Abstract
Zoster vaccines generate antibody responses against varicella-zoster virus (VZV). We compared antibody-dependent cell cytotoxicity (ADCC) elicited by zoster vaccine live (ZVL) and recombinant zoster vaccine (RZV). ADCC mediated by antibodies against VZV lysate (VZV-ADCC) and recombinant glycoprotein E (gE-ADCC) was measured using plasma from 20 RZV- and 20 ZVL-recipients, including half 50–60-years-old and half ≥70-years-old. Solid phase-bound anti-VZV antibodies stimulated TNFα in NK cells as measured by flow cytometry or ELISA. VZV-ADCC pre- and post-immunization was higher in younger vaccinees. ZVL did not appreciably increase VZV-ADCC, whereas RZV increased VZV-ADCC in older vaccinees. ELISA-measured gE-ADCC was similar across groups pre-immunization; significantly increased after ZVL; and RZV and was higher in younger RZV than ZVL recipients. IgG3 antibodies increased after RZV and ZVL, with greater anti-gE than anti-VZV responses. Moreover, gE-ADCC strongly correlated with anti-gE antibody avidity, but there were no appreciable correlations between VZV-ADCC and avidity. NK cells stimulated by anti-gE antibodies showed increased IFNγ and CD107a expression, which was not observed with anti-VZV antibodies. In conclusion, anti-gE antibodies generated more robust ADCC than anti-VZV antibodies. RZV induced higher ADCC antibodies than ZVL depending on the antigen and age of vaccinees. Older adults had lower ADCC antibodies before and after vaccination than younger adults.
Subject terms: Medical research, Translational research
Introduction
Herpes zoster (HZ), which is caused by reactivation of varicella-zoster virus (VZV) latent in cranial nerve and sensory ganglia following varicella, is prevented by the VZV-specific immune responses that develop during childhood varicella1. Abundant clinical and immunologic data indicate: that VZV-specific cell-mediated immunity (CMI) is necessary and sufficient to prevent HZ2–4; that the frequency and severity of HZ correlates inversely with these responses2,4; and that there is no such correlation with VZV-specific antibody2,5,6. However, such antibody(ies) could contribute to the prevention or recovery from HZ. For example, antibody responses after live HZ vaccine (ZVL, Zostavax, Merck) correlated with protection2. In another study with ZVL the fold-rise in the antibody response qualified as a surrogate marker for protection7. However, a mechanism involving antibody in protection against HZ remains undefined. For example, it is unlikely that neutralizing antibodies play a role in protection against VZV, which spreads readily from cell-to-cell and which circulates within immune cells8,9. Two other mechanisms have been proposed by which VZV-specific antibody might limit VZV infection in general, and HZ in particular. The first proposal is that the antibody attaches to and interferes with the function of a virally encoded fusogen within the cell membrane, thus inhibiting virion egress and cell-to-cell spread or infectivity10. This mechanism is based on in vitro model that has not been universally reproduced. A second, more plausible role for VZV-specific antibodies, is antibody-dependent cellular cytotoxicity (ADCC). It is well established that individuals with defects in NK cells, which are central to ADCC, suffer from severe and recurrent, often fatal VZV infections11,12. ADCC may also contribute to the partial clinical success achieved using high titer VZV hyperimmune globulin for passive immunization of naïve individuals13,14. Investigations of ADCC mediated by VZV antibodies were undertaken several decades ago with the methods then available15–18. In this report we apply contemporary methods to determine if ADCC antibodies are stimulated by the two licensed HZ vaccines – the live attenuated vaccine, ZVL and the adjuvanted recombinant glycoprotein E (gE) zoster vaccine (RZV). This is critical in understanding the basis of the striking differences in efficacy and applicability of these vaccines to an elderly population19. Differences in CMI in response to each vaccine have been demonstrated20. The current report investigates whether functional differences in the humoral response to the two vaccines might contribute to differences in their efficacy.
