Graphical abstract
Keywords: Stropharia rugosoannulata, Ultrasound, Umami peptide, Molecular docking, Structure-taste activity relationship
Highlights
-
•
The basic amino acids in the peptides are more easily bound to T1R1.
-
•
The acidic amino acids in the peptides are more easily bound to T1R3.
-
•
The peptide's N-terminal and C-terminal amino acids contribute significantly to forming stable complexes.
-
•
D, E, and R are key amino acid residues in peptides that are easy to bind to receptors.
-
•
The active amino acid sites the peptide binds to the receptor account for 42 % ∼ 65 %.
Abstract
Through virtual screening, electronic tongue verification, and molecular docking technology, the structure-taste activity relationship of 47 kinds of umami peptides (octapeptide - undecapeptide) from Stropharia rugosoannulata prepared by simultaneous ultrasonic-assisted directional enzymatic hydrolysis was analyzed. The umami peptides of S.rugosoannulata can form hydrogen bond interaction and electrostatic interaction with umami receptors T1R1/T1R3. The amino acid residues at the peptides' N-terminal and C-terminal play a vital role in binding with the receptors to form a stable complex. D, E, and R are the primary amino acids in the peptides that easily bind to T1R1/T1R3. The basic amino acid in the peptides is more easily bound to T1R1, and the acidic amino acid is more easily bound to T1R3. The active amino acid sites of the receptors to which the peptides bind account for 42%−65% of the total active amino acid residues in the receptors. ASP147 and ASP219 are the critical amino acid residues for T1R1 to recognize the umami peptides, and ARG64, GLU45, and GLU48 are the critical amino acid residues for T1R3 to recognize the umami peptides. The increase in the variety and quantity of umami peptides is the main reason for improving the umami taste of the substrate prepared by synchronous ultrasound-assisted directional enzymatic hydrolysis. This study provides a theoretical basis for understanding simultaneous ultrasound-assisted directional enzymatic hydrolysis for preparing umami peptides from S.rugosoannulata, enhancing the flavor of umami, and the relationship between peptide structure and taste activity.
1. Introduction
With an in-depth study of food-derived flavor substances by researchers, peptides have been found to have an essential impact on food flavor. Corresponding peptides can be found in sweet, sour, bitter, salty, umami, and other tastes. The content of peptides in food and natural extracts is also much higher than that of free amino acids, nucleotides, and other small taste molecules. The umami peptide is a small molecular weight peptide with umami-styling characteristics. It has both nutritional and flavor characteristics. It exists in animal products [1], [2], [3], [4], [5], [6], [7], [8], plant products [9], [10], edible fungi [11], [12], [13], [14], [15], aquatic products [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], and fermented foods [27], [28], [29], [30]. It can also interact with salt and sodium glutamate to improve the umami and mellow taste of food and enhance the richness and coordination of taste [2], [9], [22], [31], [32], [33], [34]. Three umami peptides (FSGLDGAK, FAGDDAPR, and FSGLDGSK) identified in porcine bone soup have a threshold of 0.1 mM to 0.89 mM. FSGLDGSK has a significant umami taste enhancement effect in 0.35 % monosodium glutamate solution [8]. The umami peptides (DQR, NNP, EGF, EDG, TESSE, and RGENSEEGAIVT) isolated from peanut protein isolate hydrolysates have an umami threshold value of 0.39 to 1.11 mM and have an umami enhancement ability (threshold value of 0.33 mM to 0.82 mM) [9]. The umami peptides ASNMSDL and LQPLNAH obtained from Volvariella volvacea have umami thresholds of 10.19 and 12.63 mmol/L. Umami peptides showed better sensory taste than the mixture of amino acids [12]. Among the umami peptides (DGF, KCGQ, and HHYE) identified from Boletus edulis, DGF and HHYE have the lowest umami taste recognition threshold, and KCGQ has the strongest synergistic umami taste effect [15]. Three umami peptides (INKPGL, SDSCI, and GPDPER) obtained from the hydrolysis of myosin of Atlantic cod (Gadus morrua) can be recognized by umami taste receptors. The umami and richness value of all three hexapeptides in 0.4 mg/mL was higher than a 0.1 % MSG solution [17]. The umami peptides (VADLMR, STELFK, FVGLQER, DALKK, and VVLNPVARVE) isolated and identified from tilapia lower jaw with the recognition threshold of umami peptides was 0.125–0.250 mg/mL [23]. Five umami peptides (ALPEEV, LPEEV, AQALQAQA, EQQQQ, and EAGIQ) separated from soy sauce showed better sensory taste than the amino acid mixture [27]. The umami peptides (TGC, GLE, VEAL, GGE, DR, DAE, EVC, and GGE) identified from the whole soybeans and the defatted soybeans fermented soy sauce also play an essential role in the umami and umami enhancement of soy sauce [28]. The revelation of the taste characteristics and taste-presenting mechanism of different umami peptides provides new insight for developing umami peptides.
Stropharia rugosoannulata, also known as the red pine mushroom, is rich in umami peptides and is a high-quality raw material for developing a base material of natural flavors [13], [35]. Our previous research found that the base material of S.rugosoannulata prepared by traditional enzymatic hydrolysis had a high proportion of peptide (461.31 mg/g dry weight) and the flavor characteristics of the base material were close to those of the umami reference (MSG) with the same concentration. Based on the best traditional enzymatic hydrolysis to prepare a peptide substrate, simultaneous ultrasonic-assisted directional enzymatic hydrolysis technology to prepare a peptide substrate of S.rugosoannulata was further optimized. Under the condition that the ultrasound power density is 120 W/L, the working frequency is 20 kHz, the substrate concentration is 48 g/L, the amount of alkaline protease added is 1 % (the enzyme activity is 200,000 U/g, w/w), the enzymatic hydrolysis temperature is 42 ℃, the ultrasonic synchronous enzymatic hydrolysis time is 40 min, the peptide content in the base material of S.rugosoannulata is 492.87 mg/g dry weight. After ultrafiltration separation (collection of samples with molecular weight less than 3000 Da) and freeze-drying (−80 °C, 48 h), the electronic tongue umami evaluation values of the sample at a concentration of 1 mg/mL were 8.21 ± 0.23 (traditional enzymatic hydrolysis preparation process) and 10.40 ± 0.18 (synchronous ultrasonic-assisted enzymatic hydrolysis preparation process), respectively. The peptide base material prepared by synchronous ultrasonic-assisted enzymatic hydrolysis is better than that prepared by traditional enzymatic hydrolysis in terms of umami taste. Therefore, analysis of the characteristic flavor structure of the umami peptide can lay a theoretical foundation for preparing the umami peptide of S.rugosoannulata by simultaneous ultrasonic-assisted directional enzymatic hydrolysis. At the same time, after the amino acid composition and sequence of the peptides are obtained, the relationship between umami peptide structure and taste characteristics cannot be accurately predicted and explained only from the primary structure of the peptides. The taste receptor T1R1/T1R3 has a high affinity for various umami substances, which may reflect the synergistic effect between umami substances. It is the primary receptor for umami peptides [36]. Therefore, taking T1R1/T1R3 as the binding target of the umami peptides, the recognition of the umami peptides by the receptors and the binding strength between the umami peptides and the receptors can be studied through molecular simulation, which can reveal the receptor perception mechanism and the relationship between umami activity and the structure of the peptide, providing theoretical support for the development and application of the umami peptides of S.rugosoannulata.
