Abstract
The avidities of Toxoplasma-specific immunoglobulin G serum antibodies were measured in immunocompromised patients presenting with cerebral or extracerebral toxoplasmosis and/or serological reactivation. Since avidity remained high and stable in 39 of 40 patients with toxoplasmosis and 27 of 28 patients with serological reactivation, we conclude that this test cannot help diagnose toxoplasmosis in these patients.
In immunocompromised patients, the titration of anti-Toxoplasma-specific antibodies is poorly contributive to the diagnosis of cerebral or extracerebral toxoplasmosis, as the usual serological markers of an acute infection, i.e., an increase in immunoglobulin G (IgG) levels and the presence of IgM and/or IgA antibodies, are frequently lacking at the time of diagnosis (3–5, 10). Besides, serological reactivations often occur in these patients but are poorly correlated to the onset of clinical symptoms (1, 3).
Since determination of the avidities of Toxoplasma-specific IgG antibodies has proved useful in differentiating between acute and chronic infections in immunocompetent patients (2, 6–9, 12, 13), our objective was to evaluate the validity of this test for the diagnosis of cerebral and extracerebral toxoplasmosis in human immunodeficiency virus (HIV)-infected patients and transplant recipients.
Patients and methods. (i) Patients.
We determined the IgG avidities of individual and sequential serum samples from 41 HIV-infected patients, 18 allogenic bone marrow transplant (BMT) recipients, and 5 organ transplant recipients (heart, 3; lung-liver, 1; kidney-liver, 1) presenting with clinical toxoplasmosis or serological reactivation (Table 1). In these patients, the diagnosis of cerebral or extracerebral toxoplasmosis was established by the presence of clinical symptoms; by radiological signs on brain-computed tomography or magnetic resonance imaging; by demonstration of Toxoplasma gondii tachyzoites on Giemsa-stained smears or by mouse inoculation or tissue culture; or by response to specific therapy. Serological reactivation was defined as at least a twofold increase in the IgG antibody titer in two sequential serum samples. In three HIV-infected patients and one heart transplant patient, serological reactivation preceded cerebral or disseminated toxoplasmosis.
TABLE 1.
Study population
| Patient group | Condition | No. of patients | No. of sera |
|---|---|---|---|
| HIV-infected patients | Cerebral toxoplasmosis | 13 | 26 |
| Pulmonary or disseminated toxoplasmosis | 10 | 15 | |
| Ocular toxoplasmosis | 8 | 8 | |
| Asymptomatic serological reactivation | 10 | 20 | |
| Bone marrow transplant recipients | Pulmonary or disseminated toxoplasmosis | 8 | 15 |
| Asymptomatic serological reactivation | 10 | 39 | |
| Organ transplant recipients (heart, 3; kidney-liver, 1; lung-liver, 1) | Disseminated toxoplasmosis | 1 | 3 |
| Asymptomatic serological reactivation | 4 | 19 |
(ii) Methods.
The determination of IgG anti-Toxoplasma antibody titers was performed by enzyme-linked immunosorbent assay (ELISA) (Platelia Toxo-IgG; Bio-Rad, Marnes la Coquette, France). Results were expressed in international units per milliliter and were considered positive for titers of >6 IU/ml. Specific IgM antibody titers were determined by ELISA (Platelia Toxo-IgM; Bio-Rad) and immunosorbent agglutination assay (ISAGA; Bio-Mérieux). Results were expressed as indices and were considered positive for values of >1 with ELISA and >9+ with ISAGA. Specific IgA antibody titers were determined by ISAGA, with a threshold of positivity of 9+.
The determination of IgG antibody avidity was performed using the Platelia Toxo-IgG kit (Bio-Rad) with 6 M urea as a dissociative agent in the washing solution preceding the incubation of the conjugate (9).
The IgG avidity index (IgG-AI) was calculated as (OD values under dissociative conditions)/(OD value of untreated serum), where OD is optical density. In a preliminary experiment, the ability of the IgG avidity test to differentiate between acute and chronic infections had been examined on 214 sera from 194 immunocompetent patients whose dates of seroconversion were known. For an IgG-AI of <0.4 or <0.5, the predictive value for an infection of less than 5 months was 79.4 or 74.5%, respectively. For an index of >0.4 or >0.5, the predictive value for an infection of more than 5 months was 94.7 or 97.9%, respectively. Thus, an IgG-AI cutoff value of 0.5 allowed us to differentiate between most cases of acute and chronic toxoplasmosis, as previously observed by others (6, 9, 12).
Results.