Results
Solid phase bound anti-VZV antibodies stimulate ADCC measured by TNFα using flow cytometry or ELISA
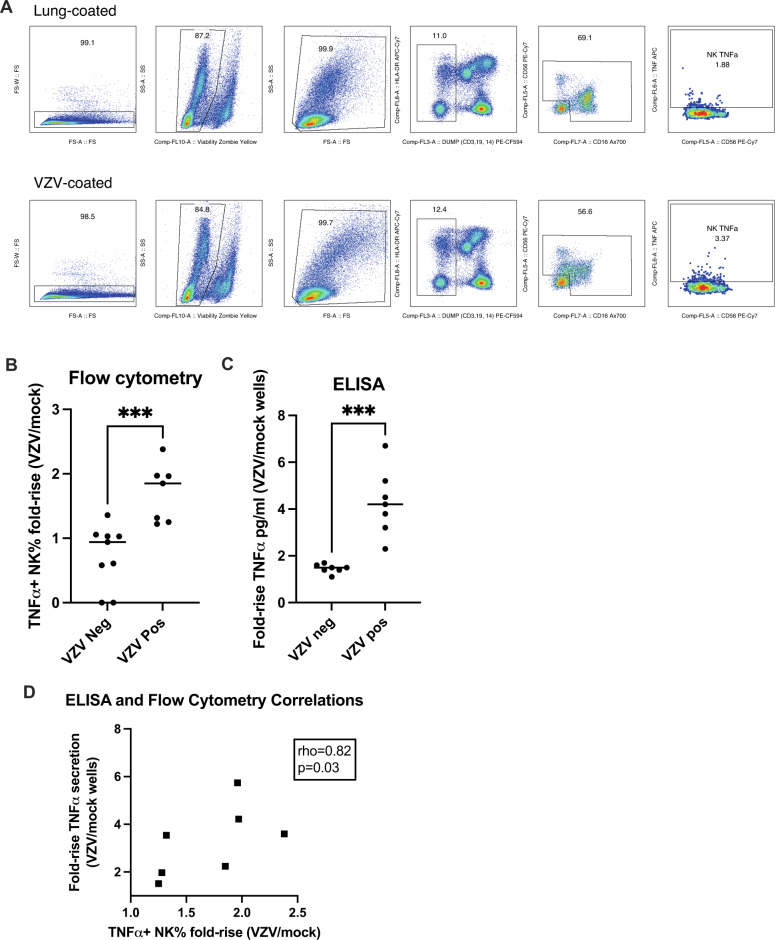
The assays were developed using a convenience sample of sera from 7 VZV-seropositive and 9 VZV-seronegative individuals characterized at the CDC. The ADCC methods were first validated for VZV and subsequently for gE as described in the Methods. For flow cytometry, NK cells in PBMC exposed to sera from VZV-seropositive donors bound to wells coated with VZV showed significantly higher expression of TNFα compared to PBMC exposed to VZV-seropositive sera in wells coated with mock-infected control antigen (median ratio of 1.9; p = 0.02, single sample Wilcoxon test; Fig. 1B). In contrast, NK cell expression of TNFα did not differ between wells coated with VZV or mock-infected control antigen when the PBMC were exposed to VZV-seronegative sera (median ratio of 1; Fig. 1B). These results indicated that the combination of VZV antibodies and VZV antigen in the wells was essential for the NK cell stimulation through ADCC. We also measured the proportion of monocytes and non-NK lymphocytes expressing TNFα in parallel with the NK cells and did not observe significant differences in their TNFα expression after exposure to VZV-seropositive compared with VZV-seronegative sera (Supplementary Fig. 1).
Fig. 1. VZV-Specific ADCC measured by the NK cell production of TNFα.
Data were derived using 9 VZV-seronegative and 7 VZV-seropositive serum samples and PBMC from a single leukopack. A Gating strategy of the flow cytometry assay. B Flow cytometry results. Data points indicate the proportions of TNFα-secreting NK cells in wells coated with VZV antigen/wells coated with mock-infected control antigen. The horizontal lines indicate the means. *** indicate p = 0.001 by unpaired T test. C ELISA results. Data points represent the proportions of TNFα-secreting NK cells in wells coated with VZV antigen/wells coated with mock-infected control antigen. The horizontal lines represent the means. *** indicate p = 0.0002 by unpaired T test. D Correlation between flow cytometric and ELISA results in VZV-seropositive samples.
For the ELISA-based measure of ADCC (VZV-ADCC), we determined the amount of TNFα produced by PBMC in the conditions described above. PBMC exposed to VZV-seropositive sera generated significantly higher concentrations of TNFα in wells coated with VZV compared with wells coated with mock-infected antigen (median ratio of 4.2; Fig. 1C). In contrast, PBMC incubated with VZV-seronegative sera showed a significantly lower median ratio of 1.5 (p = 0.0006, Mann-Whitney test; Fig. 1C), indicating that the combination of VZV antibodies and VZV was essential for the PBMC production of TNFα through ADCC. Moreover, the proportion of NK cells measured by flow cytometry that produced TNFα when exposed to VZV-seropositive sera significantly correlated with the amount of TNFα measured by ELISA in the same experimental conditions (rho = 0.82; Fig. 1D), thus validating the use of the ELISA-based assay to measure ADCC.