Based on previous research, this study focused on the structural basis of the unique umami peptides prepared by synchronous ultrasound-assisted directional enzymatic hydrolysis and clarified the critical binding sites of the receptors to recognize the umami peptides and the relationship between the structure and the taste activity of the peptides. This study provides a theoretical basis for understanding the preparation of umami peptides from S.rugosoannulata by simultaneous ultrasound-assisted directional enzymatic hydrolysis.
2. Material and methods
2.1. Preparation of the peptide base material
The optimized synchronous ultrasound-assisted directional enzymatic hydrolysis process was used to prepare the peptide base material of the S.rugosoannulata (mushroom strain certificate 2004062, NCBI strain release No. SRR14469700). The bath ultrasound working equipment is the same as in the literature [37], developed by the Institute of Food Physical Processing of Jiangsu University. After the base material of the S.rugosoannulata peptide was separated by ultrafiltration, the peptide base material with a molecular weight of less than 3000 Da was collected, freeze-dried at −80 ℃ for 48 h, and then collected for standby.
2.2. Sequence identification of peptides
The identification methods for the S.rugosoannulata peptide sequences are the same as those in the literature [37]. After desalting, dissolution (0.1 % formic acid, 5 % acetonitrile), and centrifugation (13500 rpm, centrifugation at 4 °C for 20 min) of the base material sample, 8 uL sample solution was taken for analysis and identification of peptide sequences by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The PEAKS 10.5 software is used for database retrieval.
2.3. Prediction of taste characteristics of peptides
The taste characteristics of the peptides identified by LC-MS/MS were predicted using the BIOPEP-UWM sensory peptides and amino acids module tool (https://biochemia.uwm.edu.pl/biopep-uwm/), and umami peptides and their fragments with umami taste characteristics were selected.
2.4. Verification of taste characteristics of umami peptide
The peptides with a high MS area were selected for synthesis. The synthetic peptides are entrusted to GL Biochemical (Shanghai) Ltd., and the purity of the synthetic peptides is more than 98 %. The taste characteristics of the synthetic peptides were analyzed using the SA-402B electronic tongue gustatory analysis system (Ensoul Technology Ltd., Beijing, China). 0.1 g of a synthetic peptide is weighed, 100 ml of pure water is added to dissolve it, and it is diluted step by step to obtain five concentration gradients of the umami peptide solution (0.25, 0.375, 0.5, 0.75, and 1.0 g/L). The umami strength of different concentration gradients of umami peptide solution is determined, and the taste threshold of umami peptides is calculated. The electronic tongue determination method is the same as in the literature [13].
2.5. Analysis of the structure-taste activity relationship of umami peptides
The 3D structure of the selected umami peptides of S.rugosoannulata was constructed by molecular docking software (MOE 2019), and the peptide energy was minimized. The T1R1/T1R3 receptor model constructed by our team was used [13]. The receptor binding cavity and binding site are the same as those in the report [35]. The molecular docking technique simulated the binding state between the umami peptides of S.rugosoannulata and the receptor protein. The peptide receptor tight binding complexes are screened according to the ligand-receptor binding score, the number of binding bonds, and the binding bond energy. The mode of action between the ligand and the receptor, the critical amino acid residues, and the structure-taste activity relationship of the umami peptide is determined.
2.6. Statistical analysis
The data collected by LC-MS/MS were retrieved and analyzed by Peaks software. Sankey line pathway enrichment plot was used to analyze and visualize peptide and its protein precursor data.
3. Result
3.1. Screening of umami peptides and peptide distribution assay
According to the prediction of BIOPEP-UWM, there are 46 types of umami peptides in the traditional peptide base material prepared by traditional enzyme hydrolysis, and the abundance of MS is 3.10E + 08. There are 132 types of umami peptides in the peptide base material prepared by simultaneous ultrasonic-assisted enzymatic hydrolysis, and the abundance of MS is 5.74E + 08 (Fig. 1A). In the two substrates, there are 46 kinds of common umami peptide. The abundance of common umami peptides in the peptide base material prepared by synchronous ultrasonic-assisted enzymatic hydrolysis is 3.53E + 08. The total abundance of common peptides in the two substrates is similar, with a difference in the proportion of peptide distribution (Fig. 1B). It can be inferred that ultrasound may improve the enzymatic hydrolysis sensitivity of the substrate protein, expose new sites of enzymatic hydrolysis of the protein, and increase the type and content of peptides in the hydrolysate products. The 86 unique umami peptides produced by simultaneous ultrasound-assisted enzymatic hydrolysis are the main reason for the taste improvement of the base material (Fig. 1C).
Fig. 1.
Distribution of peptides in base materials prepared by synchronous ultrasound-assisted enzymatic hydrolysis and traditional enzymatic hydrolysis. Note: A, The distribution map of peptides prepared by simultaneous ultrasound-assisted enzymatic hydrolysis. B, The distribution map of common peptides is prepared by simultaneous ultrasound-assisted enzymatic hydrolysis and traditional enzymatic hydrolysis. C, The distribution of specific peptides prepared by simultaneous ultrasound-assisted enzymatic hydrolysis. 7P−18P stands for heptapeptide - octadecapeptide. The abbreviation US_ Peptide represents simultaneous ultrasound-assisted enzymatic hydrolysis, and the abbreviation E_ Peptide stands for traditional enzymatic hydrolysis.
