIDS correlation with decreased CD28 expression. Clin Exp Immunol. 1996;105:409–415. doi: 10.1046/j.1365-2249.1996.d01-794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schellekens P T A, Roos M T L, De Wolf F, Lange J M A, Miedema F. Low T-cell responsiveness to activation via CD3/TCR is a prognostic marker for AIDS in HIV-1 infected men. J Clin Immunol. 1990;10:121–127. doi: 10.1007/BF00918194. [DOI] [PubMed] [Google Scholar]
- 34.Shearer G M, Bernstein D C, Tung K S K, Via C S, Redfield R, Salahuddin S Z, Gallo R C. A model for the selective loss of major histocompatibility complex self restricted T-cell immune responses during the development of acquired immune deficiency syndrome (AIDS) J Immunol. 1986;137:2514–2521. [PubMed] [Google Scholar]
- 35.Van Lier R A W, Boot J H A, Verhoeven A J, De Groot E R, Brouwer M A, Aarden L A. Functional studies with anti-CD3 heavy chain isotypes with variant monoclonal antibodies. J Immunol. 1987;139:2873–2879. [PubMed] [Google Scholar]
- 36.Van Lier R A W, Brouwer M, Aarden L A. Signals involved in T-cell activation. T-cell proliferation induced through the synergistic action of anti-CD28 and anti-CD2 monoclonal antibodies. Eur J Immunol. 1988;18:167–172. doi: 10.1002/eji.1830180125. [DOI] [PubMed] [Google Scholar]