Highlights
-
•
β-carotene content of genetically modified orange was 33-fold higher.
-
•
β-carotene-enriched LOJ provided greater antioxidant capacity and stress resistance.
-
•
β-carotene-enriched LOJ reduced β-amyloid proteotoxicity.
-
•
β-carotene-enriched LOJ showed higher hypolipidemic activity in glucose rich diet.
Abstract
Citrus sinensis orange juice is an excellent dietary source of β-carotene, a well-known antioxidant. However, β-carotene concentrations are relatively low in most cultivars. We developed a new orange through metabolic engineering strategy (GS) with 33.72-fold increase in β-carotene content compared to its conventional counterpart (CV). Using Caenorhabditis elegans, we found that animals treated with GS showed a greater reduction in intracellular reactive oxygen species (ROS) which is associated with a greater resistance to oxidative stress and induction of the expression of antioxidant genes. Moreover, animals treated with GS orange showed a more effective protection against β-amyloid proteotoxicity and greater hypolipidemic effect under high glucose diet compared to animals treated with CV. These data demonstrate that the increased amount of β-carotene in orange actually provides a greater beneficial effect in C. elegans and a valuable proof of principle to support further studies in mammals and humans.
1. Introduction
Orange (Citrus X sinensis L. Osbeck) is one of the most important crops in the world from an economic point of view (USDA, 2022), and its consumption has been linked to several health benefits in numerous studies (Farag et al., 2020, Favela-Hernández et al., 2016, Motallaei et al., 2021). With the aim of increasing the health properties of this fruit, an orange type enriched in β-carotene has been developed through metabolic engineering (Pons et al., 2014). This carotenoid, in addition to being a dietary precursor of vitamin A, is an antioxidant under low pressure capable of inhibiting lipid peroxidation, oxidative stress and inflammatory process (Kawata et al., 2018, Marcelino et al., 2020). Its consumption has been related to its role in defense against certain degenerative diseases, such as various types of gastric cancer (Chen et al., 2021, Lee et al., 2022, Peraita-Costa et al., 2022), type 2 diabetes (Marcelino et al., 2020, Nimbalkar et al., 2021) and cardiovascular diseases (Jayedi et al., 2019, Saini et al., 2022). In addition, carotenoids treatment is been associated with neuroprotective effects (Manochkumar, Doss, El-Seedi, Efferth, & Ramamoorthy, 2021). For instance, β-carotene supplementation ameliorates oxidative damage, activates antioxidant enzymes and attenuates β-amyloid aggregation in culture cells and mouse models (Cho et al., 2018, Hira et al., 2019, Park et al., 2020).
Although conventional orange contains suboptimal levels of β-carotene, it is rich in other carotenoids (mainly xanthophylls, which constitute >80 % of total carotenoids), as well as in a wide variety of phytonutrients including vitamin C, flavonoids and other phenolic compounds, which can enhance the beneficial effect of β-carotene, as suggested in the literature (Grosso et al., 2013, Yeum et al., 2004). The strategy carried out to address β-carotene enrichment biotechnologically consisted of silencing the endogenous gene encoding a β-carotene hydroxylase (CsβCHX), involved in the conversion of β-carotene into xanthophylls in the mature fruits, combined with overexpression of the FLOWERING LOCUS T gene from sweet orange (CsFT) in juvenile transgenic sweet orange plants, cv. Pineapple. In this way, transgenic orange seedlings were able to flower and produce regular fruits within a year. Additionally, it was possible to increase (up to 36 times) the content of β-carotene in the pulp of this orange variety through metabolic engineering (Pons et al., 2014).
Due to its high degree of homology to human genome, C. elegans has been widely used for evaluating the protective effects of dietary phytonutrients related to human diseases such as aging, neurodegeneration and obesity (Ayuda-Duran et al., 2020, Kaletta and Hengartner, 2006). Using this model, Pons et al. (2014) demonstrated that C. elegans treated with the β-carotene-enriched oranges (named HRP) exerted a greater antioxidant effect in vivo than the isogenic control oranges (CV; transformed with the empty vector). In these bioassays, the worms that were previously fed with HRP oranges showed a survival rate after acute oxidative stress (induced by treatment with hydrogen peroxide) 20 % higher than the worms previously fed with CV oranges. Recently, it has been demonstrated that C. elegans treated with oranges juices and extracts showed increased longevity (Caland et al., 2019, Wang et al., 2020). Moreover, C. elegans treated with orange juice from cultivars with higher carotenoid contents induced a stronger response against oxidative stress and β-amyloid toxicity (Caland et al., 2019).
In order to further characterize the health benefits of β-carotene enriched oranges, we developed a new transgenic adult Pineapple sweet orange line (GS) transformed with the gene encoding a β-carotene hydroxylase (CsβCHX) in intron-hairpin configuration without the transgene CsFT. We carried out investigations with C. elegans to test the effect of lyophilized orange juices (LOJ) from the new β-carotene enriched oranges (GS) compared to its convention counterpart (CV) on antioxidant status, longevity, proteostasis and fat accumulation.
2. Material and methods
2.1. Strains, chemicals and reagents
Strains: C. elegans strains used in this work was obtained at the Caenorhabditis Genetics Center (CGC), which is funded by the NIH National Center for Research Resources (NCRR): N2 (wild-type strain), CL2006 (dvIs2[pCL12(unc-54/human Abeta peptide 1–42 minigene) + pRF4]), CF1553 (muIs84 [pAD76(sod-3::GFP)]), CL2166 (dvls19[pAF15(gst-4::GFP::NLS)]), LD1171 (ldIs3[gcs-1p::GFP + rol-6(su1006)]), SJ4005 (zcIs4 [hsp-4::GFP; lin-15(n765)]).
Chemical and reagents: tert-Butyl hydroperoxide (TBHP), fluorodeoxyuridine (FUdR), and 2,7-dichlorodihydrofluorescein diacetate (H2DCFDA), Carbobenzoxy-Leu-Leu-leucinal (MG132), Oil Red O, Nile Red and Glucose were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. LOJ preparation
LOJ were obtained from CV and GS transgenic fruit (Figure S1). A total of 30 fruits per line were harvested when fully matured. Fruits were cut and pulp tissue (inner part of the fruit consisting basically on juice vesicles) was separated with a scalpel, frozen in liquid nitrogen, ground to a fine powder using a grinder and stored at −80 °C. Pulp powder samples of each line were weighed and lyophilized for three days using the Alpha 1–2 LDplus −55 °C Freeze Dryer equip (Martin Christ, Harz, GER). Lyophilized pulp samples were weighed to calculate water loss and conserved in dark at room temperature until analysis. Later, LOJ were obtained by reconstitution of lyophilized pulp samples with the corresponding volume of sterile milli-Q water and mixing by vortex. Finally, before adding to the NGM medium to perform the bioassays with C. elegans, LOJ were pretreated overnight with 7 mM Velcorin ® (Lanxess, Cologne, GER) in order to ensure proper sterilization.
2.3. LOJ characterization
2.3.1. Juice quality parameters
Total soluble solid content (SSC), titratable acidity (TA) and maturity index (MI) of LOJs were determined according to AOAC methods (AOAC: 1980. Official Methods of Analysis, 13th ed. N°46024 and N° 22061. Association of Official Analytical Chemists, Washington, DC, USA). SSC was determined in terms of Brix degrees using a refractometer PR-101 model 0–45 % (Atago, Ribeirão Preto, BR). TA was determined by titration with 0.1 N NaOH, using phenolphthalein as a visual endpoint indicator, and was expressed as mg citric acid per 100 g. The MI was estimated as the SSC/TA ratio.
