Abstract
Significant abnormalities are observed in the peripheral blood of juvenile dermatomyositis (JDM) patients with active disease. In this study, we confirm that there is a significant increase in the relative percentage of B lymphocytes in the peripheral blood of a group of untreated children with newly diagnosed active JDM compared to healthy children (P < 0.0001). In order to investigate if properties intrinsic to B cells contributed to their relative increase in JDM, the percentage of B cells expressing activation markers (CD23, CD25, CD54, and CD69) was measured and compared to pediatric controls. Compared to healthy children less than 10 years of age (not significantly different from the JDM group), the JDM patients had an increase in the proportion of lymphocytes expressing CD19 (B cells; P = 0.0017) and decreases in the percentage of lymphocytes that were CD3− CD16+ and/or CD56+ (NK cells; P = 0.01) and CD3+ CD8+ (T suppressor/cytotoxic cells; P = 0.02). There were no significant differences in any of the B-cell activation markers assessed. Of note, the percentage of CD54+ non-B lymphocytes (i.e., T cells and NK cells expressing CD54) was significantly lower in the JDM patients (25% ± 5%) than in the “age-related” healthy control group (43% ± 4%; P = 0.013). These results suggest the following for untreated children with active JDM: (i) the increase in the percentage of peripheral blood B cells is not due to intrinsic B-cell activation, and (ii) CD54/ICAM-1+ non-B cells, CD8+ T cells, and NK cells are being removed from circulation and may be participating in the pathophysiology of the disease.
Juvenile dermatomyositis (JDM) is a rare systemic vasculopathy of unknown etiology and pathogenesis, with an incidence of 3.1 of 1,000,000 children/year (19). The hallmarks of this illness include a characteristic rash, symmetrical proximal muscle weakness, and on examination of affected muscle, capillary occlusion (partially by lymphocytes). Abnormalities in the peripheral blood lymphocytes of untreated JDM with active symptoms have been reported previously. Specifically, patients are lymphopenic with a relative increase in the percentage of peripheral blood B lymphocytes (13, 20, 22). The relative increase in the percentage of circulating B lymphocytes appears to be highest in early active disease, reverts towards normal with effective therapy, and is associated with changes in clinical disease activity (10).
The purpose of this study was to assess the activation status of the B lymphocytes in the peripheral blood of JDM patients in order to investigate the underlying mechanisms associated with the relative increase in the percentage of B lymphocytes observed in patients with active disease. The percentage of B lymphocytes expressing CD23, CD25, CD54, and CD69 was determined in addition to routine immunophenotyping (B, T, T-helper, T-suppressor, and NK cells) in the peripheral blood of newly diagnosed JDM patients with untreated active disease. The results obtained from the JDM patients were compared to the results obtained simultaneously from a group of healthy control children.
MATERIALS AND METHODS
Patient populations.
Between April 1996 and August 1998, peripheral blood samples were obtained from 10 newly diagnosed, untreated JDM patients with active disease. All patients fulfilled the criteria of Bohan and Peter (2) as having definite JDM. During this time, peripheral blood samples were obtained from 37 healthy children attending outpatient clinics for well-child visits. The study was approved by the Institutional Review Board at the Children's Memorial Institute for Education and Research, and each participant or their guardian signed an informed consent.
Flow cytometry. (i) Staining.
