Abstract
The transmission of antimicrobial resistance (AMR) between food‐producing animals (poultry, cattle and pigs) during short journeys (< 8 h) and long journeys (> 8 h) directed to other farms or to the slaughterhouse lairage (directly or with intermediate stops at assembly centres or control posts, mainly transported by road) was assessed. Among the identified risk factors contributing to the probability of transmission of antimicrobial‐resistant bacteria (ARB) and antimicrobial resistance genes (ARGs), the ones considered more important are the resistance status (presence of ARB/ARGs) of the animals pre‐transport, increased faecal shedding, hygiene of the areas and vehicles, exposure to other animals carrying and/or shedding ARB/ARGs (especially between animals of different AMR loads and/or ARB/ARG types), exposure to contaminated lairage areas and duration of transport. There are nevertheless no data whereby differences between journeys shorter or longer than 8 h can be assessed. Strategies that would reduce the probability of AMR transmission, for all animal categories include minimising the duration of transport, proper cleaning and disinfection, appropriate transport planning, organising the transport in relation to AMR criteria (transport logistics), improving animal health and welfare and/or biosecurity immediately prior to and during transport, ensuring the thermal comfort of the animals and animal segregation. Most of the aforementioned measures have similar validity if applied at lairage, assembly centres and control posts. Data gaps relating to the risk factors and the effectiveness of mitigation measures have been identified, with consequent research needs in both the short and longer term listed. Quantification of the impact of animal transportation compared to the contribution of other stages of the food‐production chain, and the interplay of duration with all risk factors on the transmission of ARB/ARGs during transport and journey breaks, were identified as urgent research needs.
Keywords: antimicrobial‐resistant bacteria (ARB), antimicrobial resistance genes (ARGs), food‐producing animals, lairage, risk factors, mitigation options, data gaps, research needs
Summary
The European Parliament asked the European Food Safety Authority (EFSA) Panel on Biological Hazards (BIOHAZ) to deliver a Scientific Opinion on the transmission of antimicrobial resistance (AMR) during animal transports.
The BIOHAZ Panel was asked to answer the following questions (Terms of Reference, ToRs): ToR1: What are the most significant risk factors contributing to the spread of food‐borne zoonotic and indicator antimicrobial‐resistant bacteria (ARB) and antimicrobial resistance genes (ARGs) between food‐producing animals during short journeys (< 8 h) and long journeys (> 8 h) directed to other farms or to slaughterhouses (directly or through livestock markets)?; ToR2: What preventive measures and control options could be implemented during short journeys and long journeys directed to other farms or to slaughterhouses and during subsequent lairage to reduce the probability of spread of food‐borne zoonotic and indicator ARB/ARGs between food‐producing animals?; ToR3: What are the data gaps and what are the most urgent data needs to support the analysis of the correlation between the main risk factors identified above and the spread of food‐borne zoonotic and indicator ARB/ARGs between food‐producing animals during transport and lairage?
The Scientific Opinion focused on ARB of public health importance, including food‐borne zoonotic pathogens and indicator bacteria covered by the European Union (EU) AMR monitoring legislation Commission Implementing Decision (EU) 2020/17291 or upcoming EU baseline surveys (Salmonella spp., Campylobacter spp., Escherichia coli, Enterococcus spp., MRSA) and on ARGs. Information on the transmission of zoonotic pathogens and indicator bacteria in general was used to support the assessment when there was a lack of data on ARB/ARGs. For the purpose of this Scientific Opinion, the European Commission Council Regulation (EC) No 1/20052 definition of animal transport was used: ‘the movement of animals effected by one or more means of transport and the related operations, including loading, transfer and rest, until unloading of the animals at the place of destination is completed’. The focus was the transport of the main food‐producing animals ‐poultry, pigs and cattle‐, from one farm to another farm and/or to the slaughterhouse lairage (directly or with intermediate stops at assembly centres or control posts, mainly transported by road), within, from and to the EU/EFTA countries in compliance with current EU regulations.
The end point of the assessment was any possible variation in the AMR status (abundance and diversity of ARBs/ARGs) associated with transportation and on arrival at the destination.
To address the mandate, a qualitative assessment was undertaken based on information from international reports, European Legislation, scientific literature and expert knowledge.
Uncertainty was addressed following EFSA guidance. The certainty of the conclusions was obtained through consensus expert judgement, following discussion in the working group, informed by the collected evidence and expert knowledge.
In general, there is scarce information and lack of specific studies addressing the risk factors for AMR transmission during transport of animals, and the mitigation and control of those risks. Thus, several of the conclusions made are supported by expert knowledge on risks for bacterial transmission in general.
The following risk factors were considered 99%–100% certain (almost certain) to contribute to the probability of transmission of ARB/ARGs during food‐producing animal transport: the resistance status (presence and type of ARB/ARGs) of the animals pre‐transport, increased faecal shedding, insufficient hygiene of the areas and vehicles, exposure to other animals carrying and/or shedding ARB/ARGs (especially from different origins), duration of transport (given the presence of other risk factors) and exposure to contaminated assembly centre, control post and lairage areas.
The following risk factors were considered 66%–90% certain (likely) to contribute to the probability of transmission of ARB/ARGs during food‐producing animal transport: airborne transmission within the vehicle, ARB/ARGs carriage in workers (this is considered to have a minor contribution compared to exposure to the vehicle environment and other animals carrying ARB/ARGs), the health status of the animal, unfavourable environmental conditions (high temperature and humidity) which enhances the survival rate of bacteria in the environment as well as inadequate transport environmental conditions which can cause alteration in the microbiota of animals.
It is considered that feed withdrawal and stress are 33%–66% certain (as likely as not) to contribute to the probability of transmission of ARB/ARGs during transport. Both positive (reduction of vomiting and shedding of faecal material) and negative (increased shedding of certain bacteria) effects on ARB/ARGs transmission can be assumed, but the overall direction of the effect of feed withdrawal remains unclear. There is evidence that stress can lead to alterations in microbiota and suppression of the immune system, but the impact (positive or negative) on the transmission of ARB/ARGs is unclear.
Although most of the identified risk factors are influenced by transport duration (i.e. with longer transports the exposure to other risk factors is prolonged), there is no specific data to estimate differences between journeys shorter or longer than 8 h. Journeys that require rests in control posts are associated to specific risk factors in those temporal areas (e.g. mixing of animals, environmental contamination, new environment‐stress).
Minimising the duration of transport and organising the transport in relation to AMR criteria (transport logistics based, e.g. on AMR load, ARBs with resistance to ‘critical’ antimicrobials and ARGs conferring those resistances, epidemiological data or indirect parameters such as antimicrobial use (AMU)) were considered mitigation strategies which would reduce the probability of AMR transmission with a 95%–99% certainty (extremely likely). With the data available, no maximum journey duration can be recommended.
In general, it is considered 90%–95% certain (very likely) that any measure improving animal health and welfare and/or biosecurity just before and during transport will reduce ARB/ARGs transmission. Such measures include: good husbandry and handling practices associated with animal transport preparation interventions, animal segregation (by species, production stage, or age), minimising the number of farms visited or ensuring the thermal comfort of the animals during the transport. The same certainty level is provided for mitigation measures tackling hygiene, e.g. proper cleaning and disinfection of transport vehicles, crays, cages, and in general surfaces and equipment. Efficacy of the protocols should be validated and/or tested regularly by inspection and microbiological analyses. These measures apply to loading/unloading areas and equipment as well.
Reducing stock densities and number of animals in contact as well as avoiding the transport of sick animals were considered 66%–90% certain (likely) to mitigate the risk of AMR transmission.
It was considered 66%–100% certain (likely to extremely likely) that apart from minimising the use of assembly centres and control posts, the implementation of the measures recommended above with regard to animal handling, stocking densities, mixing/segregating animals and facilities cleaning and disinfection, are also relevant to mitigate ARB/ARGs transmission in these places as well as in the lairage. Limiting the lairage time to the minimum possible will also reduce the probability of this transmission.
The effect of the provision of bedding (quantity and type) as a mitigation strategy was considered 33%–66% certain (as likely as not) to be efficient, as it may have both beneficial and negative effects on probability of ARB/ARGs transmission.
Finally, based on the uncertainties associated with the risk for ARB/ARGs transmission linked to feeding measures (feed withdrawal and/or use of alternative substances to antimicrobials), no specific mitigation measures in relation to feeding management are proposed.
A range of data gaps relating to the risk factors and the effectiveness of mitigation measures have been identified, with consequent research needs in both the short and longer term listed. The data gaps identified included: quantification of the effect of fasting prior and during transport on ARB/ARGs in the microbiota, the effectiveness of different cleaning and disinfection protocols to reduce/eliminate ARB/ARGs, the direction of the association between transport‐related stressors and ARB/ARGs transmission, the effect of type and amount of bedding, the definition and identification of AMR criteria and the best indicators for each criterion upon which the transport logistics could be organised, the possible contribution of the airborne route during transport and lairage, the impact of mechanical vs. manual catching/loading of animals, the contribution of the health status of the animal (e.g. in relation to the shedding of ARB/ARGs, the susceptibility to colonisation or infection by ARB, or transmission of ARGs) and the effect of interventions using alternative substances to antimicrobials to mitigate transmission of ARB/ARGs.
Among the most urgent research needs, studies assessing the impact of animal transportation compared to the contribution of other stages of the food‐production chain as a contributor to dissemination of AMR between farms and/or to contamination of meat at slaughter were identified. Studies quantifying the interplay of duration with all risk factors during transport and journey breaks were particularly identified as an urgent research need. Such studies should include the determination of the time‐lag between uptake of ARB and faecal shedding and subsequent transmission of such bacteria under transport and lairage conditions. It is also considered important to define the AMR criteria and the best indicators for each criterion that could be used for transport logistics as indicated above.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
By letter dated 20 May 2021, Mr. Pascal Canfin, the Chair of the Committee on Environment, Public Health and Food Safety (ENVI) requested that the European Parliament asks the European Food Safety Authority (EFSA) to deliver a scientific opinion on the transmission of antimicrobial resistance (AMR) and zoonotic agents during animal transports. The Coordinators of the ENVI Committee endorsed this suggestion from the Committee of inquiry on the protection of animals during transport (ANIT).
The emergence and spread of AMR and other zoonotic agents are rising. During the transport, animals are subject to an environment where factors such as temperature, ventilation, and the mixing of animals from different origins contribute to the dissemination of resistant microorganisms, as well as zoonotic agents. However, important data gaps remain regarding the transmission of these microorganisms and agents. As this lack of knowledge is a public health concern, it is crucial to assess the potential role of transport of live animals in this particular matter. The European Parliament, therefore, considers it opportune to request EFSA to draw up a scientific opinion on different aspects of the transmission of AMR and zoonotic agents during animal transports, and more specifically on the three following questions:
What are the most significant risk factors contributing to the spread of foodborne zoonotic and indicator antimicrobial‐resistant bacteria (ARB) and antimicrobial resistance genes (ARG) between food‐producing animals during short journeys (< 8 h) and long journeys (> 8 h) directed to other farms or to slaughterhouses (directly or through livestock markets)?
What preventive measures and control options could be implemented during short journeys and long journeys directed to other farms or to slaughterhouses and during subsequent lairage to reduce the probability of spread of foodborne zoonotic and indicator ARB/ARG between food‐producing animals?
What are the current data gaps and what are the most urgent data needs to support the analysis of the correlation between the main risk factors identified above and the spread of foodborne zoonotic and indicator ARB/ARG between food‐producing animals during transport and lairage?
The European Parliament hereby requests this scientific opinion in accordance with Article 29 of Regulation 178/2002. As this scientific opinion will be useful for the future work not only in the ENVI committee, but also in the ANIT Committee, the European Parliament requests that the opinion be ready before the end of October 2021.
The deadline of the opinion was extended to the end of September 2022.
1.2. Interpretation of the Terms of Reference
The Scientific Opinion focuses on ARB of public health importance, including food‐borne zoonotic pathogens and indicator bacteria covered by the European Union (EU) AMR monitoring legislation Commission Implementing Decision (EU) 2020/17293 or upcoming baseline surveys (EFSA, 2022) (Salmonella spp., Campylobacter spp., Escherichia coli, Enterococcus spp., methicillin‐resistant Staphylococcus aureus – MRSA) and on ARGs. Information on general bacterial transmission will be used to support the assessment if there is a need for extrapolation due to lack of data on ARB/ARGs.
The Scientific Opinion focuses on transport of food‐producing animals carried out within, from and to the EU/EFTA countries in compliance with current EU regulations. Information from relevant studies in other countries will be used to support the assessment when appropriate.
In the European Commission Council Regulation (EC) No 1/2005 2 (henceforth referred to as EC 1/2005), animal ‘transport’ is defined as ‘the movement of animals effected by one or more means of transport and the related operations, including loading, transfer and rest, until unloading of the animals at the place of destination is completed’. By ‘means of transport’ it is meant ‘any road or rail vehicles, vessels and aircraft used for the transport of animals’. Considering that the majority of the movements are carried out by road, the Scientific Opinion main focus is on this means of transport. The term ‘vehicle’ is used for all types of transportation.
The assessment focuses on pigs, cattle and poultry transport, since they cover the majority of the live animal movements in Europe. Information on other animal species is used to support the assessment when relevant.
The impact of transport on AMR will be assessed considering any possible variation in the diversity and abundance of ARB/ARGs in the microbiota of the animals such as would happen during transport from one farm to another farm and/or slaughterhouse (directly or with intermediate stops at assembly centres or control posts). This includes the proportion of carrier or contaminated animals (e.g. as assessed by tests on faeces/intestinal content, skin, nasal swabs, saliva, etc.) and/or abundance of different ARB or ARGs. For abattoirs, both the end of the journey and the end of lairage will be considered as end points of the assessment.
The movement of living animals between premises contributes to the transfer of bacteria (carried by the animals moved) between farms or from farms to other destinations such as slaughterhouses. On the farm of destination animals are often mixed and exchange ARB/ARGs. Moreover, farm‐to‐farm transport often involves young animals, with a longer lifespan ahead. Consequently, ARB/ARGs are spread between farms, regions, countries and even continents. As a consequence of transportation of animals between farms and the commingling of animals from different origins (not commonly applied to poultry), these animals are more likely to contract infectious diseases, such as respiratory and gastrointestinal infections, and may require as a consequence antimicrobial treatment (Smith, 2019; Pokharel and Karna, 2022). While the need for these treatments can often be indirectly attributed to transport, the impact of antimicrobial usage after transport on the possible emergence and spread of AMR is outside the scope and will not be considered in this Scientific Opinion. ARB/ARGs transmission during transport to the slaughterhouse is also important from a public health and food safety perspective as ARB/ARGs present in the animals at the end of lairage may contaminate carcasses during slaughter and reach the consumer through meat, depending on the hygiene measures implemented at slaughter. When assessing risk factors related to transport on the transmission on ARB/ARGs these different aspects, as well as cross‐contamination during the slaughter process, are not considered, since stages after transport and/or after lairage (management practices on farm or slaughter procedures) are outside the scope of this Scientific Opinion.
In addition to the considerations listed above and the limits of this Scientific Opinion, the occupational exposure to ARB/ARGs by humans, such as transmission by aerosols/dust, close contact with animals or with their environments will be only referred to in the context of transportation. An assessment of the risks via these pathways is outside the remit of EFSA, since this does not relate to food‐borne infection. Moreover, management practices on farms prior to transport will not be considered unless directly related to preparation for transport (e.g. feed withdrawal, handling/catching).
The term ‘assembly centres’, as defined in EC 1/2005, refers to ‘those places such as holding centres, collection centres and markets where domestic animals originating from different holdings are grouped together to form consignments’. ‘Control posts’ are defined as the ‘places where animals are rested for at least 12 h or more (formerly called staging points) after long journeys’ (EC 1/2005).
The Terms of Reference (ToRs) of the current mandate, as provided by the requestor, have been translated into a series of Assessment Questions (AQs).
ToR 1 was transcribed into AQ1:
What are the most important risk factors contributing to the transmission of food‐borne zoonotic and indicator antimicrobial‐resistant bacteria (ARB) and antimicrobial resistance genes (ARGs) between food‐producing animals during short journeys (< 8 h) and long journeys (> 8 h) directed to other farms or to slaughterhouses (directly or through livestock assembly centres)?
ToR2 is used as the assessment question AQ2:
What preventive measures and control options could be implemented during short journeys and long journeys directed to other farms or to slaughterhouses and during subsequent lairage to reduce the probability of spread of food‐borne zoonotic and indicator ARB/ARGs between food‐producing animals?
ToR3, was reformulated into:
AQ3: What are the knowledge gaps required to assess the contribution of the risk factors identified in ToR1, the mitigation measures identified in ToR2 and to identify any factors not covered by existing studies?
AQ4: Which are the most urgent and longer‐term research requirements needed to fill the identified data gaps?
The work for the development of this Scientific Opinion was undertaken with the advice of EFSA Animal Health and Animal Welfare Panel (AHAW) which worked in parallel to answer the European Commission Mandate: ‘Request for a Scientific Opinion concerning the protection of terrestrial animals during transport’ (EFSA AHAW Panel, 2022a,b,c).
1.3. Additional information
1.3.1. Transport of animals
Live animal transportation in Europe is regulated by EC 1/2005 (this regulation is currently under revision) and the ‘Journey’ is defined as follows: ‘entire transport operation from the place of departure to the place of destination, including any unloading, accommodation and loading occurring at intermediate points in the journey’.
Millions of animals are transported daily in Europe within Member States (MSs) and across MSs and to/from third countries. While the movement of livestock across the borders of MSs of the EU is monitored using the Trade Control and Expert System (TRACES) and reported each year in the European Activity Reports (available online at https://food.ec.europa.eu/animals/traces/information-material_en), movements within each country are more difficult to quantify as they are not always registered by competent authorities, depending on the animal species.
Slaughter statistics show that in the EU 23 million cattle and 245 million pigs are slaughtered annually but only an extremely small minority at the premises where they have been raised, i.e. most of them have been transported prior to slaughter. For example, in Germany, the proportion of slaughter at the premises was below 1% of all slaughtered cattle and pigs (data for 2nd semester 2018, Destatis, 2019). Likewise, a substantial proportion of these animals have not been raised fully on the farm of birth, i.e. there has been transport from the farm of origin to the fattening unit, sometimes with some intermediate stops such as livestock assembly centres (including markets, dealers' premises or holdings that only cover a certain part of the rearing period). Likewise, 5.5 billion chickens in the EU have been transported yearly as day‐old chicks from the hatchery to the farm and to the abattoir for slaughter at the end of the fattening period or productive life, or from rearing farms to production farms (EFSA AHAW Panel, 2022a).
From a recent analysis of TRACES (Dahl‐Pedersen and Herskin, 2021), approximately 4 million cattle and 33 million pigs were transported annually across MS‐borders in Europe from 2014 to 2018. For instance, in 2018, Italy imported a total number of 1,075,895 live bovine animals from several countries and of these 1,050,319 were from Europe, mainly from France (Istat, 2021, online, last accessed 5 May 2021). The purpose of those international movements is mainly for production (about 65%), slaughter (about 15%) and breeding (20%) each year. Most of those are considered ‘long journeys’ since they exceed 8 h, starting from when the first animals of the consignment are moved (EC 1/2005).
From a recent study (Padalino et al., 2021a), it was evident that these journeys were conducted by specific commercial transport companies, using large trucks and lorries predominantly designed and equipped for the transport of a specific species, and tended to cover the same routes many times during a year. The companies were categorised into large and small companies. The transport companies were usually from the country of destination. When farm animals are transported over very long distances and have to stop at a control post, the first part of the journey is usually performed by a company from the departure country, and the second part by a company from the destination country. For logistic reasons, the animals may therefore change from one vehicle to another during the same journey. Animals transported for short distances may be transported using vehicles which belong to the slaughterhouse company (Bozzo et al., 2020). Depending on the length and/or duration of the journey, the vehicles must guarantee certain requirements to comply with EC 1/2005 (e.g. watering systems and forced ventilation are required for vehicles transporting animals for more than 8 h).
With the aim of reducing transport stress and consequently the incidence of transport‐related health welfare issues, many studies have identified risk factors for farm animals pre‐, during and post‐road transport (Marahrens et al., 2011). Pre‐journey risk factors include factors such as on‐farm handling, rearing conditions, assembly of animals, classifying, weighing, re‐penning in a new environment, re‐grouping, mixing with unfamiliar animals, fitness for transport and handling at loading (Schwartzkopf‐Genswein et al., 2007; Šímová et al., 2016). Risk factors during the journey include duration, withdrawal of feed and water, thermal, physical and hygiene conditions inside the vehicle, overcrowding, absence of partitions, driving skills, noise, vibration and road quality (Cockram and Spence, 2012; Costa et al., 2012; Padalino, 2015). Transportation by rail, sea and air also applies in some cases and may have particular risk factors associated with this type of transportation (e.g. sea sickness, fear associated with the sensation of taking off and landing) (Bhatt et al., 2021). Post‐journey risk factors include handling at unloading, duration of rest periods at lairage, recovery practices, re‐grouping and mixing with unfamiliar animals (Messori et al., 2015, 2017; Padalino et al., 2016a). The identification of risk factors is essential to help design mitigation strategies which are the basis of the current Animal Transportation Codes around the world.
In Europe, transport best practices have been recently suggested to minimise the adverse effects of transportation on health and welfare using expert knowledge elicitations (‘Animal Transport Guides’, available online at www.animaltransportguides.eu/materials/). Transport issues and practices have similarly been investigated through the use of surveys.
Antimicrobial use (AMU) before shipping or on arrival has been reported for certain animal species (Padalino et al., 2016b; Cirone et al., 2019; Pratelli et al., 2021). Nonetheless in the EU, the use of antimicrobials for prophylaxis or for metaphylaxis should be restricted as addressed by Regulation (EU) 2019/64.
One of the determining risk factors relating to animal health and welfare is journey duration (Cave et al., 2005; Nielsen et al., 2011). Consequently, EC 1/2005, includes special requirements depending on journey length and/or duration (see Figure 1). EC 1/2005 considers as ‘short journeys’ transportation that does not exceed 8 h, and ‘long journeys’ as transportation that exceeds 8 h. Journeys under 65 km are not covered in this Regulation.
Figure 1.

Description of type of journey, maximal journey duration and journey breaks in accordance with EC 1/2005
Vehicles have different requirements and consequently approval depending on the journey duration (authorisations of Type 1 or 2 for short or long journeys, respectively). The same Regulation reports that the MSs may grant derogations for means of transport by road in respect of journeys not exceeding 12 h to reach the final place of destination. Consequently, journeys of up to 12 h are made with trucks without the additional provisions required for long journeys. Journeys longer than 14 h are considered as ‘very long journeys’ and must include a rest stop without unloading the animals but must be completed within the current maximum journey duration set for the different species. For instance, maximum journey duration is 29 (14 + 1 + 14) h for weaned large and small ruminants and 24 h for pigs. After this time, animals must be unloaded for resting, watering and feeding for at least 24 h in locations approved by the competent authorities (Sossidou and de Roest, 2012). Such locations used to be called ‘staging points’ in Council Regulation (EC) 1255/19975 and have now been renamed ‘control posts’ by EC 1/2005.
EC 1255/1997 (Article 6) requires that official veterinarians inspect the transport vehicle and accompanying documents, as well as evaluate the animals' fitness for transport before the animals leave the control post again. The facilities and management at control posts have been identified as key factors in animal recovery, affecting both resting behaviour and associated biochemical parameters such as stress hormone levels, etc. (Sossidou and de Roest, 2012; Messori et al., 2015, 2017). If during the journey, there is delay and the final destination has not been reached within the maximum journey duration, but it is reachable within 2 h, the journey can continue without stopping at a control post.
Depending on species, the animals travel in containers (e.g. poultry, turkeys, rabbits) or loose (e.g. small and large ruminants, pigs and horses). Space allowance varies depending on the species and often the animal category and it is reported in the Annex 1 of EC 1/2005. For example, for bovines, depending on the weight categories, the following different minimal space allowance is envisaged, ranging from an area of 0.30 and 0.40 m2 for small calves (approximate body weight of 50 kg) to an area greater than 1.60 m2 for very heavy cattle (body weight more than 700 kg).
The legislation specifies that the minimum space allowance should be increased depending on the physical state of the animals (i.e. late pregnancy, newborn animals) and weather conditions (e.g. increase space by 20% in case of hot weather). Since space allowance is considered one of the most important risk factors for poor health and welfare, the current minimum space allowance is under revision (EFSA AHAW Panel, 2022a,b,c). Cattle are usually transported on single deck vehicles, able to move around freely. The number of animals per vehicle depends on the animal categories, and on the total available space within a vehicle (usually it is approximately 32 m2). The average number of medium size cattle transported per vehicle is 30 (Padalino et al., 2021a). The available space is sometimes divided into smaller compartments, so the group size is smaller. This practice is recommended to avoid mixing unfamiliar animals. Pigs usually travel in multi‐deck vehicles, usually with three decks. The vehicle compartment, and the type of deck floor can be risk factors for transport‐related diseases, due to different environmental conditions within the vehicle (Broom, 2008). For instance, the top level is usually hotter and the lower level colder due to different ventilation and air movements within the vehicle (EFSA AHAW Panel, 2022a,b,c). Bedding is often used to improve animal comfort and welfare, and the use of bedding is compulsory during long (8–14 h) and very long (> 14 h) journeys (see Figure 1). The quantity of bedding material may be important in controlling the microenvironment and thereby minimising cold stress. One study has reported that adding more than six bales/trailer of bedding in cold weather and more than three bales/trailer of bedding in mild weather provided no additional benefit to the pigs (McGlone et al., 2014). Different types of bedding materials (i.e. sand, feed, wood shavings, straw or hay) can be used, often straw and hay are preferred because they can be used for feeding or they can be mixed with pelleted feed.
Animals travelling in containers, such as poultry and rabbits, are manually or mechanically caught and loaded into containers. These can be of different ‘systems’ (loose crate, fixed cages on the vehicle or modular). The dimensions of the containers vary among species and animal category. For instance, the most common size of the crate used for laying hens and broilers is 85 × 66 × 30 cm, with an opening of 30 × 35 cm. The space allowance for caged animals is usually expressed as area in cm2/kg (e.g. for poultry with body weight < 1.6 kg the space is 180–200 cm2/kg). The group size per container therefore depends on the size/category/species of the animal.
Animals are transported for different purposes and one animal can be transported more than once in a lifetime, depending on the production system.
More information can be found in the previously mentioned ‘Animal Transport Guides’ (www.animaltransportguides.eu/materials/) and recently published EFSA Scientific Opinions (EFSA AHAW Panel, 2022a,b,c). In these Opinions, the journey has been split in different stages, which are different depending on the species and if the animals travel or not in containers. Various hazards, welfare consequences, preventive and corrective measures have been identified for each stage.
For animals transported in containers the stages of transport have been defined as follows:
Stage 1: Preparation includes planning of the journey and preparation of the animals by removal of feed and assessment of fitness for transport.
Stage 2: Loading includes catching the animals, placing them in containers (crating) and loading of containers onto the vehicle.
Stage 3: Journey includes the movement of animals by vehicle and intermediate stops along the way until the place of destination is reached.
Stage 4: Arrival includes the period from arrival of the vehicle, unloading of the containers from the vehicle, and waiting period (on lairage/unloading area) up to the start of the uncrating.
Stage 5: Uncrating includes the removal of animals from the containers.
For free moving animals (pigs and cattle) the stages of transport have been defined as follows:
Stage 1: Preparation includes planning of the journey, preparation of the animals (for pigs usually by removal of feed), assessment of fitness for transport, grouping them in the loading area.
Stage 2: Loading starts when the first animal is moved from the holding pen into the means of transport and ends when the last animal is loaded and until the ramp is closed.
Stage 3: Transit starts when the ramp has been closed and ends when the ramp is opened.
Stage 4: Unloading starts when the ramp is opened and the first animal exits the means of transport and ends when the last animal exits.
Stage 5: New environment, the first period when animals arrive in the new place (at slaughterhouses this stage is usually named lairage period).
During some journeys there may be ‘journey breaks’ which are periods when the truck is stopped on the side of a road, or when animals are offloaded to other facilities for feeding, watering and resting, including control posts.
1.3.1.1. Description of types of journeys and holding establishments applying to hatching eggs, chicks and later stages of rearing, breeding and commercial egg and meat production stages of poultry
Movements associated with poultry are shown in Figure 2. There are several tiers of breeding flocks from Grandparent/elite flocks down to commercial breeding flocks. Each of these supply eggs on plastic trays in vans or lorries, to breeder or commercial hatcheries which in turn supply chicks to the tier below; ending with commercial single stage meat bird flocks, rearing flocks for laying hens and some two stage rearing sites for meat birds such as turkeys and slow growing meat chickens. Chicks are transported in reusable plastic or metal crates or single use cardboard trays and birds from rearing flocks are delivered in plastic or metal crates or modules containing cages that are carried on lorries, which applies to harvested birds or spent breeding or laying flocks being transported to slaughter.
Figure 2.

Type of movements within the poultry industry. Around 95% of the meat production birds (e.g. poultry and turkey) move twice, from hatchery to farm and from farm to slaughterhouse. Solid lines display main routes of transport for production, while dotted lines show less frequent routes
Transportation of poultry between farms and to slaughter is an industrialised stressful process. It may include loud and unpredictable noise, manual or automated catching (predominantly for larger birds) and loading into crates or cages (depending on the species). Stocking densities of birds are high. Birds are usually subjected to feed and water withdrawal. The crates are loaded into modules and then onto trucks. Trucks often travel for several hours (EFSA AHAW Panel, 2022a). During the journey, animals are subjected to stress caused by microclimatic conditions, motion of the vehicle, vibration and noise. After the journey, modules are unloaded, followed by waiting in the lairage area. Finally, birds are unloaded manually for slaughter (unless gas stunning is used) or placed in a new housing environment (Wein et al., 2017). Transport of day old chicks follows different practices and regulation, and it is the only transportation carried out in fully air‐conditioned vehicles (for details see EFSA AHAW Panel, 2022a).
More information on poultry transport can be found in the ‘Guide to good practices for the transport of poultry’ (Consortium of the Animal Transport Guides Project, 2018a, available online at http://www.animaltransportguides.eu/wp-content/uploads/2021/02/EN-Guides-Poultry-final_2021.pdf) and EFSA AHAW Panel (2022a).
1.3.1.2. Description of types of journeys and holding establishments applying to pig production based on production farm, production stage and intended final use of the animals
Figure 3 shows a diagrammatic representation of pig production. This production has a pyramidal structure with several levels of breeding and multiplier farms down to the farms that primarily produce piglets for meat production. From all levels, pigs may be transported to one of the following levels, or directly to slaughter. At the lowest level are pigs primarily produced for fattening purposes. This includes the vast majority of pigs and farms. Pigs may either be raised on their farm of birth or be moved to a fattening farm, sometimes via a nursery farm stage which may specialise in rearing weaned piglets to the grower stage, thus involving an additional level of transport. Studies of the dynamics of pig transport revealed that shipments to the slaughterhouse occur at a higher frequency than farm‐to‐farm transport (Crescio et al., 2021). In addition, different studies have concluded that ‘within‐EU’ pig movements are characterised by a randomised structure (Bigras‐Poulin et al., 2007), often not restricted to an administrative region (Lentz et al., 2011).
Figure 3.

Type of movements within the pig industry. The scheme summarises the different sort of farms by production objectives. Solid lines display main routes of transport for production, while dotted lines show less frequent routes. Dotted boxes indicate the type of animal that is transported. Some individual steps may be skipped. Nursery units may be part to the commercial breeder farm (farrow to grower farms) with no transport after weaning. Likewise, fattening units may be on the same farm as well (farrow to finish farm). In those cases, less transport is involved. Additional transport may be involved in multi‐site operations. Transport to livestock assembly centres (markets, dealers, shows) occurs in most countries, albeit mostly at small scale. Most pigs move twice, from farm to farm and from farm to slaughter house/abattoir
More information on transport of pigs can be found in the ‘Guide to good practices for the transport of pigs’ (Consortium of the Animal Transport Guides Project, 2018b, available online http://animaltransportguides.eu/wp-content/uploads/2016/05/Guides-Pig-EC-Templ.pdf) and EFSA AHAW Panel (2022b).
1.3.1.3. Description of types of journeys and holding establishments applying to cattle production based on production farm, production stage and intended final use of the animals
Types of journeys within the bovine industry are presented in Figure 4. Cattle (bovine animals) are transported for various purposes. The majority of transport events occur either in early life, when animals are transported directly from their farm of birth to a further farm, or to a livestock market or dealer and subsequently to another farm. In multi‐site production systems, having specialised units for young stock raising, transport may include shipment between the unit where the calf is born and the unit where it is to be raised. In beef production, further transport events may occur between the units raising the small calves until they are weaned and the final fattening units. In suckler cow herds, transport normally takes place at weaning, either to a fattening unit run by the same farm or by another farm. For cattle, all these transport stages may additionally include assembly centres where animals are unloaded and re‐loaded. Transport of animals to and from agricultural shows or markets may play a role for some animals. Another major transport event is journeys to slaughter for animals used for meat production (beef and veal) or at the end of the production life (cull cows). A third event is trade in animals between farms, e.g. as dairy replacement heifers and trading of older animals (> 1 year of age) for other purposes than slaughter.
Figure 4.

Cattle breeding and distribution. Solid lines display main routes of transport for production, while dotted lines show less frequent routes. Dotted boxes indicate the type of animal that is transported. Most meat production cattle move at least twice, from the farm of birth to the raising/fattening farm and from that farm to slaughterhouse/abattoir. For all cattle additional movements may occur (e.g. to and from assembly centres, including markets or fairs, or between different parts of the same farm)
Conditions of transport differ substantially between these transport events. For calves, transport is known to be associated with substantial health risks (Wilson et al., 2020). In pure dairy breeds, such as Holstein Friesian or Jersey, transportation mainly involves male animals that are of very little economic value to the dairy farm and therefore may have been managed with low input. These animals usually end up in veal calf units.
After transport, such calves exhibit high levels of ARB/ARGs (Gay et al., 2019). It has been reported that upon arrival, or in the period following transport, these animals will frequently be treated with antimicrobials (Pardon et al., 2012; Jarrige et al., 2017; Bokma et al., 2020). Levels of AMR in bacteria from calves are generally high (Tenhagen et al., 2020), although designated studies on the effect of transport on ARB/ARGs in these animals are not available.
Transport for slaughter happens for veal calves at the end of the fattening period as well as for beef cattle slaughtered at older ages.
More information on cattle transport is available in the ‘Guide to good practices for the transport of cattle’ (Consortium of the Animal Transport Guides Project, 2018c, http://animaltransportguides.eu/wp-content/uploads/2016/05/Guides-Cattle-EC-Templ.pdf) and EFSA AHAW Panel (2022c).