Vitamin C quantification was performed in the Metabolomics Platform at the Instituto de Biología Molecular y Celular de Plantas (IBMCP) (UPV-CSIC) according to Chebrolu, Jayaprakasha, Yoo, Jifon, and Patil (2012) with minor modifications. Briefly, 0.5 mL of LOJ were diluted 1/10 in 2.5 % phosphoric acid on ice. The extract was filtered with 45 µm disposable filters. Two 0.5 mL aliquots were taken. To determine ascorbic acid (AA), 0.5 mL of water was added to one of them, and to determine ascorbic acid + dehydroascorbic acid (TOTAL), 0.5 mL of 5 mM tris (2-carboxy ethyl) phosphine hydrochloride (TCEP) was added to the other one. 4 µL samples were injected in Waters Acquity UPLC system (Milford, MA, USA) coupled to a photodiode array detector. The column used was a Waters BEH C18 UPLC column (particle size 1.7 µM). The mobile phase was composed by 0.1 % formic acid in water (A) and 0.1 % formic acid in acetonitrile (B). The gradient used was: in 10 min from 100 %A to 95 %A at a 0.4 mL/min flow rate. Ascorbic acid eluted at 1.3 min. The ascorbic acid peak was detected at 243 nm. Measurements were performed from three independent samples of LOJ from each orange type (GS and CV), and two-tailed Student’s t-test was performed for means comparison.
2.3.2. Carotenoid extraction and analysis
The extraction of carotenoids from LOJ followed a previously described protocol (Pons et al., 2014). Briefly, 1 mL of each LOJ was centrifuged, and the aqueous phase was removed. Carotenoids were extracted from pellet with successive washings with acetone (2.5 mL) followed by stirring for 5 min and centrifugation for 5 min at 18,000 g, until it was colorless. Saponification was performed by treatment of acetone extracts with 5 mL of methanolic KOH (10 % w/v) for 1 h under dim light at room temperature. The saponified carotenoids were subsequently re-extracted with dichloromethane (10 mL), and washed three times with water. Dichloromethane extracts were dried by rotary evaporation and stored under a nitrogen atmosphere at −20 °C until HPLC analysis. The HPLC analysis method was described previously (Alquezar, Rodrigo, & Zacarias, 2008). Dried carotenoid extracts were retrieved in 30 μL of chloroform/MeOH/acetone (5:3:2 by vol.), and a 25-μL aliquot was immediately injected. Carotenoids were identified by their retention time, absorption and fine spectra (Britton, 1998, Rodrigo et al., 2003, Rodrigo et al., 2004, Rouseff et al., 1996). The carotenoid peaks were integrated at their individual maxima wavelength and their contents were calculated using calibration curves of β-carotene (Sigma) for α- and β-carotene; β-cryptoxanthin (Extrasynthese) for α- and β-cryptoxanthin; zeaxanthin (Extrasynthese) for zeaxanthin and antheraxanthin; lutein (Sigma) for lutein and violaxanthin isomers. Phytoene and phytofluene standards for quantification were obtained from flavedo extracts of Pinalate sweet oranges, which accumulate large amounts of these compounds (Rodrigo et al., 2003), and were then purified by TLC (Pascual, Mallent, & Cuñat, 1993). Empower chromatography software (Waters Corp., Milford, MA) was used for quantification and the analyses were performed in triplicate.
2.3.3. C. elegans culture conditions
C. elegans were cultivated in Nematode Growth Medium (NGM) plates seeded with Escherichia coli OP50 at the temperature 20 °C. For the treatment, the worms were cultivated NGM plates containing 2 % LOJ. The animals of the control group non-treated (NT) were added 2 % sterile water. Synchronized populations were achieved by egg-laying.
2.4. Intracellular reactive oxygen species (ROS) quantification
Intracellular ROS levels were measured under standard and stress conditions in order to evaluate the antioxidant potential of LOJ treatment. Wild-type animals (N2) were synchronized at first-stage larvae (L1) and cultivated for 48 h in NGM plates containing 2 % LOJ. For stress condition, animals were exposed to 10 mM tert-butyl hydroperoxide (TBHP) for 1 h after LOJ treatment. A total of 120 animals were analyzed across three biological replicates for each treatment group in a 96-well microtiter plate to which 50 µM H2DCF-DA was added. For stress condition, after LOJ treatment animals were exposed to 10 mM tert-butyl hydroperoxide (TBHP) for 1 h before being transferred to the 96-well plate. Fluorescent measurements were calculated as the mean values of three wells measured by microplate reader GloMax®- Multi Detection System (Promega, Wisconsin, USA), with excitation at 490 nm and emission at 510–570 nm. Readings were performed every 30 min for 3 h and the reading selected for analysis was the fourth. This experiment was conducted three times.
2.5. Analysis of reporter genes
In order to evaluate whether LOJ treatment induce the expression of antioxidant and chaperonin genes, transgenic lines expressing γ-glutamyl cysteine synthetase (gcs-1::GFP), glutathione S-transferase 4 (gst-4::GFP), manganese superoxide dismutase 3 (sod-3::GFP), and heat shock protein 4 (hsp-4::GFP) were synchronized and then treated with 2 % of LOJ for 48 h from L1 until L4 stage. Twenty-five worms were captured by the optic microscope Olympus BX51 (Tokyo, Japan) and fluorescent signals were measured using NIH ImageJ software. This experiment was conducted three times.
2.6. Oxidative stress resistance assay
In order to evaluate whether LOJ treatment increase the worm’s survival under stress conditions, an oxidative stress resistance assay was performed. Synchronized N2 wild-type animals were cultivated in NGM plates for 48 h from L1 stage until L4 stage. Animals were transferred to either control or 2 % LOJ plates containing FudR, to avoid progeny growth, for another 48 h. Thereafter, 50 worms from each group were incubated with 10 mM TBHP to induce oxidative stress. Survival fractions were scored every-three hours until all animals were determined dead, without any pharyngeal pumping or movement. Animals with internal hatched eggs, extruded parts or those who went missing were eliminated from analysis. This experiment was conducted three times.
2.7. Lifespan assay
In order to evaluate whether LOJ treatment increase the worm’s lifespan, a lifespan assay was conducted. Synchronized N2 wild-type animals were treated with 2 % LOJ for 48 h from L1 stage until L4 stage. Ninety worms per group was distributed in three new treatment plates containing FudR, to prevent progeny growth. The survival analysis was performed by scoring dead/alive animals every 24 h starting at the first day of adulthood at 25 °C. The worms were considered dead when no pharyngeal pumping or movement was observed. This experiment was conducted three times.
2.8. Evaluation of neuromuscular parameters
In order to evaluate whether the antioxidant effects of LOJ treatment was associated with improved neuromuscular parameters, synchronized N2 wild-type animals treated with 2 % LOJ for 48 h from L1 to L4 stage were used to measure body bending and pharyngeal pumping rates. For body bending analysis, 10 animals of each experimental group were transferred to NGM plates without OP50. Body folding movements of 90° or more were considered, as well as two curvatures in a row to the same side. The body bending score was obtained by calculating the average number of bends of three 20-second intervals per animal. To evaluate pharyngeal pumping rates, 10 worms of each group were transferred to NGM without OP50. The pharyngeal pumping rate was obtained by calculating the average number of pharyngeal contractions of three 20-second intervals per animal observed on a 40x objective microscope. These experiments were conducted three times.
2.9. Paralysis-induced by β-amyloid Proteotoxicity.
In order to evaluate whether LOJ treatment could delay the toxic affect β-amyloid accumulation in C. elegans, synchronized CL2006 transgenic animals expressing β-amyloid peptide 1–42 in the muscle were treated in 2 % LOJ for 48 h from L1 until L4 stage. Thirty animals were then transferred to new plates containing 2 % LOJ at 35 °C. The analyzes were made by scoring paralyzed/alive animals every 1 h until all animals were considered paralyzed. The worms were scored paralyzed when failing to present body movement but still kept pharynx pumping. This experiment was conducted three times.