Samples were processed for flow cytometry using standard whole-blood staining methodology as prescribed by the manufacturer. Monoclonal antibodies were combined into two-color panels to measure the following activation markers on B cells: CD19-FITC/CD23-PE (low-affinity immunoglobulin E [IgE] receptor), CD19-FITC/CD25-PE (the alpha chain of the interleukin-2 [IL-2] receptor), CD19-FITC/CD54-PE (intracellular adhesion molecule 1 [ICAM-1]), and CD19-FITC/CD69-PE (early activation markers). For the measurement of the major lymphocyte subsets, premixed two-color monoclonal antibody combinations were used: CD45-FITC/CD14-PE (lymphocyte gating reagent), CD3-FITC/CD4-PE (T-helper cells), CD3-FITC/CD8-PE (T-suppressor/cytotoxic cells), CD3-FITC/CD19-PE (Pan T cells and B cells), and CD3-FITC/CD16&CD56-PE (Pan T cells and natural killer [NK] cells). All monoclonal antibodies were obtained from Becton Dickinson (Mountain View, Calif.). All samples were run and analyzed on either a FACScan or a FACScalibur flow cytometer (Becton Dickinson).
(ii) Analysis.
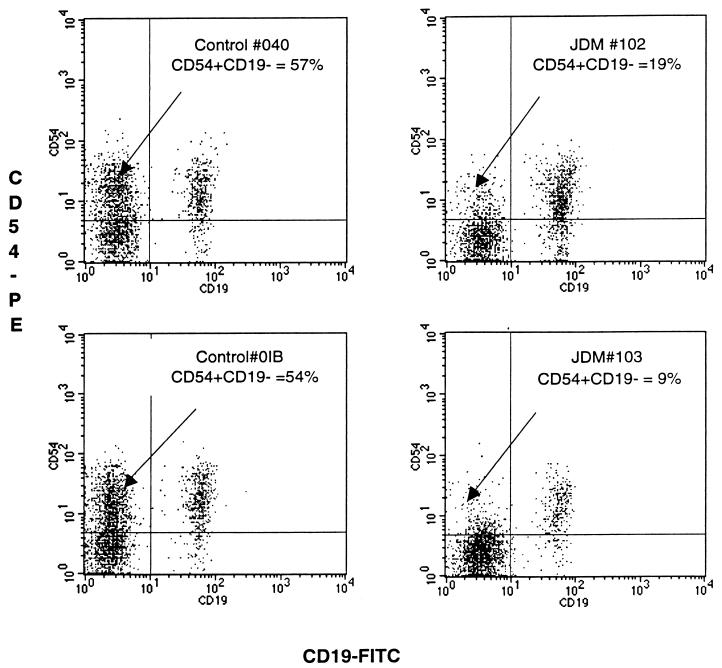
Sample data were acquired as list mode data by using the Simulset acquisition and analysis software. Both the routine subset and the B-cell activation marker analyses were run simultaneously. The first tube in the panel contained the lymphocyte reagent gating (CD45-FITC/CD14-PE), which is used to define the lymphocyte cluster and to determine the recovery and purity of the lymphocytes in the analysis region. All specimens in the study had a lymphocyte recovery of greater than 90% and lymphocyte purity greater than 85%. Measurements of all the lymphocyte subsets were corrected to 100%, based on the purity of lymphocytes within the lymphocyte analysis gate. Positive fluorescence was defined as the percentage of events which expressed fluorescence above a non-leukocyte-specific isotype and fluorochrome-matched monoclonal antibody. Data for B-cell activation markers were expressed as the percentage of CD19+ B lymphocytes expressing the activation marker of interest. For the analysis of CD54+ non-B cells, the results are expressed as percent CD54+ non-B cells calculated as the number of CD54+ CD19− events (Fig. 1) divided by CD54− CD19− plus CD54+ CD19− events (Fig. 1) times 100. When results of one subset, e.g., CD3, were obtained from more than one tube of a single patient, then the arithmetic mean of all the values was used.
FIG. 1.
Four dot plots generated from peripheral blood samples obtained from two healthy age-related control patients (left) and two representative untreated JDM patients (right) with active disease; samples were stained with CD19-FITC (x axis) and CD54-PE (y axis). The events illustrated in each dot plot were generated from lymphocytes electronically gated by characteristic forward versus right angle light scatter signals (see Materials and Methods). Note the apparent decrease in the CD54+ CD19− subset in the upper left hand quadrant of each histogram of the JDM patients (19 and 9%) compared to the two healthy controls (57 and 54%).