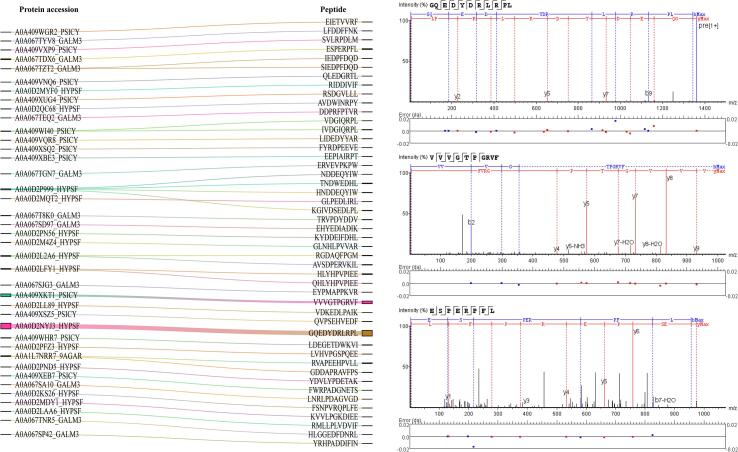
Among the 86 unique umami peptides, the number of octapeptide to undecapeptide peptides reached 47, and the MS abundance represented 82.28 % (MS abundance 1.82E + 08). The number and abundance of peptides were at a high level. Therefore, the relationship between the structure and taste activity of the 47 umami peptides of S.rugosoannulata was analyzed. The 47 types of umami peptides come from 39 protein sequences. A0A0D2P999_ HYPSF is the precursor protein with the most umami peptides identified. Information on 47 umami peptides of S.rugosoannulata is shown in Fig. 2. According to the prediction of BIOPEP-UWM, among 47 types of umami peptides of S.rugosoannulata, 22 types of amino acid combinations play a role in umami characteristics. There are many combinations of amino acids with umami characteristics composed of acidic amino acids, such as ED (12), DD (9), EE (7), and DE (6). Many previous studies have shown that the peptides contain umami amino acids D and E and their combinations, and the peptides have umami characteristics. Also, through BIOPEP-UWM screening, VD (6), DG (5), ET (4), VV (4), AD (3), DA (3), VE (3), DDE (3), EDF (2), EV (2), PE (2), VG (2), DED (1), EG (1), ES (1), EY (1), KG (1), NP (1) in the S.rugosoannulata peptides were also combinations of amino acids that promoted umami taste of the peptides.
Fig. 2.
Information of 47 umami peptides and their precursor proteins prepared by simultaneous ultrasound-assisted directional enzymatic hydrolysis.
3.2. Taste verification of umami peptides
GQEDYDRLRPL, VVVGTPGRVF, ESPERPFL, and HLYHPVPIEE are the top 10 % of the 47 kinds of umami peptides of S.rugosoannulata in terms of MS abundance. Four kinds of umami peptides were synthesized, and taste characteristics were validated. The results of the electronic tongue showed that the four synthetic peptides all had umami characteristics, and the umami taste thresholds were 116.98 umol/L, 402.96 umol/L, 254.39 umol/L, and 639.78 umol/L, respectively. It can be seen that the results of the umami peptide obtained based on the prediction and screening of BIOPEP-UWM are reliable. Therefore, further molecular docking was used to analyze the taste structure-taste activity relationship of 47 umami peptides.
3.3. Structure-taste activity relationship of umami peptide
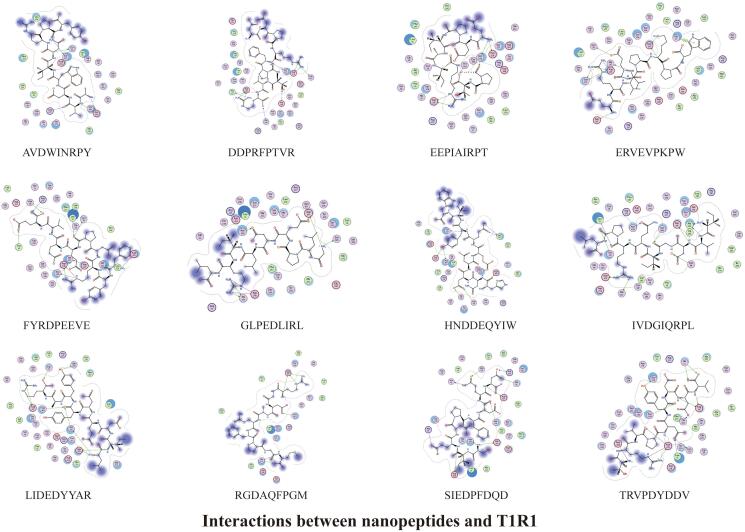
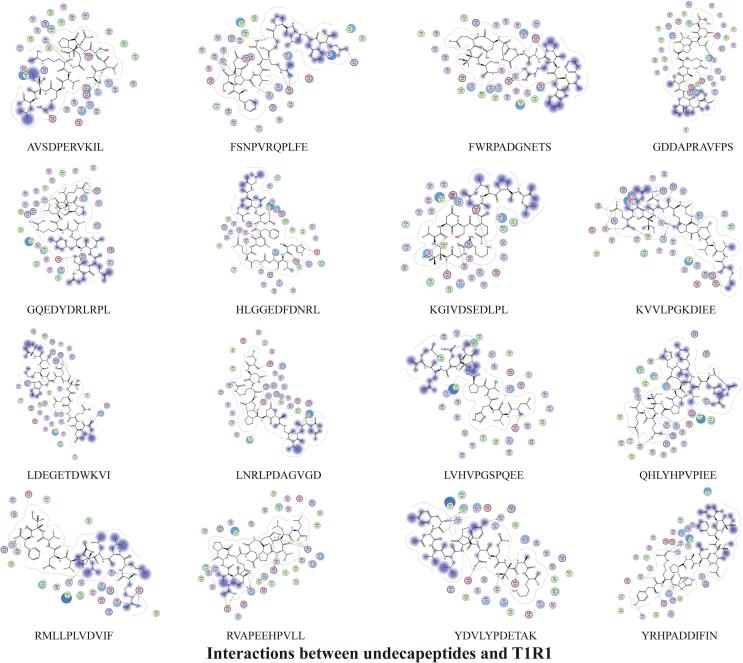
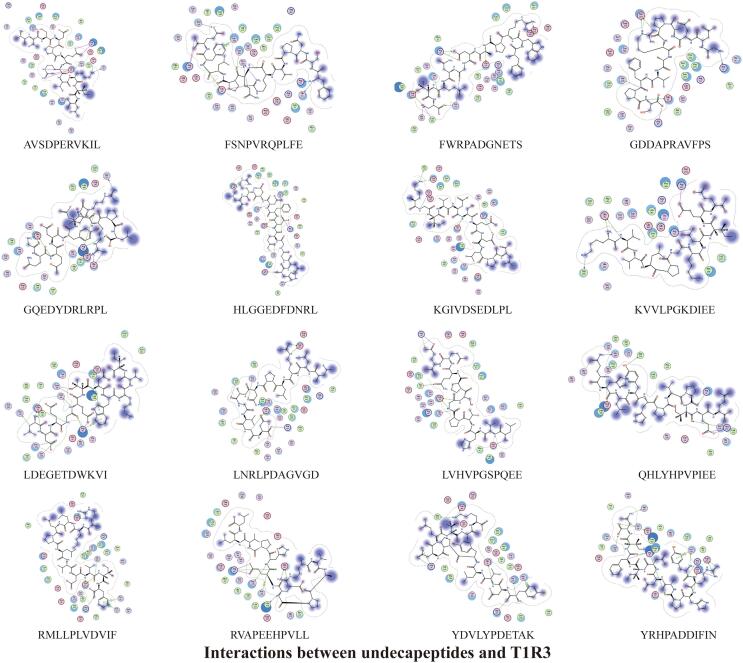
The docking results of 47 umami peptides from S.rugosoannulata with T1R1/T1R3 showed that many hydrogen bonds, ionic bonds, and a small amount of H-pi bonds could be formed between peptides and receptors. Hydrogen bonds and electrostatic interactions are the main interaction modes between the peptides and the receptors. The peptide's N-terminal and C-terminal amino acid residues play an essential role in the docking with the receptors to form stable complexes (Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, Fig. 11, Fig. 12, Fig. 13, Supplementary Tables 1–8).