2.10. Quantification of proteasome activity
In order to characterize a possible proteasome activity associated with LOJ treatment, we measured the in vitro 26S proteasome activity as described by Kisselev & Goldberg (2005). Approximately 5,000 synchronized N2 wild-type animals were treated in plates with 2 % LOJ for 48 h from L1 to L4 stage. The worms were then harvested and sonicated. Lysates were centrifuged at 20,000 × g for 30 min at 4 °C. Protein extract was quantified using the QUBIT Protein Assay Kit system (Life technologies, California, EUA). To measure the chymotrypsin-like activity of the proteasome, succinyl-Leu-Leu-Val-Tyr-4-methyl-coumaryl-7-amide (SLLVY-MCA) (Sigma-Aldrich, St. Louis, MO, USA) was used both in the presence or absence of 20 µM MG-132, a proteasome inhibitor, and incubated for 30 min at 37 °C. The fluorescence measurements were made with 380 nm excitation and 440 nm emission, using the GloMax®-Multi Detection System (Promega corporation, Wisconsin, USA), 90 min after the incubation. Proteasome activity was calculated as the difference between the total activity and the activity remaining in the presence of 20 µM MG-132. This experiment was conducted three times.
2.11. Evaluation of lipid distribution by Oil Red O staining
To evaluate whether LOJ treatment could reduce lipid distribution, synchronized N2 wild-type animals were treated with 2 % LOJ in NGM plates containing or not 4 % glucose for 48 h from L1 to L4 stage. Worms were fixed using 40 % isopropanol and stained the lipids with Oil Red O, a fat-soluble dye used for staining of neutral triglycerides and lipids, for 2 h as previously described (Escorcia, Ruter, Nhan, & Curran, 2018). Images of worms were captured using AmScope MU300 Digital Camera plugged to microscope in 10x objective. The evaluation of lipid droplet distribution was made by measuring the red intensity of each animal using ImageJ. We used 15 animals for group to each experiment. This experiment was conducted three times.
2.12. Lysosome organelles (LRO) quantification using Red Nile
To quantify lysosome-related organelles, synchronized N2 wild-type animals were treated 2 % LOJ for 72 h from L1 stage to 1-day old in NGM plates seeded with 4 mg/ml of Red Nile dye, a fluorescent hydrophobic dye used for staining acidic lysosomal organelles. Images of 30 worms were captured using optic microscope Olympus BX51 (Tokyo, Japan) and NIH ImageJ software was used to analyze fluorescent levels. This experiment was conducted three times.
2.13. Statistical analysis
Statistical analysis performed by Graph Pad Prism (v 6.0) software (CA, USA). Student’s t test and one-way ANOVA was used for comparison between pairs of groups, one-way ANOVA followed by Tukey’s posttest was also utilized to compare three or more groups, for normally distributed data. Survival curves were analyzed by the log-rank (Mantel-Cox) test. For all tests, statistical significance was considered as p < 0.05.
3. Results
3.1. Quality and phytochemical characterization of lyophilized orange juice (LOJ) from β-carotene enriched (GS) and control (CV) oranges
First, we evaluated the quality parameters ° Brix, juice acidity, maturity index and vitamin C from GS and CV lyophilized (Table S1). The lyophilized orange juices (LOJ) from GS did not show any statistically significant difference (p < 0.05) compared to CV indicating that both types of oranges are isolines and that the genetic modification introduced in the GS oranges did not affect any of the main quality parameters.
Next, we analyzed the carotenoid profile from LOJ in order to confirm and quantify the carotenoid accumulation in GS oranges (Table 1). LOJ from CV fruit presented a characteristic carotenoid profile of standard sweet orange juice: rich in xanthophylls, with Z-violaxanthin being the main carotenoid (656.77 ng/mL out of a total of 2112.44 ng/mL carotenoids), while its β-carotene content was very low (16.09 ng/mL). In comparison with CV orange, LOJ from GS orange presented a 33.72-fold increase in β-carotene content, as well as a moderate increases in other carotenoids upstream in the carotenoid biosynthesis pathway such as phytoene, phytofluene, ζ-carotene and α-carotene. Concomitantly, a substantial reduction in the content of all the carotenoids downstream in the pathway was detected, that is, in the xanthophylls α- and β-cryptoxanthin, lutein, zeaxanthin, anteraxanthin, E- and Z-violaxanthin. This characteristic profile of LOJ from GS fits well with the blocking strategy of the pathway carried out by metabolic engineering (Table 1; Fig. S1A) and coincides with the results previously reported by Pons et al. (2014).
Table 1.
Content of carotenoids in lyophilized juices from CV and GS oranges.
| Carotenoid (ng/mL) | CV | GS | Fold change (GS/CV) |
|---|---|---|---|
| Phytoene | 385.56 ± 17.15 | 459.82 ± 15.85 | 1.19 |
| Phytofluene | 42.00 ± 12.24 | 98.21 ± 2.90 | 2.34 |
| ζ-carotene | 16.85 ± 6.52 | 96.36 ± 5.41 | 5.72 |
| α-carotene b | n.d. a | 16.03 ± 2.64 | – |
| β-carotene | 16.09 ± 8.31 | 542.59 ± 33.40 | 33.72 |
| α-cryptoxanthin b | 28.41 ± 9.69 | n.d. a | – |
| Lutein | 145.84 ± 11.62 | 85.95 ± 8.95 | 0.59 |
| β-cryptoxanthin | 306.95 ± 10.06 | 99.14 ± 5.92 | 0.32 |
| Zeaxanthin | 100.18 ± 41.57 | 17.39 ± 3.43 | 0.17 |
| Anteraxanthin b | 373.30 ± 24.96 | 40.12 ± 11.16 | 0.11 |
| E-violaxanthin b | 40.48 ± 4.46 | 13.41 ± 3.66 | 0.33 |
| Z-violaxanthin b | 656.77 ± 7.39 | 66.50 ± 5.50 | 0.10 |
| Total carotenoids c | 2112.44 ± 33.58 | 1535.53 ± 66.19 | 0.73 |
n.d., Not detected.
Identified tentatively.
Total carotenoids calculated as the sum of all the carotenoids identified individually.
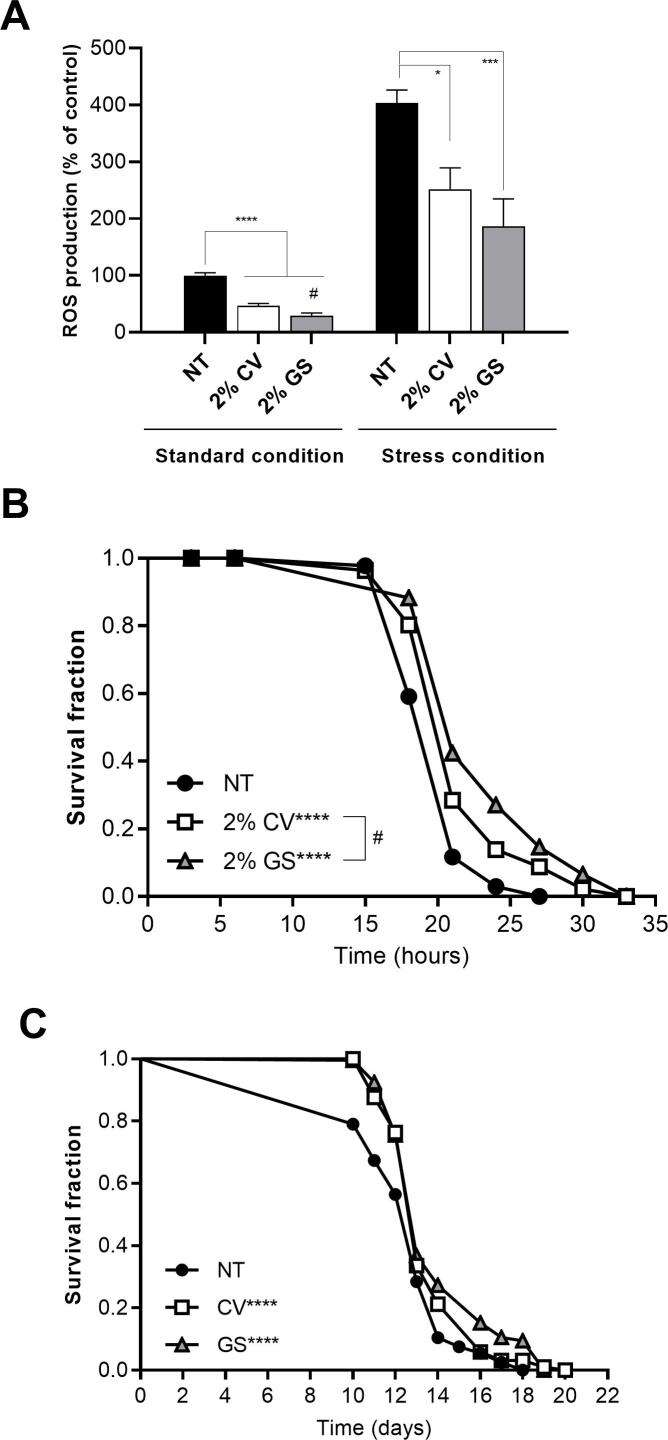
Fig. 1.