Statistics.
Since the percentages of many lymphocyte subsets are not normally distributed, nonparametric statistics (Mann-Whitney rank test) were used to compare the results obtained between groups. The two-sample t test was used to compare differences in age between the groups. To evaluate the relationship between the percentage of CD54+ non-B cells and age, simple regression analysis was performed.
RESULTS
B cells and B-cell activation markers.
The percentage of B lymphocytes in the peripheral blood of the JDM patients was significantly higher (P = 0.0001) than that observed in the control patients (Table 1). Of the B-cell activation markers measured, only the CD54+ B-cell subset was significantly different (P = 0.02) from the percentage observed in the healthy control group. It has been reported that the percentage of CD54-positive cells is associated with age (1, 21). When all of the healthy control children were included in the analysis, the mean age of the healthy controls (n = 37) was significantly greater than that of the JDM patients (11.2 ± 0.6 [mean ± standard error of the mean] years versus 5.9 ± 0.9 years, P < 0.0001). When healthy children older than 10 years of age were excluded from the analysis, there was no significant difference in age between the two groups (7.9 ± 0.6 years versus 5.9 ± 0.9 years, P = 0.7); therefore, the “age-related” healthy control group was used in all subsequent analyses. The relative percentage of B cells in the JDM patients was significantly higher than that of the age-related control group (P = 0.0017); however, none of the B-cell activation markers differed significantly (Table 1).
TABLE 1.
Comparison between healthy controls and JDM patients for the percentage of lymphocytes expressing CD19 (B cells), the proportion of B cells expressing the cell surface activation marker CD23, CD25, CD54, or CD69, and the proportion of non-B cells expressing CD54
| Group (n)a | % Lymphocytes expressing CD19 | % CD19+ cells expressing:
|
% Non-CD19+ CD54+ | |||
|---|---|---|---|---|---|---|
| CD23 | CD25 | CD54 | CD69 | |||
| Healthy controls (37) | 17 ± 1 (P < 0.0001)b | 81 ± 2 (NS) | 15 ± 1 (NS) | 85 ± 2 (p = .02) | 15 ± 2 (NS) | 50 ± 2 (P = 0.0002) |
| Healthy controls (<10 yr old) (12) | 19 ± 1 (P = 0.0017)c | 78 ± 3 (NS) | 16 ± 1 (NS) | 80 ± 4 (NS) | 15 ± 4 (NS) | 43 ± 4 (P = 0.013) |
| JDM patients (10) | 30 ± 3 | 83 ± 3 | 19 ± 3 | 77 ± 3 | 20 ± 4 | 25 ± 5 |
Healthy controls (n = 37) differed significantly in age from the JDM patients (11.2 ± 0.6 years [mean ± standard error of the mean] versus 5.9 ± 0.9 years, P < 0.0001) at the time of analysis. The healthy controls <10 years old did not differ significantly in age from the JDM group (7.9 ± 0.6 versus 5.9 ± 0.9, P = 0.07).
P values indicate the level of significance of the difference between the entire group of healthy children (n = 37) and the JDM patients. NS, not significant.
P values indicate the level of significance of the difference between the group of healthy children less than 10 years of age (n = 12) and the JDM patients. NS, not significant.
CD54+ non-B cells.