1.3.1.4. Effect of transport on susceptibility to disease and bacterial transmission
Surveys on farm animal transport have been performed to explore the epidemiological basis of transport‐related health and welfare issues worldwide. For instance, the mortality due to road transport has been calculated for beef cattle in North America (0.01%) (González et al., 2012), fattening pigs in Europe (0.07%) (Averós et al., 2010) and bobby calves in Australia (0.64%) (Cave et al., 2005). The prevalence of transport‐related health problems varies significantly even within the same species (e.g. the prevalence of bovine respiratory disease varies from about 4% to more than 80%, Timsit et al., 2016; Pratelli et al., 2021). One reason for this large variation may be the use of different criteria to assess health problems, but may relate to the journey conditions and duration, busy and winding roads (Nielsen et al., 2011; Di Martino et al., 2017), the assessment of fitness for transport (i.e. different health status before departure) and the different effects of transport stress on the single animal immune system (Padalino et al., 2018).
During loading, herding, mixing and transporting, animals will encounter different kind of stressors (e.g. motion stress, thermal stress, separation stress) which activate stress responses (see Figure 5) which may affect the immunocompetence, microbiota balance/composition (i.e. increasing the shedding of particular bacteria), and therefore bacterial transmission and susceptibility to disease.
Figure 5.

Gut–brain axis model for the chicken (adapted from Wickramasuriya et al., 2022)
The nexus between transportation and acute phase responses has been investigated in pigs (Murata, 2007), camels (Baghshani et al., 2010) and cattle (Van Engen and Coetzee, 2018). Impaired cell‐mediated immunity and release of cortisol are two signs of the acute phase response, which is an immune based reaction to non‐specific stimuli (Kushner, 1982). In broilers, the stress‐associated hormones (cathecolamines and corticoids) have been shown to promote the expression of virulence factors in pathogens, e.g. in Campylobacter spp. (Truccollo et al., 2020). The acute phase response is characterised by many systemic, metabolic and physiological alterations, including oxidative stress and the release of acute phase proteins (Kushner, 1982; Piñeiro et al., 2007; Cray et al., 2009).
Transport‐associated alterations of oxidative balance may induce an oxidative stress with cellular damage (Kirschvink et al., 2008) and increase susceptibility to disease (McCord, 2000), if not adequately mitigated by antioxidant responses. Thus, oxidative stress might be involved in the development of transport‐related diseases. Monitoring of the redox balance by reactive oxygen metabolites (ROMs) and plasma total antioxidant status (PTAS) in saliva could be a useful tool to assess the health and welfare of transported animals, as already proposed for transported ewes (Piccione et al., 2013).
Other factors (e.g. feed withdrawal) may lead to changes in the composition of the microbiota. These may potentially increase the susceptibility of animals to colonisation by pathogens or enrich commensal organisms that are more likely to carry important transmissible ARGs (e.g. increase in coliforms in calves submitted to dietary stress). This might also potentiate transmission through increased shedding (Cray et al., 1998).
Studies on the shedding of zoonotic bacteria during transport or at lairage have been conducted by different research groups, as further detailed in Section 3.2.
1.3.2. Antimicrobial resistance
AMR describes the resistance of bacteria to antimicrobial drugs. AMR exists in pathogenic as well as in commensal bacteria. It is considered a major global health threat. A recent study estimated 1·27 million deaths in 2019 directly attributable to AMR, including about 33,000 annual fatalities in the EU alone (Cassini et al., 2019; Antimicrobial Resistance Collaborators, 2022).
Humans can acquire ARB via human‐to‐human transmission, direct contact with animals, via the food chain, and from the environment.
In pathogenic bacteria, AMR may impair therapy in both humans and animals. Commensal ARB can provide a reservoir for resistance genes and may be the source of transfer of AMR to pathogens. Microbiome studies have shown that production animals usually carry ARB in the gut, on mucous membranes or on the skin and hide/fleece/fur (Von Tippelskirch et al., 2018; Keijser et al., 2019; Luiken et al., 2020; Schlattmann et al., 2020; Holman et al., 2021; Tong et al., 2022). Antimicrobial usage in both humans and animals selects for ARB and ARGs in their microbiomes. This enhances shedding through faecal material and bodily fluids. It has been shown that other substances such as heavy metals or lack of a diverse microbiome may select or enhance occurrence of ARB and ARGs (Kim et al., 2017; Wang et al., 2021).
Resistance genes can be located within or on mobile elements such as plasmids, transposons, membrane vesicles or phages (EFSA BIOHAZ Panel, 2021). Additionally, free genetic material from dead bacterial cells may include resistance genes, which could be taken up by neighbouring bacteria by natural transformation. In the intestine, there is a constant exchange of genetic information between bacteria (Frazão et al., 2019). This includes genes relevant for metabolism involved in adaptation to the intestinal environment but can be observed for AMR and virulence genes (Capozzi and Spano, 2009; Bakkeren et al., 2019). Under animal stress, e.g. thermal stress in mice, it has been shown that mobile elements can be activated in the microbiome of the intestine and in vitro studies have shown that stress hormones such as norepinephrine can activate horizontal gene transfer in bacteria (Zeng and Lin, 2017; Peterson et al., 2011). Thus, horizontal transfer of genes may be induced under conditions of stress in the host.
ARB/ARGs have been isolated from a range of sources in animal production, including from the animals, their environment and farm staff. These sources of ARB/ARGs have been reported at transport, lairage and slaughter stages (EFSA, 2021).
Bacterial pathogens, including non‐typhoidal Salmonella spp. and Campylobacter spp., MRSA, Enterococcus faecium and E. faecalis, Acinetobacter baumannii and Pseudomonas aeruginosa resistant to last resort/option antimicrobials were considered as the highest public health priority Group 1 bacteria in relation to the food‐producing environments (EFSA BIOHAZ Panel, 2021). In the same context, commensals or environmental bacteria carrying mobile ARGs conferring resistance to ‘last resort’ antimicrobials (priority Group 2 bacteria) were considered to be of highest relevance to public health as well (EFSA BIOHAZ Panel, 2021).
Among the highest priority ARGs, those conferring resistance to carbapenems (e.g. bla VIM, bla NDM, bla OXA‐48‐like, bla OXA‐23‐like), extended‐spectrum cephalosporins (e.g. bla CTX‐M, AmpC encoding genes), plazomicin (armA), colistin (mcr), methicillin (mecA, mecC), glycopeptides (vanA genes) and oxazolidinones (cfr, optrA) have been reported in the animal production sector (EFSA BIOHAZ Panel, 2021).
In accordance with Directive 2003/99/EC6 on the monitoring of zoonoses and zoonotic agents, the EU MSs must collect relevant and comparable data on the occurrence of zoonoses, zoonotic agents and of AMR in zoonotic agents. The Commission Implementing Decision 2020/1729 lays down specific technical requirements for AMR testing and reporting in representative bacterial isolates derived from randomised sampling of broilers, laying hens, fattening turkeys, fattening pigs and calves (bovine animals under 1 year of age) domestically produced, performed at farm and/or at slaughter level, and of fresh meat from broilers, turkeys, pigs and bovine animals performed at retail and at border control posts. EFSA produces, in collaboration with the ECDC, an annual EU summary report (EFSA and ECDC, 2022) that analyses all the AMR data reported by the MSs to EFSA and assesses the situation in the populations mentioned above, the targeted bacterial species are: (i) Salmonella spp.: isolates obtained from samples taken within the framework of national control programmes (for broilers, laying hens, fattening turkeys; and fattening pigs if such programmes are in place in the MS), and isolates from caecal samples at slaughter (fattening pigs, if no control programmes in place; bovine animals), (ii) C. coli and C. jejuni, indicator commensal E. coli, ESBL/AmpC/CP‐producing E. coli, and voluntarily, E. faecalis and E. faecium, from caecal samples at slaughter (e.g. broilers, fattening turkeys and pigs, bovines). Although MRSA is not currently targeted, an EU‐wide baseline survey to update previous data (EFSA, 2019) on its prevalence in slaughter pigs is planned to run over 2023 (EFSA, 2022).
1.3.3. Previous EFSA Scientific Opinion of interest to this Mandate
Previous scientific opinions and technical reports published by EFSA and collaborators have explored different aspects relevant for the current scientific opinion. The topics assessed in these documents were, among others:
In relation to AMR:
‘Foodborne antimicrobial resistance as a biological hazard’ (EFSA, 2008),
‘Analysis of the baseline survey on the prevalence of methicillin‐resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008, Part A: MRSA prevalence estimates’ (EFSA, 2009),
‘Analysis of the baseline survey on the prevalence of methicillin‐resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008, Part B: factors associated with MRSA contamination of holdings’ (EFSA, 2010),
‘The public health risks of bacterial strains producing extended‐spectrum beta (β)‐lactamases (ESBLs) and/or AmpC β‐lactamases (AmpC) in food and food‐producing animals’ (EFSA BIOHAZ Panel, 2011a),
‘Carbapenem resistance in food animal ecosystems’ (EFSA BIOHAZ Panel, 2013),
‘Measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union (EU) and the resulting impacts on food safety, taking into account the impact on public health and animal health and welfare – RONAFA’ (EMA and EFSA, 2017),
‘Technical specifications on harmonised monitoring of antimicrobial resistance in zoonotic and indicator bacteria from food‐producing animals and food’ (EFSA, 2019),
‘The role played by the environment in the emergence and spread of antimicrobial resistance (AMR) through the food chain’ (EFSA BIOHAZ, 2021),
A series of assessments of ‘animal diseases caused by bacteria resistant to antimicrobials’ in different animal species (EFSA AHAW Panel, 2021a,b,c),
Yearly EU Summary Reports on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food (EFSA and ECDC, 2022).
In relation to other bacteria relevant for the present study:
‘Quantitative microbiological risk Assessment of Salmonella in slaughter and breeder pigs’ (EFSA BIOHAZ Panel, 2010),
‘Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain’ (EFSA BIOHAZ Panel, 2011b).
‘Technical specifications on harmonised epidemiological indicators for biological hazards to be covered by meat inspection of poultry’ (EFSA, 2012) are also relevant documents.
‘Update and review of control options for Campylobacter in broilers at primary production’ (EFSA BIOHAZ, 2020).
As already indicated, in parallel with the current AMR Transport Mandate, Scientific Opinions on the welfare of different animals, including pigs, cattle and domestic birds, during transport were produced (EFSA AHAW Panel, 2022a,b,c).
2. Data and methodologies
2.1. Data
Data were extracted from the scientific literature, EU Legislation, previous EFSA Scientific Opinions and reports, other international reports (FAO/WOAH/WB, 2010, ‘Animal Transport Guides’, online http://www.animaltransportguides.eu/materials/) as well as from publicly available databases (e.g. TRACES, ISTAT, D‐Statis). Information gathered by the EFSA AHAW Panel during the development of the Scientific Opinions EFSA AHAW Panel (2022a,b,c) was shared with the AMR Transport working group (WG).
2.2. Methodologies
2.2.1. Approach to answer the ToRs
The approach to answer the ToRs was defined in advance and is described in the protocol (Annex A). It covers both the problem formulation (i.e. what the assessment aims to address) and which methods will be used for addressing the problem. The problem formulation (‘what’) includes the clarification of the mandate (see further refined in Section 1.2) and consists of the steps (1) translation of the mandate into scientifically answerable AQs, and (2) the selection of the approach for the assessment. The planning of the methods for conducting the assessment (‘how’) consists of specifying the evidence needs and the methods for answering each AQ, including the uncertainty analysis. Protocol development followed the draft framework for protocol development for EFSA's scientific assessments (EFSA, 2020).
2.2.2. Literature searches
A qualitative assessment of the transmission of AMR and zoonotic agents during animal transportation and holding in animal gathering centres such as livestock markets, animal dealers, collection centres and lairages was undertaken, based on the available literature and expert knowledge within the WG. Literature searches were extended using ‘footnote chasing’ (White et al., 1992) and supplemented by citation inputs by WG members and information about relevant publications provided by members of the EFSA BIOHAZ Panel. The relevance of the records in providing information was assessed by screening the title, keywords and the abstract and based on the knowledge and expertise of the WG members. This review included international reports and EFSA Scientific Opinions and Reports, scientific review papers, book chapters, peer‐review papers and other documents known by the experts or retrieved through non‐systematic searches as well as current European Legislation. The search strategy (search strings and databases) is included in Appendix A.
2.2.3. Uncertainty analysis
Uncertainty in this Scientific Opinion was investigated in a qualitative manner following the procedure detailed in the EFSA guidance on uncertainty analysis in scientific assessments (EFSA Scientific Committee, 2018a,b). The sources of the main uncertainties were identified by the experts in the WG and their individual impact as well as their combined impact on the certainty of the answers to the AQs were discussed. Consensus expert judgement within the WG, informed by the collected evidence and expert knowledge, was used to assess the certainty of the answers to the AQs, which was expressed using EFSA's subjective probability scale (EFSA Scientific Committee, 2018a) (Tables 1 and 2).
Table 1.
Transport‐related risk factors associated with transmission of antimicrobial resistance and associated uncertainties
| Risk factor | Supporting references (a) | Data based on (b) | Comments and uncertainties | Effect of duration (c) | Conclusions/subjective probability range (d) |
|---|---|---|---|---|---|
| 1. Resistance status pre‐transport | EMA and EFSA (2017), Munk et al. (2018) | AMR animal monitoring. |
The higher the load and diversity of the ARB/ARGs in the animals, the higher the risk for transmission. All factors that affect the abundance of ARB/ARGs in animals on the farm are of interest (e.g. AMU, hygiene, mixing of animals). Risk factors on farm are out of the scope of this Opinion. Moreover, the exact effect of presence of low amounts of ARB/ARGs in a few animals on the risk of transmission to other animals during transport is uncertain. The impact depends on the public health relevance of the ARB/ARGs as well . |
Duration of transport itself will not have any impact on the resistance status pre‐transport. The effect of the resistance status pre‐transport might be bigger when the duration of transport increases. | Considered 99–100% certain (almost certain) that the resistance status (presence and type of ARB/ARGs) of the animals pre‐transport will influence the probability of transmission of ARB/ARGs during transport. A higher load and diversity of ARB/ARGs will increase the probability of transmission. |
| 2. Factors affecting microbiota in animals. | |||||
| 2a. Feed withdrawal |
Ramirez et al. (1997), Wilson et al. (2018), Gast and Porter (2020), Shane (2000) Massacci et al. (2020) |
Increased prevalence and shedding of Salmonella spp. and Campylobacter spp. in poultry. Reduced welfare may lead to altered microbiota composition. Increased gut permeability, which can lead to diarrhoea . |
Feed withdrawal has effects on the microbiota of animals as demonstrated by increased prevalence and shedding of certain bacteria (mainly studied in pathogens). Although data are based on studies not focused on ARB/ARGs, these pathogens (e.g. Salmonella spp., Campylobacter spp.) often carry ARGs. The majority of the studies that investigate feed restriction focus on outcomes other than ARB/ARGs (e.g. increased gut permeability, disturbance of the microbiota composition) that may have an effect on the shedding of ARB/ARGs. On the other side, it has been shown that withdrawal reduces vomiting in pigs, and less faecal material would be produced. Due to the lack of evidence of the effect of withdrawal for shedding and transmission of ARB/ARGs and because both positive and negative effects on ARB/ARGs transmission can be assumed, the overall direction of the effect of feed withdrawal remains unclear . |
Longer periods of feed withdrawal may provoke hunger‐related stress responses and may be associated with changes in the composition of the microbiota. When feed withdrawal time increases, a trend of increased caecal Enterobacteriaceae and Salmonella spp. in faeces is observed . |
Considered 33–66% certain (as likely as not) that feed withdrawal can affect the probability of transmission of ARB/ARGs during transport. Feed withdrawal can increase shedding of certain bacteria (e.g. Salmonella spp., Campylobacter spp.) which are often resistant to antimicrobials, but at the same time, the reduction on vomiting and shedding of faecal material could reduce the probability of transmission of ARB/ARGs. |
| . | |||||
| . | |||||
| 2b. Stressors | Artuso‐Ponte et al. (2015), Verbrugghe et al. (2011) | Cortisol increased the concentration of Salmonella in the intestines of pigs. | There was an association reported of levels of stress hormones with shedding of Salmonella spp. There is no direct evidence of an effect on ARB/ARGs transmission. | No evidence, although theoretically expected. | Considered 33–66% certain (as likely as not) that stress can affect the probability of transmission of ARB/ARGs during transport. There is evidence that stress can lead to alterations in microbiota and suppression of the immune system, but the direction of the association with ARB/ARGs is unclear. |
| 2c. Environmental conditions | Huus and Ley, 2021; Sepulveda and Moeller, 2020; Sun et al., 2020; Woldehiwet et al., 1990 |
Changes in temperature and/or humidity can impact growth conditions for bacteria on mucosal membranes. Such changes may favour the expansion of certain ARB and shedding of bacteria . |
No specific data for ARB/ARGs are available. Transportation out of the thermoneutral zone may increase disease risk with shedding of bacteria and impairment of immunity. | No evidence, although theoretically expected. | Considered 66–90% certain (likely) that inadequate transport environmental conditions increase the probability of transmission of ARB/ARGs due to alterations in the microbiota, contributing to the expansion of certain ARB and shedding of bacteria. |
| 2d. Health status of the animal | Animals suffering bacterial infections, i.e. enteric, respiratory or skin infections are a source of potential AMR transmission. A specific effect on AMR selection can be expected if such animals need treatment before or during transport. |
Animals suffering bacterial infections are a potential source of potential ARB/ARGs transmission and of higher risk of colonisation with ARB/ARGs. In animals with an inadequate health status the likelihood of treatment increases and this will have an effect on AMR although it is outside of the scope of this Opinion . |
The probability of acquiring infections and subsequent increased shedding increases over time. The probability of the need for treatment in such animals will increase over time . |
Considered 66%–90% certain (likely) that the health status of the animal contributes to the probability of transmission of ARB/ARGs. Infections during transport contribute to the probability of transmission of ARB/ARGs through increased shedding and decreased resilience to colonisation/infection. | |
| 3. Increased faecal shedding. |
Simons et al. (2016), Callaway et al. (2006), Grønstøl et al. (1974) Kent and Ewbank (1983), Kenny and Tarrant (1987), Pempek et al. (2017) |
Increased Salmonella spp. excretion during transport in pigs and cattle. Increased shedding of faeces in cattle during transportation . |
Transport related stress can lead to increased shedding of faeces and certain bacteria. Increased faecal shedding can lead to increased shedding of ARB/ARGs, when present in the gut. No specific information for ARB/ARGs is available. This risk factor is closely linked to the ARB/ARGs load present in the animal prior to transport of ARB/ARGs . |
Although agitation at loading will diminish over time, shedding is likely to be more extensive when duration of transport increases. | Considered 99%–100% certain (almost certain) that increased faecal shedding during transport increases the probability of transmission of ARB/ARGs during transport. Any factor that increases shedding (e.g. due to different stressors) would also increase the shedding of ARB/ARGs, if present. |
| 4. Environmental exposure to ARB/ARGs. | |||||
| 4a. Cleanliness of loading and unloading areas and of vehicles. |
Mannion et al. (2008), Hurd et al. (2002), Magistrali et al. (2008) Lowe et al. (2014), Dee et al. (2005), Bronsvoort et al. (2008), Mur et al. (2012), Baker et al. (2017), VanderWaal et al. (2018) |
Salmonella and Campylobacter present in transport vehicles and crates. Inefficient cleaning and disinfection of vehicles shown by studies on viruses. Spread of pathogens between farms by contaminated vehicles, including crates . |
Contaminated transport vehicles and related equipment such as transport crates have the potential of contaminating previously clean animals with bacteria originating from animals transported before. No ARB/ARGs specific studies are available on the contribution of contaminated vehicles to the transmission. | When duration increases, the exposure time of animals to environmental contamination increases as well as the potential to contaminate the environment (for the latter see above). | Considered 99%–100% certain (almost certain) that insufficient hygiene of the loading and unloading areas and vehicles contributes to the probability of transmission of ARB/ARGs during transport, as it increases the probability of transmission of ARB/ARGs between animal batches (transmission through the environment). |
| 4b. Airborne transmission | Rule et al. (2008), Friese et al. (2013), Schulz et al. (2012), Bos et al. (2016); Dohmen et al. (2017b) |
Resistant enterococci were found in air samples behind animal transport vehicles. ESBL/MRSA are detected in dust on farms. Airborne exposure can lead to transmission in a farm . |
Airborne transmission of ARB/ARGs is not described within transport vehicles. The findings of resistant bacteria behind vehicles and within dust on farms suggest the possibility of airborne transmission within vehicles. Resistance bacteria can be found in air and dust. It is not completely clear if the duration of transport is long enough for airborne transmission to be of high relevance . |
When duration increases, the exposure time of animals to ARB/ARGs in air increases as well. | Considered 66%–90% certain (likely) that airborne transmission contributes to the probability of transmission of ARB/ARGs during transport. The importance of this effect will depend on the bacteria (e.g. higher in respiratory pathogens and MRSA than in ESBL‐producing Enterobacteriaceae) and the presence in airborne particulates. There is evidence of the presence of ARB/ARGs in the air, which can lead to subsequential transmission, although not exactly clear to what extent. The likelihood of airborne transmission is probably dependent on duration of transport and ventilation. |
| 4c. Workers | Mughini‐Gras et al. (2019) | Transmission between different reservoirs (e.g. farmers and their livestock) occurs. |
Transmission from livestock to workers is considered to be dominant compared to the vice versa route, based on a higher total load of ARB/ARGs in a group of animals compared to humans. Moreover, differences in gene types and distribution between these reservoirs provide evidence for transmission from animals to humans. Workers and their protective clothing can serve as vectors for ARB/ARGs transmission . |
The likelihood of transmission increases by a higher number of contacts between workers and animals. At a longer journey duration, there are more contact moments and therefore a higher risk. | Considered 66%–90% certain (likely) that ARB/ARGs in workers (either as carrier or vector) may contribute to the probability of transmission of ARB/ARGs during transport. This is likely of minor importance in comparison to exposure to the truck environment and other animals carrying ARB/ARGs. |
| 5. Exposure to other animals carrying and/or shedding ARB/ARGs | Broens et al. (2011) | Exposure is defined by the intensity and number of contacts. |
When contact between animals is limited, the risk of direct transmission is lower. Specific data for the transmission of ARB/ARGs during transport is not available. The information to date is extrapolated from other contexts (livestock farms, and/or non‐resistant bacteria). Most likely, mixing of animals from different batches will increase the risk of transmission. The transmission rate between mixed animals will depend on the type of bacteria . |
When the duration of transport increases, contact between animals is prolonged as well. | Considered 99%–100% certain (almost certain) that exposure to other animals carrying and/or shedding ARB/ARGs contributes to the probability of transmission during transport. A higher frequency, duration and intensity of direct contacts between animals increases the risk of faecal‐oral transmission. The diversity of transmitted ARB/ARGs increases when animals from different origins are transported together. |
| 6. Environmental conditions | Humidity and warm temperatures can increase the survival rate and multiplication of bacteria in the environment. | Increased temperature and/or humidity favour bacterial growth and will support the multiplication of the present bacteria, independent of the resistance features. | Duration of changes in temperature/humidity would impact on bacteria multiplication and likely in ABR. | Considered 66%–90% certain (likely) that unfavourable conditions (high temperature and humidity) will increase the probability of transmission due to increased multiplication and survival rate of bacteria in the environment, including ARB/ARGs. | |
| 7. Duration of transport/lairage | The duration of transport is associated with contamination of hides with Salmonella and E. coli in cattle and Salmonella infection in pigs. |
Duration is associated with many other factors (see above). Only a few studies report an effect of duration of transport on pathogen presence. Moreover, information on the association with ARB/ARGs is not available. Prolonged stress, longer period of feed withdrawal and an increased number of contacts between animals may play a role in increased transmission of ARB/ARGs between animals during longer transport and resting . |
NA |
Considered 99%–100% certain (almost certain) that the duration of transport contributes to the probability of transmission of ARB/ARGs during transport. Transmission of ARB/ARGs can occur during short duration of transport. Nevertheless, the effect of most of the identified risk factors for transmission of ARB/ARGs will increase as a result of a longer duration. Although most of the identified risk factors are influenced by duration, there is no evidence to estimate differences between journeys shorter or longer than 8 h. Journeys that require rests in control posts, will be associated with specific factors in those temporal areas (e.g. mixing of animals, environmental contamination, stress) . |
|
| Lairage, livestock assembly centres and control posts |
Boughton et al. (2007b) Arthur et al. (2008) Broens et al. (2011) |
Contamination of lairage with Salmonella. Increased contamination of hides at lairage. Identity of bacteria at lairage and on carcasses . |
Environmental contamination and exchange between animals at lairage, control posts and/or assembly centres can lead to acquisition of bacteria. An increase of the proportion of MRSA positive animals at lairage has been observed, although it was not clear whether that was due to exchange of bacteria between animals or to uptake from the environment. The environmental exposure to ARB/ARGs at lairage, assembly centres and control posts is additional to the environmental exposure during transport and is likely different in terms of variety and abundance. On the contrary, the exposure to other animals harbouring ARB/ARGs is probably only extended at lairage, assembly centres and control posts, since mostly animals are not mixed anymore at these locations. The role of environmental contamination in the transmission of ARB/ARGs during lairage is therefore probably more dominant than the role of direct exchange of ARB/ARGs between animals. Since the latter is not different, but only extended for the duration of lairage . |
When duration increases, the exposure time of animals to ARB/ARGs increases as well, both to the environment as to other animals. | Considered 99%–100% certain (almost certain) that exposure to contaminated lairage, livestock assembly centres and control posts will increase carriage of ARB/ARGs in animals and therefore the probability of transmission. Depending on the duration of stay in these locations, most of the contamination will be on the surface of the animals. |
Note: Most of the risk factors have an effect on the transmission of ARB/ARGs during multiple stages of transport.
Further references can be found in the risk factor Section 3.2.2.
Since references on ARB/ARB during transport are very limited, information is extrapolated from studies on risk factors on the transmission of non‐resistant (or not investigated) bacteria during transport. In addition, information is extrapolated from studies on risk factors on the transmission of ARB/ARB in other settings, such as livestock farms.
In this column, the interaction between the risk factor and duration of transport is explored.
The risk factors that were identified as 99%–100% certain to contribute to the probability of transmission of ARB/ARGs, were also considered to be of high importance.
Table 2.
Mitigation measures with their associated uncertainties
| Stage | Mitigation strategy. | Targeted risk(s). | Supporting references (a) | Comments and uncertainties (b) | Conclusions/subjective probability range |
|---|---|---|---|---|---|
| All | 1. Good husbandry practices. | Factors impacting negatively on health, welfare, e.g. stress (general, temperature, grouping, stress hormones), frequency of faecal shedding, or driving to an unstable microbiota. | EMA and EFSA (2017), FAO/WOAH/WB (2010), Weeks et al. (2019), Benincasa et al. (2020), EFSA AHAW Panel (2022a,b,c), USDA and CFSPH (2010) | Generally, measures which improve animal health and welfare, and those influencing the reduction of bacterial transmission, would contribute to mitigate AMR. | Most of the measures improving animal health, welfare, and/or biosecurity, immediately prior to and during transport will reduce ARB/ARGs transmission. It is considered 90%–95% certain (very likely) that good husbandry and handling practices associated to animal transport will reduce AMR transmission. |
| 2. Proper cleaning of transport vehicles, loading/unloading spaces and equipment linked to transport. | Transmission of ARB/ARGs between subsequent batches of transported animals. This can be due to failures during the cleaning process, the use of inefficient protocols (e.g. not appropriated to eliminate ARB that could be resistant to several disinfectants. | De Busser et al. (2013), Porphyre et al. (2020) | Improving cleaning and disinfection to efficiently remove bacteria from transport vessels will effectively reduce ARB/ARGs transmission between subsequent groups of animals. | Considered 90–95% certain (very likely) that proper cleaning and disinfection of transport vehicles, crays, cages, loading and unloading areas, lairage areas, assembly centres and in general surfaces and equipment will mitigate AMR transmission. Ineffective cleaning and disinfection of transport is highlighted as one of the major risks for new ARB/ARGs acquisition in this stage. Thus, cleaning and disinfection protocols should be revised and validated to ensure thorough cleaning and disinfection to effectively guarantee removal of resistant microorganisms after each animal transport. Efficacy of the protocols should be tested regularly by inspection and microbiological analyses. | |
| Pre‐transport | 3. Assessment of ‘Fitness of animals for transport’. | Avoiding transport of sick animals and their increased likelihood of shedding ARB and increased susceptibility to new infection/colonisation potentially requiring treatment. | Definition of fitness for transport is still vague, the list of pathologies is not comprehensive and focuses on severe clinical signs. In addition, potential carriers and asymptomatic animals are not detected. Implementing the assessment of the fitness of transport as guidelines recommended by EFSA AHAW Panel (see EFSA AHAW Panel, 2022a,b,c). The efficacy will also depend on the type of ‘sickness’ (e.g. gastrointestinal infections). | Considered 66–90% certain (likely) that depending on the ‘sickness’, avoiding the transportation of sick animals will contribute to decrease the levels of ARB/ARGs transmission during transport. | |
| 4. Measures associated with feeding management: | Based on the uncertainties associated with the risk for AMR transmission linked to feeding management measures (feed withdrawal and alternative substances to antimicrobials), with the data available, no specific mitigation measures in relation to feed on AMR are proposed. | ||||
| – Measures in relation to feed withdrawal (time and extent). | Microbiota imbalance, pathogen (over)‐growth. Limiting thirst and hunger and negative energy balance especially in young stock as stressors that may foster multiplication and exchange of ARB/ARGs. | Dewell et al. (2008a), Farghly et al. (2018), Sabaw and Muhammed (2021). | Precise data to evaluate the final impact of feed withdrawal in AMR is lacking. | ||
| – Intervention using alternative substances to antimicrobials i (e.g. organic acids, essential oils, bacteriophages, bacteriocins, probiotics, etc.). | Microbiota disbalance, pathogen (over)‐growth. | Walia et al. (2016), Rodrigues da Costa et al. (2021). | Lack of specific studies in transport and lairage. Thus, gap in the efficacy against pathogens and ARB. | ||
|
5. Transport logistics: transport organisation depending on AMR criteria such as e.g. AMR load, presence of certain ARB/ARGs (e.g. MRSA, carbapenemase‐ and/or ESBL‐producers, VRE, linezolid resistant Staphylococci/Enterococci) in the batch or farm, as well as AMU . |
Pathogen and AMR spread among transported animals. |
Lack of specific references on AMR transport. References for the slaughter process available: Swanenburg et al. (2001) Hotes et al. (2011), Argüello et al. (2014), Miranda‐de la Lama et al. (2014) |
Lack of specific references on the efficacy on AMR transmission during transport but measure effective for slaughter. To apply this measure either specific or epidemiological information on AMR at farm or batch level prior to transport need to be available. | Considered 95–99% (extremely likely) that AMR transport logistics (transport organisation depending on AMR criteria) would reduce AMR transmission between animals of different AMR loads and ARB/ARGs types. | |
| 6. Transport planning:. | New sources for AMR | Neumann et al. (2021) | Lack of specific studies to quantify the impact of each intervention associated to transport planning. |
Considered 90–95% (very likely) that adequate planning of the transport can limit ARB/ARGs spread. This would include interventions such as minimising the number of farms visited and number of vehicles an animal is exposed to during a journey. Animals should be segregated by species, production stage, or age avoiding the same vehicle for animals transported to farms and the slaughterhouse within the same trip. Adequate animal densities in the vehicle should be foreseen. For the journey duration, see Mitigations during transport. |
|
| – Farms visited and vehicles used to transport these animals (potential use of several vehicles during the same transport). | Transmission due to mixing of animals from different origins. | ||||
| – Transport route. | Unappropriated routes which increase transport length/duration or discomfort, i.e. stressors potentially fostering ARB/ARGs. | ||||
| – Time (day/night). | Impacting animal health by the exposure to heat and low temperatures. | ||||
| 7. Segregation, stocking density and group size: | Lack of specific references. |
Considered 90–95% certain (very likely) that animal segregation would limit animal contact and potential ARB/ARGs transmission. Considered 66–90% (likely) that reducing stock densities and number of animals in contact will have a beneficial effect reducing ARB/ARGs transmission in absence of other factors (i.e. sick animals, cleaning). |
|||
| – Effective segregation of animals of different origins. | Transmission between groups of animals. | ||||
| – Minimising group size. | Reducing number of animals in contact. | Airborne transmission difficult to avoid, even if the animals are separated in different compartments. | |||
| – Increasing space allowance (reducing stocking density). | Risk factors linked to high stock densities (animal close contact, faecal spread, welfare). | No specific data on AMR. Burden of AMR at origin may hamper the efficacy of the strategy. More space may increase the interactions among animals. | |||
| Loading, transportation (journey/transit), and unloading | 8. Ensuring thermal comfort of the animals: | Considered 90%–95% (very likely) that establishing measures that ensure the thermal comfort of the animals during the transport would have direct or indirect impact in reducing ARB/ARGs transmission. Therefore, the microclimatic conditions during the transport should be adjusted. Vehicles and/or crates should be designed accordingly. Moreover, the most appropriate times of the day should be selected for the travel. | |||
| – Ensuring that animals are transported under microclimatic conditions within their specific thermal comfort zones. | Thermal stress, environmental conditions that foster multiplication of bacteria. | Mitchell and Kettlewell (2008), Ramadiani et al. (2021), EFSA AHAW Panel (2022a,b,c) | Effect of thermal stress on AMR transmission through increased respiration rate with increased exchange of respiratory bacteria. There is as yet no experimental evidence for the efficacy against ARB/ARGs transmission. | ||
|
– Design of transport vehicles/crates to improve animal comfort (e.g. using forced ventilation and air‐conditioning). – Travelling during the most appropriate time of the day (i.e. not the coldest or the hottest) . |
Tª, humidity, comfort, ventilation. | Bhatt et al. (2021), Pinheiro et al. (2021) | Lack of specific data on AMR | ||
| 9. Bedding | Reducing spillage of faeces and urine. Tackling welfare risks (comfort, microclimatic conditions in winter). | Ray et al. (2022) |
Probably insufficient capacity to suck up liquid with long transportation. Potential introduction of AMR through organic bedding material (straw, saw dust, etc.). Risk that contaminated bedding is consumed. |
The effect of providing adequate bedding as mitigation strategy on the risk of transmission is considered 33%–66% certain (as likely as not). There are factors which may mitigate it and others which may increase it. On the positive side, during shorter journeys bedding may have hygienic benefits by soaking up liquid. During longer journeys the capacity of the bedding to soak up liquid is likely to be overwhelmed. On the negative side, bedding increases the effort needed to clean vehicles after transport thereby potentially increasing the risk of inadequate cleaning and disinfection. Likewise, bedding may provoke its ingestion thereby promoting uptake of resistant bacteria from the contaminated environment. | |
| 10. Shortening duration of journey. | Reduce time for exchange of bacteria, establishment of new infection and duration associated stressors (feed withdrawal, etc.). | Caffrey et al. (2017), Dos Santos et al. (2017) | Lack of specific data for AMR. Potential correlation between duration and AMR burden/transmission. | Considered 95%–99% certain (extremely likely) that minimising the duration of transport will reduce AMR transmission. Long journeys requiring stops or unloading are a risk for AMR acquisition through mixing of animals and uptake of ARB/ARGs from a contaminated environment. Based on the limited data available, no maximum journey duration can be recommended. | |
| Assembly centres, control posts | Measures described in transport section can be applied here with the same expected efficacy. Thus, proper management of assembly centres, e.g. clear separation of batches, adequate animal handling and housing conditions (under Tª, humidity, ventilation rates which provide physiological comfort, etc.), efficient cleaning and disinfection, space allowance. |
Avoid mixing and transmission between groups of animals. Risk of transmission by direct contact and increased defaecation . |
Lack of references |
Complex logistics necessary. Reduce animal contact and burden of faeces. Additional benefits from welfare. |
Considered 66%–100% certain (likely to extremely likely), depending on the measure taken, that implementation of mitigations against the risk factors identified linked to the use of assembly centres and control posts mentioned above would reduce the probability of transmission of AMR. The measures recommended above with regards to animal handling, stocking densities, mixing/segregating animals and facilities cleaning and disinfection, including its validation, are also relevant to mitigate AMR transmission in these places. |
| Lairage | Measures described in transport section can be applied here with the same expected efficacy: |
Microbiological monitoring of cleaning and disinfection would improve the quality check. No information about which microbiological analysis would be better. Limitation of the time required for analyses. Lairage pens are hot spots of bacterial transmission and improvement of cleaning and disinfection protocols should reduce AMR transmission. Effect of frequent disinfection on tolerance to disinfectants needs to be monitored. |
Considered 66%–100% certain (likely to extremely likely) depending on the measure taken, that implementation of the mitigations mentioned in the point above as well as limiting lairage time to the minimum required, will reduce the probability of transmission of ARB/ARGs. See conclusions about interventions using alternative substances to antimicrobials in pre‐transport. |
||
|
– Effective cleaning and disinfection in lairage facilities. – Limiting lairage time to the minimum required. – Segregation of animals by origin. – Appropriate animal husbandry in lairage. |
Transmission of ARB/ARGs between subsequent groups of animals. Limiting exposure to the risks identified. Transmission between animals from different origins. Risk associated to animals stress and poor welfare. |
Walia et al. (2017) Lack of references Lack of references Lack of references |
|||
|
– Interventions using alternatives to antimicrobials. |
Microbiota disbalance, pathogen (over)‐growth. |
Lack of specific studies in transport and lairage. Thus, gap in the efficacy against pathogens. In addition, there are no studies evaluating if they are useful for mitigating or controlling AMR spread. |
Further references can be found in the mitigation Section 3.3.