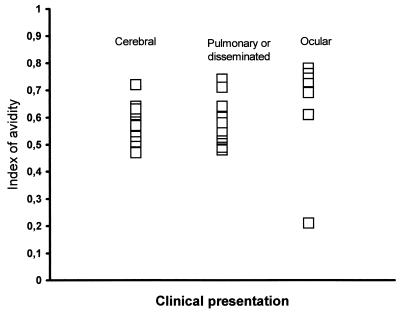
For immunocompromised patients with cerebral or extracerebral toxoplasmosis, the IgG-AI was determined on individual serum samples taken at the onset of symptoms. For HIV-infected and BMT patients, IgG-AI values at the time of diagnosis were >0.4 in 38 of 39 patients and >0.5 in 35 of 39 patients (Fig. 1). A low IgG-AI (0.21) was observed for one patient with ocular toxoplasmosis who presented serological signs of recently acquired infection, i.e., the presence of IgM and IgA antibodies and a subsequent increase in IgG antibody titers. For 22 patients, a previous serum sample was available, and the IgG-AI was >0.4 in all cases and >0.5 in 21 of 22 cases. Overall, no correlation was found between the IgG-AI and the IgG antibody titer, or between the IgG-AI and the presence of IgM or IgA antibody.
FIG. 1.
IgG avidity at the onset of symptoms of cerebral, ocular, or pulmonary/disseminated toxoplasmosis in AIDS or BMT patients.
In all cases of serological reactivation occurring in asymptomatic HIV-infected patients, the IgG-AI remained at high and stable levels, while antibody titers increased, even in three patients who further developed a cerebral toxoplasmosis. Similarly, all BMT recipients with asymptomatic serological reactivation except one had stable IgG-AI values, and no relation was found between the evolution of the IgG-AI and the serological status of the bone marrow donor. The remaining patient presented with serological reactivation 17 months after BMT, with a decrease in the IgG-AI from 0.53 to 0.18, and then an increase to 0.32 at the time when IgG antibody titers respectively increased from 24 to 196, and then to 1,390 IU/ml. This low IgG-AI persisted for several months, and no clinical symptom suggestive of toxoplasmosis was recorded during this period. The patient and the donor were both seropositive for T. gondii before BMT. In the five solid-organ transplant patients with serological reactivation, the IgG-AI remained unchanged before and after transplantation; this was true even for the patient who developed disseminated toxoplasmosis 1 month after transplantation, possibly through heart-transmitted infection (Table 2).
TABLE 2.
Clinical description and results obtained for solid organ transplant patientsa
| Patient no. | Transplant type | Serological status of donor | IgG antibody titer (IU/ml) (wks post-TP) | Clinical symptoms | IgG-AI |
|---|---|---|---|---|---|
| 1 | Heart | + | 21 (0) | 0.70 | |
| 273 (4) | Disseminated toxoplasmosisb | 0.58 | |||
| 364 (6) | Death | 0.48 | |||
| 2 | Heart | + | 753 (0) | None | 0.57 |
| 153 (2) | 0.46 | ||||
| 1,038 (11) | 0.81 | ||||
| 3 | Liver-lung | − | 212 (0) | None | 0.70 |
| 446 (10) | 0.60 | ||||
| 772 (12) | 0.50 | ||||
| 1,354 (19) | 0.52 | ||||
| 261 (24) | 0.52 | ||||
| 4 | Liver-kidney | − | 130 (0) | None | 0.72 |
| 2,260 (1) | 0.73 | ||||
| >2,400 (3) | 0.78 | ||||
| >2,400 (8) | 0.74 | ||||
| 5 | Heart | NA | 106 (0) | None | 0.50 |
| 820 (4) | 0.60 | ||||
| 1,360 (6) | 0.59 | ||||
| 1,110 (8) | 0.62 |
n = 5. Abbreviations: NA, not available; TP, transplantation.
Probably transmitted by the donor, as attested by anatomopathological signs of evolutive toxoplasmic myocarditis, detection of circulating parasites by PCR, and appearance of newly synthesized IgG isotypes by Western blot analysis (F. Robert-Gangneux et al., submitted for publication).
Discussion.
In this study, we hypothesized that the determination of IgG-AI could be useful for diagnosing reactivated toxoplasmosis in immunocompromised patients, based on the concept that neoantigens emerging from cyst rupture could induce a primary-type immune response with low-avidity IgG antibodies. Therefore, a decrease in the IgG-AI could be of diagnostic help in two situations: (i) in patients with serological reactivation, as an early marker of infection recrudescence, and (ii) in patients with symptomatic visceral reactivated toxoplasmosis.
Our results show that for all patients with asymptomatic serological reactivation, except one BMT recipient, the IgG-AI remained unchanged and at a high level in sequential sera taken before and after antibody increase. No difference was noted between patients whose serological reactivation preceded the onset of clinical symptoms and those who remained asymptomatic.