RZV and ZVL increase VZV- and gE-ADCC in vaccine- and age-dependent fashions
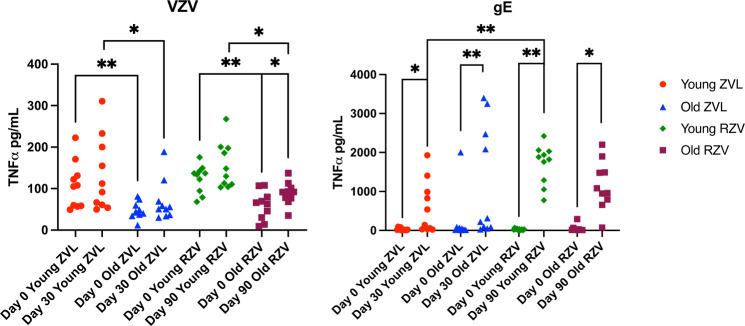
VZV-ADCC was determined using sera obtained after RZV and ZVL administration to 10 younger and 10 older vaccine recipients (Fig. 2). VZV-ADCC was higher in younger compared with older adults pre-vaccination (medians of 106 versus 43 TNFα pg/ml, p = 0.007 for ZVL; and 135 versus 65 pg/ml, p = 0.002 for RZV; Mann Whitney U test). Similar age differences were observed for peak responses after vaccination (102 versus 53 pg/ml for ZVL at 30 days after vaccination, p = 0.04; and 139 versus 92 pg/ml for RZV at 30 days after the 2nd dose of vaccine, p = 0.03). Neither ZVL nor RZV generated significant increases in VZV-ADCC in younger adults from pre- to post-vaccination. However, older ZVL recipients showed a trend toward an increase from pre-vaccination to peak response (43 versus 53 pg/ml; p = 0.08; Wilcoxon matched-pairs signed rank test), while older RZV recipients showed significant increases (65 versus 92 pg/ml; p = 0.03). The comparison of post-immunization VZV-ADCC between the two vaccines did not reveal significant differences.
Fig. 2. Effect of Zoster Vaccines on VZV-Specific ADCC.
Data were derived from 40 individuals equally distributed between young and old ZVL or RZV recipients. Shown are TNFα concentrations measured by ELISA before vaccination and at peak response to each vaccine against VZV (left panel) and gE (right panel). Bars indicate medians and quartiles. Significant differences are shown on the graph and marked by *0.01 < p < 0.05 or **0.001 < p < 0.01.
gE-ADCC prevaccination was similar across age and vaccine groups (medians of 14 to 27 pg/ml; Fig. 2). Both vaccines generated significant increases at peak response in younger and older adults with medians of 340 and 269 pg/ml in younger and older ZVL recipients, and larger medians of 1853 and 1022 pg/ml in younger and older RZV recipients. The comparison of peak responses between age groups showed no difference in ZVL recipients and a trend toward higher responses in younger versus older RZV recipients (p = 0.07, Mann-Whitney test). The comparison of responses between vaccines showed significantly higher peak responses in younger RZV recipients compared with younger ZVL recipients (p = 0.002).
RZV and ZVL preferentially increase anti-gE IgG3 compared with anti-VZV IgG3 antibodies
To define the differences between anti-gE and anti-VZV ADCC responses to vaccination, we measured the IgG subtypes composing the antibody response. IgG3 antibodies are deemed to be most effective in mediating ADCC due to their long hinge that allows optimal flexibility to accommodate both target and effector cells21. Together with IgG1 and IgG4, IgG3 is a major component of the responses to varicella (primary VZV infection), to HZ (reactivation infection), and to varicella vaccination22,23. Before and after HZ vaccination, anti-VZV and anti-gE IgG1, IgG3 and IgG4 were similar in recipients of each vaccine and both age groups (not depicted). Figure 3A shows that anti-gE IgG3 fold-rise was significantly higher than anti-VZV IgG3 fold-rise after administration of ZVL or RZV assessed by paired analysis. In contrast, gE- and VZV-specific IgG1 and IgG4 responses to vaccination did not differ in ZVL recipients. In RZV recipients IgG1 anti-gE fold-rise was significantly higher than IgG1 anti-VZV fold-rise, whereas IgG4 fold-rises against gE and VZV did not differ. The analysis of IgG3 antibody levels showed that older RZV recipients had lower peak anti-VZV responses compared to younger RZV and to older ZVL recipients (Fig. 3B). There were no significant differences in IgG3 anti-gE responses by age or vaccine group.
Fig. 3. IgG subclass antibody responses to ZVL and RZV.
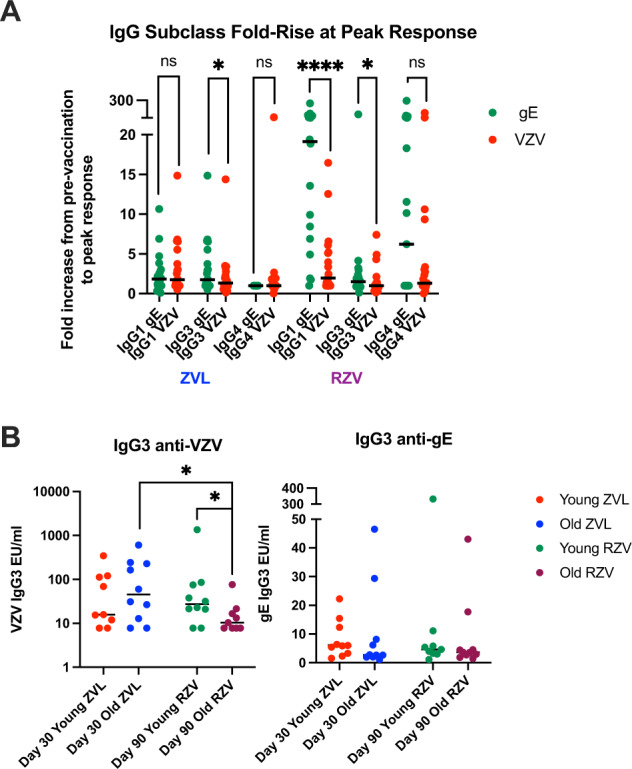
Data were derived from 20 ZVL and 19 RZV recipients. Each symbol represents a study participant. Lines represent medians. Panel A shows IgG fold-increases from pre-vaccination to peak response. Paired comparisons of IgG1, IgG3 and IgG4 anti-gE (green) and anti-VZV (red) responses measured by Wilcoxon signed rank test (nonparametric) are shown on the graph. Panel B shows IgG3 anti-VZV (left) and anti-gE (right) peak responses. Significant differences between age and vaccine groups performed by Mann-Whitney test (nonparametric) are shown on the graph. * indicates 0.01 < p < 0.05; **** indicates p < 0.001.