References are quite limited. The consideration of the efficacy of the mitigation options is based largely on expert judgement and empirical extrapolation from evidence on mitigation measures efficient to avoid/minimise bacterial transmission.
3. Assessment
3.1. Introduction
As already indicated, most production animals are transported more than once during their lifetime. Animals are transported to meet the needs of hierarchical and specialised production systems (breeding, rearing, fattening; meat, milk or egg production). Transport between production units may take short or longer times (less or more than 8 h) and might include rests or stops in control posts and/or assembly centres (collection/sorting centres and/or dealers' premises or auction markets) before animals arrive at the farm of destination or at slaughter facilities (Figure 6). Slaughter transports mainly involve short journeys by lorries (less than 8 h) but may take longer if necessary (EC 1/2005). Poultry, pigs and cattle transportation (with main focus on road transport) and holding in assembly centres or lairage will be assessed, in order to evaluate the actual or potential impact of these practices on the occurrence, level and transmission of ARB/ARGs.
Figure 6.
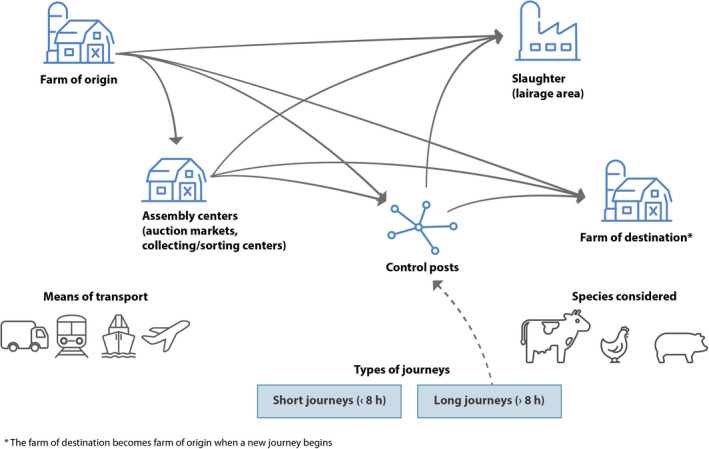
Routes and means of transport of the animal categories (cattle, pigs and poultry) covered in the current assessment. These include short and long journeys (less and more than 8 h) by different transport vehicles (trucks, trains, livestock vessels, ferries, planes). In accordance with its share among all transports, transport by trucks on the road will be the main focus. Included in the assessment are all transports of production animals from farms to other farms and/or slaughterhouses, also including control posts (stops during the journey), collection or sorting centres and/or auction markets (assembly centres)
At the point of departure, prior to loading animals harbour a specific microbiota that may contain a certain number of more or less diverse ARBs. This microbiota will reside within or on the surface of the animal, in different sites of the body (upper alimentary tract, gut, skin, upper respiratory tract, etc.). Before, during and after transportation, animals are exposed to different environments including the presence of other animals and staff handling the animals, where an uptake or exchange of ARB/ARGs is possible (Figure 7). Moreover, various stressors, such as feed withdrawal, may have an influence on the microbiota and hence lead to changes in the ARB/ARGs load without external contacts.
Figure 7.

Sources of ARB and ARGs in the transport vehicles. The main source of ARB/ARGs in the transport vehicles are the transported animals, which may be carriers and shed ARB/ARGs via their excretions and secretions (e.g. faeces, saliva, respiratory droplets, urine, and vomit). ARB/ARGs may persist in the environment between batches of animals. Other potential contamination sources are bedding material, feed, water and handling equipment. Finally, workers, including personnel involved in animal loading/unloading on farms, at control posts and assembly centres, drivers and inspectors may be sources of ARB/ARGs. Transmission may occur directly between animals, or indirectly, through direct contact with contaminated materials of the truck and the transport equipment (including crates, ramps, handling equipment and loading platforms) or may be airborne
During loading, transportation/transit, unloading and lairage, animals may exchange ARB/ARGs directly, mainly via contact with other animals or people or indirectly, via different transmission routes.
Indirect transmission can occur via oral uptake of contaminated feed and water or licking of contaminated surfaces. It may further occur through contact with fomites and finally via airborne transmission. Contamination of the environment mainly originates from other animals transported previously or concurrently by shedding of faeces, urine, saliva and vomit. The situation may be aggravated by a lack of effective cleaning and disinfection and numerous other factors as pointed out under risk factors.
The detailed assessment of risk factors affecting the transmission of AMR during transport is presented in the section below.
3.2. TOR1. Risk factors
3.2.1. Introduction to risk factors associated with ARB/ARGs transmission during transport
Documentary information regarding the different effects of factors (related to the animal itself or the environment) during transportation of livestock on the occurrence of ARB/ARGs in these animals is very limited. Information therefore needs to be extrapolated from evidence on the transmission of ARB/ARGs in animals in different contexts (e.g. livestock farms) and from risk factors for transmission of non‐resistant (including those with an unidentified status) bacteria, both commensals and pathogens. Assumptions need to be made, such as that there will not be a substantial difference in the effect of certain precipitating factors influencing the transmission and shedding of sensitive vs. ARB of the same bacterial species.
3.2.2. Overview of risk factors for transmission of ARB/ARGs during transport
Transmission of AMR during transport is subject to a broad range of risk factors. In principle, these can be categorised as:
The ARB/ARGs status of the microbiota of the animals before transport.
Factors affecting the microbiota in animals during transport.
Factors affecting the shedding of ARB/ARGs by the individual animal.
Environmental exposure to ARB/ARGs.
Exposure to other animals carrying and/or shedding ARB/ARGs.
Environmental and transport conditions.
Duration of the transportation (journey/transit).
The first three risk factors listed above are more related to the animal, while the latter four are more related to the environment. All these factors may to some degree be interrelated. For instance, when certain animals are shedding more ARB/ARGs (e.g. by increased defaecation), the exposure of the other animals in the compartment to ARB/ARGs via the environment increases. The risk factors identified are summarised in Figure 8 as well as in Table 1 in Section 3.2.4. The Table also includes the supporting data, the uncertainties associated, and the relationship of those risk factors with the duration of the transport.
Figure 8.
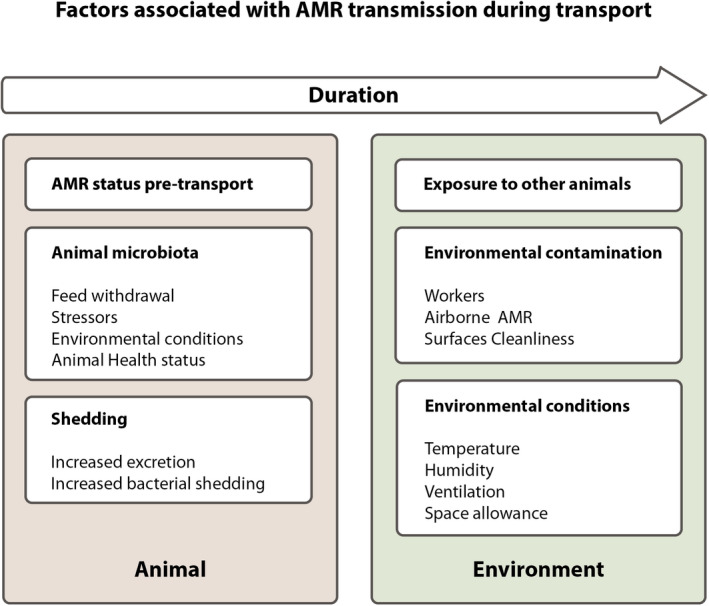
Risk factors for transmission of antimicrobial resistance during animal transport. The AMR status before transport will have a substantial effect on the likelihood of transmission of ARB/ARGs during transport. During transport, health status and environmental conditions are factors affecting the microbiota of animals and the likelihood of ARB/ARGs transmission. Feed withdrawal and stressors were also identified, although the direction of influence on ARB/ARGs transmission (decrease or increase) is unclear. Exposure to ARB/ARG in other animals (either shed or present on their skin or mucosal surfaces) is a major source of contamination for other animals during transport. Environmental exposure to ABR/ARGs is related to the cleanliness of loading and unloading areas and of vehicles, workers related to transport and their transmission through air. The environmental conditions of the transport (e.g. temperature, humidity) are also important for the survival/growth and dispersion of bacteria outside the animal. Transmission is influenced by duration since the exposure to risk factors is prolonged, although there is no evidence to estimate differences between journeys shorter or longer than 8 h
Most of the risk factors that affect ARB/ARGs transmission during transport are applicable to assembly centres, control posts and lairage as well. Some issues are specific for lairage and/or assembly centres and control posts. Risk factors specific for these locations are therefore assessed in a separate section. The duration of transport and/or lairage is most likely affecting most of the risk factors mentioned above. Duration is therefore assessed separately, and also in relation to its potential interaction with the other risk factors within this Scientific Opinion.
3.2.2.1. Antimicrobial resistance status of the microbiota of the animals before transport
The AMR status (e.g. resistome, mobilome, bacterial fitness traits) of the microbiota of the animals before transport will have a major effect on the likelihood of transmission of ARB/ARGs during transport. If ARB/ARGs are not present amongst animals, transmission and contamination of transport and lairage surfaces and equipment will not occur. The resistance status prior to transport depends on all of the factors that influence the presence of ARB/ARGs in animals on livestock farms (EMA and EFSA, 2017). Although these factors will not be discussed in this Scientific Opinion, they need to be considered if alleviation of the resistance situation is chosen as the approach to minimise potential transmission of ARGs and resistant organisms during transport.
There is ample evidence that the level and variety of ARB/ARGs differs between different categories of farm animals, among farms and between countries. Even within the different animal categories variability is highly dependent on numerous factors (Munk et al., 2018). As an example, prevalence of Campylobacter spp. in chickens, commonly resistant to antimicrobials, is prone to seasonal effects (EFSA, 2020, 2022; Lynch et al., 2022). Moreover, some animal categories such as fattening pigs, broilers, veal calves and fattening turkeys are considered more at risk for harbouring ARB/ARGs than others, e.g. sheep, dairy cows and beef cattle (EMA and EFSA, 2017; Hitch et al., 2018; Haley et al., 2020; Tello et al., 2020; AbuOun et al., 2021; Buta‐Hubeny et al., 2022; Yang et al., 2022; Zhou et al., 2022).
The prevalence of ARB/ARGs may depend on the age of the animals. In calves, there is a gradual decline in shedding of antimicrobial‐resistant and/or ESBL‐producing E. coli from birth to weaning (Hoyle et al., 2004; Bastard et al., 2021; Massé et al., 2021). This age effect was confirmed by the analysis of ARGs: a longitudinal study described a more than 6‐fold decline in the abundance of ARGs in the gut of calves during nursing. The authors associated this reduction with the shift of the microbiota composition associated with the change of diet, since the abundance of facultative anaerobes, which harbour most ARGs, declined after weaning (Liu et al., 2019). A decrease in the prevalence of ESBL‐producing E. coli has been observed in pigs during ageing (Dohmen et al., 2017a). In broilers, data about the effect of age on the prevalence of ESBL‐producing E. coli are still controversial: Dierikx et al. (2013) and Laube et al. (2013) described a rapid increase in the prevalence of ESBL‐producing E. coli in the first week of life, which then remained high until slaughter, while other authors observed a declining rate of antimicrobial‐resistant E. coli after the first week of life (Roth et al., 2017; Lee et al., 2021). In pigs, weaners are more at risk of harbouring MRSA, based on a study in Australia, probably as a consequence of the overall stress associated with weaning and the high antimicrobial consumption at this age (Sahibzada et al., 2020). High levels of MRSA as compared to sows have been observed in the environment of weaner pigs in Germany (BVL, 2015) and in milk fed dairy calves as compared to older animals (Schnitt and Tenhagen, 2020). Again, the prevalence of Clostridioides difficile, a bacterial species characterised by high levels of AMR, declines with age in piglets and calves, caused by age‐related changes in the intestinal microbiota (Zidaric et al., 2012; Proctor et al., 2021; Redding et al., 2021). In summary, young age is generally a risk factor for harbouring ARB/ARGs even though this may not be valid for all species, management conditions, bacteria and AMR determinants. Conventional farms are at greater risk for housing ARB than organic or antimicrobial‐free farms (Tenhagen et al., 2018; Pesciaroli et al., 2020; Innes et al., 2021; Mencía‐Ares et al., 2021; Weinroth et al., 2022). In all species, antimicrobial consumption is a major driver for an increase of ARB/ARGs.
If transportation is not associated with mixing of animals from different sources, the effect of these different levels of ARB/ARGs is probably less substantial although the intensity of exchange of bacteria may be similar. Animals of a similar age from the same epidemiological groups within herds or flocks are likely to have similar resistance profiles (in terms of AMR determinants and their abundance), on account of the constant exchange of bacteria within these populations. Therefore, the exchange of ARB has no major effect. If animals of the same category from different origins are mixed, it may be expected that the initial differences between the mixed animals will be reduced through exchange of bacteria. The same can apply if transport vehicles and loading or unloading ramps are used for animals of different origins or different animal categories without effective cleaning and disinfection. If, for example, transport crates that are used for transporting laying hens to the slaughterhouse are used for transporting breeding birds to the slaughterhouse, or pullets to laying farms, transfer of organisms carrying AMR, such as Salmonella spp., is possible (e.g. Slader et al., 2002).
3.2.2.2. Transport‐related factors affecting the microbiota in animals
Several factors may lead to changes in growth conditions for microorganisms and therefore affect the composition of the microbiota. This may render the microbiota more susceptible to colonisation by ARB. Intrinsic changes of AMR in the individual animal may be induced by selection pressure through use of antimicrobials prior to or during transport. This is a cause/effect relationship similar to the effect of antimicrobial treatment or exposure to certain biocidal products or heavy metals without transport and therefore will not be addressed in detail in this report. This is not the only factor potentially leading to changes in the microbiota. Other factors influencing the microbiota in animals during transport include:
Feed withdrawal.
Stressors (such as animal handling, thermal stress, hunger, loading/unloading).
Environmental conditions.
General health status of the animal.
2a. Role of feed withdrawal
Withdrawal of feed and water prior to or during transport may change environmental conditions in the animals' digestive tract as substances are digested, absorbed, etc., leading to variations in pH, release of stress hormones and other factors that prompt changes in the microbiota composition. These changes in microbial abundance may have an impact on the AMR profile (either in positive or negative direction). The effect of stress on the microbial population may include increased exchange of mobile genetic elements within or between bacterial species. This could alter the AMR profile or may increase AMR abundance in the microbiota. There is a lack of specific data to support this hypothesis. Moreover, animals may turn to eating bedding, which might lead to the uptake of resistant microorganisms from the environment.
Feed withdrawal can either occur prior to transport or/and during transport. Depending on the animal category involved, it will have an impact on the intestinal microbiota, e.g. increased caecal prevalence and shedding of specific bacteria, and on the energy balance of the animals. For some animal species, feed withdrawal is implemented prior to transport to slaughter to reduce motion stress effects and consequent vomiting (for pigs) during transport. Such measures are intended to reduce the content of the intestines and thereby simplify evisceration of the slaughtered animals by minimising intestinal rupture (including the crop in poultry) during the evisceration process.
Experimental studies have shown that restriction of feed for chickens being reared for breeding, table egg production or meat production can substantially increase the caecal prevalence and shedding of both Salmonella spp. (Joat et al., 2021) and Campylobacter spp. This holds true, even when there are no other stressors associated with catching and transporting birds (Ramirez et al., 1997; Shane, 2000; Wilson et al., 2018; Gast and Porter, 2020). Similar findings regarding the occurrence of Salmonella spp. in the crop have been reported after feed withdrawal for laying hens and broiler breeders. Feed withdrawal results in a tendency of birds to ingest contaminated bedding because they are hungry, as well as increasing levels of corticosterone and interference with immune responses which can accelerate the multiplication of certain groups of enteric microorganisms (Mahmood et al., 2007; Andreatti Filho et al., 2019; de Jong et al., 2002). Feed withdrawal has been shown to increase the prevalence of Salmonella spp. and Campylobacter spp. in the crop of market age broilers (Byrd et al., 1998; Corrier et al., 1999). As crop rupture is more frequent during slaughter than intestinal rupture, this may be associated with an increased risk of carcass contamination (Hargis et al., 1995). Stress associated with feed withdrawal can result in changes to the intestinal epithelium that can lead to greater attachment and colonisation by Salmonella spp. (Soleimani et al., 2012a).
In countries with high‐temperature conditions, feed restriction has been reported to be associated with a reduction of the impact of heat stress in broilers (Abu‐Dieyeh, 2006) and in Salmonella spp. proliferation in chicks that are subject to heat stress (Soleimani et al., 2012b) or certain intestinal infections (Tsiouris et al., 2014).
In pigs, feed withdrawal is a common preslaughter and transportation practice. There are benefits such as reduced vomiting and improved intestinal handling in the evisceration process, which reduces carcass contamination via spillage of intestinal contents. There may be potential negative aspects related to welfare, resulting in prolonged hunger (Eicher et al., 2017) or disturbance of the microbiome composition (Massacci et al., 2020), favouring increased shedding of bacterial pathogens such as Salmonella (Verbrugghe et al., 2011) and the potential transmission or exchange of mobile genetic elements such as plasmids (Guiney et al., 1995). These potential negative effects seem to be associated with the duration of feed withdrawal (Martín‐Peláez et al., 2009).
In calves, feed restriction has been associated with increased gut permeability (Pisoni et al., 2022). Increased gut permeability in turn has been associated with contracting diarrhoea (Araujo et al., 2015), i.e. increased shedding of liquid faeces, which may lead to a higher level of bacterial cross‐contamination between animals and the environment. Feed withdrawal associated with long transportation (1,200 km) has been demonstrated to result in multiple metabolic effects in steer calves, underlining the complexity of the sequelae. Some of the changes needed 7 days after arrival at the destination before they were normalised (Takemoto et al., 2017).
In cattle sent for slaughter, the duration of feed withdrawal is associated with an increase in rumen pH that may facilitate growth of Salmonella spp. and E. coli, while low pH and high volatile fatty acids in well fed animals tend to inhibit growth of Salmonella spp. and E. coli (Mattila et al., 1988; Cray et al., 1998). In line with that, starving sheep were more prone to infection with Salmonella spp. than well fed ones. Feeding starved sheep only once after inoculation, increased the multiplication of Salmonella spp., while resumption of continuous feeding inhibited the growth (Grau et al., 1969). Similar studies have not been undertaken in cows, but a similar situation is considered likely. In addition, transfer of resistance genes between E. coli and Salmonella spp. was observed in starved sheep and not in well fed sheep (Smith, 1977).
In experimental studies, fasting had no effect on shedding of E. coli O157 in weaned calves, but it did increase susceptibility to colonisation with these bacteria (Cray et al., 1998; Harmon et al., 1999). Withholding feed increased the number of E. coli O157 culture‐positive animals in an experimental study in heifers, steers and sheep. Cattle with slower rates of intestinal cell proliferation in the cecum and the distal colon were culture positive significantly longer than cattle with faster cell proliferation rates. The authors suggest that fasting is associated with a slower cell proliferation rate (Magnuson et al., 2000). These findings indicate that fasting does affect growth conditions of specific bacteria which may include ARB.
In summary, feed withdrawal can influence the microbiota and increase the shedding of certain bacteria (e.g. Salmonella spp., Campylobacter spp.) which are often resistant to antimicrobials and therefore contribute to the risk of transmission of ARB/ARGs during transport. On the contrary, the associated decreased faecal contents in the intestines may reduce the shedding of ARB/ARGs and therefore lower the risk of transmission of ARB/ARGs.
2b. Stressors
Stressors (such as animal handling, thermal stress, hunger, loading/unloading) activate the animals' hypothalamic–pituitary–adrenal activity, thereby triggering release of various stress hormones such as catecholamines and cortisol (Van Engen and Coetzee, 2018). The liberation of those hormones has been shown to have effects on the gut/blood barrier and growth of certain intestinal pathogens. In pigs starved for 24 h, cortisol levels and the salmonella concentration in the intestines increased (Verbrugghe et al., 2011). In contrast, in the same study, social stress had no effect on Salmonella spp. concentration in the gut (Verbrugghe et al., 2011).
2c. Environmental conditions
Environmental conditions such as changes in temperature and/or humidity are likely to impact the respiratory microbiota as well as the intestinal microbiota (Woldehiwet et al., 1990; Sun et al., 2020; Huus and Ley, 2021; Sepulveda and Moeller, 2020). This impact may result from direct exposure of the bacteria to temperature/humidity of the transport environment (e.g. upper respiratory mucosa) and/or be mediated through changes in body temperature of the animal.
In general, the higher the temperature and duration of the exposure to temperature the more readily can microorganisms can multiply within or on the surface of animals. Common drug‐resistant pathogens which can infect humans (e.g. Salmonella enterica) and also non‐pathogenic commensals (e.g. some types of E. coli) demonstrate an increased cell growth rate in response to increasing temperature in a central normal range of its growth temperatures (20–37°C). Such changes may favour the expansion of certain ARB and shedding of bacteria.
2d. Health status of the animal prior to transport
Animals suffering bacterial infections, i.e. enteric, respiratory or skin infections are a source of potential ARB/ARGs transmission. Transportation of these animals is discouraged from an animal health and welfare perspective, hence specific studies on the transport of such animals are lacking. Infections acquired or re‐activated during transport may cause inflammation in the gut and change the composition of the intestinal microbiota. Such changes may reduce the resistance to colonisation provided by the gut microbiota. In consequence, ingested ARB may be more likely to successfully colonise the intestines. Similar changes may also affect the respiratory system, but the organisms involved are less relevant to public health.
A specific effect on AMR selection can be expected if such animals need treatment before, during or after transport. Another effect of their transport is the potential transmission of the infection to other animals which may require antimicrobial treatment after transport. The latter is out with the scope of this Scientific Opinion.
3.2.2.3. Factors affecting shedding of bacteria
Faecal‐oral transmission during transport is well documented for food‐borne bacteria, such as Salmonella and Campylobacter in pigs and broilers (Beloeil et al., 2004; Gebreyes et al., 2004; Marin and Lainez, 2009; Quintana‐Hayashi and Thakur, 2012; Greening et al., 2021). In faecal‐oral transmission, animals shed ARB/ARGs with their faeces, which can then be ingested by other animals concurrently on the same transporter or can lead to contamination of the vehicle. If vehicles are not thoroughly cleaned after transport, there is therefore also the risk of contamination of animal groups that may subsequently be transported on the same vehicle. Other excretions, including milk, uterine discharges, urine, saliva and vomit can be contaminated by ARB and subsequently expose other animals to these bacteria.
Factors leading to an increase in the frequency and volume of defaecation and urination may increase the spread of bacteria and the cross‐contamination of other animals. Likewise, shedding of bacteria present in the respiratory system through, e.g. increased respiration rates can support exchange of respiratory pathogens potentially harbouring ARGs.
Faecal shedding of bacteria by animals per se has two components, the amount of faeces shed and the bacterial load in the faeces. The amount of faeces shed will depend on the amount of feed ingested and on an overstimulated intestinal motility in agitated or diarrhoeic animals. The bacterial load in the faeces on the other hand is determined by the carrier status of the animals (e.g. Salmonella spp. or a specific ARB present or not present) and on microbiota compositional changes under transport conditions.
The role of stress responses in pathogen shedding using Salmonella spp. as a model is described in different studies. For instance, modelling studies (Simons et al., 2016) highlight stress factors as the most important drivers in Salmonella spp. shedding during transport. The level of cortisol in saliva of pigs was associated with the shedding of Salmonella spp. after transport to slaughter (Artuso‐Ponte et al., 2015). In addition to other stressors, the social stress of mixing animals (weaned pigs) increases the shedding of Salmonella spp. as well as the shedding of coliforms (Callaway et al., 2006). Stress levels, for instance measured by cortisol concentrations, are linked to monocyte proliferation and increase of Salmonella spp. shedding (Verbrugghe et al., 2011). The observed effect can occur with other enteric or non‐enteric pathogens. Stress and anxiety have been associated with changes in the microbiota of the gut and ultimately diminished host resistance to pathogens (Petrosus et al., 2018).
Transportation has been shown to provoke shedding of Salmonella serovar Dublin in latently infected calves (Grønstøl et al., 1974). Transportation increased urination, defaecation and salivation in 6‐month‐old calves (Kent and Ewbank, 1986) and urination in 15‐month‐old bulls (Kenny and Tarrant, 1987). In a study of health outcomes in veal calves after transport, 14% suffered from diarrhoea. The prevalence of diarrhoea prior to transportation was not assessed (Pempek et al., 2017).
High arousal of animals during loading was associated with an increased risk of cattle hides being contaminated by Salmonella spp. at slaughter (Dewell et al., 2008a). Whether that is due to an increased frequency of defaecation or a specific increase in shedding of Salmonella spp. was not investigated.
One study highlighted the role of ‘super‐shedders’ of E. coli O157 for the contamination of hides (Arthur et al., 2010). Other studies have suggested that super shedding was probably a temporary phenomenon that would have a minor effect on the overall shedding (Munns et al., 2014). Moreover, shedding of E. coli O157, and E. coli per se in cattle, is highly variable between animals (Davidson and Taylor, 1978; Robinson et al., 2009).
3.2.2.4. Environmental exposure to ARB/ARGs
Exposure to ARB/ARGs in the environment may occur at all the different stages of transportation, e.g. in loading areas, on vehicle/transport crate surfaces, on surfaces at exchange points such as assembly centres, where animals are unloaded or in the receiving lairage area, as well as in contaminated drinking water in troughs or contaminated bedding. Low standards of environmental hygiene, such as suboptimal cleanliness of loading and unloading areas and interiors of the vehicles (and crates) prior to loading increase the probability of contamination of animals. Residual environmental contamination may cause indirect transmission of ABR/ARGs to other groups of animals transported by the same vehicle or coming into contact with the same premises (Quintana‐Hayashi and Thakur, 2012; Abdalla et al., 2021; Ingham et al., 2021). Environmental conditions, such as humidity and temperature, may influence the survival of ARB in the environment (Davies and Wales, 2019). Residues of organic material, including airborne and settled dust in premises, vehicles, lairage, or at markets or collection centres may provide a suitable environment for the survival and growth of ARB (Davies and Wales, 2019; Bai et al., 2022; Luiken et al., 2022). Moreover, ARB might be transmitted through air, e.g. attached to dust particles that originate from dried faeces, skin particles and feathers.
4a Cleanliness of loading and unloading areas and of vehicles
Cleaning and disinfection of trucks and control posts is compulsory in the EU after each use (EC 1/2005). Decontamination of vehicles may be impaired by the presence of rough surfaces and hidden areas where organic material can build up (Baker et al., 2017). When vehicles are not properly cleaned and disinfected prior to loading of animals (Arthur et al., 2008; Weber and Meemken, 2018; Huneau‐Salaün et al., 2020, 2022), exposure to ARB/ARGs in the following transport can occur. Within 2 h after experimental exposure to contaminated areas, Salmonella can be found in the caecal content of broilers or pigs (Rigby and Pettit, 1980; Boughton et al., 2007a). Even if trucks are visually clean after cold‐water power hosing, such washing may not be effective in removing Salmonella and other Enterobacteriaceae (Mannion et al., 2008). In this study, transport of pigs from highly Salmonella‐infected farms was associated with 6% of trucks positive for Salmonella spp. preload, 17% post‐load and 18% after washing. This indicates that contamination of trucks during transport may lead to carry over to the next batch of animals transported on the same truck. On the other hand, the figures for trucks carrying pigs from Salmonella‐free or low Salmonella prevalence origins were 11% preload, 11% post‐load and 6% after washing. This underlines that the degree of contamination after washing likely is guided by the degree of contamination during transport.
Pulsed‐field gel electrophoresis (PFGE) patterns of Salmonella spp. isolates from trucks post‐load and after washing were indistinguishable, from Salmonella spp. isolated on farm, indicating shedding by carrier pigs during transport (Mannion et al., 2008). In addition to the proven contamination of trucks by shedding animals, there is evidence that contaminated trucks and lairage pose a risk for pigs and cattle in terms of Salmonella and STEC infection (Hurd et al., 2002; Boughton et al., 2007a; Arthur et al., 2008; Dewell et al., 2008a). In a longitudinal study, a group of fattening pigs was followed along the production line until slaughter. Some Salmonella spp. isolates from slaughtered pigs belonged to serovars never detected on the farm but had been recovered from trucks preload. The preloaded and the pig‐derived isolates were indistinguishable, based upon the PFGE profiles (Magistrali et al., 2008). For MRSA it has been shown that pigs that were negative at loading were positive at unloading, making the lorry a likely source of contamination or colonisation of the pigs (Broens et al., 2011). In this study, four batches of around 30 pigs tested negative for MRSA before transportation – on arrival at the lairage, two batches contained MRSA‐positive pigs (7/27 and 5/30). In one of these batches, no pigs of other batches were transported in the same lorry, suggesting the environment as the source of MRSA. The status of the lorry prior to loading was not investigated. A further increase was observed during lairage, which could be explained both by environmental contamination and direct contact between animals.
For poultry, cleanliness of crates has been shown to play a major role in the distribution of bacteria. Contamination of washed transport crates used for taking broilers to slaughterhouses with relevant (zoonotic) pathogens and other ARB has repeatedly been shown in many studies (Rigby et al., 1982; Corry et al., 2002; Slader et al., 2002; Rasschaert et al., 2007; Peyrat et al., 2008; Zeng et al., 2021). Several studies have identified ineffective removal of Salmonella spp. (including serovars that are typically multidrug resistant) during washing and disinfection of crates, sometimes with higher levels of contamination being found after washing than before (Rigby et al., 1982; Corry et al., 2002; Slader et al., 2002; Rasschaert et al., 2007; Zeng et al., 2021).
High total microbial counts remain after standard washing procedures and ineffectively cleaned crates and modules have been shown to be a source of Campylobacter spp., including resistant strains (Natsos et al., 2021), for birds to be slaughtered (Herman et al., 2003; Sibanda et al., 2018; Rasschaert et al., 2020; Hertogs et al., 2021; Moazzami et al., 2021). Campylobacter genotyping supports contaminated crates as being a source of flock and carcass contamination (Hastings et al., 2011).
Contamination included specific ARB such as MDR isolates of bacteria such as A. baumannii, K. pneumoniae, ESBL‐producing E. coli, colistin‐resistant E. coli and vancomycin‐resistant E. faecium (ESKAPE) (Hayes et al., 2004; Von Tippelskirch et al., 2018; Molechan et al., 2019; McIver et al., 2020; Savin et al., 2020a) and it has been postulated that basically every antimicrobial‐resistant bacterial type that can be found in the animals can be found in the contaminated crates (Savin et al., 2020b), which similarly applies to bacteria such as livestock‐associated MRSA (Mulders et al., 2010; Amoako et al., 2020).
The presence of ARB in the environment also depends on the ability of the ARB to persist, e.g. by the ability to produce biofilm or resist desiccation or competition from environmental microorganisms or by other mechanisms. There are some examples of ARB that are less susceptible to disinfectants and/or may acquire antimicrobial and biocide resistance in a combined manner. Thus sanitisers may exert a selection pressure favouring the persistence of these ARB on surfaces (Davies and Wales, 2019; Han et al., 2019)
Contamination of the environment might be more relevant when animals of different origins are transported within the same vehicle (either parallel or in series), because of the expected higher variability of the microbiota of animals between different species and populations (see Sections 3.2.2.1 and 3.2.2.3). Hence, usage of equipment and vehicles for unrelated populations (e.g. laying hens and broilers) is likely to have a more substantial effect.
Bedding is currently compulsory during journeys longer than 8 h (EC 1/2005). Bedding such as straw can act as a source of microorganisms and is liable to be consumed by animals that are hungry after feed withdrawal (Ray et al., 2022). While beneficial with respect to animal welfare, organic bedding such as straw and wood shavings can support growth of potentially pathogenic bacteria making the management of bedding a complex task (Godden et al., 2008). High densities of animals on vehicles are likely to lead to massive contamination of bedding during transport. On the other hand, bedding may absorb some of the liquid during transport and thus reduce the spread of excretions on surfaces. Overall, the hygienic particularities associated with bedding during transport need to be further investigated. Most studies on bedding have been done in confined housing systems.
4b Airborne transmission of bacteria between animals
Aerosol particles carrying ARB may be inhaled by a susceptible host or deposited onto mucous membranes or environmental surfaces. During transport, very small particles may remain suspended in the air for extended periods and be disseminated by air currents through a transport vehicle (Schmithausen et al., 2018). The close proximity or contact, usually occurring during transport, as well as the survival of the ARB in the environment for extended periods, are important aspects in airborne transmission.