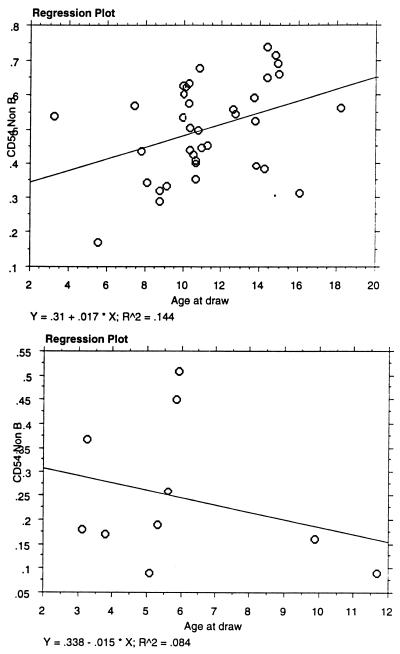
Measurement of the activation marker CD54 on non-B cells (Fig. 1) indicated that the percentages of CD54+ non-B lymphocytes in the peripheral blood of the JDM patients were significantly lower than the percentages observed in both the entire group of 37 healthy control subjects (P = 0.0002) and the group of age-related control subjects (P = 0.013). Of note, there was a positive association between the percentage of CD54-positive lymphocytes and age in the healthy control subjects whereas there was a negative association between percent CD54+ lymphocytes and age in the JDM patients (Fig. 2).
FIG. 2.
Regression plots of the percentage of CD54+ non-B lymphocytes versus the age of the healthy controls (top) and the JDM patients (bottom). Note the increase in the percentage of CD54-positive lymphocytes with age in the healthy control patients versus the decrease with age in the JDM group.
Major lymphocyte subsets.
In addition to confirming a significant increase in the relative percentage of CD19+ B lymphocytes in the peripheral blood of active untreated JDM patients, the percent total T cells (CD3+), percent NK cells (CD3−/CD16+ and/or CD56+), and percent T suppressor/cytotoxic cells (CD3+ CD8+) were significantly reduced as compared to the results obtained from the age-related control group (Table 2).
TABLE 2.
Routine immunophenotyping for the percentages of B (CD19+), T (CD3+), T-helper (CD3+ CD4+), T-suppressor/cytotoxic (CD3+ CD8+), and NK (CD3− CD16+ CD56+) lymphocytes in newly diagnosed untreated JDM patients and age-related healthy control children
| Group | Age (n) | % CD3+ | % CD4+ | % CD8+ | % CD19+ | % CD16+ CD56+ |
|---|---|---|---|---|---|---|
| Healthy controla | 7.9 ± 0.6 (12) | 71 ± 2 | 42 ± 2 | 26 ± 1 | 19 ± 1 | 10 ± 1 |
| JDM patients | 5.9 ± 0.9 (10) (P = 0.07) | 64 ± 3 (P = 0.05) | 43 ± 3 (P = 0.9) | 21 ± 1 (P = 0.01) | 30 ± 3 (P = 0.0017) | 7 ± 2 (P = 0.019) |
Includes only healthy children less than 10 years of age.
P values indicate the level of significance of the difference between the healthy control group and the JDM patient group. P values were calculated using the Wilcoxon rank sum test for nonparametric data.
DISCUSSION
This study confirms previous observations of a significant increase in the relative percentage of B lymphocytes and a corresponding decrease in the percentage of T lymphocytes in the peripheral blood of patients with untreated active JDM (13, 20, 22). In addition to the increase in B cells, over 70% of active untreated JDM patients have a positive anti-nuclear antibody test and selected (not polyclonal) elevation in Ig levels (22, 24). The peripheral lymphopenia and the relative increase in the percentage of B lymphocytes may be associated with a decrease in circulating CD8+ cells, with increased localization of CD8+ lymphocytes in the affected muscle (23); however, intrinsic B-cell activation has not been investigated. The present study was designed as a follow-up to measure the in vivo activation status of the B lymphocytes in untreated JDM patients with active early disease in order to investigate the mechanisms underlying the humoral abnormalities and the overrepresentation of B lymphocytes in the peripheral blood.