Effect of lyophilized orange juice (LOJ) on C. elegans ROS production, stress resistance and longevity. A) ROS was quantified by measuring H2DCFDA fluorescence levels. For standard condition, L1 stage wild-type animals were treated with 2 % of either CV or GS juices for 48 h. **** p < 0.0001 compared to not treated (NT) control by one-way ANOVA. #p = 0.0088 comparing 2 % GS to 2 % CV using two-tailed Student’s t-test. For stress condition, after the worms were treated with 2 % LOJ for 48 h, they were exposed to 10 mM TBHP for 1 h to induce oxidative stress. * p = 0.0130 and *** p = 0.0003 compared to NT control under stress condition by one-way ANOVA. B) Stress resistance assay. L1 stage wild-type animals were treated with 2 % LOJ for 48 h and then incubated with TBHP to induce oxidative stress. Survival fractions were scored every-three hours at 20 °C. **** p < 0.0001 compared to not treated (NT) control and #p = 0.0038 comparing LOJ GS to CV by log rank (Mantel-Cox) test. (b) Lifespan assay. Wild-type animals were treated with 2 % LOJ for 48 h starting at L1 stage. Survival fractions were scored daily at 25 °C. **** p < 0.0001 compared to not treated (NT) control by log rank (Mantel-Cox) test.
3.2. Lyophilized orange juice (LOJ) reduces intracellular ROS production and increases survival under standard and stress conditions
Given that our previously β-carotene-enriched orange juice increased oxidative stress resistance in C. elegans (Pons et al., 2014), we decided to test the effects of lyophilized juice from this new β-carotene-enriched orange on the intracellular ROS accumulation in C. elegans. Previous work has shown that treatment of 2 % of orange juice was the most efficient concentration to reduce ROS production under standard condition. Here, we used the same concentration and observed that 2 % of either CV or GS LOJ juice reduced ROS levels compared to the control group of untreated worms (Fig. 1A). Interestingly, ROS levels were significantly reduced in the animals treated with 2 % GS LOJ compared to those from worms treated with 2 % CV LOJ (Fig. 1A). Under stress conditions, both LOJ also reduced ROS production but no significant difference was observed between animals treated with either 2 % CV or 2 % GS LOJ (Fig. 1A).
We also evaluated how LOJ treatment would affect C. elegans stress resistance and longevity. Animals treated with either 2 % CV or GS LOJ showed increased mean and maximum survival under stress conditions compared to control non treated animals (Fig. 1B, Table S2). Notably, the mean survival time for the animals treated with 2 % GS LOJ were significantly increased compared to animals treated with 2 % CV LOJ (Table S2). LOJ treatment also increased mean and maximum lifespan compared to control non treated, however no statistical difference was observed between the animals treated with either 2 % CV or GS LOJ (Fig. 1C, Table S2).
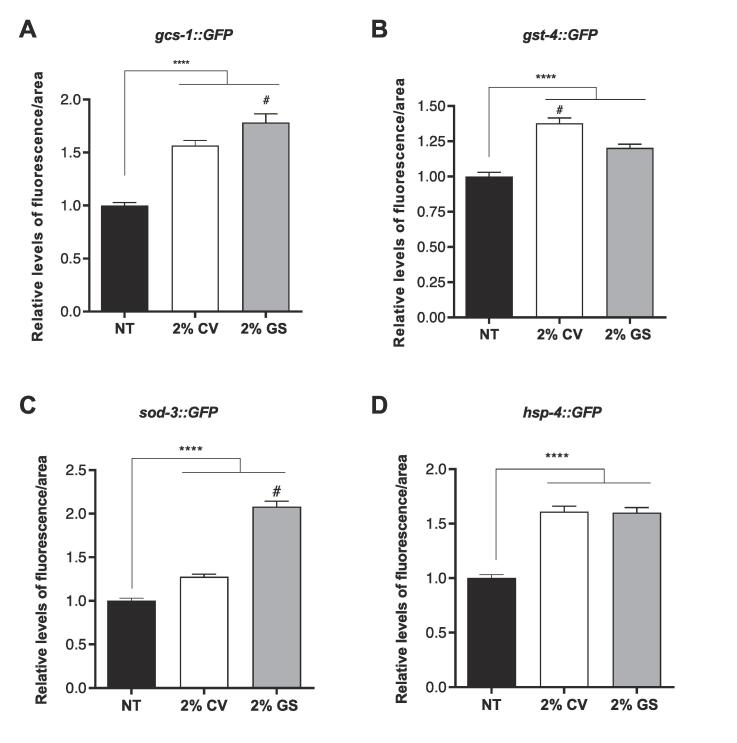
3.3. β-carotene-enriched LOJ increases expression of stress-related genes and oxidative stress resistance
To further characterize our LOJ antioxidant status, we decided to test the effects of LOJ on C. elegans antioxidant and stress-related gene expression. We analyzed the gene expression of four reporter genes associated with detoxification (γ-glutamyl cysteine synthetase, gcs-1 and glutathione S-transferase 4, gst-4), stress resistance and longevity (manganese superoxide dismutase, sod-3) and heat shock protein 4 (hsp-4). The fluorescent levels of gcs-1::GFP, gst-4::GFP, sod-3::GFP and hsp-4::GFP were significantly higher in the animals treated with 2 % LOJ from either CV or GS oranges compared to control non treated animals (Fig. 2A–D). Interestingly, fluorescent levels of gcs-1::GFP and sod-3::GFP in worms treated with 2 % GS juice were significantly increased compared to worms treated with 2 % CV juice (Fig. 2A and C). On the other hand, fluorescent signals of animals expressing gst-4::GFP were significantly higher after treatment with 2 % CV juice compared to animals treated with 2 % GS juice (Fig. 2B). No statistical difference was detected between the animals expressing hsp-4::GFP treated with 2 % LOJ from either CV or GS oranges (Fig. 2D).
Fig. 2.
Expression levels of antioxidant and stress-related genes after treatment with lyophilized oranges juices (LOJ). L1 stage transgenic worms expressing (A) gcs-1::GFP, (B) gst-4::GFP, (C) sod-3::GFP and (D) hsp-4::GFP were treated with 2 % of LOJ for 48 h until L4 stage. Images were taken using a microscope fluorescent and the measure levels fluorescent were using NIH ImageJ software. **** p < 0.0001 compared control not treated (NT) and #p < 0.03 comparing 2 % GS to 2 % CV by one-way ANOVA.
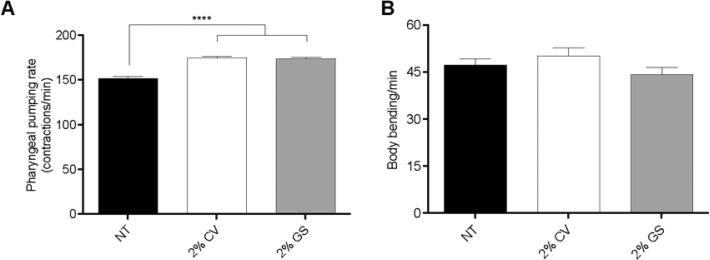
3.4. Lyophilized orange juice (LOJ) does not interfere in neuromuscular functions
In order to test whether LOJ could interfere with C. elegans neuromuscular parameters, we analyzed C. elegans pharynx pumping and body bending rates. Animals treated with either 2 % LOJ CV or GS presented increased pharynx pumping rate compared to NT animals (p < 0.0001) (Fig. 3A). We did not observe a statistical difference between 2 % CV or GS LOJ-treated animals compared to NT in body bending experiment (Fig. 3B).