Antimicrobial‐resistant enterococci were recovered from air and surface samples collected by motor vehicles driving behind poultry transport vehicles. Of the 24 resistant isolates, 62.5% were resistant to tetracycline, 41.7% to erythromycin, one to quinupristin/dalfopristin and one to streptomycin. Three of the 24 isolates were resistant to more than one antimicrobial (Rule et al., 2008). While the environmental issue with this finding is outside the scope of this Scientific Opinion, it clearly indicates that airborne transmission of enteric bacteria is possible. Transmission on the vehicle may therefore occur without direct contact between animals or indirect contact via contaminated surfaces. Another study highlighted that the position of the animal in the transport vehicle would have an effect with animals in the nose compartments being less contaminated than those in the deck or belly compartments (Stanford et al., 2011). Survival of artificially aerosolised E. coli is limited with a half‐life of 5.7 min; attachment to dust particles will prolong this and may enable transmission of E. coli across compartments within transport vehicles (Nguyen et al., 2022).
ARB can be carried by dust particles and transmitted via the airborne route, as shown for MRSA and ESBL‐producing Enterobacteriaceae after the faeces have dried (Schmithausen et al., 2018; Davies and Wales, 2019). Longitudinal studies on pig farms in the Netherlands and Germany showed that both ESBL‐producing E. coli and MRSA can be found in collected dust on the farm. Moreover, carriage of MRSA and ESBL by farmers was associated with the presence of MRSA and ESBL in dust on their pig farm (Bos et al., 2016; Dohmen et al., 2017b). MRSA was detected in indoor air and in lower concentrations in exhaust air from barns housing pigs and poultry (Schulz et al., 2012; Friese et al., 2013). In addition, bioaerosols have been shown to be involved in transmission of ARGs at farm level (Song et al., 2021). Dispersion of MDR E. faecalis and E. faecium strains, including high‐risk clonal complexes, through dust was reported in piggeries in Portugal (Novais et al., 2013). Although these studies are conducted on farms and not related to transport, this shows that transmission of ARB/ARGs by dust (as a proxy for airborne transmission) can occur and is likely to occur during transport.
4c Workers in the transport environment
Several studies have analysed the role of different reservoirs in the transmission of ARB/ARGs (e.g. Dorado‐García et al., 2018; Mughini‐Gras et al., 2019). From a theoretical perspective, the direction of transmission is not clear when similar isolates are found in both animals and humans within an epidemiologically‐linked cluster (e.g. farmers and livestock on the same farm). Certain genes or gene types appear to be much more commonin animals than in humans, such as ESBL genotype CTX‐M‐1 and MRSA ST‐398 (Aires‐de‐Sousa, 2017; Ewers et al., 2021). Transport personnel entering the farm were a risk factor for cephalosporin‐resistant E. coli in Norwegian broiler flocks (Mo et al., 2016). Human visitors were considered as a likely source of introduction of LA‐MRSA on Norwegian farms (Grøntvedt et al., 2016). Carriage of LA‐MRSA in humans can last for up to 2 days after visiting a contaminated herd (Schulz et al., 2019). Recently, drivers of lorries transporting live pigs were shown to be carriers of LA‐MRSA (Gómez et al., 2020; Ingham et al., 2021).
Moreover, the numbers and variety of ARB/ARGs present in a group of animals is assumed to be much higher than in the small number of workers expected to be occupied in the transportation in this group of animals. Transmission of ARB/ARGs from workers to animals during loading, unloading, checks etc. cannot be ruled out. Some findings hint that the transmission from humans to animals can occur (Dohmen et al., 2017a), but it is hypothesised that this potential transmission does not have a significant impact compared to the exposure to the truck environment and other animals carrying ARB/ARGs. Finally, persons handling animals and their protective clothing can serve as vectors for ARB/ARGs between concurrent or subsequent groups of animals.
3.2.2.5. Exposure to other animals carrying and/or shedding ARB/ARGs
Animals may be exposed to ARB/ARGs carried by other animals, either shed or present on their skin or mucosal surfaces. Shedding of materials harbouring bacteria such as faeces, exhaust air, saliva, urine and milk is probably a major source of contamination for other animals during transport. Because of the expected higher variability of the microbiota of these animals, transmission of ARB/ARGs between animals from different origins might be more relevant. The effect is primarily determined by the difference in antimicrobial resistance between the animals and by the amount of shedding (see Subsections 3.2.2.1 and 3.2.2.3). The stocking density and division in groups using compartments may influence the probability of transmission of ARB/ARGs between animals.
Common haulage or visiting different farms with the same transport is believed to increase the chance of transmission of bacteria, either commensals or pathogens, which may carry ARGs. For instance, a study evaluating the presence of B. hyodysenteriae in transport trucks reported that the number of farms visited in the same journey could be a risk factor increasing the chance of introduction of this pathogen and the spread of both during the transport of animals (Giacomini et al., 2018).
The importance of exposure to other animals for the transmission of ARB is supported by on‐farm epidemiological studies. Both high animal density and herd size probably facilitate the on‐farm transmission of ARB, since many studies confirmed stocking density as a risk factor for ARB in primary production. As an example, the risk of contamination by ESBL‐producing E. coli increases with animal density in broilers, dairy, and beef farms (Hille et al., 2018; Becker et al., 2022; Ferroni et al., 2022). The same effect of animal density was shown for MRSA in pigs and dairy cattle (EFSA, 2010; Schnitt and Tenhagen, 2020). Jones et al. (2013) reported an effect of farm size on AMR: turkeys from larger farms showed a higher risk of carrying fluoroquinolone‐resistant E. coli than turkeys from small farms. Along with farm size, the number of animals hosted in the same group is a risk factor for the detection of ARB.
In a study carried out on cattle farms, Hille et al. (2018) showed that the size of the sampled group was associated with being positive for ESBL‐producing and antimicrobial‐resistant E. coli. The contact between animals of different species may increase the risk for ARB, as shown for the contamination by MRSA in dairy cattle, which was positively associated with the presence of pigs on the same farm (Schnitt and Tenhagen, 2020). Even proximity to other farms was identified as a risk factor for the presence of ARB/ARGs (Hille et al., 2018; Luiken et al., 2022).
As the amount and intensity of contacts increase, total exposure to ARB/ARGs from other animals increases, thereby enhancing the risk of transmission. This factor is closely linked to other factors affecting transmission of ARB/ARGs during transport.
3.2.2.6. Environmental and transport conditions
In addition to the effect of environmental conditions on the microbiota (as described in Section 3.2.2.2 2c), an effect is expected to the presence of ARB/ARGs in the environment (e.g. surface of the truck). The survival conditions/growth conditions for bacteria outside the animal (e.g. temperature, humidity, exposure to light) are of importance. Humidity and warm temperatures can increase the survival rate and multiplication of bacteria in the environment. Poor ventilation increases the concentration of bacteria in the air. Dust may be formed and subsequently can be aerosolised and further spread due to transport vibrations. Solar radiation may, on the other hand, reduce the viability of bacterial cells in the environment, although during transport, solar radiation is considered minimal within vehicles.
For animals, depending on the intensity and duration, heat and low temperatures may negatively affect animal health, e.g. immune suppression and bacterial shedding. This effect is influenced by the resilience of the animal (species). Increased temperatures and temperature/humidity indices may increase respiration rates favouring shedding of respiratory pathogens. On the other hand, low temperatures and ‘wind chill’ may lead to cold stress in young animals (e.g. calves and piglets) and poultry.
In a recent prospective study (Padalino et al., 2021b) environmental parameters, such as temperature variance between departure and arrival, was identified as a risk factor for respiratory pathogens in beef steers transported from France to the south of Italy (29 h journey).
3.2.2.7. Duration of transport
During longer transportation the likelihood and extent of exchange of bacteria between animals is likely to increase. Such an increase may be due to prolonged contact with other animals or to the negative effects of longer transportation on clinical parameters of animals that may be associated with an increased susceptibility to colonisation or infection. Moreover, longer transportation frequently means longer periods of feed withdrawal that may cause hunger‐related stress responses with potential effects on the composition of the microbiota, which may be associated with changes in the ingesta (Mattila et al., 1988) (see chapter 2a). Long‐distance animal transport is an animal health and welfare issue because it is particularly stressful in impairing the immune system, and often triggers the onset of health problems leading mainly to infectious diseases (e.g. respiratory and gastrointestinal problems) (Schwartzkopf‐Genswein et al., 2012; Padalino et al., 2021b). On the other hand, journeys under 65 km have been demonstrated to be already very stressful for animals (Tateo et al., 2012). The loading and the first hour of journey (about 65 km in a typical livestock truck) are identified as the most stressful phases of a journey for sheep, cattle and pigs (Grandin, 2019).
The length of the journey (> 160.9 km) was associated with a higher risk of contaminated hides with Salmonella spp. (Relative risk [RR] of 2.28, 95%, CI 1.4–3.7) and E. coli O157 in beef cattle at slaughter (Dewell et al., 2008a,b). Grau et al. (1968) found an increasing percentage of cattle with Salmonella spp. in the rumen with increasing duration of time between leaving the farm and slaughter. It is reasonable to assume that the same applies for other bacteria harboured by the animals, including drug‐resistant bacteria. From a theoretical perspective, all animals are likely to carry a similar set of bacteria over time. The shape of the contamination curve over time has not explicitly been studied. Contamination of the environment accumulates over time, which may enhance transmission to the next batch of animals.
In calves, duration of transport was significantly associated with overall morbidity and mortality (Cernicchiaro et al., 2012) which may, among other aspects, be indicative of a negative effect on resilience. Long transport had a significant effect on clinical parameters in calves indicating, e.g. dehydration (Bernardini et al., 2012), although calves were generally capable of maintaining values within the physiological ranges. A preventative effect of pre‐transport diet was observed for short transports (6 h) but not for long transports (18 h) (Marcato et al., 2020).
The duration of transport has been suggested as a risk factor for Salmonella spp. infection of pigs at slaughter (Hurd et al., 2002). Field studies show that even a short transport and a limited lairage time (< 2 h) is associated with an increase in the prevalence of Salmonella spp. in batches of pigs (Hurd et al., 2002; Larsen et al., 2004; Magistrali et al., 2008). A challenge study showed that Salmonella spp. is able to infect the mesenteric lymph nodes of pigs in less than 2 h, after exposure to a contaminated environment, with an infectious dose of 1,000 cfu (Hurd et al., 2001).
The effect of rest periods, including unloading and reloading or change of the vehicle, of cattle on infection or colonisation status has not been investigated. While resting at a control post may alleviate the effects of feed and water withdrawal during transport and thus have a positive effect on welfare, it is potentially associated with exposure to a new, possibly contaminated environment and with additional commingling with animals from other sources (see section below). Moreover, unloading and loading may be considered stressful, with potential effects on the resilience of the animals. In line with this, additional stops were associated with an increased risk of carriage of most bacterial and viral respiratory pathogens (Padalino et al., 2021b).
An additional stop of 8 h with feeding and access to water increased the diversity index in the intestinal microbiota of 18 kg pigs. It is not clear what effect that would have on AMR or on AMR transmission (Williams et al., 2008).
3.2.3. Lairage, livestock assembly centres and control posts
Most of the risk factors explained in Subsections 1–7 of this section are affecting both ARB/ARGs transmission during transport as well as during the stay of animals in assembly centres, control posts and/or lairage. It can be concluded that similar risk as those exposed above can be attributed to these facilities. Specific studies describing the effect of lairage and/or assembly centres, both in pigs and cattle, have proven the link between carcass contamination and lairage. For instance, a study demonstrated that contaminated lairage pens were associated with an increased risk of contaminated hides at slaughter (RR of 1.8 for Salmonella spp. and 3.1 for E. coli O157, Dewell et al., 2008a,b). This risk can be hypothesised as well for ARB/ARGs, since these bacteria can carry ARGs frequently. Studies on the prevalence of contaminated lairages in the USA found 60% contaminated with E. coli O157 and 70% contaminated with non‐typhoidal Salmonella spp. (Arthur et al., 2008), results by which authors state that the lairage effect on hide contamination is more important than contamination acquired on farm. In a study on free‐range chickens, an increase of Campylobacter spp. after transport and especially after the holding period pre‐slaughter was reported (McCrea et al., 2006). Another study of sheep lairage showed that both Salmonella spp. and ESBL‐producing E coli were more commonly isolated from the lairage environment than from the animals themselves, demonstrating the potential importance of reservoir status of the lairage (Atlaw et al., 2022).
Several factors make lairage one of the most‐important points for contamination through the soiling of skin or gastrointestinal infection by the oral route. These factors can be grouped into two categories: cross‐contamination/cross‐infection of animals at lairage at the same time and carry over of bacteria from previous occupants of the lairage area due to the failure of lairage cleaning protocols. Depending on the duration of stay in the lairage, newly acquired infections or the recrudescence of those already present in carriers during transport can result in the shedding of AMR pathogens such as Salmonella spp. or ARB during the lairage period.
Most of the stress‐inducing factors described in animal transport, such as variations in temperature, high stocking densities, unexpected movements of animals, people and vehicles and noise, occur also during lairage (Morgan et al., 1987). A systematic review with respect to interventions to control Salmonella spp. found that abolishing lairage was the only effective measure (Wilhelm et al., 2017).
Lairage contamination can fluctuate during the working week. An Irish study underlined the contamination of lairage facilities and its evolution over the working week. While on Monday mornings 6% of samples from the lairage were positive for Salmonella spp. and the load was limited (2 cfu/100 cm2), 44% were positive on Thursday afternoons with a load of 8 cfu/100 cm2 (Boughton et al., 2007b). In pathogens and commensals, the bacterial load in the lairage environment plays a pivotal role in the likelihood of infection or establishment as commensal respectively. In pathogens with short incubation periods, such as Salmonella spp., exposed animals like pigs would be positive, infected in lymph nodes and shedding the pathogen in faeces after only 2 h of exposure to relative low concentrations (103 cfu/g of faeces) (Hurd et al., 2001; Boughton et al., 2007a), a timespan and concentration that is easily reached if transport and lairage are considered together. At lairage, bacteria that have been obtained during transport may therefore be shed. If lairage alone extends to more than 2 h, even infections with ARB obtained during lairage may lead to shedding of the ARB bacteria at the end of lairage and at stunning and scalding. The time period to shedding can be extended if the concentration of the pathogen in the environment is lower (Boughton et al., 2007a). For transport, that may imply that the risk associated with short transport can be considered smaller than with long transport.
Assembly centres include livestock markets and livestock dealers' premises. They present similar challenges as lairage, since animals are exposed to an additional environment harbouring ARB/ARGs (which also applies when animals change vehicles during transportation). In addition, use of collection centres to gather animals from different farms prior to transport to slaughter increases the time and opportunity for acquisition, multiplication and transmission of bacteria between animals and from the environment before further transport and lairage before slaughter. Assembly centres are popular in many countries for gathering and redistribution of ruminants and can be associated with dissemination of MDR pathogens such as Salmonella spp. (Wray et al., 1991; Wells et al., 2001; Matthews and Woolhouse, 2005). Such assembly centres are not used for commercial scale poultry production or pig production, although on‐farm collection points are often used on large farms to gather pigs from different parts of the farm for later onward dispatch. One peculiarity of this kind of assembly centre is that the animals are not necessarily destined for slaughter. Hence the bacteria that are exchanged in these facilities are likely to be spread further to other animal holdings where they may be a source of additional spread, but this is considered outside the scope of this Scientific Opinion.
3.2.4. Summary of risk factors and uncertainties identified
The identified risk factors for the transmission of ARB/ARGs during transport are visualised in the Figure 8. In Table 1, the risk factors are summarised, accompanied by supporting data, the uncertainties associated, and the relationship of those risk factors with the duration of the transport. The risk factors that were identified as 99%–100% certain to contribute to the probability of transmission of ARB/ARGs, were also considered to be of high importance.
3.2.5. Risk factors – concluding remarks
The AMR status (e.g. resistome, mobilome, fitness traits) of the animals' microbiota before transport is considered to have a major effect on the probability of transmission of ARB/ARGs during transport.
- During transport, the microbiota of animals might be altered which possibly contributes to the acquisition and shedding of ARB/ARGs
-
○Feed withdrawal before and during transportation and lairage of animals has effects on the microbiota of animals as demonstrated by increased susceptibility to, colonisation and shedding of certain bacteria, including Salmonella, as well as increased exchange of plasmids between bacteria. Data relating to ARB is scarce. On the other hand, feed withdrawal can be hypothesised to reduce the shedding of ARB/ARGs, since it has been shown that withdrawal decreases vomiting in pigs, and less faecal material would be produced. Due to the lack of evidence on the effect of withdrawal for shedding and transmission of ARB/ARGs and because both positive and negative effects on ARB/ARGs transmission can be assumed, the overall direction of the effect of feed withdrawal remains unclear.
-
○In addition to feed withdrawal, the microbiota in animals during transport may be altered by other stressors (such as animal handling, heat/cold, loading/unloading), environmental conditions, infectious diseases and general health status of the animal. Specific data for ARB/ARGs are not available, thus the effect and the overall direction of the effect of stressors on ARB/ARGs transmission remains unclear.
-
○
Shedding of bacteria can be altered during transport. For all the animal species considered, the effect of stress, especially during loading and transportation, is often reported to be associated with increased defaecation, leading to a higher risk of dissemination of bacteria, including ARBs if present, between transported animals and to their environment, hence increasing exposure to other animals.
- Environmental exposure to ARB/ARGs can occur during transport.
-
○When contaminated transport vehicles and related equipment such as transport crates are not cleaned and disinfected properly, they have the potential of contaminating animals with bacteria originating from animals transported before. While this contamination is likely to be predominantly superficial it may lead to colonisation and/or infection in transport > 2 h. Usage of equipment and vehicles for unrelated populations (e.g. laying hens and broilers) is likely to have a more substantial effect.
-
○Airborne transmission can contribute to the exchange of bacteria between animals during transport. Although the presence of ARB/ARGs in air during transport is described, it is unclear as to whether this leads to transmission and in what amount.
-
○Workers can be a source and vector for the transmission of ARB/ARGs between animals, but it is considered to contribute minorly compared to exposure to the truck environment and other animals carrying ARB/ARGs.
-
○
Exposure to other animals carrying and/or shedding ARB/ARGs contributes to the probability of transmission of ARB/ARGs during transport. Transmission of ARB/ARGs between animals is more likely when the duration and frequency of contacts between animals increase.
The relevance of exchange of ARB/ARGs between animals likely depends on the differences in the microbiota between the transported animals. Transport of animals of different origins is therefore likely to be more problematic than transport of animals from the same population.
Adverse environmental conditions (high temperature/humidity) are associated with the multiplication and survival rate of bacteria in the environment, including ARB/ARGs.
The impact of the duration of transport is important, allowing for greater bacterial multiplication and transmission of bacteria between animals with long transport times. When the duration of transportation increases, the exposure time to ARB/ARGs in other animals and the environment (e.g. air, truck) increases as well. The exposure to additional contaminated environment during resting periods, as well as the additional time to reach the final destination may increase the risk of multiplication and dissemination of bacteria in batches of transported animals.
Ineffective cleaning and disinfection of lairages and livestock markets has been reported. Environmental contamination at lairage can lead to the acquisition of bacteria. The exchange of ARB/ARGs between animals can occur at lairage. Animals are commonly not mixed further at the stage of lairage. The role of environmental contamination in the transmission of ARB/ARGs during lairage is therefore probably more dominant than the role of direct exchange of ARB/ARGs between animals. Moreover, as the time spent in lairage is short, the contamination will mostly occur on the surface of the animals.
3.3. TOR2. Preventive measures and control options
3.3.1. Mitigation measures in transport and lairage, brief definitions and reasoning behind the strategies described in the section
Section 3.3 addresses the potential mitigation options considered to reduce the burden and transmission of AMR in transport and lairage stages. In this Scientific Opinion, mitigation refers to any measure, strategy or action which can reduce AMR development, transmission or which can remove/eliminate or reduce the AMR burden in transport and/or lairage stages.
The mitigation strategies described in this section mostly address the risk factors described in the Section 3.2. Thus, most of the strategies aim at offering solutions to tackle the main risk factors identified in this Scientific Opinion. Due to the scarce information and the lack of specific studies addressing the mitigation of AMR transmission in these two stages of the food chain (see data gaps section for more information), the considerations provided below are based on general and specific mitigation strategies which have been shown to reduce, control or eliminate the multiplication and transmission of pathogens harbouring ARB/ARGs. This applies predominantly to pathogens, which frequently harbour AMR determinants. The interventions are grouped considering the different stages encompassed in transport: pre‐transportation including the preparation of the animals, transportation including the loading, the journey/transit and the unloading, as well as the stay of the animals in intermediate places such as control posts (for rest stops), assembly centres and lairage.
These interventions include mitigation measures which can be divided into:
those which directly help to control AMR transmission
those which exert an effect on AMR transmission indirectly, for instance improvements in animals' health and welfare.
In each part of the section, the interventions are described with reference to scientific literature in the targeted animal and bacterial species. Specific interventions which apply only to a particular animal sector, such as poultry (i.e. catching and transport in cages), are stressed at the end of each section.
3.3.2. Mitigation measures which aim at improving animal health and welfare but with a potential impact on AMR transmission
As mentioned in the previous paragraph, in general, AMR mitigation options can overlap with strategies to reduce or control pathogens. Thus, there is relevant information for this Scientific Opinion in previous EFSA reports in the AMR topic such as RONAFA (EMA and EFSA, 2017) or a recent Opinion in the role of the food‐producing environment on the emergence and spread of AMR (EFSA BIOHAZ Panel, 2021). Similarly, strategies to reduce transport stress can be useful to indirectly improve health and welfare of the animals (EFSA AHAW Panel, 2022a,b,c). Thus, relevant information to limit AMR transmission can be found among publications by FAO in their recommendations for transport and lairage (‘Antimicrobial Resistance’, online https://www.fao.org/antimicrobial-resistance/key-sectors/animal-production/en/; FAO/WOAH/WB, 2010) as well as in the ‘Animal Transport Guides' (online http://www.animaltransportguides.eu/materials/), which will be mentioned briefly within this section.
Applying good husbandry and animal handling practices while managing the animals before and during animal transport (e.g. ensuring good quality of feed and water, adequate handling, appropriate ventilation rates and space allowances) are considered as general mitigation strategies to enhance animal health and welfare. Consequently, such measures would be expected to reduce the impact of various relevant risk factors such as stress (general, temperature, stress hormones) on the of faecal shedding, or driving for the transmission of ARB/ARGs to an unstable microbiota. Other possibilities may involve the choice of locally‐adapted breeds which are more resistant to diseases and stress.
In line with the previous point, staff training and auditing in the aspects mentioned in the previous paragraph may help in guaranteeing their effective application.
3.3.3. Mitigation measures addressing animal preparation for transport
Transport preparation is important to limit any potentially stressful disturbances which may have a negative effect on animal health, immunocompromising the animals, re‐activating latent infections or increasing the shedding of pathogens and ARB/ARGs. Handling stress has been identified as a hazard for compromising welfare during preparation (see EFSA AHAW Panel, 2022a,b,c). Consequently, training of staff on handling based on animal learning theory (Boivin et al., 2003; Abramson and Kieson, 2016) is considered as a mitigation strategy. Likewise, it may be helpful to habituate the animals to humans, human handling and transport‐related practices. It may be also useful to expose animals to the required procedures and handling by staff in advance. Even if habituation might increase overall risk, ensuring animals are used to regular human contact is likely to be beneficial (Grandin, 2019; EFSA AHAW Panel, 2022a,b,c). Handling stress may favour the transmission and exchange of resistant bacteria or AMR determinants via increased defecation, shedding of specific organisms and poorly controlled contact, although further research is needed to quantify the relative risk of each potential factor identified in the risk Section 3.2. These actions can reduce AMU and the associated selection pressure, linked to the aforementioned negative health effects of transport stress (EMA and EFSA, 2017).
Pre‐transport mitigation options have some particularities in poultry, associated with stress linked to animal handling and catching. Good handling practices and training by experienced staff are important in minimising handling stress and injuries to birds during catching and loading (Bayliss and Hinton, 1990; Weeks et al., 2019: Grandin, 2020; Edwards and Hemsworth, 2021). It has been reported that well‐managed mechanical catching can be less stressful than manual procedures (Nicol and Scott, 1990; Benincasa et al., 2020). Animals that are inadvertently injured during catching should not be loaded (EFSA AHAW Panel, 2022a).
Although there are no specific studies describing a direct link between the interventions described below and AMR mitigation, their implementation may help limit pathogen and AMR spread. Housing poultry in less stressful enriched environmental conditions that include perches, straw bales, etc., can help to reduce the stress response of birds in general, so can have a calming effect prior to transportation (Li et al., 2021). Careful management of temperature during the early life of the birds can lead to improved tolerance of heat stress later in life (Goel, 2021). Stress and related adverse effects on hatched chicks can be reduced by installing feeding systems within hatcher incubators (Souza da Silva et al., 2021). Careful management of catching will reduce stress levels and thus corticosteroid levels (Kannan et al., 1997; Vosmerova et al., 2010), and hence reduce a potential disbalance on the microbiota, which could affect ARB/ARGs. Management of lighting conditions during catching of birds can help to reduce stress responses and may be applicable to conditions during transport and waiting for slaughter at the abattoir lairage (Abo‐Al‐Ela et al., 2021). The use of dimmed lights when catching broilers rather than red light can reduce the number of birds found dead on arrival at the slaughterhouse (Van Lìmbergen et al., 2020).
Similarly, pigs and cattle should be treated with care during preparation to avoid higher rates of defaecation and a consequent greater likelihood of contaminated hides and environment. Therefore, a calm approach and appropriate handling can be expected to reduce spread of ARB/ARGs (Dewell et al., 2008a).
Assessing the fitness for transport, hence, the identification and isolation of unhealthy animals is already mandatory, but the current list of pathologies is limited and focuses only on severe clinical signs (e.g. fever). Implementing the assessment of the fitness of transport as recommended by the EFSA AHAW Panel (2022a,b,c) and adequate staff training to identify unhealthy animals are crucial for this. Animals deemed unfit for transport should not be loaded and treated as soon as possible. The relevance of health checks becomes even more crucial if animals are going to be transported to collecting centres, markets and lairage areas. These places offer the opportunity for pathogen and ARB/ARGs transmission between animals from different origins.
Measures associated with feeding management have different considerations depending on the destination, i.e. other farms vs. transport to slaughter.
The potential impact of feed withdrawal on AMR dissemination is discussed in the previous section, and the impact on AMR is associated with high uncertainty. Specific data evaluating the impact of feed withdrawal on AMR is lacking. Therefore, with respect to resistance transmission, the management of feed withdrawal prior to transportation and slaughter needs to be carefully assessed and adjusted according to the expected duration of transport. With the data available, no recommendations in relation to feed withdrawal can be made in order to contain AMR transmission.
The impact of the use of alternative substances to antimicrobials (e.g. botanicals, organic acids, essential oils, bacteriophages, bacteriocins or probiotics) is reviewed in previous EFSA Scientific Opinions (EMA and EFSA, 2017). Although such interventions could be applied during on‐farm preparation, during transport or in the lairage, similarly to the conclusions in these EFSA Opinions, we lack specific data which demonstrate their usefulness in AMR mitigation. In addition, some of these interventions often rely on a long period of administration (De Busser et al., 2009; Gilson et al., 2019), limiting their potential use in short‐term procedures such as transport and lairage.
Transport logistics considering AMR criteria
Organisation of the transport considering AMR criteria, henceforth referred to as ‘transport logistics’, can be an efficient strategy to mitigate pathogen and AMR exchange among animals, at least from different farms (Figure 9). Among direct criteria, various ‘risks’ could include the AMR load, presence of ARB with resistance to ‘critical’ or last resort antimicrobials (indicators such as presence of CPEs, MRSA, VRE, linezolid‐resistant staphylococci and other bacteria included in Group 1 and Group 2 described in Section 1.3.2) and/or presence of certain genes (e.g. carbapenemase‐encoding genes, ESBLs), and/or specific mobile genetic elements (EFSA BIOHAZ Panel, 2021). These AMR indicators would permit categorisation of animal batches/farms based on their risk of ARB/ARG transmission and schedule the transport of conflictive animals accordingly. This might include transporting such animals to the slaughterhouse at the end of the day or the week and increasing the time‐lapse between transports, which reduces ARB viability and thus avoids contamination of animals subsequently transported. It also implies separating such animals to minimise the risk of transmission to other animals. This sort of intervention can similarly be applied to control posts, assembly centres and lairage, where animal batches exhibiting AMR, particularly to ‘critical’ antimicrobials, should be handled with measures which guarantee their isolation from other animals and cleaning protocols which preclude the transmission to subsequent animals kept there.
Figure 9.

Scheme of potential transport logistics based on AMR criteria. Based on AMR information (AMR load, ARB, ARGs or indirect measures) farms or batches (feedlots) can be categorised by their potential risk and specific measures can be implemented in transport, assembly centres, control posts and/or lairage
Such measures require appropriate knowledge to characterise the batches of animals, i.e. a routine schedule to test herds for a predefined set of criteria, similar to the data provided by Salmonella control programs, if information (e.g. presence of highly important ARB/ARGs as those mentioned above) is not already available.
Alternatively, other direct/indirect strategies or measures could be used to establish criteria: for instance, EU/National surveillance information provided by AMR epidemiological studies (routine or specific monitoring, baseline surveys that provide data from antimicrobial susceptibility testing or whole genome sequencing analyses). Such programmes are mostly based on a sample of herds rather than on the sampling of every herd. Also, information collected from National control programmes for specific pathogens/zoonotic bacteria (e.g. Salmonella) could be useful.
Categorisation of the transported animals could be based on the presence of risk factors for AMR on the farm of origin as well. Such a risk‐factor based approach should rely upon factors already available in public databases and with a well‐known association with AMR. Among such approaches, e.g. AMU data would be available since on‐farm monitoring systems of AMU are now mandatory across the EU (Regulation 2019/6). Other indicators might include animal species and category, animal age, farm records (i.e. outbreaks), and data about on‐farm biosecurity, where available. Nevertheless, although a risk factor‐based approach may not require generating additional data, it has lower accuracy than directly measuring AMR hazards in the animals to be transported.
Altogether, this mitigation strategy is limited by the current AMR information, lack of criteria to choose the best indicators or the low correlation between data from indirect measures such as AMU and AMR risk transmission.
Planning
During preparation, the journey should be carefully planned. The management of transport preparation is highly relevant. Some strategies linked to biosecurity, such as minimising the number of farms to be visited and the number of vehicles used per transport, limits the contacts among animals with different origins and thus the risk of relevant AMR transfer associated with pathogens or commensal bacteria. In this sense, the use of the same truck to transport different animal species or categories (e.g. broilers and laying hens) should be avoided for the same reason to account for residual contamination of trucks and transport crates. Similarly, transports with different purposes should be segregated, e.g. avoiding the same vehicle for animals transported to farms and the slaughterhouse within the same trip (Neumann et al., 2021). Additionally, stopping at different farms to load different animals or change vehicles will increase the journey duration and the cross contaminations and consequently the risk of AMR transmission. Since transport duration should be kept as short as possible, during planning unnecessary extension of the journey duration should be avoided (e.g. select for the nearest slaughterhouse). For the same reason, the movement of animals should be limited to the minimum required (e.g. check necessity of life‐breeding animal exchange). In the planning, the time of departure and arrival should be decided to travel during the times of the day suitable for optimal thermal comfort zone of the animals to minimise the risk of heat stress/unfavourable environmental conditions which will increase the risk of spreading ARB/ARGs.
Stocking density, group size and segregation
Increasing space allowance (i.e. reducing stocking density), apart from reducing group, motion, heat stress and resting problems (EFSA AHAW Panel, 2022a,b,c), is likely to reduce environmental contamination, as less faeces will be shed per surface, and limits the number of animals in contact. Moreover, limiting the number of animals in a transport vehicle or transport cages may lead to reduced exchange of bacteria by reducing both the number of potential shedders and the number of potential recipients in a compartment.
Minimising the group size (i.e. segregating the animals in different compartments) and any measure to assure effective segregation of animals of different origins, i.e. mainly from different farms, but different barns, feedlots, age groups or production goals (meat, milk, egg) during transport will likely reduce the potential exchange of bacteria, including pathogenic and AMR bacteria between animals of different origins. Jones et al., (Jones et al., 2013) reported a reduction in the spread of ESBL‐producing and fluoroquinolone‐resistant E. coli with the compartmentalisation of the flocks on turkey farms. Correspondingly, compartmentalisation and avoiding mixing veal calves from different origins have proven successful in reducing the burden of ARB in veal calves (Becker et al., 2022). Such measures will furthermore decrease social stressors associated with being confronted with unfamiliar animals of the same species. Using smaller compartments with solid divisions may contribute to reduced or less protracted exchange of bacteria between animals. Smaller compartments may increase the likelihood of the animals accessing drinkers and feeders.
3.3.4. Mitigation measures addressing loading and unloading
Loading and unloading have been highlighted as hot spots in the risk factors section. Mitigation in these procedures should involve the use of adequate technical equipment, hygiene and well‐trained staff. Loading and unloading platforms and ramps should be made of non‐slip materials that are at the same time easy to clean and disinfect. Cleaning and disinfection should be thoroughly done to avoid transmission of bacteria between subsequently loaded/unloaded animal groups. The efficacy of cleaning and disinfection should be checked at intervals and procedures should be adjusted if they are shown to be ineffective.
All procedures during loading and unloading should be carried out minimising stress, injury or any potential event which may negatively impact animal health and welfare (see EFSA AHAW Panel, 2022a,b,c).
Unloading should start as soon as the vehicles arrive at the destination. During waiting times, the temperature inside the vehicle increases and the animals tend to interact among them and be more restless. Any delay in unloading (and uncrating in case of birds) should be avoided to avoid additional transmission of bacteria and contamination of animals in this period. To avoid AMR transmission, animals which have become unhealthy or injured during the journey should be unloaded and isolated.
3.3.5. Mitigation measures during journey/transit
Animal transport implies a number of welfare hazards for which mitigation and corrective measures have been identified for cattle, pigs and poultry (EFSA AHAW Panel, 2022a,b,c). Since reducing the presence of these hazards will reduce distress, pain and frustration of the animals and their effects on the immune system, it will potentially minimise AMR spread.
Heat or cold stress can be associated with microclimatic conditions inside the vehicles. They can often be caused by transportation in confined and overcrowded conditions and result in increased faecal and pathogen contamination of animals or susceptibility to infection (Warriss et al., 2005; Soro et al., 2020; Lalonde et al., 2021; Lu et al., 2021; Ricke, 2021). Mitigation options ensuring thermal comfort include:
Ensuring animals are transported under microclimatic conditions within their specific thermal comfort zones would be a way to reduce the negative impacts of unsuitable temperatures (see EFSA AHAW Panel, 2022a,b,c for details). This can be achieved by improvements in vehicle design, using forced ventilation and air‐conditioning. The design of poultry crates and their spacing within the load is important for optimisation of ventilation, but more research is needed to define best options for various types of birds and climatic conditions (Bhatt et al., 2021; Pinheiro et al., 2021). It may also be possible to design ventilation or cooling systems linked to temperature sensors which can respond to different conditions within a load (Mitchell and Kettlewell, 2008; Ramadiani et al., 2021).
-
As discussed with regard to the transportation planning described above, not travelling during the hottest or coldest time of the day can be planned to make sure that the animals travel within their thermal comfort zone, avoiding both heat and cold stress.