Four well-characterized B-cell activation antigens were measured in the JDM patients and compared to those of the healthy control group. CD23, the low-affinity IgE receptor, is a member of the C-type lectin superfamily of signal transduction receptors and was cloned in the late 1980's (14, 15). CD23 is an early activation antigen expressed primarily on B lymphocytes, appearing within 4 h after in vitro activation with IL-4 and peaking after approximately 16 to 24 h (7). It has numerous functions as both a ligand and a receptor on B cells, and its expression is actively regulated (reviewed in reference 3). Abnormal expression of CD23 on B cells has been reported for several diseases, including rheumatoid arthritis (16), and for HIV-infected children (25). Abnormalities in CD23 expression on B cells remains an active area of investigation in rheumatoid arthritis (18).
CD69 is one of the first antigens to appear on the surface of all lymphocyte subsets following in vitro activation with a variety of stimuli and functions as a signal-transducing receptor (11). In vitro CD69 can be detected on lymphocytes as early as 2 h after stimulation, and it is expressed maximally between 18 and 30 h poststimulation and decreases thereafter (11). Baseline CD69 expression levels and abnormalities in the ability of cells to upregulate CD69 has been investigated in numerous patient populations.
CD25, the alpha chain of the IL-2 receptor, joins with the constitutively expressed beta and gamma chains to form the high-affinity IL-2 receptor. In order for B cells to proliferate and differentiate they must express the high-affinity IL-2 receptor, which requires the induction and increased surface expression of CD25 (6, 8). CD25 can be unregulated on B cells by anti-Ig (5) IL-4 and soluble CD40 ligand (6). Rodriguez et al. (25) recently assessed the expression of CD25 on B lymphocytes in HIV-infected patients and noted small but insignificant decreases compared to a healthy control group.
CD54 (ICAM-1) is a membrane glycoprotein and a member of the Ig supergene family. It is constitutively expressed on endothelial cells, epithelial cells, and fibroblasts, as well as on T cells, B cells, dendritic cells, macrophages, and eosinophils (27), and it can be actively upregulated in response to a variety of mediators, including viruses, proinflammatory cytokines, and hormones (26). CD54 plays a central role in cell-to-cell mediated immune responses and is a ligand for the leukocyte function-associated antigen LFA-1 (CD11a/CD18). CD54 can be actively upregulated on the surface of B lymphocytes by exposure to IL-1 and IL-7 (9).
The percentages of B cells expressing each of the above activation markers did not differ between the patients with active JDM and the age-related healthy children. Initial comparisons which included the entire cohort of healthy children (n = 37) had indicated that CD54-positive B cells were significantly reduced in JDM patients compared to the entire cohort of healthy children. Since the entire healthy control group was significantly older than the JDM group and since it is known that CD54 expression on lymphocytes increases with age (1, 21), we could not rule out the possibility that the increased CD54 expression in the control group was due to their being significantly older. Therefore, we performed a separate analysis that included only control children less than 10 years of age. When the groups were more closely matched for age (i.e., not significantly different), the relative percentage of B cells remained significantly higher in the JDM patients; however, there were no significant differences in any of the B-cell subsets. These results suggest that the increase in the relative percentage of B lymphocytes in the peripheral blood of the JDM patients is not due to intrinsic B-cell activation.
In addition to the increase in the proportion of B lymphocytes, there was a corresponding decrease in the proportion of T cells in JDM patients versus the age-related controls. The decrease in total T cells appeared to be due to a specific decrease in CD8+ T cells, as the proportions of CD4+ T cells did not differ significantly between the two groups. Increased B cells and decreased CD8+ cells have been previously observed in a group of adult patients with dermatomyositis (20). Not previously reported was the significant reduction in the percentage of circulating NK cells observed in the JDM group compared to the age-related healthy control group (7 versus 10%, P = 0.02).