Fig. 3.
Docking results of umami octapeptides and T1R1.
Fig. 4.
Docking results of umami octapeptides and T1R3.
Fig. 5.
Docking and overlapping results of umami octapeptides and T1R1/T1R3.
Fig. 6.
Docking results of umami nonapeptides and T1R1.
Fig. 7.
Docking results of umami nonapeptides and T1R3.
Fig. 8.
Docking and overlapping results of umami nonapeptides and T1R1/T1R3.
Fig. 9.
Docking results of umami decapeptides and T1R1/T1R3.
Fig. 10.
Docking and overlapping results of umami decapeptides and T1R1/T1R3.
Fig. 11.
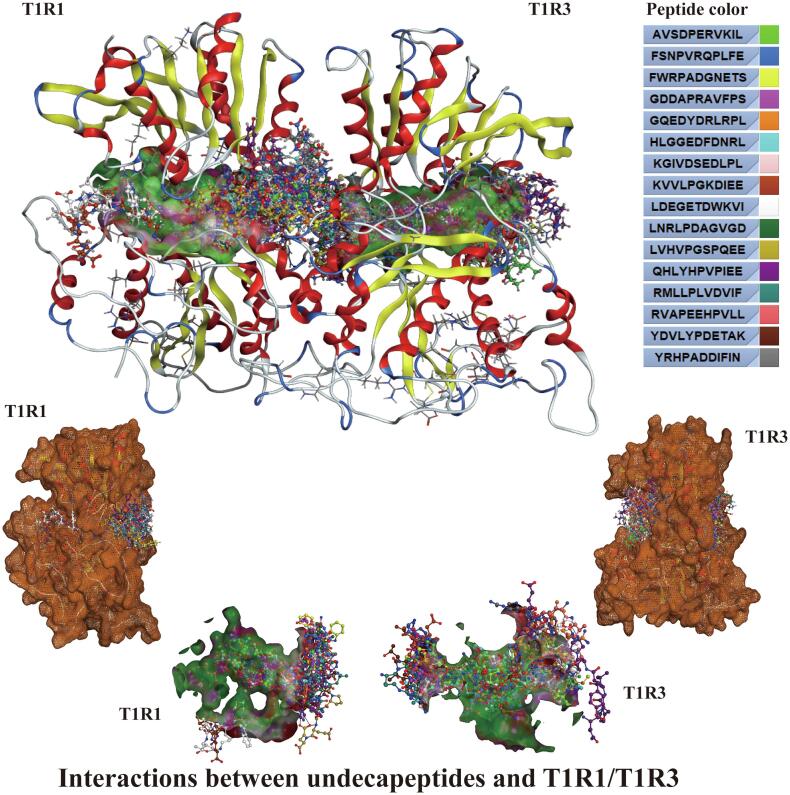
Docking results of umami undecapeptides and T1R1.
Fig. 12.
Docking results of umami undecapeptides and T1R3.
Fig. 13.
Docking and overlapping results of umami undecapeptides and T1R1/T1R3.
3.3.1. Structure-taste activity relationship of umami octapeptide
The amino acid residues E1, I1, Q1, R1, S1, V1, D8, K8, L8, and F8 at the N-terminal and C-terminal of the octapeptide have low binding energy (E < −4.5 kcal/mol) and solid binding ability with the amino acid residues in the T1R1/T1R3 receptors (the E value is low, the binding ability is strong). In the peptides, the amino acids binding to T1R1 and T1R3 differ. The amino acids in the peptide-binding T1R1 receptor are mainly D, E, and R. The amino acid residues D, E, R, V, F, N, I, and L contribute significantly to the binding of the octapeptide to T1R3. The N-terminal and C-terminal amino acid residues of the octapeptide bound to T1R1/T1R3 include acid amino acids, basic amino acids, hydrophobic amino acids, and uncharged polar amino acids. For the amino acids distributed at the N and C terminals of the peptides, the position of the peptide terminus has more influence on the binding of the peptides to the receptors than the nature of the amino acid itself. For amino acid residues in the peptide, the oxygen atom in the peptide's acidic amino acids D and E acts as the H acceptor, and the NH and NH2 groups of the basic amino acid R act as the H donor, which can combine with the receptor amino acid residues to form hydrogen bond interactions. R can also form ion bonds with receptor amino acid residues to generate electrostatic interactions. The results above indicate that the type of amino acid residues in the peptide has a more significant impact on the binding of the peptides to the receptors.
The properties and quantity of amino acid residues of peptide-binding receptors in octapeptide were counted. The number of interaction bonds between the octapeptide and T1R1 is the largest in acidic and basic amino acids, and the total number of both amino acids is 39. Hydrophobic amino acids form 30 functional bonds with T1R1, and uncharged polar amino acids form 20 functional bonds with T1R1. R is the amino acid residue that forms the most interaction bonds (34) with T1R1 in the octapeptide, followed by E (25), L (15), D (14), Q (9), F (5), and V (5). The interaction bonds between the octapeptide and T1R3 are still the most acidic amino acid, totaling 52, followed by the basic amino acid (33), the hydrophobic amino acid (27), and the uncharged polar amino acids (18). D is the amino acid residue that forms the most effective bonds (38) with T1R3 among the octapeptide, followed by R (27), E (14), and L (10). F, K, N, and Q form six binding bonds.