Solutions to avoid prolonged transport duration due to traffic density (i.e. traffic jams, traffic controls, tolls or control customs) should be provided.
Bedding can help improve the comfort of the animals. It can also soak up faeces and urine and therefore reduce exposure of animals to these. Depending on the capacity of the bedding to do so, this may limit contamination of the animals (Singh et al., 2020). Bedding is currently compulsory during journeys longer than 8 h (EC 1/2005). Straw and hay have been recommended because they can be a source of feed for cattle and pigs. From a hygienic perspective, the latter is not welcome as ingestion of contaminated bedding might support the spread of (resistant) bacteria (Ray et al., 2022). For shorter journeys, bedding is not compulsory, although its hygienic benefits (soaking up liquid) might even be more relevant than in long journeys when the capacity of the bedding to soak up liquid is likely to be overwhelmed. Use of bedding increases the efforts for cleaning and disinfection, increasing the material to be removed from trucks and lairage areas. Thus, despite the clear benefits for animal welfare, the role of bedding in AMR mitigation needs further study.
Shorter journey lengths are likely to be associated with reduced cross‐contamination between animals (Nijdam et al., 2004: Dewell et al., 2008a,b; Caffrey et al., 2017; Dos Santos et al., 2017, 2020).
Mobile slaughter units could be used to generally limit transport time and stress and the time between catching and slaughter if food hygiene and licensing requirements can be fulfilled (Cartoni Mancinelli et al., 2018).
Any other practices safeguarding and enhancing animal welfare during transport (EFSA AHAW Panel, 2022a,b,c) may help to minimise the risks of the shedding of AMR pathogens/bacteria. Control posts and other interrupted travels may at the same time increase risk of transmission by additional mixing of animals and/or uptake of bacteria from a contaminated environment. Therefore, efforts should be made to balance the effects on the different targets (i.e. comfort vs. hygiene).
3.3.6. Mitigation measures after transport
Vehicle cleaning after each transport. Cleaning and disinfection should prevent the potential transmission of bacteria to subsequent groups of animals placed in the same environment. With respect to AMR this can reduce transmission of ARB and a build‐up of a resistant microbiota in the animals' environment. It is particularly critical for haulage vehicles (Porphyre et al., 2020) to avoid cross‐contamination between different groups of animals loaded on the trucks.
The transport vehicle itself should be cleaned and disinfected after each delivery to the farm or slaughter plant as specified by EU Regulation (EC 1/2005). The same regulation applies to cleaning and disinfection of facilities for loading and unloading and intermediate stays such as control posts or assembly centres. The transporter must keep a register to keep information about the date and place of disinfection. According to the EC Regulation 853/20047, abattoirs must have separate areas for cleaning and disinfection of transport vehicles and equipment.
It is essential that cleaning and disinfection procedures are performed properly and thoroughly (De Busser et al., 2013). Effective protocols for decontamination of trucks should include washing, disinfection and drying (Baker et al., 2017; Neumann et al., 2021). A standard operating procedure (SOP) for cleaning and disinfection of trucks with certified effectiveness should be followed in areas dedicated to this task (USDA and CFSPH, 2010; Weber and Meemken, 2018). Cleaning should be carried out as soon as possible after unloading. Bedding and contaminated material should be segregated to avoid re‐contamination (Mannion et al., 2008; FAO/WOAH/WB, 2010). No visible dirt should be present on surfaces after washing. This level of cleanness may be achieved using a high‐pressure washer and detergents to help the removal of organic matter (FAO/WOAH/WB, 2010; Weber and Meemken, 2018). Both help to improve disinfection and drying effectiveness. Disinfection is a critical control step in the process as it inactivates the residual bacteria which persist after cleaning. Moreover, disinfectants like sodium hypochlorite are able to remove free‐DNA molecules (Nilsson et al., 2022). Quaternary ammonium compounds and anionic surfactants, in hatcheries are often used at a concentration that is unlikely to eliminate some strains of bacteria within species such as Serratia marcescens, E. faecalis, E. faecium, Pseudomonas spp. and Pantoea agglomerans. These species commonly include AMR strains (Willinghan et al., 1996). For disinfection, choosing an appropriate product at a suitable concentration, application rate and contact time is essential (Gosling, 2018). Use of copper alloy antimicrobial surfaces instead of polypropylene for construction of chick crates was found to reduce surface microbial counts but this was undermined by a design that inhibited drainage in the floor of the baskets (Depner et al., 2021). Moreover, resistance to copper is frequently associated with AMR (e.g. Argudín et al., 2011). The use of a copper alloy may therefore in the long term, support AMR selection.
To assure effectiveness of disinfection, the procedures and equipment (especially disinfectant metering devices) should be checked at regular intervals and in case of malfunctioning they should be adjusted.
Several authors have underlined the importance of drying vehicles after cleaning and disinfection and before transporting (Dee et al., 2005; FAO/WOAH/WB, 2010). For trucks, 8–12 h of drying are usually sufficient. Since this may be difficult to carry out under field conditions, forced air fans and heaters can be used to facilitate the process (FAO/WOAH/WB, 2010). The livestock transport industry has developed the thermo‐assisted drying decontamination method (TADD). This method is based on forcing hot air into the trailer, increasing the temperature up to 71°C for 30 min. TADD has been successfully tested for porcine reproductive respiratory syndrome virus (Dee et al., 2005), but further studies are necessary to test its efficacy for bacteria. Neumann et al. (2021) suggests extending cleaning and disinfection procedures to loading ramps and lairage areas.
Some authors suggest regularly monitoring the efficacy of cleaning and disinfection procedures through random checks (Mannion et al., 2008; Weber and Meemken, 2018). Checks based on visual inspection fail to detect microbiological deficiencies in cleaning which cannot be observed by the human eye. Microbial testing is more sensitive in detecting potential indicators of inefficient cleaning like Salmonella spp. or Enterobacteriaceae (Mannion et al., 2008; Weber and Meemken, 2018).
Although these biosecurity procedures are simple, they require a coordinated effort by those involved in the animal production chain to be effectively implemented (Lowe et al., 2014). Weber and Meemken (2018) suggested that the abattoir should have a closed washing space equipped with a pressure cleaner and sufficient lighting for nighttime. Appropriate equipment should be available on site for the protection of the driver during cleaning and disinfection procedures.
Analogous procedures should be followed after transport of animals between farms or between farms and assembly centres and/or control posts (see Section 3.3.8). Contaminated materials should be disposed of in a safe way that avoids introduction of bacteria from these materials back into the vehicles or into the herd of destination. The possibility of the main introduction of contamination occurring through animals arriving requires assessment.
Effective sanitation of poultry transport crates and prevention of recontamination of cleaned crates via aerosols and dust is important to avoid survival or introduction of pathogens. Cleaning and disinfection of crates have to be done thoroughly as insufficiently cleaned and disinfected crates have repeatedly been identified as a source of (resistant) bacteria to poultry. This was not supported by a literature review contained in a USA Quantitative Microbial Risk Assessment (QMRA) study, which did not confirm a significant effect of crate washing, in view of the high rate of pre‐existing flock infections that contribute the major burden of contamination and the limited efficacy of current crate washing methods (Kang, 2020). The impact of crate washing may therefore be more relevant in situations where other sources of Campylobacter spp. are better controlled. Decontamination can be improved by an additional disinfection stage after washing (Davies and Wray, 1994) and strict segregation of pre and post washing airspaces (Huneau‐Salaün et al., 2022). As with vehicles, Dzieciolowski et al. (2022) reported that hot air drying of crates after washing and disinfection could improve reduction of Enterobacteriaceae from just over 2 logs to nearly 3.5 logs.
A similar study reported a modest reduction on log counts associated with the use of UV light, but this could be compromised by shadowing associated with the common occurrence of poor cleaning of crates (Moazzami et al., 2021). This can lead to increased microbial load and diversity (Freeland et al., 2021). An intensive decontamination pilot study produced good results for reduction of Salmonella and coliform bacteria in broiler transport crates: after each use, the crates were cleaned with a detergent using a high‐pressure jet to remove faecal matter and reduce biofilms. The containers were then submerged in 1% sodium hypochlorite solution at 70°C for 2 min (Ramesh et al., 2003).
3.3.7. Mitigation measures at lairage
Lairage time should be kept to the minimum required (e.g. shorter exposure to the environment and/or exposure to other animals). Management procedures such as overnight resting should be avoided to limit the time available for exchange of bacteria between animals of different origin and/or the lairage environment and for multiplication of the transmitted bacteria. Animal batches should only be mixed if they are transported in the same group or come from the same herd. Whilst this would reduce relevant exchange of bacteria, mixing these animals still continues, with subsequent social stress of regrouping and re‐penning that should rather be avoided (see above). Group sizes should likewise be limited to reduce the number of potential shedders and recipients and therefore limit the probability of transmission. For the same reason, sufficient space should be provided per animal to reduce intensity of contacts.
Despite the lack of specific data on the influence of factors such as temperature, humidity, airstreams or excessive noise on AMR, these stressors should be minimised, as they may impair the immune response of the host and facilitate the transmission of bacteria (Knowles, 1999). If animals are not slaughtered immediately after arrival, water and feed should be provided to reduce the impact of feed withdrawal on animal welfare (EFSA AHAW Panel, 2022a,b,c). Feeding hay to cattle at lairage seemed to reduce hide contamination with E. coli O157 (Mather et al., 2007). Nevertheless, issues with intestinal fill during slaughter may limit the usefulness of feeding at lairage.
To reduce environmental contamination, cleaning and disinfection protocols, like in other preharvest stages mentioned in this Scientific Opinion is crucial. A study evaluating cleaning and disinfection protocols in the lairage area demonstrated that the application of power washing, a foam detergent followed by disinfection and final drying was able to reduce the presence of salmonella organisms (including resistant ones) from the environment by over 90% (Walia et al., 2017). Any protocol failing in any of the steps described, demonstrated lower efficacy in AMR pathogen removal.
Lairage pens usually provide a source of water for animals and the alternative substances to antimicrobials mentioned in transport feeding strategies could be applied in this stage. The same limitations and gaps for these products are applied here (see feeding management measures mentioned above and Section 3.4 Data gaps section).
3.3.8. Mitigation measures at assembly centres and control posts
Mitigation of AMR transmission in assembly centres may begin by minimising their use (Marquetoux et al., 2016). When used, organisation of handling and penning facilities needs to consider animal origin (farm, but additionally truck, region, etc.), species, age and any factor that can have an influence on AMR exchange among animals from different sources. Biosecurity protocols in transport related activities should include the mitigation of the risk associated with these centres. Particular attention should be paid to transmission of pathogens and ARB/ARGs between different batches of animals through minimising contact between the batches. This can be achieved by regulating animal traffic in the centres and effective cleaning and disinfection protocols. Those protocols should be evaluated regarding their effectiveness against ARB/ARGs in regular intervals.
A long rest at control posts is compulsory for pigs and cattle during very long journeys. During this stay it is crucial that animals are not mixed. It is particularly important that they keep the same group as they travelled, and do not get in contact with animals of other origins. In addition, the use of the same vehicles is highly recommended. Moreover, after the animals leave the control posts, appropriate cleaning of the facilities is recommended before another load of animals is disembarked into in the same pens. Checking the fitness for continuing to travel should also be carried out carefully. Ideally, possible sick/injured animals should not be loaded and instead isolated and treated at the control post.
3.3.9. Summary mitigation measures and uncertainties identified
3.3.10. Mitigation measures – concluding remarks
Despite the lack of scientific studies which have evaluated the efficacy of different AMR mitigation strategies in transport, this Scientific Opinion has collated information about various strategies which can help in mitigating the risks pointed out in the previous section.
Interventions improving animal health, biosecurity and welfare immediately prior to and during transport could reduce ARB/ARGs transmission. Staff training in good husbandry practices, identification of sick animals, proper handling before transport, including catching, and in animal loading and unloading could mitigate AMR exchange.
Considering that ineffective cleaning and disinfection of transport is highlighted as one of the major risks for new ARB/ARGs acquisition, the protocols used should be revised and accomplished to guarantee optimal removal of microorganisms after each animal transport. Cleaning and disinfection are not limited to the transport vehicle itself but include loading/unloading areas, and equipment such as cages and crates. The efficacy of the protocols should be tested regularly by inspection and microbiological analyses.
‘Transport logistics' meaning farm/batch transport organised based on AMR criteria (AMR load, ARBs with resistance to ‘critical’ antimicrobials and ARGs conferring those resistances, epidemiological data or indirect parameters such as AMU), would help to establish specific measures which guarantee their isolation from other animals and strict cleaning protocols to reduce AMR. This would apply to transmission to other animals in transport, lairage, control posts and assembly centres. The mitigation strategy is contingent upon choosing the best criteria and indicators for each criterion to categorise farms or batches.
Transport planning can limit ARB/ARGs spread through interventions such as minimising the number of farms visited and multiple vehicles used to transport a particular animal or batch. Reducing stocking densities and the number of animals in contact and ensuring animal segregation/ compartmentalisation by species, production stage, or age, will likewise limit transmission. Transport should ideally be completed at the times of day suitable for optimal thermal comfort for animals. Finally, avoiding the use of the same vehicle for animals transported to farms and to the slaughterhouse within the same trip and adequate animal densities in the truck, and avoiding to change vehicles during long journeys (e.g. at control post) for logistic reasons will also limit transmission.
Transport vehicles should be designed to guarantee a high standard of animal comfort (health and welfare risks, i.e. thermal comfort). Other strategies such as the use of bedding require further research to evaluate their final contribution to AMR mitigation or spreading. Based on the uncertainties associated with the risk for ARB/ARGs transmission linked to feed withdrawal, with the data available and, the scarcity data in relation to the efficacy of as the use of alternative substances to antimicrobials on AMR, no specific mitigation measures in relation to feeding management are proposed.
Transport duration should be carefully considered. Long journeys requiring stops or unloading are a risk for AMR acquisition through mixing of animals and uptake of ARB/ARGs from a contaminated environment at control posts and assembly centres. Moreover, long transports may increase the likelihood that new carriers of ARB or zoonotic pathogens become active shedders thus increasing the infection pressure from the environment.
Use of assembly centres and control posts should be the exception, i.e. when unforeseen events lengthen travel times excessively. Recommendations about animal handling, mixing and facilities cleaning and disinfection are relevant in mitigating ARB/ARGs transmission in these places. Changes of vehicles during the same journey should be limited.
Lairage is a hotspot in pathogen and potential ARB/ARGs transmission. Thus, all risk factors linked to lairage should be mitigated, e.g. limiting lairage time to the minimum required, segregating the animals by origin, ensuring proper animal handling and establishing and validating cleaning and disinfection protocols which guarantee the mitigation of AMR transmission by the lairage environment.
3.4. TOR3. Data gaps and research needs
There is little specific information on ARB and ARGs relating to animal transport.
Few studies have tested AMR in bacteria from animals immediately before and after unloading. Present knowledge is on the most part therefore based on extrapolation from data collected for other purposes, e.g. for zoonotic pathogens, assuming that drug‐resistant strains of these, as well as other bacteria, behave similarly.
As the biology and interaction with the host and environment differs between bacteria, this is an oversimplification of the true situation. Studies are therefore needed to meaningfully assess the contribution of animal transport to the overall burden of AMR in the food chain both in relation to different ARB and ARGs and to animals of different origins/species.
3.4.1. Data gaps
The most important data gaps on ARB/ARGs transmission during transport are considered to be:
the quantification of the relative contribution of the risk factors and proposed mitigation options;
the effect of fasting prior and during transport, and the duration of this fasting on AMR in the microbiota (increase/decrease of ARB, gene transfer), shedding and transmission of the most important bacterial species commonly exhibiting AMR;
the effectiveness of different cleaning and disinfection protocols to reduce/eliminate ARB/ARGs;
the direction of the association between transport‐related stressors and ARB/ARGs transmission;
the effect of type and amount of bedding;
knowledge on the dynamics of ARB/ARGs transmission over time during transport and during lairage;
definition of and identification of AMR criteria and the best indicators for each criterion upon which the transport logistics could be organised (see Section 3.3.3);
the possible contribution of the airborne route during transport and lairage;
the impact on ARB/ARGs transmission of mechanical vs. manual catching/loading of animals;
the contribution of the health status, e.g. shedding of ARB/ARGs, the susceptibility to colonisation or infection by ARB, or transmission of ARGs;
the effect of interventions using alternative substances to antimicrobials to mitigate transmission of ARB/ARGs.
3.4.2. Research needs
3.4.2.1. Most urgent
Assess the impact of animal transportation compared to the contribution of other stages of the food‐production chain, as a contributor to dissemination of AMR between farms, and/or to contamination of meat at slaughter.
- Perform studies quantifying the effect on transmission of ARB/ARGs during transport by:
-
○feed withdrawal, considering ARB transmission between animals and on horizontal gene transfer between bacteria within such animals. Such studies should also address the effect of duration of feed withdrawal;
-
○cleaning and disinfection procedures for loading and unloading areas, transport vehicles, cages, assembly centres, control posts and lairage. Identification of the best methods and their evaluation to assess their efficacy are required;
-
○various stressors individually and in combination considering transmission of ARB between animals and on horizontal gene transfer;
-
○the type and amount of bedding used during the journey, or at control posts, assembly centres and/or lairage;
-
○airborne transmission of ARB/ARGs in transport vehicles;
-
○mechanical vs. manual catching/loading of animals;
-
○the animal health status during transport, and its effect on e.g. shedding of ARB/ARGs, the susceptibility to colonisation or infection by ARB, and/or transmission of ARGs;
-
○study the interplay of duration with all risk factors during transport and journey breaks. Those studies should include the determination of the time‐lag between uptake of ARB and faecal shedding and subsequent transmission of such bacteria under transport and lairage conditions.
-
○
Define the criteria, and the best indicators for each criterion, that could be used for transport logistics.
3.4.2.2. Longer term
- Studies on intervention strategies to help mitigate:
-
○effect of stressors with a demonstrated impact on ARB/ARGs transmission, e.g. by testing different environmental management strategies – e.g. facility design, ventilation, management and handling;
-
○possible hygienic problems of transport and lairage related with surface materials and improvement of facilities design;
-
○the mixing/contact of animals during transport, at animal assembly centres, control posts and lairage (e.g. improvements of facilities design);
-
○
To assess the effect of interventions using alternative substances to antimicrobials to lessen probability of transmission of ARB/ARGs in animal transportation settings;
To define the influence of AMR acquisition on survival and persistence of bacteria in animal transportation settings, e.g. increased/decreased colonisation proficiency.
To develop dynamic models allowing a more comprehensive and accurate estimation of risks related to ARB/ARGs transmission during animal transport and the effects of potential interventions.
4. Conclusions
ToR1: What are the most significant risk factors contributing to the spread of food‐borne zoonotic and indicator antimicrobial‐resistant bacteria (ARB) and antimicrobial resistance genes (ARG) between food‐producing animals during short journeys (< 8 h) and long journeys (> 8 h) directed to other farms or to slaughterhouses (directly or through livestock markets)?
AQ1. What are the most important risk factors contributing to the transmission of food‐borne zoonotic and indicator ARB and ARGs between food‐producing animals during short journeys (< 8 h) and long journeys (> 8 h) directed to other farms or to slaughterhouses (directly or through livestock assembly centres)?
There is scarce published information and specific studies addressing the risk factors contributing to AMR transmission during transport of animals. Thus, most of the conclusions below are based on expert judgement, informed by the collected evidence as reported in this Scientific Opinion and expert knowledge on risks for bacterial transmission. Accordingly, it is considered:
- 99–100% certain (almost certain) that:
-
○the resistance status (presence and type of ARB/ARGs) of the animals pre‐transport will influence the probability of transmission of ARB/ARGs during transport. A higher load and diversity of ARB/ARGs will increase the probability of transmission.
-
○increased faecal shedding during transport increases the probability of transmission of ARB/ARGs during transport. Any factor that increases faecal shedding (e.g. due to different stressors) would also increase the shedding of ARB/ARGs if present.
-
○insufficient hygiene of the loading and unloading areas and vehicles contributes to the probability of transmission of ARB/ARGs, as it increases the probability of transmission of ARB/ARGs between animal batches (transmission through the environment).
-
○exposure to other animals carrying and/or shedding ARB/ARGs contributes to the probability of transmission during transport. A higher frequency, duration and intensity of direct contacts between animals increases the probability of faecal‐oral transmission. The diversity of transmitted ARB/ARGs increases when animals from different origins are transported together.
-
○the duration of transport contributes to the probability of transmission of ARB/ARGs during transport. Transmission of ARB/ARGs can occur during short duration of transport. Nevertheless, the effect of most of the identified risk factors for transmission of ARB/ARGs will increase as a result of a longer transport duration.
-
○exposure to contaminated lairage, livestock assembly centres and control posts will increase the risk of carriage of ARB/ARGs in animals and therefore the probability of transmission. Depending on the duration of stay in these locations, most of the contamination will be on the surface of the animals.
-
○
- 66–90% certain (likely) that:
-
○airborne transmission contributes to the probability of transmission of ARB/ARGs during transport. The importance of this effect will depend on the bacteria (e.g. higher in respiratory pathogens and MRSA than in ESBL‐producing Enterobacteriaceae) and their presence in airborne particulates. There is evidence of the presence of ARB/ARGs in the air, which can lead to subsequential transmission, although not exactly clear to what extent. The likelihood of airborne transmission is probably dependent on duration of transport and ventilation.
-
○ARB/ARGs in workers (either as carriers or vectors) may contribute to the probability of transmission of ARB/ARGs during transport. This is likely of minor importance in comparison to exposure to the truck environment and other animals carrying ARB/ARGs.
-
○the health status of the individual animal contributes to the probability of transmission of ARB/ARGs. Infections during transport contribute to the probability of transmission of ARB/ARGs through increased shedding and decreased resilience to colonisation/infection.
-
○unfavourable environmental conditions (high temperature and humidity) will increase the probability of transmission due to increased multiplication and survival rate of bacteria in the environment, including ARB/ARGs.
-
○inadequate transport environmental conditions increase the probability of transmission of ARB/ARGs due to alterations in the microbiota, thereby contributing to the expansion of certain ARB and shedding of bacteria.
-
○
- 33–66% certain (about as likely as not) that:
-
○feed withdrawal can affect the transmission of ARB/ARGs during transport. Feed withdrawal can increase shedding of certain bacteria (e.g. Salmonella spp., Campylobacter spp. which are often resistant to antimicrobials), but at the same time, the reduction on vomiting and shedding of faecal material could reduce the probability of transmission of ARB/ARGs.
-
○stress can affect the transmission of ARB/ARGs during transport. There is evidence that stress can lead to alterations in the microbiota and suppression of the immune system, but the impact (positive or negative) on ARB/ARGs is unclear.
-
○
Although most of the identified risk factors are influenced by duration, there is no evidence to estimate differences between journeys shorter or longer than 8 h. Journeys that require rests in control posts will be associated with specific risk factors in those temporal areas (e.g. mixing of animals, environmental contamination, stress).
Among the risk factors identified above, the resistance status (presence of ARB/ARGs) of the animals pre‐transport, increased faecal shedding, hygiene of the areas and vehicles, the exposure to other animals carrying and/or shedding ARB/ARGs (especially from different origins), duration of transport (given the presence of other risk factors), and exposure to contaminated lairage areas are considered particularly important for the transmission of ARB/ARGs during transport.
ToR2 (AQ2): What preventive measures and control options could be implemented during short journeys and long journeys directed to other farms or to slaughterhouses and during subsequent lairage to reduce the probability of spread of food‐borne zoonotic and indicator ARB/ARG between food‐producing animals?
There is scarce published information and specific studies addressing the mitigation of AMR transmission during transport of animals. Thus, most of the conclusions below are based on expert knowledge on efficacy against the risk factors described in this Scientific Opinion and bacterial transmission in general.
Based on the main risk factors identified and the potential mitigation options available to reduce the probability of AMR transmission in transport, assembly centres and control posts, the conclusions of this Scientific Opinion, ranked by certainty, are listed below. Accordingly, it is considered:
- 95–99% certain (extremely likely) that:
-
○minimising the duration of transport will reduce AMR transmission. Long journeys requiring stops or unloading are a particularly high risk for AMR acquisition through mixing of animals and uptake of ARB/ARGs from a contaminated environment. With the limited data available, no maximum journey duration can be recommended.
-
○AMR transport logistics, meaning transport organisation dependent on AMR criteria (e.g. AMR load, ARBs with resistance to ‘critical’ antimicrobials and ARGs conferring those resistances, epidemiological data or indirect parameters such as AMU), will reduce ARB/ARGs transmission.
-
○
- 90–95% certain (very likely) that:
-
○measures that improve animal health, welfare, and/or biosecurity, immediately prior to and during transport, will reduce ARB/ARGs transmission. Thus, good husbandry and handling practices associated with animal transport preparation interventions will reduce such transmission.
-
○proper cleaning and disinfection of transport vehicles, crays, cages, loading and unloading areas, lairage areas, assembly centres and in general surfaces and equipment will mitigate ARB/ARGs transmission. Ineffective cleaning and disinfection of transport is highlighted as one of the major risks for new AMR acquisition in this stage. Thus, cleaning and disinfection protocols should be revised and validated to ensure thorough cleaning and disinfection to effectively guarantee removal of resistant microorganisms after each animal transport. Efficacy of the protocols should be tested regularly by inspection and microbiological analyses.
-
○adequate planning of the transport can limit ARB/ARGs spread. This would include interventions such as minimising the number of farms visited and number of vehicles an animal is exposed to during a journey. Animals should be segregated by species and production stage or age avoiding using the same vehicle for animals transported to farms and the slaughterhouse within the same trip. Adequate animal densities in the vehicle should be foreseen.
-
○animal segregation will limit animal contact and thus, by extension, potential AMR transmission.
-
○establishing measures that ensure the thermal comfort of the animals during transport will have direct or indirect impact in reducing ARB/ARGs transmission. Therefore, the microclimatic conditions during transport should be adjusted. Vehicles and/ or crates should be designed accordingly. Moreover, the most appropriate times of the day should be selected for the travel.
-
○
- 66–100% certain (extremely likely to likely, depending on the measure taken) that:
-
○implementation of measures directed against the risk factors associated with the use of assembly centres and control posts will limit the probability of transmission of ARB/ARGs. The measures recommended above with regards to animal handling, stocking densities, mixing/segregating animals and facilities cleaning and disinfection, including its validation, are also relevant to mitigate AMR transmission in these places.
-
○implementation of the mitigations mentioned in the point above as well as limiting lairage time to the minimum required, will reduce the probability of transmission of ARB/ARGs in lairage.
-
○
- 66–90% certain (likely) that:
-
○reducing stock densities and number of animals in contact will have a beneficial effect reducing the probability of ARB/ARGs transmission in absence of other factors (i.e. sick animals, cleaning).
-
○depending on the ‘sickness’, avoiding the transportation of sick animals will contribute to decrease the levels of ARB/ARGs transmission during transport.
-
○
The effect of providing adequate bedding as mitigation strategy is considered 33%–66% certain (as likely as not). There are factors which may mitigate ARB/ARGs transmission and others which may increase such transmission. Thus, during shorter journeys bedding may have hygienic benefits by soaking up liquid. During longer journeys the capacity of the bedding to soak up liquid is likely to be overwhelmed. On the other hand, bedding increases the effort needed to clean vehicles after transport thereby potentially increasing the risk of inadequate cleaning and disinfection. Likewise, bedding may provoke ingestion of bacteria, thereby promoting uptake of resistant bacteria from the contaminated environment.
Based on the uncertainties associated with the risk for AMR transmission linked to feeding management measures (feed withdrawal and alternative substances to antimicrobials), with the data available, no specific mitigation measures in relation to feed on AMR are proposed.
ToR3, What are the current data gaps and what are the most urgent data needs to support the analysis of the correlation between the main risk factors identified above and the spread of food‐borne zoonotic and indicator ARB/ARGs between food‐producing animals during transport and lairage?
AQ3: What are the knowledge gaps required to assess the contribution of the risk factors identified in ToR1, the mitigation measures identified in ToR2 and to identify any factors not covered by existing studies?
The most important data gaps on ARB/ARGs transmission during transport are considered to be:
the quantification of the relative contribution of the risk factors and proposed mitigation options;
the effect of fasting prior to and during transport, and the duration of this fasting on AMR in the microbiota (increase/decrease of ARB, gene transfer), shedding and transmission of the most important bacterial species commonly exhibiting AMR;
the effectiveness of different cleaning and disinfection protocols to reduce / eliminate ARB/ARGs;
the direction of the association between transport‐related stressors and ARB/ARGs transmission;
the effect of type and amount of bedding;
knowledge on the dynamics of ARB/ARGs transmission over time during transport and during lairage;
definition of AMR criteria and the best indicators for each criterion upon which the transport logistics could be organised (see Section 3.3.3);
the possible contribution of the airborne route during transport and lairage;
the impact on ARB/ARGs transmission of mechanical vs. manual catching/loading of animals;
the contribution of the health status, e.g. on shedding of ARB/ARGs, the susceptibility to colonisation or infection by ARB, and/or transmission of ARGs;
-
the effect of interventions using alternative substances to antimicrobials to mitigate transmission of ARB/ARGs.
AQ4: Which are the most urgent and longer‐term research requirements needed to fill the data gaps?
The research needs identified as the most urgent for the different animal categories were:
The assessment of the impact of animal transportation compared to the contribution of other stages of the food‐production chain, as a contributor to dissemination of AMR between farms, and/or to contamination of meat at slaughter.
- Studies quantifying the effect on transmission of ARB/ARGs during transport by:
-
○feed withdrawal, considering ARB transmission between animals and horizontal gene transfer between bacteria within such animals. Such studies should also address the effect of duration of feed withdrawal;
-
○cleaning and disinfection procedures for loading and unloading areas, transport vehicles, cages, assembly centres, control posts and lairage. Identification of the best methods and approaches to assess their efficacy are required;
-
○various stressors individually and in combination considering transmission of ARB between animals and horizontal gene transfer;
-
○the type and amount of bedding used during the journey, or at control posts, assembly centres and/or lairage;
-
○airborne transmission of ARB/ARGs in transport vehicles;
-
○mechanical vs. manual catching/loading of animals;
-
○the animal health status during transport, and its effect on, e.g. shedding of ARB/ARGs, the susceptibility to colonisation or infection by ARB, and/or transmission of ARGs;
-
○the interplay of duration with all risk factors during transport and journey breaks. Those studies should include the determination of the time‐lag between uptake of ARB and faecal shedding and subsequent transmission of such bacteria under transport and lairage conditions.
-
○
To define the criteria, and the best indicators for each criterion, that could be used for transport logistics.
For a longer term:
- Studies on intervention strategies to help mitigate:
-
○the effect of stressors with a demonstrated impact on ARB/ARGs transmission, e.g. by testing different environmental management strategies – facility design, ventilation, management and handling;
-
○possible hygienic problems of transport and lairage related with surface materials and improvement of facilities design;
-
○the mixing/contact of animals during transport, at animal assembly centres, control posts and lairage (e.g. improvements in the facilities design);
-
○
To assess the effect of interventions using alternative substances to antimicrobials to lessen the probability of transmission of ARB/ARGs in animal transportation settings;
To define the influence of AMR acquisition on survival and persistence of bacteria in animal transportation settings – e.g. increased/decreased colonisation proficiency.
To develop dynamic models allowing a more comprehensive and accurate estimation of risks related to ARB/ARGs transmission during animal transport and the effects of potential interventions.
Abbreviations
- AHAW Panel
EFSA Panel on Animal Health and Animal Welfare Panel
- AmpC
AmpC β‐lactamases
- AMR
antimicrobial resistance
- AMU
antimicrobial use
- ANIT
Committee of inquiry on the protection of animals during transport
- AQ
Assessment Questions
- ARB
antimicrobial‐resistant bacteria
- ARG
antimicrobial resistance genes
- AST
antimicrobial susceptibility testing
- BIOHAZ Panel
EFSA Panel on Biological Hazards
- BVL
Bundesamt für Verbraucherschutz und Lebensmittelsicherheit/The Federal Office of Consumer Protection and Food Safety
- CI
coefficient interval
- CP‐producing
carbapenemase‐producing microorganisms
- CPEs
carbapenemase‐producing Enterobacteria
- Destatis
German Federal Statistical Office
- ECDC
European Centre for Disease Prevention and Control
- EFTA
European Free Trade Association
- EMA
European Medicines Agency
- ENVI Committee
Committee on the Environment, Public Health and Food Safety
- ESBL(s)
extended spectrum beta‐lactamase(s)
- ESKAPE
ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species)
- FAO
Food and Agriculture Organization of the United Nations
- GGP
great grandparents (poultry generation)
- GP
grandparents (poultry generation)
- ISTAT
Italian National Institute of Statistics
- LA‐MRSA
livestock‐associated methicillin‐resistant Staphylococcus aureus
- MDR
multidrug resistant
- MRSA
methicillin‐resistant Staphylococcus aureus
- MSs
Member States
- PFGE
pulsed‐field gel electrophoresis
- PTAS
plasma total antioxidant status
- QMRA
quantitative microbial risk assessment
- ROMs
reactive oxygen metabolites
- RONAFA
EMA and EFSA joint Scientific Opinion on measures to reduce the need to use antimicrobials agents in animal husbandry in the EU and the resulting impact on food safety
- RR
relative risk
- SOP
standard operating procedure
- STEC
Shiga toxin‐producing Escherichia coli
- TADD
thermo‐assisted drying decontamination method
- ToRs
Terms of Reference
- TRACES
Trade Control and Expert System
- USDA
United States Department of Agriculture
- UV light
ultraviolet light
- VRE
vancomycin‐resistant Enterococci
- WB
World Bank
- WG
Working Group
- WGS
whole genome sequencing
- WOAH
World Organisation for Animal Health
Appendix A – Search strings
Generic searches (PubMed, Web of Science):
((Animal transport) AND (antimicrobial resistance OR antibiotic resistance)), 7397 hits (most of them not useful)
(((shedding) AND (animal)) AND (transport)) AND (bacteria). 220 hits; 46 of possible interest
Specific targeted searches
Combinations of animal species AND resistance terms AND bacteria or genes AND transport related terms (see Table A.1).
Table A.1.