Similarly, IgG avidity determination was of no help for the diagnosis of visceral toxoplasmosis occurring in HIV-infected or transplant patients. In 39 of 40 (97.5%) symptomatic patients, the IgG-AI remained high and stable in sera taken before or after the onset of symptoms. The stability of the IgG-AI observed in our study supports the idea that most cases of cerebral or disseminated toxoplasmosis occurring in immunocompromised patients result from reactivation of a latent infection (9) and indicates a secondary immune response to recall antigens rather than a primary immune response to neoantigens emerging from cyst rupture. In transplant patients receiving organs from Toxoplasma-seropositive donors, there is a well-known risk of organ transmission of Toxoplasma (11, 14). Thus, our hypothesis was that reinfection related to a donor-transmitted strain could result in a decrease in the IgG-AI. We observed that the IgG-AI remained >0.5 in sera taken before and after transplantation, even in a patient who rapidly developed a case of disseminated toxoplasmosis that was likely due to recontamination by the heart transplant. Therefore, the IgG-AI determination could not distinguish a reactivation from an organ-transmitted toxoplasmosis.
From these results, we conclude that the determination of IgG avidity is of no help in the diagnosis of cerebral or extracerebral toxoplasmosis occurring in immunocompromised patients or for the interpretation of serological reactivations that are frequently observed in these patients.
REFERENCES
- 1.Bélanger F, Derouin F, Grangeot-Keros L, Meyer L the HEMOCO-SEROCO Study Group. Incidence and risk factors of toxoplasmosis in a cohort of HIV-infected patients: 1988–1995. Clin Infect Dis. 1999;28:575–581. doi: 10.1086/515147. [DOI] [PubMed] [Google Scholar]
- 2.Cozon G J N, Ferrandiz J, Nebhi H, Wallon M, Peyron F. Estimation of the avidity of immunoglobulin G for routine diagnosis of chronic Toxoplasma gondii infection in pregnant women. Eur J Clin Microbiol Infect Dis. 1998;17:32–36. doi: 10.1007/BF01584360. [DOI] [PubMed] [Google Scholar]
- 3.Derouin F, Devergie A, Auber P, Gluckman E, Beauvais B, Garin Y J F, Larivière M. Toxoplasmosis in bone marrow transplant recipients: report of seven cases and review. Clin Infect Dis. 1992;15:267–270. doi: 10.1093/clinids/15.2.267. [DOI] [PubMed] [Google Scholar]
- 4.Derouin F, Thulliez P, Garin Y J F. Intérêt et limites de la sérologie de la toxoplasmose chez les sujets VIH+ Pathol Biol. 1991;39:255–259. [PubMed] [Google Scholar]
- 5.Derouin F, Leport C, Pueyo S, Morlat P, Letrillart B, Chêne G, Ecobichon J L, Luft B, Aubertin J, Hafner R, Vildé J L, Salamon R ANRS 005/ACTG 154 Trial Group. Predictive value of Toxoplasma gondii antibody titres on the occurrence of toxoplasmic encephalitis in HIV-infected patients. AIDS. 1996;10:1521–1527. doi: 10.1097/00002030-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Holliman R E, Raymond R, Renton N, Johnson J D. The diagnosis of toxoplasmosis using IgG avidity. Epidemiol Infect. 1994;112:399–408. doi: 10.1017/s0950268800057812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenum P A, Stray-Pedersen B, Gerd Gundersen A. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G avidity. J Clin Microbiol. 1997;35:1972–1977. doi: 10.1128/jcm.35.8.1972-1977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lappalainen M, Koskela P, Koskiniemi M, Ammala P, Hiilesmaa V, Teramo K, Raivio K O, Remington J S, Hedman K. Toxoplasmosis acquired during pregnancy: improved serodiagnosis based on avidity of IgG. J Infect Dis. 1993;167:691–697. doi: 10.1093/infdis/167.3.691. [DOI] [PubMed] [Google Scholar]
- 9.Lécolier B, Pucheu B. Interêt de l'étude de l'avidité des IgG pour le diagnostic de la toxoplasmose. Pathol Biol. 1993;41:155–158. [PubMed] [Google Scholar]
- 10.Luft B J, Remington J S. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 11.Luft B, Naot Y, Araujo F G, Stinson E B, Remington J S. Primary and reactivated toxoplasma infection in patients with cardiac transplant. Ann Intern Med. 1983;99:27–31. doi: 10.7326/0003-4819-99-1-27. [DOI] [PubMed] [Google Scholar]
- 12.Robert-Gangneux F, Vieljeuf C, Tourte-Schaefer C, Dupouy-Camet J. Apport de l'avidité des anticorps dans la datation d'une séroconversion toxoplasmique. Ann Biol Clin. 1998;56:586–589. [PubMed] [Google Scholar]
- 13.Sensini A, Pascoli S, Marchetti D, Castronari R, Marangi M, Sbaraglia G, Cimmino C, Favero A, Castelletto M, Mottola A. IgG avidity in the serodiagnosis of acute Toxoplasma gondii infection: a multicenter study. Clin Microbiol Infect. 1996;2:25–29. doi: 10.1111/j.1469-0691.1996.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 14.Speirs G E, Hakim M, Calne R Y, Wreghitt T G. Relative risk of donor-acquired Toxoplasma gondii in heart, liver and kidney transplant recipients. Clin Transplant. 1988;2:257–260. [Google Scholar]