The information on amino acid residues in the receptor to which the octapeptide binds is counted. There are 28 active amino acid residues in T1R1 bound by octapeptide. Among them, ASP147 (19), ARG277 (15), ASP108 (10), ASP219 (14), ASN150 (8), and HIS71 (8) are the primary amino acid active sites in the T1R1 receptor to which the octapeptide binds. There are 26 active amino acid residues in T1R3 bound by octapeptide. ARG64 (26), GLU45 (26), ARG252 (17), and GLU48 (11) are the primary amino acid active sites in the T1R3 receptor to which the octapeptide binds. The active sites of the T1R1 amino acids bound by octapeptide account for 49 % of the active sites in the cavity, and the active sites of the T1R3 amino acids bound by octapeptide account for 49 % of the active sites in the cavity (Fig. 3, Fig. 4, Fig. 5, Supplementary Tables 1–2).
3.3.2. Structure-taste activity relationship of umami nonapeptide
The A1, D1, E1, F1, G1, H1, I1, R1, S1, and T1 at the N-terminal of the nonapeptides have low binding energy (E value < −4.5 kcal/mol) to the T1R1/T1R3 receptor and play a significant role in the stability of receptor-ligand conjugates. R9, Y9, and L9 are amino acid residues with strong C-terminal binding forces and outstanding contributions. R, D, and V are the peptide's primary amino acid residues that bind to T1R1. N, R, D, K, E, and V are the primary amino acid residues that bind to T1R3 in the nonapeptide, and the number of interaction bonds formed between the nonapeptide and T1R3 is more significant than that between the nonapeptide and T1R1.
Among nonapeptides, basic amino acids and T1R1 form the most active bonds (67), followed by acid amino acids (33), hydrophobic amino acids (27), and uncharged polar amino acids (27). R is the amino acid residue that forms the most interaction bonds (61) with T1R1 in the nonapeptide, followed by E (19), D (14), Y (9), and V (7). The bonds formed between the nonapeptide and T1R3 are still the most acid amino acids (66), followed by basic amino acids (47), hydrophobic amino acids (32), and uncharged polar amino acids (28). R is the amino acid residue with the most significant number of bonds (42), followed by D (40), E (26), F (8), S (7), and N (7), and V and W all form six bonds.
There are 25 active amino acid residues in T1R1 bound by nonapeptides, mainly ASP147 (39), ASP219 (33), ASP108 (12), ASN150 (10), and SER217 (7). Twenty-eight active amino acid residues in T1R3 are bound by nonapeptide. ARG64 (42), GLU45 (30), GLU48 (19), ARG252 (14), and ASP215 (7) are the central binding active amino acid residues. Active sites of T1R1 amino acids bound to nonapeptides account for 44 % of active sites in the cavity, and active sites of T1R3 amino acids bound to nonapeptide account for 53 % of active sites in the cavity (Fig. 6, Fig. 7, Fig. 8, Supplementary Tables 3–4).
3.3.3. Structure-taste activity relationship of umami decapeptide
E1, G1, H1, K1, V1, K10, R10, E10, and F10 at the decapeptide N- terminal and C-terminal are the amino acid residues that contribute more significantly to the binding of the peptides with T1R1/T1R3. The E-binding energy value of Y, H, D, E, K, V, and R in the peptides with the receptor is low (E < −4.5 kcal/mol), which is the leading force in maintaining stable binding of the receptor and ligand.
The basic amino acid in decapeptide forms the most interaction bond (39) with T1R1, followed by the acid amino acid (25), the hydrophobic amino acid (25), and the uncharged polar amino acids (14). The amino acid residues in the decapeptide that bond to T1R1 are mainly K (22), followed by E (15), R (11), D (10), and V (10). F, H, and Y all form six bonds. The interaction bonds between the decapeptide and T1R3 are the acid amino acid (48), followed by the basic amino acid (36), the hydrophobic amino acid (18), and the uncharged polar amino acids (3). In the decapeptide, E is the amino acid residue that forms the most interaction bonds (31) with T1R3, followed by D (17), R (17), K (11), V (10), and H (8).
There are 24 types of active amino acid residues that bind to the decapeptide in the T1R1 cavity, mainly including ASP219 (20), ASP147 (14), CYS50 (8), and GLN222 (6). Twenty-four active amino acid residues are bound by decapeptide in the T1R3 cavity. ARG64 (30), GLU48 (20), GLU45 (12), and GLU47 (8) are the primary amino acid residues bound by decapeptide to the T1R3 receptor. The active sites of the T1R1 amino acids bound to the decapeptide account for 42 % of the active sites in the cavity, and the active sites of the T1R3 amino acids bound to the decapeptide account for 45 % of the active sites in the cavity (Fig. 9, Fig. 10, Supplementary Tables 5–6).
3.3.4. Structure-taste activity relationship of umami undecapeptide
It can be seen from the docking results of undecapeptide with T1R1/T1R3 that amino acid residues with low binding energy E values at the N-terminal and C-terminal are mainly A1, G1, H1, K1, L1, Q1, R1, Y1, S11, E11, F11, and K11. D, K, R, I, V, E, A, L, S, and F in the peptides contribute significantly to the stable binding of the peptides to the receptors. At the same time, the number of interaction bonds between undecapeptide and T1R3 is higher than that between undecapeptide and T1R1.
The basic amino acid and T1R1 form the most active bonds (64), followed by the hydrophobic amino acid (46), the acid amino acid (42), and the uncharged polar amino acids (30). The amino acid residues in the undecapeptide that form bonds with T1R1 are mainly R (38), followed by E (22), D (20), L (19), K (16), H (10), Y (10), P (7), V (7), and A, G, and S all form six bonds. Among undecapeptides, the number of basic amino acids (71) and acid amino acids (69) that form bonds with T1R3 does not differ significantly, followed by uncharged polar amino acids (40) and hydrophobic amino acids (36). R is the amino acid residue that forms the most interaction bonds (50) with T1R3 in the undecapeptide, followed by E (41), D (28), K (16), F (13), G (12), L (11), S (9), Q (7) and Y (6).
There are 36 types of active amino acid residues that interact with undecapeptide in the cavity of the T1R1 receptor, mainly including ASP147 (28), ASP219 (28), ARG277 (15), ASP108 (12), GLN222 (9), SER276 (9), ARG281 (7), ASP218 (7) and GLN278 (7). Thirty-five types of active amino acid residues bind to undecapeptide in the cavity of the T1R3 receptor. ARG64 (46), GLU45 (32), GLU148 (21), ARG54 (15), ASP215 (13), ASN68 (9), GLU48 (9), ASP307 (8), and HIS278 (7) are the primary amino acid residues in the T1R3 receptor that interact with undecapeptide. The active sites of the T1R1 amino acids bound to undecapeptide represented 63 % of the active sites in the cavity, and the active sites of the T1R3 amino acids bound to undecapeptide represented 65 % of the active sites in the cavity (Fig. 11, Fig. 12, Fig. 13, Supplementary Tables 7–8).