Fig. 3.
Effect of lyophilized orange juice (LOJ) in C. elegans neuromuscular parameters. A) Pharyngeal pumping rate. L1 stage wild-type animals were treated with LOJ for 48 h until L4 stage. Pharyngeal pumping rate was scored by counting the movements of the pharynx terminal bulb using a microscope in 40x objective. **** p < 0.0001 compared to not treated (NT) control by one-way ANOVA. B) Body bending rate. L1 stage wild-type animals were treated with LOJ for 48 h until L4 stage. Body bending score was obtained through the counting of the animal’s body movements. No statistical difference was found.
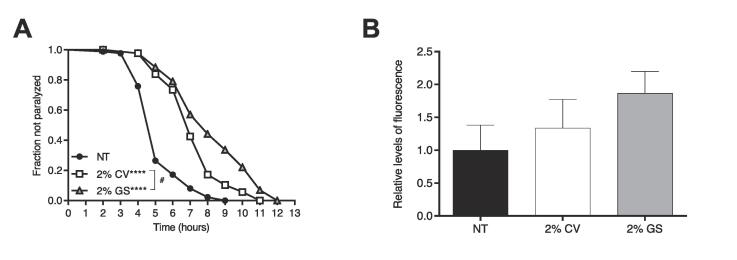
3.5. β-carotene-enriched LOJ reduces β-amyloid proteotoxicity
It is known that β-amyloid and other protein aggregation alongside oxidative stress causes several brain inflammation and neuronal loss (Chen et al., 2012, Currais et al., 2016). Also, the proteasome system plays an important role in protein degradation, contributing to the maintenance of protein homeostasis (Voges, Zwickl, & Baumeister, 1999). Therefore, we decided to test whether LOJ could affect β-amyloid accumulation in a C. elegans model with β-amyloid super expression. CL2006 worms express β-amyloid in the muscle which induces paralysis over time. Worms treated with both 2 % LOJ CV or GS showed a delayed paralysis time compared to NT animals (Fig. 4A, Table S2). Moreover, 2 % GS-treated animals demonstrated an increased mean paralysis time compared to 2 % CV-treated animals (Table S2). Thereafter, we tested whether LOJ treatment could influence C. elegans proteasome activity. Animals treated with either 2 % CV or 2 % GS LOJ showed an increased proteasome activity compared to NT animals, however, no statistical difference was found (Fig. 4B).
Fig. 4.
Effect of lyophilized orange juice (LOJ) on C. elegans proteotoxicity. A) Paralysis-induced by β-amyloid accumulation. L1 stage transgenic worms were treated with 2 % LOJ for 48 h until L4 stage. Analyses were made by scoring paralyzed animals every 1 h at 35 °C. **** p < 0.0001 compared not treated (NT) control and # p = 0.0003 comparing 2 % GS to 2 % CV by log rank (Mantel-Cox) test. B) Quantification of proteasome activity. L1 stage wild-type animals were treated with LOJ for 48 h until L4 stage. Proteasome activity was calculated as the difference between the total activity and the activity remaining in the presence of 20 µM MG-132. Experiment performed in duplicate. No statistical difference was found.
3.6. β-carotene-enriched LOJ promotes higher hypolipidemic activity under glucose rich diet compared to conventional LOJ.
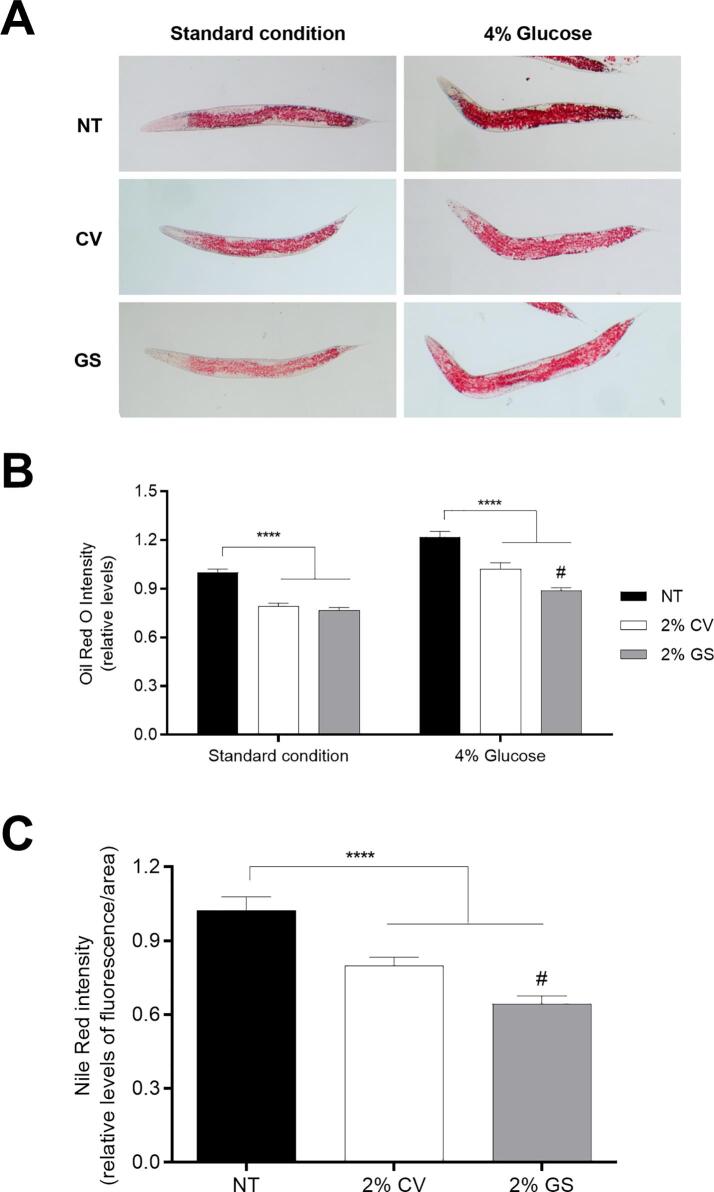
Since excessive fat accumulation could stimulate oxidative stress (Marseglia et al., 2015), we tested whether LOJ could affect fat accumulation in C. elegans under standard and high glucose diet conditions using Oil Red O dye. In standard conditions, worms treated with either 2 % CV or GS LOJ presented less fat accumulation compared to NT animals (Fig. 5A). Similarly, glucose-fed worms treated with either 2 % CV or GS LOJ showed lower fat distribution compared to NT glucose-fed worms (Fig. 5B). Interestingly, levels of lipid distribution on glucose-fed animals treated with 2 % GS LOJ were significantly lower compared to animals treated with 2 % CV LOJ (Fig. 5B). Given that lysosome-related organelles (LRO) are an intestinal compartment for cholesterol storage (Lee et al., 2015), we tested how LOJ treatment would affect LRO accumulation in C. elegans using Red Nile dye. Animals treated with either 2 % CV LOJ or 2 % GS LOJ showed reduced LRO levels compared to NT animals (Fig. 5C). Interestingly, animals treated with 2 % GS LOJ showed less LRO Nile red levels when compared to 2 % CV LOJ animals. These findings could indicate that GS LOJ has a higher hypolipidemic effect compared to CV especially in glucose rich diet.
Fig. 5.