gE-ADCC strongly correlates with antibody avidity
To further characterize the antibody responses after HZ vaccination, we performed correlation analyses of VZV- and gE-ADCC measured by ELISA with anti-VZV and anti-gE antibody avidity, which we had measured in a previous study24. gE-ADCC strongly correlated with anti-gE antibody avidity at peak response after administration of either HZ vaccine (ρ = 0.5454, p = 0.0003; Fig. 4). Moreover, the increase from pre- to postvaccination in gE-ADCC also strongly correlated with the increase in gE avidity (ρ = 0.5392, p = 0.0003; not depicted). gE-ADCC weakly correlated with neutralizing antibody titers (ρ = 0.28, p = 0.08; Supplementary Fig. 2). In contrast, VZV-ADCC did not correlate with the avidity of anti-VZV antibodies or neutralizing titers irrespective of the type of vaccine or age.
Fig. 4. gE-ADCC correlates with anti-gE antibody avidity.

Data were derived from 40 participants who received RZV or ZVL and whose ADCC responses were measured in this study. Avidity measures were previously described24. TNFα concentrations and antibody avidity were log-transformed for this analysis to improve the distribution of the data points. The correlation coefficient and p value were calculated using Spearman correlations.
gE-ADCC is characterized by increased CD107α and IFNγ in addition to TNFα expression
To determine the functional characteristics of NK cells activated by the anti-gE antibodies, we investigated CD107a, IFNγ, and TNFα expression by flow cytometry in NK cells exposed to solid phase-bound gE incubated with sera from 9 younger and 10 older RZV recipients (Fig. 5). Before and after vaccination, gE-specific antibodies elicited CD107a expression in NK cells from both younger and older adults, but significant increases in post-vaccination responses compared to pre-vaccination responses were observed only in younger adults. RZV administration generated anti-gE antibodies in younger and older adults that stimulated NK cell IFNγ production that was not detected pre-vaccination. TNFα production in NK cells stimulated with anti-gE-specific antibodies was detected with plasma only from younger adults pre- and post-vaccination. Moreover, RZV vaccination significantly increased the ability of anti-gE antibodies to elicit NK TNFα responses in younger adults.
Fig. 5. NK Functionality after Binding to gE-Specific Antibodies in RZV Recipients.
Data were derived from 9 young and 8 older RZV recipients. Graphs show proportions of NK cells expressing the marker indicated in the title of each panel. Bars represent medians and quartiles. Significant differences are shown on the graph and marked by *0.01 < p < 0.05; **0.001 < p < 0.01; or ***p < 0.001.
Discussion
ADCC is a potential mechanism of protection against viral infections, including influenza, dengue, HIV and SARS CoV225–29. We showed that administration of RZV and ZVL to adults ≥50 years of age increased ADCC elicited by antibodies against VZV lysate and gE. This was the first study of ADCC antibodies generated by HZ vaccines. Previously, studies in children with varicella detected ADCC antibodies, which preceded the appearance of neutralizing antibodies in the blood compartment, suggesting a role for ADCC in viral clearance30. After varicella vaccination ADCC antibodies were also observed in children who never developed neutralizing antibodies30.
The clinical significance of ADCC antibodies was clearly shown after RV144 vaccination for HIV, where the levels of antibodies directed toward the anti-HIV V3 loop that mediated ADCC were associated with protection31. ADCC antibodies are also elicited by influenza vaccines, but their protective role has not been established32,33. ADCC antibody responses to HZ vaccines likely play a role in protection against HZ by clearing infected cells. The comparison of RZV and ZVL showed that both vaccines increased ADCC antibodies. However, significant increases in anti-VZV ADCC antibodies post-vaccination were found only in older RZV recipients. In addition, in young adults, RZV generated higher anti-gE ADCC antibodies than did ZVL, suggesting greater ability of RZV to generate ADCC antibodies.