With respect to the activation markers, a significant decrease in the proportion of CD54+ non-B cells was observed in the JDM patients compared to the age-related control group (Fig. 1). Only 3 out of 10 (30%) JDM patients had greater than 30% CD54+ CD19− lymphocytes in their peripheral blood compared with 35 out of 37 healthy children (95%). Since the analysis was performed on total lymphocytes as defined by characteristic light scatter properties, the CD54+ non-B cell populations must represent T cells and/or NK cells. Further delineation of the specificity of the CD54+ non-B cells was not possible, but it is currently being investigated in active untreated JDM patients. It is also significant that the relationship between the expression of CD54 and age differed between the JDM patients and the healthy control children, providing further evidence that the cells expressing CD54 are involved in the pathogenesis of JDM.
The significant decrease in the CD54+ non-B lymphocytes may represent a specific loss of these cells or a selective depletion as a consequence of homing to the sites of inflammation present in JDM skin and muscle. The latter hypothesis is supported by immunohistochemical evaluations indicating a significant number of ICAM-1-positive lymphocytes located near blood vessels in muscle (17) and skin (12) biopsies of JDM patients. Our own investigations comparing lymphocyte subsets in concurrently obtained blood and muscle samples from untreated JDM patients have shown selective increases in CD8+ and CD56+ cells in the affected muscle compared to peripheral blood (23), and we are in the process of examining the expression of CD54 on these cells. The functions of ICAM-1+ T cells have not been fully elucidated. It has recently been reported that ICAM-1+ T cells may play a role in reactive airway disease (27). The increase in ICAM-1 expression on T cells in the lumen of airways in patients with asthma has led to the development of therapeutic strategies designed to interfere with ICAM-1 binding in an effort to ameliorate the symptoms of asthma (27).
The results of our investigation are consistent with the hypothesis that the increase in the proportion of B cells observed in the peripheral blood of JDM patients is most likely due to the loss of T cells and NK cells coexpressing CD54 from the circulation. This is supported by the observation that while the absolute T-cell count is lower in JDM patients than age-related controls, the B-cell count does not differ significantly (22). An alternative hypothesis proposed by Miller et al. (20) suggests that the increased proportion of B cells in the peripheral blood of JDM is related to a more “humorally mediated” disease. We did not observe any abnormalities in the B-cell activation markers studied, further supporting our hypothesis that JDM is a T-cell-mediated disease.
In summary, there is an increase in the percentage of lymphocytes expressing CD19 (B lymphocytes) in the peripheral blood of newly diagnosed untreated JDM patients with active disease. This abnormality does not appear to be intrinsic to B lymphocytes, as evidenced by a lack of B-cell activation. Rather, it appears that the relative increase in the proportion of B lymphocytes is due to a selective depletion of circulating CD8+ T cells and/or NK cells coexpressing CD54. It will be very important to characterize more specifically the CD54+ T and/or NK subsets which appear to be selectively depleted from the peripheral blood of JDM patients with active untreated disease. This information might lead to the development of therapeutic strategies to interrupt the migration of pathogenic lymphocytes into skin and muscle of JDM patients.
ACKNOWLEDGMENTS
This work was supported in part by the Greater Illinois Chapter of the Arthritis Foundation and NIH grant no. RO1 AR43978.
The technical expertise of Nicolas Bensen, Janelle Hunt, Mary Paniagua, and Sharon Mark and the data management assistance of Jennifer Kinder, Edward Mendez, and Kori Ade are gratefully acknowledged.