Search strings used in targeted searches combining animal species‐, resistance‐, bacterial species‐, resistance‐gene‐ and transport‐related terms
| Animal species | Resistance terms | Bacteria or genes | Transport | N° hits PubMed | N° hits Web of Science | |||
|---|---|---|---|---|---|---|---|---|
| "Cattle"[Mesh] OR calves[tiab] OR cattle[tiab] OR cow[tiab] OR cows[tiab] OR bovine[tiab] | AND | "Drug Resistance, Microbial"[Mesh] OR "antibiotic resistan*"[tiab] OR "resistan*"[tiab] OR "antimicrobial resistan*"[tiab] OR AMR[tiab] OR "drug resistan*"[tiab] OR multidrug resistan*[tiab] OR MDR[tiab] | AND | "Acinetobacter"[Mesh] OR "Acinetobacter Infections"[Mesh] OR "Campylobacter Infections"[Mesh] OR "Campylobacter"[Mesh] OR "Klebsiella"[Mesh] OR "Klebsiella Infections"[Mesh] OR "Enterobacteriaceae"[Mesh] OR "Enterobacteriaceae Infections"[Mesh] OR "Enterococcus"[Mesh] OR "Escherichia"[Mesh] OR "Escherichia coli Infections"[Mesh] OR "Pseudomonas"[Mesh] OR "Pseudomonas Infections"[Mesh] OR "Salmonella"[Mesh] OR "Salmonella Infections, Animal"[Mesh] OR "Staphylococcus"[Mesh] OR "Staphylococcal Infections"[Mesh] OR "Streptococcus"[Mesh] OR "Streptococcal Infections"[Mesh] OR Acinetobacter[tiab] OR Campylobacter[tiab] OR carbapenemase[tiab] OR Klebsiella[tiab] OR Enterobacteriaceae[tiab] OR Enterococc*[tiab] OR Escherichia[tiab] OR "E coli"[tiab] OR ecoli[tiab] OR Pseudomonas[tiab] OR Salmonell*[tiab] OR Staphylococc*[tiab] OR Streptococc*[tiab] OR arm[tiab] OR cfr[tiab] OR ESBL[tiab] OR mcr[tiab] OR MRSA[tiab] OR optr[tiab] OR vga[tiab] OR VRE[tiab] OR methylase[tiab] | AND | "Transportation"[Mesh] OR automobile*[tiab] OR boat[tiab] OR boats[tiab] OR car[tiab] OR cars[tiab] OR lorry[tiab] OR lorries[tiab] OR ship[tiab] OR ships[tiab] OR train[tiab] OR trains[tiab] OR transport[tiab] OR transportation*[tiab] OR transporting[tiab] OR transports[tiab] OR transported[tiab] OR truck[tiab] OR trucks[tiab] OR vehicle*[tiab] | 121 | 179 |
| "Swine"[Mesh] OR piglet[tiab] OR sow [tiab] OR porcine[tiab] OR pig[tiab] | 142 | 170 | ||||||
| “broiler*”[tiab] OR poultry[tiab] OR chicken[tiab] OR lay*[tiab] OR hen[tiab] OR hens[tiab] OR turkey*[tiab] OR chick*[tiab] OR hatch*[tiab] | 189 | 448 | ||||||
| lairage[tiab] OR slaughterhouse[tiab] | – | – | – | – | – | – | 3,885 | 8,904 |
| "Lairage"[Mesh] OR lairage[tiab] | AND | – | – | 25 | 34 |
Other search terms were used alone or in combination with the animal species and/or transportation terms included: lairage OR slaughterhouse (Table A.1), risk factors (e.g. temperature), mitigation (awareness, surveillance, control, cleaning and disinfection), microbiome, resistome, metabolome linked to stress and/or resistance bacteria terms.
Searches done by AHAW Panel for EFSA AHAW Panel, 2022a,b,c (e.g. heat stress OR hyperthermia OR cold stress OR hypothermia OR thermal stress OR hunger OR starvation OR thirst OR dehydration) were revised, and publications of possible interest were also considered for the assessment.
Annex A – Protocol for the assessment of the transmission of antimicrobial resistance (AMR) during animal transport
Annex A can be found in the online version of this output (‘Supporting Information’ section): https://doi.org/10.2903/j.efsa.2022.7586
Supporting information
Protocol for the assessment of the transmission of antimicrobial resistance (AMR) during animal transport
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Álvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Ru G, Simmons M, Skandamis P, Suffredini E, Argüello‐Rodríguez H, Dohmen W, Magistrali CF, Padalino B, Tenhagen B‐A, Threlfall J, García‐Fierro R, Guerra B, Liébana E, Stella P and Peixe L, 2022. Scientific Opinion on the transmission of antimicrobial resistance (AMR) during animal transport. EFSA Journal 2022;20(10):7586, 83 pp. 10.2903/j.efsa.2022.7586
Requestor: European Parliament
Question number: EFSA‐Q‐2021‐00435
Panel members: Ana Allende, Avelino Álvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Konstantinos Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Declarations of interest: If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements: The Panel wishes to thank the former EFSA trainee Pilar García Bello for her contributions to this scientific output, as well as to the EFSA staff Gianluca Rossi for the graphic design of the figures included, Sean Ashe and Denise Candiani for their advice and liaison with the AHAW Panel, Gina Cioacata, and Kateryna Chuzhakina (EFSA seconded National Expert from the State Service of Ukraine on Food Safety and Consumer Protection, SSUFSCP), for their support.
Adopted: 28 September 2022
Notes
Commission Implementing Decision (EU) 2020/1729 of 17 November 2020 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria and repealing Implementing Decision 2013/652/EU. OJ L 387, 19.11.2020, pp. 8–21.
Council Regulation (EC) No 1/2005 of 22 December 2004 on the protection of animals during transport and related operations and amending Directives 64/432/EEC and 93/119/EC and Regulation (EC) No 1255/97, OJ L 3, 5.1.2005, pp. 1–44.
Commission Implementing Decision (EU) 2020/1729 of 17 November 2020 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria and repealing Implementing Decision 2013/652/EU. OJ L 387, 19.11.2020, pp. 8–21.
Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC. OJ L 4, 7.1.2019, pp. 43–167.
Council Regulation (EC) No 1255/97 of 25 June 1997 concerning Community criteria for staging points and amending the route plan referred to in the Annex to Directive 91/628/EEC. OJ L 174, 2.7.1997, pp. 1–6.
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. OJ L 325, 12.12.2003, pp. 31–40.
Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. OJ L 139, 30.4.2004, pp. 55–205. Consolidated text: Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. http://data.europa.eu/eli/reg/2004/853/2021-10-28
References
- Abdalla SE, Abia ALK, Amoako DG, Perrett K, Bester LA and Essack SY, 2021. From Farm‐to‐Fork: E. coli from an intensive pig production system in South Africa shows high resistance to critically important antibiotics for human and animal use. Antibiotics, 10. 10.3390/antibiotics10020178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo‐Al‐Ela HG, El‐Kassas S, El‐Naggar K, Abdo SE, Jahejo AR and Al Wakeel RA, 2021. Stress and immunity in poultry: light management and nanotechnology as effective immune enhancers to fight stress. Cell Stress & Chaperones, 26, 457–472. 10.1007/s12192-021-01204-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson CI and Kieson E, 2016. Conditioning methods for animals in agriculture: a review. Ciência Animal Brasileira, 17, 359–375. 10.1590/1089-6891v17i341981 [DOI] [Google Scholar]
- Abu‐Dieyeh ZHM, 2006. Effect of chronic heat stress and long‐term feed restriction on broiler performance. International Journal of Poultry Science, 5, 185–190. 10.3923/ijps.2006.185.190 [DOI] [Google Scholar]
- AbuOun M, Jones H, Stubberfield E, Gilson D, Shaw LP, Hubbard ATM, Chau KK, Sebra R, Peto TEA, Crook DW, Read DS, Gweon HS, Walker AS, Stoesser N, Smith RP, Anjum MF and The Rehab C , 2021. A genomic epidemiological study shows that prevalence of antimicrobial resistance in Enterobacterales is associated with the livestock host, as well as antimicrobial usage. Microbial Genomics, 7. 10.1099/mgen.0.000630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aires‐de‐Sousa M, 2017. Methicillin‐resistant Staphylococcus aureus among animals: current overview. Clinical Microbioly and Infection, 23, 373–380. 10.1016/j.cmi.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Amoako DG, Somboro AM, Abia ALK, Molechan C, Perrett K, Bester LA and Essack SY, 2020. Antibiotic resistance in Staphylococcus aureus from poultry and poultry products in uMgungundlovu district, South Africa, using the "Farm to Fork" approach. Microbial Drug Resistance, 26, 402–411. 10.1089/mdr.2019.0201 [DOI] [PubMed] [Google Scholar]
- Andreatti Filho RL, Milbradt EL, Okamoto AS, Silva TM, Vellano IHB, Gross LS, Oro CS and Hataka A, 2019. Salmonella Enteritidis infection, corticosterone levels, performance and egg quality in laying hens submitted to different methods of molting. Poultry Science, 98, 4416–4425. 10.3382/ps/pez248 [DOI] [PubMed] [Google Scholar]
- Antimicrobial Resistance Collaborators , 2022. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet, 399, 629–655. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo G, Yunta C, Terre M, Mereu A, Ipharraguerre I and Bach A, 2015. Intestinal permeability and incidence of diarrhea in newborn calves. Journal of Dairy Science, 98, 7309–7317. 10.3168/jds.2015-9666 [DOI] [PubMed] [Google Scholar]
- Argudín MA, Tenhagen BA, Fetsch A, Sachsenröder J, Kasböhrer A, Schroeter A, Hammerl JA, Hertwig S, Helmuth R, Braunig J, Mendoza MC, Appel B, Rodicio MR and Guerra B, 2011. Virulence and resistance determinants of German Staphylococcus aureus ST398 isolates from nonhuman sources. Applied and Environmental Microbiology, 77, 3052–3060. 10.1128/AEM.02260-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argüello H, Carvajal A, Álvarez‐Ordóñez A, Jaramillo‐Torres HA and Rubio P, 2014. Effect of logistic slaughter on Salmonella contamination on pig carcasses. Food Research International, 55, 77–82. 10.1016/j.foodres.2013.10.041 [DOI] [Google Scholar]
- Arthur TM, Bosilevac JM, Brichta‐Harhay DM, Kalchayanand N, King DA, Shackelford SD, Wheeler TL and Koohmaraie M, 2008. Source tracking of Escherichia coli O157:H7 and Salmonella contamination in the lairage environment at commercial U.S. beef processing plants and identification of an effective intervention. Journal of Food Protection, 71, 1752–1760. 10.4315/0362-028x-71.9.1752 [DOI] [PubMed] [Google Scholar]
- Arthur TM, Brichta‐Harhay DM, Bosilevac JM, Kalchayanand N, Shackelford SD, Wheeler TL and Koohmaraie M, 2010. Super shedding of Escherichia coli O157:H7 by cattle and the impact on beef carcass contamination. Meat Science, 86, 32–37. 10.1016/j.meatsci.2010.04.019 [DOI] [PubMed] [Google Scholar]
- Artuso‐Ponte V, Moeller S, Rajala‐Schultz P, Medardus JJ, Munyalo J, Lim K and Gebreyes WA, 2015. Supplementation with quaternary benzo(c)phenanthridine alkaloids decreased salivary cortisol and Salmonella shedding in pigs after transportation to the slaughterhouse. Foodborne Pathogens and Disease, 12, 891–897. 10.1089/fpd.2015.2009 [DOI] [PubMed] [Google Scholar]
- Atlaw NA, Keelara S, Correa M, Foster D, Gebreyes W, Aidara‐Kane A, Harden L, Thakur S and Fedorka‐Cray PJ, 2022. Evidence of sheep and abattoir environment as important reservoirs of multidrug resistant Salmonella and extended‐spectrum beta‐lactamase Escherichia coli . International Journal of Food Microbiology, 363, 109516. 10.1016/j.ijfoodmicro.2021.109516 [DOI] [PubMed] [Google Scholar]
- Averós X, Knowles TG, Brown SN, Warriss PD and Gosálvez LF, 2010. Factors affecting the mortality of weaned piglets during commercial transport between farms. Veterinary Record, 167, 815–819. 10.1136/vr.c6226 [DOI] [PubMed] [Google Scholar]
- Baghshani H, Nazifi S, Saeb M and Saeb S, 2010. Influence of road transportation on plasma concentrations of acute phase proteins, including fibrinogen, haptoglobin, serum amyloid A, and ceruloplasmin, in dromedary camels (Camelus dromedarius). Comparative Clinical Pathology, 19, 193–198. 10.1007/s00580-009-0839-2 [DOI] [Google Scholar]
- Bai H, He LY, Wu DL, Gao FZ, Zhang M, Zou HY, Yao MS and Ying GG, 2022. Spread of airborne antibiotic resistance from animal farms to the environment: dispersal pattern and exposure risk. Environment International, 158, 106927. 10.1016/j.envint.2021.106927 [DOI] [PubMed] [Google Scholar]
- Baker KL, Thomas PR, Karriker LA, Ramirez A, Zhang J, Wang C and Holtkamp DJ, 2017. Evaluation of an accelerated hydrogen peroxide disinfectant to inactivate porcine epidemic diarrhea virus in swine feces on aluminum surfaces under freezing conditions. BMC Veterinary Research, 13, 372. 10.1186/s12917-017-1300-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkeren E, Huisman JS, Fattinger SA, Hausmann A, Furter M, Egli A, Slack E, Sellin ME, Bonhoeffer S, Regoes RR, Diard M and Hardt WD, 2019. Salmonella persisters promote the spread of antibiotic resistance plasmids in the gut. Nature, 573, 276–280. 10.1038/s41586-019-1521-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard J, Haenni M, Gay E, Glaser P, Madec JY, Temime L and Opatowski L, 2021. Drivers of ESBL‐producing Escherichia coli dynamics in calf fattening farms: a modelling study. One Health, 12, 100238. 10.1016/j.onehlt.2021.100238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss PA and Hinton MH, 1990. Transportation of broilers with special reference to mortality‐rates. Applied Animal Behaviour Science, 28, 93–118. 10.1016/0168-1591(90)90048-I [DOI] [Google Scholar]
- Becker J, Perreten V, Steiner A, Stucki D, Schupbach‐Regula G, Collaud A, Rossano A, Wuthrich D, Muff‐Hausherr A and Meylan M, 2022. Antimicrobial susceptibility in E. coli and Pasteurellaceae at the beginning and at the end of the fattening process in veal calves: comparing ‘outdoor veal calf’ and conventional operations. Veterinary Microbiology, 269(109419). 10.1016/j.vetmic.2022.109419 [DOI] [PubMed] [Google Scholar]
- Beloeil PA, Fravalo P, Fablet C, Jolly JP, Eveno E, Hascoet Y, Chauvin C, Salvat G and Madec F, 2004. Risk factors for Salmonella enterica subsp. enterica shedding by market‐age pigs in French farrow‐to‐finish herds. Preventive Veterinary Medicine, 63, 103–120. 10.1016/j.prevetmed.2004.01.010 [DOI] [PubMed] [Google Scholar]
- Benincasa NC, Sakamoto KS, da Silva IJO and Lobos CMV, 2020. Animal welfare: impacts of pre‐slaughter operations on the current poultry industry. Journal of Animal Behaviour and Biometeorology, 8, 104–110. 10.31893/jabb.20014 [DOI] [Google Scholar]
- Bernardini D, Gerardi G, Peli A, Nanni Costa L, Amadori M and Segato S, 2012. The effects of different environmental conditions on thermoregulation and clinical and hematological variables in long‐distance road‐transported calves. Journal of Animal Science, 90, 1183–1191. 10.2527/jas.2011-4113 [DOI] [PubMed] [Google Scholar]
- Bhatt N, Singh N, Mishra A, Kandpal D, Rajneesh R and Jamwal S, 2021. A detailed review of transportation stress in livestock and its mitigation techniques. International Journal of Livestock Research, 11, 30–41. 10.5455/ijlr.20201109102902 [DOI] [Google Scholar]
- Bigras‐Poulin M, Barfod K, Mortensen S and Greiner M, 2007. Relationship of trade patterns of the Danish swine industry animal movements network to potential disease spread. Preventive Veterinary Medicine, 80, 143–165. 10.1016/j.prevetmed.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Boivin X, Lensink J, Tallet C and Veissier I, 2003. Stockmanship and farm animal welfare. Animal Welfare, 12, 479–492. [Google Scholar]
- Bokma J, Boone R, Deprez P and Pardon B, 2020. Short communication: herd‐level analysis of antimicrobial use and mortality in veal calves: do herds with low usage face higher mortality? Journal of Dairy Science, 103, 909–914. 10.3168/jds.2019-16764 [DOI] [PubMed] [Google Scholar]
- Boniotti MB, Papetti A, Bertasio C, Giacomini E, Lazzaro M, Cerioli M, Faccini S, Bonilauri P, Vezzoli F, Lavazza A and Alborali GL, 2018. Porcine epidemic diarrhoea virus in Italy: disease spread and the role of transportation. Transboundary and Emerging Diseases, 65, 1935–1942. 10.1111/tbed.12974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos ME, Verstappen KM, van Cleef BA, Dohmen W, Dorado‐García A, Graveland H, Duim B, Wagenaar JA, Kluytmans JA and Heederik DJ, 2016. Transmission through air as a possible route of exposure for MRSA. Journal of Exposure Science & Environmental Epidemiology, 26, 263–269. 10.1038/jes.2014.85 [DOI] [PubMed] [Google Scholar]
- Boughton C, Egan J, Kelly G, Markey B and Leonard N, 2007a. Rapid infection of pigs following exposure to environments contaminated with different levels of Salmonella typhimurium . Foodborne Pathogens and Disease, 4, 33–40. 10.1089/fpd.2006.58 [DOI] [PubMed] [Google Scholar]
- Boughton C, Egan J, Kelly G, Markey B and Leonard N, 2007b. Quantitative examination of Salmonella spp. in the lairage environment of a pig abattoir. Foodborne Pathogens and Disease, 4, 26–32. 10.1089/fpd.2006.57 [DOI] [PubMed] [Google Scholar]
- Bozzo G, Padalino B, Bonerba E, Barrasso R, Tufarelli V, Zappaterra M and Ceci E, 2020. Pilot study of the relationship between deck level and journey duration on plasma cortisol, epinephrine and norepinephrine levels in Italian heavy pigs. Animals, 10. 10.3390/ani10091578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broens EM, Graat EA, Vand der Wolf PJ, Van de Giessen AW and De Jong MC, 2011. Transmission of methicillin resistant Staphylococcus aureus among pigs during transportation from farm to abattoir. The Veterinary Journal, 189, 302–305. 10.1016/j.tvjl.2010.08.003 [DOI] [PubMed] [Google Scholar]
- Bronsvoort BM, Alban L and Greiner M, 2008. Quantitative assessment of the likelihood of the introduction of classical swine fever virus into the Danish swine population. Preventive Veterinary Medicine, 85, 226–240. 10.1016/j.prevetmed.2008.01.013 [DOI] [PubMed] [Google Scholar]
- Broom DM, 2008. The welfare of livestock during road transport. In: Cussen V and Garces L (eds.). Long Distance Transport and Welfare of Farm Animals. CABI, Wallingford. pp. 157–181. [Google Scholar]
- Buta‐Hubeny M, Korzeniewska E, Hubeny J, Zieliński W, Rolbiecki D, Harnisz M and Paukszto Ł, 2022. Structure of the manure resistome and the associated mobilome for assessing the risk of antimicrobial resistance transmission to crops. Science of the Total Environment, 808, 152144. 10.1016/j.scitotenv.2021.152144 [DOI] [PubMed] [Google Scholar]
- BVL (Bundesamt für Verbraucherschutz und Lebensmittelsicherheit) , 2015. BVL‐report 11.2, Berichte zur Lebensmittelsicherheit. Zoonosen‐Monitoring. Available online: https://www.bvl.bund.de/SharedDocs/Downloads/01_Lebensmittel/04_Zoonosen_Monitoring/Zoonosen_Monitoring_Bericht_2015.pdf?__blob=publicationFile&v=5 [Accessed: 2016]. [Google Scholar]
- Byrd JA, Corrier DE, Hume ME, Bailey RH, Stanker LH and Hargis BM, 1998. Effect of feed withdrawal on Campylobacter in the crops of market‐age broiler chickens. Avian Diseases, 42, 802–806. [PubMed] [Google Scholar]
- Caffrey NP, Dohoo IR and Cockram MS, 2017. Factors affecting mortality risk during transportation of broiler chickens for slaughter in Atlantic Canada. Preventive Veterinary Medicine, 147, 199–208. 10.1016/j.prevetmed.2017.09.011 [DOI] [PubMed] [Google Scholar]
- Callaway TR, Morrow JL, Edrington TS, Genovese KJ, Dowd S, Carroll J, Dailey JW, Harvey RB, Poole TL, Anderson RC and Nisbet DJ, 2006. Social stress increases fecal shedding of Salmonella typhimurium by early weaned piglets. Current Issues in Intestinal Microbiology, 7, 65–71. [PubMed] [Google Scholar]
- Capozzi V and Spano G, 2009. Horizontal gene transfer in the gut: is it a risk? Food Research International, 42, 1501–1502. 10.1016/j.foodres.2009.08.001 [DOI] [Google Scholar]
- Cartoni Mancinelli A, Dal Bosco A, Mattioli S, Ranucci D and Castellini C, 2018. Mobile poultry processing unit as a resource for small poultry farms: planning and economic efficiency, animal welfare, meat quality and sanitary implications. Animals, 8. 10.3390/ani8120229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassini A, Hogberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, Colomb‐Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, Ouakrim DA, Oliveira TC, Struelens MJ, Suetens C, Monnet DL and Burden of AMR Collaborative Group , 2019. Attributable deaths and disability‐adjusted life‐years caused by infections with antibiotic‐resistant bacteria in the EU and the European Economic Area in 2015: a population‐level modelling analysis. The Lancet Infectious Diseases, 19, 56–66. 10.1016/S1473-3099(18)30605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave JG, Callinan AP and Woonton WK, 2005. Mortalities in bobby calves associated with long distance transport. Australian Veterinary Journal, 83, 82–84. 10.1111/j.1751-0813.2005.tb12203.x [DOI] [PubMed] [Google Scholar]
- Cernicchiaro N, White BJ, Renter DG, Babcock AH, Kelly L and Slattery R, 2012. Associations between the distance traveled from sale barns to commercial feedlots in the United States and overall performance, risk of respiratory disease, and cumulative mortality in feeder cattle during 1997 to 2009. Journal of Animal Science, 90, 1929–1939. 10.2527/jas.2011-4599 [DOI] [PubMed] [Google Scholar]
- Cirone F, Padalino B, Tullio D, Capozza P, Lo Surdo M, Lanave G and Pratelli A, 2019. Prevalence of pathogens related to bovine respiratory disease before and after transportation in beef steers: preliminary results. Animals, 9. 10.3390/ani9121093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockram MS and Spence JY, 2012. The effects of driving events on the stability and resting behaviour of cattle, young calves and pigs. Animal Welfare, 21, 403–417. 10.7120/09627286.21.3.403 [DOI] [Google Scholar]
- Consortium of the Animal Transport Guides Project , 2018a. Guide to Good Practices for the Transport of the Poultry. Publications Office of the European Commission, Luxembourg: http://www.animaltransportguides.eu/wp-content/uploads/2021/02/EN-Guides-Poultry-final_2021.pdf [Google Scholar]
- Consortium of the Animal Transport Guides Project , 2018b. Guide to Good Practices for the Transport of Pigs. Publications Office of the European Commission, Luxembourg: http://animaltransportguides.eu/wp-content/uploads/2016/05/Guides-Pig-EC-Templ.pdf [Google Scholar]
- Consortium of the Animal Transport Guides Project , 2018c. Guide to Good Practices for the Transport of Cattlòe. Publications Office of the European Commission, Luxembourg: http://animaltransportguides.eu/wp-content/uploads/2016/05/Guides-Cattle-EC-Templ.pdf [Google Scholar]
- Corrier DE, Byrd JA, Hargis BM, Hume ME, Bailey RH and Stanker LH, 1999. Survival of Salmonella in the crop contents of market‐age broilers during feed withdrawal. Avian Diseases, 43, 453–460. [PubMed] [Google Scholar]
- Corry JE, Allen VM, Hudson WR, Breslin MF and Davies RH, 2002. Sources of Salmonella on broiler carcasses during transportation and processing: modes of contamination and methods of control. Journal of Applied Microbiology, 92, 424–432. 10.1046/j.1365-2672.2002.01543.x [DOI] [PubMed] [Google Scholar]
- Costa LN, Sapino M, Pippione S, Mattalia G, Saracco M, Di Trani S and Zanasi C, 2012. Risk assessment in stock calf transportation from France to Italy: the contribution of road inspections. Italian Journal of Animal Science, 11, e6. 10.4081/ijas.2012.e6 [DOI] [Google Scholar]
- Cray WC Jr, Casey TA, Bosworth BT and Rasmussen MA, 1998. Effect of dietary stress on fecal shedding of Escherichia coli O157:H7 in calves. Applied and Environmental Microbiology, 64, 1975–1979. 10.1128/AEM.64.5.1975-1979.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cray C, Zaias J and Altman NH, 2009. Acute phase response in animals: a review. Comparative Medicine, 59, 517–526. 10.1016/B978-0-12-394596-9.00005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescio MI, Mastrantonio G, Bertolini S, Maurella C, Adkin A, Ingravalle F, Simons RRL, DeNardi M, Stark K, Estrada‐Pena A and Ru G, 2021. Using network analysis to identify seasonal patterns and key nodes for risk‐based surveillance of pig diseases in Italy. Transboundary and Emerging Diseases, 68, 3541–3551. 10.1111/tbed.13960 [DOI] [PubMed] [Google Scholar]
- Dahl‐Pedersen K and Herskin MS, 2021. Transportation of cattle and pigs between EU Member States 2014–2018 – can data from TRACES be used to create overview and inform about potential welfare consequences? Journal of Applied Animal Welfare Science, 1–14. 10.1080/10888705.2021.1923491 [DOI] [PubMed] [Google Scholar]
- Davidson CM and Taylor M, 1978. Variability of E. coli levels in bovine feces and its implication on guidelines for ground beef. Canadian Institute of Food Science and Technology Journal, 11, 53. 10.1016/s0315-5463(78)73161-5 [DOI] [Google Scholar]
- Davies R and Wales A, 2019. Antimicrobial resistance on farms: a review including biosecurity and the potential role of disinfectants in resistance selection. Comprehensive Reviews in Food Science and Food Safety, 18, 753–774. 10.1111/1541-4337.12438 [DOI] [PubMed] [Google Scholar]
- Davies RH and Wray C, 1994. An approach to reduction of Salmonella infection in broiler chicken flocks through intensive sampling and identification of cross‐contamination hazards in commercial hatcheries. International Journal of Food Microbiology, 24, 147–160. 10.1016/0168-1605(94)90114-7 [DOI] [PubMed] [Google Scholar]
- De Busser EV, Dewulf J, Nollet N, Houf K, Schwarzer K, De Sadeleer L, De Zutter L and Maes D, 2009. Effect of organic acids in drinking water during the last 2 weeks prior to slaughter on Salmonella shedding by slaughter pigs and contamination of carcasses. Zoonoses and Public Health, 56, 129–136. 10.1111/j.1863-2378.2008.01172.x [DOI] [PubMed] [Google Scholar]
- De Busser EV, De Zutter L, Dewulf J, Houf K and Maes D, 2013. Salmonella control in live pigs and at slaughter. Veterinary Journal, 196, 20–27. 10.1016/j.tvjl.2013.01.002 [DOI] [PubMed] [Google Scholar]
- de Jong IC, van Voorst S, Ehlhardt DA and Blokhuis HJ, 2002. Effects of restricted feeding on physiological stress parameters in growing broiler breeders. British Poultry Science, 43, 157–168. 10.1080/00071660120121355 [DOI] [PubMed] [Google Scholar]
- Dee S, Torremorell M, Thompson B, Deen J and Pijoan C, 2005. An evaluation of thermo‐assisted drying and decontamination for the elimination of porcine reproductive and respiratory syndrome virus from contaminated livestock transport vehicles. Canadian Journal of Veterinary Research, 69, 58–63. [PMC free article] [PubMed] [Google Scholar]
- Depner RFR, Pontin KP, Otutumi LK, Westenhofen M, Borges KA, Furian TQ, do Nascimento VP and Lovato M, 2021. Antimicrobial activity of poultry hatch baskets containing copper inserts. Journal of Applied Poultry Research, 30, 100183. 10.1016/j.japr.2021.100183 [DOI] [Google Scholar]
- Destatis, 2019 . 2000. Land und Forstwirtschaft. Schlachttier‐ und Fleischuntersuchung, Fachserie 3 Reihe 4.3. 377 pp. Available online: https://www.destatis.de/DE/Themen/Branchen-Unternehmen/Landwirtschaft-Forstwirtschaft-Fischerei/Tiere-Tierische-Erzeugung/Publikationen/Downloads-Tiere-und-tierische-Erzeugung/fleischuntersuchung-hj-2030430185324.pdf?__blob=publicationFile
- Dewell GA, Simpson CA, Dewell RD, Hyatt DR, Belk KE, Scanga JA, Morley PS, Grandin T, Smith GC, Dargatz DA, Wagner BA and Salman MD, 2008a. Risk associated with transportation and lairage on hide contamination with Salmonella enterica in finished beef cattle at slaughter. Journal of Food Protection, 71, 2228–2232. 10.4315/0362-028x-71.11.2228 [DOI] [PubMed] [Google Scholar]
- Dewell GA, Simpson CA, Dewell RD, Hyatt DR, Belk KE, Scanga JA, Morley PS, Grandin T, Smith GC, Dargatz DA, Wagner BA and Salman MD, 2008b. Impact of transportation and lairage on hide contamination with Escherichia coli O157 in finished beef cattle. Journal of Food Protection, 71, 1114–1118. 10.4315/0362-028x-71.6.1114 [DOI] [PubMed] [Google Scholar]
- Di Martino G, Capello K, Stefani AL, Tripepi L, Garbo A, Speri M, Trolese M, Brichese M, Marangon S and Bonfanti L, 2017. The effect of crate height on the behavior of female turkeys during commercial pre‐slaughter transportation. Animal Science Journal, 88, 1651–1657. 10.1111/asj.12823 [DOI] [PubMed] [Google Scholar]
- Dierikx CM, van der Goot JA, Smith HE, Kant A and Mevius DJ, 2013. Presence of ESBL/AmpC‐producing Escherichia coli in the broiler production pyramid: a descriptive study. PLoS One, 8, e79005. 10.1371/journal.pone.0079005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen W, Dorado‐García A, Bonten MJ, Wagenaar JA, Mevius D and Heederik DJ, 2017a. Risk factors for ESBL‐producing Escherichia coli on pig farms: a longitudinal study in the context of reduced use of antimicrobials. PLoS One, 12, e0174094. 10.1371/journal.pone.0174094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen W, Schmitt H, Bonten M and Heederik D, 2017b. Air exposure as a possible route for ESBL in pig farmers. Environmental Research, 155, 359–364. 10.1016/j.envres.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Dorado‐García A, Smid JH, van Pelt W, Bonten MJM, Fluit AC, van den Bunt G, Wagenaar JA, Hordijk J, Dierikx CM, Veldman KT, de Koeijer A, Dohmen W, Schmitt H, Liakopoulos A, Pacholewicz E, Lam T, Velthuis AG, Heuvelink A, Gonggrijp MA, van Duijkeren E, van Hoek A, de Roda Husman AM, Blaak H, Havelaar AH, Mevius DJ and Heederik DJJ, 2018. Molecular relatedness of ESBL/AmpC‐producing Escherichia coli from humans, animals, food and the environment: a pooled analysis. The Journal of Antimicrobial Chemotherapy, 73, 339–347. 10.1093/jac/dkx397 [DOI] [PubMed] [Google Scholar]
- Dos Santos VM, Dallago BSL, Racanicci AMC, Santana AP and Bernal FEM, 2017. Effects of season and distance during transport on broiler chicken meat. Poultry Science, 96, 4270–4279. 10.3382/ps/pex282 [DOI] [PubMed] [Google Scholar]
- Dos Santos VM, Dallago BSL, Racanicci AMC, Santana AP, Cue RI and Bernal FEM, 2020. Effect of transportation distances, seasons and crate microclimate on broiler chicken production losses. PLoS One, 15, e0232004. 10.1371/journal.pone.0232004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzieciolowski T, Boqvist S, Rydén J and Hansson I, 2022. Cleaning and disinfection of transport crates for poultry – comparison of four treatments at slaughter plant. Poultry Science, 101, 101521. 10.1016/j.psj.2021.101521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LE and Hemsworth PH, 2021. The impact of management, husbandry and stockperson decisions on the welfare of laying hens in Australia. Animal Production Science, 61, 944–967. 10.1071/An19664 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2008. Foodborne antimicrobial resistance as a biological hazard – Scientific Opinion of the Panel on Biological Hazards. EFSA Journal, 2008;6:765, 87 pp. 10.2903/j.efsa.2008.765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2009. Analysis of the baseline survey on the prevalence of methicillin‐resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008, Part A: MRSA prevalence estimates; on request from the European Commission. EFSA Journal 2009;7:1376, 82 pp. 10.2903/j.efsa.2009.1376 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2010. Analysis of the baseline survey on the prevalence of methicillin‐resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008, Part B: factors associated with MRSA contamination of holdings; on request from the European Commission. EFSA Journal 2010;8:1597, 67 pp. 10.2903/j.efsa.2010.1597 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2012. Technical specifications on harmonised epidemiological indicators for biological hazards to be covered by meat inspection of poultry. EFSA Journal 2012;10:2764, 87 pp. 10.2903/j.efsa.2012.2764 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2019. Aerts M, Battisti A, Hendriksen R, Kempf I, Teale C, Tenhagen B‐A, Veldman K, Wasyl D, Guerra B, Liébana E, Thomas‐López D and Belœil P‐A. Scientific report on the technical specifications on harmonised monitoring of antimicrobial resistance in zoonotic and indicator bacteria from food‐producing animals and food. EFSA Journal 2019;17:5709, 122 pp. 10.2903/j.efsa.2019.5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Martino L, Aiassa E, Halldórsson TI, Koutsoumanis PK, Naegeli H, Baert K, Baldinelli F, Devos Y, Lodi F, Lostia A, Manini P, Merten C, Messens W, Rizzi V, Tarazona J, Titz A and Vos S, 2020. Draft framework for protocol development for EFSA's scientific assessments. EFSA supporting publication 2020:EN‐1843, 46 pp. 10.2903/sp.efsa.2020.EN-1843 [DOI]