In summary, 47 types of umami peptides of S.rugosoannulata can enter the T1R1/T1R3 receptor cavity (Fig. 5, Fig. 8, Fig. 10, Fig. 13), combine with the active amino acid residues of the receptor, and form a complex of receptor-peptide dominated by hydrogen bond interaction and electrostatic interaction. The peptides' N-terminal and C-terminal amino acid residues are essential in binding with receptors to form stable complexes. The molecular characteristics of amino acids in peptides, such as the H donor or H acceptor, significantly affect the binding of peptides to receptors. D, E, and R are essential in binding the peptides (octapeptide to undecapeptide) to T1R1/T1R3. Also, peptides with different chain lengths have different binding receptor characteristics. For example, L and Q in the octapeptide, K and V in the decapeptide, and K and L in the undecapeptide significantly contribute to the number and energy of bond formation.
From the statistics of amino acids bound to T1R1/T1R3 in the octapeptide - undecapeptide, it can be seen that the basic amino acids in the peptides are more easily bound to T1R1, with the binding number of 39–67. Acidic amino acids are more easily bound to T1R3, with a binding amount of 48–69. It shows that the recognition peptides of receptor T1R1 and T1R3 have their specificity, and the same peptide will have differences in receptor recognition, receptor binding, taste presentation intensity, and taste presentation properties.
The active amino acid sites in T1R1 bound by the peptides (octapeptide - undecapeptide) accounted for 42 %−63 % of the active sites in the cavity. There are 18 common active amino acid sites in the T1R1 cavity bound by octapeptide - undecapeptide. Among them, ASP147 and ASP219 form many binding bonds with peptides, which are the critical amino acid residues for T1R1 to recognize the umami peptide of S.rugosoannulata. Active amino acid sites in T1R3 bound to the peptide accounted for 45 %−65 % of active sites in the cavity. There are 13 common active amino acid sites in the T1R3 cavity, and ARG64, GLU45, and GLU48 are the critical amino acid residue for T1R3 to recognize the umami peptide of S.rugosoannulata.
4. Discussion
The peptide has a specific stable structure in the free state. In the binding process to the receptor, there is a dynamically induced change process, which directly affects its role on the oral taste receptor and causes the perception of a sour, sweet, bitter, salty, or umami taste. For short-chain taste peptides (dipeptides and tripeptides), their taste characteristics depend mainly on their amino acid composition. Umami amino acids (D and E) are essential amino acids for short-chain umami peptides. With the extension of the peptide chain, the influence of the spatial structure of the peptides on the taste characteristics gradually deepens. Factors such as the type of amino acids that make up the peptides, the location of amino acids, the nature of amino acids distributed outside the 3D spatial structure, and their side chain groups increase the complexity of receptor recognition peptides, which has a substantial impact on the binding of peptides and receptors. Therefore, it is essential to clarify the structure-taste activity relationship of umami peptides to understand their taste mechanism and guide their development and application.
Bu et al. separated, purified, and identified the umami peptides (MTNLLEDLSFR, GFGDSCTPGKNER, YADSNIQINGTDR, AREALQELGEQAK, IQQDDCK, QAEADAR) from Thai fish sauce. The 3D-QSAR results showed that the peptide had a significant spatial effect when combined with the T1R1/T1R3 receptor [16]. Wang et al. identified the umami peptides from the pufferfish (Takifugravidus) and found that ASP147 is the primary amino acid residue of the DVILPVPAF (DF9), AMLEQVAMTDK (AK11), IGAEVYHNLK (IK10) and GGKLVVDGHAIT (GT12) peptide that binds to the T1R1 receptor. I3, A1, H7, and K3 are the binding amino acid residues in the peptide, respectively. ASP219 is the primary amino acid residue that AK11 and GT12 bind to the T1R1 receptor, and Q5 and H9 are the binding amino acid residues in the peptide [18]. ASP147 and ASP219 are also the critical amino acid residues for T1R1 to recognize the umami peptide of S.rugosoannulata. Li et al. screened the umami peptides FAGDDAPR, FSGLDGAK, and FSGLDGSK with a molecular weight of less than 1 k Da from pig bone soup by sensory analysis. The results of the molecular docking simulation show that GLU48 is the critical binding site of T1R3 to which umami peptides bind [8]. Our previous studies also found that GLU45 plays a significant role in the binding interaction between water-extracted umami peptides (EPLCNQ, SGCVNEL, PHEMQ, SEPSHF, and ESCAPQL) and T1R3 [13].
By comparing the docking simulation results of umami peptides and T1R1/T1R3 published by scholars, umami peptides from different sources have rich kinds of binding amino acid residues on the T1R1/T1R3 receptor. It indicates that the receptors recognition peptides have specificity, which leads to differences in taste intensity and taste characteristics of peptides in receptor binding and receptor perception. Hydrogen bonds and electrostatic interaction are basic modes of interaction between umami peptides and receptors [4], [6], [17], [20], [22], [23], [24], [25], [29], [30], [38], [39].
5. Conclusions
The main reason for the umami improvement of the base material prepared by synchronous ultrasound-assisted enzymatic hydrolysis compared to that of the base material prepared by traditional enzymatic hydrolysis is the increase in the type and quantity of umami peptides, which has achieved the effect of umami enhancement. Forty-seven umami peptides from S.rugosoannulata can form hydrogen bond interaction and electrostatic interaction with T1R1/T1R3. The amino acid residues at the N-terminal and C-terminal of the peptides have a solid binding ability with the receptor and make a more significant contribution to the stable complex formed by the receptors and peptides. The molecular characteristics of amino acids in peptides significantly affect the binding of peptides to receptors, and D, E, and R are the critical amino acid residues that bind to the T1R1/T1R3 receptors. Generally, the basic amino acids in the umami octapeptide-undecapeptide are more easily bound to T1R1, and the acidic amino acids are more easily bound to T1R3. The active amino acid sites of the receptors to which the umami peptides bind account for 42 %–65 % of the total active amino acid residues in the receptors. ASP147 and ASP219 are the critical amino acid residues for T1R1 to recognize the umami peptides, and ARG64, GLU45, and GLU48 are the critical amino acid residues for T1R3 to recognize the umami peptides of S.rugosoannulata.
Funding
This research was funded by Shanghai Agriculture Applied Technology Development Program, China (No. 2020-02-08-00-12-F01484), SAAS Program for Excellent Research Team (No. G2022003).