A tantalizing observation was the higher increase in anti-gE than anti-VZV ADCC antibodies, both after ZVL and RZV compared with pre-vaccination levels. Since IgG3, due to its long Fc hinge, is the main IgG subtype that mediates ADCC, we hypothesized that anti-gE antibodies contained higher proportions of IgG3 compared to total anti-VZV antibodies21. This was a novel hypothesis since the IgG subclass response to HZ vaccines had not been described. Our experiments showed that both HZ vaccines generated higher increases in anti-gE IgG3 antibodies than anti-VZV IgG3 antibodies. This difference was unique to IgG3 and did not extend to IgG1 or IgG4. We do not know if there are clinical consequences of the difference in anti-gE and anti-VZV ADCC antibodies. However, it is conceivable that this property of the anti-gE antibodies contributes to the superior protection conferred by RZV34,35. We previously showed that RZV generates superior cell-mediated immunity, neutralizing antibody titers, and antibody avidity compared to ZVL and here we add that greater amounts of ADCC antibodies are generated after RZV36,37.
Another novel observation in this study was the positive correlation between the magnitude and relative increase of gE-ADCC and anti-gE antibody avidity after vaccination. This observation expands our previous findings that anti-gE antibody avidity correlated with anti-VZV neutralizing antibodies24. Collectively, these data underscore the critical contribution of the physical characteristics of the antibodies to their functionality.
Interestingly, we did not observe appreciable associations of VZV-ADCC with anti-VZV antibody avidity. Moreover, the increase in VZV-ADCC or anti-VZV antibody avidity post-vaccination was more modest. There are several potential explanations for this phenomenon, which are not mutually exclusive: (1) before vaccination, VZV-ADCC, similar to avidity were already close to peak, whereas gE-ADCC and avidity were below their peak capacity to expand in response to vaccination; (2) gE contains more ADCC antibody binding sites per molecule than the average contained in VZV glycoproteins; and (3) the purified recombinant gE preparation used in our assays exposed antibody binding sites better than the inactivated whole virus VZV preparation.
We analyzed the effect of age on ADCC antibodies because older age is associated with decreased efficacy of many vaccines, including ZVL19. Although previous studies showed similar total IgG responses to HZ vaccines in younger and older adults, we observed a significant effect of age on ADCC, consisting of lower levels of VZV-ADCC antibodies in older compared with younger adults pre- and post-administration of either HZ vaccine. Thus, older age had similar effects on ADCC and vaccine efficacy. The investigation of IgG3 anti-VZV or anti-gE responses between younger and older adults pre-vaccination did not reveal any differences, while post-vaccination only RZV recipients showed higher levels of anti-VZV IgG3 in younger than older adults. Thus, the levels of IgG3 did not correlate with the difference in ADCC magnitude between younger and older vaccine recipients. Additional information about the physicochemical properties of the antibodies generated by the vaccines in different age groups may be required to define the age effect on ADCC. For example, the pattern of antibody glycosylation was shown to play a role in ADCC, whereby afucosylated antibodies were most likely to elicit ADCC38. Adjuvants may also affect the pattern of antibody glycosylation generated by vaccines39. In addition, our observations should be expanded with studies of other vaccines to determine if lower ADCC responses to vaccination contribute to the lower efficacy of vaccines in older than younger adults.
Our study was limited by the relatively low number of participants for the comparison of responses in younger versus older adults. Because chronological and biological age may differ in the geriatric population, hundreds of participants may be necessary to tease out the effect of age. The strength of our study was that participants were randomized to ZVL and RZV in the parent study. We also developed a new ELISA assay for ADCC based on the correlation with NK activation examined by flow cytometry. This assay is technically easier to perform and is more sensitive than the flow cytometry assay.
In conclusion, both zoster vaccines generated robust anti-gE antibodies, including IgG3. The increase in gE-ADCC post-vaccination may contribute to the protection conferred by ZVL and RZV in all age groups.
Methods
Study participants
For assay development, we used a convenience sample of sera with known VZV-seropositive or negative status. The effect of vaccination was evaluated using archived samples from 40 participants in a previously published study (NCT02114333)20. The study was approved by the Colorado Multiple Institution Review Board. All participants signed informed consent. All participants had prior varicella or had resided in the US at least 30 years, and none had prior HZ. Twenty participants were 50–59 years old; 10 received ZVL and 10 received RZV. The remaining 20 participants were 70–85 years old and were also equally divided between ZVL or RZV recipients. We used samples collected before vaccination in all participants, 30 days after ZVL administration, and 30 days after the second dose of RZV (day 90).