Effect of lyophilized orange juice (LOJ) on C. elegans lipid distribution. A) Oil Red O staining. L1 stage wild-type animals were treated with 2 % LOJ on either NGM plates or 4 % glucose NGM glucose plates for 48 h until L4 stage. Animals were fixed with 40 % isopropanol and lipid droplets were stained with Oil Red O. Images were captured using microscope in 10x objective. B) Quantification of lipid distribution was done by measuring Oil Red O dye using NIH ImageJ software. **** p < 0.0001 compared to control NT by one-way ANOVA and # p = 0.0005 comparing 2 % GS-glucose to 2 % CV-glucose by one-way ANOVA. C) Quantification of lysosome related organelles (LRO). L1 stage wild-type animals were treated with 2 % LOJ on NGM plate containing Red Nile dye for 72 h until 1-day old. Images were captured using fluorescent microscope and fluorescence levels were analyzed using NIH ImageJ software. **** p < 0.0001 comparing either CV or GS to NT animals and #p = 0.0264 comparing GS to CV treated animals by one-way ANOVA. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Orange (C. sinensis L. Osbeck) is one of the most cultivated fruits worldwide, and is rich in several phytochemical compounds with health benefits such as carotenoids and flavonoids. β-carotene is a well-known antioxidant due to its ROS scavenger and quencher capacity (Kang et al., 2017, Kawata et al., 2018, Nishino et al., 2017). However, β-carotene concentrations are relatively low in most cultivars. β-carotene antioxidant property has been associated with anti-inflammatory and neuroprotective effects (Chen et al., 2019, Zhou et al., 2018), as well as with lipid oxidation and fat accumulation inhibition (Esrefoglu et al., 2016, Harari et al., 2008). We have previously reported a β-carotene-enriched genetically-modified (GM) orange with greater antioxidant effect in vivo compared to the isogenic non-GM control oranges (Pons et al., 2014). In this work, we expand the characterization of the beneficial health properties of a new β-carotene-enriched orange obtained through a similar metabolic engineering strategy (GS) versus its conventional counterpart (CV).
Both content and profile of carotenoids observed in GS and CV lines were as expected according to the strategy used and very similar to those previously reported (Pons et al., 2014). The most important change observed was the 33.72-fold increase in β-carotene content. In addition to this, changes occurred in the content of other carotenoid compounds. Of special interest is the moderate decrease observed in the content of all xanthophylls, because they have been also described as dietary antioxidants and multiple health benefits in the protection against some chronic diseases have been attributed to them.
The physicochemical characterization of GS and CV juices revealed that ° Brix, juice acidity, maturity index and vitamin C had not changed in GS as a consequence of the genetic modification performed. So, it can be stated that GS and CV are isogenic materials (at least as regards the main characteristics of the juice quality) suitable for carrying out functional bioassays in vivo with C. elegans. Although a growing body of evidence supports the healthy properties of oranges (and other citrus fruits), in most cases, a concrete beneficial effect could not be attributed unequivocally to a particular phytonutrient. This is, in part, due to the lack of well-characterized and contrasting plant foods required to test hypotheses for the health-promoting activity of specific plant metabolites. In fact, in the vast majority of interventional (preclinical and clinical) studies performed to assess the health properties of citrus, food treatments consisted basically on: I) juice from a citrus type versus water/not treatment, II) juices from very different citrus types, or III) juice versus juice-derived metabolites dissolved in water (Miles & Calder, 2021). The bioactivity of phytonutrients is highly dependent on the food matrix in which they are supplied, due to interactions with other phytonutrients, effects on bioavailability and absorption, etc. In this regard, metabolic engineering offers the possibility of studying the beneficial role of specific phytonutrients in the context of the same food matrix. Theoretically, genetic modification through biotechnology allows the generation of isolines in which a trait or metabolic pathway has been modified without altering the rest of the food matrix’s characteristics (Martin, 2013). The fact of having confirmed in this work that the GS is an isogenic line of CV allows us to attribute, in a more precise way, their putative protective effects to the changes in the carotenoid profile that have taken place in this orange line. Next, we used C. elegans to characterize in detail its antioxidant capacity in vivo and test its protective effect against physiological processes closely related to oxidation, such as stress resistance, longevity, β-amyloid proteotoxicity and fat accumulation under glucose rich diet.
First, we confirmed that 2 % concentrations of LOJ increases the worms' antioxidant capacity as demonstrated for other oranges juices and extracts (Caland et al., 2019, Wang et al., 2020). But most importantly, we showed that animals treated with our new β-carotene-enriched genetically-modified (GS) orange juice significantly improves ROS reduction, gene expression activation (gcs-1 and sod-3) and oxidative stress resistance compared to animals treated with conventional counterpart (CV). These results are in agreement with the increased oxidative stress resistance promoted by our previous β-carotene-enriched genetically-modified (GM) orange (Pons et al., 2014). The strategy of augmenting β-carotene content by down-regulating CsβCHX gene also increased the antioxidant capacity and stress resistance of transgenic sweet potato plants (Kang et al., 2017). Likewise, C. elegans treated with orange juice from cultivars with higher carotenoid contents have stronger response against oxidative stress (Caland et al., 2019). Considering that when administered alone, β-carotene can increase cellular antioxidant defense system in ex vivo and in vivo models under stress or pathologic conditions (Chen et al., 2019, Zhou et al., 2018), our results indicates that the greater β-carotene content in our GS LOJ is able to significantly improve antioxidant capacity.
Previous work has shown that orange juice with higher carotenoid content induces stronger response against oxidative stress and promotes greater lifespan in C. elegans (Caland et al., 2019). Moreover, orange extract treatment induces a dose-dependent increase in the worms' mean lifespan (Wang et al., 2020). Surprisingly, we did not observe any significant difference related to longevity between the worms treated with either GS or CV LOJ. Despite GS LOJ having more β-carotene, CV LOJ has a higher total carotenoid content. Evidence that β-carotene supplementation can extend mean lifespan in aging Drosophila melanogaster (Lashmanova et al., 2015, Weinrich et al., 2019) but not in C. elegans has also been observed (Lashmanova et al., 2015). This suggests that anti-aging effects found in orange extracts might be associated with the total concentration of the different phytochemicals present on them rather than a higher level of a specific one.
Neurodegenerative diseases (NDD) such as Alzheimer’s disease (AD), and Parkinson’s disease (PD) are characterized by progressive damage of neurons and neuronal apoptosis leading to impaired cognitive and intellectual function. NDD shares many common risk factors such as oxidative stress, mitochondrial dysfunction, impaired bioenergetics, deficiency of the ubiquitin–proteasome–autophagy systems and neuroinflammatory processes (Liu, Zhou, Ziegler, Dimitrion, & Zuo, 2017). Use of carotenoids as neuroprotective antioxidants have been considered as a promising strategy to slow down the disease progression and to minimize the level of neuronal loss in chronic NDD and after acute brain lesions (Manochkumar et al., 2021). In the case of AD, β-carotene supplementation has a protective role by ameliorating oxidative damage, activating antioxidant enzymes, attenuating β-amyloid aggregation and inhibiting neuro-inflammation (Cho et al., 2018, Hira et al., 2019, Park et al., 2020). Caland et al. (2019) observed that the onset paralysis induced by β-amyloid toxicity in C. elegans was significantly delayed in animals treated with orange juice from those with higher carotenoid levels. Here, GS LOJ treatment provided superior protection against Aβ1–42-induced paralysis over that provided by CV LOJ. Even though it was not statistically significant, our results suggest that LOJ treatment may also act as neuroprotective by modulating proteasomal activity in addition to its antioxidant properties.
Other beneficials outcomes associated with the β-carotene supplementation are control of lipid metabolism and development of obesity in animal models and humans (Chen, Barclay, Burgoyne, & Morgan, 2015). Orange juice and pulp parts have also shown hypolipidemic effects in the diet-induced hypercholesterolemia and diabetic rats (Mallick and Khan, 2016, Miceli et al., 2007). Unlike mammals that store droplet-like lipids in adipocytes and hepatocytes, C. elegans store fat as lipid droplet primarily in their intestinal and hypodermic epidermal cells since they do not have fat cells. Triglycerides make up approximately 40–55 % of total lipids (Shen, Yue, & Park, 2018). The worms’ intestinal cells contain several different types of gut granules, including acidic lysosome-related organelles (LRO) (Bowman, Bi-Karchin, Le, & Marks, 2019). LRO presents diverse functions including storage of cholesterol, metals and xenobiotics (Lee et al., 2015, Morris et al., 2018). Here, we investigated the effect of LOJ to modulate both lipid droplet and LRO in the C. elegans intestine. We showed that LOJ reduces fat accumulation in worms cultivated in standard and glucose-rich diets. Interestingly, β-carotene-enriched LOJ exhibits significantly higher hypolipidemic activity under glucose-rich diet compared to conventional LOJ. We also observed that LOJ treatment reduced levels of LRO especially in animals treated with β-carotene-enriched LOJ. These results suggest that the increased β-carotene level in our GS orange is able to significantly change lipid and cholesterol-containing LRO granules profile in C. elegans.