REFERENCES
- 1.Amlot P L, Tahami F, Chinn D, Rawlings E. Activation antigen expression on human T cells. I. Analysis by two-colour flow cytometry of umbilical cord blood, adult blood and lymphoid tissue. Clin Exp Immunol. 1996;105:176–182. doi: 10.1046/j.1365-2249.1996.d01-722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohan A, Peter J B. Polymyositis and dermatomyositis. N Engl J Med. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 3.Bonnefoy J-Y, Lecoanet-Henchoz S, Aubry J-P, Gauchat J-F, Graber P. CD23 and B-cell activation. Curr Opin Immunol. 1995;7:355–359. doi: 10.1016/0952-7915(95)80110-3. [DOI] [PubMed] [Google Scholar]
- 4.Burlinson E L, Pringle H M, Ozanne B W, Cushley W. Anti-immunoglobulin and anti-CD40 stimulation induces CD25 expression by resting human tonsillar B lymphocytes. Immunol Lett. 1995;45:93–98. doi: 10.1016/0165-2478(94)00232-g. [DOI] [PubMed] [Google Scholar]
- 5.Burlinson E L, Graber P, Bonnefoy J-Y, Ozanne B W, Cushley W. Soluble CD40 ligand induces expression of CD25 and CD23 in resting human tonsillar B lymphocytes. Eur J Immunol. 1996;26:1069–1073. doi: 10.1002/eji.1830260517. [DOI] [PubMed] [Google Scholar]
- 6.Callard R E. Cytokines and B lymphocytes. London, England: Academic Press; 1990. [Google Scholar]
- 7.Crow M K, Jover J A, Friedman S. Direct T helper-B cell interactions induce early B cell activation antigen. J Exp Med. 1986;164:1760–1772. doi: 10.1084/jem.164.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cushley W, Harnett M M. Regulation of B lymphocyte growth and differentiation by soluble mediators. In: Snow E C, editor. Handbook of B and T lymphocytes. London, England: Academic Press; 1994. pp. 389–420. [Google Scholar]
- 9.Dennig D, Lacerda J, Yan Y, Gasparetto C, O'Reilly J. ICAM-1 (CD54) expression on B lymphocytes is associated with their co-stimulatory function and can be increased by co-activation with IL1 and IL7. Cell Immunol. 1994;156:414–423. doi: 10.1006/cimm.1994.1186. [DOI] [PubMed] [Google Scholar]
- 10.Eisenstein D M, O'Gorman M R G, Pachman L M. Correlations between change in disease activity and changes in peripheral blood lymphocyte subsets in patients with juvenile dermatomyositis. J Rheumatol. 1997;24:1830–1832. [PubMed] [Google Scholar]
- 11.Hara T, Jung L K L, Bjorndahl J M, Fu S M. Human T cell activation. III. Rapid induction of a phosphorylated 28dD/32kD disulphide-linked early activation antigen (EA-1) BY 12-O-tetradecanoyl phorbol-13-acetate, mitogens, and antigens. J Exp Med. 1986;164:1988–2005. doi: 10.1084/jem.164.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hausmann G, Mascaro J M, Jr, Herrero C, Cid M C, Palou J, Mascaro J M. Cell adhesion molecule expression in cutaneous lesions of dermatomyositis. Acta Dermato-Venereol. 1996;76:222–225. doi: 10.2340/0001555576222225. [DOI] [PubMed] [Google Scholar]
- 13.Iannone F, Aculi A, Yanni G, Kingsley G H, Isenberg D A, Corrigall V, Panyi G S. T-lymphocyte immunophenotyping in polymyositis and dermatomyositis. Br J Rheumatol. 1996;35:839–845. doi: 10.1093/rheumatology/35.9.839. [DOI] [PubMed] [Google Scholar]
- 14.Ikuta K, Takami M, Kim C W, Honjo T, Miyoshi T, Tagaya J, Kawabe T, Yodoi J. Human lymphocyte Fc receptor for IgE: sequence homology and its cloned cDNA with animal lectins. Proc Natl Acad Sci USA. 1986;84:819–823. doi: 10.1073/pnas.84.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kikutani H, Inui R, Sato E L, Barsumian H, Owaki K, Yamasaki T, Kaisho N, Uchibayashi R P, Hardy T, Hirano S, Tsunasawa F, Sakiyama M, Suemura M, Kishimoto T. Molecular structure of human lymphocyte receptor for immunoglobulin. Cell. 1986;47:657–665. doi: 10.1016/0092-8674(86)90508-8. [DOI] [PubMed] [Google Scholar]