Antibody-dependent cellular cytotoxicity (ADCC) measured by flow cytometry
Adapted from Gonzalez-Gonzalez et al., Immulon II 96-microwell ELISA plates were coated overnight either with VZV antigen obtained from VZV Oka strain-infected human lung fibroblasts at a multiplicity of infection of 1, harvested and UV-irradiated after 4 days of culture (VZV); similarly treated mock-infected lung fibroblasts (mock) diluted 1:20 in PBS; recombinant gE glycoprotein at 1ug/ml in PBS (rgE; GlaxoSmithKline); or PBS control. After overnight incubation at 4 °C, plates were washed and blocked with 5% fetal calf serum in PBS for 2 h at room temperature. Plasma or serum samples at the pre-optimized dilution of 1:100 in PBS were added at 100 µl/well to duplicate wells for 2 h at room temperature and plates were washed with PBS containing 0.5% Tween. PBMC from a Leukopack dedicated to this research were added at a preoptimized concentration of 200,000 cells/well in 200 µl of RPMI 1640 (Corning) containing 10% fetal bovine serum (FBS; Gemini Bio-Products), 1% glutamine (Gemini Bio-Products), 1% penicillin-streptomycin (Gemini Bio-Products), and HEPES buffer (Corning). After overnight incubation at 37 °C in a humidified 5% CO2 atmosphere, Brefeldin A (MilliporeSigma, 5 μg/ml), Monensin (MilliporeSigma, 5 μg/ml), and anti-CD107a (Ax488; Clone H4A3; Biolegend 328610; 1:10 dilution) were added for the last 4 hours. At the end of the incubation, PBMCs were removed, washed and stained with Zombie Yellow Viability Stain (BioLegend 423104; 1:100 dilution); antibodies against CD3 (PE-CF594; Clone UCHT1; BD 557943; 1:33 dilution), CD19 (PE-CF594; HIB19; BD 562294; 1:50 dilution), CD14 (PE-CF594; Clone MφP9; BD 562335; 1:33 dilution), granzyme B (PerCP-Cy5.5; Clone QA16A02; Biolegend 372211; 1:25 dilution), CD56 (PE-Cy7; Clone HCD56; Biolegend 318318; 1:50 dilution), CD16 (Ax700; Clone 3G8; BD 557920; 1:25 dilution); HLA-DR (APC-H7; Clone G46-6; BD 561358; 1:50 dilution), CD107a (Alexa 488; Clone H4A3; Biolegend 328610; 1:10 dilution), and perforin (BV421; Clone dG9; Biolegend 308122; 1:50 dilution). Then cells were fixed with FACS Lysing Solution (BD) and permeabilized with Permeabilizing Solution 2 (BD) and intracellular staining was performed with antibodies against granzyme B (PerCP-Cy5.5; Clone QA16A02; Biolegend 372212; 1:50 dilution), TNFα (APC, Clone Mab11; BD 551384; 1:50 dilution), perforin (BV421; Clone dG9; Biolegend 308122; 1:50 dilution) and INFγ (PE; Clone P2G10; BD 559812; 1:25 dilution). PBMC (≥200,000 events) were acquired with the Gallios instrument (Beckman Coulter). Analysis used FlowJo (Becton Dickinson) software. Gating strategy to identify activated NK cells by expression of TNFα in the ADCC assay is shown in Fig. 1A.
ADCC assay using ELISA-measured TNFα production
The assay was performed as previously described with modifications40,41. PBMC stimulation was performed in Immulon II plates coated with relevant antigens as described above, at 50,000 cells/well. After overnight incubation cells were lysed with 1% v/v TritonX-100 solution in 150 mM NaCl and 20 mM Tris 7.5. Supernatants were collected and assayed for the presence of TNFα using a commercial ELISA kit as per manufacturer’s instructions (R&D Systems, Minneapolis, MN).
IgG subclass characterization
96-well strips (Immulon 2HB; Thermo) were coated with 100μL gE at 1μg/mL (courtesy of GlaxoSmithKline) or VZV Oka strain lysate at 1:40. Plates were incubated at 4 °C overnight with 500 rpm shaking. Wells were washed 4x with PBS and blocked with 200μL PBS/.05% Tween-20/5% FBS for 2 hours. Wells were washed 4x with PBS/.05% Tween-20. Plasma samples were diluted in PBS/.05% Tween-20/5% FBS at the following preoptimized ratios: IgG1 assay 1:100; IgG3 assay 1:10; IgG4 assay 1:50. 100 μL sample/well was incubated for 2 hours at room temperature on a shaker at 500 rpm. After washing wells 4x, the following detection antibodies diluted in PBS/.05% Tween-20/5% FBS were added: Mouse anti-human IgG1 hinge-AP (Southern Biotech) 1:2000; Mouse anti-human IgG3 hinge-AP (Southern Biotech) 1:500; Mouse anti-human IgG4 Fc-AP (Southern Biotech) 1:1000. Wells were washed 4x and 100 μL pNPP 1-component AP substrate (ImmunoChemistry Technologies) were added. Color development was read at 405 nm on a MultiSkan FC (Thermo) using SkanIt software. Results were interpolated using Prism (Graph Pad).
Statistical analysis
Statistical analyses and graphs were performed using Graphpad Prism 9 (GraphPad Software Inc., La Jolla, CA). Analyses were performed using nonparametric tests. A two-tailed P value of <0.05 defined statistical significance.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This study was funded by GSK Investigator Initiated Study Program (contract 63007497 to AW) and by the NIAID grant 1U01AI141919-01 to AW. Dr. Park received support from the National Research Foundation of Korea (grant no. NRF-2017R1C1B2003336) and from the Dongguk University Research fund of 2019.