5. Conclusion
In summary, we successfully showed that our new β-carotene transgenic orange provides increased antioxidant status. We found that C. elegans treated with β-carotene-enriched pulp shows reduced endogenous ROS production, increased expression of antioxidant genes, increased resistance against oxidative stress, delayed β-amyloid-induced paralysis, and increased hypolipidemic activity under glucose-rich diet compared to animals treated with conventional orange. Taking together, we provided a valuable proof of principle to subside further studies in mammals and humans aiming degenerative diseases prevention and health promotion.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was supported by Fundecitrus, São Paulo, Brazil, and Universidade Federal do Rio Grande do Norte (UFRN). Research fellowships were sponsored by CNPq (Oliveira, R. P.).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochms.2022.100141.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Alquezar B., Rodrigo M.J., Zacarias L. Regulation of carotenoid biosynthesis during fruit maturation in the red-fleshed orange mutant Cara Cara. Phytochemistry. 2008;69(10):1997–2007. doi: 10.1016/j.phytochem.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Ayuda-Duran B., Gonzalez-Manzano S., Gonzalez-Paramas A.M., Santos-Buelga C. Caenorhabditis elegansas a model organism to evaluate the antioxidant effects of phytochemicals. Molecules. 2020;25(14) doi: 10.3390/molecules25143194. Article 3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman S.L., Bi-Karchin J., Le L., Marks M.S. The road to lysosome-related organelles: insights from Hermansky-Pudlak syndrome and other rare diseases. Traffic. 2019;20(6):404–435. doi: 10.1111/tra.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton G. In: Carotenoids, Volume 3: Biosynthesis and Metabolism. Britton G., Pfander H., Liaaen-Jensen S., editors. Springer; 1998. Biosynthesis and metabolism; pp. 13–148. [Google Scholar]
- Caland R.B.D., Cadavid C.O.M., Carmona L., Pena L., Oliveira R.D. Pasteurized orange juice rich in carotenoids protects Caenorhabditis elegans against oxidative stress and beta-amyloid toxicity through direct and indirect mechanisms. Oxidative Medicine and Cellular Longevity. 2019;2019 doi: 10.1155/2019/5046280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebrolu K.K., Jayaprakasha G.K., Yoo K.S., Jifon J.L., Patil B.S. An improved sample preparation method for quantification of ascorbic acid and dehydroascorbic acid by HPLC. Lwt - Food Science and Technology. 2012;47:443–449. doi: 10.1016/j.lwt.2012.02.004. In press. [DOI] [Google Scholar]
- Chen P.Q., Li L., Gao Y.F., Xie Z.Q., Zhang Y., Pan Z.J.…Xin X.M. beta-carotene provides neuro protection after experimental traumatic brain injury via the Nrf2-ARE pathway. Journal of Integrative Neuroscience. 2019;18(2):153–161. doi: 10.31083/j.jin.2019.02.120. [DOI] [PubMed] [Google Scholar]
- Chen Q.H., Wu B.K., Pan D., Sang L.X., Chang B. Beta-carotene and its protective effect on gastric cancer. World Journal of Clinical Cases. 2021;9(23):6591–6607. doi: 10.12998/wjcc.v9.i23.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Barclay J.W., Burgoyne R.D., Morgan A. Using C. elegans to discover therapeutic compounds for ageing-associated neurodegenerative diseases. Chemistry Central Journal. 2015;9:65. doi: 10.1186/s13065-015-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.P., Guo C.Y., Kong J.M. Oxidative stress in neurodegenerative diseases. Neural Regeneration Research. 2012;7(5):376–385. doi: 10.3969/j.issn.1673-5374.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K.S., Shin M., Kim S., Lee S.B. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxidative Medicine and Cellular Longevity. 2018;2018 doi: 10.1155/2018/4120458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currais A., Quehenberger O., Armando A.M., Daugherty D., Maher P., Schubert D. Amyloid proteotoxicity initiates an inflammatory response blocked by cannabinoids. NPJ Aging and Mechanisms of Disease. 2016;2:16012. doi: 10.1038/npjamd.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escorcia W., Ruter D.L., Nhan J., Curran S.P. Quantification of lipid abundance and evaluation of lipid distribution in Caenorhabditis elegans by Nile red and oil red O staining. Jove-Journal of Visualized Experiments. 2018;133 doi: 10.3791/57352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esrefoglu M., Akinci A., Taslidere E., Elbe H., Cetin A., Ates B. Ascorbic acid and beta-carotene reduce stress-induced oxidative organ damage in rats. Biotechnic & Histochemistry. 2016;91(7):455–464. doi: 10.1080/10520295.2016.1220019. [DOI] [PubMed] [Google Scholar]
- Farag M.A., Abib B., Ayad L., Khattab A.R. Sweet and bitter oranges: An updated comparative review of their bioactives, nutrition, food quality, therapeutic merits and biowaste valorization practices. Food Chemistry. 2020;331 doi: 10.1016/j.foodchem.2020.127306. [DOI] [PubMed] [Google Scholar]
- Favela-Hernández J.M., González-Santiago O., Ramírez-Cabrera M.A., Esquivel-Ferriño P.C., Camacho-Corona M.e.R. Chemistry and pharmacology of Citrus sinensis. Molecules. 2016;21(2):247. doi: 10.3390/molecules21020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso G., Galvano F., Mistretta A., Marventano S., Nolfo F., Calabrese G.…Scuderi A. Red orange: Experimental models and epidemiological evidence of its benefits on human health. Oxidative Medicine and Cellular Longevity. 2013;2013 doi: 10.1155/2013/157240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari A., Harats D., Marko D., Cohen H., Barshack I., Kamari Y.…Shaish A. A 9-cis beta-carotene-enriched diet inhibits atherogenesis and fatty liver formation in LDL receptor knockout mice. Journal of Nutrition. 2008;138(10):1923–1930. doi: 10.1093/jn/138.10.1923. [DOI] [PubMed] [Google Scholar]
- Hira S., Saleem U., Anwar F., Sohail M.F., Raza Z., Ahmad B. beta-Carotene: A natural compound improves cognitive impairment and oxidative stress in a mouse model of streptozotocin-induced Alzheimer’s disease. Biomolecules. 2019;9(9) doi: 10.3390/biom9090441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayedi A., Rashidy-Pour A., Parohan M., Zargar M.S., Shab-Bidar S. Dietary and circulating vitamin C, vitamin E, beta-carotene and risk of total cardiovascular mortality: A systematic review and dose-response meta-analysis of prospective observational studies. Public Health Nutrition. 2019;22(10):1872–1887. doi: 10.1017/s1368980018003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletta T., Hengartner M.O. Finding function in novel targets: C-elegans as a model organism. Nature Reviews Drug Discovery. 2006;5(5):387–398. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- Kang L., Ji C.Y., Kim S.H., Ke Q., Park S.C., Kim H.S.…Kwak S.S. Suppression of the beta-carotene hydroxylase gene increases beta-carotene content and tolerance to abiotic stress in transgenic sweetpotato plants Le Kang. Plant Physiology and Biochemistry. 2017;117:24–33. doi: 10.1016/j.plaphy.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Kawata A., Murakami Y., Suzuki S., Fujisawa S. Anti-inflammatory activity of beta-carotene, lycopene and Tri-n-butylborane, a scavenger of reactive oxygen species. In Vivo. 2018;32(2):255–264. doi: 10.21873/invivo.11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashmanova E., Proshkina E., Zhikrivetskaya S., Shevchenko O., Marusich E., Leonov S.…Moskalev A. Fucoxanthin increases lifespan of Drosophila melanogaster and Caenorhabditis elegans. Pharmacological Research. 2015;100:228–241. doi: 10.1016/j.phrs.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Lee H.J., Zhang W.D., Zhang D.L., Yang Y., Liu B., Barker E.L.