CRediT authorship contribution statement
Wen Li: Conceptualization, Data curation, Formal analysis, Writing – original draft. Wanchao Chen: Software, Validation. Haile Ma: Resources, Supervision. Jinbin Wang: Funding acquisition. Zhengpeng Li: Resources. Qian Wang: Resources. Zhong Zhang: Methodology, Validation. Di Wu: Methodology, Validation. Jingsong Zhang: Project administration. Yan Yang: Funding acquisition, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2022.106206.
Contributor Information
Haile Ma, Email: mhl@ujs.edu.cn.
Yan Yang, Email: yangyan@saas.sh.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Dang Y., Gao X., Ma F., Wu X. Comparison of umami taste peptides in water-soluble extractions of Jinhua and Parma hams. LWT - Food Sci. Technol. 2015;60:1179–1186. doi: 10.1016/j.lwt.2014.09.014. [DOI] [Google Scholar]
- 2.Yu Z., Jiang H., Guo R., Yang B., You G., Zhao M., Liu X. Taste, umami-enhance effect and amino acid sequence of peptides separated from silkworm pupa hydrolysate. Food Res. Int. 2018;108:144–150. doi: 10.1016/j.foodres.2018.02.047. [DOI] [PubMed] [Google Scholar]
- 3.Begum N., Raza A., Song H., Iftikhar M., Zhang Y., Zhang L., Liu P. Fractionation and identification of flavor peptides from bovine bone extract after enzymatic hydrolysis and Maillard reaction by consecutive chromatography. J. Food Process. Preserv. 2021;45:e15778. doi: 10.1111/jfpp.15778. [DOI] [Google Scholar]
- 4.Chen M., Gao X., Pan D., Xu S., Zhang H., Sun Y., He J., Dang Y. Taste characteristics and umami mechanism of novel umami peptides and umami-enhancing peptides isolated from the hydrolysates of Sanhuang Chicken. Eur. Food Res. Technol. 2021;247:1633–1644. doi: 10.1007/s00217-021-03734-w. [DOI] [Google Scholar]
- 5.Kong Y., Yang X., Ding Q., Zhang Y.Y., Sun B.G., Chen H.T., Sun Y. Comparison of non-volatile umami components in chicken soup and chicken enzymatic hydrolysate. Food Res. Int. 2017;102:559–566. doi: 10.1016/j.foodres.2017.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Liang L., Duan W., Zhang J., Huang Y., Zhang Y., Sun B. Characterization and molecular docking study of taste peptides from chicken soup by sensory analysis combined with nano-LC-Q-TOF-MS/MS. Food Chem. 2022;383 doi: 10.1016/j.foodchem.2022.132455. [DOI] [PubMed] [Google Scholar]
- 7.Gao B., Hu X., Xue H., Li R., Liu H., Han T., Ruan D., Tu Y., Zhao Y. Isolation and screening of umami peptides from preserved egg yolk by nano-HPLC-MS/MS and molecular docking. Food Chem. 2022;377 doi: 10.1016/j.foodchem.2021.131996. [DOI] [PubMed] [Google Scholar]
- 8.Liang L., Zhou C., Zhang J., Huang Y., Zhao J., Sun B., Zhang Y. Characteristics of umami peptides identified from porcine bone soup and molecular docking to the taste receptor T1R1/T1R3. Food Chem. 2022;387 doi: 10.1016/j.foodchem.2022.132870. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J., Zhao M., Su G., Lin L. Identification and taste characteristics of novel umami and umami-enhancing peptides separated from peanut protein isolate hydrolysate by consecutive chromatography and UPLC-ESI-QTOF-MS/MS. Food Chem. 2019;278:674–682. doi: 10.1016/j.foodchem.2018.11.114. [DOI] [PubMed] [Google Scholar]
- 10.Bao X., Ma S., Fu Y., Wu J., Zhang M. Sensory and structural characterization of umami peptides derived from sunflower seed. CyTA – J. Food. 2020;18:485–492. doi: 10.1080/19476337.2020.1778794. [DOI] [Google Scholar]
- 11.Kong Y., Zhang L.L., Zhao J., Zhang Y.Y., Sun B.G., Chen H.T. Isolation and identification of the umami peptides from shiitake mushroom by consecutive chromatography and LC-Q-TOF-MS. Food Res. Int. 2019;121:463–470. doi: 10.1016/j.foodres.2018.11.060. [DOI] [PubMed] [Google Scholar]
- 12.Xu X., Xu R., Song Z., Jia Q., Feng T., Huang M., Song S. Identification of umami-tasting peptides from Volvariella volvacea using ultra performance liquid chromatography quadrupole time-of-flight mass spectrometry and sensory-guided separation techniques. J. Chromatogr. A. 2019;1596:96–103. doi: 10.1016/j.chroma.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Chen W., Li W., Wu D., Zhang Z., Chen H., Zhang J., Wang C., Wu T., Yang Y. Characterization of novel umami-active peptides from Stropharia rugoso-annulata mushroom and in silico study on action mechanism. J. Food Compos. Anal. 2022;110:104530. [Google Scholar]
- 14.Liang J., Chen L., Li Y.-N., Hu X. Isolation and identification of umami-flavored peptides from Leccinum extremiorientale and their taste characteristic. J. Food Process. Preserv. 2021;45(3):e15255. doi: 10.1111/jfpp.15255. [DOI] [Google Scholar]
- 15.Song S., Zhuang J., Ma C., Feng T., Yao L., Ho C.T., Sun M. Identification of novel umami peptides from Boletus edulis and its mechanism via sensory analysis and molecular simulation approaches. Food Chem. 2023;398 doi: 10.1016/j.foodchem.2022.133835. [DOI] [PubMed] [Google Scholar]
- 16.Bu Y., Liu Y., Luan H., Zhu W., Li X., Li J. Characterization and structure-activity relationship of novel umami peptides isolated from Thai fish sauce. Food Funct. 2021;12:5027–5037. doi: 10.1039/d0fo03326j. [DOI] [PubMed] [Google Scholar]
- 17.Zhu W., He W., Wang F., Bu Y., Li X., Li J. Prediction, molecular docking and identification of novel umami hexapeptides derived from Atlantic cod (Gadus morhua) Int. J. Food Sci. Technol. 2020;56:402–412. doi: 10.1111/IJFS.14655. [DOI] [Google Scholar]
- 18.Wang W., Yang L., Ning M., Liu Z., Liu Y. A rational tool for the umami evaluation of peptides based on multi-techniques. Food Chem. 2022;371 doi: 10.1016/j.foodchem.2021.131105. [DOI] [PubMed] [Google Scholar]