Author contributions
A.W. designed the study, oversaw the development of laboratory assays, analyzed the data, and wrote the manuscript; S.Y.P., M.J.J., and J.C. developed and executed assays, analyzed the data, and contributed to manuscript preparation; M.J.L. contributed to study design and manuscript preparation; and DSS oversaw development of anti-gE ELISA. All authors reviewed and approved the manuscript.
Data availability
The datasets generated and analyzed in this study will be made available to other investigators following submission and approval of a Material Transfer Agreement by the University of Colorado Tech Transfer Office.
Competing interests
A.W. receives grants from G.S.K. and Merck and personal fees from Merck and Seqirus. M.J.L. received research grants from G.S.K., Merck, Moderna, and Saol Therapeutics (moneys to University of Colorado School of Medicine) and personal fees for Advisory Boards for Merck, G.S.K., Pfizer, and Curevo). S.Y.P., J.C., M.J. and D.S.S. declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41541-022-00545-2.
References
- 1.Zerboni L, Sen N, Oliver SL, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nat. Rev. Microbiol. 2014;12:197–210. doi: 10.1038/nrmicro3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin MJ, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J. Infect. Dis. 2008;197:825–835. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvin A. Aging, immunity, and the varicella-zoster virus. N. Engl. J. Med. 2005;352:2266–2267. doi: 10.1056/NEJMp058091. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg A, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J. Infect. Dis. 2009;200:1068–1077. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvin AM. Humoral and cellular immunity to varicella-zoster virus: an overview. J. Infect. Dis. 2008;197:S58–S60. doi: 10.1086/522123. [DOI] [PubMed] [Google Scholar]
- 6.Asada H. VZV-specific cell-mediated immunity, but not humoral immunity, correlates inversely with the incidence of herpes zoster and the severity of skin symptoms and zoster-associated pain: The SHEZ study. Vaccine. 2019;37:6776–6781. doi: 10.1016/j.vaccine.2019.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert PB, et al. Fold rise in antibody titers by measured by glycoprotein-based enzyme-linked immunosorbent assay is an excellent correlate of protection for a herpes zoster vaccine, demonstrated via the vaccine efficacy curve. J. Infect. Dis. 2014;210:1573–1581. doi: 10.1093/infdis/jiu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura H, et al. Comparison of quantitations of viral load in varicella and zoster. J. Clin. Microbiol. 2000;38:2447–2449. doi: 10.1128/JCM.38.6.2447-2449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satyaprakash AK, et al. Viremia in acute herpes zoster. J. Infect. Dis. 2009;200:26–32. doi: 10.1086/599381. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez JE, Moninger T, Grose C. Entry and egress of varicella virus blocked by same anti-gH monoclonal antibody. Virology. 1993;196:840–844. doi: 10.1006/viro.1993.1543. [DOI] [PubMed] [Google Scholar]
- 11.Etzioni A, et al. Fatal varicella associated with selective natural killer cell deficiency. J. Pediatr. 2005;146:423–425. doi: 10.1016/j.jpeds.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Levy O, et al. Disseminated varicella infection due to the vaccine strain of varicella-zoster virus, in a patient with a novel deficiency in natural killer T cells. J. Infect. Dis. 2003;188:948–953. doi: 10.1086/378503. [DOI] [PubMed] [Google Scholar]
- 13.Levin MJ, Duchon JM, Swamy GK, Gershon AA. Varicella zoster immune globulin (VARIZIG) administration up to 10 days after varicella exposure in pregnant women, immunocompromised participants, and infants: Varicella outcomes and safety results from a large, open-label, expanded-access program. PLoS One. 2019;14:e0217749. doi: 10.1371/journal.pone.0217749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaia JA, et al. Evaluation of varicella-zoster immune globulin: protection of immunosuppressed children after household exposure to varicella. J. Infect. Dis. 1983;147:737–743. doi: 10.1093/infdis/147.4.737. [DOI] [PubMed] [Google Scholar]
- 15.Tilden AB, et al. Demonstration of NK cell-mediated lysis of varicella-zoster virus (VZV)-infected cells: characterization of the effector cells. J. Immunol. 1986;136:4243–4248. [PubMed] [Google Scholar]
- 16.Babbage J, Sigfusson A, Souhami RL. Antibody-dependent cell-mediated cytotoxicity to Varicella zoster. Clin. Exp. Immunol. 1984;58:217–222. [PMC free article] [PubMed] [Google Scholar]
- 17.Ito M, Ihara T, Grose C, Starr S. Human leukocytes kill varicella-zoster virus-infected fibroblasts in the presence of murine monoclonal antibodies to virus-specific glycoproteins. J. Virol. 1985;54:98–103. doi: 10.1128/jvi.54.1.98-103.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gershon AA, Steinberg SP. Inactivation of varicella zoster virus in vitro: effect of leukocytes and specific antibody. Infect. Immun. 1981;33:507–511. doi: 10.1128/iai.33.2.507-511.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin MJ, Weinberg A. Adjuvanted Recombinant Glycoprotein E Herpes Zoster Vaccine. Clin. Infect. Dis. 2020;70:1509–1515. doi: 10.1093/cid/ciz770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinberg A, et al. Comparative Immune Responses to Licensed Herpes Zoster Vaccines. J. Infect. Dis. 2018;218:S81–S87. doi: 10.1093/infdis/jiy383. [DOI] [PubMed] [Google Scholar]