…Cheng J.X. Assessing cholesterol storage in live cells and C-elegans by stimulated raman scattering imaging of phenyl-diyne cholesterol. Scientific Reports. 2015;5 doi: 10.1038/srep07930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.E., Kwon M., Kim Y.S., Kim Y., Chung M.G., Heo S.C. beta-carotene regulates cancer sternness in colon cancer in vivo and in vitro. Nutrition Research and Practice. 2022;16(2):161–172. doi: 10.4162/nrp.2022.16.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.W., Zhou T.Y., Ziegler A.C., Dimitrion P., Zuo L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxidative Medicine and Cellular Longevity. 2017;2017 doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick N., Khan R.A. Antihyperlipidemic effects of Citrus sinensis, Citrus paradisi, and their combinations. Journal of Pharmacy and Bioallied Sciences. 2016;8(2):112–118. doi: 10.4103/0975-7406.171727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manochkumar J., Doss C.G.P., El-Seedi H.R., Efferth T., Ramamoorthy S. The neuroprotective potential of carotenoids in vitro and in vivo. Phytomedicine. 2021;91 doi: 10.1016/j.phymed.2021.153676. [DOI] [PubMed] [Google Scholar]
- Marcelino G., Machate D.J., Freitas K.D., Hiane P.A., Maldonade I.R., Pott A.…Guimaraes R.D.A. beta-Carotene: Preventive role for type 2 diabetes mellitus and obesity: A review. Molecules. 2020;25(24) doi: 10.3390/molecules25245803. Article 5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marseglia L., Manti S., D'Angelo G., Nicotera A., Parisi E., Di Rosa G.…Arrigo T. Oxidative stress in obesity: A critical component in human diseases. International Journal of Molecular Sciences. 2015;16(1):378–400. doi: 10.3390/ijms16010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. The interface between plant metabolic engineering and human health. Current Opinion in Biotechnology. 2013;24(2):344–353. doi: 10.1016/j.copbio.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Miceli N., Mondello M.R., Monforte M.T., Sdrafkakis V., Dugo P., Crupi M.L.…Trovato A. Hypolipidemic effects of Citrus bergamia Risso et Poiteau juice in rats fed a hypercholesterolemic diet. Journal of Agricultural and Food Chemistry. 2007;55(26):10671–10677. doi: 10.1021/jf071772i. [DOI] [PubMed] [Google Scholar]
- Miles E.A., Calder P.C. Effects of citrus fruit juices and their bioactive components on inflammation and immunity: A narrative review. Frontiers in Immunology. 2021;12 doi: 10.3389/fimmu.2021.712608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C., Foster O.K., Handa S., Peloza K., Voss L., Somhegyi H.…Hermann G.J. Function and regulation of the Caenorhabditis elegans Rab32 family member GLO-1 in lysosome-related organelle biogenesis. Plos Genetics. 2018;14(11) doi: 10.1371/journal.pgen.1007772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motallaei M., Ramezani-Jolfaie N., Mohammadi M., Shams-Rad S., Jahanlou A.S., Salehi-Abargouei A. Effects of orange juice intake on cardiovascular risk factors: A systematic review and meta-analysis of randomized controlled clinical trials. Phytotherapy Research. 2021;35(10):5427–5439. doi: 10.1002/ptr.7173. [DOI] [PubMed] [Google Scholar]
- Nimbalkar V., Joshi U., Shinde S., Pawar G. In-vivo and in-vitro evaluation of therapeutic potential of beta-Carotene in diabetes. Journal of Diabetes and Metabolic Disorders. 2021;20(2):1621–1630. doi: 10.1007/s40200-021-00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino A., Yasui H., Maoka T. Reaction and scavenging mechanism of beta-carotene and zeaxanthin with reactive oxygen species. Journal of Oleo Science. 2017;66(1):77–84. doi: 10.5650/jos.ess16107. [DOI] [PubMed] [Google Scholar]
- Park H.A., Hayden M.M., Bannerman S., Jansen J., Crowe-White K.M. Anti-apoptotic effects of carotenoids in neurodegeneration. Molecules. 2020;25(15) doi: 10.3390/molecules25153453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M., Mallent M., Cuñat P. Estudio de los carotenoides de naranjas cv. Navelina. Revista española de ciencia y tecnología de alimentos. 1993;33(2):179–196. [Google Scholar]
- Peraita-Costa I., Garcia P.C., Morales-Suarez-Varela M. Is there an association between beta-carotene and breast cancer? A systematic review on breast cancer risk. Nutrition and Cancer-an International Journal. 2022;74(1):39–54. doi: 10.1080/01635581.2020.1865422. [DOI] [PubMed] [Google Scholar]
- Pons E., Alquezar B., Rodriguez A., Martorell P., Genoves S., Ramon D.…Pena L. Metabolic engineering of beta-carotene in orange fruit increases its in vivo antioxidant properties. Plant Biotechnology Journal. 2014;12(1):17–27. doi: 10.1111/pbi.12112. [DOI] [PubMed] [Google Scholar]
- Rodrigo M.J., Marcos J.F., Alferez F., Mallent M.D., Zacarias L. Characterization of Pinalate, a novel Citrus sinensis mutant with a fruit-specific alteration that results in yellow pigmentation and decreased ABA content. Journal of Experimental Botany. 2003;54(383):727–738. doi: 10.1093/jxb/erg083. [DOI] [PubMed] [Google Scholar]
- Rodrigo M.J., Marcos J.F., Zacarias L. Biochemical and molecular analysis of carotenoid biosynthesis in flavedo of orange (Citrus sinensis L.) during fruit development and maturation. Journal of Agricultural and Food Chemistry. 2004;52(22):6724–6731. doi: 10.1021/jf049607f. [DOI] [PubMed] [Google Scholar]
- Rouseff R., Raley L., Hofsommer H.J. Application of diode array detection with a C-30 reversed phase column for the separation and identification of saponified orange juice carotenoids. Journal of Agricultural and Food Chemistry. 1996;44(8):2176–2181. doi: 10.1021/jf950631q. [DOI] [Google Scholar]
- Saini R.K., Prasad P., Lokesh V., Shang X.M., Shin J., Keum Y.S., Lee J.H. Carotenoids: Dietary sources, extraction, encapsulation, bioavailability, and health benefits-A review of recent advancements. Antioxidants. 2022;11(4) doi: 10.3390/antiox11040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P.Y., Yue Y.R., Park Y. A living model for obesity and aging research: Caenorhabditis elegans. Critical Reviews in Food Science and Nutrition. 2018;58(5):741–754. doi: 10.1080/10408398.2016.1220914. [DOI] [PubMed] [Google Scholar]
- USDA, Economics, Statistics and Market Information System Citrus: World Markets and Trade. 2022. https://downloads.usda.library.cornell.edu/usda-esmis/files/w66343603/bv73d549r/1v53m4335/citrus.pdf
- Voges D., Zwickl P., Baumeister W. The 26S proteasome: A molecular machine designed for controlled proteolysis. Annual Review of Biochemistry. 1999;68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- Wang J., Deng N., Wang H., Li T., Chen L., Zheng B.S., Liu R.H. Effects of orange extracts on longevity, healthspan, and stress resistance in Caenorhabditis elegans. Molecules. 2020;25(2) doi: 10.3390/molecules25020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich T., Xu Y.N., Wosu C., Harvey P.J., Jeffery G. Mitochondrial function, mobility and lifespan are improved in Drosophila melanogaster by extracts of 9-cis-beta-Carotene from Dunaliella salina. Marine Drugs. 2019;17(5) doi: 10.3390/md17050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeum K.J., Russell R.M., Krinsky N.I., Aldini G. Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Archives of Biochemistry and Biophysics. 2004;430(1):97–103. doi: 10.1016/j.abb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Zhou L.H., Ouyang L., Lin S.Z., Chen S., Liu Y.J., Zhou W., Wang X.C. Protective role of beta-carotene against oxidative stress and neuroinflammation in a rat model of spinal cord injury. International Immunopharmacology. 2018;61:92–99. doi: 10.1016/j.intimp.2018.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.