- 19.Yu X., Zhang L., Miao X., Li Y., Liu Y. The structure features of umami hexapeptides for the T1R1/T1R3 receptor. Food Chem. 2017;221:599–605. doi: 10.1016/j.foodchem.2016.11.133. [DOI] [PubMed] [Google Scholar]
- 20.Yang D., Li C., Li L., Chen S., Hu X., Xiang H. Taste mechanism of umami peptides from Chinese traditional fermented fish (Chouguiyu) based on molecular docking using umami receptor T1R1/T1R3. Food Chem. 2022;389 doi: 10.1016/j.foodchem.2022.133019. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z., Zhu Y., Wang W., Zhou X., Chen G., Liu Y. Seven novel umami peptides from Takifugu rubripes and their taste characteristics. Food Chem. 2020;330 doi: 10.1016/j.foodchem.2020.127204. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y., Gao X., Pan D., Zhang Z., Zhou T., Dang Y. Isolation, characterization and molecular docking of novel umami and umami-enhancing peptides from Ruditapes philippinarum. Food Chem. 2021;343 doi: 10.1016/j.foodchem.2020.128522. [DOI] [PubMed] [Google Scholar]
- 23.Shiyan R., Liping S., Xiaodong S., Jinlun H., Yongliang Z. Novel umami peptides from tilapia lower jaw and molecular docking to the taste receptor T1R1/T1R3. Food Chem. 2021;362 doi: 10.1016/j.foodchem.2021.130249. [DOI] [PubMed] [Google Scholar]
- 24.Li X., Xie X., Wang J., Xu Y., Yi S., Zhu W., Mi H., Li T., Li J. Identification, taste characteristics and molecular docking study of novel umami peptides derived from the aqueous extract of the clam meretrix meretrix Linnaeus. Food Chem. 2020;312 doi: 10.1016/j.foodchem.2019.126053. [DOI] [PubMed] [Google Scholar]
- 25.Deng X., Lin H., Ahmed I., Sui J. Isolation and identification of the umami peptides from Trachinotus ovatus hydrolysate by consecutive chromatography and Nano-HPLC-MS/MS. LWT - Food Sci Technol. 2021;141:110887. [Google Scholar]
- 26.Zhang T., Hua Y., Zhou C., Xiong Y., Pan D., Liu Z., Dang Y. Umami peptides screened based on peptidomics and virtual screening from Ruditapes philippinarum and Mactra veneriformis clams. Food Chem. 2022;394 doi: 10.1016/j.foodchem.2022.133504. [DOI] [PubMed] [Google Scholar]
- 27.Zhuang M., Lin L., Zhao M., Dong Y., Sun-Waterhouse D., Chen H., Qiu C., Su G. Sequence, taste and umami-enhancing effect of the peptides separated from soy sauce. Food Chem. 2016;206:174–181. doi: 10.1016/j.foodchem.2016.03.058. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X., Sun-Waterhouse D., Chen J., Cui C., Wang W. Comparative study on the novel umami-active peptides of the whole soybeans and the defatted soybeans fermented soy sauce. J. Sci. Food Agric. 2021;101:158–166. doi: 10.1002/jsfa.10626. [DOI] [PubMed] [Google Scholar]
- 29.Amin M.N.G., Kusnadi J., Hsu J.L., Doerksen R.J., Huang T.C. Identification of a novel umami peptide in tempeh (Indonesian fermented soybean) and its binding mechanism to the umami receptor T1R. Food Chem. 2020;333 doi: 10.1016/j.foodchem.2020.127411. [DOI] [PubMed] [Google Scholar]
- 30.Zhu W., Luan H., Bu Y., Li X., Li J., Zhang Y. Identification, taste characterization and molecular docking study of novel umami peptides from the Chinese anchovy sauce. J. Sci. Food Agric. 2021;101:3140–3155. doi: 10.1002/jsfa.10943. [DOI] [PubMed] [Google Scholar]
- 31.Dang Y., Hao L., Zhou T., Cao J., Sun Y., Pan D. Establishment of new assessment method for the synergistic effect between umami peptides and monosodium glutamate using electronic tongue. Food Res. Int. 2019;121:20–27. doi: 10.1016/j.foodres.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Dang Y., Hao L., Cao J., Sun Y., Zeng X., Wu Z., Pan D. Molecular docking and simulation of the synergistic effect between umami peptides, monosodium glutamate and taste receptor T1R1/T1R3. Food Chem. 2019;271:697–706. doi: 10.1016/j.foodchem.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Yang J., Huang Y., Cui C., Dong H., Zeng X., Bai W. Umami-enhancing effect of typical kokumi-active gamma-glutamyl peptides evaluated via sensory analysis and molecular modeling approaches. Food Chem. 2021;338 doi: 10.1016/j.foodchem.2020.128018. [DOI] [PubMed] [Google Scholar]
- 34.Zhang C., Miao Y., Feng Y., Wang J., Tian Z., Dong J., Gao B., Zhang L. Umami polypeptide detection system targeting the human T1R1 receptor and its taste-presenting mechanism. Biomaterials. 2022;287 doi: 10.1016/j.biomaterials.2022.121660. [DOI] [PubMed] [Google Scholar]
- 35.Li W., Chen W., Wu D., Zhang Z., Yang Y. Taste peptides derived from Stropharia rugosoannulata fermentation mycelium and molecular docking to the taste receptor T1R1/T1R3. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.960218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J., Sun-Waterhouse D., Su G., Zhao M. New insight into umami receptor, umami/umami-enhancing peptides and their derivatives: a review. Trends Food Sci. Technol. 2019;88:429–438. doi: 10.1016/j.tifs.2019.04.008. [DOI] [Google Scholar]
- 37.Li W., Chen W., Ma H., Wu D., Zhang Z., Yang Y. Structural characterization and angiotensin-converting enzyme (ACE) inhibitory mechanism of Stropharia rugosoannulata mushroom peptides prepared by ultrasound. Ultrason. Sonochem. 2022;88 doi: 10.1016/j.ultsonch.2022.106074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu Z., Kang L., Zhao W., Wu S., Ding L., Zheng F., Liu J., Li J. Identification of novel umami peptides from myosin via homology modeling and molecular docking. Food Chem. 2021;344 doi: 10.1016/j.foodchem.2020.128728. [DOI] [PubMed] [Google Scholar]
- 39.Zhao W., Su L., Huo S., Yu Z., Li J., Liu J. Virtual screening, molecular docking and identification of umami peptides derived from Oncorhynchus mykiss. Food Sci. Hum. Well. 2023;12:89–93. doi: 10.1016/j.fshw.2022.07.026. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.