- EFSA (European Food Safety Authority) , Aerts M, Battisti A, Hendriksen R, Larsen J, Nilsson O, Cortiñas Abrahantes J, Guerra B, Papanikolaou A and Beloeil PA, 2022. Technical specifications for a baseline survey on the prevalence of Methicillin Resistant Staphylococcus aureus (MRSA) in pigs. EFSA Journal 2022;20(10):7620, 33 pp. 10.2903/j.efsa.2022.7620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortazar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Spoolder H, Stahl K, Velarde A, Viltrop A, Winckler C, Dewulf J, Guardabassi L, Hilbert F, Mader R, Baldinelli F and Álvarez J, 2021a. Scientific Opinion on the assessment of animal diseases caused by bacteria resistant to antimicrobials: poultry. EFSA Journal 2021;19:7114, 47 pp. 10.2903/j.efsa.2021.7114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortazar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Stahl K, Velarde A, Viltrop A, Winckler C, Dewulf J, Guardabassi L, Hilbert F, Mader R, Baldinelli F and Álvarez J, 2021b. Scientific Opinion on the assessment of animal diseases caused by bacteria resistant to antimicrobials: swine. EFSA Journal 2021;19(12):7113, 66 pp. 10.2903/j.efsa.2021.7113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortazar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Spoolder H, Stahl K, Velarde A, Viltrop A, Winckler C, Dewulf J, Guardabassi L, Hilbert F, Mader R, Baldinelli F and Álvarez J, 2021c. Scientific Opinion on the assessment of animal diseases caused by bacteria resistant to antimicrobials: cattle. EFSA Journal 2021;19(12):6955, 89 pp. 10.2903/j.efsa.2021.6955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortazar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Roberts HC, Spoolder H, Stahl K, Viltrop A, Winckler C, Mitchell M, James Vinco L, Voslarova E, Candiani D, Mosbach‐Schulz O, Van der Stede Y and Velarde A, 2022a. Scientific Opinion on the welfare of domestic birds and rabbits transported in containers. EFSA Journal 2022;20(9):7441, 187 pp. 10.2903/j.efsa.2022.7441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortazar Schmidt C, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Spoolder H, Stahl K, Velarde A, Viltrop A, Winckler C, Earley B, Edwards S, Faucitano L, Marti S, Miranda de La Lama GC, Nanni Costa L, Thomsen PT, Ashe S, Mur L, Van der Stede Y and Herskin M, 2022b. Scientific Opinion on the welfare of pigs during transport. EFSA Journal 2022;20(9):7445, 108 pp. 10.2903/j.efsa.2022.7445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Álvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortazar Schmidt C, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Spoolder H, Stahl K, Velarde A, Viltrop A, Winckler C, Earley B, Edwards S, Faucitano L, Marti S, de La Lama GCM, Costa LN, Thomsen PT, Ashe S, Mur L, Van der Stede Y and Herskin M, 2022c. Welfare of cattle during transport. EFSA Journal 2022;20(9):7442, 121 pp. 10.2903/j.efsa.2022.7442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) , 2022. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2019–2020. EFSA Journal 2022;20(9):7209, 197 pp. 10.2903/j.efsa.2022.7209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2010. Scientific Opinion on a Quantitative Microbiological Risk Assessment of Salmonella in slaughter and breeder pigs. EFSA Journal 2010;8(4):1547, 90 pp. 10.2903/j.efsa.2010.1547 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2011a. Scientific Opinion on the public health risks of bacterial strains producing extended‐spectrum β‐lactamases and/or AmpC β‐lactamases in food and food‐producing animals. EFSA Journal 2011;9(8):2322, 95 pp. 10.2903/j.efsa.2011.2322 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2011b. Scientific Opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA Journal 2011;9(4):2105, 141 pp. 10.2903/j.efsa.2011.2105 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2013. Scientific Opinion on Carbapenem resistance in food animal ecosystems. EFSA Journal 2013;11(12):3501, 70 pp. 10.2903/j.efsa.2013.3501 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Álvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Alter T, Crotta M, Ellis‐Iversen J, Hempen M, Messens W, Chemaly M, 2020. Update and review of control options for Campylobacter in broilers at primary production. EFSA Journal 2022;18(4):6090, 89 pp. 10.2903/j.efsa.2020.6090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Álvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Ru G, Simmons M, Skandamis P, Suffredini E, Argüello H, Berendonk T, Cavaco LM, Gaze W, Schmitt H, Topp E, Guerra B, Liébana E, Stella P and Peixe L, 2021. Role played by the environment in the emergence and spread of antimicrobial resistance (AMR) through the food chain. EFSA Journal 2021;19(6):6651, 188 pp. 10.2903/j.efsa.2021.6651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee (European Food Safety Authority) , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018a. Guidance on uncertainty analysis in scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee (European Food Safety Authority) , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Germini A, Martino L, Merten C, Mosbach‐Schulz O, Smith A and Hardy A, 2018b. The principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher SD, Rostagno MH and Lay DC, 2017. Feed withdrawal and transportation effects on Salmonella enterica levels in market‐weight pigs. Journal of Animal Science, 95, 2848–2858. 10.2527/jas.2017.1454 [DOI] [PubMed] [Google Scholar]
- EMA and EFSA (European Medicines Agency and European Food Safety Authority) , 2017. EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFSA Journal 2017;15:4666, 245 pp. 10.2903/j.efsa.2017.4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers C, de Jong A, Prenger‐Berninghoff E, El Garch F, Leidner U, Tiwari SK and Semmler T, 2021. Genomic diversity and virulence potential of ESBL‐ and AmpC‐beta‐lactamase‐producing Escherichia coli strains from healthy food animals across Europe. Frontiers in Microbiology, 12, 626774. 10.3389/fmicb.2021.626774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WOAH/WB (Food and Agriculture Organization of the United Nations/World Organisation for Animal Health/World Bank) , 2010. Good practices for biosecurity in the pig sector – issues and options in developing and transition countries. FAO Animal Production and Health Paper No. 169. FAO, Rome. [Google Scholar]
- Farghly MFA, Mahrose KM, Galal AE, Ali RM, Ahmad EAM, Rehman ZU, Ullah Z and Ding C, 2018. Implementation of different feed withdrawal times and water temperatures in managing turkeys during heat stress. Poultry Science, 97, 3076–3084. 10.3382/ps/pey173 [DOI] [PubMed] [Google Scholar]
- Ferroni L, Albini E, Lovito C, Blasi F, Maresca C, Massacci FR, Orsini S, Tofani S, Pezzotti G, Díaz Vicuna E, Forte C and Magistrali CF, 2022. Antibiotic consumption is a major driver of antibiotic resistance in calves raised on Italian cow‐calf beef farms. Research in Veterinary Science, 145, 71–81. 10.1016/j.rvsc.2022.01.010 [DOI] [PubMed] [Google Scholar]
- Frazão N, Sousa A, Lässig M and Gordo I, 2019. Horizontal gene transfer overrides mutation in Escherichia coli colonizing the mammalian gut. Proceedings of the National Academy of Sciences of the United States of America, 116, 17906–17915. 10.1073/pnas.1906958116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeland G, Hettiarachchy N, Atungulu GG, Apple J and Mukherjee S, 2021. Strategies to combat antimicrobial resistance from farm to table. Food Reviews International. 10.1080/87559129.2021.1893744 [DOI] [Google Scholar]
- Friese A, Schulz J, Zimmermann K, Tenhagen BA, Fetsch A, Hartung J and Rösler U, 2013. Occurrence of livestock‐associated methicillin‐resistant Staphylococcus aureus in turkey and broiler barns and contamination of air and soil surfaces in their vicinity. Applied and Environmental Microbiology, 79, 2759–2766. 10.1128/AEM.03939-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gast RK and Porter RE, 2020. Salmonella infections. In: Swayne DE, Boulianne M, Logue CM, McDougald LR, Nair V, Suarez DL, de Wit S, Grimes T, Johnson D, Kromm M, Prajitno TY, Rubinoff I and Zavala G (eds). Diseases of Poultry, 14th Edition, Place Wiley, Wiley. 717–753 pp. [Google Scholar]
- Gay E, Bour M, Cazeau G, Jarrige N, Martineau C, Madec JY and Haenni M, 2019. Antimicrobial usages and antimicrobial resistance in commensal Escherichia coli from veal calves in France: evolution during the fattening process. Frontiers in Microbiology, 10, 792. 10.3389/fmicb.2019.00792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreyes WA, Davies PR, Turkson PK, Morrow WE, Funk JA, Altier C and Thakur S, 2004. Characterization of antimicrobial‐resistant phenotypes and genotypes among Salmonella enterica recovered from pigs on farms, from transport trucks, and from pigs after slaughter. Journal of Food Protection, 67, 698–705. 10.4315/0362-028x-67.4.698 [DOI] [PubMed] [Google Scholar]
- Giacomini E, Gasparrini S, Lazzaro M, Scali F, Boniotti MB, Corradi A, Pasquali P and Alborali GL, 2018. The role of transportation in the spread of Brachyspira hyodysenteriae in fattening farms. BMC Veterinary Research, 14, 10. 10.1186/s12917-017-1328-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson D, Martelli F, Jones H, Davies R and Smith R, 2019. Short duration acidified feed use as a pre‐slaughter Salmonella intervention. Safe Pork, 13, 152. [Google Scholar]
- Godden S, Bey R, Lorch K, Farnsworth R and Rapnicki P, 2008. Ability of organic and inorganic bedding materials to promote growth of environmental bacteria. Journal of Dairy Science, 91, 151–159. 10.3168/jds.2007-0415 [DOI] [PubMed] [Google Scholar]
- Goel A, 2021. Heat stress management in poultry. Journal of Animal Physiology and Animal Nutrition, 105, 1136–1145. 10.1111/jpn.13496 [DOI] [PubMed] [Google Scholar]
- Gómez P, Aspiroz C, Hadjirin NF, Benito D, Zarazaga M, Torres C and Holmes MA, 2020. Simultaneous nasal carriage by methicillin‐resistant and methicillin susceptible Staphylococcus aureus of lineage ST398 in a live pig transporter. Pathogens, 9. 10.3390/pathogens9050401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González LA, Schwartzkopf‐Genswein KS, Bryan M, Silasi R and Brown F, 2012. Relationships between transport conditions and welfare outcomes during commercial long haul transport of cattle in North America. Journal of Animal Science, 90, 3640–3651. 10.2527/jas.2011-4796 [DOI] [PubMed] [Google Scholar]
- Gosling RJ, 2018. A review of cleaning and disinfection studies in farming environments. Livestock, 23, 232–237. 10.12968/live.2018.23.5.232 [DOI] [Google Scholar]
- Grandin T, 2019. Livestock handling and transport. CABI, Wallingford. [Google Scholar]
- Grandin T, 2020. The importance of good pre‐slaughter handling to improve meat quality in cattle, pigs, sheep and poultry. The slaughter of farmed animals: practical ways of enhancing animal welfare. CABI, Wallingford. [Google Scholar]
- Grau FH, Brownlie LE and Roberts EA, 1968. Effect of some preslaughter treatments on the Salmonella population in the bovine rumen and faeces. The Journal of Applied Bacteriology, 31, 157–163. 10.1111/j.1365-2672.1968.tb00353.x [DOI] [PubMed] [Google Scholar]
- Grau FH, Brownlie LE and Smith MG, 1969. Effects of food intake on numbers of salmonellae and Escherichia coli in rumen and faeces of sheep. The Journal of Applied Bacteriology, 32, 112–117. 10.1111/j.1365-2672.1969.tb02195.x [DOI] [PubMed] [Google Scholar]
- Greening SS, Zhang J, Midwinter AC, Wilkinson DA, Fayaz A, Williamson DA, Anderson MJ, Gates MC and French NP, 2021. Transmission dynamics of an antimicrobial resistant Campylobacter jejuni lineage in New Zealand's commercial poultry network. Epidemics, 37, 100521. 10.1016/j.epidem.2021.100521 [DOI] [PubMed] [Google Scholar]
- Grønstøl H, Osborne AD and Pethiyagoda S, 1974. Experimental Salmonella infection in calves. 2. Virulence and the spread of infection. Journal of Hygiene, 72, 163–168. 10.1017/s0022172400023354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grøntvedt CA, Elstrøm P, Stegger M, Skov RL, Skytt Andersen P, Larssen KW, Urdahl AM, Angen Ø, Larsen J, Åmdal S, Løtvedt SM, Sunde M and Bjørnholt JV, 2016. Methicillin‐resistant Staphylococcus aureus CC398 in humans and pigs in Norway: a "One Health" perspective on introduction and transmission. Clinical Infectious Diseases, 63, 1431–1438. 10.1093/cid/ciw552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney DG, Fang FC, Krause M, Libby S, Buchmeier NA and Fierer J, 1995. Biology and clinical significance of virulence plasmids in Salmonella serovars. Clinical Infectious Diseases, 21(Suppl 2), S146–S151. 10.1093/clinids/21.supplement_2.s146 [DOI] [PubMed] [Google Scholar]
- Haley BJ, Kim SW, Salaheen S, Hovingh E and Van Kessel JAS, 2020. Differences in the microbial community and resistome structures of feces from preweaned calves and lactating dairy cows in commercial dairy herds. Foodborne Pathogens and Disease, 17, 494–503. 10.1089/fpd.2019.2768 [DOI] [PubMed] [Google Scholar]
- Han Y, Zhou ZC, Zhu L, Wei YY, Feng WQ, Xu L, Liu Y, Lin ZJ, Shuai XY, Zhang ZJ and Chen H, 2019. The impact and mechanism of quaternary ammonium compounds on the transmission of antibiotic resistance genes. Environmental Science and Pollution Research International, 26, 28352–28360. 10.1007/s11356-019-05673-2 [DOI] [PubMed] [Google Scholar]
- Hargis BM, Caldwell DJ, Brewer RL, Corrier DE and Deloach JR, 1995. Evaluation of the chicken crop as a source of Salmonella contamination for broiler carcasses. Poultry Science, 74, 1548–1552. 10.3382/ps.0741548 [DOI] [PubMed] [Google Scholar]
- Harmon BG, Brown CA, Tkalcic S, Mueller PO, Parks A, Jain AV, Zhao T and Doyle MP, 1999. Fecal shedding and rumen growth of Escherichia coli O157:H7 in fasted calves. Journal of Food Protection, 62, 574–579. 10.4315/0362-028x-62.6.574 [DOI] [PubMed] [Google Scholar]
- Hastings R, Colles FM, McCarthy ND, Maiden MC and Sheppard SK, 2011. Campylobacter genotypes from poultry transportation crates indicate a source of contamination and transmission. Journal of Applied Microbiology, 110, 266–276. 10.1111/j.1365-2672.2010.04883.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JR, English LL, Carr LE, Wagner DD and Joseph SW, 2004. Multiple‐antibiotic resistance of Enterococcus spp. isolated from commercial poultry production environments. Applied and Environmental Microbiology, 70, 6005–6011. 10.1128/AEM.70.10.6005-6011.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman L, Heyndrickx M, Grijspeerdt K, Vandekerchove D, Rollier I and De Zutter L, 2003. Routes for Campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiology and Infection, 131, 1169–1180. 10.1017/s0950268803001183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertogs K, Heyndrickx M, Gelaude P, De Zutter L, Dewulf J and Rasschaert G, 2021. The effect of partial depopulation on Campylobacter introduction in broiler houses. Poultry Science, 100, 1076–1082. 10.1016/j.psj.2020.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille K, Felski M, Ruddat I, Woydt J, Schmid A, Friese A, Fischer J, Sharp H, Valentin L, Michael GB, Hormansdörfer S, Messelhäusser U, Seibt U, Honscha W, Guerra B, Schwarz S, Rosler U, Kasböhrer A and Kreienbrock L, 2018. Association of farm‐related factors with characteristics profiles of extended‐spectrum beta‐lactamase‐/plasmid‐mediated AmpC beta‐lactamase‐producing Escherichia coli isolates from German livestock farms. Veterinary Microbiology, 223, 93–99. 10.1016/j.vetmic.2018.07.022 [DOI] [PubMed] [Google Scholar]
- Hitch TCA, Thomas BJ, Friedersdorff JCA, Ougham H and Creevey CJ, 2018. Deep sequence analysis reveals the ovine rumen as a reservoir of antibiotic resistance genes. Environmental Pollution, 235, 571–575. 10.1016/j.envpol.2017.12.067 [DOI] [PubMed] [Google Scholar]
- Holman DB, Gzyl KE, Mou KT and Allen HK, 2021. Weaning age and its effect on the development of the swine gut microbiome and resistome. mSystems, 6, e0068221. 10.1128/mSystems.00682-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotes S, Traulsen I and Krieter J, 2011. Salmonella control measures with special focus on vaccination and logistic slaughter procedures. Transboundary and Emerging Diseases, 58, 434–444. 10.1111/j.1865-1682.2011.01226.x [DOI] [PubMed] [Google Scholar]
- Hoyle DV, Knight HI, Shaw DJ, Hillman K, Pearce MC, Low JC, Gunn GJ and Woolhouse ME, 2004. Acquisition and epidemiology of antibiotic‐resistant Escherichia coli in a cohort of newborn calves. The Journal of Antimicrobial Chemotherapy, 53, 867–871. 10.1093/jac/dkh177 [DOI] [PubMed] [Google Scholar]
- Huneau‐Salaün A, Scoizec A, Thomas R and Le Bouquin S, 2020. Cleaning and disinfection of crates and trucks used for duck transport: field observations during the H5N8 avian influenza outbreaks in France in 2017. Poultry Science, 99, 2931–2936. 10.1016/j.psj.2019.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huneau‐Salaün A, Scoizec A, Thomas R, Martenot C, Schmitz A, Pierre I, Allée C, Busson R, Massin P, Briand FX, Guillemoto C, Louboutin K, Souchaud F, Cherbonnel‐Pansart M, Niqueux E, Grasland B, Souillard R and Bouquin SL, 2022. Avian influenza outbreaks: evaluating the efficacy of cleaning and disinfection of vehicles and transport crates. Poultry Science, 101, 101569. 10.1016/j.psj.2021.101569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd HS, McKean JD, Wesley IV and Karriker LA, 2001. The effect of lairage on Salmonella isolation from market swine. Journal of Food Protection, 64, 939–944. 10.4315/0362-028x-64.7.939 [DOI] [PubMed] [Google Scholar]
- Hurd HS, McKean JD, Griffith RW, Wesley IV and Rostagno MH, 2002. Salmonella enterica infections in market swine with and without transport and holding. Applied and Environmental Microbiology, 68, 2376–2381. 10.1128/AEM.68.5.2376-2381.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huus KE and Ley RE, 2021. Blowing hot and cold: body temperature and the microbiome. mSystems, 6, e0070721. 10.1128/mSystems.00707-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham AC, Urth TR, Sieber RN, Stegger M, Edslev SM, Angen Ø and Larsen AR, 2021. Dynamics of the human nasal microbiota and Staphylococcus aureus CC398 carriage in pig truck drivers across one workweek. Applied and Environmental Microbiology, 87, e0122521. 10.1128/AEM.01225-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes GK, Nachman KE, Abraham AG, Casey JA, Patton AN, Price LB, Tartof SY and Davis MF, 2021. Contamination of retail meat samples with multidrug‐resistant organisms in relation to organic and conventional production and processing: a cross‐sectional analysis of data from the United States National Antimicrobial Resistance Monitoring System, 2012–2017. Environmental Health Perspectives, 129, 57004. 10.1289/EHP7327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrige N, Cazeau G, Morignat E, Chanteperdrix M and Gay E, 2017. Quantitative and qualitative analysis of antimicrobial usage in white veal calves in France. Preventive Veterinary Medicine, 144, 158–166. 10.1016/j.prevetmed.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Joat NN, Khan S and Chousalkar K, 2021. Understanding the effects of intramuscular injection and feed withdrawal on Salmonella Typhimurium shedding and gut microbiota in pullets. Journal of Animal Science and Biotechnology, 12, 78. 10.1186/s40104-021-00597-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EM, Snow LC, Carrique‐Mas JJ, Gosling RJ, Clouting C and Davies RH, 2013. Risk factors for antimicrobial resistance in Escherichia coli found in GB turkey flocks. Veterinary Record, 173, 422. 10.1136/vr.101759 [DOI] [PubMed] [Google Scholar]
- Kang, DSK , 2020. Quantitative microbial risk assessment model to estimate exposure to Campylobacter from consumption of chicken in the United States. Doctoral Dissertation, Virginia Tech. Available online: http://hdl.handle.net/10919/101038
- Kannan G, Heath JL, Wabeck CJ, Souza MC, Howe JC and Mench JA, 1997. Effects of crating and transport on stress and meat quality characteristics in broilers. Poultry Science, 76, 523–529. 10.1093/ps/76.3.523 [DOI] [PubMed] [Google Scholar]
- Keijser BJF, Agamennone V, van den Broek TJ, Caspers M, van de Braak A, Bomers R, Havekes M, Schoen E, van Baak M, Mioch D, Bomers L and Montijn RC, 2019. Dose‐dependent impact of oxytetracycline on the veal calf microbiome and resistome. BMC Genomics, 20, 65. 10.1186/s12864-018-5419-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny FJ and Tarrant PV, 1987. The reaction of young bulls to short‐haul road transport. Applied Animal Behaviour Science, 17, 209–227. 10.1016/0168-1591(87)90147-x [DOI] [Google Scholar]
- Kent JE and Ewbank R, 1983. The effect of road transportation on the blood constituents and behaviour of calves. I. Six months old. Br Vet J, 139, 228–235. 10.1016/S0007-1935(17)30489-X [DOI] [PubMed] [Google Scholar]
- Kim S, Covington A and Pamer EG, 2017. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunological Reviews, 279, 90–105. 10.1111/imr.12563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschvink N, de Moffarts B and Lekeux P, 2008. The oxidant/antioxidant equilibrium in horses. Veterinary Journal, 177, 178–191. 10.1016/j.tvjl.2007.07.033 [DOI] [PubMed] [Google Scholar]
- Knowles TG, 1999. A review of the road transport of cattle. The Veterinary Record, 144, 197–201. 10.1136/vr.144.8.197 [DOI] [PubMed] [Google Scholar]
- Kushner I, 1982. The phenomenon of the acute phase response. Annals of the New York Academy of Sciences, 389, 39–48. 10.1111/j.1749-6632.1982.tb22124.x [DOI] [PubMed] [Google Scholar]
- Lalonde S, Beaulac K, Crowe TG and Schwean‐Lardner K, 2021. The effects of simulated transportation conditions on the core body and extremity temperature, blood physiology, and behavior of white‐strain layer pullets. Poultry Science, 100, 697–706. 10.1016/j.psj.2020.10.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen ST, Hurd HS, McKean JD, Griffith RW and Wesley IV, 2004. Effect of short‐term lairage on the prevalence of Salmonella enterica in cull sows. Journal of Food Protection, 67, 1489–1493. 10.4315/0362-028x-67.7.1489 [DOI] [PubMed] [Google Scholar]
- Laube H, Friese A, von Salviati C, Guerra B, Kasböhrer A, Kreienbrock L and Roesler U, 2013. Longitudinal monitoring of extended‐spectrum‐beta‐lactamase/AmpC‐producing Escherichia coli at German broiler chicken fattening farms. Applied and Environmental Microbiology, 79, 4815–4820. 10.1128/AEM.00856-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Aldeieg M, Woodward MJ, Juniper DT and Rymer C, 2021. The effect of Candida famata and Lactobacillus plantarum on the number of coliforms and the antibiotic resistance and virulence of Escherichia coli in the gut of broilers. Animal, 15, 100310. 10.1016/j.animal.2021.100310 [DOI] [PubMed] [Google Scholar]
- Lentz HH, Konschake M, Teske K, Kasper M, Rother B, Carmanns R, Petersen B, Conraths FJ and Selhorst T, 2011. Trade communities and their spatial patterns in the German pork production network. Preventive Veterinary Medicine, 98, 176–181. 10.1016/j.prevetmed.2010.10.011 [DOI] [PubMed] [Google Scholar]
- Li C, Zhang R, Wei H, Wang Y, Chen Y, Zhang H, Li X, Liu H, Li J and Bao J, 2021. Enriched environment housing improved the laying hen's resistance to transport stress via modulating the heat shock protective response and inflammation. Poultry Science, 100, 100939. 10.1016/j.psj.2020.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Taft DH, Maldonado‐Gomez MX, Johnson D, Treiber ML, Lemay DG, DePeters EJ and Mills DA, 2019. The fecal resistome of dairy cattle is associated with diet during nursing. Nature Communications, 10, 4406. 10.1038/s41467-019-12111-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J, Gauger P, Harmon K, Zhang J, Connor J, Yeske P, Loula T, Levis I, Dufresne L and Main R, 2014. Role of transportation in spread of porcine epidemic diarrhea virus infection, United States. Emerging Infectious Diseases, 20, 872–874. 10.3201/eid2005.131628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Marmion M, Ferone M, Wall P and Scannell AGM, 2021. On farm interventions to minimise Campylobacter spp. contamination in chicken. British Poultry Science, 62, 53–67. 10.1080/00071668.2020.1813253 [DOI] [PubMed] [Google Scholar]
- Luiken REC, Van Gompel L, Bossers A, Munk P, Joosten P, Hansen RB, Knudsen BE, García‐Cobos S, Dewulf J, Aarestrup FM, Wagenaar JA, Smit LAM, Mevius DJ, Heederik DJJ, Schmitt H and EFFORT Group , 2020. Farm dust resistomes and bacterial microbiomes in European poultry and pig farms. Environment International, 143, 105971. 10.1016/j.envint.2020.105971 [DOI] [PubMed] [Google Scholar]
- Luiken RE, Heederik DJ, Scherpenisse P, Van Gompel L, van Heijnsbergen E, Greve GD, Jongerius‐Gortemaker BG, Tersteeg‐Zijderveld MH, Fischer J, Juraschek K, Skarżyńska M, Zając M, Wasyl D, Wagenaar JA, Smit LA, Wouters IM, Mevius DJ, Schmitt H and EFFORT Group , 2022. Determinants for antimicrobial resistance genes in farm dust on 333 poultry and pig farms in nine European countries. Environmental Research, 208, 112715. 10.1016/j.envres.2022.112715 [DOI] [PubMed] [Google Scholar]
- Lynch H, Franklin‐Hayes P, Koolman L, Egan J, Gutiérrez M, Byrne W, Golden O, Bolton D, Reid P, Coffey A, Lucey B, O'Connor L, Unger K and Whyte P, 2022. Prevalence and levels of Campylobacter in broiler chicken batches and carcasses in Ireland in 2017–2018. International Journal of Food Microbiology, 372, 109693. 10.1016/j.ijfoodmicro.2022.109693 [DOI] [PubMed] [Google Scholar]
- Magistrali C, Dionisi AM, De Curtis P, Cucco L, Vischi O, Scuota S, Zicavo A and Pezzotti G, 2008. Contamination of Salmonella spp. in a pig finishing herd, from the arrival of the animals to the slaughterhouse. Research in Veterinary Science, 85, 204–207. 10.1016/j.rvsc.2007.12.002 [DOI] [PubMed] [Google Scholar]
- Magnuson BA, Davis M, Hubele S, Austin PR, Kudva IT, Williams CJ, Hunt CW and Hovde CJ, 2000. Ruminant gastrointestinal cell proliferation and clearance of Escherichia coli O157:H7. Infection and Immunity, 68, 3808–3814. 10.1128/IAI.68.7.3808-3814.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood S, Mehmood S, Ahmad F and Kausar A, 2007. Effects of feed restriction during starter phase on subsequent growth performance, dressing percentage, relative organ weights and immune response of broilers. Pakistan Veterinary Journal, 27, 137–141. [Google Scholar]
- Mannion C, Egan J, Lynch BP, Fanning S and Leonard N, 2008. An investigation into the efficacy of washing trucks following the transportation of pigs – a salmonella perspective. Foodborne Pathogens and Disease, 5, 261–271. 10.1089/fpd.2007.0069 [DOI] [PubMed] [Google Scholar]
- Marahrens M, Kleinschmidt N, Di Nardo A, Velarde A, Fuentes C, Truar A, Otero JL, Di Fede E and Villa PD, 2011. Risk assessment in animal welfare – especially referring to animal transport. Preventive Veterinary Medicine, 102, 157–163. 10.1016/j.prevetmed.2011.04.010 [DOI] [PubMed] [Google Scholar]
- Marcato F, van den Brand H, Kemp B, Engel B, Wolthuis‐Fillerup M and van Reenen K, 2020. Effects of pretransport diet, transport duration, and type of vehicle on physiological status of young veal calves. Journal of Dairy Science, 103, 3505–3520. 10.3168/jds.2019-17445 [DOI] [PubMed] [Google Scholar]
- Marin C and Lainez M, 2009. Salmonella detection in feces during broiler rearing and after live transport to the slaughterhouse. Poultry Science, 88, 1999–2005. 10.3382/ps.2009-00040 [DOI] [PubMed] [Google Scholar]
- Marquetoux N, Stevenson MA, Wilson P, Ridler A and Heuer C, 2016. Using social network analysis to inform disease control interventions. Preventive Veterinary Medicine, 126, 94–104. 10.1016/j.prevetmed.2016.01.022 [DOI] [PubMed] [Google Scholar]
- Martín‐Peláez S, Peralta B, Creus E, Dalmau A, Velarde A, Pérez JF, Mateu E and Martín‐Orue SM, 2009. Different feed withdrawal times before slaughter influence caecal fermentation and faecal Salmonella shedding in pigs. Veterinary Journal, 182, 469–473. 10.1016/j.tvjl.2008.08.002 [DOI] [PubMed] [Google Scholar]
- Massacci FR, Morelli A, Cucco L, Castinel A, Ortenzi R, Tofani S, Pezzotti G, Estellé J, Paniccià M and Magistrali CF, 2020. Transport to the slaughterhouse affects the Salmonella shedding and modifies the fecal microbiota of finishing pigs. Animals, 10. 10.3390/ani10040676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé J, Lardé H, Fairbrother JM, Roy JP, Francoz D, Dufour S and Archambault M, 2021. Prevalence of antimicrobial resistance and characteristics of Escherichia coli isolates from fecal and manure pit samples on dairy farms in the province of Quebec, Canada. Frontiers in Veterinary Science, 8, 654125. 10.3389/fvets.2021.654125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather AE, Innocent GT, McEwen SA, Reilly WJ, Taylor DJ, Steele WB, Gunn GJ, Ternent HE, Reid SW and Mellor DJ, 2007. Risk factors for hide contamination of Scottish cattle at slaughter with Escherichia coli O157. Preventive Veterinary Medicine, 80, 257–270. 10.1016/j.prevetmed.2007.02.011 [DOI] [PubMed] [Google Scholar]
- Matthews L and Woolhouse M, 2005. New approaches to quantifying the spread of infection. Nature Reviews. Microbiology, 3, 529–536. 10.1038/nrmicro1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila T, Frost AJ and O'Boyle D, 1988. The growth of Salmonella in rumen fluid from cattle at slaughter. Epidemiology and Infection, 101, 337–345. 10.1017/s0950268800054273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM, 2000. The evolution of free radicals and oxidative stress. The American Journal of Medicine, 108, 652–659. 10.1016/s0002-9343(00)00412-5 [DOI] [PubMed] [Google Scholar]
- McCrea BA, Tonooka KH, VanWorth C, Boggs CL, Atwill ER and Schrader JS, 2006. Prevalence of Campylobacter and Salmonella species on farm, after transport, and at processing in specialty market poultry. Poultry Science, 85, 136–143. 10.1093/ps/85.1.136 [DOI] [PubMed] [Google Scholar]
- McGlone J, Johnson A, Sapkota A and Kephart R, 2014. Establishing bedding requirements during transport and monitoring skin temperature during cold and mild seasons after transport for finishing pigs. Animals, 4, 241–253. 10.3390/ani4020241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver KS, Amoako DG, Abia ALK, Bester LA, Chenia HY and Essack SY, 2020. Molecular epidemiology of antibiotic‐resistant Escherichia coli from farm-to-fork in intensive poultry production in KwaZulu‐Natal, South Africa. Antibiotics, 9. 10.3390/antibiotics9120850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencía‐Ares O, Argüello H, Puente H, Gómez‐García M, Manzanilla EG, Álvarez‐Ordóñez A, Carvajal A and Rubio P, 2021. Antimicrobial resistance in commensal Escherichia coli and Enterococcus spp. is influenced by production system, antimicrobial use, and biosecurity measures on Spanish pig farms. Porcine Health Manag, 7, 27. 10.1186/s40813-021-00206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messori S, Pedernera‐Romano C, Magnani D, Rodríguez P, Barnard S, Dalmau A, Velarde A and Villa PD, 2015. Unloading or not unloading? Sheep welfare implication of rest stop at control post after a 29 h transport. Small Ruminant Research, 130, 221–228. 10.1016/j.smallrumres.2015.07.012 [DOI] [Google Scholar]
- Messori S, Pedernera‐Romano C, Rodríguez P, Barnard S, Giansante D, Magnani D, Dalmau A, Velarde A and Dalla Villa P, 2017. Effects of different rest‐stop durations at control posts during a long journey on the welfare of sheep. Veterinaria Italiana, 53, 121–129. 10.12834/VetIt.316.1483.3 [DOI] [PubMed] [Google Scholar]
- Miranda‐de la Lama GC, Villarroel M and María GA, 2014. Livestock transport from the perspective of the pre‐slaughter logistic chain: a review. Meat Science, 98, 9–20. 10.1016/j.meatsci.2014.04.005 [DOI] [PubMed] [Google Scholar]
- Mitchell MA and Kettlewell PJ, 2008. Engineering and design of vehicles for long distance road transport of livestock (ruminants, pigs and poultry). Veterinaria Italiana, 44, 201–213. [PubMed] [Google Scholar]
- Mo SS, Kristoffersen AB, Sunde M, Nodtvedt A and Norström M, 2016. Risk factors for occurrence of cephalosporin‐resistant Escherichia coli in Norwegian broiler flocks. Preventive Veterinary Medicine, 130, 112–118. 10.1016/j.prevetmed.2016.06.011 [DOI] [PubMed] [Google Scholar]
- Moazzami M, Fernstrom LL and Hansson I, 2021. Reducing Campylobacter jejuni, Enterobacteriaceae and total aerobic bacteria on transport crates for chickens by irradiation with 265‐nm ultraviolet light (UV‐C LED). Food Control, 119, 107424. 10.1016/j.foodcont.2020.107424 [DOI] [Google Scholar]
- Molechan C, Amoako DG, Abia ALK, Somboro AM, Bester LA and Essack SY, 2019. Molecular epidemiology of antibiotic‐resistant Enterococcus spp. from the farm‐to‐fork continuum in intensive poultry production in KwaZulu‐Natal, South Africa. Science of the Total Environment, 692, 868–878. 10.1016/j.scitotenv.2019.07.324 [DOI] [PubMed] [Google Scholar]
- Morgan IR, Krautil FL and Craven JA, 1987. Effect of time in lairage on caecal and carcass Salmonella contamination of slaughter pigs. Epidemiology and Infection, 98, 323–330. 10.1017/s0950268800062075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mughini‐Gras L, Dorado‐García A, van Duijkeren E, van den Bunt G, Dierikx CM, Bonten MJM, Bootsma MJC, Schmitt H, Hald T, Evers EG, de Koeijer A, van Pelt W, Franz E, Mevius DJ, Heederik DJJ and ESBL Attribution Consortium , 2019. Attributable sources of community‐acquired carriage of Escherichia coli containing beta‐lactam antibiotic resistance genes: a population‐based modelling study. The Lancet Planetary Health, 3, e357–e369. 10.1016/S2542-5196(19)30130-5 [DOI] [PubMed] [Google Scholar]
- Mulders MN, Haenen AP, Geenen PL, Vesseur PC, Poldervaart ES, Bosch T, Huijsdens XW, Hengeveld PD, Dam‐Deisz WD, Graat EA, Mevius D, Voss A and Van De Giessen AW, 2010. Prevalence of livestock‐associated MRSA in broiler flocks and risk factors for slaughterhouse personnel in The Netherlands. Epidemiology and Infection, 138, 743–755. 10.1017/S0950268810000075 [DOI] [PubMed] [Google Scholar]