- 21.Damelang T, Rogerson SJ, Kent SJ, Chung AW. Role of IgG3 in Infectious Diseases. Trends Immunol. 2019;40:197–211. doi: 10.1016/j.it.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Asano Y, et al. Immunoglobulin Subclass Antibodies to Varicefla-Zoster Virus. Pediatrics. 1987;80:933–936. doi: 10.1542/peds.80.6.933. [DOI] [PubMed] [Google Scholar]
- 23.Echevarr, et al. Subclass Distribution of the Serum and Intrathecal IgG Antibody Response in Varicella-Zoster Virus Infections. J. Infect. Dis. 1990;162:621–626. doi: 10.1093/infdis/162.3.621. [DOI] [PubMed] [Google Scholar]
- 24.Coignard J, et al. A case-only study to identify genetic modifiers of breast cancer risk for BRCA1/BRCA2 mutation carriers. Nat. Commun. 2021;12:1078. doi: 10.1038/s41467-020-20496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson SI, et al. IgG3 enhances neutralization potency and Fc effector function of an HIV V2-specific broadly neutralizing antibody. PLoS Pathog. 2019;15:e1008064. doi: 10.1371/journal.ppat.1008064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamin R, et al. Fc-engineered antibody therapeutics with improved anti-SARS-CoV-2 efficacy. Nature. 2021;599:465–470. doi: 10.1038/s41586-021-04017-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong W, et al. Antibody-Dependent Cell-Mediated Cytotoxicity to Hemagglutinin of Influenza A Viruses After Influenza Vaccination in Humans. Open Forum Infect. Dis. 2016;3:ofw102. doi: 10.1093/ofid/ofw102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He W, et al. Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus. Proc. Natl Acad. Sci. USA. 2016;113:11931–11936. doi: 10.1073/pnas.1609316113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun P, et al. NK cell degranulation as a marker for measuring antibody-dependent cytotoxicity in neutralizing and non-neutralizing human sera from dengue patients. J. Immunol. Methods. 2017;441:24–30. doi: 10.1016/j.jim.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Ihara T, et al. Antibody response determined with antibody-dependent cell-mediated cytotoxicity (ADCC), neutralizing antibody, and varicella skin test in children with natural varicella and after varicella immunization. Acta Paediatr. Jpn. 1991;33:43–49. doi: 10.1111/j.1442-200X.1991.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 31.Bonsignori M, et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J. Virol. 2012;86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jegaskanda S, et al. Generation and Protective Ability of Influenza Virus-Specific Antibody-Dependent Cellular Cytotoxicity in Humans Elicited by Vaccination, Natural Infection, and Experimental Challenge. J. Infect. Dis. 2016;214:945–952. doi: 10.1093/infdis/jiw262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jegaskanda S, Reading PC, Kent SJ. Influenza-specific antibody-dependent cellular cytotoxicity: toward a universal influenza vaccine. J. Immunol. 2014;193:469–475. doi: 10.4049/jimmunol.1400432. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham AL, et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N. Engl. J. Med. 2016;375:1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 35.Lal H, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 2015;372:2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 36.Levin MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J. Clin. Invest. 2018;128:4429–4440. doi: 10.1172/JCI121484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmid DS, et al. Comparative Antibody Responses to the Live-Attenuated and Recombinant Herpes Zoster Vaccines. J. Virol. 2021;95:e00240–00221. doi: 10.1128/JVI.00240-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu LL, et al. A functional role for antibodies in tuberculosis. Cell. 2016;167:e414. doi: 10.1016/j.cell.2016.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alter G, Ottenhoff THM, Joosten SA. Antibody glycosylation in inflammation, disease and vaccination. Semin. Immunol. 2018;39:102–110. doi: 10.1016/j.smim.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez-Gonzalez E, et al. Validation of an ADCC assay using human primary natural killer cells to evaluate biotherapeutic products bearing an Fc region. J. Immunol. Methods. 2019;464:87–94. doi: 10.1016/j.jim.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Tan CS, Ghofrani J, Geiger E, Koralnik IJ, Jost S. Brief Report: Decreased JC Virus-Specific Antibody-Dependent Cellular Cytotoxicity in HIV-Seropositive PML Survivors. J. Acquir Immune Defic. Syndr. 2019;82:220–224. doi: 10.1097/QAI.0000000000002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed in this study will be made available to other investigators following submission and approval of a Material Transfer Agreement by the University of Colorado Tech Transfer Office.