- 16.Kumagai S, Ishida H, Iwai K, Tsubata T, Umehara H, Ozaki S, Suginoshita T, Araya S, Imura H. Possible different mechanisms of B cell activation in systemic lupus erythematosus and rheumatoid arthritis: opposite expression of low-affinity receptors for IgE (CD23) on their peripheral B cell. Clin Exp Immunol. 1989;78:348–353. [PMC free article] [PubMed] [Google Scholar]
- 17.Liprandi A, Figarella-Branger D, Lepidi L, Cartoli C, Pellissier J-F. Expression des molecules d'adhesion dans les myopathies inflammatoires idiopathiques. Ann Pathol. 1999;19:12–18. [PubMed] [Google Scholar]
- 18.Loza E, Tinture T, Sanchez-Ibarrola A. CD5 and CD23 expression of B cells in peripheral blood and synovial fluid of rheumatoid arthritis patients: relationship with interleukin-4, soluble CD23 and tumour necrosis factor alpha levels. Rheumatology (Oxford) 1999;38:325–328. doi: 10.1093/rheumatology/38.4.325. [DOI] [PubMed] [Google Scholar]
- 19.Mendez E, Lipton R, Dyer A, Ramsey-Goldman R, Roettcher P, Bowyer S, Pachman L M. The incidence of juvenile dermatomyositis (JDM): results from the NIAMS JDM research registry. Arthritis Rheum. 1999;42:S300. doi: 10.1002/art.11122. [DOI] [PubMed] [Google Scholar]
- 20.Miller F W, Love L A, Barbieri S A, Balow J E, Plotz P H. Lymphocyte activation markers in idiopathic myositis: changes with disease activity and differences among clinical and autoantibody subgroups. Clin Exp Immunol. 1990;81:373–379. doi: 10.1111/j.1365-2249.1990.tb05341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neubert R, Delgado I, Abraham K, Schuster C, Helge H. Evaluation of the age-dependent development of lymphocyte surface receptors in children. Life Sci. 1998;62:1099–1110. doi: 10.1016/s0024-3205(98)00033-2. [DOI] [PubMed] [Google Scholar]
- 22.O'Gorman M R G, Corrochano V, Roleck J, Donovan M, Pachman L M. Flow cytometric analyses of the lymphocyte subsets in peripheral blood of children with untreated active juvenile dermatomyositis. Clin Diagn Lab Immunol. 1995;2:205–208. doi: 10.1128/cdli.2.2.205-208.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pachman L M, O'Gorman M R G, Lawton T, Liotta M, Pope R M, Green M, Wu T T. Evidence of a TCR Vβ8 motif and increased CD56+ NK cells in muscle biopsies (Mbx) from DQA1 *0501 positive untreated children with juvenile dermatomyositis (JDM) very early in their disease course. Pediatr Res. 1998;43:338A. [Google Scholar]
- 24.Pachman L M, Friedman J M, Maryjowski-Sweeny M C, Jonasson O, Radvany R M, Sharp G C, Cobb M A, Battles N D, Crowe W E, Fink C W, Hanson V, Levinson J E, Spencer C H, Sullivan D B. Immunogenetic studies of juvenile dermatomyositis. III. Study of antibody to organ-specific and nuclear antigens. Arthritis Rheum. 1985;28:151–157. doi: 10.1002/art.1780280208. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez C, Thomas J K, O'Rourke S, Stiehm E R, Plaeger S. HIV disease in children is associated with a selective decrease in CD23+ and CD62L+ B cells. Clin Immunol Immunopathol. 1996;81:191–199. doi: 10.1006/clin.1996.0176. [DOI] [PubMed] [Google Scholar]
- 26.Roebuck K A, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 27.Stanciu L A, Djukanovic R. The role of ICAM-1 on T-cells in the pathogenesis of asthma. Eur Respir J. 1998;11:949–957. doi: 10.1183/09031936.98.11040949. [DOI] [PubMed] [Google Scholar]