- Munk P, Knudsen BE, Lukjancenko O, Duarte ASR, Van Gompel L, Luiken REC, Smit LAM, Schmitt H, García AD, Hansen RB, Petersen TN, Bossers A, Ruppé E, Group E , Lund O, Hald T, Pamp SJ, Vigre H, Heederik D, Wagenaar JA, Mevius D and Aarestrup FM, 2018. Abundance and diversity of the faecal resistome in slaughter pigs and broilers in nine European countries. Nature Microbiology, 3, 898–908. 10.1038/s41564-018-0192-9 [DOI] [PubMed] [Google Scholar]
- Munns KD, Selinger L, Stanford K, Selinger LB and McAllister TA, 2014. Are super‐shedder feedlot cattle really super? Foodborne Pathogens and Disease, 11, 329–331. 10.1089/fpd.2013.1621 [DOI] [PubMed] [Google Scholar]
- Mur L, Martínez‐López B and Sánchez‐Vizcaíno JM, 2012. Risk of African swine fever introduction into the European Union through transport‐associated routes: returning trucks and waste from international ships and planes. BMC Veterinary Research, 8, 149. 10.1186/1746-6148-8-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata H, 2007. Stress and acute phase protein response: an inconspicuous but essential linkage. Veterinary Journal, 173, 473–474. 10.1016/j.tvjl.2006.05.008 [DOI] [PubMed] [Google Scholar]
- Natsos G, Mouttotou NK, Magiorkinis E, Ioannidis A, Magana M, Chatzipanagiotou S and Koutoulis KC, 2021. Antimicrobial resistance, FlaA sequencing, and phylogenetic analysis of Campylobacter isolates from broiler chicken flocks in Greece. Veterinary Sciences, 8. 10.3390/vetsci8050068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann EJ, Hall WF, Dahl J, Hamilton D and Kurian A, 2021. Is transportation a risk factor for African swine fever transmission in Australia: a review. Australian Veterinary Journal, 99, 459–468. 10.1111/avj.13106 [DOI] [PubMed] [Google Scholar]
- Nguyen XD, Zhao Y, Evans JD, Lin J and Purswell JL, 2022. Survival of Escherichia coli in airborne and settled poultry litter particles. Animals, 12. 10.3390/ani12030284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol CJ and Scott GB, 1990. Pre‐slaughter handling and transport of broiler‐chickens. Applied Animal Behaviour Science, 28, 57–73. 10.1016/0168-1591(90)90046-G [DOI] [PubMed] [Google Scholar]
- Nielsen BL, Dybkjaer L and Herskin MS, 2011. Road transport of farm animals: effects of journey duration on animal welfare. Animal, 5, 415–427. 10.1017/S1751731110001989 [DOI] [PubMed] [Google Scholar]
- Nijdam E, Arens P, Lambooij E, Decuypere E and Stegeman JA, 2004. Factors influencing bruises and mortality of broilers during catching, transport, and lairage. Poultry Science, 83, 1610–1615. 10.1093/ps/83.9.1610 [DOI] [PubMed] [Google Scholar]
- Nilsson M, De Maeyer H and Allen M, 2022. Evaluation of different cleaning strategies for removal of contaminating DNA molecules. Genes, 13. 10.3390/genes13010162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novais C, Freitas AR, Silveira E, Antunes P, Silva R, Coque TM and Peixe L, 2013. Spread of multidrug‐resistant Enterococcus to animals and humans: an underestimated role for the pig farm environment. The Journal of Antimicrobial Chemotherapy, 68, 2746–2754. 10.1093/jac/dkt289 [DOI] [PubMed] [Google Scholar]
- Padalino B, 2015. Effects of the different transport phases on equine health status, behavior, and welfare: a review. Journal of Veterinary Behavior, 10, 272–282. 10.1016/j.jveb.2015.02.002 [DOI] [Google Scholar]
- Padalino B, Raidal SL, Hall E, Knight P, Celi P, Jeffcott L and Muscatello G, 2016a. Survey of horse transportation in Australia: issues and practices. Australian Veterinary Journal, 94, 349–357. 10.1111/avj.12486 [DOI] [PubMed] [Google Scholar]
- Padalino B, Raidal SL, Hall E, Knight P, Celi P, Jeffcott L and Muscatello G, 2016b. A survey on transport management practices associated with Injuries and health problems in horses. PLoS One, 11, e0162371. 10.1371/journal.pone.0162371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padalino B, Tullio D, Cannone S and Bozzo G, 2018. Road transport of farm animals: mortality, morbidity, species and country of origin at a Southern Italian control post. Animals (Basel), 8, 155. 10.3390/ani8090155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padalino B, Menchetti L, Mininni V, Tullio D and Costa LN, 2021a. Transport certifications of cattle moved from France to Southern Italy and Greece: do they comply with Reg. EC 1/2005? Italian Journal of Animal Science, 20, 1870–1881. 10.1080/1828051x.2021.1971573 [DOI] [Google Scholar]
- Padalino B, Cirone F, Zappaterra M, Tullio D, Ficco G, Giustino A, Ndiana LA and Pratelli A, 2021b. Factors affecting the development of bovine respiratory disease: a cross‐sectional study in beef steers shipped from France to Italy. Frontiers in Veterinary Science, 8, 627894. 10.3389/fvets.2021.627894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon B, Catry B, Dewulf J, Persoons D, Hostens M, De Bleecker K and Deprez P, 2012. Prospective study on quantitative and qualitative antimicrobial and anti‐inflammatory drug use in white veal calves. The Journal of Antimicrobial Chemotherapy, 67, 1027–1038. 10.1093/jac/dkr570 [DOI] [PubMed] [Google Scholar]
- Pempek J, Trearchis D, Masterson M, Habing G and Proudfoot K, 2017. Veal calf health on the day of arrival at growers in Ohio. Journal of Animal Science, 95, 3863–3872. 10.2527/jas2017.1642 [DOI] [PubMed] [Google Scholar]
- Pesciaroli M, Magistrali CF, Filippini G, Epifanio EM, Lovito C, Marchi L, Maresca C, Massacci FR, Orsini S, Scoccia E, Tofani S and Pezzotti G, 2020. Antibiotic‐resistant commensal Escherichia coli are less frequently isolated from poultry raised using non‐conventional management systems than from conventional broiler. International Journal of Food Microbiology, 314, 108391. 10.1016/j.ijfoodmicro.2019.108391 [DOI] [PubMed] [Google Scholar]
- Peterson G, Kumar A, Gart E and Narayanan S, 2011. Catecholamines increase conjugative gene transfer between enteric bacteria. Microbial Pathogenesis, 51, 1–8. 10.1016/j.micpath.2011.03.002 [DOI] [PubMed] [Google Scholar]
- Petrosus E, Silva EB, Lay D Jr and Eicher SD, 2018. Effects of orally administered cortisol and norepinephrine on weanling piglet gut microbial populations and Salmonella passage. Journal of Animal Science, 96, 4543–4551. 10.1093/jas/sky312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrat MB, Soumet C, Maris P and Sanders P, 2008. Phenotypes and genotypes of Campylobacter strains isolated after cleaning and disinfection in poultry slaughterhouses. Veterinary Microbiology, 128, 313–326. 10.1016/j.vetmic.2007.10.021 [DOI] [PubMed] [Google Scholar]
- Piccione G, Casella S, Giannetto C, Bazzano M, Giudice E and Fazio F, 2013. Oxidative stress associated with road transportation in ewes. Small Ruminant Research, 112, 235–238. 10.1016/j.smallrumres.2012.11.001 [DOI] [Google Scholar]
- Piñeiro M, Piñeiro C, Carpintero R, Morales J, Campbell FM, Eckersall PD, Toussaint MJ and Lampreave F, 2007. Characterisation of the pig acute phase protein response to road transport. Veterinary Journal, 173, 669–674. 10.1016/j.tvjl.2006.02.006 [DOI] [PubMed] [Google Scholar]
- Pinheiro DG, Machado NAF, Barbosa JAD and Da Silva IJO, 2021. Computational analysis of load ventilation in broiler transport. Engenharia Agricola, 41, 9–18. 10.1590/1809-4430-Eng.Agric.v41n1p9-18/2021 [DOI] [Google Scholar]
- Pisoni L, Devant M, Blanch M, Pastor JJ and Marti S, 2022. Simulation of feed restriction and fasting: effects on animal recovery and gastrointestinal permeability in unweaned Angus‐Holstein calves. Journal of Dairy Science, 105, 2572–2586. 10.3168/jds.202 [DOI] [PubMed] [Google Scholar]
- Pokharel B and Karna SR, 2022. Antimicrobials in livestock production and its cross‐domain dynamics. In: Akhtar N, Singh KS and Goyal D (eds). Emerging Modalities in Mitigation of Antimicrobial Resistance. Springer International Publishing, Cham. pp. 3–21. [Google Scholar]
- Porphyre T, Bronsvoort BMC, Gunn GJ and Correia‐Gomes C, 2020. Multilayer network analysis unravels haulage vehicles as a hidden threat to the British swine industry. Transboundary and Emerging Diseases, 67, 1231–1246. 10.1111/tbed.13459 [DOI] [PubMed] [Google Scholar]
- Pratelli A, Cirone F, Capozza P, Trotta A, Corrente M, Balestrieri A and Buonavoglia C, 2021. Bovine respiratory disease in beef calves supported long transport stress: an epidemiological study and strategies for control and prevention. Research in Veterinary Science, 135, 450–455. 10.1016/j.rvsc.2020.11.002 [DOI] [PubMed] [Google Scholar]
- Proctor A, Cornick NA, Wang C, Mooyottu S, Arruda PA, Kobs K and Phillips GJ, 2021. Neonatal piglets are protected from Clostridioides difficile infection by age‐dependent increase in intestinal microbial diversity. Microbiology Spectrum, 9, e0124321. 10.1128/Spectrum.01243-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana‐Hayashi MP and Thakur S, 2012. Longitudinal study of the persistence of antimicrobial‐resistant Campylobacter strains in distinct swine production systems on farms, at slaughter, and in the environment. Applied and Environmental Microbiology, 78, 2698–2705. 10.1128/AEM.07723-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadiani R, Setio Budianto EW, Widada D, Widiastuti M and Labib Jundillah M, 2021. Temperature and humidity control system for broiler chicken coops. Indonesian Journal of Electrical Engineering and Computer Science, 22, 1327. 10.11591/ijeecs.v22.i3.pp1327-1333 [DOI] [Google Scholar]
- Ramesh N, Joseph SW, Carr LE, Douglass LW and Wheaton FW, 2003. Serial disinfection with heat and chlorine to reduce microorganism populations on poultry transport containers. Journal of Food Protection, 66, 793–797. 10.4315/0362-028x-66.5.793 [DOI] [PubMed] [Google Scholar]
- Ramirez GA, Sarlin LL, Caldwell DJ, Yezak CR Jr, Hume ME, Corrier DE, Deloach JR and Hargis BM, 1997. Effect of feed withdrawal on the incidence of Salmonella in the crops and ceca of market age broiler chickens. Poultry Science, 76, 654–656. 10.1093/ps/76.4.654 [DOI] [PubMed] [Google Scholar]
- Rasschaert G, Houf K and De Zutter L, 2007. Impact of the slaughter line contamination on the presence of Salmonella on broiler carcasses. Journal of Applied Microbiology, 103, 333–341. 10.1111/j.1365-2672.2006.03248.x [DOI] [PubMed] [Google Scholar]
- Rasschaert G, De Zutter L, Herman L and Heyndrickx M, 2020. Campylobacter contamination of broilers: the role of transport and slaughterhouse. International Journal of Food Microbiology, 322, 108564. 10.1016/j.ijfoodmicro.2020.108564 [DOI] [PubMed] [Google Scholar]
- Ray T, Gaire TN, Dean CJ, Rowe S, Godden SM and Noyes NR, 2022. The microbiome of common bedding materials before and after use on commercial dairy farms. Animal Microbiome, 4, 18. 10.1186/s42523-022-00171-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding LE, Berry AS, Indugu N, Huang E, Beiting DP and Pitta D, 2021. Gut microbiota features associated with Clostridioides difficile colonization in dairy calves. PLoS One, 16, e0251999. 10.1371/journal.pone.0251999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke SC, 2021. Strategies to improve poultry food safety, a landscape review. Annual Review of Animal Biosciences, 9, 379–400. 10.1146/annurev-animal-061220-023200 [DOI] [PubMed] [Google Scholar]
- Rigby CE and Pettit JR, 1980. Changes in the Salmonella status of broiler chickens subjected to simulated shipping conditions. Canadian Journal of Comparative Medicine, 44, 374–381. [PMC free article] [PubMed] [Google Scholar]
- Rigby CE, Pettit JR, Bentley AH, Spencer JL, Salomons MO and Lior H, 1982. The relationships of salmonellae from infected broiler flocks, transport crates or processing plants to contamination of eviscerated carcases. Canadian Journal of Comparative Medicine, 46, 272–278. [PMC free article] [PubMed] [Google Scholar]
- Robinson SE, Brown PE, Wright EJ, Hart CA and French NP, 2009. Quantifying within‐ and between‐animal variation and uncertainty associated with counts of Escherichia coli O157 occurring in naturally infected cattle faeces. Journal of the Royal Society Interface, 6, 169–177. 10.1098/rsif.2008.0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues da Costa M, Pessoa J, Meemken D and Nesbakken T, 2021. A systematic review on the effectiveness of pre‐harvest meat safety interventions in pig herds to control Salmonella and other foodborne pathogens. Microorganisms, 9. 10.3390/microorganisms9091825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth N, Mayrhofer S, Gierus M, Weingut C, Schwarz C, Doupovec B, Berrios R and Domig KJ, 2017. Effect of an organic acids based feed additive and enrofloxacin on the prevalence of antibiotic‐resistant E. coli in cecum of broilers. Poultry Science, 96, 4053–4060. 10.3382/ps/pex232 [DOI] [PubMed] [Google Scholar]
- Rule AM, Evans SL and Silbergeld EK, 2008. Food animal transport: a potential source of community exposures to health hazards from industrial farming (CAFOs). Journal of Infection and Public Health, 1, 33–39. 10.1016/j.jiph.2008.08.001 [DOI] [PubMed] [Google Scholar]
- Sabaw AB and Muhammed TS, 2021. Meat quality and carcass characteristics assessments in broiler chickens subjected to different pre‐slaughter feed withdrawal times. IOP Conference Series: Earth and Environmental Science, 761, 012112. 10.1088/1755-1315/761/1/012112 [DOI] [Google Scholar]
- Sahibzada S, Pang S, Hernández‐Jover M, Jordan D, Abraham S, O'Dea M and Heller J, 2020. Prevalence and antimicrobial resistance of MRSA across different pig age groups in an intensive pig production system in Australia. Zoonoses and Public Health, 67, 576–586. 10.1111/zph.12721 [DOI] [PubMed] [Google Scholar]
- Savin M, Bierbaum G, Hammerl JA, Heinemann C, Parcina M, Sib E, Voigt A and Kreyenschmidt J, 2020a. ESKAPE bacteria and extended‐spectrum‐beta‐lactamase‐producing Escherichia coli isolated from wastewater and process water from German poultry slaughterhouses. Applied and Environmental Microbiology, 86. 10.1128/AEM.02748-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin M, Bierbaum G, Blau K, Parcina M, Sib E, Smalla K, Schmithausen R, Heinemann C, Hammerl JA and Kreyenschmidt J, 2020b. Colistin‐resistant Enterobacteriaceae isolated from process waters and wastewater from German poultry and pig slaughterhouses. Frontiers in Microbiology, 11, 575391. 10.3389/fmicb.2020.575391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlattmann A, von Lutzau K, Kaspar U and Becker K, 2020. The porcine nasal microbiota with particular attention to livestock‐associated methicillin‐resistant Staphylococcus aureus in Germany‐a culturomic approach. Microorganisms, 8. 10.3390/microorganisms8040514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithausen RM, Schulze‐Geisthoevel SV, Heinemann C, Bierbaum G, Exner M, Petersen B and Steinhoff‐Wagner J, 2018. Reservoirs and transmission pathways of resistant indicator bacteria in the biotope pig stable and along the food chain: a review from a One Health perspective. Sustainability, 10, 3967. 10.3390/su10113967 [DOI] [Google Scholar]
- Schnitt A and Tenhagen BA, 2020. Risk Factors for the occurrence of methicillin‐resistant Staphylococcus aureus in dairy herds: an update. Foodborne Pathogens and Disease, 17, 585–596. 10.1089/fpd.2019.2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz J, Friese A, Klees S, Tenhagen BA, Fetsch A, Rosler U and Hartung J, 2012. Longitudinal study of the contamination of air and of soil surfaces in the vicinity of pig barns by livestock‐associated methicillin‐resistant Staphylococcus aureus . Applied and Environmental Microbiology, 78, 5666–5671. 10.1128/AEM.00550-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz J, Boklund A, Toft N and Halasa T, 2019. Effects of control measures on the spread of LA‐MRSA among Danish pig herds between 2006 and 2015 – a simulation study. Scientific Reports, 9, 691. 10.1038/s41598-018-37075-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkopf‐Genswein KS, Booth‐McLean ME, Shah MA, Entz T, Bach SJ, Mears GJ, Schaefer AL, Cook N, Church J and McAllister TA, 2007. Effects of pre‐haul management and transport duration on beef calf performance and welfare. Applied Animal Behaviour Science, 108, 12–30. 10.1016/j.applanim.2006.11.012 [DOI] [Google Scholar]
- Schwartzkopf‐Genswein KS, Faucitano L, Dadgar S, Shand P, Gonzalez LA and Crowe TG, 2012. Road transport of cattle, swine and poultry in North America and its impact on animal welfare, carcass and meat quality: a review. Meat Science, 92, 227–243. 10.1016/j.meatsci.2012.04.010 [DOI] [PubMed] [Google Scholar]
- Sepulveda J and Moeller AH, 2020. The effects of temperature on animal gut microbiomes. Frontiers in Microbiology, 11, 384. 10.3389/fmicb.2020.00384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane SM, 2000. Campylobacter infection of commercial poultry. Revue Scientifique et Technique, 19, 376–395. 10.20506/rst.19.2.1224 [DOI] [PubMed] [Google Scholar]
- Sibanda N, McKenna A, Richmond A, Ricke SC, Callaway T, Stratakos AC, Gundogdu O and Corcionivoschi N, 2018. A Review of the effect of management practices on Campylobacter prevalence in poultry farms. Frontiers in Microbiology, 9, 2002. 10.3389/fmicb.2018.02002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RR, Hill AA, Swart A, Kelly L and Snary EL, 2016. A transport and lairage model for Salmonella transmission between pigs applicable to EU Member States. Risk Analysis, 36, 482–497. 10.1111/risa.12390 [DOI] [PubMed] [Google Scholar]
- Šímová V, Večerek V, Passantino A and Voslářová E, 2016. Pre‐transport factors affecting the welfare of cattle during road transport for slaughter – a review. Acta Veterinaria Brno, 85, 303–318. 10.2754/avb201685030303 [DOI] [Google Scholar]
- Singh A, Kumari T, Rajput M, Baishya A, Bhatt N and Roy S, 2020. Review on effect of bedding material on production, reproduction and health of dairy animals. International Journal of Livestock Research, 7, 11–20. 10.5455/ijlr.20200207073618 [DOI] [Google Scholar]
- Slader J, Domingue G, Jørgensen F, McAlpine K, Owen RJ, Bolton FJ and Humphrey TJ, 2002. Impact of transport crate reuse and of catching and processing on Campylobacter and Salmonella contamination of broiler chickens. Applied and Environmental Microbiology, 68, 713–719. 10.1128/AEM.68.2.713-719.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MG, 1977. Transfer of R factors from Escherichia coli to salmonellas in the rumen of sheep. Journal of Medical Microbiology, 10, 29–35. 10.1099/00222615-10-1-29 [DOI] [PubMed] [Google Scholar]
- Smith DR, 2019. Herd Immunity. Veterinary Clinics of North America‐Food Animal Practice, 35, 593. 10.1016/j.cvfa.2019.07.001 [DOI] [PubMed] [Google Scholar]
- Soleimani AF, Zulkifli I, Hair‐Bejo M, Ebrahimi M, Jazayeri SD, Hashemi SR, Meimandipour A and Goh YM, 2012a. Epigenetic modification: possible approach to reduce Salmonella enterica serovar enteritidis susceptibility under stress conditions. Avian Pathology, 41, 351–354. 10.1080/03079457.2012.691155 [DOI] [PubMed] [Google Scholar]
- Soleimani AF, Zulkifli I, Hair‐Bejo M, Omar AR and Raha AR, 2012b. The role of heat shock protein 70 in resistance to Salmonella enteritidis in broiler chickens subjected to neonatal feed restriction and thermal stress. Poultry Science, 91, 340–345. 10.3382/ps.2011-01703 [DOI] [PubMed] [Google Scholar]
- Song L, Wang C, Jiang G, Ma J, Li Y, Chen H and Guo J, 2021. Bioaerosol is an important transmission route of antibiotic resistance genes in pig farms. Environment International, 154, 106559. 10.1016/j.envint.2021.106559 [DOI] [PubMed] [Google Scholar]
- Soro AB, Whyte P, Bolton DJ and Tiwari BK, 2020. Strategies and novel technologies to control Campylobacter in the poultry chain: a review. Comprehensive Reviews in Food Science and Food Safety, 19, 1353–1377. 10.1111/1541-4337.12544 [DOI] [PubMed] [Google Scholar]
- Sossidou EN and de Roest K, 2012. Assuring quality and welfare at control posts: a European perspective. Scientific Papers Animal Science and Biotechnologies, 45, 263–267. [Google Scholar]
- Souza da Silva C, Molenaar R, Giersberg MF, Rodenburg TB, van Riel JW, De Baere K, Van Dosselaer I, Kemp B, van den Brand H and de Jong IC, 2021. Day‐old chicken quality and performance of broiler chickens from 3 different hatching systems. Poultry Science, 100, 100953. 10.1016/j.psj.2020.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanenburg M, van der Wolf PJ, Urlings HA, Snijders JM and van Knapen F, 2001. Salmonella in slaughter pigs: the effect of logistic slaughter procedures of pigs on the prevalence of Salmonella in pork . International Journal of Food Microbiology, 70, 231–242. [DOI] [PubMed] [Google Scholar]
- Stanford K, Bryan M, Peters J, González LA, Stephens TP and Schwartzkopf‐Genswein KS, 2011. Effects of long‐ or short‐haul transportation of slaughter heifers and cattle liner microclimate on hide contamination with Escherichia coli O157. Journal of Food Protection, 74, 1605–1610. 10.4315/0362-028X.JFP-11-154 [DOI] [PubMed] [Google Scholar]
- Sun J, Liao XP, D'Souza AW, Boolchandani M, Li SH, Cheng K, Luis Martinez J, Li L, Feng YJ, Fang LX, Huang T, Xia J, Yu Y, Zhou YF, Sun YX, Deng XB, Zeng ZL, Jiang HX, Fang BH, Tang YZ, Lian XL, Zhang RM, Fang ZW, Yan QL, Dantas G and Liu YH, 2020. Environmental remodeling of human gut microbiota and antibiotic resistome in livestock farms. Nature Communications, 11, 1427. 10.1038/s41467-020-15222-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto S, Tomonaga S, Funaba M and Matsui T, 2017. Effect of long‐distance transportation on serum metabolic profiles of steer calves. Animal Science Journal, 88, 1970–1978. 10.1111/asj.12870 [DOI] [PubMed] [Google Scholar]
- Tateo A, Padalino B, Boccaccio M, Maggiolino A and Centoducati P, 2012. Transport stress in horses: effects of two different distances. Journal of Veterinary Behavior, 7, 33–42. 10.1016/j.jveb.2011.04.007 [DOI] [Google Scholar]
- Tello M, Ocejo M, Oporto B and Hurtado A, 2020. Prevalence of cefotaxime‐resistant Escherichia coli isolates from healthy cattle and sheep in Northern Spain: phenotypic and genome‐based characterization of antimicrobial susceptibility. Applied and Environmental Microbiology, 86. 10.1128/AEM.00742-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhagen BA, Alt K, Pfefferkorn B, Wiehle L, Kasböhrer A and Fetsch A, 2018. Short communication: methicillin‐resistant Staphylococcus aureus in conventional and organic dairy herds in Germany. Journal of Dairy Science, 101, 3380–3386. 10.3168/jds.2017-12939 [DOI] [PubMed] [Google Scholar]
- Tenhagen BA, Kasböhrer A, Grobbel M, Hammerl J and Kaspar H, 2020. Antimicrobial resistance in E. coli from different cattle populations in Germany. Tierärztliche Praxis. Ausgabe G, Grosstiere/Nutztiere, 48, 218–227. 10.1055/a-1197-5701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timsit E, Dendukuri N, Schiller I and Buczinski S, 2016. Diagnostic accuracy of clinical illness for bovine respiratory disease (BRD) diagnosis in beef cattle placed in feedlots: a systematic literature review and hierarchical Bayesian latent‐class meta‐analysis. Preventive Veterinary Medicine, 135, 67–73. 10.1016/j.prevetmed.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Tong C, Xiao D, Xie L, Yang J, Zhao R, Hao J, Huo Z, Zeng Z and Xiong W, 2022. Swine manure facilitates the spread of antibiotic resistome including tigecycline‐resistant tet(X) variants to farm workers and receiving environment. Science of the Total Environment, 808, 152157. 10.1016/j.scitotenv.2021.152157 [DOI] [PubMed] [Google Scholar]
- Truccollo B, Whyte P and Bolton DJ, 2020. An investigation of the effect of catecholamines and glucocorticoids on the growth and pathogenicity of Campylobacter jejuni . Pathogens, 9. 10.3390/pathogens9070555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiouris V, Georgopoulou I, Batzios C, Pappaioannou N, Ducatelle R and Fortomaris P, 2014. Temporary feed restriction partially protects broilers from necrotic enteritis. Avian Pathology, 43, 139–145. 10.1080/03079457.2014.889278 [DOI] [PubMed] [Google Scholar]
- USDA (United States Department of Agriculture) and CFSPH (Center for Food Security & Public Health) , 2010. NAHEMS guidelines: biosecurity. FAD PReP/NAHEMS guidelines. Available online: https://www.aphis.usda.gov/animal_health/emergency_management/downloads/nahems_guidelines/fadprep_nahems_guidelines_biosecurity.pdf
- Van Engen NK and Coetzee JF, 2018. Effects of transportation on cattle health and production: a review. Animal Health Research Reviews, 19, 142–154. 10.1017/S1466252318000075 [DOI] [PubMed] [Google Scholar]
- Van Lìmbergen T, Sarrazin S, Chantziaras I, Dewulf J, Ducatelle R, Kyriazakis I, McMullin P, Méndez J, Niemi JK, Papasolomontos S, Szeleszczuk P, Van Erum J, Maes D and PROHEALTH Consortium , 2020. Risk factors for poor health and performance in European broiler production systems. BMC Veterinary Research, 16, 287. 10.1186/s12917-020-02484-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWaal K, Pérez A, Torremorrell M, Morrison RM and Craft M, 2018. Role of animal movement and indirect contact among farms in transmission of porcine epidemic diarrhea virus. Epidemics, 24, 67–75. 10.1016/j.epidem.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugghe E, Boyen F, Van Parys A, Van Deun K, Croubels S, Thompson A, Shearer N, Leyman B, Haesebrouck F and Pasmans F, 2011. Stress induced Salmonella Typhimurium recrudescence in pigs coincides with cortisol induced increased intracellular proliferation in macrophages. Veterinary Research, 42, 118. 10.1186/1297-9716-42-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Tippelskirch P, Gölz G, Projahn M, Daehre K, Friese A, Roesler U, Alter T and Orquera S, 2018. Prevalence and quantitative analysis of ESBL and AmpC beta‐lactamase producing Enterobacteriaceae in broiler chicken during slaughter in Germany. International Journal of Food Microbiology, 281, 82–89. 10.1016/j.ijfoodmicro.2018.05.022 [DOI] [PubMed] [Google Scholar]
- Vosmerova P, Chloupek J, Bedanova I, Chloupek P, Kruzikova K, Blahova J and Vecerek V, 2010. Changes in selected biochemical indices related to transport of broilers to slaughterhouse under different ambient temperatures. Poultry Science, 89, 2719–2725. 10.3382/ps.2010-00709 [DOI] [PubMed] [Google Scholar]
- Walia K, Argüello H, Lynch H, Leonard FC, Grant J, Yearsley D, Kelly S, Duffy G, Gardiner GE and Lawlor PG, 2016. Effect of feeding sodium butyrate in the late finishing period on Salmonella carriage, seroprevalence, and growth of finishing pigs. Preventive Veterinary Medicine, 131, 79–86. 10.1016/j.prevetmed.2016.07.009 [DOI] [PubMed] [Google Scholar]
- Walia K, Argüello H, Lynch H, Grant J, Leonard FC, Lawlor PG, Gardiner GE and Duffy G, 2017. The efficacy of different cleaning and disinfection procedures to reduce Salmonella and Enterobacteriaceae in the lairage environment of a pig abattoir. International Journal of Food Microbiology, 246, 64–71. 10.1016/j.ijfoodmicro.2017.02.002 [DOI] [PubMed] [Google Scholar]
- Wang XM, Lan BR, Fei HX, Wang SY and Zhu GB, 2021. Heavy metal could drive co‐selection of antibiotic resistance in terrestrial subsurface soils. Journal of Hazardous Materials, 411, 124848. 10.1016/j.jhazmat.2020.124848 [DOI] [PubMed] [Google Scholar]
- Warriss PD, Pagazaurtundua A and Brown SN, 2005. Relationship between maximum daily temperature and mortality of broiler chickens during transport and lairage. British Poultry Science, 46, 647–651. 10.1080/00071660500393868 [DOI] [PubMed] [Google Scholar]
- Weber L and Meemken D, 2018. Hygienic measures during animal transport to abattoirs – a status quo analysis of the current cleaning and disinfection of animal transporters in Germany. Porcine Health Management, 4, 1. 10.1186/s40813-017-0078-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks CA, Tuyttens FAM and Grandin T, 2019. Poultry handling and transport. Livestock Handling and Transport. CABI. pp. 404–426. ISBN 9781786399151. 10.1079/9781786399151.0000 [DOI] [Google Scholar]
- Wein Y, Geva Z, Bar‐Shira E and Friedman A, 2017. Transport‐related stress and its resolution in turkey pullets: activation of a pro‐inflammatory response in peripheral blood leukocytes. Poultry Science, 96, 2601–2613. 10.3382/ps/pex076 [DOI] [PubMed] [Google Scholar]
- Weinroth MD, Thomas KM, Doster E, Vikram A, Schmidt JW, Arthur TM, Wheeler TL, Parker JK, Hanes AS, Alekoza N, Wolfe C, Metcalf JL, Morley PS and Belk KE, 2022. Resistomes and microbiome of meat trimmings and colon content from culled cows raised in conventional and organic production systems. Anim Microbiome, 4, 21. 10.1186/s42523-022-00166-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells SJ, Fedorka‐Cray PJ, Dargatz DA, Ferris K and Green A, 2001. Fecal shedding of Salmonella spp. by dairy cows on farm and at cull cow markets. Journal of Food Protection, 64, 3–11. 10.4315/0362-028x-64.1.3 [DOI] [PubMed] [Google Scholar]
- White HD, Bates MJ and Wilson P, 1992. For information specialists: interpretations of reference and bibliographic work. Ablex Pub. ISBN 0893919837. [Google Scholar]
- Wickramasuriya SS, Park I, Lee K, Lee Y, Kim WH, Nam H and Lillehoj HS, 2022. Role of physiology, immunity, microbiota, and infectious diseases in the gut health of poultry. Vaccines, 10. 10.3390/vaccines10020172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm BJ, Young I, Cahill S, Desmarchelier P, Nakagawa R and Rajic A, 2017. Interventions to reduce non‐typhoidal Salmonella in pigs during transport to slaughter and lairage: systematic review, meta‐analysis, and research synthesis based infection models in support of assessment of effectiveness. Preventive Veterinary Medicine, 145, 133–144. 10.1016/j.prevetmed.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Williams JL, Minton JE, Patterson JA, Marchant Forde J and Eicher SD, 2008. Lairage during transport of eighteen‐kilogram pigs has an impact on innate immunity and commensal bacteria diversity in the intestines. Journal of Animal Science, 86, 1232–1244. 10.2527/jas.2007-0592 [DOI] [PubMed] [Google Scholar]
- Willinghan EM, Sander JE, Thayer SG and Wilson JL, 1996. Investigation of bacterial resistance to hatchery disinfectants. Avian Diseases, 40, 510–515. [PubMed] [Google Scholar]
- Wilson KM, Bourassa DV, McLendon BL, Wilson JL and Buhr RJ, 2018. Impact of skip‐a‐day and every‐day feeding programs for broiler breeder pullets on the recovery of Salmonella and Campylobacter following challenge. Poultry Science, 97, 2775–2784. 10.3382/ps/pey150 [DOI] [PubMed] [Google Scholar]
- Wilson DJ, Stojkov J, Renaud DL and Fraser D, 2020. Risk factors for poor health outcomes for male dairy calves undergoing transportation in western Canada. The Canadian Veterinary Journal, 61, 1265–1272. [PMC free article] [PubMed] [Google Scholar]
- Woldehiwet Z, Mamache B and Rowan TG, 1990. The effects of age, environmental temperature and relative humidity on the bacterial flora of the upper respiratory tract in calves. The British Veterinary Journal, 146, 211–218. 10.1016/s0007-1935(11)80004-7 [DOI] [PubMed] [Google Scholar]
- Wray C, Todd N, McLaren IM and Beedell YE, 1991. The epidemiology of Salmonella in calves: the role of markets and vehicles. Epidemiology and Infection, 107, 521–525. 10.1017/S0950268800049219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Tong C, Xiao D, Xie L, Zhao R, Huo Z, Tang Z, Hao J, Zeng Z and Xiong W, 2022. Metagenomic insights into chicken gut antibiotic resistomes and microbiomes. Microbiology Spectrum, 10, e0190721. 10.1128/spectrum.01907-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X and Lin J, 2017. Factors influencing horizontal gene transfer in the intestine. Animal Health Research Reviews, 18, 153–159. 10.1017/S1466252317000159 [DOI] [PubMed] [Google Scholar]
- Zeng H, De Reu K, Gabriël S, Mattheus W, De Zutter L and Rasschaert G, 2021. Salmonella prevalence and persistence in industrialized poultry slaughterhouses. Poultry Science, 100, 100991. 10.1016/j.psj.2021.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fu H, Yang H, Wu J, Chen Z, Jiang H, Liu M, Liu Q, Huang L, Gao J and Chen C, 2022. Extensive metagenomic analysis of the porcine gut resistome to identify indicators reflecting antimicrobial resistance. Microbiome, 10, 39. 10.1186/s40168-022-01241-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidaric V, Pardon B, Dos Vultos T, Deprez P, Brouwer MS, Roberts AP, Henriques AO and Rupnik M, 2012. Different antibiotic resistance and sporulation properties within multiclonal Clostridium difficile PCR ribotypes 078, 126, and 033 in a single calf farm. Applied and Environmental Microbiology, 78, 8515–8522. 10.1128/AEM.02185-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol for the assessment of the transmission of antimicrobial resistance (AMR) during animal transport


